Abstract
Diabetes mellitus is the leading cause of end-stage renal disease (ESRD) in the U.S. and many countries globally. The role of improved glycemic control in ameliorating the exceedingly high mortality risk of diabetic dialysis patients is unclear. The treatment of diabetes in ESRD patients is challenging, given changes in glucose homeostasis, the unclear accuracy of glycemic control metrics, and the altered pharmacokinetics of glucose-lowering drugs by kidney dysfunction, the uremic milieu, and dialysis therapy. Up to one-third of diabetic dialysis patients may experience spontaneous resolution of hyperglycemia with hemoglobin A1c (HbA1c) levels <6%, a phenomenon known as “Burnt-Out Diabetes,” which remains with unclear biologic plausibility and undetermined clinical implications. Conventional methods of glycemic control assessment are confounded by the laboratory abnormalities and comorbidities associated with ESRD. Similar to more recent approaches in the general population, there is concern that glucose normalization may be harmful in ESRD patients. There is uncertainty surrounding the optimal glycemic target in this population, although recent epidemiologic data suggest that HbA1c ranges of 6% to 8%, as well as 7 to 9%, are associated with increased survival rates among diabetic dialysis patients. Lastly, many glucose-lowering drugs and their active metabolites are renally metabolized and excreted, and hence, require dose adjustment or avoidance in dialysis patients.
Keywords: Burnt-out diabetes, ESRD, hypoglycemia
Diabetes mellitus is the leading cause of chronic kidney disease (CKD) in the U.S., accounting for approximately 44% and 38% of incident and prevalent cases of end-stage renal disease (ESRD), respectively.1 While the total number of new patients with ESRD due to diabetes continues to rise (i.e., 49,603 new cases in 2011), there has been a plateau in the incidence rate over the past decade (i.e., 159 new cases per million in 2011). Over the past decade, the mortality rates for diabetic dialysis patients have also declined (i.e., 90 vs. 71 deaths per 1000 patient-years of at-risk time in 2000 vs. 2011, respectively). However, diabetic dialysis patients continue to have poor survival (i.e., 34% over 5 years), worse than those with ESRD due to hypertension and glomerular disease. Thus, there is a compelling need to determine if improved glycemic control with well-managed diabetic pharmacotherapies may ameliorate this exceedingly high mortality risk, or even perhaps be associated with adverse outcomes. In this review, we will discuss: 1) alterations in glucose homeostasis conferred by the uremic milieu; 2) the strengths and limitations of diagnostic tools used to evaluate intermediate- and long-term glycemic control in dialysis patients; 3) existing literature on glycemic targets and outcomes in the dialysis population; and 4) the safety and effectiveness of various diabetic pharmacotherapy regimens in diabetic dialysis patients.
Effects of Kidney Dysfunction and Dialysis on Glucose Homeostasis
Maintenance dialysis patients, with or without diabetes, may experience both hyper- and hypoglycemia through multifactorial mechanisms relating to kidney dysfunction, the uremic environment, and dialysis.2–5
Hyperglycemia
In CKD patients without overt diabetes, including those on dialysis, hyperglycemia and impaired glucose tolerance may ensue as a result of increased insulin resistance and decreased insulin secretion.3,4,6 The pathogenesis and exact site of insulin resistance in dialysis patients has not been fully elucidated4; however, uremic toxins are thought to be contributory, as insulin sensitivity improves with dialysis.7–10 Secondary hyperparathyroidism and vitamin D deficiency may impair insulin secretion, and vitamin D repletion has been shown to improve insulin secretion independent of its effects on parathyroid hormone levels.4,11,12 While limited data suggest that peritoneal dialysis (PD) confers improved insulin sensitivity compared to hemodialysis,13 PD may result in significantly greater dialysate glucose exposure, particularly if higher glucose dialysate concentrations are required to achieve ultrafiltration goals. For example, dialysis solutions used by PD patients contain glucose concentrations ranging from 1360 to 3860 mg/dL,14 and the glucose load delivered by PD may confer as much as 10 to 30% of a patient’s total caloric intake.15
Hypoglycemia and the “Burnt-Out Diabetes” Phenomenon
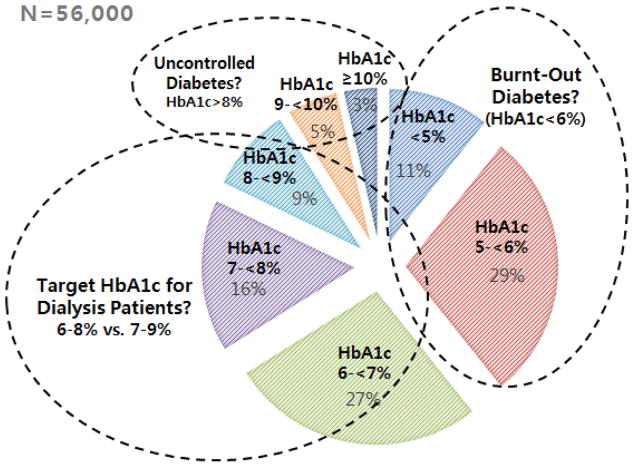
In diabetic dialysis patients, spontaneous resolution of hyperglycemia and the apparent normalization of glycated hemoglobin (hemoglobin A1c [HbA1c]) levels, independent of treatment, is commonly observed and referred to as “Burnt-Out Diabetes.”2–5 In one study of 23,618 diabetic dialysis patients from a large U.S. dialysis organization, up to one-third were observed to have HbA1c levels <6% (Figure 1).16 Frequent hypoglycemic episodes may result in the discontinuation of insulin and oral anti-diabetic medications in dialysis patients.3–5,16
Figure 1.
Approximately one-third of diabetic dialysis patients have an average HbA1c<6%, referred to as “Burnt-Out Diabetes” (data based on Ricks et al., Diabetes 61(30): 708–715, 2012).53
Multiple factors may contribute to this condition. First, malnutrition, protein-energy wasting, and diabetic gastroparesis are frequently observed complications in dialysis patients, which heighten the risk of hypoglycemia.2–4,17 Second, the clearance and degradation of exogenous insulin is reduced in kidney dysfunction, which results in prolongation of insulin half-life.18 Third, there is a decline in the hepatic clearance of insulin in kidney dysfunction, which may improve after initiation of dialysis.6 Fourth, decreased nephron mass and kidney function also lead to a reduction in renal gluconeogenesis.19,20 Finally, the accumulation of some uremic toxins, such as guanidino compounds, may act similar to biguanide agents used for the treatment of type 2 diabetes, thus mitigating or even “curing” diabetes.21–23 At this time, the biologic plausibility of burnt-out diabetes as a distinct clinical condition is debatable, and its clinical significance remains unclear.
Monitoring of Glycemic Control in Dialysis Patients
Laboratory abnormalities and comorbidities associated with the uremic state may impact the accuracy of various methods used for assessing intermediate- and long-term glycemic control, including glycated hemoglobin (HbA1c), fructoasmine, and glycated albumin (Table 1). Despite these limitations, the Kidney Disease Quality Outcomes Initiative (KDOQI) and Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines recommend routine measurement of long-term glycemic control using HbA1c, in combination with home blood glucose monitoring, as a cornerstone of diabetes management in CKD and ESRD patients.
Table 1.
Comparison of Methods of Glycemic Control Assessment
| Glycemic Metric | Period of Assessment | Confounders | Strengths | Limitations |
|---|---|---|---|---|
| Hemoglobin A1c | 2–3 months |
Falsely increase:
Falsely decrease:
|
|
|
| Fructosamine | 2 weeks |
|
|
|
| Glycated Albumin | 2 weeks |
|
|
|
Adapted from Kovesdy CP, Sharma K, Kalantar-Zadeh K. AJKD 2008; 52: 766–777.
