Abstract
Several refractive and therapeutic treatments as well as several ocular or systemic diseases might induce changes in the mechanical resistance of the cornea. Furthermore, intraocular pressure measurement, one of the most used clinical tools, is also highly dependent on this characteristic. Corneal biomechanical properties can be measured now in the clinical setting with different instruments. In the present work, we review the potential role of the biomechanical properties of the cornea in different fields of ophthalmology and visual science in light of the definitions of the fundamental properties of matter and the results obtained from the different instruments available. The body of literature published so far provides an insight into how the corneal mechanical properties change in different sight-threatening ocular conditions and after different surgical procedures. The future in this field is very promising with several new technologies being applied to the analysis of the corneal biomechanical properties.
1. Introduction
Corneal biomechanics is a branch of science that studies deformation and equilibrium of corneal tissue under the application of any force [1]. The structure and hence the properties of a soft tissue, such as the cornea, are dependent on the biochemical and physical nature of the components present and their relative amounts. The mechanical properties of a tissue depend on how the fibres, cells, and ground substance are organized into a structure [2]. Collagen and elastin are responsible for the strength and elasticity of a tissue, while the ground substance is responsible for the viscoelastic properties. All these terms are important because the cornea is considered a viscoelastic material and some devices try to measure and even differentiate between the different components of the biomechanical behavior of the living corneal tissue [3]. In the specific case of the human cornea, collagen in Bowman's layer and stroma accounting for over 80% of the dry weight of the cornea would be the major contributor to corneal elasticity. The ground substance, formed mostly by proteoglycans and keratocytes or fibroblasts, would provide the viscous behaviour. The corneal epithelium accounting for 10% of the central corneal thickness could also contribute to the viscous behaviour. It is important to bear in mind that the corneal epithelium is easily deformable and is the reference surface for most of the biomechanical corneal measurements.
Over the past two decades, researchers have developed a variety of techniques that can alter corneal surface for refractive purposes or even for halting disease progression in corneas with mechanical decompensation. Beside geometric corneal parameters, the additional influence of the biomechanical corneal properties has received little attention, mostly because of the lack of appropriate in vivo measurement techniques. However, in recent years, increasing interest has arisen in corneal biomechanics to predict corneal response to surgical or therapeutic interventions and to assist in the detection of early keratoconus [4–6]. Additionally, increasing interest has also arisen in corneal biomechanical properties and glaucoma once corneal biomechanics have been shown to influence intraocular pressure (IOP) measurements and may be also indicative of ocular globe biomechanics that could also be predictive of glaucoma susceptibility [7].
Corneal biomechanics have been assessed in in vitro studies by measuring stress-strain and Young's modulus in isolated corneas [8]. In the recent years, two devices have been marketed: the Ocular Response Analyser (ORA, Reichert, Depew, NJ) since 2005 and the Corneal Visualization Scheimpflug Technology (Corvis ST, Oculus, Wetzlar, Germany) since 2011. Many studies covering a wide range of topics have been conducted and published using the ORA.
The aim of the present review is to provide an overview of published results on corneal biomechanics obtained with ORA under different ocular and systemic conditions. Knowledge accumulated to date on this field will potentially help the ophthalmic community to gain a better understanding of the changes that the corneal tissue undergoes during different ocular and systemic conditions as well as to predict the outcomes of therapeutic and refractive therapies. New technologies under development will also be discussed briefly since there is currently a wide range of instrumentation under development to provide a better understanding of the biomechanical nature of the cornea and its implications in visual care, with particular relevance to the detection and management of sight-threatening conditions.
2. Biomechanical Descriptors and Their Physical Meaning
To better understand the results of corneal biomechanical measurements, it is important to remember the meaning of some corneal properties such as elastic, viscous, or viscoelastic response, hysteresis, and stiffness, among other concepts.
The elastic response of a material is attributed to the instantaneous and reversible deformation under an external load [2]. In elastic materials, the deformation is proportional to the force applied and it is recovered instantly upon unloading. Thus, the stress-strain relationship would be a straight line [9]. Figure 1(a) shows the typical stress-strain diagram of an elastic material. The constant of proportionality between stress and strain is the elastic modulus, also called Young's modulus. Young's modulus is defined as the ratio of the stress (load per unit area) and the strain (deformation/displacement per unit length) [10]. A high modulus indicates a stiffer material (i.e., not easy to bend). This also leads us to the definition of resistance, which is the capacity of a material to hold stress without deformation.
Corneal Young's modulus, measured in vitro, varies from 0.1 to 57 MPa [8, 11–20] that might be explained by variations in testing conditions and methods used. More recently, Hamilton and Pye [21], using the Orssengo-Pye algorithm, reported on 100 healthy eyes with mean Young's modulus being 0.29 ± 0.06 MPa (range 0.13 to 0.43 MPa). Modulus was positively correlated with the IOP measured with GAT, assuming that Young's modulus itself affects the IOP measurement.
A material shows a viscous behaviour when the deformation velocity is faster than the relaxation rate. The slow relaxation is due to configurational rearrangement of the material during deformation [2].
-
Viscoelastic materials exhibit elastic and viscous behaviour at the same time, so they present characteristics of elastic and viscous materials [2]. Figure 1(c) shows the typical stress-strain diagram of a viscoelastic material. Their particular characteristics make it possible to define characteristic properties including one known as “hysteresis”.
- Hysteresis in viscoelastic materials under periodic loading and unloading, curves in the stress-strain diagram (Figure 1(c)) are not coincident with each other; the gap between them is called hysteresis [22].
- The energy stored over one full loading and unloading cycle in a material is zero since the material returns to its initial configuration (elastic behavior). The area within the hysteresis loop represents the energy per volume dissipated in the material per cycle [23].
Figure 1.
