Abstract
The aim of the current study was to evaluate the effectiveness of three nonthermal light technologies (NUV-Vis, continuous UV, and HILP) on their ability to inactivate Escherichia coli K12 and Listeria innocua. E. coli K12 was selected as a representative microorganism for the enterohaemorrhagic foodborne pathogen E. coli O157:H7 and L. innocua as a surrogate microorganism for the common foodborne pathogen Listeria monocytogenes, respectively. The liquid matrix used for the disinfection experiments was a liquid matrix (MRD solution). The results of the present study show that the HILP treatment inactivated both E. coli and L. innocua more rapidly and effectively than either continuous UV-C or NUV-vis treatment. With HILP at 2.5 cm from the lamp, E. coli and L. innocua populations were reduced by 3.07 and 3.77 log10 CFU/mL, respectively, after a 5 sec treatment time, and were shown to be below the limit of detection (<0.22 log10 CFU/mL) following 30 sec exposure to HILP (106.2 J/cm2). These studies demonstrate the bactericidal efficacy of alternative nonthermal light technologies and their potential as decontamination strategies in the food industry.
1. Introduction
In recent years, nonthermal technologies have shown potential as alternatives to conventional pasteurization, with scope for inactivating pathogens and spoilage microorganisms without any of the adverse effects on product quality associated with thermal treatments such as reduced nutritional value or altered sensory attributes [1, 2].
Some pathogenic bacteria such as Listeria monocytogenes and other psychrotrophic bacteria can grow at low temperatures, threatening public health and shortening the shelf life of raw foods [3]. Many outbreaks associated with fresh ready-to-eat produce have been reported previously with E. coli, Listeria, and Salmonella identified as implicated pathogens [3–8].
Currently fresh produce, fruit, and vegetables, are washed with aqueous sanitizers such as chlorine, hydrogen peroxide, and trisodium phosphate in order to reduce the microbial load of fresh produce. However, the use of aqueous sanitizers alone has not been successful in controlling foodborne pathogens [9] and treatment of produce with chlorine has adverse effects, such as formation of trihalomethanes [10, 11]. Organic acids, mainly citric, lactic, and acetic acid, which are in GRAS (Generally Recognized As Safe) status, have been also used as disinfectants because of their bactericidal activity [12]. Hydrogen peroxide (H2O2), also referred to as hydrogen dioxide, has also been used as bleaching agent due to its strong oxidizing power [12]. As a result there is a need for the development of additional effective hurdles for these raw foods which can eliminate or significantly reduce microbial contamination, be environmentally friendly while not impacting on the quality of foods [4]. A range of nonthermal technologies (Ultrasound, UV-C, Ozone, and HHP) have already been successfully implemented on a number of ready-to-eat fruits and vegetables [11, 13–17].
NUV-vis light 395 ± 5 nm is a safe, non-UV based decontamination technology which is thought to act by stimulating endogenous microbial porphyrin molecules to produce oxidizing reactive oxygen species (ROS), predominantly singlet oxygen (1O2) that damages cells leading to microbial death [18–21]. Exposure of microorganisms to visible light particularly at wavelengths of 405 nm, has been shown to be effective in inactivating a range of bacteria, including Gram- positive and Gram-negative bacterial species and antibiotic-resistant microorganisms such as Methicillin-resistant Staphylococcus aureus, and its use has been suggested for a range of decontamination applications [22–26].
The inactivation mechanism of UV light is the formation of photoproducts in the DNA of target microorganisms. Of these photoproducts, the most important is the pyrimidine dimer which forms between adjacent pyrimidine molecules on the same strand of DNA and can interrupt both DNA transcription and translation [27]. The DNA damage inflicted by UV-C radiation leads to cell death by altering the microbial DNA through dimer formation between neighbouring pyrimidine nucleoside bases in the same DNA strand [28, 29].
High-intensity light pulses (HILP) is a nonthermal technology which uses short (100–400 μs) high-power pulses of broad-spectrum (200–1100 nm) and has been used to inactivate bacteria (vegetative cells and spores), yeasts, moulds, and even viruses [30, 31]. The mode of action of HILP on microorganisms is likely the photochemical action of the UV-C part of the light spectrum that causes thymine dimerization in the DNA chain preventing replication and ultimately leading to cell death [2, 32–34]. Microbial inactivation using HILP has gained attention in recent years due to lower energy consumption compared to conventional thermal processes [35]. Depending on the energy delivered through each flash, the distance between the lamps and the contaminated matrix, the targeted microorganism, and even the nature of the contaminated matrix itself, HILP has been reported to result in a 0.5 to 8 log10 CFU/mL bacterial reduction [36]. The germicidal action of HILP has been also attributed to the localized elevated temperature due to the UVs and IR radiations leading to bacterial disruption [33, 37–40].
The objective of the present work was to evaluate the effectiveness of three nonthermal light technologies (NUV-vis, Continuous UV, and HILP) to reduce microbial populations in a liquid matrix. Different treatment intensities and times were selected in order to investigate the inactivation capacity of each light technology on one Gram-negative (E. coli K12) and one Gram- positive bacteria (L. innocua NCTC 11288). E. coli K12 and L. innocua were selected as surrogate organisms for Escherichia coli O157:H7 and Listeria monocytogenes, respectively [2]. To the authors' knowledge, this is the first paper where all these three nonthermal light technologies were compared for their ability to inactivate possible foodborne pathogens.
2. Material and Methods
2.1. Microorganisms and Culture Preparation
Experiments were conducted using E. coli K12 (DSM 1607) and L. innocua (NCTC 11288). The strains were maintained at 4°C on Tryptone Soya Agar, TSA (Oxoid, Hampshire, UK). For inoculation of the model solutions, cultures of E. coli or L. innocua grown overnight at 37°C in Tryptone Soya Broth, TSB (Oxoid), were used. The 24 h cultures were then centrifuged for 10 min at 30,000 ×g and the resulting pellets were washed and centrifuged twice in Maximum Recovery Diluent (MRD, Oxoid) before being mixed together by resuspending in a final volume of 10 mL MRD. This resulted in mixed culture cell suspensions of ~108 colony forming units per milliliter (CFU/mL). The suspensions containing both E. coli and L. innocua inoculates were assessed for susceptibility to three light technologies in a liquid matrix (MRD). Mixed pure cultures (in MRD) were prepared as described previously. Samples (10 mL) were then placed into Petri dishes (50 mm diameter). They were then positioned at different distances from the lamp source. After removal of covers, Petri dishes containing the MRD solutions were subjected to different light doses ranging from 0.18 to 106.2 J/cm2.
