Abstract
Background:
Interleukin-2 (IL-2) treatment for patients with metastatic melanoma has shown remarkable durable responses. Systemic administration of IL-2 may cause severe side effects, whereas local administration is considered to be a safe alternative. The lungs are common sites of metastases in melanoma patients causing considerable respiratory problems. We sought to evaluate the potential antitumoral effect of a low-dose inhalative IL-2 (lh-IL-2) regimen for patients with melanoma lung metastases. In addition, we explored the prophylactic potential of Ih-IL-2 after surgical removal of lung metastases in a study carried out in an outpatient setting.
Methods:
Twenty patients with American Joint Committee on Cancer stage-IV (M1b and M1c) melanoma were enrolled in this study and treated with 3 × 3 million IU inhalative IL-2 q.d. together with monthly dacarbazine bolus injections. Five patients received lh-IL-2 after surgical resection of lung metastases to prevent recurrence of the disease (prophylaxis group, N=5). All other patients were enrolled in the treatment group (N=15). Clinical evaluations were carried out monthly and radiological follow-up was performed every third month.
Results:
Nine patients in the treatment group had a clinical benefit with partial regression (27%) or stable disease (33%). Four patients had progression of lung metastases (26.7%) and two patients were not evaluable (13.3%). In the prophylaxis group, none of the patients developed new lung metastases during lh-IL-2 therapy. The median follow-up period was 7.8 months in the treatment group and 25.7 months in the prophylaxis group. In the majority of patients, treatment was well tolerated.
Conclusions:
Low-dose IL-2 inhalation might offer an effective and safe treatment option for lung metastases in melanoma patients. In addition, lh-IL-2 may have a prophylactic potential to prevent recurrence in the lungs after pulmonary melanoma metastasectomy. Administration can easily be performed in an outpatient setting, thus offering an attractive treatment option.
Keywords: melanoma, inhalation, pulmonary, interleukin-2, prophylaxis, treatment
Melanoma is the deadliest form of skin cancer with increasing prevalence and incidence over the last decades (http://seer.cancer.gov/statfacts/html/melan.html). The identification of specific mutations in several oncogenes (BRAF, NRAS, KIT and GNAQ) that drive melanoma development and progression has led to remarkable improvements in the treatment of melanoma. Specific inhibitors have shown tremendous preclinical results, some of which could be translated into successful clinical trials. However, despite the fact that the overall response rate of targeted inhibition was reported in up to 50–81%, the median progression free survival (PFS) is only 6–8 months and durable remissions are rare (6%) (Flaherty et al, 2010; Sosman et al, 2012; Posch et al, 2013). A smaller subset of patients respond to immunotherapy, such as systemic interleukin-2 (IL-2) therapy, interferon (IFN)-α or immune-modulating antibodies such as anti-CTLA-4 (ipilimumab) and anti-PD1(L). In contrast to targeted therapy, ∼70% of complete responders to IL-2 treatment display long-term regression and, in many cases, can be considered cured, emphasising the role of IL-2 and immunotherapy in melanoma treatment (Smith et al, 2008; Hodi et al, 2010; Coventry and Ashdown, 2012; Dillman et al, 2012; Simeone and Ascierto, 2012; Lipson et al, 2013).
A common problem in melanoma is lung metastasis, which can be present in up to 89% of patients with American Joint Committee on Cancer (AJCC) stage-IV disease, often resulting in severe respiratory problems (Neuman et al, 2007). Inhalation therapy with high-dose IL-2 (32.5–36 million IU q.d.) has shown activity for the treatment of lung metastases in patients with melanoma and renal cell carcinoma (Enk et al, 2000; Huland et al, 2003).
In this study, we report data from 20 stage-IV (M1b and M1c) melanoma patients, who received daily low-dose IL-2 inhalations and monthly bolus injections with dacarbazine.
