Since the classical, many years ignored, and by current standards haphazard experiments of Sidney Ringer on isolated rat hearts,1 we have come a long way in understanding the role of Ca2+ in the contraction of cardiac muscle. Ringer found that suspending the hearts in a saline solution prepared with tap water (which contained high amounts of calcium carbonate from limestone) sustained robust contractions for a long time; but in an attempt to professionalize his art, Ringer replaced tap water for distilled water, only to observe that in this “clean” medium, heart contractions declined quickly after only a few beats. By systematically adding different salts to the distilled saline medium, Ringer discovered that calcium, until then considered exclusively a structural element of bones and teeth, was essential for cardiac muscle contraction. Since this serendipitous discovery, many others, in smaller or greater scale, kept adding to the inescapable notion that calcium ions (Ca2+) play a critical role as a relay signal (a messenger) in many biological processes not only of cardiac myocytes, but of virtually every living cell. Continuing with the story of Ca2+ in the heart, technically challenging experiments by Alexander Fabiato defined, almost singlehandedly, the process of Ca2+-induced Ca2+ release (CICR) in cardiac cells, whereby a small amount of Ca2+ (in his case injected by a microsyringe on skinned cardiac tissue) caused a much larger release of Ca2+ from the sarcoplasmic reticulum (SR), inducing vigorous contractions.2 Fabiato’s experiments, therefore, set the basis for a functional coupling between the sarcolemma (and its invaginations, the t-tubules) which “injected” Ca2+ by the voltage-induced opening of L-type Ca2+ channels (DHPRs) and the SR, which elicited massive Ca2+ release upon binding of the incoming Ca2+ to ryanodine receptors (RyRs). Electron microscopy analysis of frozen skeletal and cardiac microsections, mainly the work of Clara Franzini-Armstrong and colleagues (see, for example, ref. 3), painstakingly reconstructed the structural arrangement of DHPRs and RyRs and helped define the microarchitecture of triads in skeletal muscle (SR-t tubule-SR apposition) and dyads in cardiac muscle (SR separated from t-tubule by a tiny gap of ~15–20 nm) in a mesoscopic scale. The concept of “couplon” was logically derived from these functional and structural interactions,4 and reaffirmed the association of voltage sensors in t-tubules (DHPRs) with Ca2+ release channels in the junctional SR (RyRs) in an inseparable functional unit. Thus, in an interesting saga from tap water to couplons (and many other intermediate steps omitted here for lack of space) the initial question of Ringer (What ions are necessary for heart contractions?) has been refined to other questions such as those involving precise, nanoscale interactions between DHPRs and RyRs, the elusive Ca2+ gradient resulting from their almost simultaneous opening, the process quenching the regenerative nature of CICR, the all-or-none versus graded recruitment of RyRs in a single dyad during normal e-c coupling, etc.
The sophistication of the current questions in e-c coupling would have not been possible without the recording of intracellular Ca2+ signals in cardiac cells. The first visualization of intracellular Ca2+ transients was reported by Allen and Blinks in aequorin-injected frog cardiac muscle.5 The results were groundbreaking and revealed with fair approximation the cytosolic Ca2+ gradients achieved during single contractions for the first time. However, aequorin, a ~22 kDa Ca2+-sensitive chemiluminescent protein, is membrane impermeable and uses coelenterazine, which is irreversibly consumed to produce light, hence necessitating continuous addition of fresh protein into the media. These technical difficulties complicated the use of aequorin and the widespread application of this technique never materialized. The arrival in 1985 of BAPTA-based Ca2+ indicators with capacity to permeate membranes, high Ca2+ affinity and fast kinetics6 made