Abstract
Background:
Oestrogen (E2) induces apoptosis in long-term E2-deprived MCF7 cells (MCF7:5C). Taxanes have been used extensively in the treatment of early and advanced breast cancer. We have interrogated the sequence of events that involve the apoptotic signalling pathway induced by E2 in comparison with paclitaxel.
Methods:
DNA quantification and cell cycle analysis were used to assess proliferation of cancer cells. Apoptosis was evaluated using annexin V and DNA staining methods. Regulation of apoptotic genes was determined by performing PCR-based arrays and RT–PCR.
Results:
E2-induced apoptosis is a delayed process, whereas paclitaxel immediately inhibits the growth and induces death of MCF7:5C cells. The cellular commitment for E2-triggered apoptosis occur after 24 h. Activation of the intrinsic pathway was observed by 36 h of E2 treatment with subsequent induction of the extrinsic apoptotic pathway by 48 h. Paclitaxel exclusively activated extramitochodrial apoptotic genes and caused rapid G2/M blockade by 12 h of treatment. By contrast, E2 causes an initial proliferation with elevated S phase of cell cycles followed by apoptosis of the MCF7:5C cells. Most importantly, we are the first to document that E2-induced apoptosis can be reversed after 24 h treatment.
Conclusions:
These data indicate that E2-induced apoptosis involves a novel, multidynamic process that is distinctly different from that of a classic cytotoxic chemotherapeutic drug used in breast cancer.
Keywords: E2, oestrogen receptor, 4-hydroxy tamoxifen, RT–PCR, TNF, endoplasmic reticulum stress, paclitaxel, chemotherapy
Endocrine therapy remains the standard of care in the treatment of oestrogen receptor (ER)-positive breast cancer (Jordan, 2009). Tamoxifen inhibits estradiol (E2)-induced tumour growth; but continuous tamoxifen treatment of nude mice with transplantable ER-positive tumours results in tumour growth with either E2 or tamoxifen (Osborne et al, 1987; Gottardis and Jordan, 1988). After 5 years of re-transplantation and tamoxifen treatment, these serially transplanted tamoxifen-stimulated tumours grow in response to tamoxifen, but paradoxically rapidly regress with physiological E2 treatment (Yao et al, 2000). Development of acquired resistance to long-term (5 years) antihormonal therapy in breast cancer causes a reconfiguration of the tumour cells that now makes them vulnerable to physiological E2-induced apoptosis. MCF7 breast cancer cells that are resistant to long-term oestrogen withdrawal undergo apoptosis in response to E2 (Lewis et al, 2005a, 2005b). Clinical trials (Lønning et al, 2001; Ellis et al, 2009) have evaluated this concept, and their results show that about 30% of patients with advanced breast cancer who have acquired resistant to antihormone therapy show an objective clinical response with oestrogen therapy. The Women Health Initiative trial (WHI, 1998), which compared conjugated equine oestrogen (CEE) therapy with placebo in hysterectomised postmenopausal women, noted a paradoxical decrease in incidence of breast cancer compared with combination of CEE and progestin (Rossouw et al, 2002; Chlebowski et al, 2013), and this observation was subsequently supported by results obtained from the Million Women Study (Beral et al, 2011). In neither clinical study was a molecular mechanism offered to explain the apparent anomaly that CEE alone does not induce a profound significant increase in breast cancer risk. However, reanalysis of the mature data from the Women Health Initiative CEE alone study (Anderson et al, 2012) now demonstrates a persistent and sustained decrease in the incidence and mortality of breast cancer in women who received E2 alone therapy. We recently reported that constituents of CEE cause apoptosis in long-term E2-deprived MCF7 cells (Obiorah and Jordan, 2013). Given that these laboratory observations translate to clinical benefit for patients, it is appropriate to investigate the molecular events that precede the induction of apoptosis by E2.
Cancer chemotherapy induces rapid death of neoplastic cells (Kaufmann and Earnshaw, 2000; Makin and Dive, 2001), but E2-induced apoptosis, in contrast, is a delayed event. Ariazi et al (2011) recently identified the total gene activation sequence that occurs over a 7-day period during E2-induced apoptosis. Endoplasmic reticulum stress is induced by E2 that activates unfolded protein response leading to upregulation of mitochondrial proapoptotic genes. Involvement of the extrinsic pathway in E2-induced apoptosis have been implicated, but its exact role is not clearly defined (Song et al, 2001; Osipo et al, 2003). However, nothing is known on the effect of cytotoxic chemotherapy in the MCF7:5C cells. Paclitaxel, a member of the drug family, the taxanes, is a mitotic spindle inhibitor that prevents destabilization of microtubules (Jordan et al, 1993; Yvon et al, 1999). Taxanes are used extensively as part of combination therapy in metastatic breast cancer (Robert et al, 2011; Kelly et al, 2012), and are the gold standard in the adjuvant therapy of early breast cancer where they decrease risk of cancer recurrence and mortality(Ward et al, 2007; Gines et al, 2011).
The goal of this paper is to determine the critical trigger point for E2-induced apoptosis. We have explored the differential gene expression as a prelude to determine the early molecular events in E2-induced apoptosis in comparison with classic cytotoxic chemotherapy-induced apoptosis. Induction of mRNA levels of proapoptotic genes confirmed whether mitochondrial and tumour necrosis factor (TNF) apoptotic pathways were activated. We compared and contrasted the ability of E2 and paclitaxel with arrest cell cycle to advance the molecular understanding of the new biology of E2-induced apoptosis in therapy.
Materials and Methods
Cell culture and reagents
Cell culture media were purchased from Invitrogen Inc. (Grand Island, NY, USA), and fetal calf serum was obtained from HyClone Laboratories (Logan, UT, USA). Compounds E2, 4-hydroxytamoxifen (4OHT) and paclitaxel were obtained from Sigma (St Louis, MO, USA). MCF7:5C cells were derived from MCF7 cells obtained from the Dr Dean Edwards (San Antonio, TX, USA) as reported previously (Jiang et al, 1992). It was long-term cultured in E2-deprived medium. MCF7 cells were maintained in RPMI media supplemented with 10% fetal calf serum, 6 ng ml−1 bovine insulin and penicillin and streptomycin. MCF7:5C cells were maintained in phenol-red-free RPMI media containing 10% charcoal dextran-treated fetal calf serum, 6 ng ml−1 bovine insulin and penicillin and streptomycin. The cells were treated with indicated compounds (with media changes every 48 h) for the specified time, and were subsequently harvested for tissue culture experiments.