We initiated this study to evaluate the activity of a low-dose inhalative IL-2 (lh-IL-2, 3 × 3 million IU q.d) regimen for the treatment of lung metastases in a population of advanced melanoma patients. A subset of patients (N=5) had metastasectomy before the study treatment regimen and was followed-up to investigate whether treatment would prevent recurrence of lung metastasis.
Materials and methods
Patients
Twenty patients (10 males, 10 females) with histologically confirmed AJCC stage-IV (M1b and M1c) metastatic melanoma were enrolled in this open cohort study at The Rudolfstiftung Hospital between 2003 and 2011. Patients under the age of 18 years, pregnant women and patients with previously diagnosed infectious or inflammatory lung disease were excluded. Informed consent to participate was obtained from all eligible patients. All patients had progressive disease (PD) on study entry. Most patients also had metastatic lesions in other organs and had previously received other systemic treatment such as dacarbazine (DTIC), fotemustine, paclitaxel/carboplatin, high-dose (hd) or low-dose (ld) IFN-α. A detailed characterisation of all patients is given in Table 1. Initial staging included computed tomography (CT) scans of the torso, sonographic examinations of axillary and inguinal lymph nodes, magnetic resonance imaging scans of the brain and blood examinations. The low-dose IL-2 regimen was the same for all the patients consisting of daily inhalations of 3 × 3 million IU recombinant IL-2 (Proleukin, Chiron International, Ratingen, Germany). Treatment was initiated at the Department of Dermatology, The Rudolfstiftung Hospital and patients were closely monitored for adverse effects for 4 days. Established lh-IL-2 was then continued in an outpatient setting. IL-2 solution was prepared as previously described (Enk et al, 2000). Briefly, IL-2 was dissolved in a solution containing 5% (vol/vol) glucose and 2% (vol/vol) human albumin and administered with the use of the Jetair Gamma 20C Inhalator. Patients with metastatic sites other than the lung at the beginning of the study, or patients, who progressed during the follow-up, also received monthly intravenous bolus injections of 850 mg m−2 dacarbazine (DTIC). Physical examinations and blood testing (whole-blood counts, liver and renal function tests, and electrolytes) were performed every month; radiological follow-up with CT scans, X-rays or magnetic resonance imaging scans were carried out every 3 months. Responses were determined by the criteria of the World Health Organisation and applied only to the lung: a complete remission (CR) indicating the disappearance of all metastases for more than 3 months, a partial remission (PR) showing the decrease of indicator lesions of more than 25%, a stable disease (SD) indicating changes of less than 25%, and PD showing a growth of indicator lesions of more than 25%. Radiological images were analysed by an independent radiologist. Patients with CR, PR or SD continued treatment. Criteria considered for discontinuation of treatment were disease progression in the lung or the occurrence of unmanageable side effects. The study was approved by the ethical review committee of the city of Vienna.
Table 1. Characteristics of patients.
| Characteristics | Treatment group (N=15) | Prophylaxis groupa (N=5) |
|---|---|---|
|
Sex, no. (%) | ||
| Male | 8 (54) | 2 (40) |
| Female |
7 (46) |
3 (60) |
| Mean age at diagnosis of MM (years) |
59.0 |
60.9 |
|
Site of primary melanoma, no. (%) | ||
| Trunk | 8 (53) | 4 (80) |
| Head and neck | 2 (13) | 1 (20) |
| Mucosal | 1 (7) | — |
| Uveal | 1 (7) | — |
| Other |
3 (20) |
— |
| Ulceration, no. (%) |
4 (27) |
1 (20) |
|
Breslow (mm), no. (%) | ||
| <1 mm | 1 (7) | — |
| 1–2 mm | 1 (7) | 1 (20) |
| 2–4 mm | 4 (27) | 2 (40) |
| >4 mm | 6 (40) | 2 (40) |
| NA |
3 (20) |
— |
|
Site of disease in addition to the lung, no. (%) | ||
| Cutaneous or subutaneous | 7 (47) | 1 (20) |
| Bone | 2 (13) | 2 (40) |
| Liver | 3 (20) | 1 (20) |
| Brain | 4 (27) | 2 (40) |
| Otherb |
3 (20) |
1 (20) |
|
Disease stage, no. (%)c | ||
| M1b | 3 (20) | 1 (20) |
| M1c |
12 (80) |
4 (80) |
|
Previous treatment, no. (%) | ||
| Dacarbazine | 14 (93) | 5 (100) |
| Low-dose interferon-α | 7 (47) | 5 (100) |
| High-dose interferon-α | 3 (20) | — |
| Fotemustine | 8 (53) | 3 (60) |
| Carboplatin/paclitaxel | 2 (13) | 1 (20) |
Abbreviations: MM=metastatic melanoma; NA=not applicable.