Glycated Hemoglobin (HbA1c)
HbA1c is formed by a non-enzymatic reaction between glucose and hemoglobin’s beta chain.24 It measures the concentration of circulating glucose over a 120-day exposure period, and it is the index upon which current standard therapeutic targets for glycemic control are based in the general population.25–27 The glycation rate of hemoglobin is influenced by various factors, including: 1) length of glucose exposure, 2) glucose concentration, 3) hemoglobin level, 4) pH, and 5) temperature.3 Hence, numerous ESRD-related factors may result in aberrant HbA1c levels (Table 1). Spuriously elevated HbA1c levels may be observed in the context of elevated blood urea nitrogen (BUN) levels and metabolic acidosis.28,29 Exposure to high urea concentrations promotes formation of carbamylated hemoglobin, which cannot be distinguished from glycated hemoglobin in certain assays (e.g., electric charge-based assays).4,29 In contrast, boronate-agarose affinity chromatography and thiobarbituric acid methods provide more robust measurement of HbA1c in dialysis patients.30–32
Conversely, both spuriously and truly low HbA1c levels may be observed in the context of anemia, blood transfusions, and conditions associated with shortened erythrocyte life span (e.g., hemoglobinopathies, erythrocyte fragility due to uremia, erythrocyte lysis due to the dialysis procedure), which may consequently lead to underestimation of long-term glucose control and undertreatment of hyperglycemia.3–5 The frequent utilization of erythropoietin-stimulating agents in dialysis patients also falsely lower HbA1c levels by accelerating erythropoiesis and increasing the proportion of young circulating erythrocytes that have limited time for hemoglobin glycosylation.33 To address these limitations, various equations accounting for hemoglobin and other laboratory covariates have been developed to better characterize the HbA1c and blood glucose relationship in hemodialysis and PD patients.34,35
Fructosamine
Fructosamine is a metric of intermediate-term glycemic control (i.e., 7 to 14 days) and is a measure of the ketoamines formed by the nonenzymatic glycation of serum proteins.36 While fructosamine may be a more accurate glycemic control metric in anemic dialysis patients, it may also be confounded by a number of conditions, particularly dysproteinemias (Table 1).3,4 Hence, falsely low fructosamine levels may be observed in PD patients with protein losses in the peritoneal dialysate and in patients with hypoalbuminemia due to protein-energy wasting. Some, but not all, studies have shown that fructosamine is a more accurate measure of glycemic control than HbA1c. In a prospective study of 100 diabetic hemodialysis patients, fructosamine was observed to be a more potent predictor of hospitalization and infections compared to HbA1c.37 In a more recent study of 503 incident hemodialysis patients from the CHOICE cohort, a doubling of fructosamine levels was associated with a two-fold higher risk of all-cause and cardiovascular mortality.38
Glycated Albumin
Whereas fructosamine is a measure of all glycated serum proteins, glycated albumin is formed by a non-enzymatic reaction between glucose and albumin.3,4,39 Glycated albumin measures short-term glucose control (i.e., 7 to 14 days), is robust in anemia and conditions of shortened erythrocyte lifespan, but may be confounded by similar pathologic conditions as fructosamine (Table 1). Several studies comparing the interrelationship between blood glucose, glycated albumin, and HbA1c in diabetic dialysis patients vs. control patients without kidney disease have reported a correlation between blood glucose and glycated albumin levels in these two groups.40–43 However, for any glucose level, HbA1c was lower in dialysis patients vs. control patients, raising the concern that HbA1c underestimates glycemic levels compared to glycated albumin in uremic states.
Elevated glycated albumin levels have also been associated with adverse cardiovascular surrogates (e.g., increased arterial stiffness, vascular calcification)3,44,45 and hard outcomes. In a prospective study of 444 diabetic dialysis patients by Freedman et al., glycated albumin was observed to be a more potent predictor of death risk compared to HbA1c and glucose levels.46 For every 5% increase in glycated albumin level, there was a 14% higher risk of all-cause death, whereas no associations between HbA1c, glucose level, and mortality were observed. These findings stand in contrast to a number of other studies that have observed an incremental increase in mortality risk with higher HbA1c.14,16,47–58 The discrepant findings in the Freedman et al. study may have been due to lack of cumulative glycemic exposure assessment (i.e., lack of time-dependent or time-averaged exposure analysis); an infrequent number of HbA1c measures compared to glycated albumin; and a sparse number of events resulting in limited power.42 Nonetheless, this important study has prompted interest in glycated albumin as a novel metric of glycemic control and outcomes in dialysis patients. Further studies are needed prior to the adoption of glycated albumin and fructosamine as routinely-used intermediate-term glycemic control metrics in diabetic dialysis patients.
Glycemic Control and Outcomes
In the general population, the landmark Diabetes Control and Complications Trial (DCCT) and the follow-up Epidemiology of Diabetes Interventions and Complications (EDIC) studies demonstrated that intensive vs. standard glycemic control reduces microvascular complications and cardiovascular disease, respectively, in type 1 diabetic patients.59,60 In the United Kingdom Prospective Diabetes Study (UKPDS), intensive treatment was observed to reduce microvascular complications among type 2 diabetic patients61,62; in the 10-year post-trial follow-up study, a reduction in myocardial infarction and all-cause death was also observed in the intensive treatment group despite an attenuation in glycemic differences between the intensive and standard treatment groups over time.63
More recently, three randomized controlled trials - ADVANCE, ACCORD, and Veterans Affairs Diabetes Trial (VADT) – sought to examine the impact of tight glycemic control on macrovascular outcomes in type 2 diabetic patients.64–66 Neither ADVANCE nor VADT showed an improvement in cardiovascular outcomes, while ACCORD, which consisted of patients with underlying cardiovascular disease, reported that intensive treatment was associated with higher cardiovascular mortality risk. There are several explanations for these discrepant findings between the earlier DCCT/EDIC and UKPDS studies and the more recent ADVANCE, ACCORD, and VADT trials, which include: 1) lower cardiovascular risk profiles among the DCCT/EDIC and UKPDS cohorts; 2) comparatively less intensive glycemic control in the DCCT/EDIC and UKPDS studies (i.e., in the earlier UKPDS study, intensive glycemic control was analogous to conventional glycemic control in the ADVANCE, ACCORD, and VADT trials); and 3) longer follow-up with which to observe hard outcomes in the DCCT/EDIC and UKPDS studies.67
These recent data have prompted concern that attempts to normalize glycemic control in populations with high underlying cardiovascular risk may be harmful. This has contributed to the uncertainty surrounding the optimal glycemic target in dialysis patients, in whom there are higher risks of cardiovascular morbidity and mortality. A number of observational studies examining the association between degree of glycemic control and mortality in dialysis patients have shown mixed findings (Table 2). From 1993 to 2006, several studies of small cohort size (<250 patients/study) largely observed that higher HbA1c levels were associated with increased mortality in hemodialysis and PD patients.47,50,51,56,58
Table 2.
