Abstract
Natural killer (NK) cell-mediated cytotoxicity is governed by the formation of a lytic immune synapse in discrete regulated steps, which give rise to an extensive array of cellular checkpoints in accessing NK cell-mediated cytolytic defense. Appropriate progression through these cell biological steps is critical for the directed secretion of specialized secretory lysosomes and subsequent target cell death. Here we highlight recent discoveries in the formation of the NK cell cytolytic synapse as well as the molecular steps and cell biological checkpoints required for this essential host defense process.
Keywords: cytotoxicity, innate immunity, natural killer cell
Natural killer (NK) cells are the innate immune system's cytolytic effector cells. Their function is critical to human host defense, as described by the severe viral infection and malignancy attributed to their absence or dysfunction.1 NK cell cytotoxicity has long been recognized as a stepwise, highly regulated process that is requisite to exercising control over their powerful ‘armed and ready' state.2, 3 Central to this process is the immunological synapse (IS), a finely tuned, specialized interface formed by an immune cell.4, 5 Originally described in the context of T-cell activation, the IS has now been described in many forms, and is critical for NK cell cytotoxicity, inhibition, regulation and co-stimulation (reviewed in Orange3, Barreira and Munz6 and Huse et al.7). In the case of NK cell cytotoxicity, a primary purpose of the IS is to enable the secretion of preformed lytic granules, large specialized secretory lysosomes containing lytic effector molecules. As mediators primarily of innate immunity (a discussion of NK cells adaptive properties is beyond the scope of this review but is discussed in detail elsewhere8), NK cells integrate signals from germline-encoded activating and inhibitory receptors that have evolved to detect stressed, malignant or virally infected cells. Upon the detection of a susceptible target, it is eliminated swiftly through directed secretion of lytic granules. This requires the coordinated regulation of cytoskeletal elements, signaling molecules, cellular organelles and the lytic granules themselves. We have previously proposed four steps of NK cell lytic synapse formation and function.3 Recent advances in technology have resulted in an increased understanding of the molecular coordination of each regulated step of NK cell cytotoxicity. Here we redefine these steps as we now see them, in three stages (recognition, effector and termination) comprising 48 steps and potential checkpoints in accessing cytolytic function (Table 1). With a thorough understanding of this process, the understanding of NK cells in health and disease can be uniquely advanced and potentially more specifically exploited for therapeutic potential.
Table 1. Stages and steps of NK cell cytotoxicity.
| Stage | Step | Description | Recent references | Model image (Figure 1) |
|---|---|---|---|---|
| Recognition | 1 | Approximation | 9–11 | a |
| 2 | Cell tethering | |||
| 3 | Adhesion | 12 | ||
| 4 | Nanotube formation | 13,14 | ||
| 5 | Integrin-mediated signaling and arrest | 18–22 | b | |
| 6 | Degranulation of pre-docked granules | 26,27,29 | ||
| 7 | Dynein motor activation | 30–32 | ||
| 8 | Minus-ended lytic granule movement | 30,31 | c | |
| 9 | Inhibitory signaling and potentially cell release | 24,25,33 | d | |
| Effector | 10 | Actin polymerization | 47–54 | e |
| 11 | Firm adhesion | 12 | ||
| 12 | Actin force generation | 55,56 | ||
| 13 | Cell shape change | 2,46,57,58 | ||
| 14 | Microcluster formation | 66–69 | f | |
| 15 | Lipid raft coalescence | 73 | ||
| 16 | Sustained activating receptor signaling | Reviewed in 74,75 | ||
| 17 | Actin-dependent activation signaling | Reviewed in 74,75 | ||
| 18 | Ion channel activation | 81,83 | ||
| 19 | Lytic cleft formation | 84 | g | |
| 20 | Membrane transfer | 85–88 | ||
| 21 | Granule conduit/hypodensity formation | 90–92 | h | |
| 22 | Transcription factor activation | 100 | i | |
| 23 | Gene transcription | 100 | ||
| 24 | Protein synthesis | |||
| 25 | Microtubule insertion | 107,113 | j | |
| 26 | MTOC polarization | 54,76,104–107,110,111,112 | ||
| 27 | Nuclear reorientation | |||
| 28 | Golgi polarization | 115 | ||
| 29 | Mitochondria reorientation | 118 | ||
| 30 | MTOC anchoring | k | ||
| 31 | Plus-ended lytic granule movement? | 119 | ||
| 32 | Lytic granule diffusion/motility | 92,122 | l | |
| 33 | Hypodensity identification | |||
| 34 | Lytic granule transit through cortex (hypodensities) | m | ||
| 35 | Lytic granule docking | 123,126 | n | |
| 36 | Lytic granule priming | 74 | ||
| 37 | Lytic granule fusion | 128–131 | o | |
| 38 | Externalization of granule contents | 124,133 | p | |
| 39 | Force generation? | 122 | ||
| 40 | Persistence of degranulations | 122 | ||
| 41 | Lytic granule endocytosis | 28,137 | ||
| Termination | 42 | Relative inactivity | q | |
| 43 | Granule biogenesis | 26 | ||
| 44 | Downmodulation | 144,145 | ||
| 45 | Detachment | 146 | r | |
| 46 | Serial killing | 147 | ||
| 47 | Exhaustion (granule and energy depletion) | |||
| 48 | Recycling | 28,137 |
Abbreviation: MTOC, microtubule-organizing center.
Three main stages (recognition, effector and termination) are shown with discrete steps and recent, primarily NK cell-specific references from the text. Model images refer to those shown in Figure 1.
STAGES OF LYTIC SYNAPSE FORMATION
Recognition
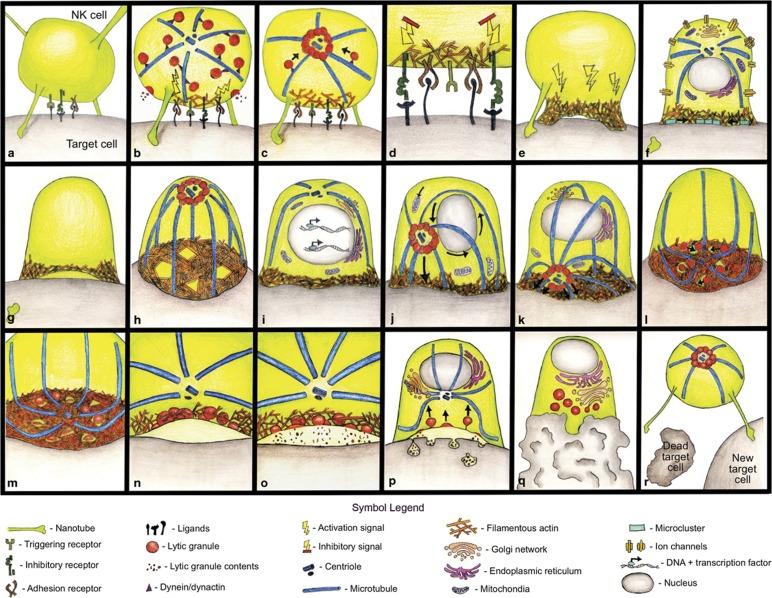
The motility of NK cells in tissue or tumor microenvironment is likely driven by chemotactic signaling resulting in an approximation to key sites of interest, although NK cell motility in tumors is also driven by the expression of NKG2D ligands.9, 10, 11 Therefore, similar to a T-cell sampling dendritic cells, NK cells undergo brief, exploratory interactions with multiple cell types, which may represent their initial encounter with a target10 (Figure 1a). It is not known what molecules specifically mediate these interactions, although there are a number of candidate receptors expressed on resting or primed NK cells, including a variety of ‘tethering' receptors as well as potentially more robust adhesion receptors. The former potentially include CD62L and PSGL-1; the latter include CD2, DNAM-1, NKG2D, the natural cytotoxicity receptors and lymphocyte function associated antigen-1 (LFA-1). Unlike T cells, a subset (∼10%) of freshly isolated human NK cells express the activated conformation of LFA-1 at rest, which presumably allows adhesion to a target before the engagement of other activating receptors.12 The transmission of a second activating signal through a receptor such as NKG2D, DNAM-1, 2B4 (CD244) or CD2 strengthens these interactions by further activating LFA-1, resulting in firm adhesion to the target and potentially providing minimal requirements for progression to mediating cytotoxicity.12
Figure 1.
Stepwise molecular progression through NK cell cytotoxicity. NK cell cytotoxicity can be broken down into three main stages: recognition (a–d), effector (d–p) and termination (q, r). During the recognition stage, the NK cell is particularly sensitive to inhibitory signaling (d). Key events include lytic granule convergence (c), the actin-dependent firm adhesion of the NK cell (e) and subsequent F-actin conduit formation (h), MTOC polarization (j) and LG fusion and exocytosis (o). This is followed by vesicle recycling (p), a period of relative inactivity (q) and detachment and subsequent serial killing (r). See text for further details.
We are still learning of surprising interactions that NK cells engage in, likely in part due to the complex microenvironment that they patrol. One such unconventional form of communication is the formation of membrane nanotubes (Figure 1a). Nanotubes appear to serve as intercellular tethers formed rapidly after contact with the target cell and can help guide the NK cell to the target cell or provide traction during its initial interaction.13 Rather than hollow tubes that mediate free diffusion, NK cell nanotubes are heterogeneous structures that transmit signals over long distances and can even deliver lytic granules from an NK cell to a target.13 Importantly, their formation enhances cytotoxicity.13, 14 NKG2D and downstream activation molecules including DAP10 and Vav1 accumulate at the nanotube junction, resulting in the description of this junction as a nanoscale synapse.13 However, blocking either LFA-1 or 2B4 does not reduce the frequency of nanotube formation, suggesting that this is not simply a stretched conventional lytic synapse.13 CD2 expression specifically increases the frequency of NK target cell nanotubes, suggesting a role for specific receptor–ligand interactions in their formation14 and partially explaining the observation that CD2 enhances NK cell cytotoxicity.15 The mechanism by which this occurs, and the prevalence of nanotubes during routine physiological NK surveillance, remains unclear.
