Abstract
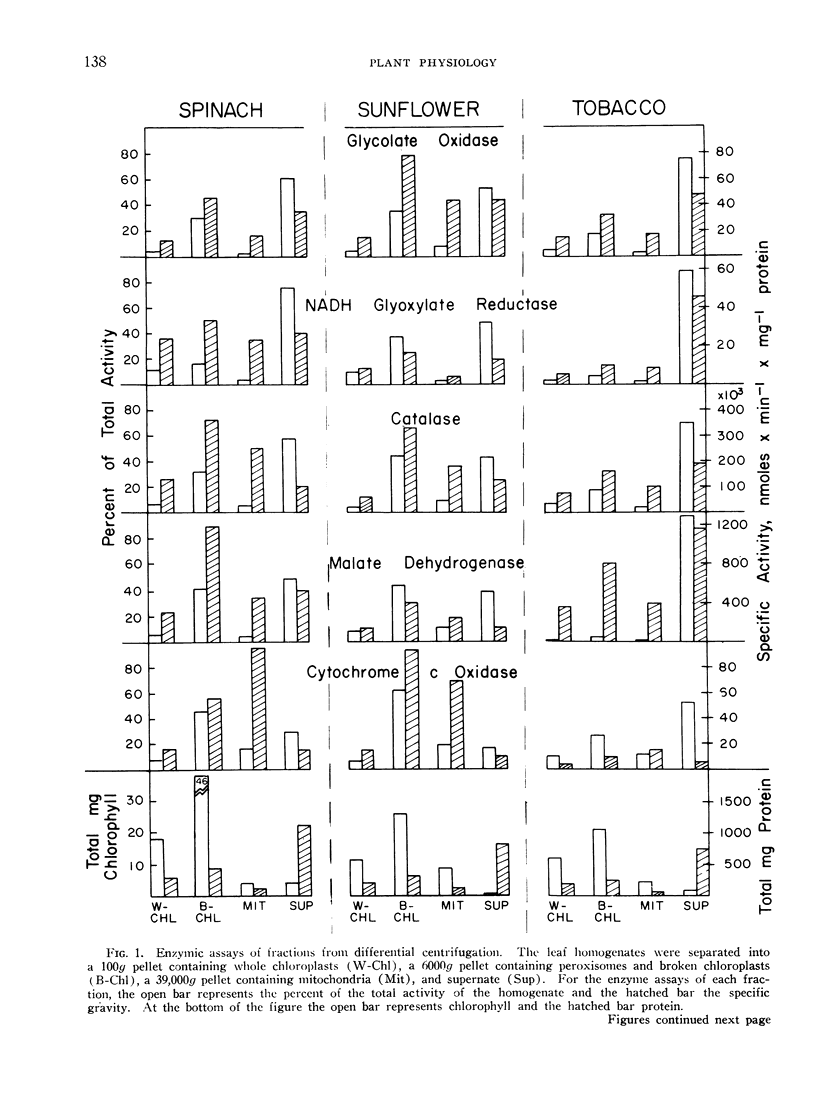
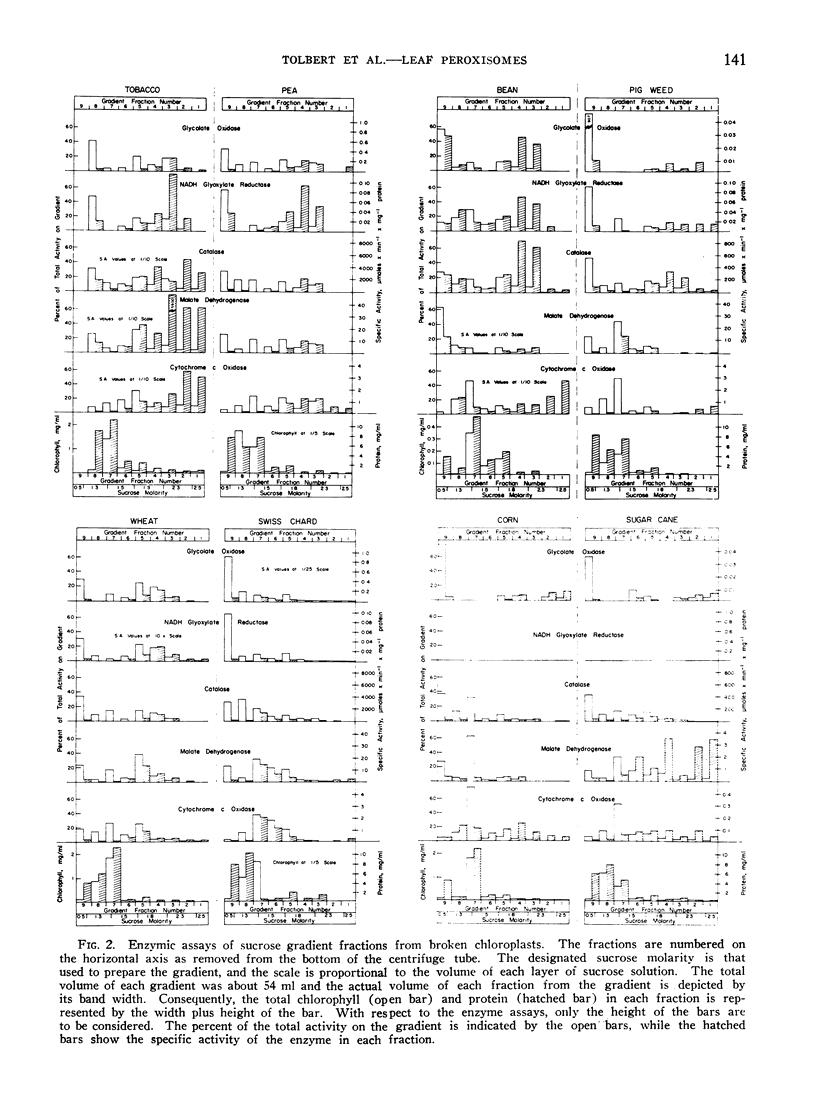
Leaves of 10 plant species, 7 with photorespiration (spinach, sunflower, tobacco, pea, wheat, bean, and Swiss chard) and 3 without photorespiration (corn, sugarcane, and pigweed), were surveyed for peroxisomes. The distribution pattern for glycolate oxidase, glyoxylate reductase, catalase, and part of the malate dehydrogenase indicated that these enzymes exist together in this organelle. The peroxisomes were isolated at the interface between layers of 1.8 to 2.3 m sucrose by isopycnic nonlinear sucrose density gradient centrifugation or in 1.95 m sucrose on a linear gradient. Chloroplasts, located by chlorophyll, and mitochondria by cytochrome c oxidase, were in 1.3 to 1.8 m sucrose.
In leaf homogenates from the first 7 species with photorespiration, glycolate oxidase activity ranged from 0.5 to 1.5 μmoles × min−1 × g−1 wet weight or a specific activity of 0.02 to 0.05 μmole × min−1 × mg−1 protein. Glyoxylate reductase activity was comparable with glycolate oxidase. Catalase activity in the homogenates ranged from 4000 to 12,000 μmoles × min−1 × g−1 wet weight or 90 to 300 μmoles × min−1 × mg−1 protein. Specific activities of malate dehydrogenase and cytochrome oxidase are also reported. In contrast, homogenates of corn and sugarcane leaves, without photorespiration, had 2 to 5% as much glycolate oxidase, glyoxylate reductase, and catalase activity. These amounts of activity, though lower than in plants with photorespiration, are, nevertheless, substantial.
