Abstract
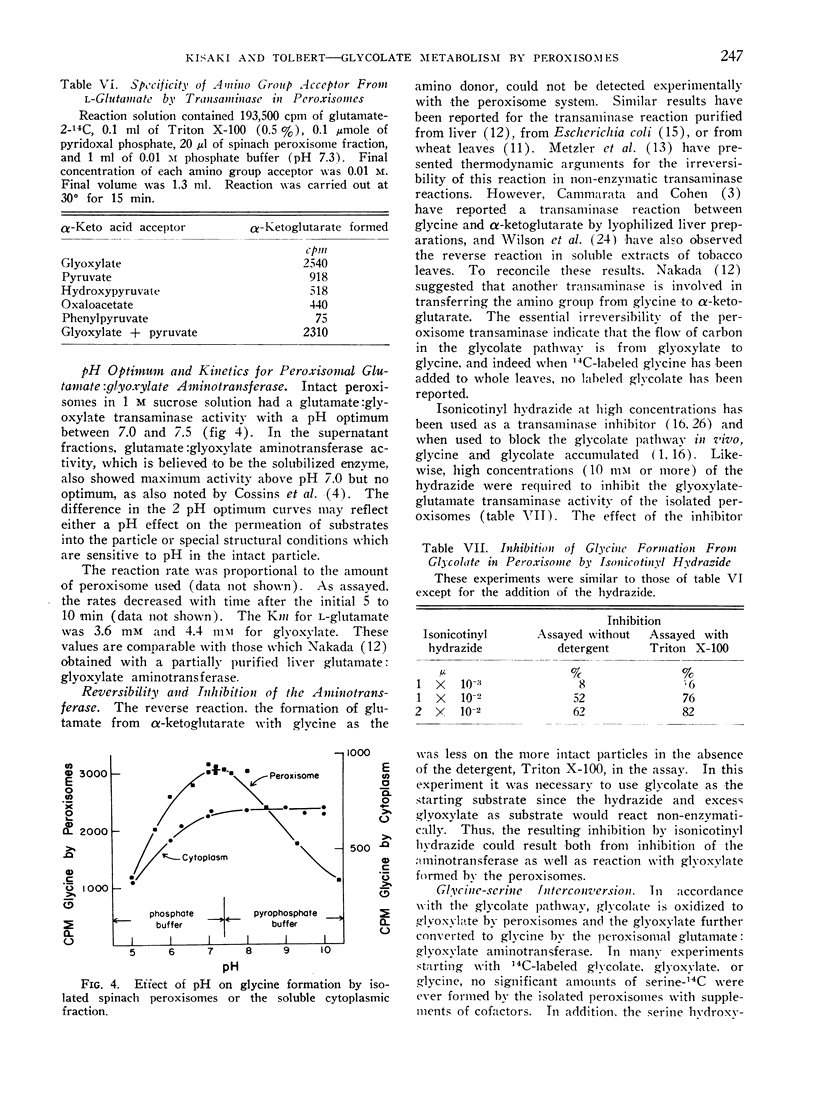
Chloroplasts, mitochondria, and peroxisomes from leaves were separated by isopycnic sucrose density gradient centrifugation. The peroxisomes converted glycolate-14C or glyoxylate-14C to glycine, and contained a glutamate: glyoxylate aminotransferase as indicated by an investigation of substrate specificity. The pH optimum for the aminotransferase was between 7.0 and 7.5, and the Km for l-glutamate was 3.6 mm and for glyoxylate, 4.4 mm. The reaction of glutamate plus glyoxylate was not reversible. The isolated peroxisomes did not convert glycine to glyoxylate nor glycine to serine.
