Abstract
Background
Hormonal resuscitation, specifically administration of levothyroxine (T4) and methylprednisolone (steroid, i.e. the “T4 Protocol”) in organ transplant donors, is becoming increasingly used. Previous studies have shown that this maximizes the number of usable organs by reducing metabolic disturbances post-brain death. However, anecdotal evidence has shown that steatotic livers are adversely affected by this protocol. Therefore, we sought to investigate the hypothesis that the use of T4 and steroid is detrimental to steatotic livers in a model of total hepatic warm ischemia and reperfusion (I/R).
Methods
We subjected male 8–10 week old C57BL/6 and ob/ob mice to injections of T4 and steroid 48 hours prior to 15 minutes of total hepatic ischemia, followed by 24 hours of reperfusion.
Results
We saw a significant decrease in survival in ob/ob animals given T4 and steroid as compared to single treated or vehicle-treated animals. This decrease in survival was accompanied by a dramatic increase in liver necrosis (as measured on a scale from 0–3) in these animals as compared to controls. Previous work in our lab has shown that UCP2 is a major mediator of I/R in steatotic animals, as it upsets normal energy homeostasis. Following with this hypothesis, we see a dramatic increase in UCP2 levels in the combination treated animals, which is accompanied by a concomitant decrease in ATP levels after reperfusion.
Conclusions
The T4 protocol is detrimental to steatotic livers subjected to I/R, likely due to a decreased ability to recover after reperfusion due to decreased ability to form ATP.
Keywords: Papworth, ischemia/reperfusion, liver, steatosis, UCP2
Introduction
The use of hormonal resuscitation to restore hemodynamic stability is a common practice in the management of cadaveric donors. Specifically, protocols that include the use of levothyroxine (T4) and methylprednisolone (steroid), known as the T4 protocol or the “Papworth cocktail,” were pioneered by Novitzky, et. al(1–4). The use of these has become commonplace, although the biological mechanisms governing their effectiveness have not been elucidated. The rationale for the use of these agents is that after brain death, the donor undergoes hormonal and metabolic changes secondary to neuro-hormonal axis failure, which leads to hormonal dysregulation and hemodynamic instability. In a dog model, brain death was followed in each subject by metabolic acidosis, increased circulating catecholamines and glucagon, and decreased corticosteroid and thyroid hormone levels (5). The Papworth cocktail, which includes a bolus of levothyroxine, methylprednisolone, insulin, and 50% dextrose, with continuous infusion of levothyroxine, was designed to restore this hormonal imbalance, thus stabilizing the donor and maximizing the number of organs procured. Previous data have shown that the use of similar formulations including a steroid and T4 results in improved procurement of viable grafts with improved function when used in heart transplants (6). However, published data and unpublished data collected from liver transplants at the Medical University of South Carolina suggest that organs subjected to this protocol had higher peak transaminases, a greater rate of graft dysfunction and failure, as well as a greater need for post-operative plasmapheresis (7–9). Further complicating an evaluation of the effectiveness of these protocols is that little is known about the effect that these cocktails have on more marginal donor organs, such as steatotic livers.
Hepatic steatosis (hepatocyte lipid accumulation) is one of the major factors determining the usability of a donor liver for transplantation. Higher levels of steatosis are correlated with poor performance of the donor liver in the recipient (10) (11), and a large number of cadaveric donor livers are discarded due to excess steatosis. Thus, the prevalence of asymptomatic steatosis has been identified and recognized as a determining factor in the prequalification criteria of living and cadaveric donors (12). However, the recipient pool is also progressively growing; thus, many transplant programs are accepting more “expanded criteria” donor livers that would have been previously rejected (13). Still, the pathophysiology of the poor performance and possible subsequent failure of these fatty livers remains unclear, and further understanding of the metabolic alterations taking place is necessary.
As a result of an ever-increasing population of obese individuals, these issues associated with the use of the Papworth cocktail in the procurement of steatotic livers require investigation. Principally, this necessitates the need for the understanding of the effects of pharmacologic manipulation of hemodynamically unstable donors in order to increase the number of usable organs harvested per donor. To this end, the whole organ and cellular effect of the pharmacologic manipulation on “marginal” or extended criteria livers will be evaluated to address the hypothesis that the T4 protocol is detrimental to steatotic liver allografts because of their heightened sensitivity to I/R injury, secondary to the increased upregulation of Uncoupling Protein-2 (UCP2).