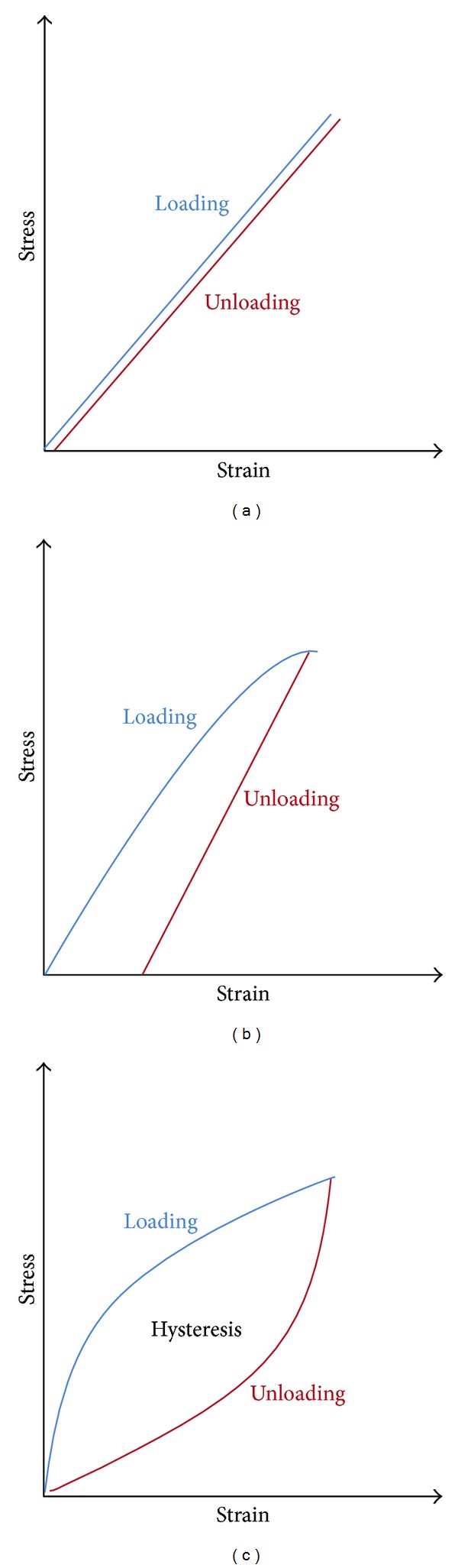
Stress-strain response diagrams of different materials showing elastic (a), plastic (b), and viscoelastic behaviour (c).
2.1. Parameters Derived from Ocular Response Analyzer
The ORA is a noncontact tonometer introduced in clinical practice in 2005 [3]. It uses a rapid air pulse to indent the cornea and an electrooptical system to record corneal deformation. It records mainly two applanation measurements: one while the cornea moves inward, reaching a first applanation, when the first pressure (P 1) is registered and the other as the cornea recovers from a slight concavity as the air pump decreases pressure at an inverse rate so that the cornea moves outward passing through a second applanation (P 2). Therefore, these two values, P 1 and P 2, indicate the pressure necessary to flatten the cornea during the loading and unloading cycle (Figure 2).
Figure 2.
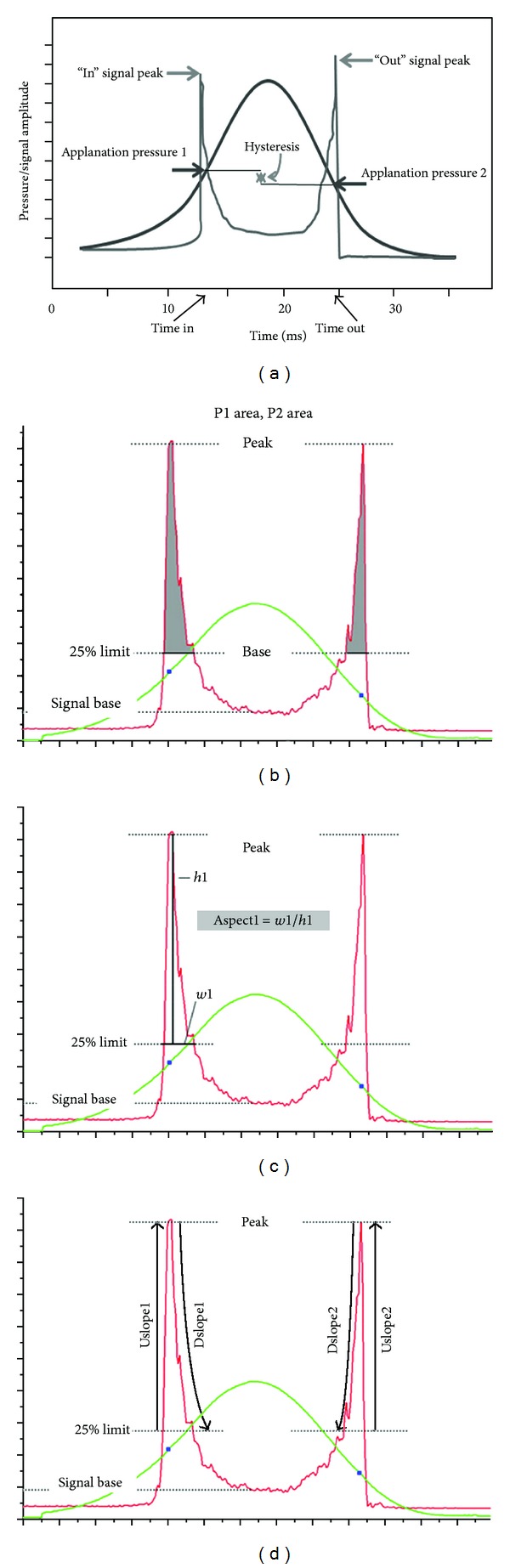
Applanation and pressure plot as determied by the ORA (Ocular Response Analyzer). See text for definition of the different variables indicated in the graphs.
Thus, below we define one by one the terms and parameters that are relevant to the understanding and interpretation of the outcomes obtained by the ORA according to the literature.
P 1 and P 2: air pressures corresponding with the two applanation states of the cornea.
Corneal hysteresis (CH) is considered an indicator of corneal viscosity and is obtained by the difference between the 2 pressures: CH = P 1 − P 2 [3].