2.2. UV Equipment
2.2.1. NUV-Vis Light Unit
The NUV-vis light was produced by a light-emitting diode (LED) array (OD-2049) (Opto Diode Corp, sourced from AP Technologies, Bath, UK) with a central wavelength of 395 ± 5 nm, a bandwidth of 12 nm full-width at half maximum (FWHM), and a half intensity beam angle of 30° [40]. The irradiance (J∗cm−2) of light emitted from the LED unit was measured using a UV-VIS Radiometer (model no. RM12, Dr. Gröbel UV Electronik, GmbH, Ettlington, Germany) fitted with a RM UV-A sensor (part no. 811030, Dr. Gröbel UV Electronik). Distances of 3, 12, and 23 cm from the light source were chosen for treatments. The corresponding energy intensities and time needed to achieve them are presented in Table 1. These distances represented the most extreme to the least extreme treatments according to the study of Haughton et al. [41]. Construction of the LED unit was as previously described [41]. Sample temperatures were measured during the treatment using a K-type thermocouple attached to a Grant Data Logger (Squirrel 2040; Grant Instruments) to ensure that the maximum temperature reached was nonlethal to the bacteria under the treatment times investigated (<50°C) (Figure 6).
Table 1.
Calculated exposure time (sec) of nonthermal light technologies at selected distances from the light source.
| Dose per treatment (J/cm2) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.18 | 0.36 | 0.72 | 1.44 | 2.832 | 6 | 17.7 | 27 | 36 | 54 | 106.2 | |||
| Distance from light source (cm) | 3 | NUV-VIS | 6 | 11 | 22 | 45 | 88 | 186 | 548 | 836 | 1115 | ∗ | ∗ |
| 12 | 28 | 55 | 110 | 221 | 435 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ||
| 23 | 149 | 298 | 595 | 1190 | 2341 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ||
| 6.5 | UV | 30 | 60 | 120 | 240 | 472 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | |
| 17 | 36 | 72 | 144 | 288 | 566 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ||
| 28.5 | 45 | 90 | 180 | 360 | 708 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ||
| 2.5 | HILP | NT | NT | NT | NT | 0.8 | NT | 5 | NT | NT | NT | 30 | |
| 8 | 0.1 | 0.2 | 0.4 | 0.8 | NT | NT | NT | NT | NT | 30 | NT | ||
| 11.5 | NT | 0.3 | 0.6 | NT | NT | 5 | NT | NT | 30 | NT | NT | ||
| 14 | 0.2 | 0.4 | 0.8 | NT | NT | NT | NT | 30 | NT | NT | NT | ||
NUV-Vis: near UV-vis light; UV: ultraviolet light; HILP: high-intensity light pulses.
(∗) Samples that are not analyzed due to high temperature, NT: not tested samples.
Distance from light source (cm).
HILP was applied in pulses 360 μs duration at a frequency of 3 Hz.
Figure 6.
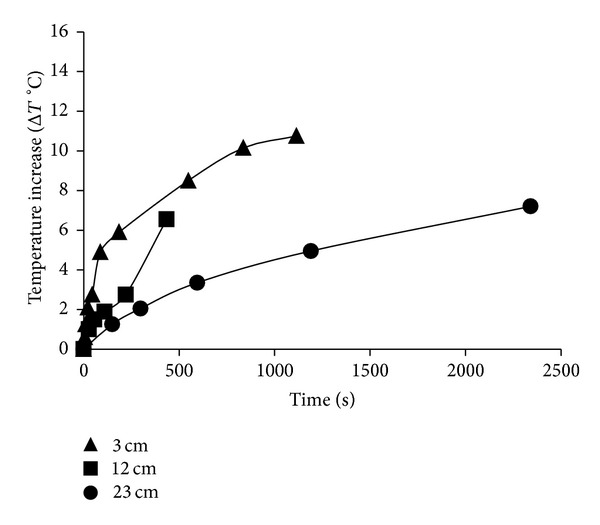
Mean Temperature increase (ΔT°C) for NUV-vis light technology at distances.
2.2.2. Continuous UV Equipment
The UV unit was a custom-made unit with intimal dimensions (length × width × height) of 790 × 390 × 345 mm and consisting of four 95-W bulbs (Baro Applied Technology Limited, Athens, Greece) 500 mm in length. The UV dose (D) was calculated by using the following equation:
| (1) |
where D is the dose (J/cm2), I 254 nm is the dosage rate, and t is the retention time (in seconds). The UV dosages (J/cm2) were varied by altering the distance of the sample (6.5, 17, and 28.5 cm) from the light source and by changing the treatment time (Table 1). Sample temperatures were measured during the treatment using a K-type thermocouple attached to a Grant Data Logger (Squirrel 2040; Grant Instruments) to ensure that the maximum temperature reached was nonlethal to the bacteria under the treatment times investigated (<50°C) (Figure 7).
Figure 7.
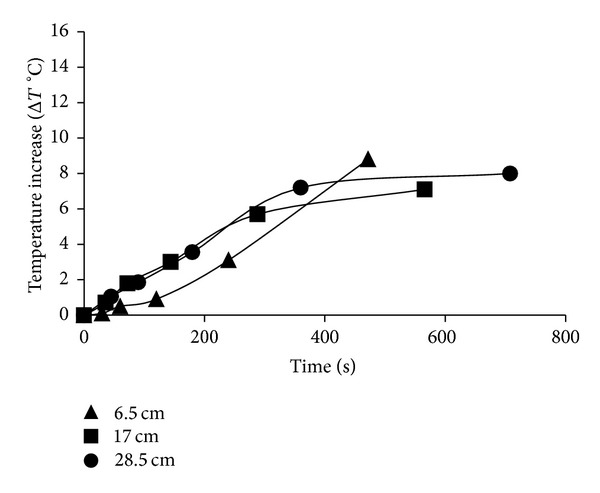
Mean Temperature increase (ΔT°C) for UV light technology at distances.