Cell growth assay
The cell growth was monitored by measuring the total DNA content per well in 24-well plates.
Fifteen thousand cells were plated per well, and treatment with indicated concentrations of compounds was started after 24 h in triplicates. Media containing the specific treatments was changed every 48 h. On day 7, the cells were harvested and total DNA was assessed using a fluorescent DNA quantification kit (cat. no. 170-2480; Bio-Rad, Hercules, CA, USA) and was performed as previously described (Lewis et al, 2005a).
RNA isolation and real-time PCR
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and RNAeasy kit according to the manufacturer's instructions. Real-time PCR was performed as previously described (Sengupta et al, 2010). The sequences for all primers were as follows: BCL2L11 (Bim) forward: 5′-TCGGACTGAGAAACGCAAG-3′ reverse: 5′-CTCGGTCACTCAGAACTTAC-3′. TNF forward: 5′-ACTTTGGAGTGATCGGCC-3′ reverse: 5′-GCTTGAGGGTTTGCTACAAC-3′. The change in expression of transcripts was determined as described previously and used the ribosomal protein 36B4 mRNA as the internal control (Sengupta et al, 2010).
Real-time profiler assay for apoptosis
RT–PCR profiler assay kits for apoptosis was used from a commercial vendor that used 384-well plates to profile the expression of 370 apoptosis-related human genes (Qiagen; SABiosciences Corp., Fredrick, MD, USA; cat. no. 330231 PAHS-3012E). All the procedures were followed as previously described(Sengupta et al, 2013). Briefly, MCF7:5C cells were treated with control or with indicated compounds (in triplicates), and total RNA was isolated using the method mentioned earlier. Two micrograms of total RNA was reverse transcribed and RT–PCR was performed using ABI 7900HT (Foster City, CA, USA). We created an apoptotic gene signature throughout these time points after comparing them with control treatment. This gene signature was generated by comparing the expression level of all the genes with vehicle treatment and selecting the genes that were at least 2.5-fold over- or under-expressed as compared with vehicle-treated cells at a statistical significance of P-value of 0.05. The fold change was calculated by ΔΔCt method and volcano plots were generated using the web-based tool, RT2 profile PCR array data analysis version 3.5 (Qiagen; SABiosciences Corp.).
Apoptosis assay
The concentration of paclitaxel was based on the publication by Gines et al (2011). The concentration of E2 was based on the growth curve with different doses and our previous publication (Lewis et al, 2005a, 2005b). MCF7:5C cells (1 × 106 cells per ml) were seeded in 100-mm dishes and cultured overnight in oestrogen-free RPMI 1640 medium containing 10% SFS. The next day, cells were treated with vehicle (0.1% ethanol) as control, E2 (1 nM) for 48 and 72 h or with paclitaxel (1 μM) for 12 and 24 h, and then harvested in cold PBS (Invitrogen, Grand Island, NY, USA) and collected by centrifugation for 2 min at 500 g. Cells were then resuspended and stained simultaneously with either with FITC-labeled annexin V and propidium iodide (PI; Pharmingen, San Diego, CA, USA) or with DNA-binding dye, YO-PRO-1 and PI (Life Technologies, Grand Island, NY, USA). Apoptosis was verified based on loss of plasma membrane integrity. Viable cells excluded these dyes, whereas apoptotic cells allowed moderate staining. Cells were analysed using a fluorescence-activated cell sorter flow cytometer (Becton Dickinson, San Jose, CA, USA). Experiments were repeated three times with similar results.
Cell cycles analysis
MCF7:5C cells were cultured in dishes and were treated with vehicle (0.1% ethanol), E2 (1 nM) and paclitaxel (1 μM). Cells were harvested and gradually fixed with 75% EtOH on ice. After staining with PI, cells were analysed using a fluorescence-activated cell sorter flow cytometer (Becton Dickinson), and the data were analysed with CellQuest software (BD Biosciences, San Jose, CA, USA).
Statistical analysis
All data were expressed as the mean of at least three determinations, unless otherwise stated. The differences between the treatment groups and the control group were determined by one-factor or two-way analysis of variance.
