Abstract
Ca2+ oscillations are a hallmark of mammalian fertilization and play a central role in the activation of development. The calcium required for these oscillations is primarily derived from the endoplasmic reticulum (ER), which accumulates in clusters at the microvillar subcortex during oocyte maturation. The migration of the ER to the cortex during maturation is thought to play an important role in rendering the ER competent to generate the calcium transients, and the redistribution of ER is believed to be primarily mediated by microtubules and microfilaments. We have previously shown that the oocyte- and early embryo-restricted maternal effect gene Mater (Nlrp5) localizes to, and is required for, formation of the oocyte cytoplasmic lattices, a tubulin-containing structure that appears to play an important role in organelle positioning and distribution during oocyte maturation. Given these observations, we hypothesized that Mater may also be required for ER redistribution and Ca2+ homeostasis in oocytes. To test this hypothesis, we first investigated ER localization in metaphase-II Matertm/tm (hypomorph) oocytes and found ER clusters to be less abundant at the microvillar cortex when compared to wild type oocytes. To examine the potential mechanisms by which MATER mediates ER redistribution, we tested whether tubulin expression levels and localization were affected in the mutant oocytes and found that the Triton-insoluble fraction of tubulin was significantly decreased in Matertm/tm oocytes. To identify potential functional defects associated with these ER abnormalities, we next set out to investigate if the pattern of Ca2+ oscillations was altered in Matertm/tm oocytes after fertilization in vitro. Intriguingly, Ca2+ oscillations in Matertm/tm oocytes exhibited a significantly lower first peak amplitude and a higher frequency when compared to wild type oocytes. We then found that the Ca2+ oscillation defect in Matertm/tm oocytes was likely caused by a reduced amount of Ca2+ in the ER stores. Taken together, these observations support the hypothesis that MATER is required for ER distribution and Ca2+ homeostasis in oocytes, likely due to defects in lattice-mediated ER positioning and/or redistribution.
Keywords: MATER, Calcium homeostasis, Endoplasmic reticulum, Oocyte maturation, Tubulin
Introduction
Ca2+ oscillations are a hallmark of mammalian fertilization and regulate the transition of oocytes into early embryos (Cuthbertson and Cobbold, 1985; Stricker, 1999; Whitaker, 2006). The prevailing model of calcium regulation at egg activation involves initial release when sperm release phospholipase C zeta (PLCz) into the oocyte cytoplasm (Saunders et al., 2002). PLCz then cleaves phosphatidylinositol 4, 5-bisphosphate (PIP2) into 1, 4, 5-trisphosphate (IP3) and diacylglycerol (DAG). Binding of IP3 to IP3 receptors (IP3Rs) located at the ER membranes elicits the release of Ca2+ from the ER resulting in a rise of intracellular Ca2+ ([Ca2+]i) (Miyazaki et al., 1992). This initial rise in [Ca2+]i is followed by repetitive [Ca2+]i transients, termed Ca2+oscillations, which stimulate egg activation and embryonic development (Runft et al., 2002; Miyazaki and Ito, 2006).
Metaphase II (MII) arrested oocytes show a higher level of IP3-induced Ca2+ release at fertilization compared to GV stage oocytes (Fujiwara et al., 1993; Mehlmann and Kline, 1994). This finding indicates that changes occur during oocyte maturation to promote optimized IP3- induced Ca2+ transients. These changes include upregulation and post-translational modification of IP3Rs, an increase of Ca2+ stores, and a redistribution of the endoplasmic reticulum (ER) to the microvillar cortex. More specifically, Ca2+ stores in the ER have been shown to increase during maturation and these increases are directly correlated with optimized Ca2+ oscillation patterns at fertilization (Tombes et al., 1992; Jones et al., 1995). Additionally, expression levels and phosphorylation of IP3R-1, the primary IP3R isoform in mammalian oocytes, increases during maturation and these increases are related to IP3R-1 sensitivity (Mehlmann et al., 1996; Parrington et al., 1998; Xu et al., 2003). Such molecular changes are accompanied by a redistribution of the ER from a more evenly distributed pattern at the GV stage to more polarized pattern that is concentrated at the vegetal cortex in metaphase II-arrested oocytes (Kline et al., 1999; FitzHarris et al., 2007). Given that the initial fertilization-induced Ca2+ rise is seen to propagate from this region after sperm-egg fusion, it is generally believed that these cortical ER clusters are the primary source of calcium for the oscillation. Importantly, the redistribution of ER during oocyte maturation is conserved across a wide range of species, suggesting that ER re-targeting is critical for functional calcium oscillations (Jaffe and Terasaki, 1994; Shiraishi et al., 1995; Stricker et al., 1998; Terasaki et al., 2001; FitzHarris et al., 2007; Mann et al., 2010).
Regarding the mechanisms by which the ER redistributes during oocyte maturation, previous studies have shown that microtubules (MTs) appear to be essential for this process (Mehlmann et al., 1995; FitzHarris et al., 2003; FitzHarris et al., 2007). MTs, along with microfilaments, mediate two major redistributions of ER during oocyte maturation from a dispersed ER pattern at the GV stage, to a congression of ER around the nucleus during germinal vesicle breakdown (GVBD), and finally to an accumulation of ~1–2 μm clusters of ER appearing at the cortex of mature MII-arrested oocytes. However, studies investigating the role of the cytoskeleton in ER redistribution remain limited. Given that the dynamic redistribution of organelles during oocyte maturation occurs within an exceedingly large volume (relative to somatic cells), it stands to reason that the oocyte may have evolved a specific structure to help facilitate organelle positioning and distribution. Recent studies suggest that the oocyte cytoplasmic lattices might represent just such a structure. The cytoplasmic lattices first appear as oocytes start to grow and eventually become a dominant feature of the mature metaphase II-arrested mouse oocyte (Wassarman and Josefowicz, 1978; Garcia et al., 1979; Sternlicht and Schultz, 1981). More recently, the maternal effect gene, peptidylarginine deiminase 6 (PADI6), was found to localize to the lattices, thus providing insight into the molecular nature of this structure (Wright et al., 2003). PADI6 is an oocyte and early embryo abundant maternal effect gene, whose genes are produced and stored in the oocytes, and required for embryonic development (Esposito et al., 2007). Through use of Padi6 knockout mice, we have found that PADI6 is required for lattice formation and that the lattices appear to contain or regulate a stable form of non-spindle associated microtubules (Kan et al., 2011). Furthermore, we found that targeting of the ER and mitochondria to the oocyte cortex and the peri-spindle regions during maturation was defective in Padi6 mutant oocytes, thus suggesting that PADI6 and the lattices play a vital role in microtubule-mediated organelle redistribution.