2.2.3. HILP (High-Intensity Light Pulses) Unit
The HILP unit was a benchtop SteriPulse-XL system (Xenon, USA). The system comprised a high-energy pulsed ultraviolet-visible flash lamp (Type C, 190 nm spectral cut-off point) delivering a maximum of 1.27 J/cm2. The pulse width produced was 360 μs at a fixed pulse rate of 3 Hz. The pulse energy delivered to the sample varied depending on its distance from the quartz window within the HILP chamber. The HILP dose of treatments applied in the present study was calculated in accordance with the manufacturer's instructions [42]. Distances of 2.5, 8, 11.5, and 14 cm were selected for treatments, in order to achieve a wide spectrum of dosages varying between 0.18 and 106.2 J/cm2. The corresponding dosages and time needed to achieve them are presented in Table 1. During HILP treatment, samples were placed in an iced bath to minimize heating. Sample temperatures were measured during the treatment using a K-type thermocouple attached to a Grant Data Logger (Squirrel 2040; Grant Instruments, Cambridge, UK) to ensure that the maximum temperature reached was nonlethal to the bacteria under the treatment times investigated (<50°C) (Figure 8).
Figure 8.
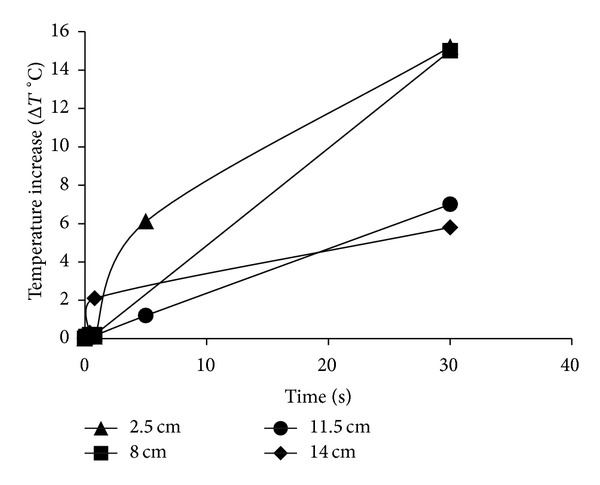
Mean Temperature increase (ΔT°C) for HILP light technology at distances.
2.3. Microbiological Analysis
After treatment of liquid samples, the contents of each Petri dish were transferred to sterile containers. Tenfold dilution series were prepared in MRD and 0.1 mL of each dilution was pour plated in duplicate using TBX (CM0945, Oxoid) for E. coli and Listeria Selective Agar (Oxford formulation, CM0856, Oxoid) for L. innocua. The plates were incubated at 44°C and 37°C for 24 and 48 h, respectively. Mean counts for each treatment were calculated and converted to log10 CFU/mL values with results for surviving numbers of microorganisms in MRD expressed per mL (CFU/mL). The plates were then used to enumerate viable cells in untreated controls and in samples following processing. The survival of bacterial cells following illumination was monitored by counting their viable number after exposure of the suspended bacteria to light. Bacterial cultures grown under the same conditions but without light exposure served as controls. The results were expressed as the logarithmic reduction (log10N/N 0), where N 0 is the initial microbial load and N the number remaining after treatment. All experiments were repeated at least three times.
2.4. Statistical Analysis
All experiments were carried out in triplicate. During each experiment two samples were taken at any time to conduct microbial counts. The microbiological data were analyzed in terms of log10(N/N 0), where N is the microorganism load at a given time, and N 0 corresponds to the initial microbial load of untreated samples. The data for inactivation of E. coli and L. innocua by NUV-vis light, continuous UV and HILP were analyzed for statistical significance using SPSS 21.0 (SPSS Inc., Chicago, USA). Results were compared by an analysis of variance followed by Tukey's pairwise comparison of the means with significance defined at the P < 0.05 level. Moreover, Pearson coefficient was used for measuring correlation between values.
3. Results
3.1. Inactivation Using NUV-Vis 395 ± 5 nm
The inactivation rate of E. coli and L. innocua was dose dependent. Generally, it was observed that as the distance from the lamp was increased, the time needed for inactivation was longer (Figure 1). Corresponding doses delivered by this method are illustrated in Table 1.
Figure 1.
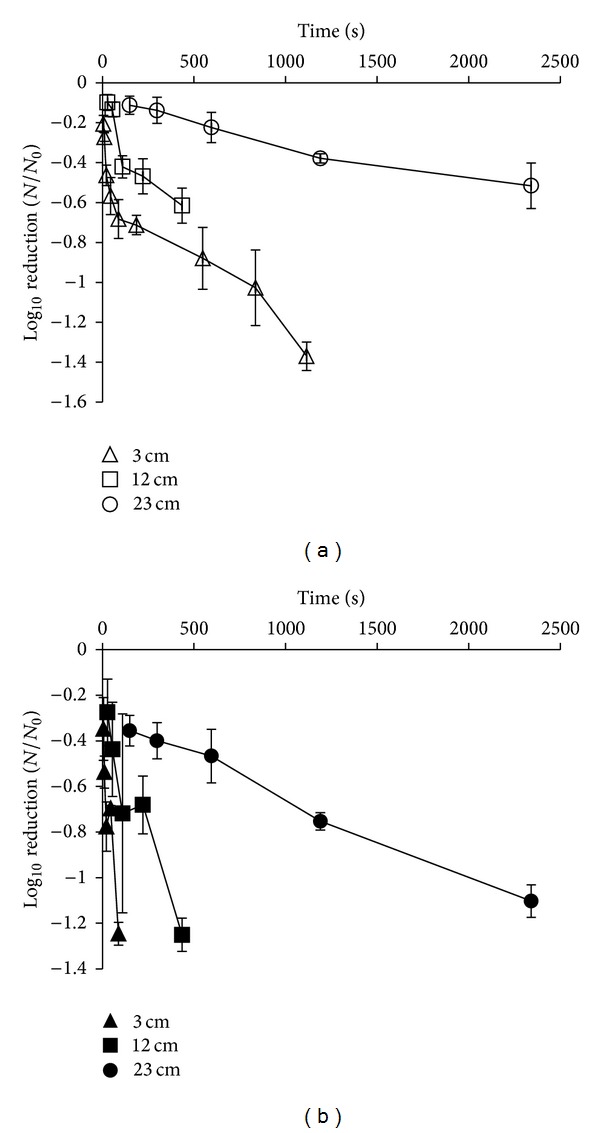
Survival curves of E. coli suspended in maximum recovery diluent (MRD) placed at 3 cm (∆), 12 cm (□), and 23 cm (○) and L. innocua placed at 3 cm (▲), 12 cm (■), and 23 cm (●) from the high-intensity near ultraviolet/visible (NUV-vis) 395 ± 5 nm light source (results expressed as mean log10 CFU/mL).