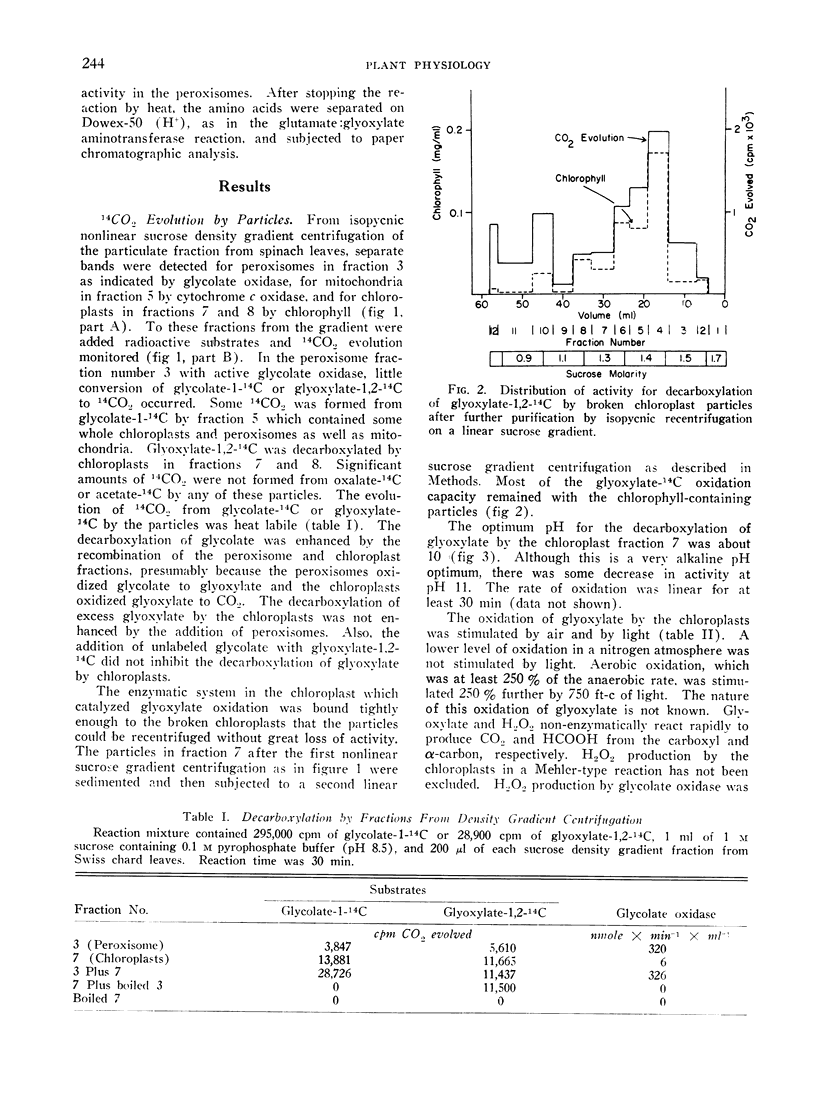
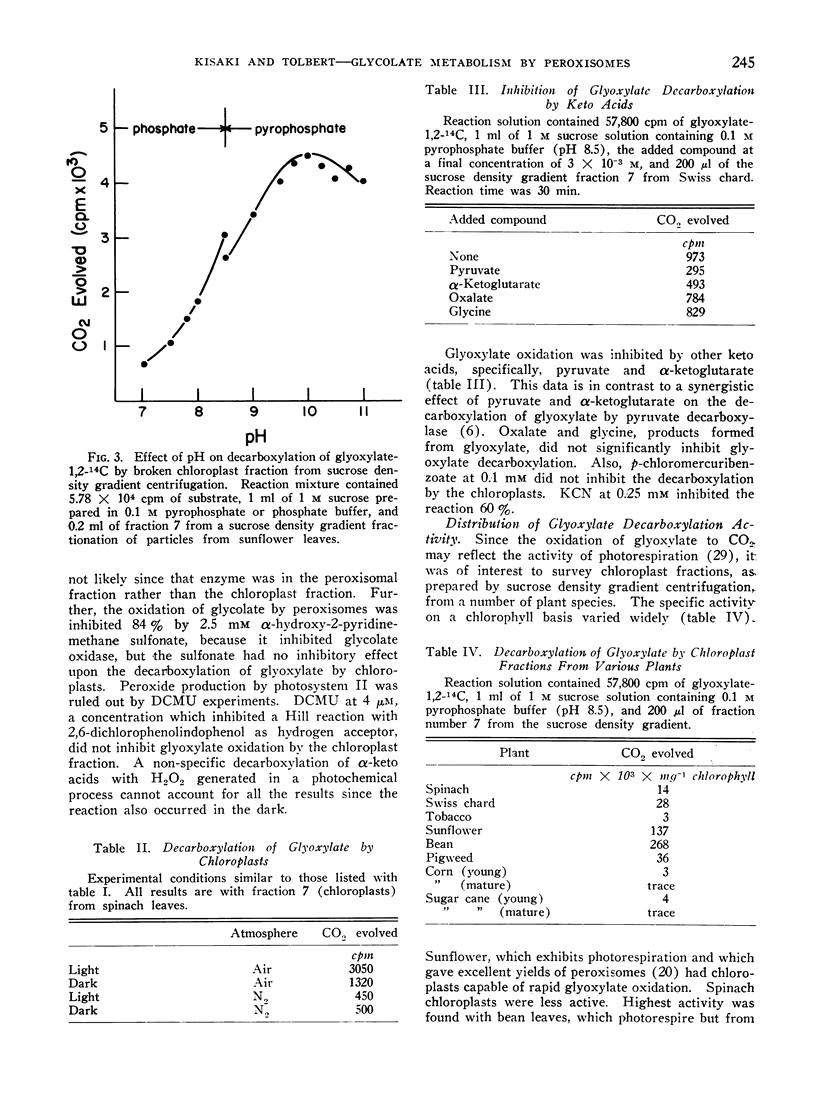
Peroxisomes did not oxidize glycolate or glyoxylate to CO2. Chloroplasts could very slowly oxidize glyoxylate, but not glycolate, to CO2. Chloroplast oxidation of glyoxylate was heat labile and widely distributed among plants. Oxidation was stimulated by light and oxygen. but was not inhibited by 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU).
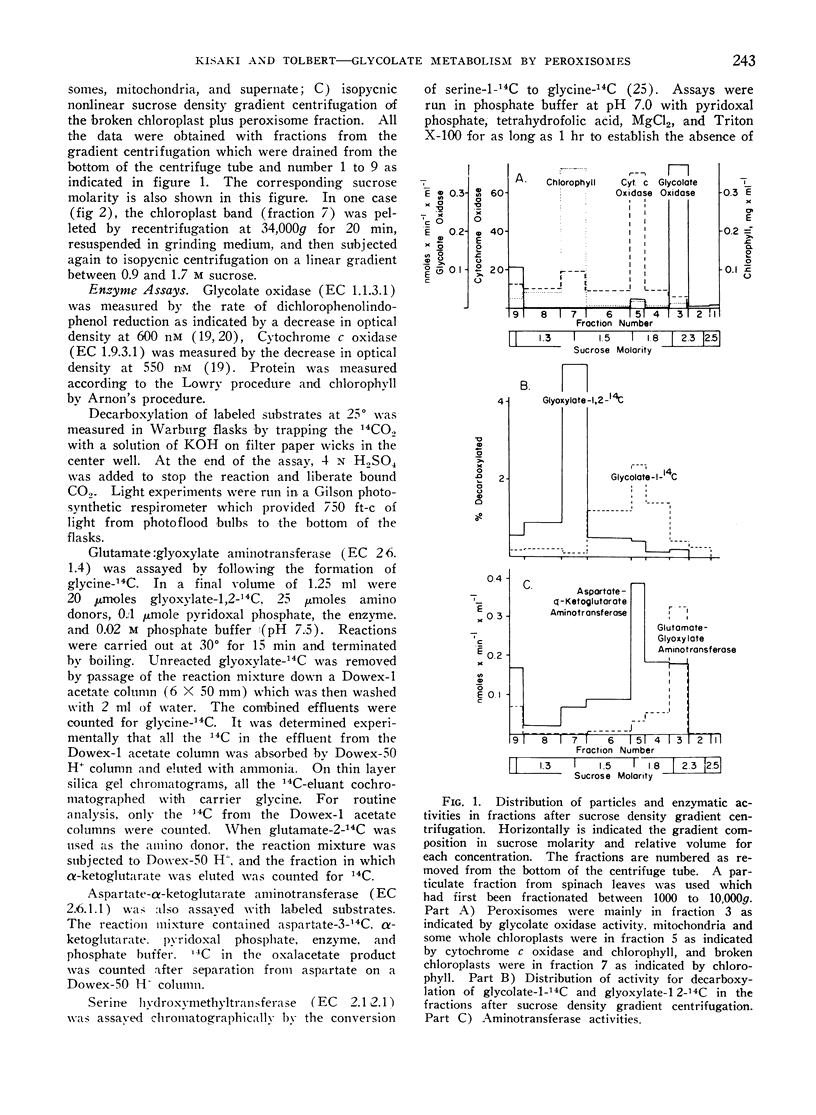
A scheme for the distribution of enzymes associated with glycolate metabolism and photorespiration is presented. Glycolate biosynthesis occurs in the chloroplasts. In the peroxisomes, glycolate is oxidized with O2 uptake to glyoxylate by glycolate oxidase, and the glyoxylate is converted to glycine by glutamate:glyoxylate aminotransferase. Further metabolism of glycine does not occur in the peroxisomes. It is possible that excess glyoxylate from the peroxisomes could return to the chloroplasts to be reduced to glycolate or oxidized to account for part of the CO2 loss during photorespiration.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAMMARATA P. S., COHEN P. P. The scope of the transamination reaction in animal tissues. J Biol Chem. 1950 Nov;187(1):439–452. [PubMed] [Google Scholar]