MATER represents another oocyte- and embryo-abundant maternal effect gene that is essential for female fertility (Tong et al., 2000). We previously showed that, similar to PADI6, MATER also localize to the oocyte’s cytoplasmic lattices and is required for lattice formation (Kim et al., 2010). Given our findings with PADI6, here we decided to test whether similar organelle redistribution defects occur in mutant, hypomorphic Mater oocytes. Furthermore, given the requirement of cortical ER clustering for optimal calcium signaling in mature oocytes, we also tested whether Ca2+ homeostasis was defective in mutant Mater oocytes. Outcomes from our study indicate that both ER positioning and Ca2+ signaling do appear to be significantly altered in mutant Mater oocytes. These findings provide new insight into the molecular mechanisms driving ER positioning and function in the mammalian oocyte.
Materials and methods
Mice
Oocytes were collected from Mater+/+ and Matertm/tm mice. The generation of MATER transgenic mice has been described elsewhere (Tong et al., 2000). Originally, Mater tm/tm mice were identified as knockout mice, but the advanced molecular techniques later identified residual amounts of MATER protein in Mater tm/tm oocytes, and therefore, these mice are now called Mater tm/tm or Mater hypomorphs (Ohsugi et al., 2008). CD-1 male mice were purchased from commercial vendors. Mouse colonies were housed in the ECRF mouse facility at Cornell University’s College of Veterinary Medicine in accordance with the “NIH Guidelines for the Care and Use of Laboratory Animals,” and all experiments were performed with permission of Cornell University’s Institutional Animal Care and Use Committee.
Collection of gametes
Oocytes
Germinal vesicle stage oocytes were collected from 4–6 week female mice in M2 media (supplemented with 200 μM IBMX) approximately 46–48 h after injection of 2.5–5 IU pregnant mare serum gonadotrophin (PMSG). Metaphase II oocytes were collected 12.5–14 h after injection of ~5 IU of human chorionic gonadotrophin (hCG) and cumulus cells were removed using 0.1% Hyaluronidase. For zona-free oocytes, MII eggs were collected in Tyrode-HEPES buffer with PVA. To remove the zona pellucida, eggs were briefly treated with acid tyrode solution (pH 1.6) and washed 3X in Tyrode-HEPES with PVA.
Sperm
For in vitro fertilization, cauda epididymal sperm were collected into 900 μl of HTF media (supplemented with 4 mg/ml BSA) from retired CD1 breeding males. To capacitate sperm, 100 μl of the sample was further diluted in 200 μl HTF media and incubated for 2–3 h in 37 °C incubator with 5% CO2.
Ca2+ imaging
For Ca2+ imaging, oocytes were loaded with 1.5 μM Fluo-4 AM (molecular probes) and 0.2% Pluronic F-127 in Tyrode-HEPES buffer with PVA for 20 min at 37 ºC or room temperature. Oocytes were washed 3X with Ca2+ free Tyrode-HEPES (containing 1 mM EGTA) and attached to poly-l-lysine coated Mattek dish for imaging. After baseline signals were recorded, thapsigargin (5 μM) or ionomycin (1 μM) was added to the media to release Ca2+ from stores. Images were taken at room temperature every 1.573 second using an Argon laser-equipped Zeiss 510 confocal microscope (objective = 20x, filter set: BP 505-550). For imaging Ca2+ oscillations, eggs were attached to Mattek dish in 50 μl of HTF media (without BSA) under mineral oil following brief washes in Tyrode-HEPES and HTF media. 50 μl of HTF media (with 8 mg/ml BSA) was then added to the dish and the eggs were inseminated with ~3000 sperm. Upon insemination, eggs were imaged for 2 h every 20 sec using a Zeiss LSM 510 confocal microscope with 20x objective. Amplitude and frequency of Ca2+ oscillations were recorded from 21 Mater+/+ and 28 Matertm/tm oocytes in 4 independent experiments and analyzed as indicated in the statistical analysis section.
Lentiviral infection
Flag-tagged Mater was subcloned into a TetO-FUW vector and viral packaging plasmids psPAX2 and pMD2.G (plasmids were gifts from Dr. John Schimenti) were co-transfected with TetO-FUW-Mater, rtTA into 293T cells. Viral supernatants were collected 48–72 h after transfection, and rtTA and TetO-FUW-Mater were pooled together and concentrated. 3×105 Cos-1 cells were seeded into 6 well plates one day prior to infection and cells were infected for 24 h. 2 μg/ml doxycycline was added into viral infected cells and assayed 48–72 h later.
Immunoprecipitation
HEK293 cells were transfected with MATER-pcDNA3.1 construct or pcNDA3.1 empty vector as a control (plasmids were gifts from Dr. Lawrence Nelson) using X-tremeGENE9 transfection reagent (Roche). 24 hours post transfection, cells were collected and lysed in RIPA buffer. The lysate was centrifuged and supernatants were pre-cleared with pre-imunue serum and protein A Sepharose beads. After clearing, supernatants were then incubated either with monoclonal anti-3-Tubulin or normal mouse IgG for 2 hours at 4 °C and further incubated with protein A Sepharose (GE Healthcare). The immune complex was pelleted, washed, and resolved by SDS-PAGE and immunoblotted as described below.
Western blotting
Western blotting procedure was described elsewhere (Kim et al., 2010). Briefly, oocytes were collected, lysed in Laemmli buffer, and boiled for 10 min at 100°C. For western blotting, PVDF membranes were run in 7.5% or 10% PAGE gel, transferred overnight, blocked in TBST with 5% milk, and incubated 1 h with anti-GRP78 (1:500, Abcam), MATER (1:8000), Tubulin (1: 10000, Sigma), Actin (1:5000, Abcam) antibodies, or overnight with anti-Rbt03 antibody at 1:1000. Rbt03 antibodies were kindly gifted from Rafael Fissore (University of Massachusetts, Amherst) and MATER polyclonal antibodies have been generated in rabbit against the C-terminal peptides (VIDGDWYASDEDDRNWWKN, proteintech). Blots were developed using Immobilion Western HRP Chemiluminescent Substrate (Millipore) and Chemidoc MP (Biorad).