Results
Cell growth and apoptotic effects of E2 and paclitaxel on MCF7:5C cells
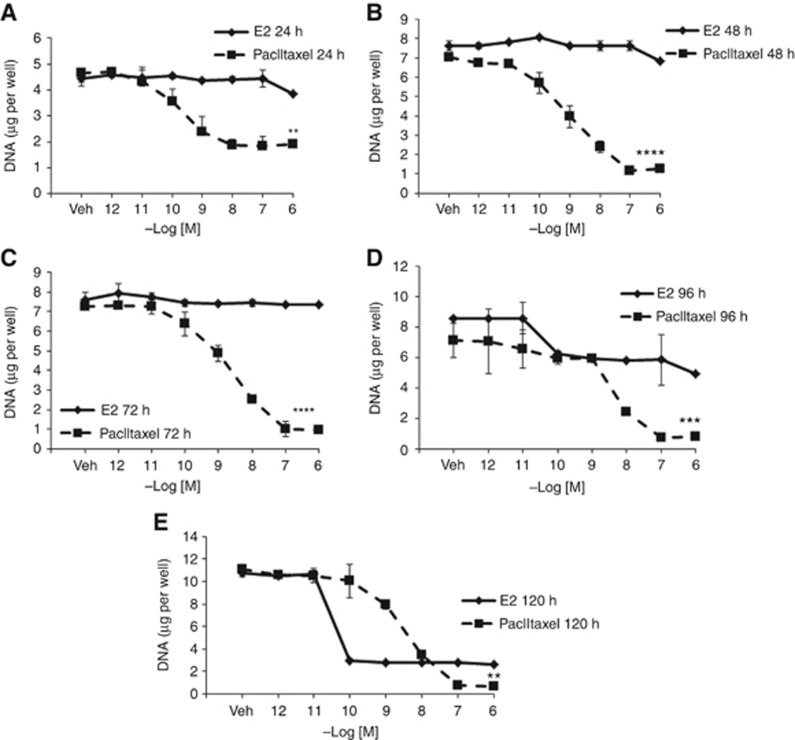
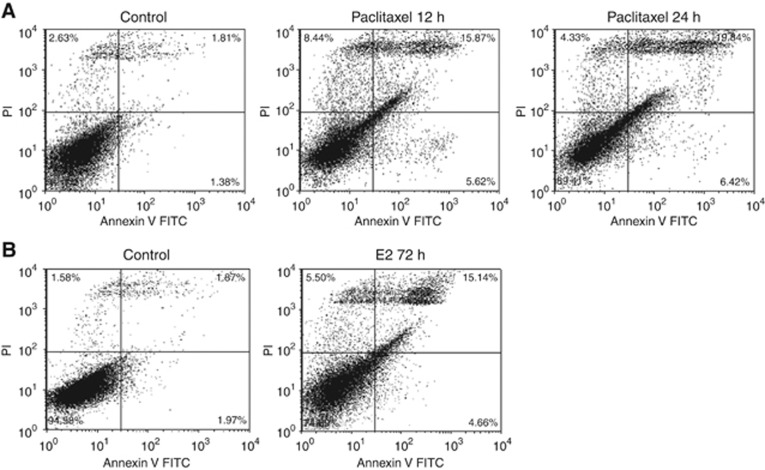
We sought to compare the antiproliferative activity between paclitaxel and E2 in the MCF7:5C cell line and explore their potential to induce apoptosis. Paclitaxel induced rapid inhibition of growth in a concentration-dependent manner with maximum inhibition at 0.1 μM. Fifty percent growth inhibition was achieved in 24 h (Figure 1A), which increased to almost 100% after 48 h of treatment (Figure 1B). In contrast, E2 achieved maximal growth inhibition at 0.1 nM, and did not quantitatively prevent cell proliferation until after 72 h (Figure 1C). Twenty-five percent of growth inhibition occurred at 96 h with E2 treatment (Figure 1D) and this increased to 80% at the 120-h time point (Figure 1E). The decrease in cell number observed with E2 and paclitaxel was further investigated to determine whether the growth inhibition was due to apoptosis. An increased apoptotic response (Figure 2A) was detected by increasing the percentage of annexin V staining from control 3.92–21.49% by paclitaxel after 12 h treatment, whereas an apoptotic effect was observed at 72 h with E2 (Figure 2B). An apoptotic response was not detected after 24 h treatments with E2 through annexin V staining (Supplementary Figure S1A). Experiments were repeated three times and a summary of results are represented in Supplementary Figure S1. Similar results were observed with a DNA-binding dye, YO-PRO-1 (Supplementary Figure S2).
Figure 1.
Effect of E2 and paclitxel on the growth characteristics in the MCF7:5C cells. MCF5C cells were seeded in 24-well plate treated with the control vehicle (Veh) or E2(♦) and paclitaxel (▪) over a range of doses and cells were harvested after (A) 24 h, (B) 48 h, (C) 72 h, (D) 96 h and (E) 120 h. Data points shown are the average of three replicates ±s.d. (**P<0.02,***P<0.0003,****P<0.0001).
Figure 2.
Differential apoptotic effects of E2 and paclitaxel. MCF7:5C cells were treated with control or (A) paclitaxel (1 μM) for 12 and 24 h or (B) E2 (1 nM) for 72 h, and then stained with annexin V-FITC and PI and analysed by flow cytometry. Viable cells (left lower quadrant) are annexin V-FITC− and PI−, early apoptotic cells (right lower quadrant) are annexin V-FITC+ and PI−, dead cells (left upper quadrant) are PI+ and late apoptotic cells (right upper quadrant) are annexin V-FITC+ and PI+. Increased staining for apoptosis is observed maximally in the right upper quadrant.
Determination of the critical trigger point of estradiol-induced apoptosis
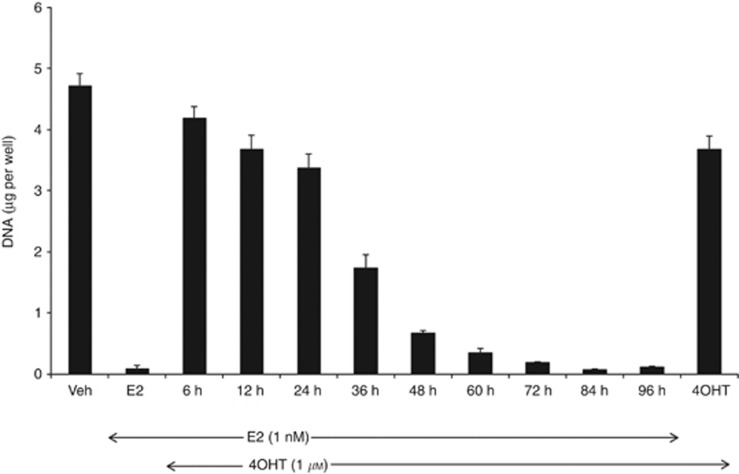
Although E2 treatment induces apoptosis of MCF7:5C cells in a concentration-dependent manner, the cells are unresponsive to the anti-oestrogen, 4OHT. Rather 4OHT blocks E2-mediated apoptosis (Maximov et al, 2011). To further investigate the delayed response to E2-mediated apoptosis and to determine the critical trigger point for E2-induced apoptosis, we used 4OHT to block and rescue the cells from the apoptotic effect of E2. In this way, we established when the cells are committed to cell death. MCF7:5C cells were treated with 1 nM of E2, and subsequently 1 μM of 4OHT was used to block the apoptotic effects of E2 at the indicated time points over a range of 96 h after the addition of E2. Cells were then all collected for DNA assay on day 7. Apoptosis triggered by E2 was competitively inhibited and rescued for up to 24 h, and thereafter it lost the ability to rescue cells committed to E2-induced apoptosis (Figure 3). Between 24 and 36 h, the cells are committed to apoptosis despite the anti-oestrogenic action of 4OHT.These data suggest that the critical trigger for the commitment of the cell to the induction of apoptosis by E2 lies between 24 and 36 h.