Observational Studies of Glycemic Control and Mortality in Dialysis Patients
| Author | Study Cohort (Country) | Period of exposure definition | Results |
|---|---|---|---|
| Tzamaloukas (1993)47 | 226 dialysis (PD and HD) patients with type 1 and 2 DM (USA) |
Glycemic control during the 1st six months of study |
|
| Wu (1997)56 | 137 HD patients with type 2 DM (Taiwan) |
Pre-dialysis glycemic control within six months prior to starting HD |
|
| Yu (1997)58 | 60 PD patients with type 2 DM (Taiwan) |
Pre-dialysis glycemic control within 6 months before starting HD (measured monthly) |
|
| Morioka (2001)50 | 150 incident HD patients with type 1 and 2 DM (Japan) |
HbA1c before HD initiation |
|
| McMurray (2002)97 | 83 dialysis (HD and PD) patients with type 1 and 2 DM (USA) |
Nonrandomized interventional trial of intensive education/care vs. control |
|
| Oomichi (2006)51 | 114 HD patients with type 1 and 2 DM (Japan) |
Mean HbA1c during the 3 month period prior to study entry |
|
| Williams (2006)68 | 24,875 HD patients with type 1 and 2 DM (USA – Fresenius) |
Baseline HbA1c during the 3 month period prior to study entry |
|
| Kalantar-Zadeh (2007)16 | 23,618 HD patients with DM (USA – DaVita) |
Time-dependent HbA1c |
|
| Okada (2007)98 | 78 HD patients with type 2 DM (Japan) |
Mean HbA1c during 1 year period after HD initiation AND Mean HbA1c over 3 months prior to study entry |
|
| Ishimura (2009)49 | 122 HD patients with type 1 and 2 DM (Japan) |
Mean A1c of 3 values measured during 3 months prior to study entry |
|
| Dreschsler (2009)48 | 1255 HD patients with type 2 DM (Germany) |
Baseline HbA1c |
|
| Williams (2010)55 | 24,875 HD patients with type 1 and 2 DM (USA – Fresenius) |
Time dependent HbA1c |
|
| Shurraw (2010)99 | 1454 HD patients type 1 and 2 DM (Canada) |
Monthly HbA1c averaged over 3 months pre- and post-HD initiation |
|
| Shima (2010)100 | 245 HD patients with type 1 and 2 DM (Japan) |
Time averaged HbA1c (measured monthly) |
|
| Duong (2011)14 | 2798 PD patients with DM (USA – DaVita) |
Baseline and time-averaged HbA1c |
|
| Sturm (2011)54 | 78 dialysis (PD and HD) patients with type 1 and 2 DM | Time-varying HbA1c (measured every 3 months) |
|
| Ricks (2012)53 | 54,757 HD patients with DM (USA – DaVita) |
Baseline and time-averaged HbA1c |
|
| Ramirez (2012)52 | 9201 HD patients with type 1 and 2 DM (USA DOPPS only) |
Mean HbA1c during 1st eight months after study entry |
|
| Yoo (2012)57 | 140 PD patients with DM (Korea) |
Averaged monthly or quarterly HbA1c levels during 1st year after PD initiation |
|
| Kim (2013)101 | 347 HD patients with DM (USA) |
Baseline HbA1c |
|
Abbreviations: PD, peritoneal dialysis; HD, hemodialysis; DM, diabetes; HbA1c, hemoglobin A1c; QOL, quality of life; DOPPS, Dialysis Outcomes and Practice Patterns Study; CV, cardiovascular
In a study of 24,875 Fresenius Medical Care diabetic hemodialysis patients by Williams et al., there was no association between HbA1c level and mortality after 1 year.68 However, these data were limited by its short-term follow-up; lack of repeated HbA1c measures and time-dependent survival models; and residual confounding by malnutrition, inflammation, anemia, and comorbidities.
In a subsequent study of 23,618 DaVita Inc. diabetic hemodialysis patients followed for up to 3 years by Kalantar-Zadeh et al., lower time-varying HbA1c levels were initially associated with increased mortality in unadjusted analyses.16 However, in subsequent analyses that adjusted for case-mix and malnutrition-inflammation markers and hemoglobin level, higher time-varying HbA1c levels were incrementally associated with higher mortality risk. Using an analogous design with time-varying HbA1c levels, comprehensive adjustment for confounders, and more extended follow-up (i.e., up to three years) Williams et al. then showed that extremes of glycemia (HbA1c<6.5% and >11%) were associated with increased death risk in the Fresenius hemodialysis cohort.55
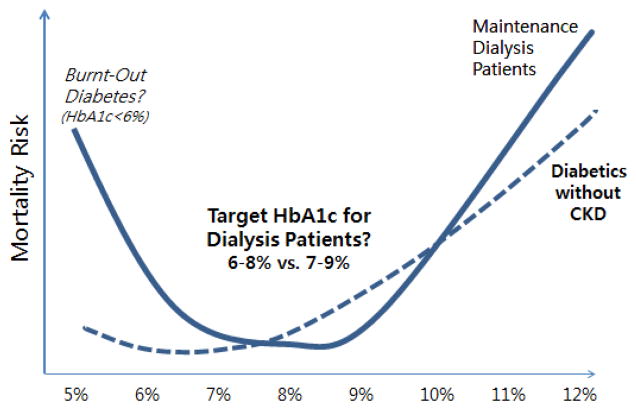
More recently, data from 9201 hemodialysis patients from the U.S. Dialysis Outcomes and Practice Pattern Study (DOPPS) cohort showed a U-shaped association between HbA1c levels and death risk (i.e., HbA1c <6% and ≥9% were each associated with increased mortality risk).52 These findings were corroborated by an even larger study by Ricks et al. of 54,757 DaVita hemodialysis patients among whom HbA1c levels <6% and >8% were associated with increased mortality (Figure 2).53 Despite extensive adjustment for confounders, lower HbA1c may have been a marker of illness and/or malnutrition in these latter observational studies.
Figure 2.
The optimal target hemoglobin A1c (HbA1c) range for diabetic dialysis patients appears to be different from the general population, e.g. 6% to 8% or 7% to 9% (data based on Ricks et al., Diabetes 61(30): 708–715, 2012).53
Hence, these data might suggest that targeting a moderate HbA1c range is associated with greater survival in dialysis patients with lower comorbidity burden and favorable nutritional status, whereas targeting lower HbA1c levels may exacerbate mortality risk in dialysis patients with underlying illness and malnutrition.
At this time, KDOQI and KDIGO clinical practice guidelines recommend that the HbA1c target should be raised to >7% in patients with comorbidities, limited life expectancy, and those at risk for hypoglycemia, the latter of which include patients with advanced CKD, including those receiving dialysis.69 However, in the opinion of some of the coauthors of this review,70 the most reasonable target range for diabetic dialysis patients should be limited to 6 to 8% or 7% to 9%, given higher mortality risks observed with HbA1c<6% and the potential implications of burnt-out diabetes and the high death risk associated with hypoglycemia in these patients.53,70 Large clinical trials are needed to determine whether intensive vs. moderate vs. liberal glycemic control optimizes morbidity and mortality in dialysis patients.
Treatment of Diabetes in the Dialysis Population
Insulin
Whereas endogenously secreted insulin is degraded by the liver, exogenous insulin is primarily excreted by the kidneys.18 After being freely filtered by the glomerulus, insulin is reabsorbed principally by the proximal tubule and to a lesser degree by peritubular endothelial cells, where it is degraded into peptide fragments. While there are no absolute guidelines regarding dose adjustments for insulin based on estimated glomerular filtration rate (eGFR), experts recommend an insulin dose reduction of 50% when eGFR is <10ml/min/1.73m2.18,71 Upon initiation of dialysis, peripheral insulin resistance may improve, further reducing insulin requirements.7–9
PD patients have the option of insulin administration via a subcutaneous (SC) or intraperitoneal (IP) route. IP insulin administration stimulates endogenous insulin secretion and inhibits hepatic gluconeogenesis and ketogenesis,72 but may also necessitate higher insulin doses due to losses into the dialysate and adsorption to the plastic surface of dialysis solution delivery systems.73 In a meta-analysis of three trials in diabetic PD patients, IP vs. SC insulin administration was associated with two-fold higher insulin requirements and a greater degree of optimal glycemic control.74 However, IP insulin regimens may also carry the risk of 1) bacterial contamination during their injection into dialysate bags,75 2) peritoneal fibroblastic proliferation,76 and 3) hepatic subcapsular steatonecrosis.77 Further study of the safety and effectiveness of long-term IP vs. SC insulin regimens in PD patients is needed.
