Abstract
Background:
To evaluate the effects of elective nodal irradiation (ENI) in clinical stage II–III breast cancer patients with pathologically negative lymph nodes (LNs) (ypN0) after neoadjuvant chemotherapy (NAC) followed by breast-conserving surgery (BCS) and radiotherapy (RT).
Methods:
We retrospectively analysed 260 patients with ypN0 who received NAC followed by BCS and RT. Elective nodal irradiation was delivered to 136 (52.3%) patients. The effects of ENI on survival outcomes were evaluated.
Results:
After a median follow-up period of 66.2 months (range, 15.6–127.4 months), 26 patients (10.0%) developed disease recurrence. The 5-year locoregional recurrence-free survival and disease-free survival (DFS) for all patients were 95.5% and 90.5%, respectively. Pathologic T classification (0−is vs 1 vs 2–4) and the number of LNs sampled (<13 vs ⩾13) were associated with DFS (P=0.0086 and 0.0012, respectively). There was no significant difference in survival outcomes according to ENI. Elective nodal irradiation also did not affect survival outcomes in any of the subgroups according to pathologic T classification or the number of LNs sampled.
Conclusions:
ENI may be omitted in patients with ypN0 breast cancer after NAC and BCS. But until the results of the randomised trials are available, patients should be put on these trials.
Keywords: breast cancer, neoadjuvant chemotherapy, breast conserving surgery, radiotherapy, elective nodal irradiation
Neoadjuvant chemotherapy (NAC) is an effective treatment modality for patients with locally advanced breast cancer (Gralow et al, 2008; Rastogi et al, 2008). Patients with operable disease may also benefit from NAC in terms of breast-conserving surgery (BCS). A certain proportion of patients receive BCS after NAC, though this varies widely according to inclusion and selection criteria (Chen et al, 2004). According to the combined analysis of National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 and B-27 trials, the 10-year cumulative incidence of locoregional recurrence (LRR) after BCS plus breast radiotherapy (RT) was 10.3%, which was similar to that observed after mastectomy (12.3%) (Mamounas et al, 2012).
Although adjuvant RT has a significant role in breast-conserving treatment (Darby et al, 2011), it is also indicated for patients receiving BCS after NAC. It remains unclear, however, whether the addition of regional irradiation is needed. After NAC, the pathologic extent of disease is modified in 80–90% of patients, whereas pathologic complete response (CR) in both the breast and axilla was achieved in 13–26% of patients (Rastogi et al, 2008). In addition, about 20–40% of patients with axillary nodal involvement at the time of diagnosis were without pathologic lymph node (LN) involvement after NAC (Fisher et al, 1997; Bear et al, 2003). Along with age, clinical tumour characteristics before NAC, breast tumour responses after NAC, and pathologic nodal status are predictive factors for LRR after NAC (Mamounas et al, 2012). In two NSABP NAC trials that previously used breast RT without regional irradiation after BCS, the LRR rate was <10% in patients with pathologically negative LN (ypN0). Although the LRR rate was low after breast RT only, whether further benefit could be achieved from the addition of regional irradiation is unknown. According to the National Comprehensive Cancer Network (NCCN) guidelines for patients treated with NAC, adjuvant RT should be based on the worst stage pre-treatment or post-treatment tumour characteristics (NCCN, 2014). In contrast, a recent study compared the outcomes of patients with ypN0 after NAC plus BCS according to whether they also received regional irradiation (Daveau et al, 2010). The 5-year LRR-free survival (LRRFS) rates were 89.4% and 86.2% with and without regional irradiation, respectively, which were not significantly different from each other regardless of pre-chemotherapy tumour status.
Notably, a prospective randomised trial has not yet been conducted to address this issue. In order to gather preliminary data, the Korean Radiation Oncology Group (KROG) conducted a multicentre retrospective study to evaluate the effects of regional irradiation in patients with ypN0 after NAC followed by BCS and RT.
Materials and methods
Patients
A total of 417 patients with clinical stage II–III breast cancer who achieved ypN0 after NAC followed by surgery at nine institutions in Korea between 1998 and 2009 were retrospectively identified. Patients with distant metastases, inflammatory breast cancer, involvement of supraclavicular or internal mammary LN, history of previous chemotherapy or RT or history of previous or concurrent malignancy except for thyroid cancer at the time of diagnosis were not included. Among the 417 patients, 266 underwent BCS and the remaining 151 received mastectomy. We excluded six patients who did not receive adjuvant RT after BCS. Then, 260 patients who received both BCS and RT were included in this study. The role of post-mastectomy RT for the 151 patients receiving mastectomy is described in a separate publication (Shim et al, 2014). After approval by the KROG (KROG 12-05), the medical and RT records of the patients were retrospectively reviewed.