Figure 3.
Deciphering the trigger point for E2-induced apoptosis. Cells were treated with vehicle (Veh) or E2 (1 nM) alone, and 1 μM 4OHT was added and used to block and reverse E2 action at 6, 12, 24, 36, 48, 60, 72, 84 and 96 h. The cells were harvested after 7 days of treatment. The extent of apoptosis was determined by measuring the DNA content of the remaining cells in each well. The experiment was done in triplicates, and the data represent the mean of three independent experiments with 95% confidence intervals. The trigger point for E2-mediated apoptosis was elucidated at the time when the apoptotic effects of E2 could not be blocked by 4OHT.
Differential gene expression of E2-mediated apoptosis at the critical trigger point
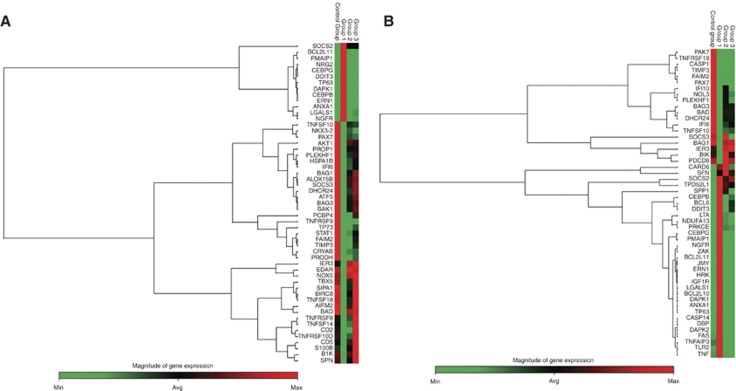
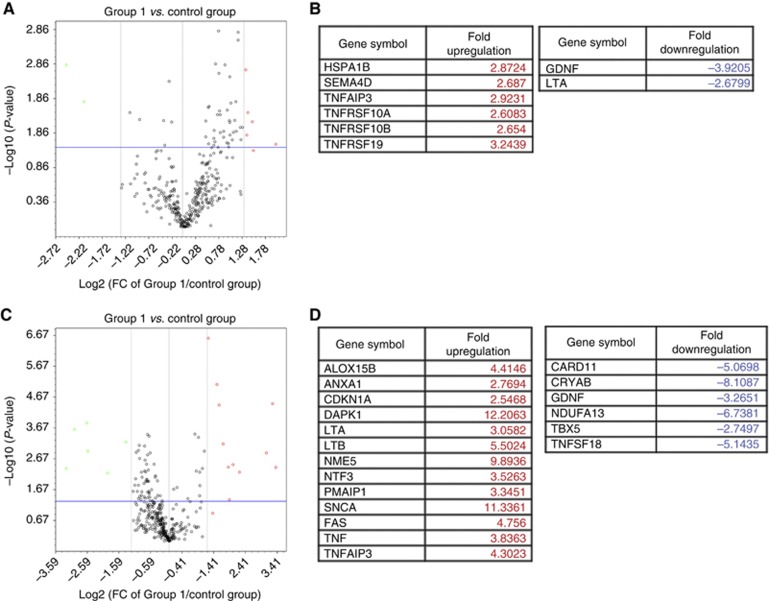
To identify genes associated with E2-induced apoptosis with a particular focus on the critical trigger time point, differential regulation of apoptotic gene expression in response to E2 was interrogated in the MCF7:5C cells. At 24 h, as expected, significant evidence of apoptotic gene induction is not apparent, rather proapoptotic genes such as BAD and BCL2L10, and Caspases 1, 9 and 10 are differentially downregulated by E2 (Supplementary Table S1). TNF-related genes, TNFRSF8 and TNFSF14, are induced by both E2 and 4OHT, and they do not have a definitive role in the TNF-mediated apoptosis but rather are involved in the T-cell response. Interestingly, at 36 h (Figure 4A), which represents the trigger point for apoptosis, E2 induces proinflammatory genes such as CEBPB, CEBPG and DAPK1, and endoplasmic reticulum stress-related genes such as DDIT3 and ERN 1. BCL2L11 (BIM), an important member of the mitochondrial pathway and an apoptosis activator, is also upregulated by E2, suggesting an early involvement of the intrinsic pathway. Following 48 h of E2 treatment (Figure 4B), the gene expression expands to involve the TNF-related genes such as FAS, TNFRSF21 and TNF, and continued increased expression of endoplasmic reticulum stress and proinflammatory-related genes. In addition, p53 expression is increased at 48 h. PMAIP 1 (also known as NOXA), a Bcl-2 homology (BH3) only family and a p53-regulated gene is also upregulated by E2. 4OHT acted as an anti-oestrogen and was able to block most of the effects of E2. The identified apoptosis-related genes are listed in Supplementary Tables S1–S3.
Figure 4.
Heat map of E2-mediated apoptotic genes that are differentially expressed by 36 and 48 h of treatment. Cells were parsed into groups of three replicates per treatment per time point, and then treated with either 0.1% ethanol (control group), 1 nM E2 (group 1), 1 μM 4OHT (group 2), in the presence (group 3) or in the absence of E2 over a period of 48 h. Total RNA was extracted and reverse transcribed as described in Materials and Methods section. Samples were loaded onto customised PCR array plates with primers for indicated apoptotic genes. Gene expression values were obtained and analysed in comparison with the controls at (A) 36 h and (B) 48 h. The maximum expressed level of any given gene is represented by red colour and minimum levels are represented as green colour.