Patients with surgical removal of pulmonary metastases prior to study treatment regimen.
Other sites of metastasis were the urogenital tract, gastrointestinal tract and breast.
The stage was determined according to the criteria of the AJCC based on the sites of disease.
Sequencing
Five 10-μm sections of formalin-fixed paraffin-embedded tissue were hand microdissected under a dissecting microscope. A corresponding haematoxylin and eosin-stained reference slide was used for precise location of tumour cells. DNA was extracted using the QIAamp DNA FFPE Tissue Kit (Qiagen, Germantown, MD, USA; 56404). Touchdown PCR was performed sequentially, using M13 primers for BRAF (exon 15), NRAS (exons 2 and 3), KIT (exons 9, 11, 13, 17, 18), GNAQ (exon 5) and GNA11 (exon 5). Sanger sequencing was carried out after clean-up of PCR products with exonuclease I and Shrimp alkaline phosphatase. Sequences were then analysed with the Mutation Surveyor Version 4.0.9 (Softgenetics, State College, PA, USA).
Results
Low-dose IL-2 inhalation reduces the size of pulmonary melanoma metastasis in patients with unresectable disease
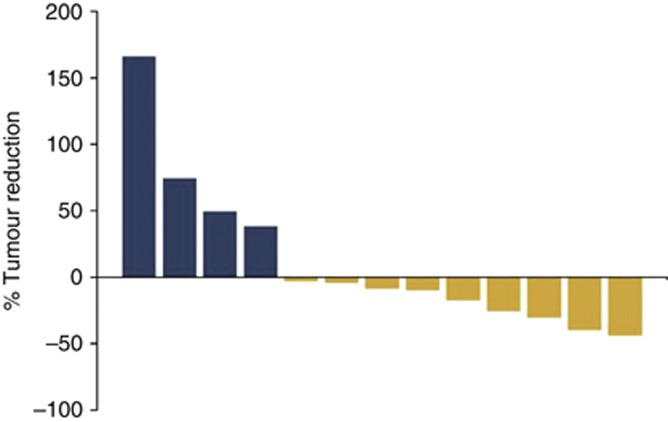
Thirteen of fifteen patients (6 males, 7 females) were available for radiological analysis in the treatment group of the study. Two patients (13.3%) had to discontinue treatment before the first staging: one owing to respiratory problems and the other owing to rapid progression of brain metastases. The median time to the onset of pulmonary metastases after the initial diagnosis of melanoma was 23.4 months. The median follow-up period after initiation of the study treatment regimen was 7.8 months. CT scans of the lung revealed that nine patients (60%) had a clinical benefit from the therapy showing either PR (27%) or SD (33%). None of the patients had a CR (Figure 1, Table 2). Four patients showed PD (26.7%) and discontinued treatment after 3 months. All patients who had a clinical benefit from the therapy regimen (PR+SD) had previously received DTIC, but progressed. Two out of four patients with PD (50%) succumbed to disease-related respiratory problems. In contrast, 2 out of 9 patients with clinical benefit (22%) developed new pulmonary metastases, but displayed only minor tumour-related respiratory problems, and deceased owing to extrapulmonary disease.