The corneal resistance factor (CRF) is considered an indicator of the overall resistance of the cornea and is expressed by the equation: CRF = (P 1 − 0.7∗P 2) [24]. It is significantly correlated with central corneal thickness (CCT) and Goldmann applanation tonometry (GAT) [3]. It has been also suggested that the CRF could be mainly related to the elastic properties of the cornea [25]. Other authors suggested modifications on the original formula to CRF = k 1∗(P 1 −0.7∗P 2) + k 2, where k 1 and k 2 are constants [26, 27]. Moreover, some authors evaluated the difference between CH and CRF, but the meaning of this “new” parameter [28, 29] is not clear.
IOPg is an IOP value equivalent to GAT, which is an average of the two pressure values measured by ORA, P 1, and P 2 and obtained by the following equation: IOPg = (P 1 + P 2)/2 [24].
IOPcc is a new IOP value called Corneal Compensated IOP and is obtained by the equation IOPcc = P 1 − 0.43P 2. It is less affected by corneal properties than by the IOP obtained with other tonometers and it is not correlated with the CCT [24] but it is correlated with CH [30, 31].
Corneal constant factor (CCF) is claimed to be an IOP-independent corneal factor introduced by Kotecha et al. [26] and was derived from the changes of P 1 and CH for every 1 mm Hg of change in GAT IOP. It describes an IOP-independent biomechanical property that increases with thicker CCT and decreases with aging and yet explains more of the interindividual variation in GAT IOP than does CCT. It is very similar to CRF proposed by Reichert and is expressed by the equation: CCF = P 1 −0.79∗P 2.
The deformation signal waveform produced by the corneal deformation signal (characteristic shape illustrated in Figure 2) can provide a unique description of each eye. Further analysis of the waveform signal delivered by the electrooptical system of the instrument has provided more parameters with potential interest to allow a refined evaluation of the corneal properties [32]. Recently, 37 new parameters were derived from the new ORA software allowing a detailed analysis of the deformation signal waveform. Each one of these parameters describes a morphological feature of the waveform and 23 parameters are derived from the upper 75% of applanation peak height and 14 are derived from the upper 50% of the applanation peak height (Figure 2). These new parameters are defined in Appendix A. Most of these parameters depend on P 1 and P 2 defined at the beginning so, in some way, these parameters could be intrinsically linked and their clinical significance and the manner in which these individual parameters represent biomechanical properties are currently unknown. Several studies have investigated the clinical relevance of the new waveform parameters and reported that they could be more useful in diagnosis and prognosis after refractive surgery, and as stated in the following sections, some of these parameters seem to be promising as being more sensitive than others to detect corneal changes in specific corneal conditions [28, 33–36].
3. Factors Affecting Corneal Biomechanical Properties
The possibility to evaluate the biomechanical properties of the cornea provides a new diagnostic tool that will allow detecting differences in corneal biomechanics between normal eyes and pathological eyes and eventually detecting weaker corneas at a subclinical state before they evolve in some kind of ectasia or avoiding postsurgical ecstatic disease. Since the introduction of ORA in clinical practice, many research studies have been conducted looking for associations between both CH and CRF and different parameters like age, corneal thickness, IOP, progress of glaucoma, or presence and severity of a given condition such as keratoconus [37]. According to Luce [3], corneas with low CH are less capable of absorbing energy than normal eyes and they may be candidates for several ocular diseases. Moreover, low CRF indicates that the overall corneal rigidity is lower than normal.
Table 1 shows results of different studies on healthy eyes. It is observed that both CH and CRF vary in a rather wide range in the normal population and that a comparison between studies for both parameters is difficult.
Table 1.
Summary of studies of corneal hysteresis (CH) and corneal resistance factor (CRF) in healthy patients.
| Study | Number of eyes | Number of patients | Sample country | Age (years) |
CH (mmHg) | CRF (mmHg) |
|---|---|---|---|---|---|---|
| Shah et al. 2006 [50] | 207 | — | United Kingdom | 62.1 ± 18.1 | 10.7 ± 2.0 | 10.3 ± 2.0 |
| Kirwan et al. 2006 [38] | 91 | 42 | Ireland | (4–18) | 12.5 ± 1.4 | — |
| Shah et al. 2007 [39] | 207 | — | United Kingdom | 62.1 ± 18.1 | 10.7 ± 2.0 | — |
| Lu et al. 2007 [73] | — | 20 | China | 19.7 ± 1.1 | 11.5 ± 1.4 | 9.6 ± 1.9 |
| Lam et al. 2007 [24] | — | 125 | China | 23.1 ± 3.3 | 10.9 ± 1.5 | 11.0 ± 1.7 |
| Lim et al. 2008 [40] | — | 271 | Singapore | 14.0 ± 0.9 (12–15) | 11.8 ± 1.6 | 11.9 ± 1.7 |
| Touboul et al. 2008 [65] | — | 122 | France | 48.0 (17–80) | 10.3 | 11.1 |
| Song et al. 2008 [59] | — | 1233 | China | 14.7 ± 0.8 | 10.7 ± 1.5 | — |
| Kirwan and O'Keefe 2008 [6] | 84 | 84 | Ireland | 36 ± 10 | 10.8 | — |
| González-Meijome et al. 2008 [68] | 58 | 58 | Portugal | 22.95 ± 3.92 | 10.7 ± 1.9 | 11.4 ± 1.5 |
| Shen et al. 2008 [41] | 90 | China | 33.7 ± 12.4 | 11.11 ± 1.49 | ||
| Chen et al. 2009 [77] | — | 20 | Hong Kong | 24.1 ± 2.6 | 11.1 ± 1.1 | 10.7 ± 1.3 |
| Franco and Lira 2009 [30] | 63 | — | Portugal | 33.2 ± 12.2 | 10.8 | 10.6 |
| Kamiya et al. 2009 [43] | 204 | 204 | Japan | 46.7 ± 19.4 | 10.1 ± 1.5 | 10.1 ± 1.6 |
| Abitbol et al. 2010 [42] | — | 75 | France | 61.44 ± 10.6 (45–85) | 10.46 ± 1.6 | — |
| Kaushik et al. 2012 [90] | — | 71 | India | >18 | 9.5 ± 1.4 | 9.2 ± 1.5 |
CH: corneal hysteresis; CRF: corneal resistance factor.