The median age of the 260 patients was 46 years (range, 25–72 years). According to the American Joint Committee on Cancer (AJCC) 7th edition cancer staging manual, 203 (78.1%) patients were classified as T1–2 and 57 (21.9%) patients were classified as T3–4. Clinical involvement of regional LNs was present in 251 (96.5%) patients, 50 of which were confirmed by cytologic evaluation. For the remaining 201 patients with clinical involvement of regional LNs, either ultrasonography (US; n=171), magnetic resonance imaging (MRI; n=98), or positron emission tomography (PET; n=140) were used for clinical diagnosis.
Molecular subtypes were classified according to the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2) status: luminal (ER- or PR-positive), triple-negative (ER-, PR-, and HER2-negative), or HER2 overexpressing (ER- and PR-negative, and HER2-positive).
Treatments
The most common NAC regimen used in patients included in this study was anthracycline plus taxane (AT, n=107, 41.2%), followed by anthracycline plus cyclophosphamide (AC, n=72, 27.7%), and taxane only (n=30, 11.5%). Anti-HER2 targeted agent was administered to 24 (9.2%) patients.
All patients underwent BCS. Seven patients (2.7%) had positive resection margins, and six (2.3%) patients had close resection margin of <1 mm. Axillary LN dissection (ALND) was performed in 236 (90.8%) patients, while the remaining 24 (9.2%) patients underwent sentinel LN biopsy (SLNB) only. The median number of sampled LNs was 15 (range, 0–42).
Adjuvant chemotherapy was delivered to 199 (76.5%) patients. The most common regimen was AT (n=86, 33.1%), followed by taxane only (n=46, 17.7%), and AC (n=43, 16.5%). One-hundred and three (39.6%) patients received adjuvant hormone treatment.
The RT doses delivered to the whole breast were 50.4 Gy in 28 fractions (n=217, 83.5%) or 50 Gy in 25 fractions (n=37, 14.2%). The primary tumour bed was boosted in 252 (96.9%) tumours, most commonly with 9 Gy in five fractions (n=109, 43.3%), followed by 10 Gy in five fractions (n=95, 37.7%). Elective nodal irradiation (ENI) in the supraclavicular area was performed in 136 (52.3%) patients. The decision for ENI was made individually based on institutional or physician's preference. There were 14 (5.4%) and 86 (33.1%) patients who received internal mammary irradiation and posterior axillary boost, respectively.
Statistical analysis
The primary endpoint of this study was LRRFS and disease-free survival (DFS) according to ENI. The secondary endpoint was overall survival (OS) and patterns of disease recurrence according to ENI. Locoregional recurrence was defined as disease recurrence in the ipsilateral breast or ipsilateral axillary, supraclavicular, infraclavicular, or internal mammary LNs. The time from the initiation of NAC to LRR or death was defined as LRRFS. Disease-free survival was defined as the time from the initiation of NAC to relapse or death. Overall survival was defined as the time from the initiation of NAC to death from any cause.
To compare clinicopathologic characteristics according to ENI, chi-square or Fisher's exact tests were used. T-tests were used to compare continuous variables such as age and the number of sampled LNs. The LRRFS, DFS, and OS were estimated using the Kaplan–Meier method and were compared using log-rank tests. Factors that showed a probability value of <0.2 or that were thought to be relevant were entered into a Cox proportional hazards regression analysis. A P-value ⩽0.05 was considered to be statistically significant, and SAS software (SAS 9.1.3; SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses.
Results
Patient characteristics according to ENI
Clinicopathologic characteristics according to ENI are presented in Table 1. Patients who did not receive ENI were significantly younger than patients who did receive the treatment (P=0.0018). Those who did not receive ENI also tended to have more advanced T classification and a triple-negative phenotype. About half (53.2%) of the patients without ENI received AT as NAC, while the regimen was administered to approximately one-third (30.2%) of patients who received ENI. As more patients received SLNB only, the number of sampled LNs was smaller in patients without ENI (median 13, range 0–42) than in patients with ENI (median 15, range 2–40) (P=0.0129). Pathologic CR, including ypT0 and ypTis, was achieved in 102 (39.2%) patients, which was not different between the two groups.