Paclitaxel induces TNF family of apoptosis-related genes in MCF7:5C cells
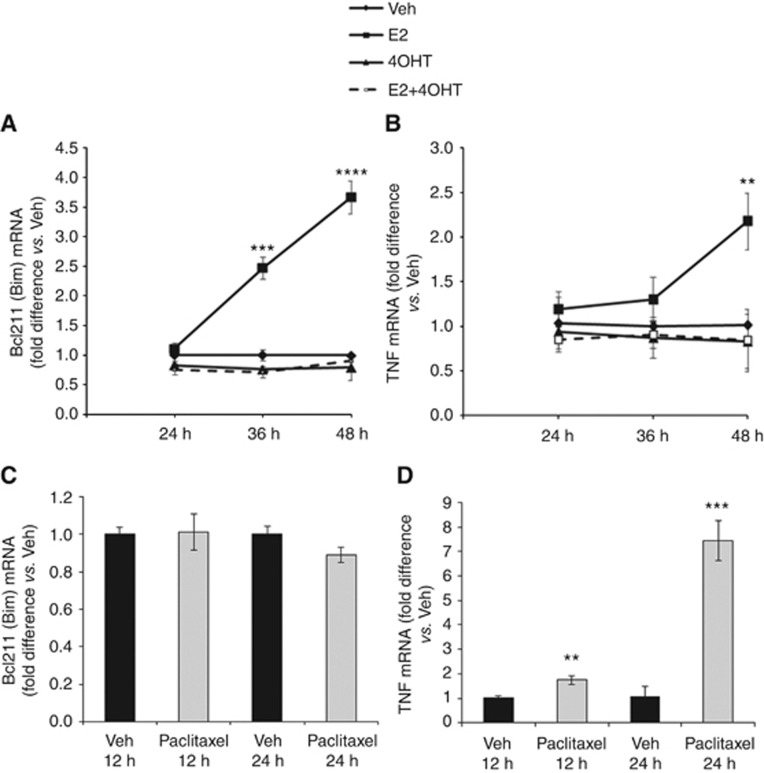
We further investigated expressed genes activated by paclitaxel that may define a molecular mechanism. Based on the biological experiments shown above (Figures 1 and 2B), paclitaxel-induced apoptotsis happened after 12 h treatment and reached to a peak at 24 h. We mainly focused on detecting gene regulation by paclitaxel at these two time points. Paclitaxel selectively activated the TNF family of apoptosis-related genes. After an initial 12 h of treatment (Figure 5A and B), paclitaxel stimulated TNFRSF10A (TNF receptor superfamily, member 10a) and TNFRSF10B (TNF receptor superfamily, member 10b), which are known to be activated by the ligand TNF-related apoptosis inducing ligand (TNFSF10/TRAIL), and causes death through the extramitochondrial pathway.TNFRSF19 (TNF receptor superfamily, member 19) induces apoptosis in a caspase-independent manner. In addition, TNF proapoptotic genes, including FAS and TNF, and other TNF proinflammatory genes such as LTA, LTB and TNFAIP3, are activated by 24 h of treatment with paclitaxel (Figure 5C and D). Paclitaxel further induces NOXA and CDKN1A (p21) that are known to inhibit the activity of cyclin-CDK2 or -CDK4 complexes at the G1 phase. Although these two p53-regulated genes were upregulated by paclitaxel, p53 induction was not observed at 24 h. Unlike E2, which increases BIM and TNF mRNA levels (Figure 6A and B), paclitaxel was only able to induce TNF expression (Figure 6C and D). These results highlight the differences in apoptosis-related genes induced by the two treatments.
Figure 5.
Determination of apoptotic genes induced by a cytotoxic chemotherapy in MCF7:5C cells. MCF7: 5C cells were treated with either 0.1% ethanol (control), or 1 μM paclitaxel (group 1) for 12 and 24 h. Gene expression values were obtained and analysed in comparison with the controls, and volcano plots were generated at 12 h of treatment (A) and the expressed genes listed (B). Similarly, gene expression levels are analysed after 24 h of paclitaxel treatment (C) and genes are listed in D. The genes selected were at least 2.5-fold overexpressed or under-expressed as compared with vehicle at P-value=0.05. Genes upregulated are represented in red and downregulated genes are represented in green.
Figure 6.
E2 activates both mitochondrial and extrinsic pathway of apoptosis, whereas paclitaxel activates only the extrinsic pathway. MCF7:5C cells were treated with vehicle (Veh), 1 nM E2, 1 μM 4OHT or combination treatment of E2 and 4OHT for 24, 36 and 48 h. Total RNA was reverse transcribed and assessed for (A) BIM and (B) TNF gene expression. Induction of (C) BIM and (D) TNF mRNA was determined in MCF7:5C cells treated with either Veh or 1 μM paclitaxel for 12 and 24 h using RT–PCR. PCR data values are presented as fold difference versus Veh-treated cells±s.e.m. (**P<0.02, ***P<0.0003, ****P<0.0001).