Microinjection
To label the endoplasmic reticulum, a saturated solution of DiI 18 (1,1’-dihexadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate 18) (Molecular Probes) was prepared in soybean oil (Sigma). The DiI solution was loaded into a Femtotips II injection pipette (Eppendorf) with a microloader (Eppendorf). Oocytes were pressure-injected with the micropipette and Eppendorf TransferMan NK2 manipulator mounted on an inverted microscope (Nikon). MII- oocytes were injected immediately after collection in M2. Oocytes were then placed in a drop of Hepes buffered CZB+IBMX covered with mineral oil during the injection. A holding pipette (Humagen) was used to immobilize the oocytes and the injection pipette was pushed through the zona pellucida and plasma membrane. Each oocyte received ~5 pl of DiI solution via Eppendorf CellTram Vario microinjector. After injection, oocytes were cultured in CZB+IBMX for 0.5-2 h followed by laser-scanning confocal microscopy analyses (Carl Zeiss).
Confocal microscopy
Immunostaining and Triton extraction procedures for oocytes have been described previously (Kim et al., 2010). For tubulin (1:1000, sigma) staining, oocytes were incubated with extraction buffer containing 0.1% Triton X-100 for 10 min prior to 4% paraformaldehyde fixation. For IP3R-1 staining, zona pellucida-free M-II oocytes were fixed with 4% PFA, permeabilized, and incubated with CT1 (1:10, Gift from Richard Wojcikiewicz, SUNY Upstate Medical University, Syracuse, NY) antibody overnight. For live staining, GV and MII oocytes were stained with ER tracker (Molecular Probes) for 25 min in MEM-alpha supplemented with 5% FBS, washed, and imaged using a Zeiss LSM 510 confocal microscop. Cos-1 cells were fixed with −20 °C methanol for 2 min, and washed in PBS. Cells were subsequently permeabilized in 0.5% Triton X-100 for 10 min, washed, and incubated with rabbit anti-MATER (1:500) or anti-alpha Tubulin (1:500, Sigma) in PBS with 1% BSA followed by 1 h incubation with appropriate Alexa Fluor conjugated secondary antibodies (1:500). Cells were mounted on slides with Fast Gold antifade agent (Molecular Probes) and imaged using a Confocal Microscope (Zeiss).
Statistical analysis
All experiments were repeated at least three times. The intensity of Fluo-4 was normalized to baseline and calculated using ImageJ. Values are given as mean ±STDEV. Values of amplitude and frequency were analyzed using student’s t-test. Differences of p<0.05 were defined as significant.
Results
MATER is required for proper ER distribution in metaphase II oocytes
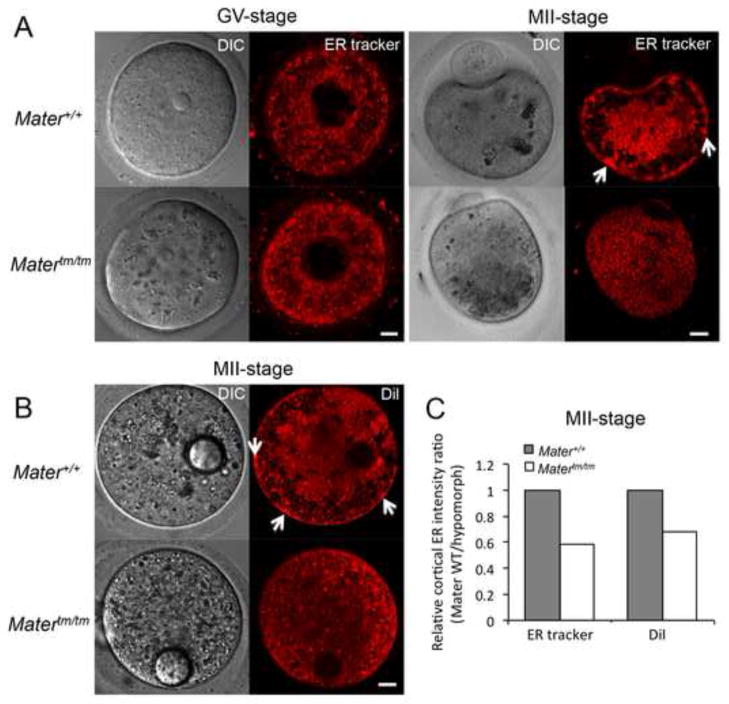
Our previous findings with PADI6 (Kan et al., 2011) suggested that organelle positioning defects in Matertm/tm oocytes might only become manifest after the onset of oocyte maturation. To test this hypothesis, we first stained fully-grown GV stage Matertm/tm oocytes with ER tracker and imaged the oocytes by confocal fluorescence microscopy. Results showed that the ER staining patterns appeared similar between Mater+/+ and Matertm/tm GV oocytes (Fig. 1A). This result suggested that, similar to PADI6, ER positioning in immature GV stage oocytes does not require MATER. We then evaluated ER distribution in mature Matertm/tm MII oocytes using ER tracker and found that, as opposed to the more polarized ER distribution pattern in wild type oocytes, a more diffuse ER distribution pattern was observed in mature Matertm/tm MII oocytes (Fig. 1A and C). To further test whether ER localization was affected in mutant Mater oocytes, we next microinjected MII oocytes with the dicarbocyanine dye DiI18 (DiI), a fluorescent lipophilic dye, and imaged DiI-injected oocytes using confocal microscopy (Mehlmann et al., 1995). We found that the DiI staining in Mater+/+ oocytes was more polarized with cortical clusters of ER being seen at the oocyte cortex, which is in line with the previous reports (Mehlmann et al., 1995; FitzHarris et al., 2007; Kan et al., 2011). However, in Matertm/tm MII oocytes, the ER was distributed in a more non-polarized, diffuse manner with the reduced number of cortical ER clusters (Fig. 1B and C). In order to gain insight into the dynamics of ER movements in mature Matertm/tm oocytes, we first took advantage of the characteristics of lipid droplets in that they are closely apposed to ER (Szymanski et al., 2007) and readily visualized by light microscopy. We tracked the movement of lipid droplets by imaging wild-type and mutant GV oocytes going through oocyte maturation over 12 h (Supplemental Fig. 1 and Movie 1). The movie showed that lipid droplets aggregated around the nucleus, and then the spindle apparatus in Mater+/+ oocytes; however, they appeared to move randomly in Matertm/tm oocytes during oocyte maturation. To specifically monitor the dynamics of ER movements in mature oocytes, we then imaged the DiI-stained oocytes by time-lapse microscopy at 10 second intervals for 20 minutes. Results demonstrated that that the ER cortical clusters appear to be stably localized to the cortex of wild-type oocytes (Supplemental Fig. 2 and Movie 2). However, in the mutant oocytes, ER clusters are seen throughout the interior of the oocyte cytoplasm and they do not appear to be targeted to the oocyte cortex.
Fig 1.