- COSSINS E. A., SINHA S. K. OCCURRENCE AND PROPERTIES OF L-AMINOACID :2-GLYOXYLATE AMINOTRANSFERASE IN PLANTS. Can J Biochem. 1965 Apr;43:495–506. doi: 10.1139/o65-058. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEARNEY P. C., TOLBERT N. E. Appearance of glycolate and related products of photosynthesis outside of chloroplasts. Arch Biochem Biophys. 1962 Jul;98:164–171. doi: 10.1016/0003-9861(62)90162-5. [DOI] [PubMed] [Google Scholar]
- King J., Waygood E. R. Glyoxylate aminotranferases from wheat leaves. Can J Biochem. 1968 Aug;46(8):771–779. doi: 10.1139/o68-118. [DOI] [PubMed] [Google Scholar]
- NAKADA H. I. GLUTAMIC-GLYCINE TRANSAMINASE FROM RAT LIVER. J Biol Chem. 1964 Feb;239:468–471. [PubMed] [Google Scholar]
- PITTS J. D., STEWART J. A., CROSBIE G. W. Observations on glycine metabolism in Escherichia coli. Biochim Biophys Acta. 1961 Jun 24;50:361–363. doi: 10.1016/0006-3002(61)90338-9. [DOI] [PubMed] [Google Scholar]
- PRITCHARD G. G., WHITTINGHAM C. P., GRIFFIN W. J. Effect of isonicotinyl hydrazide on the path of carbon in photosynthesis. Nature. 1961 May 6;190:553–554. doi: 10.1038/190553a0. [DOI] [PubMed] [Google Scholar]