Methods
Animal Studies
Adult 8–10 week-old male lean and ob/ob (B6.V-Lepob/J) mice were purchased from Jackson Laboratories (Bar Harbor, Maine). Mice designated as lean were the littermates of the ob/ob mice and are either homozygous wild-type (C57BL/6J) or heterozygous at the Lepob locus. These heterozygous mice produce leptin normally and are phenotypically indistinguishable from the homozygous wild-type C57BL/6J mice. Mice were housed 4 per cage in temperature and light controlled chambers on a 12h:12h light-dark cycle and provided with rodent chow (Labdiet #5001; St. Louis, MO) and water ad libitum. Mice described as “baseline” did not receive any treatment or surgery and were used for baseline measurements. Lean groups of mice contained 5–6 animals each, and ob/ob groups contained 8–10 animals so that the surviving animals comprised a large enough group from which we could draw statistical conclusions.
Levothyroxine (T4) and Methylprednisolone Administration (“Papworth Cocktail)”
T4 (Bedford Laboratories, Bedford, OH) and methylprednisolone (Sigma, St. Louis, MO) were reconstituted in normal saline for injection. Mice were injected 48 hours prior to ischemia with 500 μg/kg of T4 and 34 mg/kg of methylprednisolone i.p. These dosages were based on the Papworth formulation administered at human pediatric dosages(14).
Warm Ischemia/Reperfusion (I/R)
Warm hepatic I/R with bowel congestion was performed as previously described (15, 16). Briefly, mice were anesthetized with nembutal (50mg/kg body weight). A small incision was made opening the peritoneal cavity exposing the liver. A vessel loop was positioned around the portahepatis to induce total hepatic ischemia. Ischemia was performed for 15 minutes, after which the vessel loop was removed and the incision was closed. The animal was taken to a temperature controlled recovery cage and given free access to food and water. Mice were sacrificed at 24 hours after reperfusion. Whole blood was collected from the right ventricle after thoracotomy under anesthesia. Liver tissue was divided with a portion being preserved in 10% buffered formalin and the remainder being snap frozen in liquid nitrogen.
Serum Alanine Aminotransferase (ALT)
Whole blood was collected from the right ventricle after thoracotomy under anesthesia. Whole blood was allowed to clot at room temperature for 15 minutes followed by centrifugation at 3500g for 5 minutes at room temperature to collect serum and was then evaluated for serum ALT and expressed as IU/L (Clinical Laboratory Services, Medical University of South Carolina).
Histological Analysis
Hemotoxylin and Eosin (H&E) staining was performed as previously described (17, 18). Slides were graded for necrosis (necrotic index) as previously described on a modified scale from 0 to 3, where 0 shows no necrosis, 1 shows individual hepatocyte dropout, 2 shows small foci of hepatocyte dropout 2–3 cells across, and 3 shows confluent foci >3 cells in diameter(19). Ten high-powered fields per section were analyzed in relation to the central vein.
Uncoupling Protein-2 (UCP2) Expression via Northern Blot
Total cellular liver mRNA was extracted using RNA-Bee (Tel-Test, Inc.; Friendswood, TX) reagent according to the manufacturer’s instructions. RNA was fractionated by electrophoresis, transferred to a Nytran™ membrane, and the nucleic acid was fixed by UV cross-linking. UCP2 cDNA was used as previously described (Clone 531) (15). Blots were first prehybridized with QuikHyb™ buffer (Stratagene ; La Jolla, CA) for fifteen minutes at 65°C and then hybridized overnight at 65°C with 32P-UCP2 cDNA probe (Stratagene Random Priming Kit) in a rotisserie oven. Blots were washed twice for fifteen minutes with 2x SSC + 0.1% SDS at 55°C and once for thirty minutes with 0.1x SSC + 0.1% SDS at 55°C. The blots were exposed to Kodak film for 24 hours. For normalization, the blots were stripped and re-hybridized in a similar manner substituting an 18S cDNA probe.
ATP Content Assay
Whole liver ATP content was analyzed in duplicate using an ATP Assay Kit from Thermo Labsystems (Finland) as previously described (20). All samples were standardized to total protein, as determined with the bicinchoninic acid assay.
Statistical Analysis
Results are presented as mean±SEM. Statistical analyses of collected data were interpreted by paired student’s t-test for comparison of two groups or ANOVA for analysis of three or more groups with Newman-Keuls for post-hoc analysis. An alpha<0.05 was considered significant in this study.