intracellular Ca2+ measurements the mainstay of many laboratories. Only two years after the introduction of Fura-2, Cannell et al.7 not only determined the magnitude of the Ca2+ transients in patch-clamped rat cardiomyocytes, but also their voltage-[Ca2+]i relationship and the resting (diastolic) [Ca2+], establishing for the first time some of the most critical parameters of e-c coupling and revealing voltage ranges for maximal DHPR/RyR coupling efficiency. Finally, the introduction of fluorescein- and rhodamine-based Ca2+ indicators of high dynamic range and the advent of low-cost versatile confocal microscopes greatly facilitated the discovery of “Ca2+ sparks”, the localized, transient, and presumably elemental Ca2+ signaling events first detected in ventricular myocytes by Cheng et al.8 Initially, Ca2+ sparks were believed to emanate from the opening of a single or a few RyR channels, but later studies pinpointed their origin to a cluster of RyRs, perhaps all those present in a single dyad. Although a single Ca2+ spark is an all-or-none or quantal event (but see below), recruitment of variable numbers of Ca2+ sparks allows for graded global Ca2+ release and hence, contraction. Thus, the study of Ca2+ sparks provided direct evidence to the local control theory of e-c coupling9 and helped resolve the conundrum pertaining to the high-gain, regenerative nature of CICR that predicted an all-or-none (instead of graded) Ca2+ release upon cell depolarization. Fluo-3, the Ca2+ dye mostly used to detect Ca2+ sparks, displays fast Ca2+ association and dissociation kinetics (700 µM s−1 and 369 s−1, respectively) and could, in principle, return information on the Ca2+ dynamics of the dyadic cleft, but its fast diffusion coefficient distorts the spatial profile of the dyadic Ca2+ gradient, allows spatial blurring due to out-of-focus sampling, and precludes accurate estimation of the local peak Ca2+ level. Similarly, the use of intracellular solutions containing a fast, low-affinity Ca2+ indicator (such as Oregon Green 488 BAPTA 5N) and a slow, high-affinity Ca2+ buffer (EGTA) allows for detection of spatially-restricted Ca2+ signals (“Ca2+ spikes”)10 that approximate the waveform of Ca2+ release flux in a dyad but, owing to the high [EGTA] and the diffusion of the Ca2+ indicator as mentioned above, this method also fails to return accurate information on the magnitude of the dyadic Ca2+ gradient.
In this issue of Circulation Research, Shang et al.11 made clever use of a non-diffusible, dyad-targeted Ca2+ biosensor to shed light, literally, on the Ca2+ dynamics that occur in the nanodomain of the dyadic cleft. The authors used GCaMP6f, a genetically-encoded Ca2+ indicator composed of circularly permuted enhanced green fluorescent protein (cpEGFP) coupled to the Ca2+-sensing protein calmodulin (CaM) and to a CaM-binding peptide (the M13 fragment of myosin light chain kinase),12 and fused it to the N-terminal of triadin (T) or junctin (J), two proteins that traffic to the junctional SR (jSR) and apparently interact with the RyR. GCaMP6f is itself of bigger mass than triadin or junctin (~32 and ~26 KDa for the most common cardiac isoforms, respectively, see Fig. 1) and is remarkable that junctin and triadin correctly target to the jSR despite such disproportionate cargo. Nevertheless, rat cardiac myocytes transfected with GCaMP6f-T/J display punctate fluorescence that partially overlaps with Di-4, an external membrane-bound dye, and completely merges with RyR2 fluorescence, as expected if GCaMP6f-T/J was correctly trafficked to the jSR. In intact cells, the GCaMP6f-T/J fluorescence is spatially fixed, does not appear to interfere with normal Ca2+ signaling, and yields Ca2+ transients that are ~50 times smaller in volume than customary Ca2+ sparks. Because these signals presumably arise from the nanodomain pertaining to a single dyadic cleft, the authors dubbed them “Ca2+ nanosparks”.11
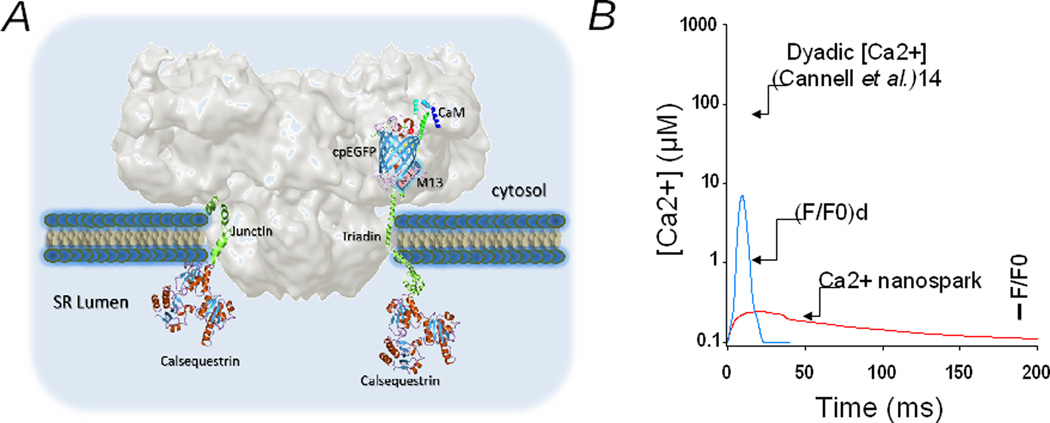
Figure 1. Ca2+ nanospark: molecules that detect it, and its relationship to other dyadic Ca2+ parameters.