Figure 1.
Waterfall plot displaying the response of patients in the treatment group to the study treatment regimen.Out of 15 patients, 13 were available for analysis of pulmonary metastases. Two patients discontinued treatment before the first evaluation. (yellow=SD and PR; blue=PD; x axis: every bar represents one patient, y axis: percentage of size reduction of pulmonary metastases, N=13). The full colour version of this figure is available at British Journal of Cancer online.
Table 2. Response in treatment group.
| Response treatment group | No. (%) (N=15) |
|---|---|
| Complete |
— |
| Partial |
4 (27) |
| Stable disease |
5 (33) |
| Partial and stable |
9 (60) |
| Progressive disease |
4 (27) |
| NAa | 2 (13) |
Abbreviation: NA=not applicable.
Two patients discontinued treatment before the first evaluation: one owing to dyspnoea and cough and one owing to rapid progression of brain metastases.
Analyses for overall survival (OS) and PFS failed to be statistically significant owing to the small number of patients in this study. However, we noticed that patients with a clinical benefit from Ih-IL-2 showed a trend to having a longer OS compared with patients with PD (median OS in months: PR=42.1 (21.9–53.5), SD=42.3 (19.1–86.2), PD=33.7 (19.2–45.0)). There was also a tendency to a longer PFS (analysis applied to the lungs only) in patients with PR compared with patients with SD or PD (median in months: PR=29.5 (11.9–42.8), SD=13.0 (4–22.9), PD=12.7 (10.4–15.9)). We noticed that patients with a clinical benefit from the study treatment regimen tended to be older than non-responders (median age at diagnosis of melanoma in years: patients with clinical benefit=59.8 (47–77.4), non-responders=52.5 (40.1–68.3)). Sentinel lymph node involvement or histology of the primary tumour was not statistically associated with the response to lh-IL-2 treatment. Nevertheless, we noticed that all four patients with nodular primary melanoma were in the group that had a benefit from lh-IL-2 (Table 3). Also, all patients with tumours of an unknown primary site displayed a clinical benefit from Ih-IL-2 therapy, one displaying PR and one SD. One patient with uveal melanoma carrying a GNAQ(Q209L) mutation had PR. We noticed that more patients who experienced a clinical benefit from Ih-IL-2 had prior (adjuvant) immunotherapy (6 out of 9, 67%) compared with patients that did not respond to the study treatment regimen (2 out of 4, 50%). Sequencing of patient samples revealed that 5 out of 13 (38.5%) patients carried a BRAF(V600) mutation. One tumour carried a NRAS(Q61R) mutation and one tumour the novel mutation in KIT(P838S). For one patient, tumour tissue was not available and one sample failed to amplify. Sequencing results are listed in Table 4. Owing to the small sample size, the mutation status was not statistically correlated with a response to the study treatment regimen.
Table 3. Comparison of clinical characteristics between patients displaying a clinical benefit from Ih-IL-2 and non-responders in the treatment group (N=13, descriptive analysis; results not statistically significant).
| Characteristics | Clinical benefit (N=9) | Non-responder (N=4) |
|---|---|---|
| Age—average years (range) |
59.8 (47–77.4) |
52.5 (40.1–68.3) |
| Prior Immunotherapy—no. (%) |
6 (67%) |
2 (50%) |
| Nodular primary melanoma—no. (%) |
4 (44%) |
0 (0%) |
| BRAF(V600) mutations—no. (%) | 3 (33%) | 2 (50%) |
Table 4. Sequencing analysis of melanomas.
| Response to treatment | Mutation status |
|---|---|
|
Treatment group (N=15) | |
| PD | Failed to amplify |
| PR | BRAF(V600E) |
| PR | BRAF(V600E) |
| PR | WT |
| SD | KIT(P838S)a |
| SD | No tissue available |
| NAb | — |
| SD | BRAF(V600E) |
| PD | WT |
| SD | WT |
| SD | NRAS(Q61R) |
| PD | BRAF(V600E) |
| PR | GNAQ(Q209L) |
| NAb | — |
| PD |
BRAF(V600K) |
|
Prophylaxis group (N=5) | |
| SD | WT |
| NAb | — |
| SD | WT |
| SD | BRAF(V600E) |
| SD | NRAS(Q61R) |
Abbreviations: PD=progressive disease; PR=partial response; SD=stable disease; WT=wild type.