Oral Agents
The armamentarium of therapeutic agents used for the treatment of diabetes has expanded over the past decade (Table 3). However, the pharmaocokinetic properties of many of these drugs are altered in kidney dysfunction and may thus require dose adjustment or avoidance in dialysis patients.18,78
Table 3.
Oral Diabetic Agents
| Medication Class | Mechanism of action | Medication | Usage in dialysis patients | Side effects |
|---|---|---|---|---|
|
| ||||
| Sulfonylureas |
|
1st generation: Acetohexamide Chlorpropamide Tolazamide Tolbutamide |
Avoid use of 1st generation agents in dialysis patients |
|
|
2nd generation: Glipizide Gliclazide Glimepiride Glyburide |
Glipizide is the agent of choice | |||
|
| ||||
| Meglitinides |
|
Repaglinide Nateglinide |
Dose reductions not specified, but no clear guidelines Avoid use in dialysis patients |
|
|
| ||||
| Biguanides |
|
Metformin | Avoid use in dialysis patients |
|
|
| ||||
| Thiazolidine-diones |
|
Rosiglitazone (soley PPAR-gamma agonist) Pioglitazone (also has PPAR-alpha effects) |
Restricted use by manufacturer Dose adjustment not required |
|
|
| ||||
| Dipeptidyl Peptidase-4 (DPP-4) Inhibitors |
|
Sitagliptin Saxagliptin Linagliptin |
Dose reduction by 75% Dose reduction to 25 mg po qday, given after dialysis Not well studied in dialysis patients |
|
|
| ||||
| Glucagon-like Peptide 1 Analogues |
|
Exenatide Liraglutide |
Avoid use in dialysis patients Avoid use in dialysis patients |
|
|
| ||||
| Amylin Analogues |
|
Pramlintide | Avoid use in dialysis patients |
|
|
| ||||
| Alpha-glucosidase inhibitors |
|
Acarbose Miglitol |
Avoid use in dialysis patients due to limited study |
|
|
| ||||
| Sodium-glucose cotransporter 2 inhibitors |
|
Canagliflozin Dapagliflozin |
Avoid use in dialysis patients |
|
Sulfonylureas (SUs) stimulate insulin secretion by binding to a receptor on the pancreatic beta cells that is a component of the ATP-dependent potassium channel.18 The older first generation SUs (e.g., acetohexamide, chlorpropamide, tolazamide, tolbutamide) are rarely used and should not be used in dialysis patients, given their long half-life and risk of hypoglycemia among this population. Among the newer, second generation SUs, short-acting glipizide is the preferred agent in dialysis patients, as it is largely metabolized by the liver, has inactive or weakly active metabolites that are excreted in the urine, and has a lower risk of hypoglycemia compared to other SUs (e.g., glyburide, glimepiride).18,78 Most clinicians, however, avoid the use of SUs in the elderly and in dialysis patients, due to the hypoglycemia risk.
Meglitinides include repaglinide and nateglinide, which are structurally different that SUs but similarly stimulate insulin secretion by regulating ATP-dependent potassium channels on pancreatic beta cells.79 Repaglinide is the preferred agent in dialysis patients, as it is completely metabolized by the liver, has inactive or weakly active metabolites that are excreted in the urine, and has lower risk of hypoglycemia compared with other agents.18,78 Nateglinide, while also hepatically metabolized, has renally-excreted active metabolites that may result in hypoglycemia in dialysis patients.
Biguanides consist of metformin, phenformin, and buformin which inhibit hepatic gluconeogenesis, decrease intestinal glucose absorption, and improve peripheral insulin sensitivity.80 Phenformin was removed from the U.S. market due to its high frequency of severe lactic acidosis, but it is still available in other countries; buformin is also only available outside of the U.S. Ninety-percent of metformin is renally excreted,81 and accumulation in kidney dysfunction causes type B (nonhypoxic) lactic acidosis due to 1) enhanced conversion of glucose to lactate in the small intestine, and 2) inhibition of hepatic gluconeogenesis by lactate, pyruvate, and alanine.82,83 The mortality rate of metformin-associated lactic acidosis is as high as 50%. Hence, U.S. FDA guidelines advise against metformin use when the creatinine in men and women is ≥1.5mg/dL and ≥1.4 mg/dL, respectively.18 While some experts have recommended metformin dose reduction (i.e., 50% reduction, or half of maximal dose) when the eGFR is 30–45ml/min/1.73m2,84 the most recent KDIGO guidelines recommend that metformin use should be reevaluated at this range of eGFR, and discontinued when eGFR is <30ml/min/1.73m2 and hence should not be used in dialysis patients.69
Thiazolidinediones (TZDs) bind to the peroxisome proliferator-activated receptor-gamma (PPAR-γ) receptor and improves peripheral insulin sensitivity and suppresses hepatic gluconeogenesis.18 TZDs are wholly metabolized in the liver, and neither the parent drug nor its major metabolites are renally excreted. TZDs may promote edema and congestive heart failure via PPAR-γ-mediated stimulation of distal tubular sodium channels and sodium reabsorption, but this risk may be irrelevant in oliguric and anuric dialysis patients.85–87 TZDs may also decrease bone formation and increase bone loss and fracture risk, which may bear consequence in patients with underlying CKD-mineral bone disease.88 However, TZDs may also favorably impact health by improving lipid (e.g., triglyceride, HDL) and adiponectin levels; reducing visceral adiposity; decreasing inflammation; and reducing muscle catabolism and protein-energy wasting.89–91
In the general population, observational data and meta-analyses suggest that TZD safety and effectiveness may be dependent on the specific agent used. Whereas studies of rosiglitazone have shown an increased risk of cardiovascular events,92 studies of pioglitazone have demonstrated a reduced risk of cardiovascular morbidity and mortality.93
To date, two rigorous studies examining TZDs and mortality in the dialysis population have shown mixed findings. In a study of 5290 incident dialysis patients with diabetes from the ArMORR cohort, Brunelli et al. showed that TZD use was associated with lower all-cause mortality among insulin-free patients, but not in those who were insulin-requiring.89 This was irrespective of the type of TZD agent used, and findings were robust in a number of sensitivity analyses that accounted for confounding by indication, severity of disease, reverse causation, and time-varying exposure status. On the basis of these data, it was posited that the benefits of TZDs on peripheral insulin sensitivity may be annulled among those exposed to exogenous insulin. In contrast, Ramirez et al. showed that rosiglitazone use was associated with increased all-cause and cardiovascular mortality among 2393 diabetic hemodialysis patients from the U.S. DOPPS cohort, irrespective of insulin use.87 However, similar associations were not observed among those who received pioglitazone, and emerging data suggest that pioglitazone is associated with improved survival in dialysis patients.94
Dipeptidyl Peptidase-4 Inhibitors (DPP-4 inhibitors) are incretin system compounds and include linagliptin, sitagliptin, and saxagliptin;18,78 several additional agents in this class have been approved in recent years or are in development. DPP-4 is an enzyme expressed on the surface of various types of cells and deactivates glucagon-like peptide-1 (GLP-1), an incretin hormone which stimulates glucose-dependent insulin secretion. By increasing GLP-1 availability, DPP-4 inhibitors promote insulin release and reduce postprandial glucose levels. Linagliptin is minimally excreted in the urine, but it has not been well studied in the dialysis population. Sitagliptin is largely excreted in the urine, and the recommended dose in dialysis patients is 25 mg orally per day. Saxagliptin and its primary active metabolite are cleared by hemodialysis and thus should be administered using a reduced dose (2.5 mg orally once a day) after dialysis.