3.1. AGE
Several studies investigated the associations between changes in corneal biomechanical parameters and aging. Several studies found no significant differences in ORA measurements with ageing [38–42]. Lim et al. [40], in a study with 271 children, reported that CH and CRF did not vary significantly with age but the range of ages was quite narrow. Notwithstanding, as the authors observed, the values of CH and CRF measured were slightly higher than those in other adult studies. The same was observed by Kirwan et al. [38] in children and adolescents who also found no correlation between age and CH. However, when compared with other studies, the values of CH were again slightly higher. On the other hand, some studies have shown that CH significantly decreases with age [4, 26, 43–45]. Kamiya et al. [43] evaluated 204 eyes of healthy subjects and found a small but statistically significant negative correlation between CH and CRF with age without significant differences in central corneal thickness (CCT) or IOP across the sample. Ortiz et al. [4] only found significant differences in CH and CRF between subjects younger than 14 and older than 60, but a linear correlation between these two biomechanical parameters and ageing did not exist. Kotecha et al. [26] observed a reduction in CH of approximately −0.28 mm Hg/decade, while Foster et al. [46] found that the CRF declined significantly with age at a rate of −0.31 mm Hg/decade, as did CH by −0.34 mm Hg/decade.
In any case, due to the potential limitations of these studies, we should be careful to extrapolate their results to the general population. For instance, in one of these studies the sample was quite limited, with only fifteen subjects [44]. In another study, the changes are possibly confounding because of the proportion of the participants affected by ocular hypertension, glaucoma, or pigment dispersion syndrome [26]. Due to age-related changes in corneal structure such as an increase in collagen fibril diameter or intermolecular Bragg spacing [47], it would be expected that corneal biomechanical properties change with ageing. In fact, ex vivo studies have shown an increase in corneal stiffness with ageing [48] and that Young's modulus of the human cornea approximately doubles between the ages of 25 and 100 [49]. Considering this, if the CRF is a real indicator of corneal rigidity, it should change with ageing as well. Nevertheless, due to the intersubject variability and the differences among the results published in the different studies, we cannot conclude, based on present data, that CH and CRF parameters are able to confirm in vivo and in the clinical routine the expected changes towards a stiffening of the cornea.
3.2. Central Corneal Thickness (CCT)
Several studies investigated the potential effect of CCT on the biomechanical properties of the cornea measured with ORA. In fact, many studies reported a positive correlation between CCT and CH [3, 24, 30, 42, 50, 51] and also with CRF [24, 30, 40, 44, 51]. These studies included healthy subjects from different races/ethnicities and with a wide range of age. Recently, Leite et al. [52] found that black subjects had lower CH values compared to white subjects, but although they attributed those differences in CH to differences in corneal thickness between the two groups, they did observe a statistical trend towards lower CH among black subjects even when adjusting for CCT. A similar result was observed in a study with a strong statistical power by Haseltine et al. [53].
These results are in agreement with the expected response because a thinner cornea will be easier to deform, while a thicker healthy cornea containing more collagen fibers and ground substance will present a higher resistance against deformation and a higher damping capacity. Consequently, the stronger the corneal tension, the faster the cornea recovers its original position following deformation. CCT also suffers circadian changes and this might affect the biomechanical properties measured. There are a couple of articles where the 24-hour changes of CCT and corneal biomechanical properties were analysed [44, 54]. Despite a significant change between the nocturnal and diurnal CCT values, a significant change in the CH and CRF was not observed. These results could be explained considering that nocturnal CCT increase is related to increase in corneal hydration instead of collagen fibril or ground substance changes that would potentially reflect more directly on the biomechanical behaviour of the cornea.
3.3. Refractive Error and Axial Length
The degree of myopia is correlated with axial length (AL). Furthermore, it has been claimed that longer eyes are associated with flat corneal curvature and thinner corneas [55]. Furthermore, longer eyes had thinner sclera walls and possible thinner choroidal structure. In this way, according to previous section, if the highly myopic eyes have thinner corneas and if corneal biomechanical response might be somewhat related to the whole-eye biomechanical response, it would be expected that that more myopic eyes have lower CH values. It has been the goal of some studies to test the hypothesis that the weaker scleral structure of highly myopic eyes might be reflected and quantified in some way through the biomechanical analysis of the cornea.
Studies performed in Chinese subjects [41, 56] and Caucasian subjects [57] with a wide range of refractive errors observed a significant negative correlation between CH and myopia. Shen et al. [41] found lower CH in highly myopic eyes (−9 D) and no statistically significant differences in CH between emmetropes and low myopes (+0.25 to −2.75 D) or moderate myopes (>−3.00 to −6.00 D). Similar results were reported by Jiang et al. [56], but the reason of this decrease was not fully explained. However, although variation was not observed neither in CCT nor in CRF among subjects with different myopia degree, it is possible that the changes are related to the different characteristics of the cornea rather than weaker sclera structure which is characteristic of the highly myopic eyes. Recently, Xu et al. [58], in a study of subjects with myopic anisometropia, reported a significant lower CH in high myopic eyes compared to contralateral normal eyes. In this study, the difference in AL between the two eyes that resulted in anisometropia and CH was correlated with AL and CCT in high myopic eyes, whereas in the contralateral eyes, it was only correlated with CCT. Additionally, since differences in IOPg and IOPcc between the high myopic and contralateral eye were not observed, the authors suggest that the difference in AL does not occur by virtue of higher IOP, but it is possible that eyes with lower CH and thinner scleral structure may be easier to elongate [58, 59]. However, these studies do not permit elucidation if the lower CH and thinner scleral structure are the cause or the consequence of the increasing myopia of those eyes.
Yet, despite above studies indicate that the mechanical strength of the anterior segment of the eye is somehow compromised in high myopia, other previous studies did not show a correlation between refractive error and ORA measurements [40, 59, 60]. The study conducted by Radhakrishnan et al. [60] evaluated 95 normal myopic adult subjects (19 to 48 years) and found that CH was not significantly correlated with refractive error, while CRF showed a statistically significant but very weak correlation with spherical equivalent refractive error (r 2 = 0.04). However, the mean spherical refractive error was −1.78 ± 2.26 D and both parameters showed a considerable scatter across the sample under analysis.