Table 1. Clinicopathologic characteristics according to ENI.
| Characteristics | No ENI (n=124) | ENI (n=136) | P |
|---|---|---|---|
| Median age (years, range) |
44 (23–69) |
48 (25–72) |
0.0018 |
|
Histologic type | |||
| IDC | 115 (92.7%) | 128 (94.1%) | 0.6540 |
| Others |
9 (7.3%) |
8 (5.9%) |
|
|
Clinical T | |||
| 1–2 | 92 (74.2%) | 112 (82.4%) | 0.0809 |
| 3–4 |
32 (25.8%) |
24 (17.6%) |
|
|
Clinical N | |||
| Negative | 5 (4.0%) | 4 (2.9%) | 0.6307a |
| Positive |
119 (96.0%) |
132 (97.1%) |
|
|
Molecular subtypes | |||
| Luminal | 42 (33.9%) | 53 (39.0%) | 0.0544 |
| Triple-negative | 38 (30.6%) | 24 (17.6%) | |
| HER2-positive | 31 (25.0%) | 42 (30.9%) | |
| Unknown |
13 (10.5%) |
17 (12.5%) |
|
|
NAC | |||
| AT | 66 (53.2%) | 41 (30.2%) | 0.0014 |
| AC | 27 (21.8%) | 45 (33.1%) | |
| Taxane | 9 (7.3%) | 21 (15.4%) | |
| Others |
22 (17.7%) |
29 (21.3%) |
|
|
Pathological T | |||
| ypT0-is | 47 (37.9%) | 55 (40.4%) | 0.1254 |
| ypT1 | 51 (41.1%) | 65 (47.8%) | |
| ypT2-4 |
26 (21.0%) |
16 (11.8%) |
|
|
Axilla surgery | |||
| ALND | 102 (82.3%) | 134 (98.5%) | <0.0001 |
| SLNB |
22 (17.7%) |
2 (1.5%) |
|
|
Adjuvant chemotherapy | |||
| AT | 58 (46.8%) | 28 (20.6%) | <0.0001 |
| AC | 11 (8.9%) | 32 (23.5%) | |
| Taxane | 12 (9.7%) | 34 (25.0%) | |
| Others | 14 (11.3%) | 10 (7.4%) | |
| No |
29 (23.4%) |
32 (23.5%) |
|
|
Adjuvant hormone treatment | |||
| Yes | 45 (36.3%) | 58 (42.7%) | 0.2952 |
| No |
79 (63.7%) |
78 (57.3%) |
|
|
Primary tumour bed boost | |||
| Yes | 119 (96.0%) | 133 (97.8%) | 0.3944 |
| No | 5 (4.0%) | 3 (2.2%) | |
Abbreviations: AC=anthracycline plus cyclophosphamide; ALND=axillary lymph node dissection; AT=anthracycline plus taxane; ENI=elective nodal irradiation; HER2=human epidermal growth factor receptor-2; IDC=invasive ductal carcinoma; NAC=neoadjuvant chemotherapy; SLNB=sentinel lymph node biopsy.
Fisher's exact test.
Failure patterns
The median follow-up duration, which was calculated from the time that NAC was initiated, was 66.2 months (range, 15.6–127.4 months). During the follow-up period, 26 (10.0%) patients experienced disease recurrence, with a median time to recurrence of 22.6 months (range, 7.9–78.2 months). Patterns of disease recurrence are shown in Table 2. Locoregional recurrence occurred in 13 (5.0%) patients, with a median latency of 25.1 months (range, 8.5–78.2 months). Among the patients who did not receive ENI, ipsilateral breast, axillary, and supraclavicular recurrences occurred in 3, 1, and 2 patients, respectively. There were no supraclavicular recurrences in patients who received ENI, whereas five and two patients experienced local and axillary recurrences, respectively. Among the patients with pathologic CR, only three (2.9%) experienced disease recurrence, two with local recurrence, and one with distant metastasis.
Table 2. Patterns of disease recurrence according to elective nodal irradiation and pathologic T classification.
| Variables | n | Locoregional | Distant | Overall |
|---|---|---|---|---|
|
ENI | ||||
| No | 124 | 6 (4.8%) | 10 (8.1%) | 13 (10.5%) |
| Yes |
136 |
7 (5.1%) |
8 (5.9%) |
13 (9.6%) |
|
Pathologic T classification | ||||
| ypT0-is | 102 | 2 (2.0%) | 1 (1.0%) | 3 (2.9%) |
| ypT1 | 116 | 9 (7.8%) | 12 (10.3%) | 17 (14.7%) |
| ypT2-4 | 42 | 2 (4.8%) | 5 (11.9%) | 6 (14.3%) |
| Total recurrences | 260 | 13 (5.0%) | 18 (6.9%) | 26 (10.0%) |
Abbreviation: ENI=elective nodal irradiation.