Another example of the complex initial interaction may be the newly observed phenomenon of NK cell-derived exosomes: NK surface marker-expressing, FasL and perforin-containing nanovesicles (50–100 nm) that, upon release from NK cells, can mediate target cell killing independent of IS formation.16 They may also potentially transfer key ligands to target cells to enable more robust interactions in environments in which exosome concentrations are high. This concept, in concert with nanotubes, harkens to a provocative early proposal of ‘harpooning' as a contributor to NK cell cytolytic function.17 Despite their apparent constitutive secretion by human NK cells isolated from peripheral blood, the relative contribution of exosomes to an NK-mediated cytotoxic response is poorly understood but compelling.
Following target cell contact, LFA-1 engagement initializes the first steps of IS formation, including protein tyrosine kinase activation, PIP(4,5)P2 generation and F-actin reorganization (Figure 1b).18, 19, 20, 21 In synergy with activating receptor ligation, LFA-1 also induces an arrest signal to migrating cells, with a strong activating signal resulting in a stable radially symmetrical synapse and inhibitory signaling resulting in asymmetry and subsequent resumption of migration.22 Whether NK cells kill through ‘kinapses', or moving synapses, as has been described in T cells, remains to be determined.23
LFA-1-mediated outside-in signaling for F-actin polymerization brings together all the requisite components for F-actin polymerization and initiates this process.19, 20 This initial signaling also includes the phosphorylation of Vav1 by a Src family kinase, its recruitment to lipid rafts and subsequent further activation as discussed in subsequent steps below.21 Therefore, initial engagement of LFA-1 in concert with a second signal can help to ready an NK cell for target cell lysis. At this point the NK cell is likely still highly sensitive to a balance of activating and inhibitory signaling, and control of Vav1 phosphorylation and subsequent actin accumulation is thought to be a critical axis in the regulation of cytotoxicity.24, 25 At this stage, microclusters are likely present, may be functional and are also described further below.
Despite the tightly regulated steps leading to NK cell cytotoxicity, there is evidence that pre-docked granules are present in the cell cortex and can be accessed rapidly by NK cells.26 Lytic granule secretion at the IS before microtubule-organizing center (MTOC) polarization has been described in primary human cytotoxic T lymphocytes (CTLs).27 Although it is conceivable that antigen specificity of T cells allows for the circumvention of further checkpoints to cytotoxicity, lytic granules are present in the cortex of resting NK cells and initial exocytosis bursts are detected (Figure 1b).26, 28, 29 Despite these observations, imaging of both NK cell lines and primary NK cells by both live and fixed cell microscopy indicates that the majority of NK cells go through the multistep, highly regulated steps to cytotoxicity (Figure 2).2 Therefore, it is unclear as to what the significance of the presence and potential exocytosis of pre-docked granules is. In this light, it is also still unclear how many lytic granules are required to kill specific types of target cells.
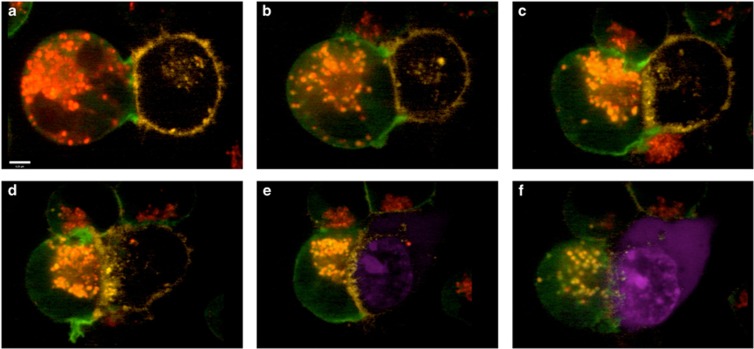
Figure 2.
Stepwise stages during NK cell-mediated cytotoxicity: YTS GFP-actin (green) cells were labeled with Lysotracker Red (red) to selectively label lytic granules and co-incubated with CellMask-labeled (Life Technologies, Carlsbad, CA, USA) (yellow) 721.221 target cells in the presence of SYTOX Blue (Life Technologies) (blue) for detection of cell death. Conjugates were imaged by spinning disk confocal microscopy for a total of 90 min. The images shown are frames acquired approximately every 15 min. (a) The NK cell is conjugated to the target cell and lytic granules are dispersed. (b) Granules are converged at the MTOC. (c) The MTOC along with converged lytic granules are polarized at the immune synapse. F-actin is evidently reorganized from a to c. (d) More F-actin reorganization is observed and lytic granules are docked at the plasma membrane. (e) Target cell death begins as SYTOX Blue enters the cell. (f) Target cell undergoes apoptosis and NK prepares for detachment as shown by reversal of shape change. Scale bar=4 μm.
Polarization of the MTOC and lytic granules to the IS is required for NK cell cytotoxicity and occurs in the effector stage. However, preceding MTOC polarization, lytic granules rapidly move along microtubules and converge upon the MTOC (Figure 1c).30, 31 This minus-ended lytic granule movement depends on dynein motor function but is independent of actin polymerization or microtubule dynamics. It also occurs whether the NK cell is conjugated to a susceptible or resistant target cell, as it is observed in the presence of an inhibitory synapse.30 This suggests that granule convergence is a step that precedes NK cell commitment to cytotoxicity, instead preparing the cell for it. Accordingly, ligation of CD28 or LFA-1 alone is sufficient to induce lytic granule convergence, as is activation by soluble interleukin-2.30 Disruption of LAMP-1 (CD107a) expression also results in impaired lytic granule motility, which corresponds with a decrease in association of the dynein component p150glued with lytic granules.32 Although this suggests that LAMP-1 itself may mediate the interaction of granules with dynein, the mechanism of this is unknown. Interestingly, MTOC polarization in LAMP-1-deficient cells is intact, suggesting that convergence is not a prerequisite for polarization.32
Lytic granule convergence before MTOC polarization likely represents the final juncture at which NK cells are susceptible to inhibitory signaling before a commitment to cytotoxicity. Signaling through immunoreceptor tyrosine-based inhibitory motif (ITIM)-containing inhibitory receptors induces formation of the inhibitory synapse, which is functionally dominated by phosphatases, particularly SHP-1, leading to exclusion of the downstream signaling molecules from the contact region (Figure 1d).33, 34 Inhibitory signaling acts swiftly to disrupt multiple points of activation, including conjugate formation and F-actin accumulation,34 LFA-1 activation,12 activating receptor clustering at the IS35 and critically, Ca2+ flux.36, 37 Dephosphorylation of the activating phospho-tyrosine residues of Vav1 appears to be a critical molecular switch for this control but this response is likely fine-tuned by other factors.25 These include the adaptor protein Crk, which is also phosphorylated following inhibitory signaling and recruited to the inhibitory synapse.35, 38 Crk, through its association with C3G and subsequent control of Rap1, likely has a role in the regulation of LFA-1 activation and subsequent target cell adhesion.38, 39, 40, 41, 42 Interestingly, inhibitory receptors also serve to ‘license' NK cells during their developmental process, and NK cells that lack the recognition of MHC molecules during maturation are rendered hyporesponsive, at least partially by reduced signaling from activating receptors to LFA-1. Unlicensed cells form fewer conjugates, but those that do conjugate mediate lytic granule polarization normally.43 Other mechanisms by which these ‘unlicensed' or ‘anergic' NK cells are restrained in their cytotoxic capacity represents an unknown cellular checkpoint that will be of great value in understanding full access to lytic capacity.
Effector
At the beginning of the effector stage, the NK cell commits to cytotoxicity and proceeds toward degranulation. This stage is marked largely by significant cytoskeletal rearrangements, beginning with significant de novo F-actin polymerization and reorganization (Figure 1e). Although the importance of the F-actin cytoskeleton in NK cell cytotoxicity has been appreciated for some time, recently many new contributors to the regulation of F-actin have been highlighted. One of these is the Wiskott-Aldrich syndrome protein (WASp) homolog WAVE2.44 Interestingly, while both WASp and WAVE2 are expressed in T and NK cells, WAVE2 may be critical for F-actin polymerization in CTLs but is normally not accessed by NK cells.45 In NK cells, WASp appears to be the predominantly utilized family member. As an illustration, Wiskott-Aldrich syndrome patients who are deficient in WASp have severe NK cell functional impairment linked to an inability to rearrange F-actin.46 This can be overcome with interleukin-2 treatment, which activates WAVE2 in NK cells and restores F-actin rearrangement in WAS patient NK cells in vitro and in vivo.47 This not only offers therapeutic options for treatment of WAS but highlights an important difference between NK- and T-cell cytotoxicity. It also illustrates the potential cooperativity between adaptive and innate immunity via the ability of T cell-produced interleukin-2 to access distinct cell biology in NK cells.
The Rho GTPase Cdc42 is also critical for F-actin polymerization by Arp2/3 through direct binding and activation of WASp. Recently, a role for the Cdc42 effector DOCK8 has been described in NK cells.48, 49, 50 Patients deficient in DOCK8 have decreased NK cell function with accompanying lytic synapse assembly and function defects that we now recognize as hallmarks of defective actin polymerization, including decreased LFA-1 recruitment, loss of granule polarization and accompanying decrease in cytotoxic function.48, 50 Importantly, DOCK8 deficiency does not affect overall NK cell F-actin content, but more specifically the targeted accumulation and polarization of F-actin at the synapse. This is in contrast to WASp deficiency in which NK cells have a reduced total F-actin content in addition to an inability to target actin accumulation.46 Loss of DOCK2 function in mouse NK cells also results in impaired Rac activation and subsequent loss of NK cell cytotoxic function,51 suggesting this family is an important regulator of NK cell cytotoxicity.