3.4. Intraocular Pressure (IOP)
The Goldmann applanation tonometer (GAT) is the reference method to measure the IOP but when the IOP is measured with GAT it is assumed that the cornea is uniformly thick and perfectly elastic and behaves like a thin and perfectly flexible membrane [61]. Actually, none of these assumptions applies to the anatomical structure and physical behaviour of the living cornea under applanation forces. The pressure required to applanate the cornea depend on the IOP and the corneal rigidity [42], and it is well known that the IOP measures are influenced by CCT with thicker corneas requiring stronger force to applanate than thinner corneas, independent of IOP [10]. Many published articles have proposed linear correction factors to convert measured IOP into “true” IOP, on the basis of CCT. However, reported correction factors are different and mostly dependent on the population under study and can lead to corrections that may be wrong in magnitude and in direction such as correcting down when the true pressure is actually higher [62]. In fact, corneal biomechanical properties seem to be stronger predictors of IOP measurement error than does CCT alone [10]; this might explain the success of the ORA over the last 8 years for the IOP measurement in several corneal conditions.
IOPg provided by ORA is analogous to standard noncontact tonometry IOP measurements whereas IOPcc takes into account the biomechanical properties and is independent of the CCT as explained above. Although some studies find no mean difference between GAT and both ORA IOP measurements [24, 31, 63], other studies found poor agreement between GAT and IOPg and IOPcc with a significant overestimation of IOPg and IOPcc compared to GAT [27, 64]. Medeiros and Weinreb [31] found that GAT IOP was significantly correlated with CCT and significantly influenced by CRF, while IOPcc was not, and similar results have been confirmed by others [27, 64, 65]. Therefore, the effect of CCT on IOP overestimation may be explained by CRF and the resistance against deformation of the cornea which is also higher in eyes with higher IOP values [27]. In contrast, some studies reported the lack of association between CH and both GAT and IOPg [30, 64, 65], suggesting that CH is independent of IOP, while other studies suggest a relationship between CH and IOP. CH has been shown to decrease as the IOPcc increases [30, 46, 66, 67]. Kamiya et al. [66] found IOP as a significant explanatory variable relevant to CH, while González-Meijome et al. [68] found a significant correlation between changes in IOP and changes in CH during the day in healthy eyes. Also, CH has been shown to increase when IOP was lowered to normal range in patients with chronic primary angle-closure glaucoma [69].
Considering the previous results and despite some controversy, it is expected that in corneas with higher CH and higher CRF and therefore higher resistance to deformation, the values of GAT IOP or IOPg may be higher than the actual values and IOPcc could be a more reliable measure in those cases. The opposite might hold true in cases of lower CH and lower CRF where the actual IOP might be higher than actually measured by conventional methods. Again, IOPcc might provide a more realistic measure of the intraocular pressure.
3.5. Soft Contact Lens Wear
Reduced oxygenation of the cornea during contact lens (CL) wear is known to produce corneal edema that is reflected in an increase in corneal thickness (swelling). In fact, in a recent study, it was observed that the myopic subjects wearing soft contact lenses have higher values of CH and CRF than noncontact lens wearers [70]. The corneal swelling response with contact lens wear and eye closure averaged from ∼3% to ∼10% [71, 72] and some studies have analysed these effects on ORA measurements [70, 73, 74]. Lau and Pye induced corneal edema wearing soft contact lens for three hours and found no change in CH even with 13.1% corneal swelling, while CRF was elevated by a maximum of 0.6 mm Hg immediately after lens removal and was followed by a gradual recovery to normal values. Additionally, there were significant increases in IOPg but not in IOPcc and there were significant but weak correlations between changes of CCT and IOPg and IOPcc and CRF. Lau and Pye [74] found that CH and CRF respond to corneal swelling in dissimilar ways: CH was reduced by 0.6 mm Hg immediately after lens wear before returning to baseline, while CRF was elevated by a maximum of 0.6 mm Hg. In addition, the ability of CCT to predict both CH and CRF was significantly different between control and monocular closed-eye contact lens wear and the GAT overestimation observed is associated with an overall increase in CRF caused by small amounts of corneal swelling. Differences in the study population as well as in the amount of corneal swelling induced are likely contributors to the differences in the results between the two studies. However, the results suggest that ORA-generated parameters may be different in subjects with and without contact lens wear when significant amounts of edema are present. This kind of response, commonly observed in aphakic patients with overnight wear of thick CL, is not expected with regular use of silicone hydrogel contact lenses under daily wear conditions by patients within the normal range of refractive errors.
3.6. Orthokeratology
Orthokeratology (OK) is a technique that uses special gas permeable CL to temporarily reduce myopia by flattening the cornea. Therefore, the epithelial corneal thickness profile is changed and the cornea is significantly flattened by the use of these CL [75, 76] and the corneal biomechanical properties could be affected by these changes. Biomechanical properties of the cornea may help to understand the different responses to OK among different subjects. A study published in 2008 [5] investigated the changes of ORA measurements, CCT, and topography in subjects three hours after wearing OK lenses and three hours after removing the CL in order to assess the effect of corneal biomechanical properties on response (corneal flattening) and recovery (corneal steepening) during OK lens wear and after removal, respectively. The authors found that corneas with high values of CH showed a slower response and slower recovery to the OK treatment in the short-term treatment (3 hours of treatment). In another study, during short-term OK treatment, CRF was shown to decrease with increasing duration of lens wear, while there was no significant change in CH [77]. On the other hand, a significant decrease in CH and CRF was reported within the first week of OK treatment [78]. However, CRF and CH returned to original values and remained unchanged thereafter. According to the authors, the early reduction in CH and CRF may be due to a temporal response of reshaping of the corneal surface, rather than changes in the corneal microstructure. This may explain why there is a trend for CH and CRF to be reduced during the first month of treatment and after 1 year of treatment; when this is interrupted, CH and CRF show a trend to return to baseline values [79].
