Abstract
Steatotic livers represent a growing proportion of marginal organs available for transplantation. These livers are highly prone to primary nonfunction following transplant, and are therefore routinely turned down for surgery. Given the elevated levels and sensitivity for reactive oxygen species (ROS) in these livers, we evaluated whether pretreatment with a targeted ROS scavenger, vitamin E succinate, could increase survival and decrease injury after ischemia/reperfusion (I/R). For this study, ob/ob mice received 50 I.U. of vitamin E succinate per day in supplemented vs. control chow for seven days and were subjected to 15 minutes of total hepatic ischemia, and 24 hours of reperfusion. Treatment resulted in a 5-fold decrease in ALT levels after reperfusion, mirrored by significant decreases in hepatocellular necrosis. These results suggest that targeted antioxidants such as vitamin E succinate may prove to be highly applicable for the pretreatment of steatotic donor livers, increasing their tolerance for I/R and the transplantation process.
Keywords: liver, transplantation, ischemia, steatosis, vitamin E
Given the current discrepancy between available donor livers and the length of the waiting list in the U.S., “marginal” livers are being used for transplantation leading to greater rates of graft primary nonfunction (PNF), retransplantation, and recipient mortality.1, 2 Livers approaching or exceeding 30% steatosis are considered marginal and carry a high rate of PNF.3 The hepatocellular condition of steatosis creates a state in which the cells are swollen due to the excessive fat accumulation, leading to sinusoidal obstruction. Sinusoidal lumens are narrowed in steatotic livers and disrupted during reperfusion, therefore reducing local perfusion, and possibly affecting hepatocellular recovery.4–6 This results in relative ischemia of fatty hepatocytes, which have been shown to have an increased sensitivity to hypoxia in culture.7 The mechanisms are not entirely understood, but the combination of altered sinusoidal perfusion along with the steatotic phenotype leads to increased reactive oxygen species (ROS) injury, mainly in the form of lipid peroxidation.8–10 The result is that steatotic livers are more sensitive to ischemia/reperfusion (I/R), exhibiting increased injury in the form of hepatocellular necrosis.11
A wide array of studies have shown promise using antioxidants to reduce liver I/R injury, however, the vast majority were conducted in normal lean livers and little is known in the steatotic condition.12 The benefits of ROS scavengers, such as vitamin E, have generally been attributed to its ability to inhibit ischemic ROS-induced mitochondrial damage and cellular lipid peroxidation.13 Esterified forms of vitamin E have become increasingly popular for use in virtually all settings. α-Tocopherol (vitamin E) is usually esterified to acetate or succinate in order to protect the phenolic functional group from premature oxidation, and to facilitate its uptake. These esterified forms, such as vitamin E succinate (VE) have specifically been shown to exhibit superior rates of absorption, especially to the liver, compared to nonesterified forms.12, 14, 15 We propose that VE-mediated ROS scavenging will ultimately protect the steatotic liver from the stressors of I/R.
MATERIALS AND METHODS
Animals
For these survival studies, 6–7 week old male ob/ob (leptin−/−) and control C57Bl6 mice (Jackson Labs) were used. Seven days prior to experimentation, mice were placed on either Purina #5001 chow or chow supplemented with 1g/kg of d-alpha-tocopherol succinate (VE) prepared by Dyets (Bethlehem, PA). Total daily consumption was approximately 50 I.U. per animal each day. Mice were either sacrificed or subjected to ischemia at the end of the 7-day treatment. All animals subjected to I/R were placed on control diet following ischemia. For all groups, n=5, and I/R was conducted as previously described.16 This experimental series complied with the protocols approved by the MUSC IACUC.
Analysis of Injury
Following 24 hours of reperfusion, blood was collected by sterile cardiac puncture, under isoflurane inhalation anesthesia, and serum was separated. Serum ALT concentrations were measured by a Synchron LX20 system (Beckman Coulter) and expressed as IU/L (Clinical Laboratory Services, MUSC). Hemotoxylin and eosin (H&E) staining was performed by MUSC university pathological services. Slides were blinded, then graded for centrilobular necrosis in H&E stained slides on a 0 to 3 scale as described by Neil et al.17 Ten central veins, each under high-powered magnification, were graded and the average was used as the individual grade for each animal. Representative photomicrographs were captured digitally and image brightness and size were adjusted using Adobe Photoshop® CS Version 8. All values are expressed as mean ± standard deviation (s.d.). An alpha value of 0.05 was established prior to experimentation as the limit for statistical significance. For each single pairwise comparison, a two-tailed t-test was used.