When low dosages were implemented (0.18, 0.36, 0.72, and 1.44 J/cm2), the observed inactivation rates were similar for both E. coli and L. innocua (P > 0.05). However, when a higher dose of 2.832 J/cm2 was delivered, L. innocua exhibited a higher log reduction (1.25 log10 CFU/mL) compared to E. coli (0.68 log10 CFU/mL) after 88 sec of treatment (P < 0.05), around 2 times the inactivation log of the more resistant bacterium of E. coli. Moreover, when high dosages were achieved, the inactivation rates of L. innocua remained significantly higher with a maximum average log10 CFU/mL reduction of 2.74 achieved after 1115 sec of treatment, compared to that of E. coli where the maximum average log reduction after the same time was 1.37 log10 CFU/mL (P < 0.05). Moreover, the log reduction achieved for L. innocua remained higher than that of E. coli after the longest exposure time. At 23 cm from the light source, a higher susceptibility was observed for the L. innocua strain, giving a log reduction at the highest dose (2.832 J/cm2) of 1.10 log10 CFU/mL, significantly greater (P > 0.05) than the corresponding reduction for E. coli (0.52 log10 CFU/mL reduction). It has to be mentioned that temperatures remained below 50°C for all treatments used in the study.
3.2. Inactivation Using Continuous UV Light
There was an increase between dosage and log10 CFU/mL reduction of both microorganisms. Reductions of 2.66 log10 CFU/mL and 3.04 log10 CFU/mL were achieved for E. coli and L. innocua, respectively. The highest reductions were achieved at the shortest distance from the lamp (6.5 cm) and at the shortest treatment time (472 sec) (Figure 2) when a dose of 2.832 J/cm2 was implemented (Table 1). However, the susceptibility of two microorganisms when this light technology was used was not significantly different (P = 0.749). Temperatures remained below 50°C for all treatments used in the study.
Figure 2.
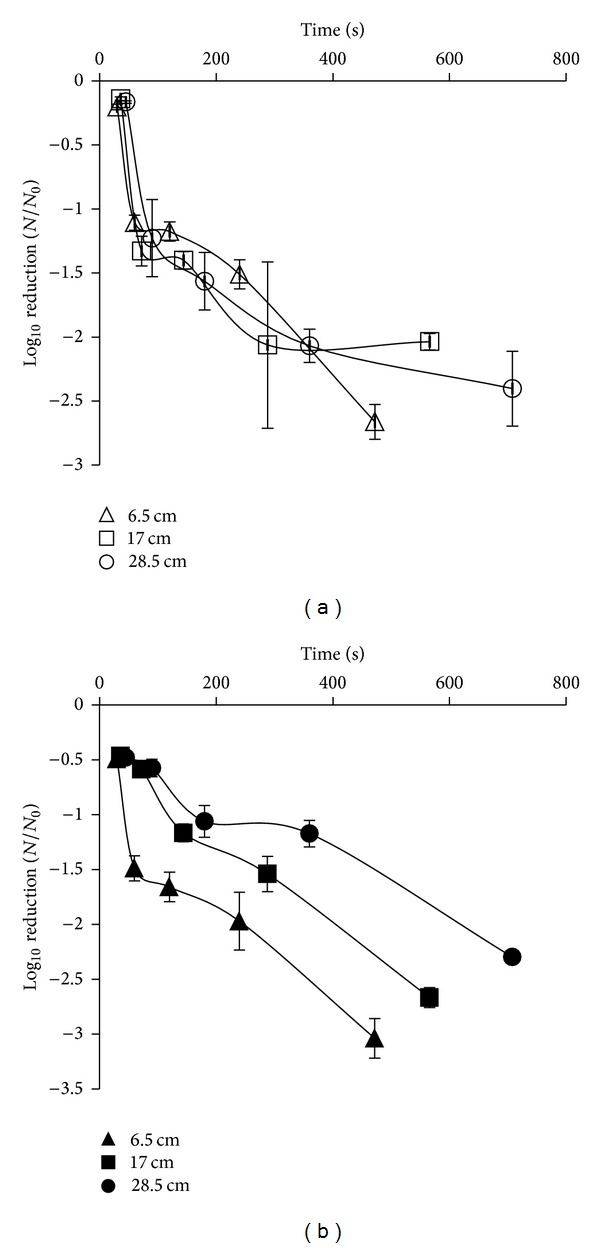
Survival curves of E. coli suspended in maximum recovery diluent (MRD) placed at 6.5 cm (∆), 17 cm (□), and 28.5 cm (○) and L. innocua placed at 6.5 cm (▲), 17 cm (■), and 28.5 cm (●) from continuous UV light source (results expressed as mean log10 CFU/mL).
3.3. Inactivation Using HILP
The measured dosages delivered with this light method and the time needed to achieve them is illustrated in Table 1. In general, increased dosage resulted in the greater reductions for both E. coli and L. innocua. The least susceptible microorganism was E. coli (Figure 3). A dosage of 17.7 J/cm2 resulted in log reductions of E. coli and L. innocua populations (3.07 and 3.77 log10 CFU/mL, resp.) and were both below the limit of detection (<0.22 log10 CFU/mL) following exposure to HILP at 106.2 J/cm2. When a dosage of 54 J/cm2 was implemented, reductions of 4.81 and 5.56 log10 CFU/mL were achieved for E. coli and L. innocua, respectively. At a dosage of 36 J/cm2, a degree of variation was observed between the two tested microorganisms. For example, E. coli was reduced by 3.85 log10 CFU/mL, whereas L. innocua was reduced by 5.30 log10 CFU/mL (P < 0.05). The susceptibility of two microorganisms when this light technology was used was significantly different (P < 0.05). Temperatures did not exceed 50°C for any of the HILP treatments used in the current study.
Figure 3.