In addition to the WASp and WAVE/Scar family members, the Arp2/3 complex can be activated by cortactin.52 The hematopoietic-specific homolog of cortactin, HS1, is localized to the NK cell lytic synapse, where it is critical for signaling to and from LFA-1.53 In NK cells, the formin homolog hDia is not required for lytic synapse branched actin, but instead is required for NK cell cytotoxicity by targeting microtubules to the synapse and subsequent lytic granule polarization, suggesting its function as the poorly understood link between the microtubule and actin cytoskeleton.54
Once F-actin polymerization has been initiated, firm adhesion to the target cell is achieved and sustained (Figure 1e). As F-actin polymerization is required for LFA-1 recruitment and maintenance at the synapse, this synergy between actin and integrins provides firm adhesion throughout the period of NK cell activation. This transmembrane linkage results in the generation of significant force, which has been measured directly through the application of single-cell force spectroscopy. Engagement of 2B4 leads to rapid, LFA-1- and actin-dependent increase in the force sufficient to disengage an NK cell from a susceptible target.55 Therefore, the mechanical forces generated at the synapse also have an important role in adhesion activation signaling. This observation is supported by the increased LFA-1-mediated adhesion of NK cells bound to targets in which intercellular adhesion molecule-1 (ICAM-1) is tethered and restrained, as opposed to freely diffusible.56
Firm adhesion to the target cell and F-actin polymerization leads to the NK cell flattening and extending the diameter of the synapse (Figure 1f).2, 46, 57, 58 This ‘cell shape change' is an actin-dependent process, as NK cells treated with actin inhibitors or from WAS patients fail to show this characteristic shape change.46 Similarly, NK cells engaging in inhibitory synapses remain rounder, further underscoring the regulation of F-actin as a mechanism of NK cell inhibition.
Engagement of activating receptors leads to dynamic microcluster formation (Figure 1f). These can be defined as a critical mass of receptors that in concert can promote signal transduction. In T cells, F-actin-dependent microcluster movement occurs toward an actin-poor sink, with productive signaling occurring in the periphery of the synapse and terminating in the center.59, 60, 61, 62, 63, 64 Myosin IIA also is required for centripetal microcluster movement and this sustained retrograde flow of microclusters is required for sustained Ca2+ release and activation signaling.64, 65 Although the kinetics may be different in NK cells, microclusters of both activating and inhibitory receptors are present and appear to have similar dynamics compared with those in T cells.66, 67, 68, 69 Studies using NK target cell conjugates show that phosphorylated KIR2DL1-containing clusters originate in the periphery and coalesce in the center, thus reaffirming both the presence of microclusters and suggesting their utility in NK cells.67, 69 Furthermore, highly motile NKG2D-DAP10-containing microclusters are also seen in the periphery of the synapse, and contain high levels of phospho-tyrosine, suggesting that, as in T cells, the periphery may also be the predominant site of activating signaling in NK cells.66 2B4-containing microclusters are found centrally, suggesting segregation of activating receptors.28 Interestingly, KIR2DL1 microclusters are found on resting NK cells, but become smaller and denser when NKG2D is ligated.66, 68 This surprising observation was made possible using super-resolution microscopy, which allows for measurement of microcluster density and size (9–20 proteins/cluster and 120 nm, respectively). It is unclear what the significance of this microcluster compression actually is, but may reflect an interplay required for NK cell licensing, as speculated previously.68, 70
The lipid microenvironment may also have a role in the spatial organization of receptors (Figure 1f). The role of higher-ordered microdomains, or lipid rafts, has been somewhat contentious. The use of multivalent labels, such as cholera toxin, always raises the possibility that aggregation of these probes may result in inaccurate detection. Early studies, however, have implicated the recruitment of activating receptors to, and exclusion of inhibitory receptors from, lipid rafts.71, 72 Recent experiments in live Jurkat T cells using a novel phase-sensitive membrane dye and high-resolution imaging have resolved higher-order membrane at the periphery of the synapse, suggesting that these domains may support microcluster or signaling platform formation.73 It remains to be seen whether this is also the case with NK cells, and what the effect of inhibitory signaling is on the membrane order at the synapse.
Activating signaling leading to NK cell activation has been extensively reviewed recently elsewhere.74, 75 Two main axes of signaling are initiated and sustained through cytotoxicity once appropriate receptor–ligand pairs are in place, namely, the PI3K and phospholipase Cγ pathways.76, 77, 78, 79 Engagement of activating receptors leads to signaling through ITAM and YINM motifs and recruitment and activation of both membrane-proximal and distal proteins signaling and adaptor proteins. It is worth emphasizing the interdependence of activating signaling with the cytoskeleton, as disruption of one affects the other and prevents functional cytotoxicity. That said, the exact cellular mechanism of signaling protein localization and re-localization in NK cells remains unknown.
One key outcome of activating receptor signaling is mobilization of ions, particularly calcium (Figure 1f). Initial calcium flux occurs as a result of rapid release from endoplasmic reticulum (ER) calcium stores mediated by IP3 channels downstream of PLCγ signaling.80 Depletion of ER Ca2+ stores is sensed first by STIM1 on the ER surface. Following activation, STIM1 complexes reposition proximal to the plasma membrane (visualized as puncta on the ER surface) and activate plasma membrane-associated ORA1.80 Human genetic immunodeficiency has defined critical roles for both ORAI1 and STIM1 in NK cell cytotoxicity. One manifestation of these patients' disease is profound susceptibility to viral infection, a hallmark of reduced NK cell function, although they also present with many other signs and symptoms and have substantive defects in T-cell and B-cell function.1, 81 Importantly, while ORAI1-deficient NK cells form conjugates with target cells and polarize the MTOC and lytic granules, they fail to degranulate.81 Similarly, NK cells from STIM-1-deficient patients fail to mediate cytotoxicity.81
Although the two Ca2+ flux systems are the best characterized, there are other ion channels expressed and utilized by NK cells. Recently, a critical role for intracellular Mg2+ has been described by a rare primary immune deficiency affecting the Mg2+ transporter MAGT1.82, 83 In these patients, intracellular levels of free Mg2+ are decreased, accompanied by defective NK cell and CTL killing and subsequent uncontrolled Epstein–Barr virus infection.83 Interestingly, the role of Mg2+ in both CTL and NK cell control of Epstein–Barr virus is through maintaining the stability of NKG2D at the cell surface, suggesting an unappreciated role for NKG2D in controlling Epstein–Barr virus infection. In addition, potassium and zinc channels are expressed and utilized by T cells (reviewed in Feske et al.80), prompting the question of whether NK cells utilize similar mechanisms.
Presumably, in and around these steps a lytic cleft forms (Figure 1g). Once thought to be simply a gasket to prevent the leakage of lytic granules to surrounding tissues, it is now appreciated that the cleft is dynamic and complex. Much of the activity that takes place in the cleft is critical to cytotoxic function, yet remains mysterious because of its inaccessibility. Measurement of the lytic cleft by transmission electron microscopy in inhibitory and activating synapses shows that both have a similar range of distances between the NK cell and the apposing target cell membrane (10–30 nm), which are proposed to account for distribution of proteins of different sizes at the synapse.84
Electron micrographs of the NK cell synapse also show significant membrane ruffling and protrusions. These synaptic membrane features may also be a source of membrane transfer (trogocytosis) between an NK cell and a target (Figure 1g), which has been defined for a number of receptors.85, 86, 87, 88 This process is reciprocal, as transfer of both ligand from the target cell and receptor from the NK cell is observed. Additional transmission electron microscopy experiments demonstrate that the target cell-derived receptors enter the NK cells in small, enclosed pits.88 Both inhibitory and activating receptor exchange occurs across the NK cell synapse, although there is controversy as to whether cognate ligand engagement is required.85, 88 The functional outcome of trogocytosis and intercellular receptor transfer is unclear, although transfer of NKG2D and MICB at the synapse results in lower NK cell cytotoxic function.87 Fascinatingly, NK cells that acquire NKG2D ligand from target cells become targets themselves, and are victims of fratricide by neighboring NK cells.86 This suggests that this ligand transfer may act to both enable and even control NK cell activity during infection, again suggesting the theory of harpooning.17
Following firm adhesion and the formation of a cleft, F-actin continues to undergo substantial remodeling at the synapse. Traditionally, this remodeling was thought to result in a large, central clearing of F-actin to enable centrosomal docking and lytic granule secretion.58, 89 Recent advances in imaging technology, namely, the adoption of super-resolution microscopy, have revealed that ligation of activating receptors creates granule-sized conduits through which granules are secreted (Figure 1h).90, 91, 92 Interestingly, viral influenza particles can also result in conduit formation, presumably through the ligation of NKp46, but this signal requires co-stimulation through LFA-1.93 Other activating receptors, such as NKG2D or CD16, can signal solely for clearance formation. Although not entirely understood, this seems to be an elegant mechanism by which an NK cell could discern a free viral particle from one bound to a target cell. Whether proteins on the granules themselves form lytic granule conduits, they are a result of the inherent dynamism at the synapse, or occur in specific hotspots of signaling remains to be determined. It seems likely, based on the presence of actin nucleating factors at the synapse, that F-actin is dynamic and is likely undergoing continual remodeling. This may contribute to conduit kinetics, as has been suggested by analyses of synaptic plane actin dynamics using total internal reflection fluorescence imaging.92
The process of remodeling and specifically breaking down as opposed to building is of particular interest as conduits in F-actin are a requirement for degranulation.91, 92 Cofilin, which severs and depolymerizes F-actin, works in concert with Arp2/3 to remodel actin to enable secretion in muscle cells.94 Coronin 1A, which promotes the turnover of actin through binding to both Arp2/3 and cofilin, regulates Arp2/3 localization to the T-cell synapse, although a role for this important immune cell regulator in NK cells has yet to be defined.95, 96 One appealing hypothesis is that hypodensity formation occurs at regions of high activating receptor density as a result of highly localized signaling events, such as is seen in mast cells.97 However, this is difficult to reconcile with what is likely the dynamic movement of microclusters of activating receptors themselves. An alternative explanation is that lytic granules create their own conduits, enabled by the presence of actin-remodeling proteins on their surface. This would be compatible with the observation that Myosin IIA is present on lytic granules and appears required for their penetrance through F-actin,98, 99 but this hypothesis has not been directly tested.