Survival
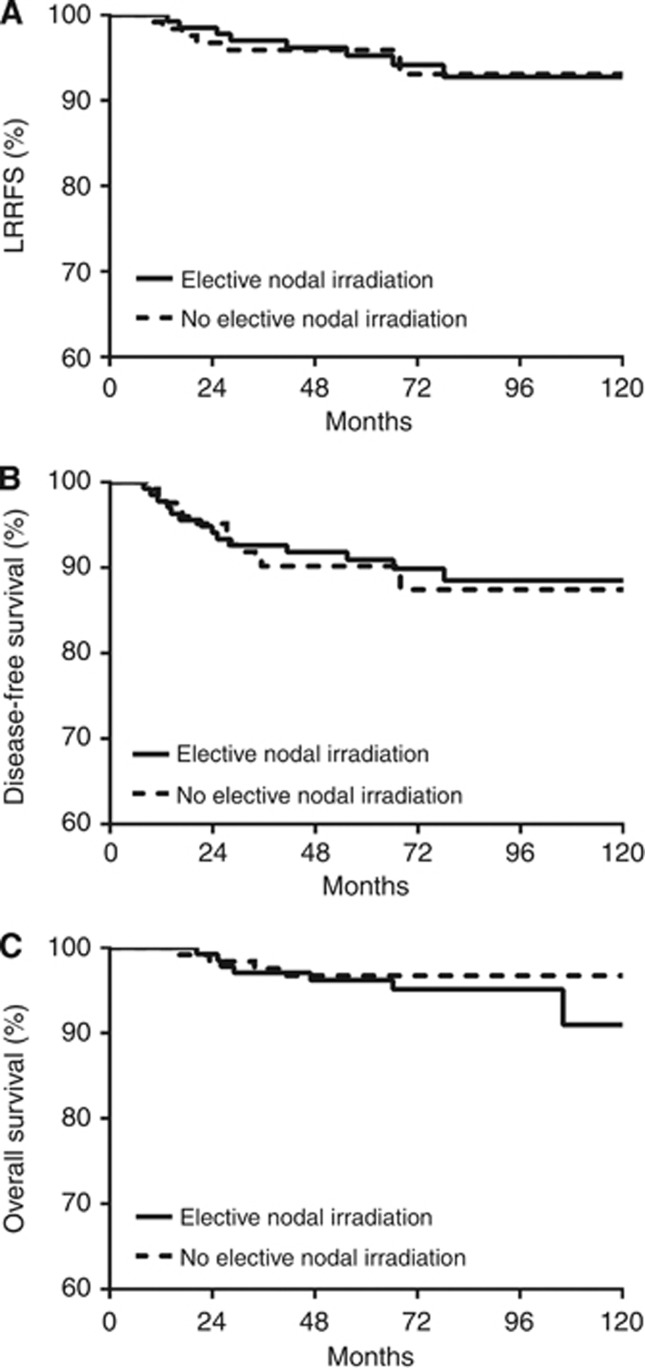
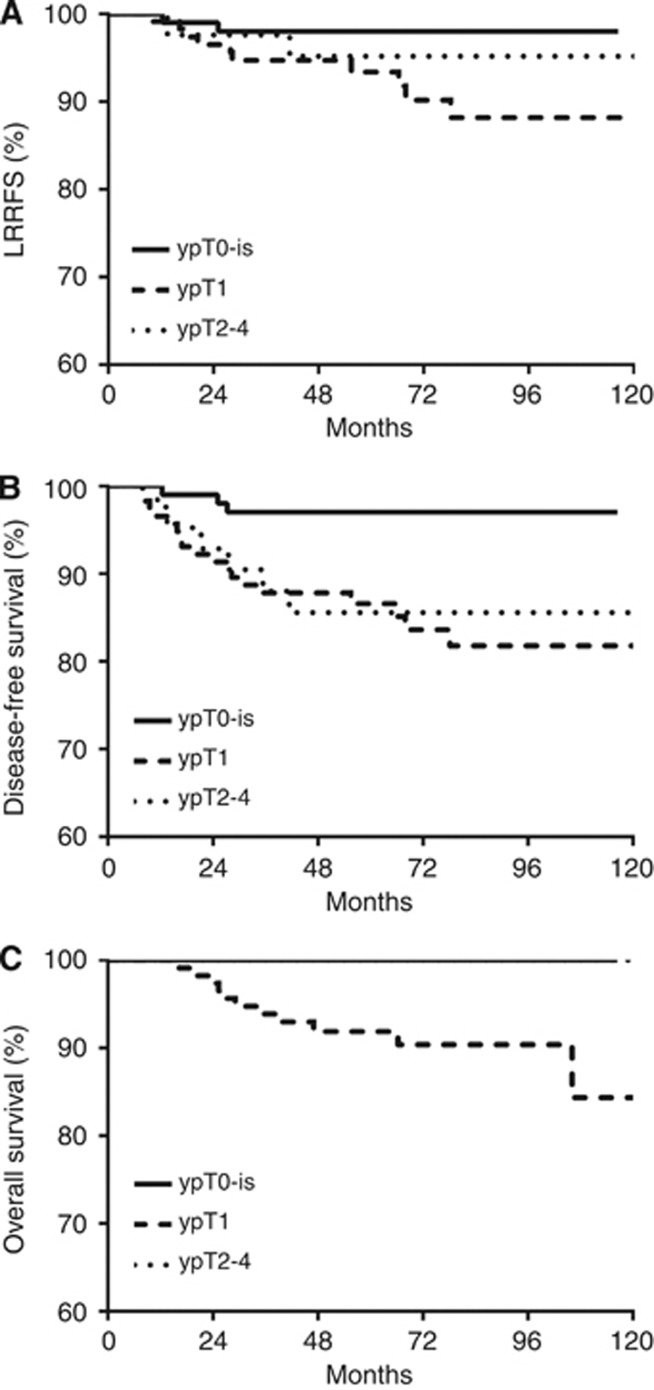
The 5-year LRRFS, DFS, and OS for all patients were 95.5%, 90.5%, and 96.4%, respectively. Survival outcomes were not different with respect to ENI (Figure 1). Disease-free survival and OS were affected by pathologic T classification (P=0.0086 and 0.0012, respectively, Figure 2). In patients with pathologic CR, the 5-year LRRFS, DFS, and OS were 98.0%, 97.0%, and 100%, respectively. When the number of LNs sampled was <13, the median number of LNs sampled in patients without ENI, DFS was significantly reduced (P=0.0099). When the patients were divided into subgroups according to pathologic T classification or number of sampled LNs, ENI did not affect survival outcomes in any of the subgroups (data not shown). Age, clinical T classification, molecular subtype, NAC regimen, and hormone treatment also did not affect survival outcomes (Table 3). On multivariate analysis, pathologic T classification and the number of LNs sampled of independent prognostic factors for DFS (Table 4), with hazard ratios were 2.025 (95% confidence interval (CI), 1.171–3.503; P=0.0116) and 0.374 (95% CI, 0.170–0.823; P=0.0145), respectively.
Figure 1.
LRRFS (A), DFS (B), and OS (C) according to the ENI. No differences in treatment outcomes were observed.
Figure 2.
LRRFS (A), DFS (B), and OS (C) according to pathologic T classification. Disease-free survival and OS were significantly affected by pathologic T classification.
Table 3. Prognostic factors affecting survival outcomes on univariate analysis.
| |
|
LRRFS |
DFS |
OS |
|||