Peroxisomes were detected in leaf homogenates of all plants tested; however, significant yields were obtained only from the first 5 species mentioned above. From spinach and sunflower leaves, a maximum of about 50% of the marker enzyme activities was found to be in these microbodies after homogenization. The specific activity for peroxisomal glycolate oxidase and glyoxylate reductase was about 1 μmole × min−1 × mg−1 protein; for catalase. 8000 μmoles × min−1 × mg−1 protein, and for malate dehydrogenase, 40 μmoles × min−1 × mg−1 protein. Only small to trace amounts of marker enzymes for leaf peroxisomes were recovered on the sucrose gradients from the last 5 species of plants. Bean leaves, with photorespiration, had large amounts of these enzymes (0.57 μmole of glycolate oxidase × min−1 × g−1 tissue) in the soluble fraction, but only traces of activity in the peroxisomal fraction. Low peroxisome recovery from certain plants was attributed to particle fragility or loss of protein as well as to small numbers of particles in such plants as corn and sugarcane.
Homogenates of pigweed leaves (no photorespiration) contained from one-third to one-half the activity of the glycolate pathway enzymes as found in comparable preparations from spinach leaves which exhibit photorespiration. However, only traces of peroxisomal enzymes were separated by sucrose gradient centrifugation of particles from pigweed. Data from pigweed on the absence of photorespiration yet abundance of enzymes associated with glycolate metabolism is inconsistent with current hypotheses about the mechanism of photorespiration.