RESULTS
To investigate whether VE protects against I/R injury in 6–7 week old ob/ob mice, we treated with VE supplemented food for 7 days, after which time, the mice were subjected to 15 minutes of warm, total hepatic ischemia, followed by 24 hours of reperfusion. VE is specifically designed for intracellular hydrolysis and the esterified tag targets it for specific and increased uptake in the liver compared to other forms of vitamin E.15, 18 All ob/ob mice receiving VE survived I/R, while only 67% of the control ob/ob animals survived, although this difference was not significant (p=0.17)(Fig. 1a). Liver injury was assessed through the measurement of serum alanine aminotransferase (ALT) concentrations. At 24 hours following reperfusion, ALT levels in the VE treated mice were 5-fold lower than the surviving ob/ob controls (p<0.05)(Fig. 1b). As an additional indicator of hepatic injury, hepatocellular necrosis was measured histologically. Graded necrosis was significantly reduced in the VE treated mice on the 0 to 3 scale (Figure 6.2c, p<0.05). Large necrotic foci were observed in the control livers (Fig. 2a), extending well beyond the centrilobular space (zone 3), while necrosis in the VE treated livers was generally mild (Fig. 2b), and confined on average to a few cells adjacent to the central veins (Fig. 2c).
Figure 1.
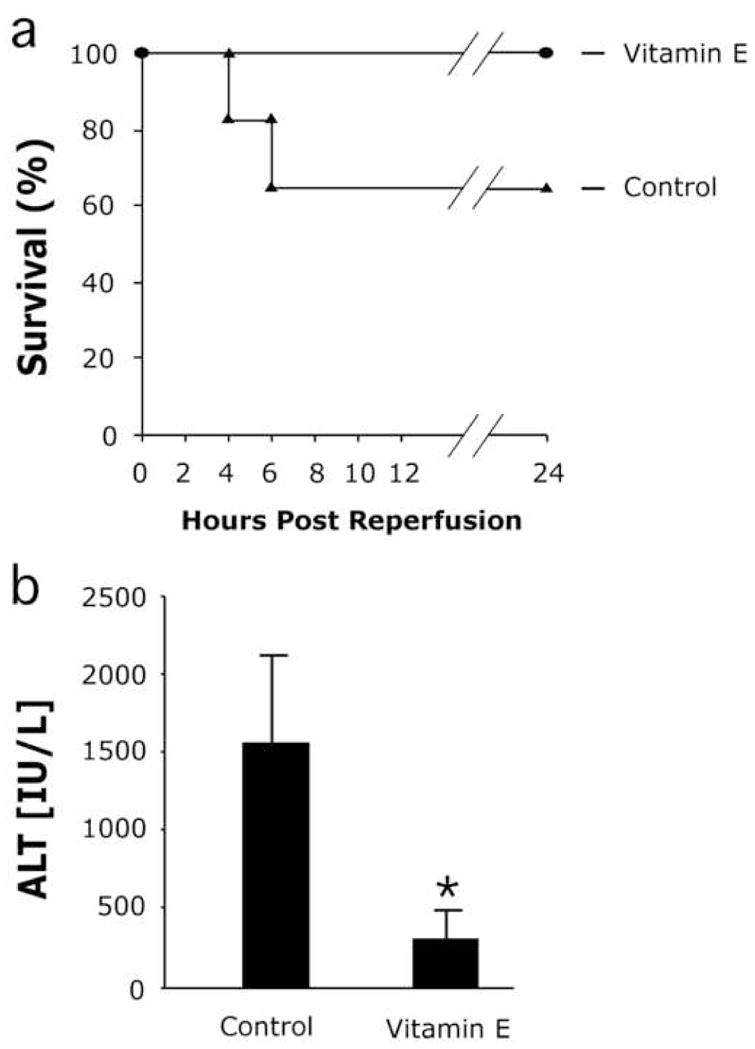
VE decreases hepatic injury following ischemia/reperfusion. (a) VE effects on animal survival after I/R (n.s.) (b) Hepatic injury was significantly reduced in UCP2 deficient ob/ob mice compared to ob/ob controls when assessed by measuring circulating levels of alanine aminotransferase (ALT). Animals were sacrificed and serum was collected 24 hours post-reperfusion, initial n=5/group. Mean values for each group are expressed as IU/L and are ± s.d. *p<0.05
Figure 2.