|---|---|---|---|---|---|---|---|
| Variables | n | 5-year rate | P | 5-year rate | P | 5-year rate | P |
| ENI |
|
|
0.9716 |
|
0.7328 |
|
0.6686 |
| No | 124 | 95.9% | 90.2% | 96.7% | |||
| Yes |
136 |
95.3% |
|
90.9% |
|
96.2% |
|
| Age |
|
|
0.3540 |
|
0.0770 |
|
0.9058 |
| <40 years | 71 | 95.8% | 87.3% | 97.1% | |||
| ⩾40 years |
189 |
95.5% |
|
91.8% |
|
96.2% |
|
| Clinical T |
|
|
0.5083 |
|
0.7017 |
|
0.0804 |
| cT1–2 | 203 | 94.8% | 89.9% | 95.4% | |||
| cT3–4 |
57 |
98.2% |
|
92.7% |
|
100% |
|
| Molecular subtype |
|
|
0.8601 |
|
0.7186 |
|
0.7447 |
| Luminal | 95 | 95.4% | 92.2% | 97.9% | |||
| Triple-negative | 62 | 96.8% | 88.7% | 96.8% | |||
| HER2-positive |
73 |
95.7% |
|
93.0% |
|
95.5% |
|
| NAC regimen |
|
|
0.7028 |
|
0.3752 |
|
0.7439 |
| AT | 107 | 96.2% | 90.6% | 97.2% | |||
| AC | 72 | 97.0% | 92.8% | 97.1% | |||
| Taxane | 30 | 92.1% | 92.1% | 96.7% | |||
| Others |
51 |
93.8% |
|
86.1% |
|
93.3% |
|
| No. of sampled LNs |
|
|
0.1609 |
|
0.0099 |
|
0.0984 |
| <13 | 105 | 93.5% | 85.0% | 94.9% | |||
| ⩾13 |
155 |
96.7% |
|
94.1% |
|
97.4% |
|
| Pathologic T |
|
|
0.1074 |
|
0.0086 |
|
0.0012 |
| ypT0-is | 102 | 98.0% | 97.0% | 100% | |||
| ypT1 | 116 | 93.4% | 86.6% | 91.9% | |||
| ypT2-4 |
42 |
95.2% |
|
85.6% |
|
100% |
|
| Hormone treatment |
|
|
0.8870 |
|
0.4400 |
|
0.7767 |
| Yes | 103 | 94.8% | 91.8% | 96.9% | |||
| No | 157 | 96.1% | 89.7% | 96.1% | |||
Abbreviations: DFS=disease-free survival; ENI=elective nodal irradiation; HER2=human epidermal growth factor receptor-2; LN=lymph node; LRRFS=locoregional recurrence-free survival; NAC=neoadjuvant chemotherapy; OS=overall survival.