Results
T4 protocol leads to decreased ob/ob survival
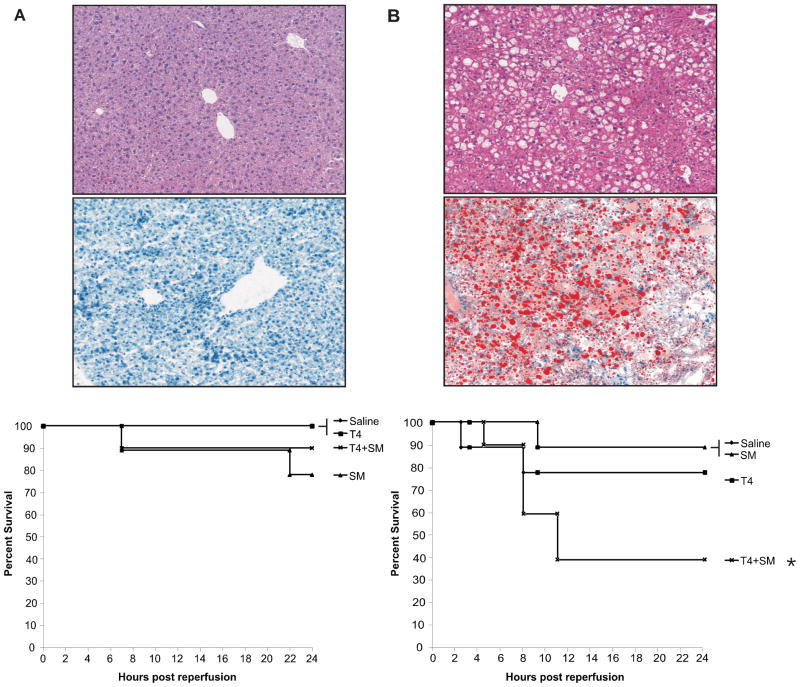
We have previously shown that ob/ob steatotic mice respond poorly to I/R injury as compared with their age-matched lean counterparts. In order to ascertain whether T4 decreases the survival of animals subjected to an I/R insult, we hypothesized that administration of the Papworth cocktail to both lean and ob/ob animals prior to ischemic insult would result in increased damage to hepatocytes after ischemia, thus decreasing animal survival. Consistent with this hypothesis, the data argued that pretreatment once daily for 48 hours prior to ischemic insult resulted in a 90% survival rate in the lean animals treated with T4/steroid and a 100% survival rate in the animals treated with T4 alone as compared with a 40% survival rate (p<0.05) in the ob/ob animals receiving T4/steroid and 80% receiving the T4 alone (Figure 1). There was no significant difference in survival rates among lean groups. Consequently, these results show that pre-administration of the Papworth cocktail to animals, prior to liver ischemia/reperfusion, significantly decreases survival of the steatotic animals.
Figure 1. Baseline Histology and Kaplan-Meier Survival Curves.
At baseline, lean animals had normal appearing histology, and had very little Oil-Red O (ORO) staining, indicating they had very little intracellular fat accumulation. ob/ob animals had significant fat artifact on H&E and had significant red staining on ORO, indicating a high level of intracellular fat. Animals were pretreated for 48 hours as indicated. Animals were ischemicized for 15 minutes and allowed to reperfuse for 24 hours. Above are the respective survival curves for each treatment. Lean animals (A, n=5/group) all had similar survival rates. ob/ob animals (B, n=10/group) had decreased survival in the T4/steroid group (*p<0.05).
Histological evaluation
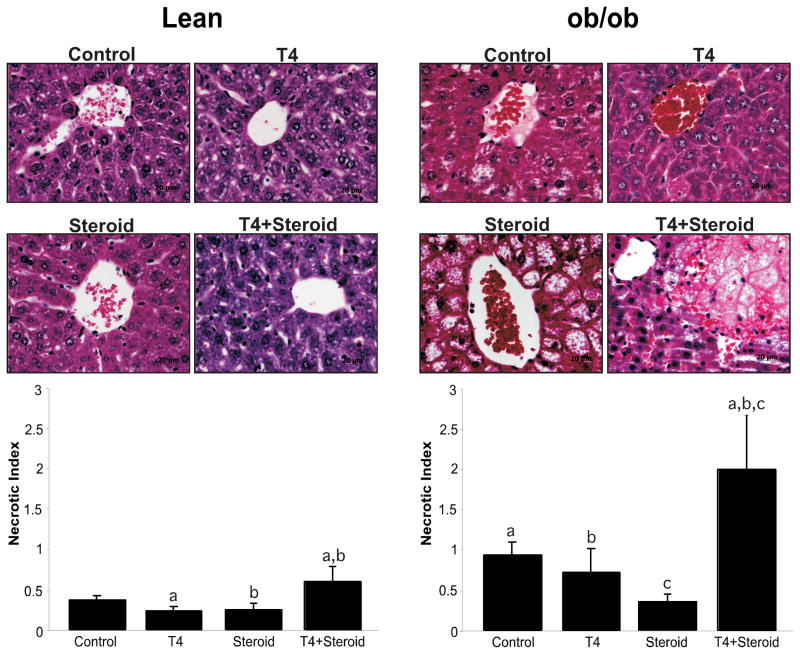
Frozen sections were stained with Oil Red O to confirm equality in the levels of fat between groups. Obese animals had approximately 60–70% fat, and there was no difference among groups. Lean animals had 0–5% fat, and there were no differences between groups (Figure 2). Histological analysis was performed to determine if the pre-treatment of both ob/ob and lean mice with levothyroxine and methylprednisolone was detrimental to hepatocytes following I/R injury. Representative images are presented in Figure 2. Samples were compared with vehicle treated animals also subjected to I/R. Evaluation of the tissues revealed increased centrilobular necrosis and a decreased area of viable tissue in the T4 and steroid treated groups as compared with their vehicle-treated counterparts. To further evaluate the histology, the H&E stained specimens were analyzed using a modification of a previously described method to measure the extent of necrosis (19). Values ranged from zero (no apoptosis) to three (confluent foci) using ten high-powered fields per section. In the animals treated with T4 and steroid, necrotic index values were mildly elevated to 0.6±0.18 in lean animals and markedly elevated to 2.0±0.67 in ob/ob as compared with their respective vehicle treated counterparts, showing significantly lower values [0.37±0.06 (lean) and 0.94±0.16 (ob/ob), (p<0.05)] (Figure 2). These results confirmed that in both the lean and obese animals, the T4 and steroid treated groups had a greater degree of cellular damage and necrosis as compared with vehicle treated animals.