Perhaps, in preparation for potential serial killing or NK cell recycling, lytic synapse formation also activates transcription factors and gene transcription (Figure 1i). Ligation of NKp30 results in rapid translocation of nuclear factor κB to the nucleus and de novo protein synthesis.100 Perhaps, not surprisingly, perforin and granzyme is refilled in CTL lytic granules while they kill.101, 102 The newly produced perforin then reaches the synapse independently of conventional lysosomal granules and mediates cytotoxicity.103 Whether NK cells employ such a mechanism remains to be determined, as does the precise program of gene transcription that is activated during cytotoxicity.
Following the dynamic rearrangement of the actin cytoskeleton, microtubule dynamics result in a dramatic reorientation of the MTOC and associated lytic granules toward the synapse (Figure 1j). Requirements for centrosome polarization include LFA-1, Pyk2, ERK2, CIP4, the formin hDia and Vav1.54, 76, 104, 105, 106, 107 However, it is important to note that F-actin polymerization is required for MTOC polarization;46, 53, 54, 58, 108, 109 therefore, any interference with F-actin dynamics will subsequently impair MTOC and granule polarization.
One consideration in MTOC polarization is the significant amount of force likely needed to generate this reorientation. It is assumed from studies in other systems that microtubule insertion and anchoring in the cell cortex lead to either pushing (microtubule growth) or pulling (microtubule shrinkage) forces that can reposition the centrosome. Dynein may again have a role, as a minus-ended motor it can generate significant pulling forces on shrinking microtubules when anchored in the cortex, and may contribute to the fine-tuning and positioning of microtubule asters.110 Accordingly, it was recently shown that in T cells, MTOC repositioning occurs as a result of end-on capture shrinkage of microtubule focused at the center of the IS and anchored to cortical dynein.111 Interestingly, in NK cells, it appears that kinesin-1 has a role in the initial movement of the MTOC to the synapse, mediated through interactions with the small GTPase Arl8b.112 IQ motif containing GTPase-activating protein 1 (IQGAP1) may act as a linker between CLIP-170 on the plus ends of microtubule and specific regions of cortical actin. Loss of IQGAP1 results in a failure of NK cells to polarize the MTOC and degranulate.113 Cip4 has also been implicated as a link between microtubules and F-actin at the cortex.107 Although in T cells the MTOC docks in contact with the plasma membrane at the synapse, this has not been directly observed in NK cells.54, 114
As the MTOC polarizes to the synapse, cellular organelles also reposition with some moving toward and others away from the synapse (Figure 1j). Reorientation of the Golgi along with microtubules toward the IS presumably aids in directed secretion of granules toward the target cell.115 In T-helper cells, the mitochondria polarize toward the synapse to maintain Ca2+ flux across the plasma membrane for T-cell activation.116, 117 In NK cells, the mitochondria reposition toward the NK cell IS following NK stimulation with anti-NKGD2 antibodies but not with anti-KIR2DL1 antibodies, suggesting that the mitochondrial dynamics are triggered as a result of NK cell activation.118 The polarization of these organelles is important for sufficient Ca2+ influx for signaling and granule exocytosis. It is conceivable that polarized mitochondria further serve as local sources of energy to power synaptic function, although this needs to be proven.
Following MTOC polarization to the IS and anchoring at the plasma membrane, the delivery of the polarized lytic granules to the synapse occurs (Figure 1k). In T cells, this process requires plus-ended, kinesin-1-dependent movement of lytic granules upon microtubules to the membrane.119 In NK cells, a role for kinesin-1 in this process has not been described but is conceivable. Other cellular machinery could also have a role in lytic granule movement. As mentioned above, Myosin IIA is associated with lytic granules and required for NK cell cytotoxicity.98, 99, 120 Whether this is through the facilitation of short runs across F-actin following MTOC polarization, the penetration of granules through the actin meshwork or the exocytosis of lytic granules121 is unclear, although obviously these scenarios are not mutually exclusive.
Following delivery to the plasma membrane, lytic granules have significant dynamic movement, which is followed by arrest, and subsequent degranulation in only a subset of granules (Figure 1l).92, 122 This movement is not dependent upon F-actin dynamic rearrangement,122 but a role for microtubule dynamics has not been excluded. Granule movement may be undirected rolling on synaptic actin, seeking regions of hypodensity. Alternatively, it may represent the requirement for granules to find a primed fusion/tethering complex to mediate their arrest, as is suggested by high-resolution imaging of lytic granules in CTL utilizing Munc 13-4 Rab27a complexes for granule arrest and subsequent degranulation.123
At some point, lytic granules must traverse the pervasive actin network at the synapse (Figure 1m). Lytic granule may mechanistically identify areas of hypodense F-actin regions to pass through, or directed F-actin remodeling at the activating synapse may be required for clearing the way for lytic granules to transit through minimally permissive F-actin hypodensities. However, even in the presence of F-actin clearances, it is likely that lytic granule transit through the cortex requires the generation of force as was suggested by experiments evaluating degranulation in the absence of an F-actin meshwork.122 Myosin IIA may mechanically facilitate lytic granule motility through the F-actin-rich area of the IS or may be involved in inducing local changes in the F-actin structure to allow polarized lytic granules access to the plasma membrane.74, 98, 99
After polarizing to the IS and before degranulation, lytic granules dock at the IS and fuse with the plasma membrane (Figure 1n).26, 120, 124, 125 Docking is likely mediated primarily by Munc 13-4 and Rab27a, both of which are recruited to lytic granules following activating receptor ligation.126 In CTL, lytic granules become fully mature only at late stages of the exocytic pathway, when Munc13-4 and Rab27a associate with perforin- and granzyme-containing lysosomal components.127 Upon interacting with Rab27a, Munc13-4 may also regulate the interaction between vesicle and target soluble NSF Attachment Protein REceptor (SNARE) required for lytic granule fusion with the plasma membrane.123 Alternatively, this may be mediated by the interaction of Syntaxin 11 and Munc18-2 with R-SNAREs (such as VAMP7) on the lytic granule membrane.74 Priming then occurs as a result of Munc13-4-mediated activation of Syntaxin 11 or bridging of the plasma membrane with lytic granule. Fusion occurs as a result of SNARE complex formation, and although this complex has not been defined in NK cells, mutations in the SNARE components Syntaxin 11, VAMP4 and VAMP7 prevent NK cell degranulation.128, 129, 130, 131
In mast cells, coordinated oscillations of calcium in concert with N-WASP and PIP(4,5)P2 dynamics trigger granule fusion with the plasma membrane.97 Whether such localized signaling dynamics contribute to NK cell exocytosis remains to be seen, although this hypothesis would complement the localized generation of actin hypodensities. Vesicular exocytosis is also triggered by calcium flux. In addition to the global calcium reserves such as the ER and the store-operated reservoirs, local Ca2+ nanodomains created by channels on the lytic granule membrane in T cells are essential for their exocytosis.132 Thus, local synaptic membrane nanoalterations may factor into a final step in allowing the release of lytic granule contents.
Granule exocytosis may be an additional mechanism of regulating cytotoxicity (Figure 1o). NK cell lytic granules use at least two distinct modes of fusion: (1) complete fusion where granule content is completely discharged and the contents diffuse rapidly at the plasma membrane and (2) incomplete fusion, whereby formation of a transient fusion pore at the plasma membrane is accompanied by release of some but retention of most of the granule contents.124, 133 This latter form of degranulation is suggestive of alternate forms of granule fusion seen in other secretory cells such as chromaffin cells and neurons. These are descriptively named ‘kiss and run',134 in which a granule pore is formed and resealed, preventing full release of granule contents, ‘kiss and stay',135 in which granules remain at the membrane and are re-acidified, presumably for rapid recycling or reuse and ‘crash fusion', in which granule contents are released without stable docking and priming.136 The true existence and significance of these alternate forms of granule fusion in NK cells is incompletely understood, although it is conceivable that it could be a means of facilitating serial killing through the rapid recycling of granule contents. As such, two-way vesicular trafficking occurs at the NK cell synapse, and rapid Munc13-4-clathrin mediated endocytosis of granule membrane proteins exposed at the plasma membrane is necessary for the recycling of a pool of endocytic vesicles, allowing the serial killing event (Figure 1p).28, 137 F-actin dynamics may have a role in the extrusion of granule contents and the persistence of lytic granules at the membrane following exocytosis.122 Although this is presumably owing to force generation, the direct proof is presently unavailable.
After degranulation, a period of relative inactivity begins and is presumably required as lytic granule contents cross the lytic cleft to act upon the target cell (Figures1o and p). The sustained presence of fused granules at the synaptic membrane, however, may serve additional functions as well. Interestingly, the exposure of LAMP-1 on the NK cell surface protects the NK cell from cytotoxicity-induced suicide,138 although this is likely not the only mechanism.139, 140, 141 Subsequently, uptake of lytic granule contents by the target cell initiates apoptosis and marks the end of the effector stage of cytotoxicity, as the lytic hit has been administered.
Termination
The termination stage of the synapse refers to the steps that immediately follow the release of lytic granules and up to either the reinitiating of a subsequent recognition or return to a resting state. Initially, there is a longer time of apparent relative inactivity. During this time the target cell continues its death process, primarily by apoptosis (Figure 1q). Perforin induces membrane flipping on the target cell, resulting in exposure of phosphatidylserine.142 Phosphatidylserine on the surface of the cell may provide signaling to the NK cell to terminate the response, as NK cells express proteins that have been shown to bind to phosphatidylserine such as the ITIM-containing molecule CD300a.143 A further mechanism by which the NK cell could relax its activation status is through receptor downmodulation as has been documented for CD16 and NKG2D.144, 145 Perhaps, this could apply to other activating receptors and cell adhesion molecules.3 Ongoing is new LG biogenesis during this relatively quiet period as a likely requirement for NK cell ‘recharging' and subsequent serial kills.26
The final stage of cytotoxicity is defined by detachment from the target cell, a process that is not entirely arbitrary. Kinetic studies show that the decision to end stable contacts with target cells is made faster when killing occurrs.146 Interestingly, detachment was sometimes seen along with the formation of nanotubes connecting the NK and target cells (Figure 1r). The purpose of these connections in the context of a dying cell, however, is unclear and they may just represent the default behavior of an NK cell to tether to activating objects of interest.