The knowledge of these associations could help to have a better predictability of the OK effect [5, 80] and then to choose the suitable patients to undergo OK treatment or to predict the speed of onset and recovery of the effect.
3.7. Refractive Surgical Treatments
3.7.1. Refractive Surgery
Laser corneal ablation might have significant implications on corneal mechanical resistance. Several studies showed invariably a significant reduction of CH and CRF by about 1 to 3 mm Hg approximately after different laser refractive treatments [4, 6, 25, 30, 81–87]. The results from these studies are summarized in Table 2. Studies comparing different laser refractive techniques showed a higher decrease in both CH and CRF in LASIK eyes when compared with photorefractive keratectomy (PRK) [83]. Similar decrease in CH has been documented for LASIK and laser-assisted subepithelial keratectomy (LASEK) [6]. This biomechanical effect was correlated with deeper ablation because more central collagen and matrix material would be removed [4, 81] or with the potential effect of flap preparation that itself causes a reduction in both CH and CRF [82, 88, 89]. Ortiz et al. [4] found a moderate correlation between the refractive error correction and the change in CH (r = 0.5, P = .007) and CRF (r = 0.6, P = .001) in myopic LASIK, while a smaller decrease in CH and CRF was found in hyperopic LASIK eyes than in myopic LASIK and LASEK eyes, supporting the predominant effect of tissue ablation [89]. Gatinel et al. [88] found a reduction in both CH and CRF with microkeratome-assisted flap creation alone. Qazi et al. [82] found that despite similar changes in CH and CRF in the myopic LASIK and myopic LASEK groups, there were significantly greater postoperative changes in the ORA waveforms in the LASIK groups than in the LASEK group with the amplitude of Peak 1 being less reduced in the group of LASEK, suggesting that the creation of a flap has a greater effect on these waveform parameters than the depth or location of the stromal ablation. Similar results were reported by Franco and Lira [30] who found that, as a result of induced changes in viscous and elastic properties by LASIK, the time needed for the first applanation of the cornea (Time in) was higher in normal than in post-LASIK eyes and that the post-LASIK eyes needed more time to recover their shape (Time out parameter).
Table 2.
Summary of studies evaluating the influence of refractive surgery on biomechanical parameters.
| Author | Sample (eyes) | Outcomes | |||
|---|---|---|---|---|---|
| CH (mmHg) | CRF (mmHg) | ||||
| Pepose et al. (2007) [25] | 66 LASIK | Pre | Post | Pre | Post |
| 9.7 ± 1.8 | 8.0 ± 1.6 | 9.5 ± 1.9 | 6.7 ± 1.7 | ||
|
| |||||
| Ortiz et al. (2007) [4] | 65 LASIK | Pre | Post | Pre | Post |
| 10.44 ± 1.74 | 9.3 ± 1.9 | 10.07 ± 1.97 | 8.1 ± 1.8 | ||
|
| |||||
| Hamilton et al. (2008) [81] | 32 LASIK (flap with femtosecond): | CH decreased 1.9 mmHg | |||
| 33 LASIK (flap with microkeratome): | CH decreased 2.2 mmHg | ||||
| 32 PRK: | CH decreased 2.3 mmHg | ||||
|
| |||||
| Franco and Lira (2009) [30] | 63 control | 10.8 ± 1.53 | 10.6 ± 1.71 | ||
| 20 LASIK | 8.5 ± 1.22 | 7.7 ± 0.97 | |||
|
| |||||
| Qazi et al. (2009) [82] | Pre | Post | Pre | Post | |
| 15 LASEK | 9.06 ± 1.56 | 7.16 ± 1.99 | 8.61 ± 1.76 | 5.95 ± 2.41 | |
| 14 LASIK | 10.00 ± 1.77 | 8.57 ± 2.25 | 9.87 ± 1.97 | 7.35 ± 2.49 | |
|
| |||||
| Kamiya et al. (2009) [84] | 36 LASIK | Pre: | 10.6 | Pre: | 10.0 |
| Post: | Post: | ||||
| 1 week: | 8.6 | 1 week: | 7.3 | ||
| 1 month: | 9.0 | 1 month: | 7.6 | ||
| 3 months: | 9.0 | 3 months: | 7.8 | ||
| 6 months: | 8.9 | 6 months: | 7.7 | ||
|
| |||||
| Kamiya et al. (2009) [83] | Pre | Post | Pre | Post | |
| 27 LASIK | 10.8 | 8.6 | 10.3 | 7.7 | |
| 31 PRK | 10.8 | 9.2 | 10.3 | 8.4 | |
|
| |||||
| Shah and Laiquzzaman (2009) [85] | 110 LASIK | Pre: | Post: | Pre | Post |
| 11.4 | 9.2 | 10.0 | 7.6 | ||
|
| |||||
| Chen et al. (2010) [86] | 60 LASIK | Pre: | 10.59 ± 1.02 | Pre: | 8.80 ± 1.45 |
| Post: | Post: | ||||
| 1 day: | 8.16 ± 0.84 | 1 day: | 5.02 ± 1.16 | ||
| 10 days: | 8.14 ± 0.77 | 10 days: | 4.96 ± 0.98 | ||
| 1 month: | 8.33 ± 0.88 | 1 month: | 5.08 ± 1.31 | ||
| 3 months: | 8.47 ± 0.78 | 3 months: | 5.26 ± 0.96 | ||
|
| |||||
| Ryan et al. (2011) [87] | 102 epi-LASIK | Pre: | 10.22 | Pre: | 10.01 ± 1.80 |
| Post: | Post: | ||||
| 1 month: | 8.17 ± 1.25 | 1 month | 7.82 ± 1.68 | ||
| 3 months: | 8.46 ± 1.44 | 3 months: | 8.03 ± 1.85 | ||
| 6 months: | 8.63 ± 1.31 | 3 months: | 7.77 ± 1.50 | ||
| 12 months: | 8.53 ± 1.49 | 3 months: | 7.80 ± 1.66 | ||
CH: corneal hysteresis; CRF: corneal resistance factor.