Exenatide and liraglutide are GLP-1 analogues that not only facilitate insulin secretion, but also decrease glucagon secretion, delay gastric empying, and promote early satiety and weight loss.18 Exenatide is an injectable, renally-excreted drug and not recommended in patients with an eGFR<30/ml/min/1.73m2. Although liraglutide is not metabolized or eliminated by the kidney, there are few data of its use in dialysis patients, and manufacturers caution against administration in mild to severe kidney dysfunction.69
Pramlintide is an analogue of amylin, a pancreatic beta cell hormone that delays gastric emptying, increases satiety, and suppresses postprandial rises in glucagon levels.78 It is co-secreted with insulin, primarily renally metabolized and excreted, and has not been studied in dialysis patients.
Alpha-glucosidase inhibitors function by delaying gastrointestinal glucose absorption and reducing postprandial blood glucose peaks.18,69,78 Gastrointestinal side effects (e.g., abdominal pain, diarrhea, flatulence) has rendered its use infrequent in the general diabetic population. While <2% of acarbose and its active metabolites are renally excreted, its use in dialysis patients is not recommended given inadequate study in this population. Miglitol is renally excreted, and administration is also not advised in patients with kidney dysfunction.
The sodium-glucose cotransporter 2 (SGLT2) inhibitors include canagliflozin and dapagliflozin and are a new line of diabetic medications that modestly lower elevated blood glucose and HbA1c levels by inhibiting reabsorption of the filtered glucose load and hence promote the renal excretion of glucose.95 In animal studies, SGL2 inhibitors have also been shown to reduce albuminuria.96 At this time, there is a lack of long-term safety and effectiveness data supporting their use in the general type 2 diabetic population, and their use is contraindicated in dialysis patients.
Conclusion
There have been substantial advances in our understanding of the unique glycemic milieu, limitations of contemporary glucose-monitoring methods, and the complex pharmacokinetics of glucose-lowering therapeutic agents in dialysis patients. However, many unanswered questions remain: What is the target range of glycemic control in diabetic dialysis patients? Is diabetes management in diabetic dialysis patients as important as it is in diabetics without advanced CKD, and what is the relative relevance of optimizing hyperglycemia in the context of other diabetic comorbidities among dialysis patients? Does burnt-out diabetes have clinical significance?
Based on existing observational data, intensive glycemic control does not appear to be associated with improved outcomes in dialysis patients who are prone to hypoglycemia and the burnt-out diabetes phenomenon. However, there remains substantial uncertainty with regards to 1) the optimal method for glycemic monitoring in dialysis patients, 2) the impact of these respective methods on glycemic control and hard outcomes in this population, 3) ideal glycemic targets that confer improved morbidity and mortality, and 4) the comparative safety and effectiveness of various glucose-lowering drugs in dialysis patients.
At this time, the critical next steps in closing these knowledge gaps will be to define 1) an accurate and broadly applicable glycemic metric in CKD, 2) the optimal glycemic target ranges in this population (and whether this differs from the general population), and 3) whether our understanding of the natural course of the burnt-out diabetes phenomenon can be used to ameliorate diabetic complications prior to end-organ damage. Given the high mortality rate among this population, there is compelling need for further investigation of how to optimally manage diabetes in dialysis patients.
Acknowledgments
Support:
This work was supported by NIH/NIDDK K24-DK091419 and R01-DK078106 (KKZ), NIH/NICHD 7K23HD06855204 (AML), and a philanthropic grant from Mr. Harold Simmons.
Footnotes
Conflicts of Interest:
None declared.
Bibliography
- 1.U.S. Renal Data System. USRDS 2013 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2013. [Google Scholar]
- 2.Kalantar-Zadeh K, Derose SF, Nicholas S, Benner D, Sharma K, Kovesdy CP. Burnt-out diabetes: impact of chronic kidney disease progression on the natural course of diabetes mellitus. J Ren Nutr. 2009;19(1):33–37. doi: 10.1053/j.jrn.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovesdy CP, Park JC, Kalantar-Zadeh K. Glycemic control and burnt-out diabetes in ESRD. Semin Dial. 2010;23(2):148–156. doi: 10.1111/j.1525-139X.2010.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovesdy CP, Sharma K, Kalantar-Zadeh K. Glycemic control in diabetic CKD patients: where do we stand? Am J Kidney Dis. 2008;52(4):766–777. doi: 10.1053/j.ajkd.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Park J, Lertdumrongluk P, Molnar MZ, Kovesdy CP, Kalantar-Zadeh K. Glycemic control in diabetic dialysis patients and the burnt-out diabetes phenomenon. Curr Diab Rep. 2012;12(4):432–439. doi: 10.1007/s11892-012-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mak RH. Impact of end-stage renal disease and dialysis on glycemic control. Semin Dial. 2000;13(1):4–8. doi: 10.1046/j.1525-139x.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- 7.DeFronzo RA, Tobin JD, Rowe JW, Andres R. Glucose intolerance in uremia. Quantification of pancreatic beta cell sensitivity to glucose and tissue sensitivity to insulin. J Clin Invest. 1978;62(2):425–435. doi: 10.1172/JCI109144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dzurik R, Spustova V, Lajdova I. Inhibition of glucose utilization in isolated rat soleus muscle by pseudouridine: implications for renal failure. Nephron. 1993;65(1):108–110. doi: 10.1159/000187450. [DOI] [PubMed] [Google Scholar]
- 9.McCaleb ML, Izzo MS, Lockwood DH. Characterization and partial purification of a factor from uremic human serum that induces insulin resistance. J Clin Invest. 1985;75(2):391–396. doi: 10.1172/JCI111712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz O. Insulin-mediated glucose uptake in nondialyzed and dialyzed uremic insulin-dependent diabetic subjects. Diabetes. 1985;34(11):1152–1159. doi: 10.2337/diab.34.11.1152. [DOI] [PubMed] [Google Scholar]
- 11.Mak RH. Intravenous 1,25 dihydroxycholecalciferol corrects glucose intolerance in hemodialysis patients. Kidney Int. 1992;41(4):1049–1054. doi: 10.1038/ki.1992.159. [DOI] [PubMed] [Google Scholar]