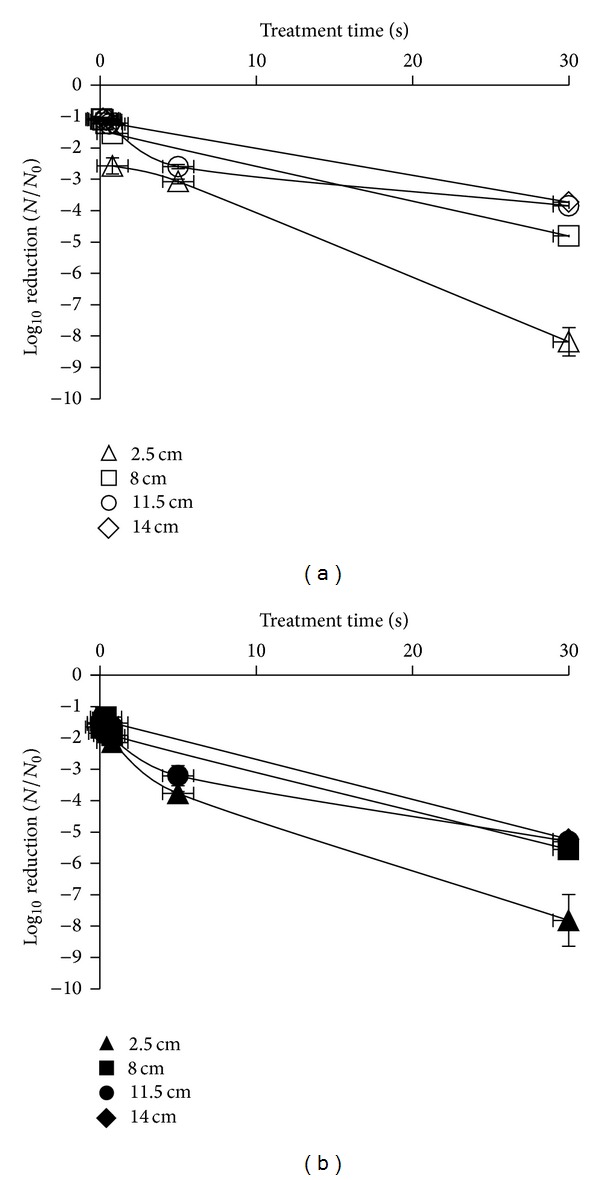
Survival curves of E. coli suspended in maximum recovery diluent (MRD) placed at 2.5 cm (∆), 8 cm (□), 11.5 cm (○), and 14 cm (◊) and L. innocua placed at 2.5 cm (▲), 8 cm (■), 11.5 cm (●), and 14 cm (♦) from HILP source (results expressed as mean log10 CFU/mL).
3.4. Comparisons between Three Light Technologies
The three light technologies were tested for their disinfection capacity on two microorganisms (Figures 4 and 5). At low dosages (0.18, 0.36, and 0.72 J/cm2) the results between the two microorganisms, when the three light technologies were used, were all significant (P < 0.05). When 1.44 J/cm2 was implemented, the log10 (CFU/mL) reduction at NUV-vis light and continuous UV light for both microorganisms was significant (P < 0.05), whereas when comparisons with HILP light were done, the differences between the susceptibility of the tested microorganisms did not differ (P > 0.05). When 2.832 J/cm2 was implemented in both continuous UV light technology and HILP, the disinfection efficiency of E. coli and L. innocua did not differ significantly (P = 0.306 and P = 0.116, resp.). Finally, at higher dosages, the correlations between log10 (CFU/mL) reduction of NUV-vis and PUV, as the two organisms are concerned, were all significant (P < 0.05). Generally, it was observed that in all light technologies (NUV-vis, continuous UV, and HILP) a significant correlation (P < 0.05) between doses and microbes log reduction existed (Figures 4 and 5).
Figure 4.
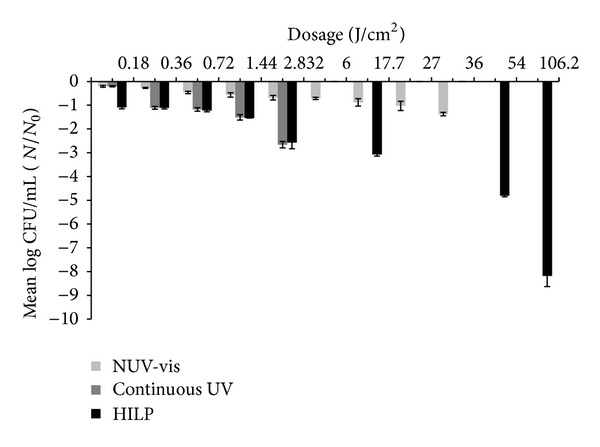
Mean log10 CFU/mL E. coli on MRD after treatment at the same dosages at shortest distance with 3 different light equipment.
Figure 5.
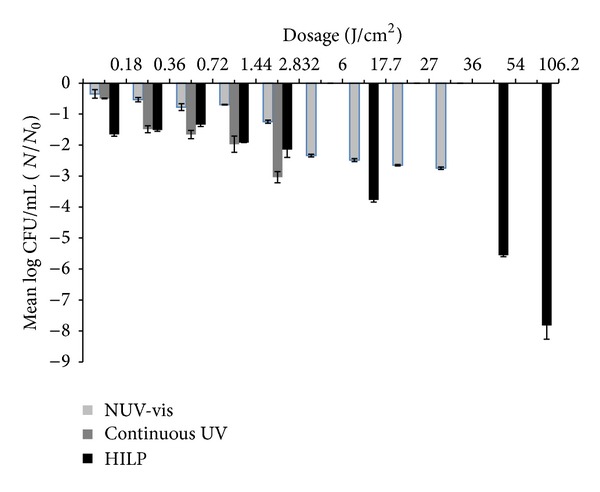
Mean log10 CFU/mL L. innocua on MRD after treatment at the same dosages at shortest distance with 3 different light equipment.
4. Discussion
The current study demonstrated that both E. coli and L. innocua are susceptible to all three light technologies investigated. Previous studies have investigated the lethal effects of high-intensity ultraviolet 405 nm light on Escherichia, Salmonella, Shigella, Listeria, and mycobacteria as well as on Saccharomyces cerevisiae, Candida albicans, and spores of Aspergillus niger [21, 26]. In a study [43], the inactivation of E. coli and T4 and T7 phages after exposure to HILP was recorded. The evaluation of the effectiveness of HILP for the inactivation of E. coli and L. innocua in citric acid-disodium phosphate buffer solution was studied from Muñoz et al. [2]. However, the authors are unaware of any studies which directly compared the differences in susceptibility of E. coli and L. innocua to three different nonthermal light technologies (a NUV-vis light, a continuous UV, and a HILP light). So, the current study set out with two aims: (1) to test the relative susceptibility of two bacteria using three different light techniques; (2) to determine the effectiveness of each light equipment for inactivation of selected types of bacteria when different dosages are implemented.