- RICHARDSON K. E., TOLBERT N. E. Phosphoglycolic acid phosphatase. J Biol Chem. 1961 May;236:1285–1290. [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Yamazaki R. K., Hageman R. H., Kisaki T. A survey of plants for leaf peroxisomes. Plant Physiol. 1969 Jan;44(1):135–147. doi: 10.1104/pp.44.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON A. P., DAVIES D. D. Serine-glycine interconversion in plant tissues. Nature. 1958 Apr 12;181(4615):1070–1071. doi: 10.1038/1811070a0. [DOI] [PubMed] [Google Scholar]
- WILSON D. G., KING K. W., BURRIS R. H. Transamination reactions in plants. J Biol Chem. 1954 Jun;208(2):863–874. [PubMed] [Google Scholar]
- Wang D., Waygood E. R. Carbon metabolism of C-labeled amino acids in wheat leaves. I. A pathway of glyoxylate-serine metabolism. Plant Physiol. 1962 Nov;37(6):826–832. doi: 10.1104/pp.37.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUATT J. The action of isoniazid on the transaminases of Mycobacterium tuberculosis (B.C.G.). Biochem J. 1958 Feb;68(2):193–197. doi: 10.1042/bj0680193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. L., Tolbert N. E., Orth G. M. Isolation and Distribution of Phosphoglycolate Phosphatase. Plant Physiol. 1964 Jul;39(4):643–647. doi: 10.1104/pp.39.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELITCH I. The role of glycolic acid oxidase in the respiration of leaves. J Biol Chem. 1958 Dec;233(6):1299–1303. [PubMed] [Google Scholar]