Figure 2. Necrotic Index of Livers post-I/R.
After 24 hours of reperfusion, H&E stained slides were graded for necrosis on a scale of 0–3 as indicated. Both lean and ob/ob animals show significantly more necrosis in the T4 plus steroid-treated groups (p<0.05). Minimal necrosis was seen in the majority of the lean groups. There was marked necrosis in the saline and T4 treated ob/ob groups, with a reduction in the steroid-treated group and a dramatic increase in necrosis in the combination treated group, as compared to the other three groups. Groups marked with ‘a,’ ‘b,’ or ‘c’ are significant from each other at p<0.05. Representative histological pictures are shown that corresond to the graphs. Of note are the singular hepatocyte dropouts near the central veins in the ob/ob control and T4 treated animals, versus the very large area of necrosis in the combination treated, ob/ob animals.
Biochemical Evaluation
To further characterize and confirm the damage done to the livers of both the lean and obese animals by the ischemic insult, we next focused our attention on hepatocyte injury, as assessed by ALT levels. While not significant, the data showed that there was an increase in injury in both lean and ob/ob animals in the T4/steroid treated group. A more pronounced trend was observed in the ob/ob animals that closely paralleled the necrotic index values observed (data not shown). However, these data are somewhat skewed because the serum available at 24 hours for evaluation only represented the surviving animals. It is likely that the results would have been more striking had the non-surviving animals, presumably the ones with more severe liver damage, been included in the data set analyzed.
Quantification of ATP levels
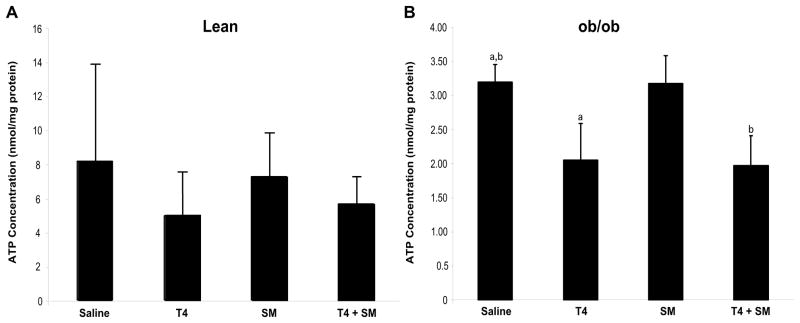
To determine if the ischemic injury correlated with a reduction in the concentration of ATP, levels of ATP were measured. A lower concentration of intracellular ATP would confer susceptibility of the insulted tissue to I/R injury that would then render it unable to return to normal following the I/R injury, resulting in the subsequent oncotic cell death. ATP levels were measured, normalized to total protein concentration, and compared to vehicle-treated groups. Particularly noteworthy was the significant depletion of ATP stores post-I/R in the obese animals treated with both T4 and T4 plus steroid (both 0.20 ummol/ng protein) as compared with the saline treated mice (0.32 ummol/ng protein, p<0.05, Figure 3). Although not as striking, the results in the lean group showed similar trends with the T4 and the T4 plus steroid groups having depleted ATP stores as compared with the control group (p=0.12 and p=0.19, respectively).
Figure 3. ATP Concentrations.