Table 4. Cox proportional hazards multivariate model for survival outcomes.
| |
LRRFS |
DFS |
OS |
|||
|---|---|---|---|---|---|---|
| Variables | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| ENI |
1.187 (0.399–3.531) |
0.7580 |
1.178 (0.543–2.560) |
0.6782 |
1.670 (0.470–5.931) |
0.4279 |
| Age |
– |
– |
0.542 (0.250–1.175) |
0.1208 |
– |
– |
| No. of sampled LNs |
0.468 (0.161–1.363) |
0.1638 |
0.374 (0.170–0.823) |
0.0145 |
0.336 (0.097–1.164) |
0.0853 |
| Pathologic T classification | 1.619 (0.764–3.429) | 0.2085 | 2.025 (1.171–3.503) | 0.0116 | 1.685 (0.710–4.001) | 0.2369 |
Abbreviations: CI=confidence interval; DFS=disease-free survival; ENI=elective nodal irradiation; HR=hazard ratio; LN=lymph node; LRRFS=locoregional recurrence-free survival; OS=overall survival.
Discussion
In this study, ENI did not affect survival outcomes in patients with breast cancer who achieved ypN0 after NAC followed by BCS and RT. Although clinicopathologic characteristics such as patient age, clinical T classification, and number of sampled LNs were unfavourable, whole breast irradiation alone showed outcomes that were comparable with that of ENI. As discussed by Haffty et al (2011), standard tangential field irradiation delivers substantial doses to the lower axilla (Reznik et al, 2005). Axillary coverage by whole breast irradiation, on the other hand, seems to be sufficient in terms of locoregional control in ypN0 patients after NAC followed by BCS regardless of pre-chemotherapy tumour status.
The current NCCN guidelines recommend that RT indications and fields should be based on the worst stage pre-treatment or post-treatment tumour characteristics (NCCN, 2014). In patients treated with NAC followed by surgery for locally advanced breast cancer, it is recommended that the RT field encompass whole breast/chest wall and ipsilateral regional LNs. This panel recommendation relies on randomised clinical trials that have shown a survival advantage for patients with axillary LN metastasis that received chest wall and regional LN RT after surgery (Overgaard et al, 1997; Ragaz et al, 2005), as well as retrospective studies that have shown that comprehensive RT after NAC followed by surgery benefits patients with clinical T3 or stage III–IV disease regardless of their response to NAC (Huang et al, 2004; McGuire et al, 2007). In contrast, a recent study by Daveau et al (2010) showed no differences in treatment outcomes whether or not additional ENI was performed. The 5-year DFS and OS were 83% and 89.3% in patients who received ENI, and 85% and 94.5% in patients who did not receive ENI, respectively. That report was similar to the present study, in that a similar number of patients with ypN0 after NAC and BCS were analysed. However, in the former study, clinical characteristics such as patient age and clinical N classification were more favourable in the group that did not receive ENI compared with the group that underwent the treatment, whereas the opposite was observed in our study. Clinical node involvement at diagnosis might influence decision for ENI, as recommended by the current NCCN guidelines (NCCN, 2014). Along with pathologic CR, it was independently predictive of OS in the study. Additional differences between the present and former study were the median follow-up period (66 vs 88 months), the number of LNs dissected (15 vs 11), and the proportion of patients receiving primary tumour bed boost (96.9% vs 25.8%). The LRRFS and DFS were 95.5% and 90.5% in the present study, and 88.3% and 85.8% in the previous study, respectively. These differences may be due to variability in patient characteristics, treatment, or duration of the follow-up period. Notably, however, there were no differences in treatment outcomes according to the additional ENI in either study.
In addition to ypN0, remission of the primary tumour was achieved in 102 (39.2%) patients. This was also associated with excellent treatment outcomes regardless of ENI. Only 2.9% of the patients experienced disease recurrence, and none of the patients died during the course of the study. Also, in patients with residual tumour after NAC, omission of ENI did not worsen their treatment outcomes. This implies that ENI may not be necessary for patients with ypN0 after NAC, and that whole breast irradiation following BCS may be a feasible local treatment.