- 12.Mak RH. 1,25-Dihydroxyvitamin D3 corrects insulin and lipid abnormalities in uremia. Kidney Int. 1998;53(5):1353–1357. doi: 10.1046/j.1523-1755.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 13.Mak RH. Insulin resistance in uremia: effect of dialysis modality. Pediatr Res. 1996;40(2):304–308. doi: 10.1203/00006450-199608000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Duong U, Mehrotra R, Molnar MZ, Noori N, Kovesdy CP, Nissenson AR, Kalantar-Zadeh K. Glycemic control and survival in peritoneal dialysis patients with diabetes mellitus. Clin J Am Soc Nephrol. 2011;6(5):1041–1048. doi: 10.2215/CJN.08921010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grodstein GP, Blumenkrantz MJ, Kopple JD, Moran JK, Coburn JW. Glucose absorption during continuous ambulatory peritoneal dialysis. Kidney Int. 1981;19(4):564–567. doi: 10.1038/ki.1981.53. [DOI] [PubMed] [Google Scholar]
- 16.Kalantar-Zadeh K, Kopple JD, Regidor DL, Jing J, Shinaberger CS, Aronovitz J, McAllister CJ, Whellan D, Sharma K. A1C and survival in maintenance hemodialysis patients. Diabetes Care. 2007;30(5):1049–1055. doi: 10.2337/dc06-2127. [DOI] [PubMed] [Google Scholar]
- 17.Mak RH, Ikizler AT, Kovesdy CP, Raj DS, Stenvinkel P, Kalantar-Zadeh K. Wasting in chronic kidney disease. J Cachexia Sarcopenia Muscle. 2011;2(1):9–25. doi: 10.1007/s13539-011-0019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reilly JB, Berns JS. Selection and dosing of medications for management of diabetes in patients with advanced kidney disease. Semin Dial. 2010;23(2):163–168. doi: 10.1111/j.1525-139X.2010.00703.x. [DOI] [PubMed] [Google Scholar]
- 19.Arem R. Hypoglycemia associated with renal failure. Endocrinol Metab Clin North Am. 1989;18(1):103–121. [PubMed] [Google Scholar]
- 20.Peitzman SJ, Agarwal BN. Spontaneous hypoglycemia in end-stage renal failure. Nephron. 1977;19(3):131–139. doi: 10.1159/000180877. [DOI] [PubMed] [Google Scholar]
- 21.De Deyn PP, Vanholder R, Eloot S, Glorieux G. Guanidino compounds as uremic (neuro)toxins. Semin Dial. 2009;22(4):340–345. doi: 10.1111/j.1525-139X.2009.00577.x. [DOI] [PubMed] [Google Scholar]
- 22.Eloot S, van Biesen W, Dhondt A, de Smet R, Marescau B, De Deyn PP, Verdonck P, Vanholder R. Impact of increasing haemodialysis frequency versus haemodialysis duration on removal of urea and guanidino compounds: a kinetic analysis. Nephrol Dial Transplant. 2009;24(7):2225–2232. doi: 10.1093/ndt/gfp059. [DOI] [PubMed] [Google Scholar]
- 23.Meglasson MD, Wilson JM, Yu JH, Robinson DD, Wyse BM, de Souza CJ. Antihyperglycemic action of guanidinoalkanoic acids: 3-guanidinopropionic acid ameliorates hyperglycemia in diabetic KKAy and C57BL6Job/ob mice and increases glucose disappearance in rhesus monkeys. J Pharmacol Exp Ther. 1993;266(3):1454–1462. [PubMed] [Google Scholar]
- 24.Bunn HF, Haney DN, Kamin S, Gabbay KH, Gallop PM. The biosynthesis of human hemoglobin A1c. Slow glycosylation of hemoglobin in vivo. J Clin Invest. 1976;57(6):1652–1659. doi: 10.1172/JCI108436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Standards of medical care in diabetes--2013. Diabetes Care. 2013;36 (Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35(6):1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Marchi S, Cecchin E, Camurri C, Quaia P, Raimondi A, Donadon W, Lippi U, Tesio F. Origin of glycosylated hemoglobin A1 in chronic renal failure. Int J Artif Organs. 1983;6(2):77–82. [PubMed] [Google Scholar]
- 29.Fluckiger R, Harmon W, Meier W, Loo S, Gabbay KH. Hemoglobin carbamylation in uremia. N Engl J Med. 1981;304(14):823–827. doi: 10.1056/NEJM198104023041406. [DOI] [PubMed] [Google Scholar]
- 30.Bruns DE, Lobo PI, Savory J, Wills MR. Specific affinity-chromatographic measurement of glycated hemoglobins in uremic patients. Clin Chem. 1984;30(4):569–571. [PubMed] [Google Scholar]
- 31.Paisey R, Banks R, Holton R, Young K, Hopton M, White D, Hartog M. Glycosylated haemoglobin in uraemia. Diabet Med. 1986;3(5):445–448. doi: 10.1111/j.1464-5491.1986.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 32.Scott MG, Hoffmann JW, Meltzer VN, Siegfried BA, Chan KM. Effects of azotemia on results of the boronate-agarose affinity and ion-exchange methods for glycated hemoglobin. Clin Chem. 1984;30(6):896–898. [PubMed] [Google Scholar]
- 33.Nakao T, Matsumoto H, Okada T, Han M, Hidaka H, Yoshino M, Shino T, Yamada C, Nagaoka Y. Influence of erythropoietin treatment on hemoglobin A1c levels in patients with chronic renal failure on hemodialysis. Intern Med. 1998;37(10):826–830. doi: 10.2169/internalmedicine.37.826. [DOI] [PubMed] [Google Scholar]
- 34.Hoshino J, Mehrotra R, Rhee CM, Yamagata K, Ubara Y, Takaichi K, Kovesdy CP, Molnar MZ, Kalantar-Zadeh K. Using hemoglobin A1c to derive mean blood glucose in peritoneal dialysis patients. Am J Nephrol. 2013;37(5):413–420. doi: 10.1159/000349929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoshino J, Molnar MZ, Yamagata K, Ubara Y, Takaichi K, Kovesdy CP, Kalantar-Zadeh K. Developing an HbA(1c)-based equation to estimate blood glucose in maintenance hemodialysis patients. Diabetes Care. 2013;36(4):922–927. doi: 10.2337/dc12-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem. 1987;33(12):2153–2163. [PubMed] [Google Scholar]
- 37.Mittman N, Desiraju B, Fazil I, Kapupara H, Chattopadhyay J, Jani CM, Avram MM. Serum fructosamine versus glycosylated hemoglobin as an index of glycemic control, hospitalization, and infection in diabetic hemodialysis patients. Kidney Int Suppl. 2010;(117):S41–45. doi: 10.1038/ki.2010.193. [DOI] [PubMed] [Google Scholar]
- 38.Shafi T, Sozio SM, Plantinga LC, Jaar BG, Kim ET, Parekh RS, Steffes MW, Powe NR, Coresh J, Selvin E. Serum fructosamine and glycated albumin and risk of mortality and clinical outcomes in hemodialysis patients. Diabetes Care. 2013;36(6):1522–1533. doi: 10.2337/dc12-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dolhofer R, Wieland OH. Improvement of the thiobarbituric acid assay for serum glycosylprotein determination. Clin Chim Acta. 1981;112(2):197–204. doi: 10.1016/0009-8981(81)90378-8. [DOI] [PubMed] [Google Scholar]
- 40.Freedman BI, Shenoy RN, Planer JA, Clay KD, Shihabi ZK, Burkart JM, Cardona CY, Andries L, Peacock TP, Sabio H, Byers JR, Russell GB, Bleyer AJ. Comparison of glycated albumin and hemoglobin A1c concentrations in diabetic subjects on peritoneal and hemodialysis. Perit Dial Int. 2010;30(1):72–79. doi: 10.3747/pdi.2008.00243. [DOI] [PubMed] [Google Scholar]
- 41.Inaba M, Okuno S, Kumeda Y, Yamada S, Imanishi Y, Tabata T, Okamura M, Okada S, Yamakawa T, Ishimura E, Nishizawa Y. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol. 2007;18(3):896–903. doi: 10.1681/ASN.2006070772. [DOI] [PubMed] [Google Scholar]
- 42.Mehrotra R, Kalantar-Zadeh K, Adler S. Assessment of glycemic control in dialysis patients with diabetes: glycosylated hemoglobin or glycated albumin? Clin J Am Soc Nephrol. 2011;6(7):1520–1522. doi: 10.2215/CJN.04210511. [DOI] [PubMed] [Google Scholar]