Insight into the steps of the termination stage can be seen during the process of NK cell serial killing. Serial killing can demonstrate burst kinetics, with a delayed first kill followed by more rapid subsequent kills.147 A proposed mechanism for burst kinetics is the idea of kinetic priming, which suggests that NK target interactions depend upon recent killing events. It is proposed that kinetic priming is a continuity of signaling, as interactions with old targets remain until a new target is found.147 As such, lytic granule may remain converged to the MTOC following target cell lysis in preparation for subsequent kills, as previously proposed.30, 31
A consequence of serial killing is a near depletion of lytic granules and cytotoxic effector molecules. This depletion can leave the NK cells in an exhausted state until they detach from the target cell that has depleted their contents. After separation, exposure to locally available activating factors such as interleukin-2 can restore their cytotoxic function.148 New granule biogenesis and new effector molecule synthesis presumably mediate this restoration. Interestingly, in CD8+ cytotoxic T cells, it was found that rapid upregulation of nongranule-restricted perforin can be transported to the IS and participate in cytotoxicity.103 As this form of perforin was identified in NK cells,103 perhaps this observation carries over to NK cells and contributes to the reacquisition of effector function after exhaustion. Although seemingly the least is known about the specifics of the termination phase of the synapse, it can be interpreted as one of the most important parts, as recycling of NK cells in vivo in diseased tissue environments is likely to be of the essence in host defense.
Summary and future thoughts
In recent years, explosive advances in technology have driven us deeper within the NK cell to better understand the mechanism of its critical function. In doing so, we have a much better understanding of the tightly regulated steps leading to cytotoxicity. There are critical and fundamental questions that remain to be answered, some of which require substantive technology development. These include, but are not limited to the following: (1) the exact number of, and temporospatial requirements for, degranulation events needed to kill particular target cells; (2) how both inhibitory signaling and ‘anergy' restrain progression through the cell biological steps of synaptic formation; (3) the mechanism by which conduits in the cell cortex are created to enable lytic granules to reach the NK cell synaptic membrane; (4) the true purpose of lytic granule convergence to the MTOC, (5) the mechanism of delivery of lytic granules from MTOC to plasma membrane; and (6) what specifically governs the release of an NK cell from its target cell. In addition, we still have much to learn about the fine-tuning of the cytotoxic response and the subversion of it by viruses and malignant cells. These represent only a sampling of the many questions begotten by the last 15 years of discovery, the answers to which will provide the tools to manipulate and control cytolytic host defense. Better understanding of these facets of NK cells will enable more effective harnessing of NK cell functions and therapeutic intervention.
Acknowledgments
This work was supported by NIH grant AI067946 to JSO.
References
- Orange JS. Natural killer cell deficiency. J Allergy Clin Immunol. 2013;132:515–525. doi: 10.1016/j.jaci.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulfing C, Purtic B, Klem J, Schatzle JD. Stepwise cytoskeletal polarization as a series of checkpoints in innate but not adaptive cytolytic killing. Proc Natl Acad Sci USA. 2003;100:7767–7772. doi: 10.1073/pnas.1336920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. [PubMed] [Google Scholar]
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- Barreira da Silva R, Munz C. Natural killer cell activation by dendritic cells: balancing inhibitory and activating signals. Cell Mol Life Sci. 2011;68:3505–3518. doi: 10.1007/s00018-011-0801-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse M, Catherine Milanoski S, Abeyweera TP. Building tolerance by dismantling synapses: inhibitory receptor signaling in natural killer cells. Immunol Rev. 2013;251:143–153. doi: 10.1111/imr.12014. [DOI] [PubMed] [Google Scholar]
- Rolle A, Pollmann J, Cerwenka A. Memory of infections: an emerging role for natural killer cells. PLoS Pathog. 2013;9:e1003548. doi: 10.1371/journal.ppat.1003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguine J, Breart B, Lemaitre F, Di Santo JP, Bousso P. Intravital imaging reveals distinct dynamics for natural killer and CD8(+) T cells during tumor regression. Immunity. 2010;33:632–644. doi: 10.1016/j.immuni.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Deguine J, Bousso P. Dynamics of NK cell interactions in vivo. Immunol Rev. 2013;251:154–159. doi: 10.1111/imr.12015. [DOI] [PubMed] [Google Scholar]
- Gregoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, et al. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson YT, Ljunggren HG, Long EO. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood. 2009;114:2657–2666. doi: 10.1182/blood-2009-01-201632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauveau A, Aucher A, Eissmann P, Vivier E, Davis DM. Membrane nanotubes facilitate long-distance interactions between natural killer cells and target cells. Proc Natl Acad Sci USA. 2010;107:5545–5550. doi: 10.1073/pnas.0910074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comerci CJ, Mace EM, Banerjee PP, Orange JS. CD2 promotes human natural killer cell membrane nanotube formation. PLOS One. 2012;7:e47664. doi: 10.1371/journal.pone.0047664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNerney ME, Kumar V. The CD2 family of natural killer cell receptors. Curr Topics Microbiol Immunol. 2006;298:91–120. doi: 10.1007/3-540-27743-9_5. [DOI] [PubMed] [Google Scholar]
- Lugini L, Cecchetti S, Huber V, Luciani F, Macchia G, Spadaro F, et al. Immune surveillance properties of human NK cell-derived exosomes. J Immunol. 2012;189:2833–2842. doi: 10.4049/jimmunol.1101988. [DOI] [PubMed] [Google Scholar]
- Kuznetsov VA. "Harpoon" model for cell-cell adhesion and recognition of target cells by the natural killer cells. J Theor Biol. 1996;180:321–342. doi: 10.1006/jtbi.1996.0106. [DOI] [PubMed] [Google Scholar]
- Barber DF, Faure M, Long EO. LFA-1 contributes an early signal for NK cell cytotoxicity. J Immunol. 2004;173:3653–3659. doi: 10.4049/jimmunol.173.6.3653. [DOI] [PubMed] [Google Scholar]
- Mace EM, Monkley SJ, Critchley DR, Takei F. A dual role for talin in NK cell cytotoxicity: activation of LFA-1-mediated cell adhesion and polarization of NK cells. J Immunol. 2009;182:948–956. doi: 10.4049/jimmunol.182.2.948. [DOI] [PubMed] [Google Scholar]
- Mace EM, Zhang J, Siminovitch KA, Takei F. Elucidation of the integrin LFA-1-mediated signaling pathway of actin polarization in natural killer cells. Blood. 2010;116:1272–1279. doi: 10.1182/blood-2009-12-261487. [DOI] [PubMed] [Google Scholar]
- Riteau B, Barber DF, Long EO. Vav1 phosphorylation is induced by beta2 integrin engagement on natural killer cells upstream of actin cytoskeleton and lipid raft reorganization. J Exp Med. 2003;198:469–474. doi: 10.1084/jem.20021995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culley FJ, Johnson M, Evans JH, Kumar S, Crilly R, Casasbuenas J, et al. Natural killer cell signal integration balances synapse symmetry and migration. PLoS Biol. 2009;7:e1000159. doi: 10.1371/journal.pbio.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML. Hunter to gatherer and back: immunological synapses and kinapses as variations on the theme of amoeboid locomotion. Ann Rev Cell Dev Biol. 2008;24:577–596. doi: 10.1146/annurev.cellbio.24.110707.175226. [DOI] [PubMed] [Google Scholar]
- Stebbins CC, Watzl C, Billadeau DD, Leibson PJ, Burshtyn DN, Long EO. Vav1 dephosphorylation by the tyrosine phosphatase SHP-1 as a mechanism for inhibition of cellular cytotoxicity. Mol Cell Biol. 2003;23:6291–6299. doi: 10.1128/MCB.23.17.6291-6299.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesecke S, Urlaub D, Busch H, Eils R, Watzl C. Integration of activating and inhibitory receptor signaling by regulated phosphorylation of Vav1 in immune cells. Sci Signal. 2011;4:ra36. doi: 10.1126/scisignal.2001325. [DOI] [PubMed] [Google Scholar]
- Liu D, Xu L, Yang F, Li D, Gong F, Xu T. Rapid biogenesis and sensitization of secretory lysosomes in NK cells mediated by target-cell recognition. Proc Natl Acad Sci USA. 2005;102:123–127. doi: 10.1073/pnas.0405737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand F, Muller S, Roh KH, Laurent C, Dupre L, Valitutti S. An initial and rapid step of lytic granule secretion precedes microtubule organizing center polarization at the cytotoxic T lymphocyte/target cell synapse. Proc Natl Acad Sci USA. 2013;110:6073–6078. doi: 10.1073/pnas.1218640110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Bryceson YT, Meckel T, Vasiliver-Shamis G, Dustin ML, Long EO. Integrin-dependent organization and bidirectional vesicular traffic at cytotoxic immune synapses. Immunity. 2009;31:99–109. doi: 10.1016/j.immuni.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Meckel T, Long EO. Distinct role of rab27a in granule movement at the plasma membrane and in the cytosol of NK cells. PLOS One. 2010;5:e12870. doi: 10.1371/journal.pone.0012870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentlik AN, Sanborn KB, Holzbaur EL, Orange JS. Rapid lytic granule convergence to the MTOC in natural killer cells is dependent on dynein but not cytolytic commitment. Mol Biol Cell. 2010;21:2241–2256. doi: 10.1091/mbc.E09-11-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AM, Hsu HT, Dongre P, Uzel G, Mace EM, Banerjee PP, et al. Rapid activation receptor- or IL-2-induced lytic granule convergence in human natural killer cells requires Src, but not downstream signaling. Blood. 2013;121:2627–2637. doi: 10.1182/blood-2012-06-437012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzewski K, Gil-Krzewska A, Nguyen V, Peruzzi G, Coligan JE. LAMP1/CD107a is required for efficient perforin delivery to lytic granules and NK-cell cytotoxicity. Blood. 2013;121:4672–4683. doi: 10.1182/blood-2012-08-453738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas YM, Mehta KM, Morgan M, Maniar H, Butros L, Jung S, et al. Spatial organization of signal transduction molecules in the NK cell immune synapses during MHC class I-regulated noncytolytic and cytolytic interactions. J Immunol. 2001;167:4358–4367. doi: 10.4049/jimmunol.167.8.4358. [DOI] [PubMed] [Google Scholar]