Studies reporting the time course of ORA parameters after different surgical techniques showed that the largest changes occurred within the first few weeks after surgery and then became nearly stable or even showed a slight recovery in the medium and longer term [84, 86, 87]. Surgically induced corneal ectasia is a rare complication of refractive surgery and is thought to be a result of biomechanical decompensation due to an insufficient residual stromal bed thickness after the surgery or when surgery is performed on unidentified subclinical keratoconic cornea. Thus, the possibility of using ORA parameters for assisting in the detection of corneas at risk has been very promising since the ORA was marketed. Although a low CH (<8 mm Hg) might be a predictive index of a preectatic conditions [3, 33], the overlap in the distribution of both CH and CRF values within the normal population does not support a role for CH and CRF measurement as single predictors to detect early ectasia or to predict its onset before surgery [91]. Instead, waveform analysis of ORA signals [33, 82, 92] has shown that the morphology of the signal may provide additional information. For instance, in a case of iatrogenic ectasia after LASIK, Kerautret et al. [33] found a lower Peak 1 height in the ectatic eye than in the fellow nonectatic eye, despite the similar CH and CRF values in the 2 eyes. These findings may suggest that a higher Peak 1 is associated with a stiffer cornea [82]. Considering that recent studies seem to indicate that the new ORA parameters represent a significant improvement over CH and CRF alone, more research is needed to confirm and improve the sensitivity and specificity for preoperative detection of at-risk corneas.
3.7.2. Cross-Linking (CXL)
Cross-linking (CXL) is a minimally invasive procedure which presumably induces the formation of new molecular bonds between the corneal collagen fibrils and lamellae using riboflavin and UV light [93]. This procedure of reinforcing the collagen meshwork with CXL has shown to be effective in the treatment of surgically induced ectasia and in halting progression of keratoconus [94–96]. In corneal CXL, the cornea is stiffened and a high increase is observed in Young's modulus by nearly 300% [93]. It would be expected that the biomechanical properties of the cornea will change as a result of the treatment, particularly corneal rigidity parameters. Differences in CH and CRF were observed during the first weeks after CXL treatment that returned to baseline values later. The effect of matrix reorganization or CCT changes immediately after the procedure may explain these differences in CH and CRF [97, 98]; however, sustainable changes in CH and CRF parameters alone that can be correlated with the assumed increase in corneal stiffness induced by CXL [34, 36, 97, 99] were not found and the clinical results did not confirm the ex vivo results. From the analysis of the new ORA parameters based on waveform signal analysis, a significant increase (35%) in area under Peak 1 and Peak 2 was observed after six months of treatment, suggesting that this can be the result of a modified corneal surface after CXL, which provides better reflectivity due to an improvement of corneal homogeneity [34, 97]. These recent studies seem to indicate that additional parameters derived from signal analysis provide supplemental information to evaluate the potential positive effect of CXL and to measure the long-term effects of this procedure.
3.7.3. Intrastromal Corneal Ring Segments
Intrastromal corneal ring segments (ICRS) are primarily used for the treatment of primary keratoconus [100] and secondary keratectasia following refractive surgery [101]. The insertion of the ICRS induces a flattening of the central cornea by adding extra material within the corneal paracentral area [102], improving regularity of the corneal shape, and preventing additional degradation of vision [103]. Knowledge of the biomechanical properties of the corneal might help to decide the best treatment approach, predict the success of the treatment, and eventually monitor the postsurgical corneal behaviour. No significant differences were found in CH in the short-term (<3rd month) postoperative period [104–106] which may indicate that the ICRS alter corneal curvature without changing the viscoelastic response of the corneal tissue. A study conducted on 20 patients with keratoconus showed a stable corneal flattening and a decrease of the astigmatism with no statistically significant changes in ORA parameters, 18 months after ICRS implantation [107]. Better visual outcomes could be expected for corneas with lower biomechanical corneal resistance due to easier deformation by the ring implantation. Piñero et al. [108] reported significant changes in CH, 6 months after ICRS implantation, and the authors suggested that these changes may limit the prediction of the ring segment effect in the long term. However, this hypothesis could not be confirmed by a recent study [109], contradicting previous results obtained by the same authors. Although the authors claim in the second publication that prediction of visual acuity (VA) by ORA parameters is feasible in the short term, they could not confirm that in the first study using the same follow-up time of 6 months. Regarding CRF value, significant transient decrease was found during the first 3-month period after the femtosecond laser-assisted ICRS implantation with no significant changes thereafter [105]. New waveform parameters such as the amplitude Peak 2 [104], aplhf, uslope11, w11, path11, time1, and deltatime [110] showed significant differences with respect to the preoperative conditions but those changes were not attributed to a modification of the biomechanical properties induced by the treatment but rather to corneal stabilization. Interestingly, from the waveform analysis provided by Ambrosio et al. [111], it has been recently reported that the corrected and uncorrected distance visual acuity improved more as the pre-ICRS implant biomechanical properties were weaker or less resistant before treatment. This might provide useful information to predict the visual outcomes of ICRS implantation in keratoconus [111].
3.7.4. Keratoplasty
Studies that evaluated corneal biomechanics by ORA showed that corneas after penetrating keratoplasty (PK) or deep anterior lamellar keratoplasty (DALK) present weaker CH and CRF than normal corneas [29, 112–114]. Additionally, Yenerel et al. [112] found that CH and CRF were higher in PK eyes than in forme fruste (FF) or advanced keratoconus (KC) eyes and both CH and CRF parameters approach the range of normal eyes after corneal transplantation. On the other hand, Shin et al. [29] analysed the results of 26 subjects that had undergone PK for different reasons (bullous keratopathy, herpes keratitis, trauma, etc.) in one eye and compared the results with the contralateral nonoperated eye. They reported lower CH and higher CRF post-PK compared with the fellow healthy eye, although these differences were not statistically significant. The effect of different keratoplasty techniques showed that post-PK eyes had lower CH and CRF when compared with post-DALK eyes and post-DALK eyes had CH and CRF values similar to normal eyes. This may be due to the action of Descemet's membrane which is preserved in DALK, which acts as a strong foundation for the rest of the corneal stroma which rests above it. Opposite findings were reported by Jafarinasab et al. [115] that found lower values of CH and CRF in the DALK group compared to PK group, but those differences were not observed 30 months after surgery. Differences between the indications for keratoplasty or graft-related differences [116] may explain the difference in the results of different studies.