Differential effect of paclitaxel in induction of G2 blockade in comparison with E2
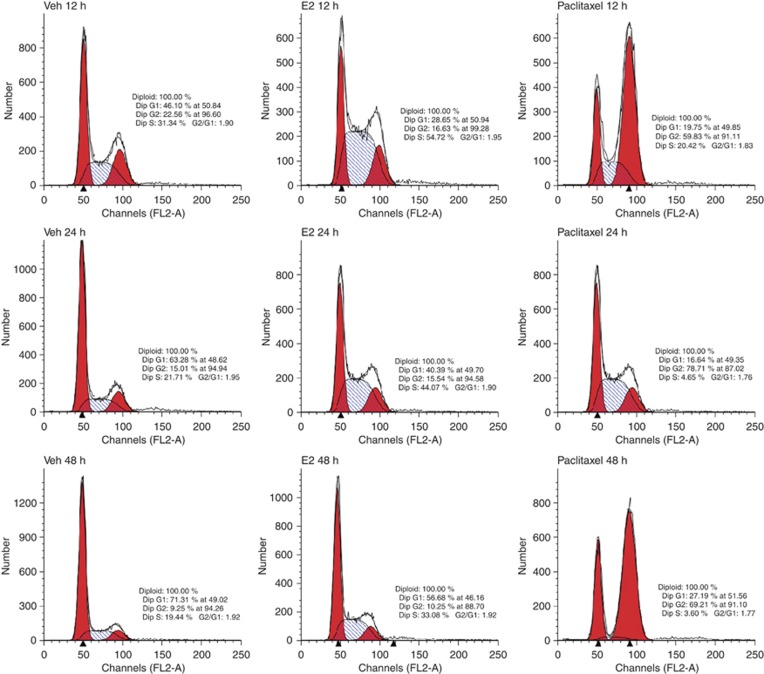
Paclitaxel prevents progression of mitosis and activates the mitotic checkpoint, paving a path for apoptosis. To elucidate whether the apoptotic effects of paclitaxel in comparison with E2 were mediated through cell cycle arrest, we performed cell cycle analysis in MCF7:5C cells using flow cytometry. Our results reveal that paclitaxel treatment causes accumulation of cells in G2/M phase with a concomitant reduction in the number of cells in G1 and S phase (Figure 7) Cell cycle arrest in G2/M phase was about threefold higher compared with control. In contrast, a G1 or G2 blockade was not observed with E2 treatment. E2 dramatically enhanced S phase at 12 h and rapidly increased to sevenfold by 48 h. This is consistent with our recent publication (Fan et al, 2013) that E2 increased S phase after 72 h treatment. Based on these observations, we hypothesise that the apoptotic effects of paclitaxel in MCF7:5C cells results from a perturbation in the cell cycle check points, whereas E2 induces cell proliferation finally resulting in apoptosis.
Figure 7.
Cell cycle analysis of the effects of E2 and paclitaxel in the MCF7:5C cells. Representative cell cycle profiles of MCF7:5C cells treated with either 0.1% ethanol (Veh), E2 (1 nM) or paclitaxel (1 μM) for 12, 24 and 48 h. FL2-A represents the intensity of PI, and the y-axis represents the cell number.
Discussion
The molecular sequence of events resulting in either E2-induced apoptosis or paclitaxel-induced apoptosis is completely different. E2-induced apoptosis appears to be unique. Paclitaxel rapidly induces apoptosis of MCF7:5C cells, whereas E2 shows a delayed process for the induction of apoptosis. Using 4OHT to block and rescue E2-induced events necessary for an apoptotic response, we observed that the trigger for apoptosis occurs after 24 h and the cells become committed to apoptosis by and after 36 h. There is activation by E2 of endoplasmic reticulum stress-related genes and proinflammatory genes at 36 h. Activation of the mitochondrial pathway was indicated by increased expression of BCL2L11, BIM, that continued to be upregulated at 48 h. Involvement of the extrinsic pathway was evidenced by induction of FAS, TNFRSF21 and TNF, and TNFAIP3 at 48 h. The TNF family genes are a group of cytokines that are involved in a number of processes including apoptosis (Micheau and Tschopp, 2003; Thorburn, 2004) and inflammation (De Paepe et al, 2012). The increased involvement of endoplasmic reticulum stress and inflammatory genes in E2-induced apoptosis is not surprising because both pathways are known to intersect (Hu et al, 2006; Zhang and Kaufmann, 2008). Multiple genes induced by E2 are NF-κB responsive that is a major regulator of inflammatory response (Baldwin, 1996; Dobrovolskaia and Kozlov, 2005). Upregulation of the observed genes provide a potential mechanism for E2 to target a variety of inflammatory and apoptotic genes.
The importance of BIM and Bax have previously been noted and verified by selective increased expression of both proteins by E2 (Lewis et al, 2005a). Involvement of the extrinsic signalling pathway in E2-induced apoptosis has been also observed. Osipo et al (2003) showed that E2-induced regression of tamoxifen-stimulated breast cancer tumours by activating the death receptor Fas and inhibiting the antiapoptotic/prosurvival factors NF-κB and HER2/neu. In addition, the growth of raloxifene-resistant MCF7 cells in vitro and in vivo was inhibited by E2 by increasing Fas expression and reduced NF-κB activity (Liu et al, 2003). However, unlike the present study, none of the previous studies investigated a time course of the intrinsic and extrinsic pathway in the MCF7:5C cells in E2-induced apoptosis. Similar to our PCR array results, RNA sequencing of E2-treated MCF7:5C cells revealed induction of multiple apoptosis-related genes (Fan et al, 2013); therefore, deletion of a single gene is unlikely to significantly affect E2-mediated apoptosis in the MCF7:5C cells. E2 induces apoptosis in osteoclasts within 24 h (Kameda et al, 1997) and is associated with upregulation of TGF-β and inhibition of E2-treated cells with anti-TGF-β antibody inhibited E2-induced apoptosis (Hughes et al, 1996). Therefore, this study show a unique sequential activation of endoplasmic reticulum stress, inflammatory-response genes as well as the intrinsic and extrinsic apoptosis-related genes in E2-mediated apoptosis.
Paclitaxel, a cytotoxic chemotherapy extensively used in the treatment of breast cancer was used as a comparator to E2 to demonstrate differences in the expression of apoptosis-related genes. Paclitaxel selectively induces the TNF proapoptotic genes, but BIM expression was not noted. On the other hand, paclitaxel kills the MCF7 cells by displacement of BIM from the BIM/BCL2 complex (Kutul and Lethai, 2010). Knockdown of BIM with siRNA significantly impairs the ability of paclitaxel to cause apoptosis in MCF7 cells (Kutul and Lethai, 2010; Ajabnoor et al, 2012). In contrast, another study(Czernick et al, 2009) showed that BIM was not required for paclitaxel-mediated apoptosis in MCF7 cells, and these apparent discrepancies could be because of differences that exist from MCF7 cell lines obtained from different sources. However, long-term deprivation of E2 from the MCF7 cells may have induced changes in the microenvironment that may be responsible for the taxane to activate the TNF apoptosis-related genes. Flow cytometry studies show that E2 causes both proliferation and apoptosis of the MCF7:5C cells, indicating that before the trigger for apoptosis occurs, the cells grow in response to E2. Because cells continue to divide with elevated S phase of cell cycles, the reduction of cell number by E2 do not become evident until after 4 days of treatment. In contrast, paclitaxel causes an immediate G2 blockade by 12 h that may explain the rapid reduction of cell number.