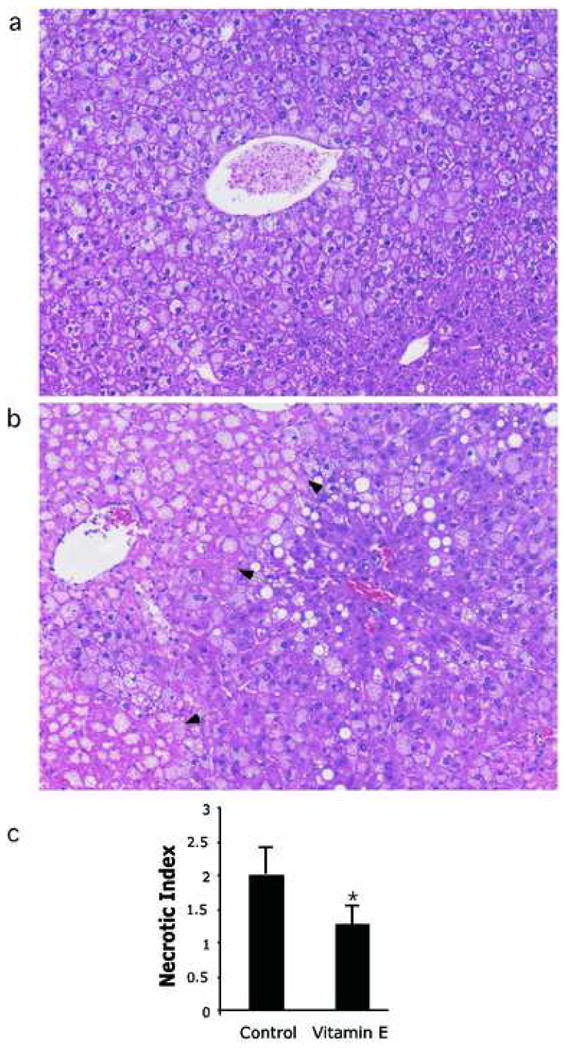
VE decreases hepatic necrosis following ischemia/reperfusion. At 24 hours following reperfusion, necrosis was measured in (a) control ob/ob livers and (b) VE treated ob/ob livers. Arrows indicate the boundary of necrosis. (c) Grading of centrilobular necrosis for each animal was conducted on a 0–3 scale, and scored per high-powered field, and represented graphically. Necrosis was significantly greater in the ob/ob control livers compared to the VE treated group. Mean values are expressed ± s.d. *p<0.05.
DISCUSSION
Although able to carry out their functions at rest, the hepatocytes of steatotic livers are acutely susceptible to injury. This observation is especially evident when the steatotic organ is exposed to the ischemia and reperfusion associated with transplantation.19, 20 Steatotic livers are more prone to necrosis, and more specifically oncotic cell death following I/R instead of apoptosis.11 In this study, we demonstrate that the pretreatment of animals with steatotic livers with VE reduced the level of injury following I/R.
VE may provide an ideal candidate for the pretreatment of steatotic livers in the clinical setting. For example, in vitro studies show that VE can enter into cells as the intact ester and then be hydrolyzed intracellularly back to alpha-tocopherol. Importantly, bypassing the gut results in delaying VE hydrolysis until cellular uptake, thus improving rate of delivery and even mitochondrial localization, especially in hepatocytes.21–23 Therefore, VE may in fact perform better when administered by a more clinically applicable route such as i.v. Additional studies are necessary to determine the effectiveness of VE treatment by these alternative routes and for a more clinically appropriate window of time.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fukumori T, Ohkohchi N, et al. The mechanism of injury in a steatotic liver graft during cold preservation. Transplantation. 1999;67:195. doi: 10.1097/00007890-199901270-00002. [DOI] [PubMed] [Google Scholar]
- 2.Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651. doi: 10.1053/jlts.2003.50105. [DOI] [PubMed] [Google Scholar]
- 3.D’Alessandro AM, Kalayoglu M, et al. The predictive value of donor liver biopsies for the development of primary nonfunction after orthotopic liver transplantation. Transplantation. 1991;51:157. doi: 10.1097/00007890-199101000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Hakamada K, Sasaki M, et al. Sinusoidal flow block after warm ischemia in rats with diet-induced fatty liver. J Surg Res. 1997;70:12. doi: 10.1006/jsre.1997.5077. [DOI] [PubMed] [Google Scholar]
- 5.Sato N, Eguchi H, et al. Hepatic microcirculation in zucker fatty rats. Adv Exp Med Biol. 1986;200:477. doi: 10.1007/978-1-4684-5188-7_59. [DOI] [PubMed] [Google Scholar]
- 6.Selzner M, Rudiger HA, et al. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology. 2000;32:1280. doi: 10.1053/jhep.2000.20528. [DOI] [PubMed] [Google Scholar]
- 7.Caraceni P, Ryu HS, et al. Rat hepatocytes isolated from alcohol-induced fatty liver have an increased sensitivity to anoxic injury. Hepatology. 1997;25:943. doi: 10.1002/hep.510250426. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson DM, Gores GJ, et al. Uw solution protects against reperfusion injury by inhibiting lipid peroxidation. Transplant Proc. 1991;23:1552. [PubMed] [Google Scholar]