Altered endoplasmic reticulum localization and decreased cortical ER clustering in metaphase II-arrested Matertm/tm oocytes. (A) GV stage and MII stage Mater+/+ and Matertm/tm oocytes were stained with ER tracker and imaged using confocal microscopy. (B) DiI was microinjected into mature MII-arrested Mater+/+ and Matertm/tm oocytes and the localization of DiI (an ER marker) was documented using confocal microscopy. DIC images of microinjected oocytes highlight the DiI-containing oil-drop. Arrows highlight the cortical ER clusters in Mater+/+ oocytes, which were markedly reduced in Matertm/tm oocytes. Scale bar: 10 μm. (C) Semi-quantitative analysis of the cortical ER immunofluorescence signals (relative intensity) with an arbitrary number of the intensity ratio of Mater+/+ MII oocytes compared to Matertm/tm MII oocytes using Image J software.
MATER plays an important role in microtubule dynamics in oocytes
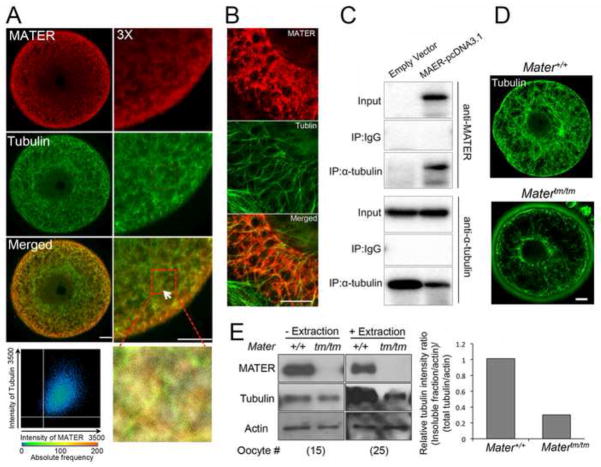
Previous studies have shown that microtubules are major drivers of ER redistribution during oocyte maturation. We demonstrated previously that PADI6 interacts with tubulin at the oocyte lattices and that PADI6 and the lattices appear to play a critical role in ER redistribution during oocyte maturation (Kan et al., 2011). These findings suggested that the lattices play an important role in microtubule-mediated organelle redistribution during maturation. Therefore, to gain insight into the potential mechanisms behind the observed organelle redistribution failure in Matertm/tm oocytes, we assessed whether the localization and/or levels of tubulin were altered in Matertm/tm oocytes. We first investigated whether MATER co-localized with tubulin in Triton X-100 extracted Mater+/+ oocytes. GV oocytes were extracted for 10 minutes with 0.1% Triton X-100, fixed, and stained with anti-tubulin and anti-MATER antibodies. Confocal imaging of the oocytes revealed that, similar to PADI6, MATER co-localized with non-MT fraction of tubulin in the extracted GV oocytes (Fig. 2A). The co-localization of MATER with tubulin was then further investigated by overexpressing MATER in Cos-1 cells and testing whether recombinant MATER (recMATER) co-localized with microtubules in this cell line. Results show that recMATER appeared to form aggregates and decorate the Cos-1 cell microtubules (Fig. 2B). We then tested for a direct interaction between recMATER and tubulin in HEK293 cells and found that an anti-tubulin mAb can immunoprecipitate recMATER from the lysate, thus confirming a physical interaction between these two proteins (Fig. 2C). We next investigated by confocal IIF (Fig. 2D) and western blotting (Fig. 2E) whether depletion of MATER affected the microtubule/tubulin abundance or distribution in oocytes. Surprisingly, we found that, while the total amount of tubulin did not appear to be affected by depletion of MATER, the amount of Triton-insoluble tubulin was markedly reduced in Matertm/tm GV stage oocytes (Fig. 2D and E). Taken together, these data support the hypothesis that MATER plays an important role in microtubule stability and/or dynamics in oocytes.
Fig 2.
MATER appears to associate with the non-microtubule fraction of tubulin in oocytes. (A) Confocal microscopy imaging of Triton X-100-extracted GV-stage oocytes stained with anti-MATER (red) and anti-3-tubulin (green). Merged images show that MATER co-localized with tubulin in the non-microtubule (MT) fraction of Triton extracted oocytes. Tubulin and MATER co-localization is highlighted by the arrow in the 3x zoom image at left. Scale bar: 10 μm. Scatter plot indicates a degree of co-localization of MATER and Tubulin shown on the lower right. Overlap coefficient: 0.89 and correlation R: 0.33. (B) Recombinant MATER appears to decorate microtubules in Cos-1 cells. Cos-1 cells were infected with a lentivirus that contained MATER cDNA and MATER expression was induced by addition of doxycycline. After 48–72 h, cells were fixed with methanol, and stained with anti-MATER (red), and anti-Tubulin (green) antibodies. Scale bar = 10 μm. (C) MATER and tubulin physically interact. MATER was overexpressed in HEK293 cells using a pcDNA3.1 vector that contained the MATER cDNA. Immuoprecipitation analysis was then carried out using either a tubulin monoclonal antibody or normal mouse IgG. Western blot analysis was then carried out using anti-MATER and anti-tubulin antibodies. (D) Confocal microscopy images of Mater+/+ and Matertm/tm oocytes following Triton X-100 extraction. The amount of Triton-insoluble tubulin (green) was decreased in Matertm/tm oocytes compared to that of Mater+/+. (E) Western blotting indicates that the Triton-insoluble fraction of tubulin was sharply reduced in Matertm/tm oocytes compared to wild type oocytes. Total tubulin levels, however did not differ between mutant and control oocytes. Relative staining intensity was shown in the lower panel.