- Burshtyn DN, Shin J, Stebbins C, Long EO. Adhesion to target cells is disrupted by the killer cell inhibitory receptor. Curr Biol. 2000;10:777–780. doi: 10.1016/s0960-9822(00)00568-6. [DOI] [PubMed] [Google Scholar]
- Liu D, Peterson ME, Long EO. The adaptor protein Crk controls activation and inhibition of natural killer cells. Immunity. 2012;36:600–611. doi: 10.1016/j.immuni.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman DS, Schoon RA, Robertson MJ, Leibson PJ. Inhibition of selective signaling events in natural killer cells recognizing major histocompatibility complex class I. Proc Natl Acad Sci USA. 1995;92:6484–6488. doi: 10.1073/pnas.92.14.6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiante NM, Phillips JH, Lanier LL, Parham P. Killer cell inhibitory receptor recognition of human leukocyte antigen (HLA) class I blocks formation of a pp36/PLC-gamma signaling complex in human natural killer (NK) cells. J Exp Med. 1996;184:2243–2250. doi: 10.1084/jem.184.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson ME, Long EO. Inhibitory receptor signaling via tyrosine phosphorylation of the adaptor Crk. Immunity. 2008;29:578–588. doi: 10.1016/j.immuni.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodniewicz D, Klemke RL. Regulation of integrin-mediated cellular responses through assembly of a CAS/Crk scaffold. Biochim Biophys Acta. 2004;1692:63–76. doi: 10.1016/j.bbamcr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Arai A, Nosaka Y, Kohsaka H, Miyasaka N, Miura O. CrkL activates integrin-mediated hematopoietic cell adhesion through the guanine nucleotide exchange factor C3G. Blood. 1999;93:3713–3722. [PubMed] [Google Scholar]
- Katagiri K, Maeda A, Shimonaka M, Kinashi T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat Immunol. 2003;4:741–748. doi: 10.1038/ni950. [DOI] [PubMed] [Google Scholar]
- Ichiba T, Hashimoto Y, Nakaya M, Kuraishi Y, Tanaka S, Kurata T, et al. Activation of C3G guanine nucleotide exchange factor for Rap1 by phosphorylation of tyrosine 504. J Biol Chem. 1999;274:14376–14381. doi: 10.1074/jbc.274.20.14376. [DOI] [PubMed] [Google Scholar]
- Thomas LM, Peterson ME, Long EO. Cutting edge: NK cell licensing modulates adhesion to target cells. J Immunol. 2013;191:3981–3985. doi: 10.4049/jimmunol.1301159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki H, Suetsugu S, Takenawa T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 1998;17:6932–6941. doi: 10.1093/emboj/17.23.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolz JC, Nacusi LP, Segovis CM, Medeiros RB, Mitchell JS, Shimizu Y, et al. The WAVE2 complex regulates T cell receptor signaling to integrins via Abl- and CrkL-C3G-mediated activation of Rap1. J Cell Biol. 2008;182:1231–1244. doi: 10.1083/jcb.200801121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange JS, Ramesh N, Remold-O'Donnell E, Sasahara Y, Koopman L, Byrne M, et al. Wiskott-Aldrich syndrome protein is required for NK cell cytotoxicity and colocalizes with actin to NK cell-activating immunologic synapses. Proc Natl Acad Sci USA. 2002;99:11351–11356. doi: 10.1073/pnas.162376099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange JS, Roy-Ghanta S, Mace EM, Maru S, Rak GD, Sanborn KB, et al. IL-2 induces a WAVE2-dependent pathway for actin reorganization that enables WASp-independent human NK cell function. J Clin Invest. 2011;121:1535–1548. doi: 10.1172/JCI44862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham H, Guerrier S, Kim J, Schoon RA, Anderson EL, Hamann MJ, et al. Dedicator of cytokinesis 8 interacts with talin and Wiskott-Aldrich syndrome protein to regulate NK cell cytotoxicity. J Immunol. 2013;190:3661–3669. doi: 10.4049/jimmunol.1202792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada Y, Tanaka Y, Terasawa M, Pieczyk M, Habiro K, Katakai T, et al. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood. 2012;119:4451–4461. doi: 10.1182/blood-2012-01-407098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizesko MC, Banerjee PP, Monaco-Shawver L, Mace EM, Bernal WE, Sawalle-Belohradsky J, et al. Defective actin accumulation impairs human natural killer cell function in patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2013;131:840–848. doi: 10.1016/j.jaci.2012.12.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y, Tanaka Y, Yanagihara T, Watanabe M, Duan X, Terasawa M, et al. The Rac activator DOCK2 regulates natural killer cell-mediated cytotoxicity in mice through the lytic synapse formation. Blood. 2013;122:386–393. doi: 10.1182/blood-2012-12-475897. [DOI] [PubMed] [Google Scholar]
- Uruno T, Liu J, Zhang P, Fan Y, Egile C, Li R, et al. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat Cell Biol. 2001;3:259–266. doi: 10.1038/35060051. [DOI] [PubMed] [Google Scholar]
- Butler B, Kastendieck DH, Cooper JA. Differently phosphorylated forms of the cortactin homolog HS1 mediate distinct functions in natural killer cells. Nat Immunol. 2008;9:887–897. doi: 10.1038/ni.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler B, Cooper JA. Distinct roles for the actin nucleators Arp2/3 and hDia1 during NK-mediated cytotoxicity. Curr Biol. 2009;19:1886–1896. doi: 10.1016/j.cub.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann SC, Cohnen A, Ludwig T, Watzl C. 2B4 engagement mediates rapid LFA-1 and actin-dependent NK cell adhesion to tumor cells as measured by single cell force spectroscopy. J Immunol. 2011;186:2757–2764. doi: 10.4049/jimmunol.1002867. [DOI] [PubMed] [Google Scholar]
- Gross CC, Brzostowski JA, Liu D, Long EO. Tethering of intercellular adhesion molecule on target cells is required for LFA-1-dependent NK cell adhesion and granule polarization. J Immunol. 2010;185:2918–2926. doi: 10.4049/jimmunol.1000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gismondi A, Jacobelli J, Mainiero F, Paolini R, Piccoli M, Frati L, et al. Cutting edge: functional role for proline-rich tyrosine kinase 2 in NK cell-mediated natural cytotoxicity. J Immunol. 2000;164:2272–2276. doi: 10.4049/jimmunol.164.5.2272. [DOI] [PubMed] [Google Scholar]
- Orange JS, Harris KE, Andzelm MM, Valter MM, Geha RS, Strominger JL. The mature activating natural killer cell immunologic synapse is formed in distinct stages. Proc Natl Acad Sci USA. 2003;100:14151–14156. doi: 10.1073/pnas.1835830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman KD, Campi G, Groves JT, Dustin ML. Altered TCR signaling from geometrically repatterned immunological synapses. Science. 2005;310:1191–1193. doi: 10.1126/science.1119238. [DOI] [PubMed] [Google Scholar]
- Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- Beemiller P, Jacobelli J, Krummel MF. Integration of the movement of signaling microclusters with cellular motility in immunological synapses. Nat Immunol. 2012;13:787–795. doi: 10.1038/ni.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babich A, Li S, O'Connor RS, Milone MC, Freedman BD, Burkhardt JK. F-actin polymerization and retrograde flow drive sustained PLCgamma1 signaling during T cell activation. J Cell Biol. 2012;197:775–787. doi: 10.1083/jcb.201201018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Fay NC, Smoligovets AA, Wu HJ, Groves JT. Myosin IIA modulates T cell receptor transport and CasL phosphorylation during early immunological synapse formation. PloS ONE. 2012;7:e30704. doi: 10.1371/journal.pone.0030704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeyweera TP, Merino E, Huse M. Inhibitory signaling blocks activating receptor clustering and induces cytoskeletal retraction in natural killer cells. J Cell Biol. 2011;192:675–690. doi: 10.1083/jcb.201009135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddos S, Dunsby C, Purbhoo MA, Chauveau A, Owen DM, Neil MA, et al. High-speed high-resolution imaging of intercellular immune synapses using optical tweezers. Biophys J. 2008;95:L66–L68. doi: 10.1529/biophysj.108.143198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pageon SV, Cordoba SP, Owen DM, Rothery SM, Oszmiana A, Davis DM. Superresolution microscopy reveals nanometer-scale reorganization of inhibitory natural killer cell receptors upon activation of NKG2D. Sci Signal. 2013;6:ra62. doi: 10.1126/scisignal.2003947. [DOI] [PubMed] [Google Scholar]
- Treanor B, Lanigan PM, Kumar S, Dunsby C, Munro I, Auksorius E, et al. Microclusters of inhibitory killer immunoglobulin-like receptor signaling at natural killer cell immunological synapses. J Cell Biol. 2006;174:153–161. doi: 10.1083/jcb.200601108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guia S, Jaeger BN, Piatek S, Mailfert S, Trombik T, Fenis A, et al. Confinement of activating receptors at the plasma membrane controls natural killer cell tolerance. Sci Signal. 2011;4:ra21. doi: 10.1126/scisignal.2001608. [DOI] [PubMed] [Google Scholar]
- Endt J, McCann FE, Almeida CR, Urlaub D, Leung R, Pende D, et al. Inhibitory receptor signals suppress ligation-induced recruitment of NKG2D to GM1-rich membrane domains at the human NK cell immune synapse. J Immunol. 2007;178:5606–5611. doi: 10.4049/jimmunol.178.9.5606. [DOI] [PubMed] [Google Scholar]