Wild type for indicated exons in BRAF, NRAS and KIT.
Not previously described mutation identified in proximity to hotspots D816, N822 and F848.
Patients had to discontinue treatment prior to first evaluation.
Low-dose IL-2 inhalation prevents post-resectional recurrence of melanoma lung metastases
Four out of five patients were available for analysis in the prophylaxis group of the study. One patient had to discontinue treatment before the first staging owing to respiratory problems. None of the patients showed recurrence of lung metastases during the treatment period (median 24.5 months). One patient, who underwent surgical removal of the right upper lobe of the lung, developed one solitary metastasis before lh-IL-2 therapy was initiated. This metastasis was stable under lh-IL-2 treatment for 46 months. All patients in the prophylaxis group developed extrapulmonary disease progression and started systemic chemotherapy. The median OS was 77.3 months in the prophylaxis group. Two patients in the prophylaxis group were wild type for BRAF, NRAS and KIT mutations, one patient carried a BRAF(V600E) and one patient a NRAS(Q61R) mutation (Table 4).
Discussion
IL-2 was approved for the systemic treatment of advanced renal cancer in 1992 and melanoma in 1998. Since then, complete durable responses with long-term survival for systemic IL-2 therapy have been reported consistently (Atkins et al, 1999). Research has determined a bimodal role of IL-2 in the immune homoeostasis, replacing the initial assumption of IL-2 just being a stimulator of T effector cells (Coventry and Ashdown, 2012). IL-2 is capable of interacting with the immune system in two different ways: induction/activation by stimulation of T effector cells or tolerance/inhibition by interaction with T regulatory cells (Ahmadzadeh and Rosenberg, 2006; Höfer et al, 2012). IL-2 not only interacts with the immune system. It has been reported that in some situations, cancer cells express IL-2 receptors or secrete IL-2, adding another level of complexity on how antitumoral responses might be explained (Plaisance et al, 1993; Rangel-Corona et al, 2010). Despite the low response rates to exogenous IL-2, the antitumoral effects of this cytokine are well documented. Our current understanding on how IL-2 induces antitumoral activity is based on its ability to (re-) activate the suppressed tumour-specific immune responses by upregulation of proinflammatory cytokines, expansion of CD4+ cells and mainly by enhancement of natural killer cell activity and induction of a Th1 immune response (Henney et al, 1981). Interestingly, other more common epithelial malignancies fail to respond to IL-2 treatment, thus it seems that melanoma generates endogenous antitumour T-cell populations that can be activated by exogenous stimuli.
Constraints of systemic IL-2 treatment are due to acute and sometimes severe toxicities that are capable of affecting every organ system (Poust et al, 2013). In addition, it has not been possible to determine reliable markers that would predict a clinical response to IL-2 treatment.
A majority of patients with AJCC stage-IV metastatic melanoma suffer from pulmonary metastases, which can cause considerable physical and psychological distress. Only a small subset of patients with lung metastases can be selected for pulmonary metastasectomy, which has been shown to improve survival (Neuman et al, 2007; Petersen et al, 2007).