Interestingly, the number of LNs examined was associated with treatment outcomes after NAC when the cutoff number was defined as 13. There was a 9.1% difference in the 5-year DFS according to the number of LNs sampled in the present study. The prognostic significance of the number of LNs sampled in patients with LN-negative breast cancer has been well established (Weir et al, 2002). Although small dissected LNs were counted after NAC was administered (Neuman et al, 2006), conflicting results have also been reported (Boughey et al, 2010). These studies concluded that the number of LNs sampled was not affected by NAC, and that surgeons needed to remove enough LNs to provide precise prognostic information. As ENI did not improve treatment outcomes in subgroups according to the number of LNs sampled, a small sample may potentially result in the understaging of disease. Because pathologic N classification after NAC is one of the most significant prognostic factors (Mamounas et al, 2012), adequate axillary staging with sufficient LN sampling is important (Boughey et al, 2010). Although at least 10 LNs are recommended to accurately stage the axilla (NCCN, 2014), we used the different cutoff number defined as 13. The optimal number of sampled LNs after NAC should be further investigated to predict prognostic influence more precisely.
There were 24 (9.2%) patients that underwent SLNB only in the present study. Although a small number of LNs was examined, treatment outcomes were not inferior in those patients (data not shown). The feasibility of SLNB after NAC has been widely discussed, though it is not recommended according to the current guidelines (Lyman et al, 2005; Fontein et al, 2013). The American College of Surgeons Oncology Group Z0011 trial showed similar treatment outcomes between SLNB and ALND in patients with SLN metastasis who underwent BCS and tangential whole breast irradiation (Giuliano et al, 2011). The trial did not include patients receiving NAC, and the authors noted that ALND remains a standard practice in patients with positive SLNs after NAC. Thus, future studies with negative SLNs after NAC followed by BCS and whole breast irradiation need to be conducted in order to optimise locoregional treatment strategies.
This retrospective study has several limitations. Because the data were collected from multiple institutions, chemotherapeutic regimen was heterogeneous and the decision for ENI was not based on clear guidelines. In addition, cytologic confirmation of regional LN involvement at diagnosis was performed in limited number (50 of 251) of patients. Although several imaging studies such as US, MRI, and PET were used for clinical diagnosis, potential inaccuracy in axillary staging could not be excluded. Furthermore, the numbers of patients and events do not seem to be enough to derive a definitive interpretation, including subgroup analysis.
In conclusion, whole breast irradiation without ENI may be a sufficient and feasible local treatment in patients with ypN0 breast cancer after NAC followed by BCS. Pathologic CR of the primary tumour and the number of LNs sampled were significant prognostic factors affecting DFS. To overcome the potential limitations of this retrospective analysis and to confirm the feasibility of omitting ENI in ypN0 patients after NAC for breast cancer, prospective randomised trials are warranted.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, Margolese R, Theoret H, Soran A, Wickerham DL, Wolmark N. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21:4165–4174. doi: 10.1200/JCO.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Boughey JC, Donohue JH, Jakub JW, Lohse CM, Degnim AC. Number of lymph nodes identified at axillary dissection: effect of neoadjuvant chemotherapy and other factors. Cancer. 2010;116:3322–3329. doi: 10.1002/cncr.25207. [DOI] [PubMed] [Google Scholar]
- Chen AM, Meric-Bernstam F, Hunt KK, Thames HD, Oswald MJ, Outlaw ED, Strom EA, McNeese MD, Kuerer HM, Ross MI, Singletary SE, Ames FC, Feig BW, Sahin AA, Perkins GH, Schechter NR, Hortobagyi GN, Buchholz TA. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol. 2004;22:2303–2312. doi: 10.1200/JCO.2004.09.062. [DOI] [PubMed] [Google Scholar]
- Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y, Peto R. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daveau C, Stevens D, Brain E, Berges O, Villette S, Moisson P, Gardner M, De la Lande B, Lasry S, Labib A, Le Scodan R. Is regional lymph node irradiation necessary in stage II to III breast cancer patients with negative pathologic node status after neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2010;78:337–342. doi: 10.1016/j.ijrobp.2009.08.053. [DOI] [PubMed] [Google Scholar]
- Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, Cruz AB, Jr, Fisher ER, Wickerham DL, Wolmark N, DeCillis A, Hoehn JL, Lees AW, Dimitrov NV. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–2493. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- Fontein DB, van de Water W, Mieog JS, Liefers GJ, van de Velde CJ. Timing of the sentinel lymph node biopsy in breast cancer patients receiving neoadjuvant therapy – recommendations for clinical guidance. Eur J Surg Oncol. 2013;39:417–424. doi: 10.1016/j.ejso.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM, Morrow M. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralow JR, Burstein HJ, Wood W, Hortobagyi GN, Gianni L, von Minckwitz G, Buzdar AU, Smith IE, Symmans WF, Singh B, Winer EP. Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol. 2008;26:814–819. doi: 10.1200/JCO.2007.15.3510. [DOI] [PubMed] [Google Scholar]
- Haffty BG, Hunt KK, Harris JR, Buchholz TA. Positive sentinel nodes without axillary dissection: implications for the radiation oncologist. J Clin Oncol. 2011;29:4479–4481. doi: 10.1200/JCO.2011.36.1667. [DOI] [PubMed] [Google Scholar]
- Huang EH, Tucker SL, Strom EA, McNeese MD, Kuerer HM, Buzdar AU, Valero V, Perkins GH, Schechter NR, Hunt KK, Sahin AA, Hortobagyi GN, Buchholz TA. Postmastectomy radiation improves local-regional control and survival for selected patients with locally advanced breast cancer treated with neoadjuvant chemotherapy and mastectomy. J Clin Oncol. 2004;22:4691–4699. doi: 10.1200/JCO.2004.11.129. [DOI] [PubMed] [Google Scholar]
- Lyman GH, Giuliano AE, Somerfield MR, Benson AB, 3rd, Bodurka DC, Burstein HJ, Cochran AJ, Cody HS, 3rd, Edge SB, Galper S, Hayman JA, Kim TY, Perkins CL, Podoloff DA, Sivasubramaniam VH, Turner RR, Wahl R, Weaver DL, Wolff AC, Winer EP. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–7720. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Mamounas EP, Anderson SJ, Dignam JJ, Bear HD, Julian TB, Geyer CE, Jr, Taghian A, Wickerham DL, Wolmark N. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol. 2012;30:3960–3966. doi: 10.1200/JCO.2011.40.8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Gonzalez-Angulo AM, Huang EH, Tucker SL, Kau SW, Yu TK, Strom EA, Oh JL, Woodward WA, Tereffe W, Hunt KK, Kuerer HM, Sahin AA, Hortobagyi GN, Buchholz TA. Postmastectomy radiation improves the outcome of patients with locally advanced breast cancer who achieve a pathologic complete response to neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1004–1009. doi: 10.1016/j.ijrobp.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network 2014. NCCN Clinical Practice Guidelines in Oncology; Breast Cancer - v.1, 2014 [cited 2013 December 29]. Available from http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf .
- Neuman H, Carey LA, Ollila DW, Livasy C, Calvo BF, Meyer AA, Kim HJ, Meyers MO, Dees EC, Collichio FA, Sartor CI, Moore DT, Sawyer LR, Frank J, Klauber-DeMore N. Axillary lymph node count is lower after neoadjuvant chemotherapy. Am J Surg. 2006;191:827–829. doi: 10.1016/j.amjsurg.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, Kjaer M, Gadeberg CC, Mouridsen HT, Jensen MB, Zedeler K. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- Ragaz J, Olivotto IA, Spinelli JJ, Phillips N, Jackson SM, Wilson KS, Knowling MA, Coppin CM, Weir L, Gelmon K, Le N, Durand R, Coldman AJ, Manji M. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil SR, Tamkus D, King KM, Pajon ER, Wright MJ, Robert J, Paik S, Mamounas EP, Wolmark N. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- Reznik J, Cicchetti MG, Degaspe B, Fitzgerald TJ. Analysis of axillary coverage during tangential radiation therapy to the breast. Int J Radiat Oncol Biol Phys. 2005;61:163–168. doi: 10.1016/j.ijrobp.2004.04.065. [DOI] [PubMed] [Google Scholar]
- Shim SJ, Park W, Huh SJ, Choi DH, Shin KH, Lee NK, Suh CO, Keum KC, Kim YB, Ahn SD, Kim SS, Ha SW, Chie EK, Kim K, Shin HS, Kim JH, Lee HS. The Role of Postmastectomy Radiation Therapy After Neoadjuvant Chemotherapy in Clinical Stage II-III Breast Cancer Patients With pN0: A Multicenter, Retrospective Study (KROG 12-05) Int J Radiat Oncol Biol Phys. 2014;88:65–72. doi: 10.1016/j.ijrobp.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Weir L, Speers C, D'Yachkova Y, Olivotto IA. Prognostic significance of the number of axillary lymph nodes removed in patients with node-negative breast cancer. J Clin Oncol. 2002;20:1793–1799. doi: 10.1200/JCO.2002.07.112. [DOI] [PubMed] [Google Scholar]