After 24 hours of reperfusion, tissue was ground and intracellular ATP levels were determined via a luciferase assay system. Intracellular ATP levels are significantly decreased in the ob/ob T4 and T4/steroid-treated groups versus the vehicle-treated and steroid-treated groups at 24 hours post-I/R (B, p<0.05, n=10/group). Though the trend was not significant, lean animals showed a similar trend (A). Additionally, overall the lean animals showed a much higher level of intracellular ATP versus the ob/ob animals, which is consistent with previous reports. Groups marked with ‘a’ or ‘b’ are significantly different from one another at p<0.05.
Evaluation of UCP2 levels
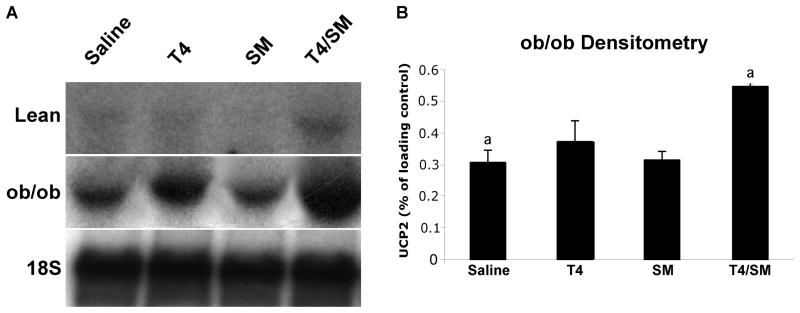
Previous research has shown UCP2 mRNA levels to be upregulated in tissues exposed to increased concentrations of levothyroxine (21) and other studies have shown it to be important in steatotic liver injury (22). Understandably, an increase in UCP2 levels could cause an increase in UCP2 protein levels, thereby uncoupling of the electron transport system, which would be consistent with our observation of a reduction in the concentration of intracellular ATP. To ascertain if the ATP depletion correlated with the levels of mitochondrial UCP2, we performed Northern Blot analysis of the tissue mitochondria and normalized the results to 18S rRNA. As expected in the ob/ob groups, there was an upregulation of UCP2 in both the T4 and T4 plus steroid treatment groups as compared with the vehicle treated groups as determined by densitometry (Figure 4, p<0.05). The T4 groups in the lean and ob/ob animals, as well as the T4 plus steroid group in the lean animals, also showed an increase in UCP2 levels, even though this was not significant. Normally, UCP2 is detected at very low levels, if at all. Here, we see very low levels in the saline and T4 group, nearly absent levels in the steroid-treated group, and we see an induction in the T4 plus steroid group (Figure 4, lean). These changes mirror the ob/ob findings, although at much lower levels that appear to have few physiological consequences.
Figure 4. UCP2 Levels.
Total RNA was isolated from liver tissue at 24 hours of reperfusion. Above is shown a representative Northern blot for UCP2 on the tissue from the lean and ob/ob animals (A). There is an upregulation of UCP2 in the T4 ob/ob group, and a more dramatic upregulation in the T4 plus steroid ob/ob group. There are very low levels of UCP2 in all the lean groups, with the exception of the T4 plus steroid lean group, which shows an upregulation mirroring the ob/ob findings. Densitometric analysis was performed to quantify the significant differences in the ob/ob animals (B). There is a significant increase n UCP2 levels in ob/ob animals (normalized to 18S rRNA) vs. vehicle-treated animals (a=p<0.05). Groups with the same letter superscripts are significant from each other at p<0.05. Groups with different letter superscripts are not different from each other.
Discussion
The prevalence of obesity in the American population has increased to pandemic levels and shows no signs of abatement. Coupled with this, we see an increased incidence of hepatic steatosis. Because of the rise in hepatic steatosis and reduction in the available donor pool, transplant centers are increasingly willing to consider “expanded criteria” donors for transplantation (23). Furthermore, additional donors are needed to address the needs of the ever-increasing list of eligible transplant recipients. However, efforts to improve the hemodynamic stability of the donor and maximize the number of usable organs procured may be detrimental to the viability of the procured organ and the function of the organ. Specifically, evidence presented here suggests that this may be true of the steatotic liver exposed to the Papworth cocktail.
To test the effects of the cocktail on steatotic livers, we have chosen a total hepatic ischemia model with bowel congestion. We have chosen this model for various reasons. First, and most importantly, it is a clinically relevant model for transplantation, as it includes a period of complete hepatic ischemia, coupled with a congestion of blood in the bowel that occurs during the anhepatic phase of transplantation. There are important studies showing a relationship between UCP2 and endotoxin, so eliminating this congestion from our model would not represent what occurs clinically (24, 25).