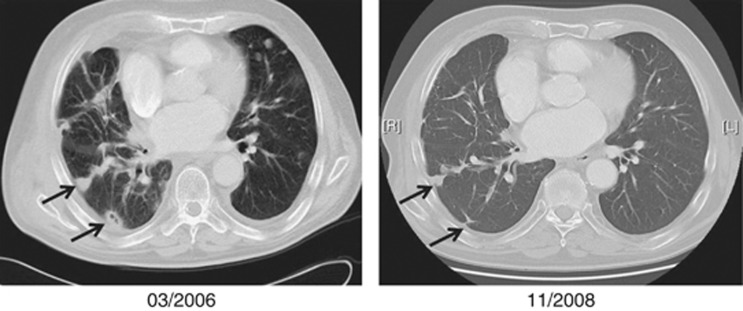
In this study, we show that inhalation therapy with IL-2 might offer an effective treatment option for patients with unresectable melanoma lung metastases. Figure 2 shows the CT scans of a patient who had a durable PR of lung metastasis. Despite the remarkable number of patients benefiting from the study regimen in the treatment group of this study, it is to mention that we did not observe patients with CR, whereas previous studies using a high-dose regimen of inhalative IL-2 (36 million IU q.d.) noticed 18.5% complete responders (Enk et al, 2000). This difference could be due to the lower amounts of IL-2 inhaled in our study, but might also be explained by differences in the patient characteristics: only two patients (10%) in our study had metastases restricted to the lungs in contrast to 51% of patients in the high-dose study. There is some evidence that patients with melanoma metastases restricted to the lungs have a better prognosis and are more likely to respond to therapy (Neuman et al, 2007). This is suggestive of differences in the biological behaviour of tumours in this subset of melanoma patients and might in part explain the differences in outcome in the two studies.
Figure 2.
Computed tomography scans of a patient displaying durable, partial regression of pulmonary metastases after 31 months of treatment with low-dose inhalation of IL-2.
We were further able to confirm results from previous studies that IL-2 inhalation is safe and can easily be performed in an outpatient setting (Enk et al, 2000; Huland et al, 2003). Side effects such as dyspnoea and cough are rare, usually occurring in the first days of treatment and resolve completely after withdrawal of IL-2 (Loppow et al, 2007).
To the best of our knowledge, we report the first results of the adjuvant used for IL-2 inhalation to prevent post-resectional recurrence of melanoma lung metastases. It is remarkable that we did not observe any recurrence of pulmonary metastases in these patients during the treatment period, which has been reported to occur in up to 39% of patients with metastasectomy only (Neuman et al, 2007). Although our patient cohort is small, we saw improved OS in patients with lh-IL-2 treatment compared with historical data with surgery alone (77.3 months compared with 40 months). Certainly, analysis needs to be evaluated with caution as other factors might have rendered these results: most patients in our study had prior (adjuvant) immunotherapies and received DTIC after developing extrapulmonary disease progression. There have been reports of possible delayed effects of immunotherapies, and indeed we noticed that more patients who had a clinical benefit from the study treatment regimen received prior immunotherapies (6 out of 9, 67%) compared with patients who had PD (2 out of 4, 50%) (Table 3). However, all patients had PD at the start of the study and the clinical benefit was timely correlated to lh-IL-2 therapy.
We did not observe a correlation between a response to lh-IL-2 and the response to other immunotherapies in our cohort: two patients, who initially responded to the study treatment regimen, one with SD and the other with PR, failed to respond to anti-CTLA-4 therapy in the later course of their disease.
Although, several new immunotherapies such as anti-PD1(L) therapy as well as its combination with other immune-modulating and -targeted drugs will potentially enrich the clinician's armamentarium soon, we conclude that lh-IL-2 therapy offers a safe, easy to perform and effective treatment option for patients with pulmonary melanoma metastases (Ascierto et al, 2013; Robert et al, 2013). We further hypothesise that lh-IL-2 could help prevent the recurrence of melanoma metastases in the lungs after pulmonary metastasectomy.
Given the high prevalence of pulmonary metastases in advanced stage melanoma patients, the favourable side effect profile and the presumed prophylactic activity of lh-IL-2, one could ask whether lh-IL-2 therapy may generally be considered to prevent pulmonary disease spread in melanoma patients.