Although longer treatment times resulted in significant temperature increases in all three light technologies, the dosages that were selected for this study did not result in temperature arise of more than 45°C (Figures 6, 7, and 8).
As the mechanism of inactivation by visible light is believed to be through the production of ROS, the susceptibility of both E. coli and L. innocua to ROS may play an important role in the inactivation of these organisms by NUV-vis light of 405 nm stimulates endogenous microbial porphyrin molecules to produce oxidizing reactive oxygen species (ROS), predominantly singlet oxygen (1O2) that damages cells leading to microbial death [20]. Specifically, 405 nm light has been shown to be capable of inactivating a range of predominantly nosocomial pathogens and also Gram-negative food-related pathogens [21, 23].
When NUV-vis light was implemented, L. innocua proved to be the most readily inactivated organism compared to E. coli (P < 0.05). Murdoch et al. [21] found that L. monocytogenes was most readily inactivated in suspension, whereas S. enterica was most resistant. They concluded that 395 ± 5 nm light inactivates diverse types of bacteria in liquids and on surfaces, in addition to the safety advantages of this visible (non-UV wavelength) light [21]. In addition, it is reported [26] that fungal organisms may be somewhat more resistant to 405 nm light than bacteria. In this study a correlation between dose (J/cm2) and microbes' inactivation was found. Other studies have reported that Gram-positive species, in general, were more susceptible to 405 nm light inactivation than Gram-negative species, which is generally consistent with the results obtained in the current study [44]. The prokaryotic bacteria also exhibit considerable variability in susceptibility with values, to achieve 5-log10 order reductions, as low as 18 J/cm2 with Campylobacter jejuni [45] but most typically around 50–300 J/cm2, with Gram-positive species being generally more susceptible than Gram-negatives [44]. Microbial inactivation by 405 nm light exposure has been found to be dose-dependent [21]. In applications where rapid inactivation is desirable, the use of a much higher power light source would significantly reduce the exposure times required for effective treatment. In our study, at the highest dosage (36 J/cm2), 1.37 log10 CFU/mL reduction was achieved for E. coli and a greater log reduction (2.74 log10 CFU/mL) was achieved for L. innocua. Our results are in accordance with another study [21], where they found that L. monocytogenes was completely inactivated at an average dosage of 128 J/cm2, whereas a 2.18 log10 reduction was achieved for E. coli at 192 J/cm2 dosage.
In the present study it was shown that, in order to achieve 2.66 log10 CFU/mL reductions for E. coli and 3.04 log10 CFU/mL for L. innocua, respectively, a dosage of 2.832 J/cm2 with continuous UV equipment was needed. However, the samples were not treated further due to the temperature arise. Our results are not in agreement with other studies [46] where better reductions (7.2 log10 CFU/mL reduction and 4.6 log10 CFU/mL reduction for E. coli and L. innocua, respectively, at 1.2 kJ/cm2) were achieved, perhaps due to different E. coli and L. innocua strains that were used. UV light creates mutated bases that compromise cell functionality, but bacteria have developed DNA repair mechanisms to restore DNA structure and functionality [47]. This phenomenon is reflected in the shape of the inactivation curves of our experiment [48].
The killing effects of HILP are caused by the rich and broad-spectrum UV content, the short duration, and the high peak power of the pulsed light produced by the multiplication of the flash power manifold [32, 49]. Other researchers found that a significant reduction of 3.6 log10 CFU/mL for E. coli Κ 12 and 2.7 log10 CFU/mL for L. innocua (P < 0.001) was achieved with HILP (3.3 J/cm2) [2]. Our results are similar to that of study [2] as 2.57 log10 CFU/mL reduction for E. coli and 2.14 log10 CFU/mL reduction for L. innocua were achieved when 2.832 J/cm2 dosage was implemented. To the best of the authors' knowledge three studies referring to the application of high-intensity light pulses in a continuous system [42, 50, 51].
5. Conclusions
The results of the present study show that HILP treatments were more effective for the inactivation of both E. coli and L. innocua. Furthermore, this technology resulted in more rapid and extensive inactivation than either continuous UV-C and NUV-vis treatments. These observations associated with HILP may be attributable to the comparatively higher penetration depth and emission power compared to continuous UV-C and NUV-vis. Moreover it has a high peak power produced by the multiplication of the flash power manifold, producing a light intensity at least 100 times greater than that of other two light technologies during the same operating time. However, research must be performed in real food matrixes, as it is known that HILP light generates off flavors. It can be concluded that short treatment times for decontamination efficiency would be an important factor related to productivity in food industry. The findings presented here suggest the expansion of the aforementioned light technologies on food decontamination. Thus these alternative nonthermal disinfection light techniques could find potential applications for decontamination in the food industry.
Abbreviations
- NUV-vis:
Near ultraviolet/visible light
- UV:
Ultraviolet light
- HILP:
High-intensity light pulses
- ROS:
Reactive oxygen species
- MRD:
Maximum recovery diluent.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Mosqueda-Melgar J, Raybaudi-Massilia RM, Martín-Belloso O. Non-thermal pasteurization of fruit juices by combining high-intensity pulsed electric fields with natural antimicrobials. Innovative Food Science & Emerging Technologies. 2008;9(3):328–340. [Google Scholar]
- 2.Muñoz A, Caminiti IM, Palgan I, et al. Effects on Escherichia coli inactivation and quality attributes in apple juice treated by combinations of pulsed light and thermosonication. Food Research International. 2012;45(1):299–305. [Google Scholar]
- 3.Andersen JK, Sorensen R, Glensbjerg M. Aspects of the epidemiological of Yersinia enterocolitica: a review. International Journal of Food Microbiology. 1991;13(3):231–238. doi: 10.1016/0168-1605(91)90007-c. [DOI] [PubMed] [Google Scholar]
- 4.Ghate V, Ng KS, Zhou W, et al. Antibacterial effect of light emitting diodes of visible wavelengths on selected foodborne pathogens at different illumination temperatures. International Journal of Food Microbiology. 2013;166:399–406. doi: 10.1016/j.ijfoodmicro.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Beuchat LR. Pathogenic microorganisms associated with fresh produce. Journal of Food Protection. 1996;59(2):204–216. doi: 10.4315/0362-028X-59.2.204. [DOI] [PubMed] [Google Scholar]
- 6.Park E-J, Alexander E, Taylor GA, Costa R, Kang D-H. Fate of foodborne pathogens on green onions and tomatoes by electrolysed water. Letters in Applied Microbiology. 2008;46(5):519–525. doi: 10.1111/j.1472-765X.2008.02351.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhou B, Feng H, Luo Y. Ultrasound enhanced sanitizer efficacy in reduction of Escherichia coli O157:H7 population on spinach leaves. Journal of Food Science. 2009;74(6):M308–M313. doi: 10.1111/j.1750-3841.2009.01247.x. [DOI] [PubMed] [Google Scholar]
- 8.CDC. Centers for Disease Control and Prevention. Reports of Selected Outbreak Investigations, 2010, http://www.cdc.gov/foodsafety/outbreaks/multistate-outbreaks/outbreaks-list.html.