(A) Approximate three-dimensional relationship between CaM-cpEGFP-M13 peptide (GCaMP6f), triadin and the RyR. The faded gray structure is the cryo-EM surface representation of the RyR1 protein at 10 Å resolution (courtesy of M. Samsó). Triadin and junctin were generated by Song et al.16 using homology modeling. (B) Estimated temporal relationship between the Ca2+ nanospark, the deconvolved Ca2+ nanospark signal (F/F0)d, and the estimated local Ca2+ gradient at the dyadic cleft (used with permission from Cannell et al.14). See text for details.
It is pertinent to remark some attributes of GCaMP6f-T/J and its Ca2+ transient to fully appreciate what the term “Ca2+ nanospark” really defines. Because of the multiple steps involved in fluorescence generation upon Ca2+ binding, GCaMPs display slow response kinetics (τon = 20 ms – 1.4 s)13 compared with BAPTA-based indicators (τon = <1 ms). GCaMP6f is one of the fastest GCaMPs and it was first used in neurons,12 where it faithfully tracked single synapse events that occurred in the sub-second time scale. Here Shang et al.11 found that GCaMP6f-T/J fusion to triadin increased its off rate ~4 fold compared to native GCaMP6f, to 17 s−1. Still, the on and off rates of GCaMP6f-T/J appear too slow for the rapidly rising and fast-decaying Ca2+ gradient that has been inferred by mathematical modeling for dyadic clefts of several animal species (see for example, ref 14). Upon “injection” of a few Ca2+ ions into the dyadic cleft by DHPRs, RyRs almost instantly open (τon ≤ 1 ms)15 generating CICR and recruiting additional RyRs within the couplon. The merging of Ca2+ influx (ICa) and SR Ca2+ release generates local Ca2+ gradients that peak in ~5 ms, persists for ~15 ms, and reach levels upwards of 100 µmol/L.14 In notorious disparity, calibration of GCaMP6f-T/J in situ yielded a Ca2+ dissociation constant (Kd) = 0.63 µmol/L, clearly too high an affinity for the peak Ca2+ gradient of the dyadic cleft. Thus, GCaMP6f-T/J, although correctly targeted and probably monitoring Ca2+ fluxes from the nano-vicinity of RyRs, appears too slow and too avid for Ca2+ to accurately report the fast Ca2+ gradient that occurs in a typical dyadic cleft. As a consequence of its slow kinetics, GCaMP6f-T/J acts as a low-pass filter, severely attenuating the amplitude of the peak Ca2+ gradient (Fig. 1). Therefore, the most defining features of these Ca2+ nanosparks are their reduced volume and their spatial immobility, but they should not be used to surmise on the magnitude of the dyadic Ca2+ gradient, one of the most elusive of the e-c coupling parameters of current times.
How much farther will the Ca2+ nanosparks take the e-c coupling field? Are we witnessing a breakthrough of proportions akin to those of Allen & Blinks5 and Cannell et al.,7 who introduced Ca2+ imaging to a field that had relied on electrical signals to infer Ca2+ movements, or Cheng et al.,8 who ushered in an era of Ca2+ microdomains and took e-c coupling to the level of single couplons? Only time will tell. But even now, some advances are evident and need not wait for the verdict of time. By making straightforward assumptions on its on and off kinetics that allowed for deconvolution of its raw signal, Shang et al.11 obtained fair estimates of the Ca2+ fluxes that occur in the dyad, which are in turn a fair approximation of the RyR channels’ open time. In essence, then, this new information is telling us for how long a dyad is activated, which had not been possible using diffusible Ca2+ indicators. Also, by virtue of the biosensor’s spatial confinement, researchers will now be able to infer when and where a dyad is activated and, since GCaMP6f-T/J does not appear to interfere with normal Ca2+ signaling, these parameters may be obtained even in contracting cells. Perhaps more importantly, information derived from these signals is already challenging long-established dogmas of e-c coupling: if the Ca2+ nanosparks truly represent Ca2+ signals from single dyads, then different amplitudes (or “substructures”)11 within a single Ca2+ nanospark may indeed represent different RyR clusters opening asynchronously (as postulated by the authors), which would not be expected from current local control models of couplon activation. Thus, there is uncontested merit in this novel approach. Although Ca2+ nanosparks do not report junctional Ca2+ levels with accuracy, neither do Ca2+ sparks report local cytoplasmic levels faithfully, yet, the latter have revolutionized our understanding of e-c coupling in fundamental ways.