To further improve treatment and characterise patients that are likely to respond, it will be important to meliorate our understanding of the dynamics and coordinated interactions of cytokines and their receptors. Although we were unable to find a correlation between the mutation status of the tumours and the response to the study treatment regimen, promising results from a recent study point in that direction. Response rates and OS might also be improved by a deeper knowledge of the genetic background of the primary tumours and metastases, as NRAS mutant melanoma seems to be predictive for greater response rates to IL-2 therapy (Joseph et al, 2012).
Acknowledgments
We are grateful to Adil Daud, Jeffrey Ma, Gary Green and Mitchell Zekhtser for their helpful advice and discussion in the elaboration of this manuscript. We would like to acknowledge Jingly Weier, Sonia Mirza and Swapna Vemula from UCSF Dermatopathology Service for their expertise and help in DNA sequencing. We also thank Marianne Überlacher for her help with preparation of the graphic design. This work has been supported by the Max Kade Foundation (New York), by the Verein für Dermatologie und Venerologie Krankenanstalt Rudolfstiftung (Vienna) and a grant from Family Honzak (Vienna).
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascierto PA, Kalos M, Schaer DA, Callahan MK, Wolchok JD. Biomarkers for immunostimulatory monoclonal antibodies in combination strategies for melanoma and other tumor types. Clin Cancer Res. 2013;19:1009–1020. doi: 10.1158/1078-0432.CCR-12-2982. [DOI] [PubMed] [Google Scholar]
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- Coventry BJ, Ashdown ML. The 20th anniversary of interleukin-2 therapy: bimodal role explaining longstanding random induction of complete clinical responses. Cancer Manag Res. 2012;4:215–221. doi: 10.2147/CMAR.S33979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillman RO, Barth NM, VanderMolen LA, Mahdavi K, McClure SE. Should high-dose interleukin-2 still be the preferred treatment for patients with metastatic melanoma. Cancer Biother Radiopharm. 2012;27:337–343. doi: 10.1089/cbr.2012.1220. [DOI] [PubMed] [Google Scholar]
- Enk AH, Nashan D, Rübben A, Knop J. High dose inhalation interleukin-2 therapy for lung metastases in patients with malignant melanoma. Cancer. 2000;88:2042–2046. doi: 10.1002/(sici)1097-0142(20000501)88:9<2042::aid-cncr9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O'Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henney CS, Kuribayashi K, Kern DE, Gillis S. Interleukin-2 augments natural killer cell activity. Nature. 1981;291:335–338. doi: 10.1038/291335a0. [DOI] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJM, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfer T, Krichevsky O, Altan-Bonnet G. Competition for IL-2 between regulatory and effector T Cells to chisel immune responses. Front Immunol. 2012;3:268. doi: 10.3389/fimmu.2012.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huland E, Burger A, Fleischer J, Fornara P, Hatzmann E, Heidenreich A, Heinzer H, Heynemann H, Hoffmann L, Hofmann R, Huland H, Kämpfer I, Kindler M, Kirchner H, Mehlhorn G, Moniak TH, Rebmann U, Roigas J, Schneider TH, Schnorr D, Schmitz HJ, Wenisch R, Varga Z, Vinke J. Efficacy and safety of inhaled recombinant interleukin-2 in high-risk renal cell cancer patients compared with systemic interleukin-2: an outcome study. Folia Biol (Praha) 2003;49:183–190. [PubMed] [Google Scholar]