- 43.Peacock TP, Shihabi ZK, Bleyer AJ, Dolbare EL, Byers JR, Knovich MA, Calles-Escandon J, Russell GB, Freedman BI. Comparison of glycated albumin and hemoglobin A(1c) levels in diabetic subjects on hemodialysis. Kidney Int. 2008;73(9):1062–1068. doi: 10.1038/ki.2008.25. [DOI] [PubMed] [Google Scholar]
- 44.Kumeda Y, Inaba M, Shoji S, Ishimura E, Inariba H, Yabe S, Okamura M, Nishizawa Y. Significant correlation of glycated albumin, but not glycated haemoglobin, with arterial stiffening in haemodialysis patients with type 2 diabetes. Clin Endocrinol (Oxf) 2008;69(4):556–561. doi: 10.1111/j.1365-2265.2008.03202.x. [DOI] [PubMed] [Google Scholar]
- 45.Yamada S, Inaba M, Shidara K, Okada S, Emoto M, Ishimura E, Nishizawa Y. Association of glycated albumin, but not glycated hemoglobin, with peripheral vascular calcification in hemodialysis patients with type 2 diabetes. Life Sci. 2008;83(13–14):516–519. doi: 10.1016/j.lfs.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Freedman BI, Andries L, Shihabi ZK, Rocco MV, Byers JR, Cardona CY, Pickard MA, Henderson DL, Sadler MV, Courchene LM, Jordan JR, Balderston SS, Graham AD, Mauck VL, Russell GB, Bleyer AJ. Glycated albumin and risk of death and hospitalizations in diabetic dialysis patients. Clin J Am Soc Nephrol. 2011;6(7):1635–1643. doi: 10.2215/CJN.11491210. [DOI] [PubMed] [Google Scholar]
- 47.Tzamaloukas AH, Murata GH, Zager PG, Eisenberg B, Avasthi PS. The relationship between glycemic control and morbidity and mortality for diabetics on dialysis. ASAIO. 1993 Oct-Dec;39(4):880–5. [PubMed] [Google Scholar]
- 48.Drechsler C, Krane V, Ritz E, Marz W, Wanner C. Glycemic control and cardiovascular events in diabetic hemodialysis patients. Circulation. 2009;120(24):2421–2428. doi: 10.1161/CIRCULATIONAHA.109.857268. [DOI] [PubMed] [Google Scholar]
- 49.Ishimura E, Okuno S, Kono K, Fujino-Kato Y, Maeno Y, Kagitani S, Tsuboniwa N, Nagasue K, Maekawa K, Yamakawa T, Inaba M, Nishizawa Y. Glycemic control and survival of diabetic hemodialysis patients--importance of lower hemoglobin A1C levels. Diabetes Res Clin Pract. 2009;83(3):320–326. doi: 10.1016/j.diabres.2008.11.038. [DOI] [PubMed] [Google Scholar]
- 50.Morioka T, Emoto M, Tabata T, Shoji T, Tahara H, Kishimoto H, Ishimura E, Nishizawa Y. Glycemic control is a predictor of survival for diabetic patients on hemodialysis. Diabetes Care. 2001;24(5):909–913. doi: 10.2337/diacare.24.5.909. [DOI] [PubMed] [Google Scholar]
- 51.Oomichi T, Emoto M, Tabata T, Morioka T, Tsujimoto Y, Tahara H, Shoji T, Nishizawa Y. Impact of glycemic control on survival of diabetic patients on chronic regular hemodialysis: a 7-year observational study. Diabetes Care. 2006;29(7):1496–1500. doi: 10.2337/dc05-1887. [DOI] [PubMed] [Google Scholar]
- 52.Ramirez SP, McCullough KP, Thumma JR, Nelson RG, Morgenstern H, Gillespie BW, Inaba M, Jacobson SH, Vanholder R, Pisoni RL, Port FK, Robinson BM. Hemoglobin A(1c) levels and mortality in the diabetic hemodialysis population: findings from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Diabetes Care. 2012;35(12):2527–2532. doi: 10.2337/dc12-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ricks J, Molnar MZ, Kovesdy CP, Shah A, Nissenson AR, Williams M, Kalantar-Zadeh K. Glycemic control and cardiovascular mortality in hemodialysis patients with diabetes: a 6-year cohort study. Diabetes. 2012;61(3):708–715. doi: 10.2337/db11-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sturm G, Lamina C, Zitt E, Lhotta K, Haider F, Neyer U, Kronenberg F. Association of HbA1c values with mortality and cardiovascular events in diabetic dialysis patients. The INVOR study and review of the literature. PLoS One. 2011;6(5):e20093. doi: 10.1371/journal.pone.0020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams ME, Lacson E, Jr, Wang W, Lazarus JM, Hakim R. Glycemic control and extended hemodialysis survival in patients with diabetes mellitus: comparative results of traditional and time-dependent Cox model analyses. Clin J Am Soc Nephrol. 2010;5(9):1595–1601. doi: 10.2215/CJN.09301209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu MS, Yu CC, Yang CW, Wu CH, Haung JY, Hong JJ, Fan Chiang CY, Huang CC, Leu ML. Poor pre-dialysis glycaemic control is a predictor of mortality in type II diabetic patients on maintenance haemodialysis. Nephrol Dial Transplant. 1997;12(10):2105–2110. doi: 10.1093/ndt/12.10.2105. [DOI] [PubMed] [Google Scholar]
- 57.Yoo DE, Park JT, Oh HJ, Kim SJ, Lee MJ, Shin DH, Han SH, Yoo TH, Choi KH, Kang SW. Good glycemic control is associated with better survival in diabetic patients on peritoneal dialysis: a prospective observational study. PLoS One. 2012;7(1):e30072. doi: 10.1371/journal.pone.0030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu CC, Wu MS, Wu CH, Yang CW, Huang JY, Hong JJ, Fan Chiang CY, Leu ML, Huang CC. Predialysis glycemic control is an independent predictor of clinical outcome in type II diabetics on continuous ambulatory peritoneal dialysis. Perit Dial Int. 1997;17(3):262–268. [PubMed] [Google Scholar]
- 59.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 60.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.United Kingdom Prospective Diabetes Study (UKPDS) 13: Relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ. 1995;310(6972):83–88. [PMC free article] [PubMed] [Google Scholar]
- 62.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–865. [PubMed] [Google Scholar]
- 63.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 64.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 65.Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, Cuddihy R, Cushman WC, Genuth S, Grimm RH, Jr, Hamilton BP, Hoogwerf B, Karl D, Katz L, Krikorian A, O’Connor P, Pop-Busui R, Schubart U, Simmons D, Taylor H, Thomas A, Weiss D, Hramiak I. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419–430. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 67.Alicic RZ, Tuttle KR. Management of the diabetic patient with advanced chronic kidney disease. Semin Dial. 2010;23(2):140–147. doi: 10.1111/j.1525-139X.2010.00700.x. [DOI] [PubMed] [Google Scholar]
- 68.Williams ME, Lacson E, Jr, Teng M, Ofsthun N, Lazarus JM. Hemodialyzed type I and type II diabetic patients in the US: Characteristics, glycemic control, and survival. Kidney Int. 2006;70(8):1503–1509. doi: 10.1038/sj.ki.5001789. [DOI] [PubMed] [Google Scholar]
- 69.KDIGO. Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International Supplements 2013. 2012;3(1):1–163. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 70.Kalantar-Zadeh K. A critical evaluation of glycated protein parameters in advanced nephropathy: a matter of life or death: A1C remains the gold standard outcome predictor in diabetic dialysis patients. Counterpoint. Diabetes Care. 2012;35(7):1625–1628. doi: 10.2337/dc12-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Charpentier G, Riveline JP, Varroud-Vial M. Management of drugs affecting blood glucose in diabetic patients with renal failure. Diabetes Metab. 2000;26 (Suppl 4):73–85. [PubMed] [Google Scholar]