Supplementary Material
Acknowledgments
Sources of funding: HHV is a recipient of National Institutes of Health grants RO1-HL055438 and PO1 HL094291. Y-TZ has no grant support.
Footnotes
Conflict of interest statement: Y-TZ and HHV declare that they have no conflict of interest.
REFERENCES
- 1.Ringer S. A further Contribution regarding the influence of the different Constituents of the Blood on the Contraction of the Heart. J Physiol. 1883;4(1):29–42.3. doi: 10.1113/jphysiol.1883.sp000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- 3.Franzini-Armstrong C, Protasi F, Ramesh V. Comparative ultrastructure of Ca2+ release units in skeletal and cardiac muscle. Ann NY Acad Sci. 1998;853:20–30. doi: 10.1111/j.1749-6632.1998.tb08253.x. [DOI] [PubMed] [Google Scholar]
- 4.Stern MD, Pizarro G, Ríos E. Local control model of excitation-contraction coupling in skeletal muscle. J Gen Physiol. 1997;110(4):415–440. doi: 10.1085/jgp.110.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen DG, Blinks JR. Calcium transients in aequorin-injected frog cardiac muscle. Nature. 1978;273:509–513. doi: 10.1038/273509a0. [DOI] [PubMed] [Google Scholar]
- 6.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260(6):3440–3450. [PubMed] [Google Scholar]
- 7.Cannell MB, Berlin JR, Lederer WJ. Intracellular calcium in cardiac myocytes: Calcium transients measured using fluorescence imaging. Soc Gen Physiol series. 1987;42:201–214. [PubMed] [Google Scholar]
- 8.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: Elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 9.Stern MD. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992;63:497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song LS, Sham JS, Stern MD, Lakatta EG, Cheng H. Direct measurement of SR release flux by tracking “Ca2+ spikes” in rat cardiac myocytes. J Physiol. 1998;512:677–691. doi: 10.1111/j.1469-7793.1998.677bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shang W, Lu F, Sun T, Xu J, Li LL, Wang Y, Wang G, Chen L, Wang X, Cannell MB, Wang SQ, Cheng H. Imaging Ca2+ Nanosparks in Heart with a New Targeted Biosensor. Circ Res. 2014;114:xxx–xxx. doi: 10.1161/CIRCRESAHA.114.302938. [in this issue]. [DOI] [PubMed] [Google Scholar]
- 12.Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun XR, Badura A, Pacheco DA, Lynch LA, Schneider ER, Taylor MP, Hogue IB, Enquist LW, Murthy M, Wang SS. Fast GCaMPs for improved tracking of neuronal activity. Nat Commun. 2013;4:2170. doi: 10.1038/ncomms3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cannell MB, Kong CH, Imtiaz MS, Laver DR. Control of sarcoplasmic reticulum Ca2+ release by stochastic RyR gating within a 3D model of the cardiac dyad and importance of induction decay for CICR termination. Biophys J. 2013;104(10):2149–2159. doi: 10.1016/j.bpj.2013.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gyorke S, Fill M. Ryanodine receptor adaptation: control mechanism of Ca2+-induced Ca2+ release in heart. Science. 1993;260:807–809. doi: 10.1126/science.8387229. [DOI] [PubMed] [Google Scholar]
- 16.Song DW, Lee JG, Youn HS, et al. Ryanodine receptor assembly: a novel systems biology approach to 3D mapping. Prog Biophys Mol Biol. 2011;105(3):145–161. doi: 10.1016/j.pbiomolbio.2010.09.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.