- 9.Gao W, Connor HD, et al. Primary nonfunction of fatty livers produced by alcohol is associated with a new, antioxidant-insensitive free radical species. Transplantation. 1995;59:674. doi: 10.1097/00007890-199503150-00005. [DOI] [PubMed] [Google Scholar]
- 10.Kanno M, Ohkohchi N, et al. Lipid peroxidation of parenchymal hepatocytes during cold preservation and after reoxygenation in rats. Transplant Proc. 1993;25:2716. [PubMed] [Google Scholar]
- 11.Malhi H, Gores GJ, et al. Apoptosis and necrosis in the liver: A tale of two deaths? Hepatology. 2006;43:S31. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- 12.Glantzounis GK, Salacinski HJ, et al. The contemporary role of antioxidant therapy in attenuating liver ischemia-reperfusion injury: A review. Liver Transpl. 2005;11:1031. doi: 10.1002/lt.20504. [DOI] [PubMed] [Google Scholar]
- 13.Packer L. Protective role of vitamin e in biological systems. Am J Clin Nutr. 1991;53:1050S. doi: 10.1093/ajcn/53.4.1050S. [DOI] [PubMed] [Google Scholar]
- 14.Fariss MW, Merson MH, et al. Alpha-tocopheryl succinate protects hepatocytes from chemical-induced toxicity under physiological calcium conditions. Toxicol Lett. 1989;47:61. doi: 10.1016/0378-4274(89)90086-6. [DOI] [PubMed] [Google Scholar]
- 15.Fariss MW, Nicholls-Grzemski FA, et al. Enhanced antioxidant and cytoprotective abilities of vitamin e succinate is associated with a rapid uptake advantage in rat hepatocytes and mitochondria. Free Radic Biol Med. 2001;31:530. doi: 10.1016/s0891-5849(01)00615-3. [DOI] [PubMed] [Google Scholar]
- 16.Evans ZP, Ellett JD, et al. Mitochondrial uncoupling protein-2 mediates steatotic liver injury following ischemia/reperfusion. J Biol Chem. 2008;283:8573. doi: 10.1074/jbc.M706784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neil DA, Hubscher SG. Are parenchymal changes in early post-transplant biopsies related to preservation-reperfusion injury or rejection? Transplantation. 2001;71:1566. doi: 10.1097/00007890-200106150-00014. [DOI] [PubMed] [Google Scholar]
- 18.Teng XW, Davies NM, et al. Pharmacokinetics and tissue distribution of d-alpha-tocopheryl succinate formulations following intravenous administration in the rat. Biopharm Drug Dispos. 2005;26:195. doi: 10.1002/bdd.451. [DOI] [PubMed] [Google Scholar]
- 19.Chavin KD, Yang S, et al. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver atp depletion. J Biol Chem. 1999;274:5692. doi: 10.1074/jbc.274.9.5692. [DOI] [PubMed] [Google Scholar]
- 20.Birsner JH, Wan C, et al. Steatotic liver transplantation in the mouse: A model of primary nonfunction. J Surg Res. 2004;120:97. doi: 10.1016/j.jss.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 21.Zhang JG, Nicholls-Grzemski FA, et al. Vitamin e succinate protects hepatocytes against the toxic effect of reactive oxygen species generated at mitochondrial complexes i and iii by alkylating agents. Chem Biol Interact. 2001;138:267. doi: 10.1016/s0009-2797(01)00278-2. [DOI] [PubMed] [Google Scholar]
- 22.Ray SD, Fariss MW. Role of cellular energy status in tocopheryl hemisuccinate cytoprotection against ethyl methanesulfonate-induced toxicity. Arch Biochem Biophys. 1994;311:180. doi: 10.1006/abbi.1994.1224. [DOI] [PubMed] [Google Scholar]
- 23.Fariss MW. Oxygen toxicity: Unique cytoprotective properties of vitamin e succinate in hepatocytes. Free Radic Biol Med. 1990;9:333. doi: 10.1016/0891-5849(90)90008-7. [DOI] [PubMed] [Google Scholar]