Intracellular Ca2+ stores are decreased in Matertm/tm oocytes
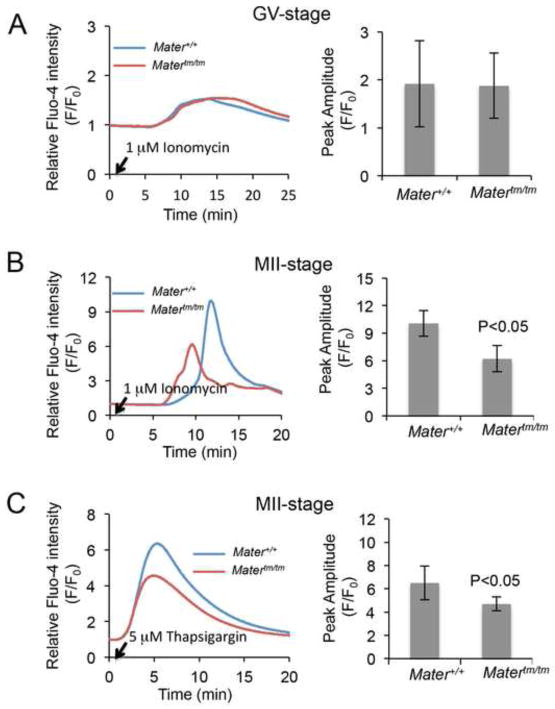
Targeting of the ER to subcortical clusters during oocyte maturation is thought to play a major role in ER-mediated Ca2+ signaling at fertilization. The finding that mature MII-arrested Matertm/tm oocytes contain a more diffuse ER distribution pattern, led to the hypothesis that ER function, particularly as a Ca2+ source, might be compromised in Matertm/tm oocytes. Therefore, we measured Ca2+ release from intracellular stores in GV and MII oocytes to determine if Ca2+ storage was affected in Matertm/tm oocytes at either of these stages. Oocytes were loaded with the Ca2+ sensitive dye, Fluo-4AM (Fluo-4) and treated with ionomycin (1 μM) to release Ca2+ from stores. Calcium dynamics were then imaged as described above. Changes in Fluo-4 intensity were normalized to resting Ca2+ levels and presented as F/F0. Results demonstrated that GV-stage Matertm/tm oocytes responded to ionomycin to a similar degree as Mater+/+ oocytes (Fig. 3A). However, we found that, while treatment of wild-type metaphase II-arrested oocytes with ionomycin led to a ~10-fold increase of the Fluo4 intensity, the hypomorph oocytes showed only a ~6 fold increased signal, indicating that depletion of MATER lead to a ~1.7 fold decrease in the level of stored Ca2+ (Fig. 3B). To investigate more directly the changes in ER Ca2+ stores in hypomorph oocytes, we treated MII oocytes with 5 μM thapsigargin, which specifically blocks the ER Ca2+-ATPase pump (SERCA) (Thastrup et al., 1990). Similar to ionomycin treatment, we found that Matertm/tm MII oocytes showed a 30% decrease in Ca2+release from ER in response to thapsigargin treatment compared to wild type oocytes (Fig. 3C). These data raise the possibility that the observed organelle redistribution defect in Mater hypomorph oocytes during oocyte maturation might lead to improper calcium storage in the ER in mature oocytes.
Fig 3.
Matertm/tm MII oocytes demonstrated a decreased Ca2+release from ER compared to Mater+/+ MII oocytes. (A) GV oocytes were loaded with Fluo-4 AM (1 μM), treated with ionomycin (arrow indicates time of treatment) and imaged at ~1.6 second intervals using 20x objective. Histogram of Fluo-4 intensity shows that GV-stage Matertm/tm oocytes responded to ionomycin to a similar degree as Mater+/+ oocytes. (B) MII-arrested Mater+/+ and Matertm/tm oocytes were treated with ionomycin (1 μM) as above. Results show that Matertm/tm oocytes responded to ionomycin to a significantly lesser degree compared to Mater+/+ oocytes. p<0.05 (C) MII Mater+/+ and Matertm/tm oocytes were treated with thapsigargin (5 μM) as above. Similar to ionomycin treatment, the responses of Matertm/tm oocytes to thapsigargin were significantly decreased when compared to Mater+/+ oocytes (p<0.05).
IP3R-1 distribution is altered in Matertm/tm oocytes
Several lines of evidence indicate that the IP3R-1 is a major receptor for releasing Ca2+ from the ER in mammalian oocytes (Parrington et al., 1998; Fissore et al., 1999). Given that IP3R-1 is primarily thought to localize to the ER and that ER distribution was found to be defective in mutant oocytes, we next investigated the localization of IP3R-1 in Mater+/+ and Mater tm/tm MII oocytes by confocal microscopy. Zona pellucida-free MII oocytes were fixed and stained with IP3R-1 specific antibody as described previously. Results showed that, compared to wild type oocytes, IP3R-1 appeared to be diffusely localized throughout the cytoplasm in Matertm/tm oocytes with the levels of IP3R-1 clusters at the oocyte cortex appearing to be reduced (Fig. 4A). We then sought to determine the abundance of IP3R-1 protein in Mater+/+ and Mater tm/tm MII oocytes by Western blot analysis. Oocytes were collected, lysed, and subjected to immunoblot analysis. The blots were then incubated with the anti-Rbt03 antibody to detect IP3R-1, and an anti-Actin antibody was employed as a loading control. Results indicated that the expression level of IP3R-1 in Matertm/tm oocytes was equivalent to that of Mater+/+ oocytes (Fig. 4B). These findings suggest that, while IP3R-1 protein levels were not affected by MATER depletion, the localization of IP3R-1 does appear to be altered in these oocytes, thus supporting our finding that ER localization is defective in Mater hypomorph oocytes.
Fig 4.
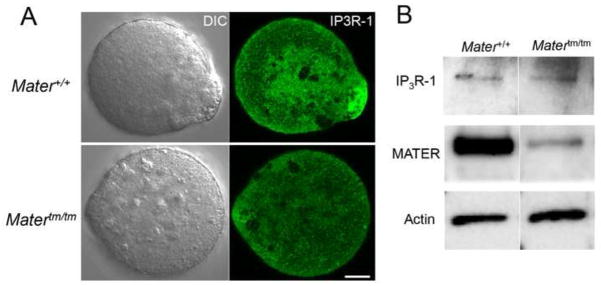
IP3R-1 distribution is altered in Matertm/tm oocytes. (A) MII-arrested Mater+/+ and Matertm/tm oocytes were first treated with acid Tyrode to remove the zona pellucida and then fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton-X 100, stained with an anti-IP3R-1 antibody. Results show that the localization of IP3R-1 was altered in the Matertm/tm oocytes when compared to wild type oocytes. Scale bar: 10 μm. (B) Proteins from Mater+/+and Matertm/tm oocytes were resolved by SDS-PAGE, and transferred to PVDF membrane. The membrane was probed with antibodies against IP3R-1, MATER, and actin. Results show that IP3R-1 levels were not significantly reduced in Matertm/tm oocytes when compared to wild type oocytes.