- Masilamani M, Nguyen C, Kabat J, Borrego F, Coligan JE. CD94/NKG2A inhibits NK cell activation by disrupting the actin network at the immunological synapse. J Immunol. 2006;177:3590–3596. doi: 10.4049/jimmunol.177.6.3590. [DOI] [PubMed] [Google Scholar]
- Owen DM, Oddos S, Kumar S, Davis DM, Neil MA, French PM, et al. High plasma membrane lipid order imaged at the immunological synapse periphery in live T cells. Mol Membr Biol. 2010;27:178–189. doi: 10.3109/09687688.2010.495353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzewski K, Coligan JE. Human NK cell lytic granules and regulation of their exocytosis. Front Immunol. 2012;3:335. doi: 10.3389/fimmu.2012.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson YT, Chiang SC, Darmanin S, Fauriat C, Schlums H, Theorell J, et al. Molecular mechanisms of natural killer cell activation. J Innate Immun. 2011;3:216–226. doi: 10.1159/000325265. [DOI] [PubMed] [Google Scholar]
- Chen X, Allan DS, Krzewski K, Ge B, Kopcow H, Strominger JL. CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells. Proc Natl Acad Sci USA. 2006;103:10346–10351. doi: 10.1073/pnas.0604236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Trivedi PP, Ge B, Krzewski K, Strominger JL, Many NK. cell receptors activate ERK2 and JNK1 to trigger microtubule organizing center and granule polarization and cytotoxicity. Proc Natl Acad Sci USA. 2007;104:6329–6334. doi: 10.1073/pnas.0611655104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, Zhong B, Gilvary DL, Corliss BC, Hong-Geller E, Wei S, et al. Pivotal role of phosphoinositide-3 kinase in regulation of cytotoxicity in natural killer cells. Nat Immunol. 2000;1:419–425. doi: 10.1038/80859. [DOI] [PubMed] [Google Scholar]
- Li C, Ge B, Nicotra M, Stern JN, Kopcow HD, Chen X, et al. JNK MAP kinase activation is required for MTOC and granule polarization in NKG2D-mediated NK cell cytotoxicity. Proc Natl Acad Sci USA. 2008;105:3017–3022. doi: 10.1073/pnas.0712310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Skolnik EY, Prakriya M. Ion channels and transporters in lymphocyte function and immunity. Nat Rev. Immunol. 2012;12:532–547. doi: 10.1038/nri3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul-Pavicic A, Chiang SC, Rensing-Ehl A, Jessen B, Fauriat C, Wood SM, et al. ORAI1-mediated calcium influx is required for human cytotoxic lymphocyte degranulation and target cell lysis. Proc Natl Acad Sci USA. 2011;108:3324–3329. doi: 10.1073/pnas.1013285108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, et al. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 2011;475:471–476. doi: 10.1038/nature10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaigne-Delalande B, Li FY, O'Connor GM, Lukacs MJ, Jiang P, Zheng L, et al. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science. 2013;341:186–191. doi: 10.1126/science.1240094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann FE, Vanherberghen B, Eleme K, Carlin LM, Newsam RJ, Goulding D, et al. The size of the synaptic cleft and distinct distributions of filamentous actin, ezrin, CD43, and CD45 at activating and inhibitory human NK cell immune synapses. J Immunol. 2003;170:2862–2870. doi: 10.4049/jimmunol.170.6.2862. [DOI] [PubMed] [Google Scholar]
- Carlin LM, Eleme K, McCann FE, Davis DM. Intercellular transfer and supramolecular organization of human leukocyte antigen C at inhibitory natural killer cell immune synapses. J Exp Med. 2001;194:1507–1517. doi: 10.1084/jem.194.10.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Nakayama M, Kawano M, Amagai R, Ishii T, Harigae H, et al. Fratricide of natural killer cells dressed with tumor-derived NKG2D ligand. Proc Natl Acad Sci USA. 2013;110:9421–9426. doi: 10.1073/pnas.1300140110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roda-Navarro P, Vales-Gomez M, Chisholm SE, Reyburn HT. Transfer of NKG2D and MICB at the cytotoxic NK cell immune synapse correlates with a reduction in NK cell cytotoxic function. Proc Natl Acad Sci USA. 2006;103:11258–11263. doi: 10.1073/pnas.0600721103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GS, Collinson LM, Brzostek J, Eissmann P, Almeida CR, McCann FE, et al. Membranous structures transfer cell surface proteins across NK cell immune synapses. Traffic. 2007;8:1190–1204. doi: 10.1111/j.1600-0854.2007.00603.x. [DOI] [PubMed] [Google Scholar]
- Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- Mace EM, Orange JS. Dual channel STED nanoscopy of lytic granules on actin filaments in natural killer cells. Commun Integr Biol. 2012;5:184–186. doi: 10.4161/cib.18818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AC, Oddos S, Dobbie IM, Alakoskela JM, Parton RM, Eissmann P, et al. Remodelling of cortical actin where lytic granules dock at natural killer cell immune synapses revealed by super-resolution microscopy. PLoS Biol. 2011;9:e1001152. doi: 10.1371/journal.pbio.1001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak GD, Mace EM, Banerjee PP, Svitkina T, Orange JS. Natural killer cell lytic granule secretion occurs through a pervasive actin network at the immune synapse. PLoS Biol. 2011;9:e1001151. doi: 10.1371/journal.pbio.1001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AC, Dobbie IM, Alakoskela JM, Davis I, Davis DM. Super-resolution imaging of remodeled synaptic actin reveals different synergies between NK cell receptors and integrins. Blood. 2012;120:3729–3740. doi: 10.1182/blood-2012-05-429977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C, Beltzner CC, Pollard TD. Cofilin dissociates Arp2/3 complex and branches from actin filaments. Curr Biol. 2009;19:537–545. doi: 10.1016/j.cub.2009.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow LR, Roadcap DW, Paris K, Watson SR, Grigorova IL, Lebet T, et al. The actin regulator coronin 1A is mutant in a thymic egress-deficient mouse strain and in a patient with severe combined immunodeficiency. Nat Immunol. 2008;9:1307–1315. doi: 10.1038/ni.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugnier B, Nal B, Verthuy C, Boyer C, Lam D, Chasson L, et al. Coronin-1A links cytoskeleton dynamics to TCR alpha beta-induced cell signaling. PLOS One. 2008;3:e3467. doi: 10.1371/journal.pone.0003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman R, Meyer T. Coordinated oscillations in cortical actin and Ca2+ correlate with cycles of vesicle secretion. Nat Cell Biol. 2012;14:1261–1269. doi: 10.1038/ncb2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanborn KB, Mace EM, Rak GD, Difeo A, Martignetti JA, Pecci A, et al. Phosphorylation of the myosin IIA tailpiece regulates single myosin IIA molecule association with lytic granules to promote NK-cell cytotoxicity. Blood. 2011;118:5862–5871. doi: 10.1182/blood-2011-03-344846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanborn KB, Rak GD, Maru SY, Demers K, Difeo A, Martignetti JA, et al. Myosin IIA associates with NK cell lytic granules to enable their interaction with F-actin and function at the immunological synapse. J Immunol. 2009;182:6969–6984. doi: 10.4049/jimmunol.0804337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey R, DeStephan CM, Madge LA, May MJ, Orange JS. NKp30 ligation induces rapid activation of the canonical NF-kappaB pathway in NK cells. J Immunol. 2007;179:7385–7396. doi: 10.4049/jimmunol.179.11.7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersperger AR, Makedonas G, Betts MR. Flow cytometric detection of perforin upregulation in human CD8 T cells. Cytometry A. 2008;73:1050–1057. doi: 10.1002/cyto.a.20596. [DOI] [PubMed] [Google Scholar]
- Isaaz S, Baetz K, Olsen K, Podack E, Griffiths GM. Serial killing by cytotoxic T lymphocytes: T cell receptor triggers degranulation, re-filling of the lytic granules and secretion of lytic proteins via a non-granule pathway. Eur J Immunol. 1995;25:1071–1079. doi: 10.1002/eji.1830250432. [DOI] [PubMed] [Google Scholar]
- Makedonas G, Banerjee PP, Pandey R, Hersperger AR, Sanborn KB, Hardy GA, et al. Rapid up-regulation and granule-independent transport of perforin to the immunological synapse define a novel mechanism of antigen-specific CD8+ T cell cytotoxic activity. J Immunol. 2009;182:5560–5569. doi: 10.4049/jimmunol.0803945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202:1001–1012. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DB, Cella M, Giurisato E, Fujikawa K, Miletic AV, Kloeppel T, et al. Vav1 controls DAP10-mediated natural cytotoxicity by regulating actin and microtubule dynamics. J Immunol. 2006;177:2349–2355. doi: 10.4049/jimmunol.177.4.2349. [DOI] [PubMed] [Google Scholar]
- Sancho D, Nieto M, Llano M, Rodriguez-Fernandez JL, Tejedor R, Avraham S, et al. The tyrosine kinase PYK-2/RAFTK regulates natural killer (NK) cell cytotoxic response, and is translocated and activated upon specific target cell recognition and killing. J Cell Biol. 2000;149:1249–1262. doi: 10.1083/jcb.149.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee PP, Pandey R, Zheng R, Suhoski MM, Monaco-Shawver L, Orange JS. Cdc42-interacting protein-4 functionally links actin and microtubule networks at the cytolytic NK cell immunological synapse. J Exp Med. 2007;204:2305–2320. doi: 10.1084/jem.20061893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzewski K, Chen X, Strominger JL. WIP is essential for lytic granule polarization and NK cell cytotoxicity. Proc Natl Acad Sci USA. 2008;105:2568–2573. doi: 10.1073/pnas.0711593105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzewski K, Chen X, Orange JS, Strominger JL. Formation of a WIP-, WASp-, actin-, and myosin IIA-containing multiprotein complex in activated NK cells and its alteration by KIR inhibitory signaling. J Cell Biol. 2006;173:121–132. doi: 10.1083/jcb.200509076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laan L, Pavin N, Husson J, Romet-Lemonne G, van Duijn M, Lopez MP, et al. Cortical dynein controls microtubule dynamics to generate pulling forces that position microtubule asters. Cell. 2012;148:502–514. doi: 10.1016/j.cell.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J, Wu X, Chung AH, Chen JK, Kapoor TM, Hammer JA. Centrosome repositioning in T cells is biphasic and driven by microtubule end-on capture-shrinkage. J Cell Biol. 2013;202:779–792. doi: 10.1083/jcb.201301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuli A, Thiery J, James AM, Michelet X, Sharma M, Garg S, et al. Arf-like GTPase Arl8b regulates lytic granule polarization and natural killer cell-mediated cytotoxicity. Mol Biol Cell. 2013;24:3721–3735. doi: 10.1091/mbc.E13-05-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar N, Wilkins JA. IQGAP1 involvement in MTOC and granule polarization in NK-cell cytotoxicity. Eur J Immunol. 2011;41:2763–2773. doi: 10.1002/eji.201040444. [DOI] [PubMed] [Google Scholar]
- Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- Kupfer A, Dennert G, Singer SJ. Polarization of the Golgi apparatus and the microtubule-organizing center within cloned natural killer cells bound to their targets. Proc Natl Acad Sci USA. 1983;80:7224–7228. doi: 10.1073/pnas.80.23.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baixauli F, Martin-Cofreces NB, Morlino G, Carrasco YR, Calabia-Linares C, Veiga E, et al. The mitochondrial fission factor dynamin-related protein 1 modulates T-cell receptor signalling at the immune synapse. EMBO J. 2011;30:1238–1250. doi: 10.1038/emboj.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana A, Schwindling C, Wenning AS, Becherer U, Rettig J, Schwarz EC, et al. T cell activation requires mitochondrial translocation to the immunological synapse. Proc Natl Acad Sci USA. 2007;104:14418–14423. doi: 10.1073/pnas.0703126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abarca-Rojano E, Muniz-Hernandez S, Moreno-Altamirano MM, Mondragon-Flores R, Enriquez-Rincon F, Sanchez-Garcia FJ. Re-organization of mitochondria at the NK cell immune synapse. Immunol Let. 2009;122:18–25. doi: 10.1016/j.imlet.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Kurowska M, Goudin N, Nehme NT, Court M, Garin J, Fischer A, et al. Terminal transport of lytic granules to the immune synapse is mediated by the kinesin-1/Slp3/Rab27a complex. Blood. 2012;119:3879–3889. doi: 10.1182/blood-2011-09-382556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andzelm MM, Chen X, Krzewski K, Orange JS, Strominger JL. Myosin IIA is required for cytolytic granule exocytosis in human NK cells. J Exp Med. 2007;204:2285–2291. doi: 10.1084/jem.20071143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masedunskas A, Sramkova M, Parente L, Sales KU, Amornphimoltham P, Bugge TH, et al. Role for the actomyosin complex in regulated exocytosis revealed by intravital microscopy. Proc Natl Acad Sci USA. 2011;108:13552–13557. doi: 10.1073/pnas.1016778108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace EM, Wu WW, Ho T, Mann SS, Hsu HT, Orange JS. NK cell lytic granules are highly motile at the immunological synapse and require F-actin for post-degranulation persistence. J Immunol. 2012;189:4870–4880. doi: 10.4049/jimmunol.1201296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstak ED, Neeft M, Nehme NT, Voortman J, Cheung M, Goodarzifard M, et al. The munc13-4-rab27 complex is specifically required for tethering secretory lysosomes at the plasma membrane. Blood. 2011;118:1570–1578. doi: 10.1182/blood-2011-02-339523. [DOI] [PubMed] [Google Scholar]
- Liu D, Martina JA, Wu XS, Hammer JA, 3rd, Long EO. Two modes of lytic granule fusion during degranulation by natural killer cells. Immunol Cell Biol. 2011;89:728–738. doi: 10.1038/icb.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SM, Bryceson YT. Lymphocyte cytotoxicity: tug-of-war on microtubules. Blood. 2012;119:3873–3875. doi: 10.1182/blood-2012-02-410381. [DOI] [PubMed] [Google Scholar]
- Wood SM, Meeths M, Chiang SC, Bechensteen AG, Boelens JJ, Heilmann C, et al. Different NK cell-activating receptors preferentially recruit Rab27a or Munc13-4 to perforin-containing granules for cytotoxicity. Blood. 2009;114:4117–4127. doi: 10.1182/blood-2009-06-225359. [DOI] [PubMed] [Google Scholar]
- Menager MM, Menasche G, Romao M, Knapnougel P, Ho CH, Garfa M, et al. Secretory cytotoxic granule maturation and exocytosis require the effector protein hMunc13-4. Nat Immunol. 2007;8:257–267. doi: 10.1038/ni1431. [DOI] [PubMed] [Google Scholar]
- Arneson LN, Brickshawana A, Segovis CM, Schoon RA, Dick CJ, Leibson PJ. Cutting edge: syntaxin 11 regulates lymphocyte-mediated secretion and cytotoxicity. J Immunol. 2007;179:3397–3401. doi: 10.4049/jimmunol.179.6.3397. [DOI] [PubMed] [Google Scholar]
- Bryceson YT, Rudd E, Zheng C, Edner J, Ma D, Wood SM, et al. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. 2007;110:1906–1915. doi: 10.1182/blood-2007-02-074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcet-Palacios M, Odemuyiwa SO, Coughlin JJ, Garofoli D, Ewen C, Davidson CE, et al. Vesicle-associated membrane protein 7 (VAMP-7) is essential for target cell killing in a natural killer cell line. Biochem Biophys Res Commun. 2008;366:617–623. doi: 10.1016/j.bbrc.2007.11.079. [DOI] [PubMed] [Google Scholar]
- Krzewski K, Gil-Krzewska A, Watts J, Stern JN, Strominger JL. VAMP4- and VAMP7-expressing vesicles are both required for cytotoxic granule exocytosis in NK cells. Eur J Immunol. 2011;41:3323–3329. doi: 10.1002/eji.201141582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LC, Morgan AJ, Chen JL, Snead CM, Bloor-Young D, Shenderov E, et al. NAADP activates two-pore channels on T cell cytolytic granules to stimulate exocytosis and killing. Curr Biol. 2012;22:2331–2337. doi: 10.1016/j.cub.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina JA, Wu XS, Catalfamo M, Sakamoto T, Yi C, Hammer JA., 3rd Imaging of lytic granule exocytosis in CD8+ cytotoxic T lymphocytes reveals a modified form of full fusion. Cell Immunol. 2011;271:267–279. doi: 10.1016/j.cellimm.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Wu LG. The debate on the kiss-and-run fusion at synapses. Trends Neurosci. 2007;30:447–455. doi: 10.1016/j.tins.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Ann Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Verhage M, Sorensen JB. Vesicle docking in regulated exocytosis. Traffic. 2008;9:1414–1424. doi: 10.1111/j.1600-0854.2008.00759.x. [DOI] [PubMed] [Google Scholar]
- Capuano C, Paolini R, Molfetta R, Frati L, Santoni A, Galandrini R. PIP2-dependent regulation of Munc13-4 endocytic recycling: impact on the cytolytic secretory pathway. Blood. 2012;119:2252–2262. doi: 10.1182/blood-2010-12-324160. [DOI] [PubMed] [Google Scholar]
- Cohnen A, Chiang SC, Stojanovic A, Schmidt H, Claus M, Saftig P, et al. Surface CD107a/LAMP-1 protects natural killer cells from degranulation-associated damage. Blood. 2013;122:1411–1418. doi: 10.1182/blood-2012-07-441832. [DOI] [PubMed] [Google Scholar]
- Sun J, Bird CH, Sutton V, McDonald L, Coughlin PB, De Jong TA, et al. A cytosolic granzyme B inhibitor related to the viral apoptotic regulator cytokine response modifier A is present in cytotoxic lymphocytes. J Biol Chem. 1996;271:27802–27809. doi: 10.1074/jbc.271.44.27802. [DOI] [PubMed] [Google Scholar]
- Hirst CE, Buzza MS, Bird CH, Warren HS, Cameron PU, Zhang M, et al. The intracellular granzyme B inhibitor, proteinase inhibitor 9, is up-regulated during accessory cell maturation and effector cell degranulation, and its overexpression enhances CTL potency. J Immunol. 2003;170:805–815. doi: 10.4049/jimmunol.170.2.805. [DOI] [PubMed] [Google Scholar]
- Balaji KN, Schaschke N, Machleidt W, Catalfamo M, Henkart PA. Surface cathepsin B protects cytotoxic lymphocytes from self-destruction after degranulation. J Exp Med. 2002;196:493–503. doi: 10.1084/jem.20011836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metkar SS, Wang B, Catalan E, Anderluh G, Gilbert RJ, Pardo J, et al. Perforin rapidly induces plasma membrane phospholipid flip-flop. PLOS One. 2011;6:e24286. doi: 10.1371/journal.pone.0024286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simhadri VR, Andersen JF, Calvo E, Choi SC, Coligan JE, Borrego F. Human CD300a binds to phosphatidylethanolamine and phosphatidylserine, and modulates the phagocytosis of dead cells. Blood. 2012;119:2799–2809. doi: 10.1182/blood-2011-08-372425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romee R, Foley B, Lenvik T, Wang Y, Zhang B, Ankarlo D, et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17) Blood. 2013;121:3599–3608. doi: 10.1182/blood-2012-04-425397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann FE, Eissmann P, Onfelt B, Leung R, Davis DM. The activating NKG2D ligand MHC class I-related chain A transfers from target cells to NK cells in a manner that allows functional consequences. J Immunol. 2007;178:3418–3426. doi: 10.4049/jimmunol.178.6.3418. [DOI] [PubMed] [Google Scholar]
- Vanherberghen B, Olofsson PE, Forslund E, Sternberg-Simon M, Khorshidi MA, Pacouret S, et al. Classification of human natural killer cells based on migration behavior and cytotoxic response. Blood. 2013;121:1326–1334. doi: 10.1182/blood-2012-06-439851. [DOI] [PubMed] [Google Scholar]
- Choi PJ, Mitchison TJ. Imaging burst kinetics and spatial coordination during serial killing by single natural killer cells. Proc Natl Acad Sci USA. 2013;110:6488–6493. doi: 10.1073/pnas.1221312110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R, Watzl C. Serial killing of tumor cells by human natural killer cells—enhancement by therapeutic antibodies. PLOS One. 2007;2:e326. doi: 10.1371/journal.pone.0000326. [DOI] [PMC free article] [PubMed] [Google Scholar]