In conclusion, the initial target site of E2 is ER. E2 induces endoplasmic reticulum stress and mitochondrial apoptotic genes and a later recruitment of the TNF family of apoptotic genes, whereas paclitaxel induces a G2/M blockade and rapidly induces TNF apoptosis-related genes. The unique delayed aspect of E2-induced apoptosis in antihormone-resistant breast cancer creates a new dimension in our opportunities to apply the knowledge for this targeted therapy of clinical significance (Ellis et al, 2009; Anderson et al, 2012; Obiorah and Jordan, 2013). This natural process of E2-induced apoptosis may have significant applications in the further understanding of the cellular biology of cancer.
Acknowledgments
This work (VCJ) was supported by the Department of Defense Breast Program under Award number W81XWH-06-1-0590. Center of Excellence; the Susan G Komen for the Cure Foundation under Award number SAC100009 and the Lombardi Comprehensive Cancer 1095 Center Support Grant (CCSG) Core Grant NIH P30 CA051008. The views and opinions of the author(s) do not reflect those of the United States Army or the Department of Defense.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Ajabnoor GM, Crook T, Coley H. Paclitaxel resistance is associated with switch from apoptotic to autophagic cell death in MCF-7 breast cancer cells. Cell Death Dis. 2012;3 (1:e260. doi: 10.1038/cddis.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GL, Chlebowski RT, Aragaki AK, Kuller LH, Manson JE, Gass M, Bluhm E, Connelly S, Hubbell FA, Lane D, Martin L, Ockene J, Rohan T, Schenken R, Wactawski-Wende J. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women's Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476–486. doi: 10.1016/S1470-2045(12)70075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariazi E, Cunliffe H, Lewis-Wambi JS, Slifker M, Willis A, Ramos P, Tapia C, Kim H, Yerrum S, Sharma C, Nicolas E, Balagurunathan Y, Ross EA, Jordan V. Estrogen induces apoptosis in estrogen deprivation-resistant breast cancer through stress responses as identified by global gene expression across time. Proc Natl Acad Sci USA. 2011;108:18879–18886. doi: 10.1073/pnas.1115188108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Beral V, Reeves G, Bull D, Green J. Breast Cancer Risk in Relation to the Interval Between Menopause and Starting Hormone Therapy. J Natl Cancer Inst. 2011;103:296–305. doi: 10.1093/jnci/djq527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski R, Manson J, Anderson G, Cauley J, Aragaki A, Stefanick M, Lane D, Johnson K, Wactawski-Wende J, Chen C, Qi L, Yasmeen S, Newcomb P, Prentice R. Estrogen plus progestin and breast cancer incidence and mortality in the Women's Health Initiative Observational Study. J Natl Cancer Inst. 2013;105:526–535. doi: 10.1093/jnci/djt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernick M, Rieger A, Goping I. Bim is reversibly phosphorylated but plays a limited role in paclitaxel cytotoxicity of breast cancer cell lines. Biochem Biophys Res Commun. 2009;379 (1:145–150. doi: 10.1016/j.bbrc.2008.12.025. [DOI] [PubMed] [Google Scholar]
- De Paepe B, Creus KK, De Bleecker JL. The tumor necrosis factor superfamily of cytokines in the inflammatory myopathies: potential targets for therapy. Clin Dev Immunol. 2012;369432:23–33. doi: 10.1155/2012/369432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolskaia MA, Kozlov SV. Inflammation and cancer: when NF-kappaB amalgamates the perilous partnership. Curr Cancer Drug Targets. 2005;5:325–344. doi: 10.2174/1568009054629645. [DOI] [PubMed] [Google Scholar]
- Ellis M, Gao F, Dehdashti F, Jeffe D, Marcom P, Carey L, Dickler M, Silverman P, Fleming P, Kommareddy A, Jamalabadi-Majidi S, Crowder R, Siegel B. Lower-dose vs high-dose oral estradiol therapy of hormone receptor–positive, aromatase inhibitor–resistant advanced breast cancer: A phase 2 randomized study. JAMA. 2009;302:774–780. doi: 10.1001/jama.2009.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan P, Griffith OL, Agboke FA, Anur P, Zou X, McDaniel RE, Creswell K, Kim SH, Katzenellenbogen JA, Gray JW, Jordan VC. c-Src modulates estrogen-induced stress and apoptosis in estrogen-deprived breast cancer cells. Cancer Res. 2013;73:4510–4520. doi: 10.1158/0008-5472.CAN-12-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gines J, Sabater E, Martorell C, Grau M, Monroy M, Casado M. Efficacy of taxanes as adjuvant treatment of breast cancer: a review and meta-analysis of randomised clinical trials. Clin Transl Oncol. 2011;13:485–498. doi: 10.1007/s12094-011-0686-x. [DOI] [PubMed] [Google Scholar]
- Gottardis MM, Jordan VC. Development of tamoxifen-stimulated growth of MCF-7 tumors in athymic mice after long-term antiestrogen administration. Cancer Res. 1988;48:5183–5187. [PubMed] [Google Scholar]
- Hu P, Han Z, Couvillon A, Kaufman R, Exton J. Autocrine tumor necrosis factor a links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1α-mediated NF-κB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DE, Dai A, Tiffee JC, Li HH, Mundy GR, Boyce BF. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat Med. 1996;2:1132–1136. doi: 10.1038/nm1096-1132. [DOI] [PubMed] [Google Scholar]
- Jiang SY, Wolf DM, Yingling JM, Chang C, Jordan VC. An estrogen receptor positive MCF-7 clone that is resistant to antiestrogens and estradiol. Mol Cell Endocrinol. 1992;90:77–86. doi: 10.1016/0303-7207(92)90104-e. [DOI] [PubMed] [Google Scholar]