Mechanistically, the effect of the Papworth formulation is unclear, both in normal, as well as steatotic livers. Data have also shown that T4 increases mitochondrial uncoupling protein levels in the hepatocyte, further contributing to the decreased hepatocyte energy stores (21). Further exacerbating this problem is that previous research has shown that livers with increased steatosis have increased expression of mitochondrial UCP2, leading to depletion of intracellular energy stores (26). Recent data from our group has also shown that in steatotic animals, UCP2 is a major mediator of I/R injury, and knocking this protein out confers significant protection after I/R (22). By upregulating uncoupling protein 2 (UCP-2) on the membrane, the electromotive gradient is decreased as protons flow into the mitochondrial matrix without ADP phosphorylation, thus decreasing ATP synthesis. While the mechanism is not entirely clear, it is thought that the increased levels of uncoupling protein alleviates the availability of excess metabolic substrate and reduces the likelihood of the production of reactive oxygen species (ROS). However, a depletion of ATP results in a diminutive response to the stresses of ischemia/reperfusion (I/R) injury, leading to an increased incidence of primary non-function (PNF) (26, 27). Thus, adding an agent which further exacerbates ATP depletion and mitochondrial uncoupling may prove detrimental following I/R.
As part of this investigation, we explored the potential impact of the continued use of the “T4 protocol” on liver viability, a treatment modality that we have observed to be detrimental in terms of post-transplant graft function of steatotic livers in clinical practice. Originally, the Papworth cocktail was designed to restore the hormonal imbalances that occur post-brain death. Early studies proved that administration of thyroid hormone was capable of returning the tissues from anaerobic metabolism to an aerobic state, reducing the detrimental effects of prolonged anoxia and metabolic acidosis (1). These studies also showed that administration of the thyroid hormone increased donor temperature and mean arterial pressure, thus decreasing pressor requirements. There was also a decrease in central venous pressure, bicarbonate requirements, and heart rate, owing to the positive inotropic effects of the cocktail (28). The Papworth cocktail is also routinely used to increase the average number of organs that can be procured per donor. Despite this original data, there has been little effort to investigate the effects that the use of thyroid hormones would have on the viability of organs procured from expanded criteria liver donors.
Previous work has shown that T4 increases the levels of mitochondrial UCP2 in hepatocytes (21). Previous research in our laboratory has also shown that UCP2 levels are increased above basal levels in steatotic livers (26). Again, additional research in our laboratory has shown that knocking out UCP2 in obese animals confers significant protection after ischemia/reperfusion (22). These observations are consistent with the results obtained in this study. This provides a basis for an initial hypothesis for developing a mechanistic understanding of the damage incurred in steatotic livers subjected to I/R insult. UCP2 decreases the electromotive potential in the steatotic cells, thus decreasing the force available for generation of ATP. It has been shown that recovery of ATP levels in the hepatocyte was important for proper graft function, and, specifically, decreased ATP levels were implicated in the decreased survival of grafts (29). In addition, decreased membrane potential and ATP levels have been shown to contribute to necrotic cell death (30). We saw a decrease in survival that correlated well with the decrease in ATP levels in our steatotic animals treated with the Papworth cocktail. From this and previous studies, we conclude that the decreased potential to synthesize ATP in steatotic livers, especially those treated with T4, would result in a decreased ability of the organ to recover from I/R injury post-transplantation. Consequently, these data now serve as a foundation from which to base an explanation for the differences observed for the occurrence of PNF between normal and steatotic livers.
Previous work with cardiac allografts showed a significant increase in survival associated with donor organs treated with the steroid/T4 combination (6). However, this work failed to address the effect such treatments had on extra-cardiac organs. Further, the results were contradictory to results reported here with respect to observations made in steatotic mouse livers in terms of liver function and recovery of the animal. A retrospective study of the UNOS database showed that T4 and glucocorticoids were beneficial when used separately. These results were similar to those presented here with respect to the fact that the steroid-treated groups had improved survival and graft function. However, in the T4-treated group, and especially in the animals treated with both T4 and steroid, a significantly poorer performance was observed in their livers. Novitzky and others showed that administration of levothyroxine shifted the metabolism from an anaerobic mode to an aerobic form (1). This, coupled with the increased oxygenation provided by the methylprednisolone administration, likely results in a short-circuiting of the electron transport chain, leading to decreased ATP in the combination treated group. It is also suspected that the methylprednisolone might convert the T4 into the more active T3 compound, accounting for the more pronounced effect seen in this treatment group. Recent data has also been published citing the active form of T4, T3, as a stimulator of liver regeneration after a partial and subtotal hepatectomy. This further implicates T4 as a stimulus for energy consuming activities that may not be well tolerated in an ATP depleted state (31).