- Joseph RW, Sullivan RJ, Harrell R, Stemke-Hale K, Panka D, Manoukian G, Percy A, Bassett RL, Ng CS, Radvanyi L, Hwu P, Atkins MB, Davies MA. Correlation of NRAS mutations with clinical response to high-dose IL-2 in patients with advanced melanoma. J Immunother. 2012;35:66–72. doi: 10.1097/CJI.0b013e3182372636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, Xu H, Yao S, Pons A, Chen L, Pardoll DM, Brahmer JR, Topalian SL. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19:462–468. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loppow D, Huland E, Heinzer H, Grönke L, Magnussen H, Holz O, Jörres RA. Interleukin-2 inhalation therapy temporarily induces asthma-like airway inflammation. Eur J Med Res. 2007;12:556–562. [PubMed] [Google Scholar]
- Neuman HB, Patel A, Hanlon C, Wolchok JD, Houghton AN, Coit DG. Stage-IV melanoma and pulmonary metastases: factors predictive of survival. Ann Surg Oncol. 2007;14:2847–2853. doi: 10.1245/s10434-007-9448-y. [DOI] [PubMed] [Google Scholar]
- Petersen RP, Hanish SI, Haney JC, Miller CC, 3rd, Burfeind WR, Jr, Tyler DS, Seigler HF, Wolfe W, D'Amico TA, Harpole DH., Jr Improved survival with pulmonary metastasectomy: an analysis of 1720 patients with pulmonary metastatic melanoma. J Thorac Cardiovasc Surg. 2007;133:104–110. doi: 10.1016/j.jtcvs.2006.08.065. [DOI] [PubMed] [Google Scholar]
- Plaisance S, Rubinstein E, Alileche A, Han DS, Sahraoui Y, Mingari MC, Bellomo R, Rimoldi D, Colombo MP, Jasmin C. Human melanoma cells express a functional interleukin-2 receptor. Int J Cancer. 1993;55:164–170. doi: 10.1002/ijc.2910550129. [DOI] [PubMed] [Google Scholar]
- Posch C, Moslehi H, Feeney L, Green GA, Ebaee A, Feichtenschlager V, Chong K, Peng L, Dimon MT, Phillips T, Daud AI, McCalmont TH, Leboit PE, Ortiz-Urda S. Combined targeting of MEK and PI3K/mTOR effector pathways is necessary to effectively inhibit NRAS mutant melanoma in vitro and in vivo. Proc Natl Acad Sci USA. 2013;110 (10:4015–4020. doi: 10.1073/pnas.1216013110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poust JC, Woolery JE, Green MR. Management of toxicities associated with high-dose interleukin-2 and biochemotherapy. Anticancer Drugs. 2013;24:1–13. doi: 10.1097/CAD.0b013e32835a5ca3. [DOI] [PubMed] [Google Scholar]
- Rangel-Corona R, Corona-Ortega T, Soto-Cruz I, López-Labra A, Pablo-Arcos T, Torres-Guarneros CF, Weiss-Steider B. Evidence that cervical cancer cells secrete IL-2, which becomes an autocrine growth factor. Cytokine. 2010;50:273–277. doi: 10.1016/j.cyto.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Robert C, Soria JC, Eggermont AMM. Drug of the year: programmed death-1 receptor/programmed death-1 ligand-1 receptor monoclonal antibodies. Eur J Cancer. 2013;49:2968–2971. doi: 10.1016/j.ejca.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Simeone E, Ascierto PA. Immunomodulating antibodies in the treatment of metastatic melanoma: the experience with anti-CTLA-4, anti-CD137, and anti-PD1. J Immunotoxicol. 2012;9:241–247. doi: 10.3109/1547691X.2012.678021. [DOI] [PubMed] [Google Scholar]
- Smith FO, Downey SG, Klapper JA, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Restifo NP, Levy CL, White DE, Steinberg SM, Rosenberg SA. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res. 2008;14:5610–5618. doi: 10.1158/1078-0432.CCR-08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ, Flaherty KT, Hersey P, Kefford R, Lawrence D, Puzanov I, Lewis KD, Amaravadi RK, Chmielowski B, Lawrence HJ, Shyr Y, Ye F, Li J, Nolop KB, Lee RJ, Joe AK, Ribas A. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]