- 72.Duckworth WC. Insulin degradation: mechanisms, products, and significance. Endocr Rev. 1988;9(3):319–345. doi: 10.1210/edrv-9-3-319. [DOI] [PubMed] [Google Scholar]
- 73.Quellhorst E. Insulin therapy during peritoneal dialysis: pros and cons of various forms of administration. J Am Soc Nephrol. 2002;13 (Suppl 1):S92–96. [PubMed] [Google Scholar]
- 74.Almalki MH, Altuwaijri MA, Almehthel MS, Sirrs SM, Singh RS. Subcutaneous versus intraperitoneal insulin for patients with diabetes mellitus on continuous ambulatory peritoneal dialysis: meta-analysis of non-randomized clinical trials. Clin Invest Med. 2012;35(3):E132–143. doi: 10.25011/cim.v35i3.16589. [DOI] [PubMed] [Google Scholar]
- 75.Selgas R. Comparative study of two different routes for insulin administration in CAPD patients: A multicenter study. Adv Perit Dial. 1988;4:126. [PubMed] [Google Scholar]
- 76.Selgas R, Lopez-Rivas A, Alvaro F, Tarduchy GR, Rueda P, Muñoz J, Vara F. Insulin influence (used as an additive to dialysate) on the mitogenic-induced effect of the peritoneal effluent in CAPD patients. Adv Perit Dial. 1989;5:161–4. [PubMed] [Google Scholar]
- 77.Wanless IR, Bargman JM, Oreopoulos DG, Vas SI. Subcapsular steatonecrosis in response to peritoneal insulin delivery: a clue to the pathogenesis of steatonecrosis in obesity. Mod Pathol. 1989;2(2):69–74. [PubMed] [Google Scholar]
- 78.Flynn C, Bakris GL. Noninsulin glucose-lowering agents for the treatment of patients on dialysis. Nat Rev Nephrol. 2013;9(3):147–153. doi: 10.1038/nrneph.2013.12. [DOI] [PubMed] [Google Scholar]
- 79.Fuhlendorff J, Rorsman P, Kofod H, Brand CL, Rolin B, MacKay P, Shymko R, Carr RD. Stimulation of insulin release by repaglinide and glibenclamide involves both common and distinct processes. Diabetes. 1998;47(3):345–351. doi: 10.2337/diabetes.47.3.345. [DOI] [PubMed] [Google Scholar]
- 80.Bailey CJ. Biguanides and NIDDM. Diabetes Care. 1992;15(6):755–772. doi: 10.2337/diacare.15.6.755. [DOI] [PubMed] [Google Scholar]
- 81.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334(9):574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 82.Kalantar-Zadeh K. Case 23-2013: a 54-year-old woman with metformin toxicity. N Engl J Med. 2013;369(18):1769. doi: 10.1056/NEJMc1310560. [DOI] [PubMed] [Google Scholar]
- 83.Kalantar-Zadeh K, Uppot RN, Lewandrowski KB. Case records of the Massachusetts General Hospital. Case 23-2013. A 54-year-old woman with abdominal pain, vomiting, and confusion. N Engl J Med. 2013;369(4):374–382. doi: 10.1056/NEJMcpc1208154. [DOI] [PubMed] [Google Scholar]
- 84.Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes care. 2011 Jun;34(6):1431–1437. doi: 10.2337/dc10-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lindenfeld J, Masoudi FA. Fluid retention with thiazolidinediones: does the mechanism influence the outcome? J Am Coll Cardiol. 2007;49(16):1705–1707. doi: 10.1016/j.jacc.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 86.Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation. 2003;108(23):2941–2948. doi: 10.1161/01.CIR.0000103683.99399.7E. [DOI] [PubMed] [Google Scholar]
- 87.Ramirez SP, Albert JM, Blayney MJ, Tentori F, Goodkin DA, Wolfe RA, Young EW, Bailie GR, Pisoni RL, Port FK. Rosiglitazone is associated with mortality in chronic hemodialysis patients. J Am Soc Nephrol. 2009;20(5):1094–1101. doi: 10.1681/ASN.2008060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grey A. Skeletal consequences of thiazolidinedione therapy. Osteoporos Int. 2008;19(2):129–137. doi: 10.1007/s00198-007-0477-y. [DOI] [PubMed] [Google Scholar]
- 89.Brunelli SM, Thadhani R, Ikizler TA, Feldman HI. Thiazolidinedione use is associated with better survival in hemodialysis patients with non-insulin dependent diabetes. Kidney Int. 2009;75(9):961–968. doi: 10.1038/ki.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology. 2006;147(9):4160–4168. doi: 10.1210/en.2006-0251. [DOI] [PubMed] [Google Scholar]
- 91.Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351(11):1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 92.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 93.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298(10):1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 94.Lynch KE, Rhee CM, Brunelli SM. Thiazolidinedione use is associated with improved all-cause mortality compared with sulfonylureas among non-insulin dependent diabetic hemodialysis patients. Abstract presented at American Society of Nephrology 2014 Annual Kidney Week Meeting; Atlanta, Georgia. [Google Scholar]
- 95.Raskin P. Sodium-glucose cotransporter inhibition: therapeutic potential for the treatment of type 2 diabetes mellitus. Diabetes Metab Res Rev. 2013;29(5):347–356. doi: 10.1002/dmrr.2403. [DOI] [PubMed] [Google Scholar]
- 96.Kojima N, Williams JM, Takahashi T, Miyata N, Roman RJ. Effects of a new SGLT2 inhibitor, luseogliflozin, on diabetic nephropathy in T2DN rats. J Pharmacol Exp Ther. 2013;345(3):464–472. doi: 10.1124/jpet.113.203869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McMurray SD, Johnson G, Davis S, McDougall K. Diabetes education and care management significantly improve patient outcomes in the dialysis unit. Am J Kidney Dis. 2002;40(3):566–575. doi: 10.1053/ajkd.2002.34915. [DOI] [PubMed] [Google Scholar]
- 98.Okada T, Nakao T, Matsumoto H, Shino T, Nagaoka Y, Tomaru R, Wada T. Association between markers of glycemic control, cardiovascular complications and survival in type 2 diabetic patients with end-stage renal disease. Intern Med. 2007;46(12):807–814. doi: 10.2169/internalmedicine.46.6355. [DOI] [PubMed] [Google Scholar]
- 99.Shurraw S, Majumdar SR, Thadhani R, Wiebe N, Tonelli M. Glycemic control and the risk of death in 1,484 patients receiving maintenance hemodialysis. Am J Kidney Dis. 2010;55(5):875–884. doi: 10.1053/j.ajkd.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 100.Shima K, Komatsu M, Kawahara K, Minaguchi J, Kawashima S. Stringent glycaemic control prolongs survival in diabetic patients with end-stage renal disease on haemodialysis. Nephrology (Carlton) 2010;15(6):632–638. doi: 10.1111/j.1440-1797.2010.01273.x. [DOI] [PubMed] [Google Scholar]
- 101.Kim Y, Park JC, Molnar MZ, Shah A, Benner D, Kovesdy CP, Kopple JD, Kalantar-Zadeh K. Correlates of low hemoglobin A1c in maintenance hemodialysis patients. Int Urol Nephrol. 2013;45(4):1079–1090. doi: 10.1007/s11255-012-0208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]