Ca2+ oscillation patterns are altered in Mater hypomorph oocytes following fertilization
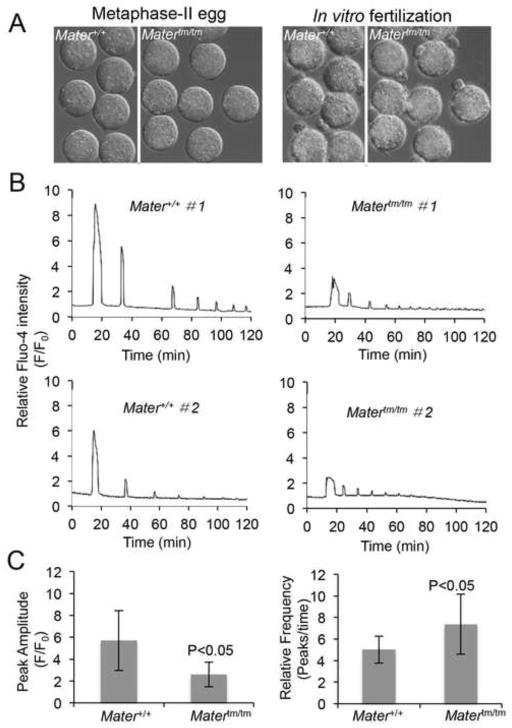
Ca2+ oscillations are dependent on intracellular Ca2+ stores and are believed to have a spatio-temporal relationship with clustering of these stores in the oocyte cortex. Given the observed decrease in ER cortical clusters and intracellular Ca2+ stores in Matertm/tm oocytes, we next tested whether Ca2+ oscillation patterns might be altered in Mater hypomorph oocytes following fertilization. Zona-free MII oocytes were loaded with Fluo-4 and were then inseminated (~100 sperm/μl) with capacitated epididymal sperm and the Fluo-4 signal was captured at 20 seconds intervals for 2 h. In vitro fertilization was confirmed by the visualization of the extruded second polar body ~2 h following fertilization (Fig. 5A). [We note that delays in polar body extrusion were not observed in Matertm/tm oocytes.] The Ca2+ oscillation profiles of fertilized Mater+/+ and Matertm/tm oocytes were then analyzed using ImageJ and the amplitude of the first peak was averaged. Results showed that the initial Fluo-4 peak in Matertm/tm oocytes was reduced by ~46% compared to Mater+/+ oocytes. Additionally, we found that the frequency of subsequent Ca2+ oscillations was about 50% higher in Matertm/tm oocytes compared to Mater+/+ oocytes (Fig. 5B and C). These results suggest that depletion of Mater severely hampers the ability of oocytes to generate functional Ca2+ oscillations. Alternatively, it is also possible that the observed decrease in first peak Ca2+ amplitude in mutant oocytes is due to preferential sequestration of Fluo-4 into organelles in Matertm/tm oocytes. However, given that baseline fluorescence levels were similar between wild type and mutant oocytes (data not shown), this alternate hypothesis seems less likely.
Fig 5.
Matertm/tm oocytes display an altered Ca2+ oscillation pattern following fertilization in vitro. (A) Zona-free metaphase II-arrested Mater+/+ and Matertm/tm oocytes were loaded with Fluo-4 AM, fertilized in vitro and Ca2+ oscillations were recorded every 20 second for 2 h following insemination. Fertilization was confirmed by visualization of the second polar body ~2 h after insemination. Images were analysed by ImageJ and the representative graphs from each group are shown in (B). The bar graphs of amplitude and frequency in (C) indicate that Matertm/tm oocytes displayed a lower amplitude and higher frequency of Ca2+ oscillation patterns when compared to Mater+/+ oocytes (p<0.05).
Discussion
In this report, we first document that MATER is required for the proper targeting of the ER to the microvillar cortex in mature metaphase II-arrested oocytes. While the precise mechanisms behind this targeting defect remain unclear, our finding that MATER interacts with tubulin and appears to play an important role in microtubule dynamics suggests that the ER positioning defect in mutant Mater oocytes is likely caused by defects in the oocyte cytoskeleton. Regarding how MATER may regulate microtubule dynamics, we have previously documented that MATER is required for the formation of the oocyte cytoplasmic lattices (Kim et al., 2010), a PADI6 and tubulin containing structure that is required for organelle positioning and movement during oocyte maturation (Kan et al., 2011). These observations suggest that MATER works in concert with PADI6 and tubulin to orchestrate organelle redistribution during oocyte maturation.
Previous studies have shown that ER redistribution during oocyte maturation is required for the generation of functional long-lasting calcium oscillations following fertilization (Kline et al., 1999; Stricker, 1999). Our studies found that ionophore-induced calcium release was reduced by ~40% in mature MII-arrested Matertm/tm oocytes compared to wild type oocytes. This observation supports the hypothesis that disregulated ER targeting and clustering in Matertm/tm oocytes compromises ERs ability to sequester and store calcium during oocyte maturation. We next tested the effect of MATER mutation on fertilization-induced calcium oscillations and found a ~50% decrease in the initial calcium peak in mutant Mater oocytes compared to controls. Previous reports using Mater hypomorphs have demonstrated that MATER is essential for progression beyond the two-cell stage of development (Tong et al., 2000). By coupling this finding with our current study, we hypothesize that the Matertm/tm oocytes are capable of generating calcium transients that are sufficient for the initial stage of development but are insufficient for development beyond the two-cell stage. This prediction is supported by previous studies which show that manipulation of Ca2+ oscillation parameters (including amplitude and frequency) in parthenogenetically activated oocytes results in decreased developmental potential (Vitullo and Ozil, 1992; Bos-Mikich et al., 1997). While our report has focused on the role of MATER in ER positioning and distribution, another group has recently investigated the role of MATER in mitochondrial positioning and function. This study found that, similar to the ER defect described in our report, Matertm/tm metaphase II-arrested oocytes also display an altered mitochondrial distribution pattern (Fernandes et al., 2012). Also, this group showed that mitochondria in 2-cell embryos were dysfunctional. Given the well-documented interplay between mitochondria and ER with respect to Ca2+ homeostasis, it is possible that the Ca2+ store defect described in our study could be attributed, in part, to the loss of a functional interaction between the ER and mitochondria in mutant oocytes. Importantly, this study also found that Matertm/tm oocytes/zygotes appear to be under mitochondrial stress, as documented by the upregulation of the phosphorylation in oxidative stress marker, p66Shc (Gertz and Steegborn, 2010). Therefore, in this study we assessed whether Matertm/tm oocytes are under ER stress by measuring protein levels of a canonical marker for ER stress, GRP78 (HSPA5), (Hendershot, 2004) (Supplemental Fig. 3). Results show that there is no discernible effect on GRP78 expression level between Mater+/+ and Matertm/tm MII oocytes, implying the defects of Ca2+ homeostasis shown in Matertm/tm MII oocytes are not likely due to ER stress.
In conclusion, our findings suggest that MATER plays an important role in microtubule dynamics and ER redistribution during oocyte maturation. Additionally, we demonstrate that MATER appears to be required for the proper storage of Ca2+ within the ER in mature MII-arrested oocytes. Lastly, we predict that the observed Ca2+ oscillation defects in mutant Mater oocytes is likely one cause of the subsequent 2-cell developmental arrest in these embryos. Future experiments will directly test these hypotheses.