- Jordan M, Toso R, Thrower D, Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc Natl Acad Sci USA. 1993;90:9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan VC. A century of deciphering the control mechanisms of sex steroid action in breast and prostate cancer: the origins of targeted therapy and chemoprevention. Cancer Res. 2009;69:1243–1254. doi: 10.1158/0008-5472.CAN-09-0029. [DOI] [PubMed] [Google Scholar]
- Kameda T, Mano H, Yuasa T, Mori Y, Miyazawa K, Shiokawa M, Nakamaru Y, Hiroi E, Hiura K, Kameda A, Yang NN, Hakeda Y, Kumegawa M. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J Exp Med. 1997;186:489–495. doi: 10.1084/jem.186.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000;256:42–49. doi: 10.1006/excr.2000.4838. [DOI] [PubMed] [Google Scholar]
- Kelly C, Green M, Broglio K, Thomas E, Brewster A. Phase III trial evaluating weekly paclitaxel versus docetaxel in combination with capecitabine in operable breast cancer. J Clin Oncol. 2012;30:930–935. doi: 10.1200/JCO.2011.36.2079. [DOI] [PubMed] [Google Scholar]
- Kutul O, Lethai A. Displacement of Bim by BmF and Puma rather than increase in Bim level mediates paclitaxel induced apoptosis in breast cancer cells. Cell Death Differ. 2010;17 (10:1624–1635. doi: 10.1038/cdd.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JS, Meeke K, Osipo C, Ross EA, Kidawi N, Li T, Jordan VC. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Natl Cancer Inst. 2005;97:1746–1759. doi: 10.1093/jnci/dji400. [DOI] [PubMed] [Google Scholar]
- Lewis JS, Osipo C, Meeke K, Jordan VC. Estrogen-induced apoptosis in a breast cancer model resistant to long-term estrogen withdrawal. J Steroid Biochem Mol Biol. 2005;94:131–141. doi: 10.1016/j.jsbmb.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Liu H, Lee E-S, Gajdos C, Pearce ST, Chen B, Osipo C, Loweth J, McKian K, De Los Reyes A, Wing L, Jordan VC. Apoptotic action of 17β-estradiol in raloxifene-resistant MCF-7 cells in vitro and in vivo. J Natl Cancer Inst. 2003;95:1586–1597. doi: 10.1093/jnci/djg080. [DOI] [PubMed] [Google Scholar]
- Lønning P, Taylor P, Anker G, Iddon J, Wie L. High-dose estrogen treatment in postmenopausal breast cancer patients heavily exposed to endocrine therapy. Breast Cancer Res Treat. 2001;67:111–116. doi: 10.1023/a:1010619225209. [DOI] [PubMed] [Google Scholar]
- Makin G, Dive C. Apoptosis and cancer chemotherapy. Trends Cell Biol. 2001;11:22–26. doi: 10.1016/s0962-8924(01)02124-9. [DOI] [PubMed] [Google Scholar]
- Maximov P, Sengupta S, Lewis-Wambi JS, Kim HR, Curpan RF, Jordan VC. The conformation of the estrogen receptor directs estrogen-induced apoptosis in breast cancer: a hypothesis. Horm Mol Biol Clin Invest. 2011;5:27–34. doi: 10.1515/HMBCI.2010.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- Obiorah I, Jordan VC. 2012 NAMS/PFIZER- Wulf H. utian endowed lecture. The scientific rationale for a delay after menopause in the use of conjugated equine estrogens in postmenopausal women that causes a reduction in breast cancer incidence and mortality. Menopause. 2013;20:372–382. doi: 10.1097/GME.0b013e31828865a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CK, Coronado EB, Robinson J. Human breast cancer in the athymic nude mouse: cytostatic effects of long-term antiestrogen therapy. Eur J Cancer Clin Oncol. 1987;23:1189–1196. doi: 10.1016/0277-5379(87)90154-4. [DOI] [PubMed] [Google Scholar]
- Osipo C, Gajdos C, Liu H, Chen B, Jordan VC. Paradoxical action of fulvestrant in estradiol-induced regression of tamoxifen-stimulated breast cancer. J Natl Cancer Inst. 2003;95:1597–1608. doi: 10.1093/jnci/djg079. [DOI] [PubMed] [Google Scholar]
- Robert N, Saleh M, Paul D, Generali D, Gressot L, Copur M, Brufsky A, Minton S, Giguere J, Smith J, Richards P, Gernhardt D, Huang X, Liau K, Kern K, Davis J. Sunitinib plus paclitaxel versus bevacizumab plus paclitaxel for first-line treatment of patients with advanced breast cancer: a phase III, randomized, open-label trial. Clin Breast Cancer. 2011;11:82–92. doi: 10.1016/j.clbc.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Obiorah I, Maximov PY, Curpan R, Jordan VC. Molecular mechanism of action of bisphenol and bisphenol A mediated by oestrogen receptor alpha in growth and apoptosis of breast cancer cells. Br J Pharmacol. 2013;169:167–178. doi: 10.1111/bph.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Sharma C, Jordan VC. Estrogen regulation of X-box binding protein-1 and its role in estrogen induced growth of breast and endometrial cancer cells. Horm Mol Biol Clin Invest. 2010;2:235–243. doi: 10.1515/HMBCI.2010.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Mor G, Naftolin F, McPherson R, Song J, Zhang Z, Yue W, Wang J, Santen R. Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17β-estradiol. J Natl Cancer Inst. 2001;93:1714–1723. doi: 10.1093/jnci/93.22.1714. [DOI] [PubMed] [Google Scholar]
- Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Ward S, Simpson E, Davis S, Hind D, Rees A, Wilkinson A. Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation. Health Technol Assess. 2007;11:1–144. doi: 10.3310/hta11400. [DOI] [PubMed] [Google Scholar]
- WHI Design of the Women's Health Initiative Clinical Trial and Observational Study. Controlled Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- Yao K, Lee E, Bentrem D, England G, Schafer J, O'Regan R, Jordan VC. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6:2028–2036. [PubMed] [Google Scholar]
- Yvon AC, Wadsworth P, Jordan MA, Toso RJ, Thrower D, Wilson L. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol Biol Cell. 1999;10:947–959. doi: 10.1091/mbc.10.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Kaufmann R. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.