- 9.Annous BA, Sapers GM, Mattrazzo AM, Riordan DCR. Efficacy of washing with a commercial flatbed brush washer, using conventional and experimental washing agents, in reducing populations of Escherichia coli on artificially inoculated apples. Journal of Food Protection. 2001;64(2):159–163. doi: 10.4315/0362-028x-64.2.159. [DOI] [PubMed] [Google Scholar]
- 10.Richardson SD, Thruston AD, Jr., Caughran TV, Collette TW, Patterson KS, Lykins BW., Jr. Chemical by-products of chlorine and alternative disinfectants. Food Technology. 1998;52(4):58–61. [Google Scholar]
- 11.Sagong H-G, Lee S-Y, Chang P-S, et al. Combined effect of ultrasound and organic acids to reduce Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh lettuce. International Journal of Food Microbiology. 2011;145(1):287–292. doi: 10.1016/j.ijfoodmicro.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Ölmez H, Kretzschmar U. Potential alternative disinfection methods for organic fresh-cut industry for minimizing water consumption and environmental impact. LWT—Food Science and Technology. 2009;42(3):686–693. [Google Scholar]
- 13.Alexandre EMC, Brandão TRS, Silva CLM. Efficacy of non-thermal technologies and sanitizer solutions on microbial load reduction and quality retention of strawberries. Journal of Food Engineering. 2012;108(3):417–426. [Google Scholar]
- 14.Allende A, Artés F. UV-C radiation as a novel technique for keeping quality of fresh processed “Lollo Rosso” lettuce. Food Research International. 2003;36(7):739–746. [Google Scholar]
- 15.Bermúdez-Aguirre D, Barbosa-Cánovas GV. Disinfection of selected vegetables under nonthermal treatments: chlorine, acid citric, ultraviolet light and ozone. Food Control. 2013;29:82–90. [Google Scholar]
- 16.Birmpa A, Sfika V, Vantarakis A. Ultraviolet light and ultrasound as non-thermal treatments for theinactivation of microorganisms in fresh ready-to-eat foods. International Journal of Food Microbiology. 2013;167:96–102. doi: 10.1016/j.ijfoodmicro.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Syamaladevi RM, Lu X, Sablani SS, et al. Inactivation of Escherichia coli population on fruit surfaces using ultraviolet-C light: influence on fruit surface characteristics. Food Bioprocess Technology. 2013;6(11):2959–2973. [Google Scholar]
- 18.Elman M, Lebzelter J. Light therapy in the treatment of Acne vulgaris. Dermatologic Surgery. 2004;30(2):139–146. doi: 10.1111/j.1524-4725.2004.30053.x. [DOI] [PubMed] [Google Scholar]
- 19.Feuerstein O, Ginsburg I, Dayan E, Veler D, Weiss EI. Mechanism of visible light phototoxicity on Porphyromonas gingivalis and Fusobacterium nucleatum . Photochemistry and Photobiology. 2005;81(5):1186–1189. doi: 10.1562/2005-04-06-RA-477. [DOI] [PubMed] [Google Scholar]
- 20.Maclean M, MacGregor SJ, Anderson JG, Woolsey GA. The role of oxygen in the visible-light inactivation of Staphylococcus aureus . Journal of Photochemistry and Photobiology B. 2008;92(3):180–184. doi: 10.1016/j.jphotobiol.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Murdoch LE, Maclean M, Endarko E, MacGregor SJ, Anderson JG. Bactericidal effects of 405 nm light exposure demonstrated by inactivation of Escherichia, Salmonella, Shigella, Listeria, and Mycobacterium species in liquid suspensions and on exposed surfaces. The Scientific World Journal. 2012;2012:8 pages. doi: 10.1100/2012/137805.137805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai T, Gupta A, Huang Y, et al. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: efficacy, safety, and mechanism of action. Antimicrobial Agents and Chemotherapy. 2013;57:1238–1245. doi: 10.1128/AAC.01652-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enwemeka CS, Williams D, Hollosi S, Yens D, Enwemeka SK. Visible 405-nm SLD light photo-destroys methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers in Surgery and Medicine. 2008;40(10):734–737. doi: 10.1002/lsm.20724. [DOI] [PubMed] [Google Scholar]
- 24.Guffey JS, Wilborn J. In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomedicine and Laser Surgery. 2006;24(6):684–688. doi: 10.1089/pho.2006.24.684. [DOI] [PubMed] [Google Scholar]
- 25.Maclean M, MacGregor SJ, Anderson JG, et al. Environmental decontamination of a hospital isolation room using high-intensity narrow-spectrum light. Journal of Hospital Infection. 2010;76(3):247–251. doi: 10.1016/j.jhin.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Murdoch LE, McKenzie K, Maclean M, Macgregor SJ, Anderson JGK. Lethal effects of high-intensity violet 405-nm light on Saccharomyces cerevisiae, Candida albicans, and on dormant and germinating spores of Aspergillus niger . Fungal Biology. 2013;117:519–527. doi: 10.1016/j.funbio.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Franz CMAP, Specht I, Cho G-S, Graef V, Stahl MR. UV-C-inactivation of microorganisms in naturally cloudy apple juice using novel inactivation equipment based on Dean vortex technology. Food Control. 2009;20(12):1103–1107. [Google Scholar]