Supplementary Material
Highlights.
MATER is required for ER redistribution in metaphase II oocytes
MATER plays an important role in microtubule dynamics in oocytes
MATER is required for Ca2+ homeostasis in oocytes
Acknowledgments
We would like to thank Carmen Williams for technical advice and support, Rafael Fissore for technical advice and the gift of antibody, Richard Wojcikiewicz for the generous gift of antibody, Lawrence M. Nelson for the gift of plasmid and Kelly Sams for assistance with in vitro fertilization experiments. This work was supported by NIH RO1 HD38353 (to S.A.C.), and X.Z. was supported by a Postdoctoral Fellowship KG101303 from Susan G. Komen for the Cure. Additional support for R.C. and C.M. was provided by NIH DP1-EB016541 (to A.J.T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bos-Mikich A, Whittingham DG, Jones KT. Meiotic and Mitotic Ca2+ Oscillations Affect Cell Composition in Resulting Blastocysts. Dev Biol. 1997;182:172–179. doi: 10.1006/dbio.1996.8468. [DOI] [PubMed] [Google Scholar]
- Cuthbertson KS, Cobbold PH. Phorbol Ester and Sperm Activate Mouse Oocytes by Inducing Sustained Oscillations in Cell Ca2+ Nature. 1985;316:541–542. doi: 10.1038/316541a0. [DOI] [PubMed] [Google Scholar]
- Esposito G, Vitale AM, Leijten FP, Strik AM, Koonen-Reemst AM, Yurttas P, Robben TJ, Coonrod S, Gossen JA. Peptidylarginine Deiminase (PAD) 6 is Essential for Oocyte Cytoskeletal Sheet Formation and Female Fertility. Mol Cell Endocrinol. 2007;273:25–31. doi: 10.1016/j.mce.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Fernandes R, Tsuda C, Perumalsamy AL, Naranian T, Chong J, Acton BM, Tong ZB, Nelson LM, Jurisicova A. NLRP5 Mediates Mitochondrial Function in Mouse Oocytes and Embryos. Biol Reprod. 2012;86, 138:1–10. doi: 10.1095/biolreprod.111.093583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fissore RA, Longo FJ, Anderson E, Parys JB, Ducibella T. Differential Distribution of Inositol Trisphosphate Receptor Isoforms in Mouse Oocytes. Biol Reprod. 1999;60:49–57. doi: 10.1095/biolreprod60.1.49. [DOI] [PubMed] [Google Scholar]
- FitzHarris G, Marangos P, Carroll J. Changes in Endoplasmic Reticulum Structure during Mouse Oocyte Maturation are Controlled by the Cytoskeleton and Cytoplasmic Dynein. Dev Biol. 2007;305:133–144. doi: 10.1016/j.ydbio.2007.02.006. [DOI] [PubMed] [Google Scholar]
- FitzHarris G, Marangos P, Carroll J. Cell Cycle-Dependent Regulation of Structure of Endoplasmic Reticulum and Inositol 1,4,5-Trisphosphate-Induced Ca2+ Release in Mouse Oocytes and Embryos. Mol Biol Cell. 2003;14:288–301. doi: 10.1091/mbc.E02-07-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Nakada K, Shirakawa H, Miyazaki S. Development of Inositol Trisphosphate-Induced Calcium Release Mechanism during Maturation of Hamster Oocytes. Dev Biol. 1993;156:69–79. doi: 10.1006/dbio.1993.1059. [DOI] [PubMed] [Google Scholar]
- Garcia RB, Pereyra-Alfonso S, Sotelo JR. Protein-Synthesizing Machinery in the Growing Oocyte of the Cyclic Mouse. A Quantitative Electron Microscopic Study. Differentiation. 1979;14:101–106. doi: 10.1111/j.1432-0436.1979.tb01017.x. [DOI] [PubMed] [Google Scholar]
- Gertz M, Steegborn C. The Lifespan-Regulator p66Shc in Mitochondria: Redox Enzyme Or Redox Sensor? Antioxid Redox Signal. 2010;13:1417–1428. doi: 10.1089/ars.2010.3147. [DOI] [PubMed] [Google Scholar]
- Hendershot LM. The ER Function BiP is a Master Regulator of ER Function. Mt Sinai J Med. 2004;71:289–297. [PubMed] [Google Scholar]
- Jaffe LA, Terasaki M. Structural Changes in the Endoplasmic Reticulum of Starfish Oocytes during Meiotic Maturation and Fertilization. Dev Biol. 1994;164:579–587. doi: 10.1006/dbio.1994.1225. [DOI] [PubMed] [Google Scholar]
- Jones KT, Carroll J, Whittingham DG. Ionomycin, Thapsigargin, Ryanodine, and Sperm Induced Ca2+ Release Increase during Meiotic Maturation of Mouse Oocytes. J Biol Chem. 1995;270:6671–6677. doi: 10.1074/jbc.270.12.6671. [DOI] [PubMed] [Google Scholar]
- Kan R, Yurttas P, Kim B, Jin M, Wo L, Lee B, Gosden R, Coonrod SA. Regulation of Mouse Oocyte Microtubule and Organelle Dynamics by PADI6 and the Cytoplasmic Lattices. Dev Biol. 2011;350:311–322. doi: 10.1016/j.ydbio.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Kan R, Anguish L, Nelson LM, Coonrod SA. Potential Role for MATER in Cytoplasmic Lattice Formation in Murine Oocytes. PLoS One. 2010;5:e12587. doi: 10.1371/journal.pone.0012587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline D, Mehlmann L, Fox C, Terasaki M. The Cortical Endoplasmic Reticulum (ER) of the Mouse Egg: Localization of ER Clusters in Relation to the Generation of Repetitive Calcium Waves. Dev Biol. 1999;215:431–442. doi: 10.1006/dbio.1999.9445. [DOI] [PubMed] [Google Scholar]