In conclusion, the administration of the “T4 protocol” prior to ischemia and reperfusion decreased animal survival in ob/ob animals. This observation was correlated with an increased necrotic index, increased UCP2 levels, and a subsequent decrease in the concentration of ATP within the cell. We believe that taken together, the data suggest a potential mechanism for an increased incidence of PNF in steatotic livers in the setting of the Papworth cocktail, and argue that further review of the benefits of this treatment modality should be carried out in relation to extra-cardiac organs.
Acknowledgments
This work was partially supported by NIH P20RR017677-06 and 5R01DK069369-02 to KDC.
Abbreviations
- ALT
Alanine Aminotransferase
- ATP
Adenosine tri-phosphate
- I/R
Ischemia/Reperfusion
- PNF
Primary Non-function
- SM
methylprednisolone (Solumedrol)
- T4
Levothyroxine
- UCP2
Uncoupling Protien-2
- UNOS
United Network for Organ Sharing
Footnotes
The authors have no conflicts of interest.
References
- 1.Novitzky D, Cooper DK, Morrell D, Isaacs S. Change from aerobic to anaerobic metabolism after brain death, and reversal following triiodothyronine therapy. Transplantation. 1988;45 (1):32. doi: 10.1097/00007890-198801000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Novitzky D, Cooper DK, Reichart B. Hemodynamic and metabolic responses to hormonal therapy in brain-dead potential organ donors. Transplantation. 1987;43 (6):852. [PubMed] [Google Scholar]
- 3.Novitzky D, Cooper DK, Reichart B. The value of hormonal therapy in improving organ viability in the transplant donor. Transplant Proc. 1987;19 (1 Pt 3):2037. [PubMed] [Google Scholar]
- 4.Novitzky D, Wicomb WN, Cooper DK, Tjaalgard MA. Improved cardiac function following hormonal therapy in brain dead pigs: relevance to organ donation. Cryobiology. 1987;24 (1):1. doi: 10.1016/0011-2240(87)90002-2. [DOI] [PubMed] [Google Scholar]
- 5.Bittner HB, Kendall SW, Chen EP, Van Trigt P. Endocrine changes and metabolic responses in a validated canine brain death model. J Crit Care. 1995;10 (2):56. doi: 10.1016/0883-9441(95)90017-9. [DOI] [PubMed] [Google Scholar]
- 6.Rosendale JD, Kauffman HM, McBride MA, et al. Hormonal resuscitation yields more transplanted hearts, with improved early function. Transplantation. 2003;75 (8):1336. doi: 10.1097/01.TP.0000062839.58826.6D. [DOI] [PubMed] [Google Scholar]
- 7.Chavin KD, Kirtz J, Rogers J, Lin A, Baillie GM, Baliga P. T4 Protocol: Donor stability at what price? Am J Transplant. 2002;3 (2):422. [Google Scholar]
- 8.Mandal AK, King KE, Humphreys SL, Maley WR, Burdick JF, Klein AS. Plasmapheresis: an effective therapy for primary allograft nonfunction after liver transplantation. Transplantation. 2000;70 (1):216. [PubMed] [Google Scholar]
- 9.Skerrett D, Mor E, Curtiss S, Mohandas K. Plasmapheresis in primary dysfunction of hepatic transplants. J Clin Apher. 1996;11 (1):10. doi: 10.1002/(SICI)1098-1101(1996)11:1<10::AID-JCA2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 10.D'Alessandro AM, Kalayoglu M, Sollinger HW, et al. The predictive value of donor liver biopsies on the development of primary nonfunction after orthotopic liver transplantation. Transplant Proc. 1991;23 (1 Pt 2):1536. [PubMed] [Google Scholar]
- 11.Marsman WA, Wiesner RH, Rodriguez L, et al. Use of fatty donor liver is associated with diminished early patient and graft survival. Transplantation. 1996;62 (9):1246. doi: 10.1097/00007890-199611150-00011. [DOI] [PubMed] [Google Scholar]
- 12.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21 (1):105. doi: 10.1055/s-2001-12933. [DOI] [PubMed] [Google Scholar]
- 13.Salizzoni M, Franchello A, Zamboni F, et al. Marginal grafts: finding the correct treatment for fatty livers. Transpl Int. 2003;16 (7):486. doi: 10.1007/s00147-003-0572-8. [DOI] [PubMed] [Google Scholar]
- 14.Ullah S, Zabala L, Watkins B, Schmitz ML. Cardiac organ donor management. Perfusion. 2006;21 (2):93. doi: 10.1191/0267659106pf851oa. [DOI] [PubMed] [Google Scholar]