- 28.Bintsis T, Litopoulou-Tzanetaki E, Robinson RK. Existing and potential applications of ultraviolet light in the food industry—a critical review. Journal of the Science of Food and Agriculture. 2000;80:637–645. doi: 10.1002/(SICI)1097-0010(20000501)80:6<637::AID-JSFA603>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Yaun BR, Sumner SS, Eifert JD, Marcy JE. Inhibition of pathogens on fresh produce by ultraviolet energy. International Journal of Food Microbiology. 2004;90(1):1–8. doi: 10.1016/s0168-1605(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 30.Marquenie D, Geeraerd AH, Lammertyn J, et al. Combinations of pulsed white light and UV-C or mild heat treatment to inactivate conidia of Botrytis cinerea and Monilia fructigena . International Journal of Food Microbiology. 2003;85(1-2):185–196. doi: 10.1016/s0168-1605(02)00538-x. [DOI] [PubMed] [Google Scholar]
- 31.Woodling SE, Moraru CI. Effect of spectral range in surface inactivation of Listeria innocua using broad-spectrum pulsed light. Journal of Food Protection. 2007;70(4):909–916. doi: 10.4315/0362-028x-70.4.909. [DOI] [PubMed] [Google Scholar]
- 32.Gómez-López VM, Ragaert P, Debevere J, Devlieghere F. Pulsed light for food decontamination: a review. Trends in Food Science and Technology. 2007;18(9):464–473. [Google Scholar]
- 33.Nicorescu I, Nguyen B, Moreau-Ferret M, Agoulon A, Chevalier S, Orange N. Pulsed light inactivation of Bacillus subtilis vegetative cells in suspensions and spices. Food Control. 2013;31:151–157. [Google Scholar]
- 34.Rajkovic A, Smigic N, Devlieghere F. Contemporary strategies in combating microbial contamination in food chain. International Journal of Food Microbiology. 2010;141(supplement):S29–S42. doi: 10.1016/j.ijfoodmicro.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 35.Barbosa-Cánovas GV, Góngora-Nieto MM, Swanson BG. Non thermal electrical methods in food preservation. Food Science and Technology International. 1998;4(5):363–370. [Google Scholar]
- 36.Hsu L, Moraru CI. Quantifying and mapping the spatial distribution of fluence inside a pulsed light treatment chamber and various liquid substrates. Journal of Food Engineering. 2011;103(1):84–91. [Google Scholar]
- 37.Dunn J. Pulsed-light treatment of food and packaging. Food Technology. 1995;49(9):95–98. [Google Scholar]
- 38.Takeshita K, Shibato J, Sameshima T, et al. Damage of yeast cells induced by pulsed light irradiation. International Journal of Food Microbiology. 2003;85(1-2):151–158. doi: 10.1016/s0168-1605(02)00509-3. [DOI] [PubMed] [Google Scholar]
- 39.Uesugi AR, Moraru CI. Reduction of Listeria on ready-to-eat sausages after exposure to a combination of pulsed light and nisin. Journal of Food Protection. 2009;72(2):347–353. doi: 10.4315/0362-028x-72.2.347. [DOI] [PubMed] [Google Scholar]
- 40.Wekhof A. Disinfection with flash lamps. PDA Journal of Pharmaceutical Science and Technology. 2000;54(3):264–276. [PubMed] [Google Scholar]
- 41.Haughton P, Gomez Grau E, Lyng J, Cronin D, Fanning S, Whyte P. Susceptibility of Campylobacter to high intensity near ultraviolet/visible 395 ± 5 nm light and its effectiveness for the decontamination of raw chicken andcontact surfaces. International Journal of Food Microbiology. 2012;159:267–273. doi: 10.1016/j.ijfoodmicro.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Haughton PN, Lyng JG, Morgan DJ, Cronin DA, Fanning S, Whyte P. Efficacy of high-intensity pulsed light for the microbiological decontamination of chicken, associated packaging, and contact surfaces. Foodborne Pathogens and Disease. 2011;8(1):109–117. doi: 10.1089/fpd.2010.0640. [DOI] [PubMed] [Google Scholar]
- 43.Bohrerova Z, Shemer H, Lantis R, Impellitteri CA, Linden KG. Comparative disinfection efficiency of pulsed and continuous-wave UV irradiation technologies. Water Research. 2008;42(12):2975–2982. doi: 10.1016/j.watres.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Maclean M, MacGregor SJ, Anderson JG, Woolsey G. Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array. Applied and Environmental Microbiology. 2009;75(7):1932–1937. doi: 10.1128/AEM.01892-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murdoch LE, MacLean M, MacGregor SJ, Anderson JG. Inactivation of Campylobacter jejuni by exposure to high-intensity 405-nm visible light. Foodborne Pathogens and Disease. 2010;7(10):1211–1216. doi: 10.1089/fpd.2010.0561. [DOI] [PubMed] [Google Scholar]
- 46.Schenk M, Raffellini S, Guerrero S, Blanco GA, Alzamora SM. Inactivation of Escherichia coli, Listeria innocua and Saccharomyces cerevisiae by UV-C light: study of cell injury by flow cytometry. LWT—Food Science and Technology. 2011;44(1):191–198. [Google Scholar]
- 47.Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. Washington, DC, USA: ASN Press; 2006. [Google Scholar]
- 48.Gayán E, Álvarez I, Condón S. Inactivation of bacterial spores by UV-C light. Innovative Food Science & Emerging Technologies. 2013;19:140–145. [Google Scholar]
- 49.Cheigh CI, Hwang HJ, Chung MS. Intense pulsed light (IPL) and UV-C treatments for inactivating Listeria monocytogenes on solid medium and seafoods. Food Research International. 2013;54:745–752. [Google Scholar]
- 50.Krishnamurthy K, Demirci A, Irudayaraj JM. Inactivation of Staphylococcus aureus in milk using flow-through pulsed UV-light treatment system. Journal of Food Science. 2007;72(7):M233–M239. doi: 10.1111/j.1750-3841.2007.00438.x. [DOI] [PubMed] [Google Scholar]
- 51.Chun HH, Kim JY, Lee BD, Yu DJ, Song KB. Effect of UV-C irradiation on the inactivation of inoculated pathogens and quality of chicken breasts during storage. Food Control. 2010;21(3):276–280. [Google Scholar]