Most of the catalase and part of the malate dehydrogenase activity was located in the peroxisomes. Contrary to previous reports, the chloroplast fractions from plants with photo-respiration did not contain a concentration of these 2 enzymes, after removal of peroxisomes by isopycnic sucrose gradient centrifugation.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- Downton W. J., Tregunna E. B. Photorespiration and Glycolate Metabolism: A Re-examination and Correlation of Some Previous Studies. Plant Physiol. 1968 Jun;43(6):923–929. doi: 10.1104/pp.43.6.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester M. L., Krotkov G., Nelson C. D. Effect of Oxygen on Photosynthesis, Photorespiration and Respiration in Detached Leaves. II. Corn and other Monocotyledons. Plant Physiol. 1966 Mar;41(3):428–431. doi: 10.1104/pp.41.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester M. L., Krotkov G., Nelson C. D. Effect of oxygen on photosynthesis, photorespiration and respiration in detached leaves. I. Soybean. Plant Physiol. 1966 Mar;41(3):422–427. doi: 10.1104/pp.41.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R., Johnson H. S. Further studies on a new pathway of photosynthetic carbon dioxide fixation in sugar-cane and its occurrence in other plant species. Biochem J. 1967 Feb;102(2):417–422. doi: 10.1042/bj1020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. Photosynthesis by sugar-cane leaves. A new carboxylation reaction and the pathway of sugar formation. Biochem J. 1966 Oct;101(1):103–111. doi: 10.1042/bj1010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe P. A., Tregunna E. B. Effect of Temperature, CO(2) Concentration, and Light Intensity on Oxygen Inhibition of Photosynthesis in Wheat Leaves. Plant Physiol. 1968 Jun;43(6):902–906. doi: 10.1104/pp.43.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. N. High activity of the glycolic Acid oxidase system in tobacco leaves. Plant Physiol. 1967 Oct;42(10):1463–1464. doi: 10.1104/pp.42.10.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll C. R., Burris R. H. Nature and Distribution of Glycolic Acid Oxidase in Plants. Plant Physiol. 1954 May;29(3):261–265. doi: 10.1104/pp.29.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIERPOINT W. S. Mitochondrial preparations from the leaves of tobacco (Nicotiana tabacum). 4. Separation of some components by density-gradient centrifuging. Biochem J. 1962 Jan;82:143–148. doi: 10.1042/bj0820143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABSON R., TOLBERTNE, KEARNEY P. C. Formation of serine and glyceric acid by the glycolate pathway. Arch Biochem Biophys. 1962 Jul;98:154–163. doi: 10.1016/0003-9861(62)90161-3. [DOI] [PubMed] [Google Scholar]
- Rocha V., Mukerji S. K., Ting I. P. Chloroplast-malic dehydrogenase: a new malic dehydrogenase isozyme from spinach. Biochem Biophys Res Commun. 1968 Jun 28;31(6):890–894. doi: 10.1016/0006-291x(68)90535-4. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Hatch M. D. Comparative studies on the activity of carboxylases and other enzymes in relation to the new pathway of photosynthetic carbon dioxide fixation in tropical grasses. Biochem J. 1967 Jun;103(3):660–665. doi: 10.1042/bj1030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOLBERT N. E., BURRIS R. H. Light activation of the plant enzyme which oxidizes glycolic acid. J Biol Chem. 1950 Oct;186(2):791–804. [PubMed] [Google Scholar]
- TOLBERT N. E., COHAN M. S. Activation of glycolic acid oxidase in plants. J Biol Chem. 1953 Oct;204(2):639–648. [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]
- Tregunna B. Flavin mononucleotide control of glycolic Acid oxidase and photorespiration in corn leaves. Science. 1966 Mar 11;151(3715):1239–1241. doi: 10.1126/science.151.3715.1239. [DOI] [PubMed] [Google Scholar]
- ZELITCH I., OCHOA S. Oxidation and reduction of glycolic and glyoxylic acids in plants. I. Glycolic and oxidase. J Biol Chem. 1953 Apr;201(2):707–718. [PubMed] [Google Scholar]
- ZELITCH I. The isolation and action of crystalline glyoxylic acid reductase from tobacco leaves. J Biol Chem. 1955 Oct;216(2):553–575. [PubMed] [Google Scholar]
- ZELITCH I. The role of glycolic acid oxidase in the respiration of leaves. J Biol Chem. 1958 Dec;233(6):1299–1303. [PubMed] [Google Scholar]
- Zelitch I. Increased rate of net photosynthetic carbon dioxide uptake caused by the inhibition of glycolate oxidase. Plant Physiol. 1966 Dec;41(10):1623–1631. doi: 10.1104/pp.41.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]