- Mann JS, Lowther KM, Mehlmann LM. Reorganization of the Endoplasmic Reticulum and Development of Ca2+ Release Mechanisms during Meiotic Maturation of Human Oocytes. Biol Reprod. 2010;83:578–583. doi: 10.1095/biolreprod.110.085985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM, Mikoshiba K, Kline D. Redistribution and Increase in Cortical Inositol 1,4,5-Trisphosphate Receptors After Meiotic Maturation of the Mouse Oocyte. Dev Biol. 1996;180:489–498. doi: 10.1006/dbio.1996.0322. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Terasaki M, Jaffe LA, Kline D. Reorganization of the Endoplasmic Reticulum during Meiotic Maturation of the Mouse Oocyte. Dev Biol. 1995;170:607–615. doi: 10.1006/dbio.1995.1240. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Kline D. Regulation of Intracellular Calcium in the Mouse Egg: Calcium Release in Response to Sperm Or Inositol Trisphosphate is Enhanced After Meiotic Maturation. Biol Reprod. 1994;51:1088–1098. doi: 10.1095/biolreprod51.6.1088. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Ito M. Calcium Signals for Egg Activation in Mammals. J Pharmacol Sci. 2006;100:545–552. doi: 10.1254/jphs.cpj06003x. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Yuzaki M, Nakada K, Shirakawa H, Nakanishi S, Nakade S, Mikoshiba K. Block of Ca2+ Wave and Ca2+ Oscillation by Antibody to the Inositol 1,4,5-Trisphosphate Receptor in Fertilized Hamster Eggs. Science. 1992;257:251–255. doi: 10.1126/science.1321497. [DOI] [PubMed] [Google Scholar]
- Ohsugi M, Zheng P, Baibakov B, Li L, Dean J. Maternally Derived FILIA-MATER Complex Localizes Asymmetrically in Cleavage-Stage Mouse Embryos. Development. 2008;135:259–269. doi: 10.1242/dev.011445. [DOI] [PubMed] [Google Scholar]
- Parrington J, Brind S, De Smedt H, Gangeswaran R, Lai FA, Wojcikiewicz R, Carroll J. Expression of Inositol 1,4,5-Trisphosphate Receptors in Mouse Oocytes and Early Embryos: The Type I Isoform is Upregulated in Oocytes and Downregulated After Fertilization. Dev Biol. 1998;203:451–461. doi: 10.1006/dbio.1998.9071. [DOI] [PubMed] [Google Scholar]
- Runft LL, Jaffe LA, Mehlmann LM. Egg Activation at Fertilization: Where it all Begins. Dev Biol. 2002;245:237–254. doi: 10.1006/dbio.2002.0600. [DOI] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLC Zeta: A Sperm-Specific Trigger of Ca(2+) Oscillations in Eggs and Embryo Development. Development. 2002;129:3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- Shiraishi K, Okada A, Shirakawa H, Nakanishi S, Mikoshiba K, Miyazaki S. Developmental Changes in the Distribution of the Endoplasmic Reticulum and Inositol 1,4,5-Trisphosphate Receptors and the Spatial Pattern of Ca2+ Release during Maturation of Hamster Oocytes. Dev Biol. 1995;170:594–606. doi: 10.1006/dbio.1995.1239. [DOI] [PubMed] [Google Scholar]
- Sternlicht AL, Schultz RM. Biochemical Studies of Mammalian Oogenesis: Kinetics of Accumulation of Total and Poly(A)-Containing RNA during Growth of the Mouse Oocyte. J Exp Zool. 1981;215:191–200. doi: 10.1002/jez.1402150209. [DOI] [PubMed] [Google Scholar]
- Stricker SA. Comparative Biology of Calcium Signaling during Fertilization and Egg Activation in Animals. Dev Biol. 1999;211:157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- Stricker SA, Silva R, Smythe T. Calcium and Endoplasmic Reticulum Dynamics during Oocyte Maturation and Fertilization in the Marine Worm Cerebratulus Lacteus. Dev Biol. 1998;203:305–322. doi: 10.1006/dbio.1998.9058. [DOI] [PubMed] [Google Scholar]
- Szymanski KM, Binns D, Bartz R, Grishin NV, Li WP, Agarwal AK, Garg A, Anderson RG, Goodman JM. The Lipodystrophy Protein Seipin is found at Endoplasmic Reticulum Lipid Droplet Junctions and is Important for Droplet Morphology. Proc Natl Acad Sci U S A. 2007;104:20890–20895. doi: 10.1073/pnas.0704154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M, Runft LL, Hand AR. Changes in Organization of the Endoplasmic Reticulum during Xenopus Oocyte Maturation and Activation. Mol Biol Cell. 2001;12:1103–1116. doi: 10.1091/mbc.12.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a Tumor Promoter, Discharges Intracellular Ca2+ Stores by Specific Inhibition of the Endoplasmic Reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombes RM, Simerly C, Borisy GG, Schatten G. Meiosis, Egg Activation, and Nuclear Envelope Breakdown are Differentially Reliant on Ca2+, Whereas Germinal Vesicle Breakdown is Ca2+ Independent in the Mouse Oocyte. J Cell Biol. 1992;117:799–811. doi: 10.1083/jcb.117.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong ZB, Gold L, Pfeifer KE, Dorward H, Lee E, Bondy CA, Dean J, Nelson LM. Mater, a Maternal Effect Gene Required for Early Embryonic Development in Mice. Nat Genet. 2000;26:267–268. doi: 10.1038/81547. [DOI] [PubMed] [Google Scholar]
- Vitullo AD, Ozil JP. Repetitive Calcium Stimuli Drive Meiotic Resumption and Pronuclear Development during Mouse Oocyte Activation. Dev Biol. 1992;151:128–136. doi: 10.1016/0012-1606(92)90220-b. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Josefowicz WJ. Oocyte Development in the Mouse: An Ultrastructural Comparison of Oocytes Isolated at various Stages of Growth and Meiotic Competence. J Morphol. 1978;156:209–235. doi: 10.1002/jmor.1051560206. [DOI] [PubMed] [Google Scholar]
- Whitaker M. Calcium at Fertilization and in Early Development. Physiol Rev. 2006;86:25–88. doi: 10.1152/physrev.00023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright PW, Bolling LC, Calvert ME, Sarmento OF, Berkeley EV, Shea MC, Hao Z, Jayes FC, Bush LA, Shetty J, Shore AN, Reddi PP, Tung KS, Samy E, Allietta MM, Sherman NE, Herr JC, Coonrod SA. ePAD, an Oocyte and Early Embryo-Abundant Peptidylarginine Deiminase-Like Protein that Localizes to Egg Cytoplasmic Sheets. Dev Biol. 2003;256:73–88. doi: 10.1016/s0012-1606(02)00126-4. [DOI] [PubMed] [Google Scholar]
- Xu Z, Williams CJ, Kopf GS, Schultz RM. Maturation-Associated Increase in IP3 Receptor Type 1: Role in Conferring Increased IP3 Sensitivity and Ca2+ Oscillatory Behavior in Mouse Eggs. Dev Biol. 2003;254:163–171. doi: 10.1016/s0012-1606(02)00049-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.