- 15.Chavin KD, Fiorini RN, Shafizadeh S, et al. Fatty acid synthase blockade protects steatotic livers from warm ischemia reperfusion injury and transplantation. Am J Transplant. 2004;4 (9):1440. doi: 10.1111/j.1600-6143.2004.00546.x. [DOI] [PubMed] [Google Scholar]
- 16.Fiorini RN, Shafizadeh SF, Polito C, et al. Anti-endotoxin monoclonal antibodies are protective against hepatic ischemia/reperfusion injury in steatotic mice. Am J Transplant. 2004;4 (10):1567. doi: 10.1111/j.1600-6143.2004.00549.x. [DOI] [PubMed] [Google Scholar]
- 17.Preece A. A Manual for Histologic Technicians. 3. Boston: Carolina Press; 1985. [Google Scholar]
- 18.Luna L. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. Army Medical Museum (US); New York: McGraw-Hill Co; 1968. [Google Scholar]
- 19.Neil DA, Hubscher SG. Are parenchymal changes in early post-transplant biopsies related to preservation-reperfusion injury or rejection? Transplantation. 2001;71 (11):1566. doi: 10.1097/00007890-200106150-00014. [DOI] [PubMed] [Google Scholar]
- 20.Fiorini RN, Donovan JL, Rodwell D, et al. Short-term administration of (-)-epigallocatechin gallate reduces hepatic steatosis and protects against warm hepatic ischemia/reperfusion injury in steatotic mice. Liver Transpl. 2005;11 (3):298. doi: 10.1002/lt.20348. [DOI] [PubMed] [Google Scholar]
- 21.Jekabsons MB, Gregoire FM, Schonfeld-Warden NA, Warden CH, Horwitz BA. T(3) stimulates resting metabolism and UCP-2 and UCP-3 mRNA but not nonphosphorylating mitochondrial respiration in mice. Am J Physiol. 1999;277 (2 Pt 1):E380. doi: 10.1152/ajpendo.1999.277.2.E380. [DOI] [PubMed] [Google Scholar]
- 22.Evans ZP, Ellett JD, Schmidt MG, Schnellmann RG, Chavin KD. Mitochondrial uncoupling protein-2 mediates steatotic liver injury following ischemia/reperfusion. J Biol Chem. 2007 doi: 10.1074/jbc.M706784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levinsky NG. Organ donation by unrelated donors. N Engl J Med. 2000;343 (6):430. doi: 10.1056/NEJM200008103430610. [DOI] [PubMed] [Google Scholar]
- 24.Busquets S, Alvarez B, Van Royen M, Figueras MT, Lopez-Soriano FJ, Argiles JM. Increased uncoupling protein-2 gene expression in brain of lipopolysaccharide-injected mice: role of tumour necrosis factor-alpha? Biochim Biophys Acta. 2001;1499 (3):249. doi: 10.1016/s0167-4889(00)00126-9. [DOI] [PubMed] [Google Scholar]
- 25.Faggioni R, Shigenaga J, Moser A, Feingold KR, Grunfeld C. Induction of UCP2 gene expression by LPS: a potential mechanism for increased thermogenesis during infection. Biochem Biophys Res Commun. 1998;244 (1):75. doi: 10.1006/bbrc.1998.8219. [DOI] [PubMed] [Google Scholar]
- 26.Chavin KD, Yang S, Lin HZ, et al. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J Biol Chem. 1999;274 (9):5692. doi: 10.1074/jbc.274.9.5692. [DOI] [PubMed] [Google Scholar]
- 27.Birsner JH, Wan C, Cheng G, et al. Steatotic liver transplantation in the mouse: a model of primary nonfunction. J Surg Res. 2004;120 (1):97. doi: 10.1016/j.jss.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 28.Novitzky D, Cooper DK. Results of hormonal therapy in human brain-dead potential organ donors. Transplant Proc. 1988;20 (5 Suppl 7):59. [PubMed] [Google Scholar]
- 29.He XS, Ma Y, Wu LW, et al. Safe time to warm ischemia and posttransplant survival of liver graft from non-heart-beating donors. World J Gastroenterol. 2004;10 (21):3157. doi: 10.3748/wjg.v10.i21.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prabhakaran K, Li L, Mills EM, Borowitz JL, Isom GE. Up-regulation of uncoupling protein 2 by cyanide is linked with cytotoxicity in mesencephalic cells. J Pharmacol Exp Ther. 2005;314 (3):1338. doi: 10.1124/jpet.105.088625. [DOI] [PubMed] [Google Scholar]
- 31.Bockhorn M, Frilling A, Benko T, et al. Tri-iodothyronine as a stimulator of liver regeneration after partial and subtotal hepatectomy. Eur Surg Res. 2007;39 (1):58. doi: 10.1159/000098443. [DOI] [PubMed] [Google Scholar]