3.8. Ocular Disease
3.8.1. Glaucoma
Differences in CCT have been considered as a risk factor for glaucoma [117, 118] and given the correlation between low CCT and glaucomatous changes in the optic disc, a biological association shared by the cornea, sclera, and lamina cribrosa is conceivable [119, 120]. A number of recent reports have suggested a relationship between CH, CRF, and glaucoma with evidence that CH is lower in glaucomatous eyes compared with normal eyes and eyes with ocular hypertension [3, 7, 42, 45, 90, 121–124]. Furthermore, normal tension glaucomatous (NTG) eyes show the lowest value among glaucomatous eyes according to some studies [121, 125]. Even after pharmacologic IOP lowering, CH was shown to be lower in glaucomatous eyes than in normal eyes [126]. This suggests that eyes with lower CH and/or thinner than normal CCT might exhibit structural weakness [42] and it is possible that CCT and CH could be considered as risk factors for glaucoma, independent of IOP [121, 122, 127]. Conversely, CRF was found to be significantly higher in patients with ocular hypertension and in patients with primary open-angle glaucoma and low in NTG patients [90, 123]. This implies that GAT IOP should be expected to be overestimated as a greater force required to applanate a cornea with higher CRF. This could suggest that CRF could be also useful to differentiate between subjects with ocular hypertension and glaucoma [123].
As both the sclera and the cornea are formed from continuous extracellular matrix, this might have some effect on the biomechanical relationship between the two tissues [128]. Bochmann et al. [120] compared CH in glaucomatous eyes with and without acquired pit of the optic nerve and reported that CH was lower in glaucomatous eyes with an acquired pit and hypothesized the possibility that corneal biomechanical properties reflect the attributes of the lamina cribrosa [120, 121]. Several studies found that eyes with low CH are associated with increased severity of glaucomatous visual field defects [45, 122, 129, 130]. In contrast, Wells et al. [124] found a relationship between CH and deformation of optic nerve head with higher CH being strongly correlated with higher deformability of the optic nerve head. In untreated newly diagnosed POAG patients, CH was the only factor significantly associated with both mean cup depth (r = −0.34) and cup-to-disc ratio (r = −0.41) [131].
In conclusion, as the elastic properties of the cornea are believed to reflect the elasticity of collagen fibres in the eyeball as a whole, there might be an opportunity to consider corneal biomechanics as an indicator of overall globe biomechanical properties in glaucoma [132]. If this is true, corneal biomechanical properties seem to be a promising addendum to the complex issues of glaucoma and may constitute a pressure-independent risk factor for glaucoma detection, prognosis, and treatment.
3.8.2. Keratoconus
In keratoconus (KC), the normal corneal collagen-fibril meshwork is disrupted leading to a localized reduction of corneal radius of curvature and tissue thinning. A significant weaker stress versus strain response in KC eyes compared to normal eyes and a more disorganised collagen fibber network as well been shown [16, 133]. Thus, changes in corneal biomechanics in KC eyes might be expected and it has been suggested that KC progression is characterized by a reduction of material properties that lead to a progressive thinning, increasing strain and stress redistribution, and lower keratocyte densities [134, 135]. CH and CRF measurements have been shown to be reduced in KC eyes [4, 28, 39, 85, 136–138] with stronger decrease as KC severity increases [110, 139–141] even after controlling for differences in age, sex, and CCT [141, 142]. This suggests that other structural alterations different from CCT lead to lower lamellar adhesion and lower shear modulus and may be responsible for these lowering effects in ORA measurements [143]. However, there is large overlap of CH and CRF between normal and KC corneas and both ORA parameters showed low sensitivity and specificity in differentiating KC or suspecting KC from healthy corneas [137, 138, 140, 142, 144, 145]. Recent studies demonstrated that the new parameters derived from waveform analysis of ORA signals represent a significant improvement in detection and differentiation of the keratoconic cornea [28, 92, 110, 145, 146]. In fact, characteristics of the air pressure corneal deformation profile are more affected by keratoconus than the traditionally extracted CH and CRF factors; keratoconic eyes have significantly lower elasticity coefficient compared to normal eyes [92] and the area under the second peak of the signal curve has been shown to produce the best results and seems more promising in distinguishing between normal and KC eyes [110, 137].
3.8.3. Fuchs Corneal Dystrophy
Fuchs corneal dystrophy (FCD) is a genetic disorder of the corneal endothelium. When the disease progresses, the number of endothelial cells decreases and corneal oedema increases affecting visual acuity [147]. Both CRF and CH parameters were found to be lower in FCD eyes compared to normal eyes [3, 51, 148]. del Buey et al. [51] reported that CRF was positively correlated with CCT in control eyes while this correlation was negative in FCD eyes. According to the authors, these results may be related not only to corneal hydration but also to other aspects of corneal biomechanics since patients with FCD have decreased endothelial cell density and thicker Descemet's membrane, and the corneal central region is usually involved which can lead to reductions of viscous damping within corneal tissues and, consequently, viscosity reduces. Additionally, the authors found that the lower the CH, the higher the IOPcc in FCD eyes, but these results may be due to an underestimation error in IOP measurement caused by the observed diminished CH and elevated CCT [51]. Similar results were reported by Clemmensen and Hjortdal [148] who found a CH and CRF reduction in FCD eyes and that IOPcc appears to overestimate IOP in those patients. Altogether, corneas affected by FCD point to a paradoxical condition in which thicker corneas are not related as expected to higher CRF as shown in normal eyes. This might also point to a mechanistic explanation to interpret CRF values. According to this, CRF increases with increase in CCT as long as this increase is justified by an increase in collagen material. Conversely, when the increase is due to a massive hydration of cornea as in FCD, the effect is the opposite as the ground substance becomes more relevant in the overall context of the mechanical behaviour of the cornea.
3.9. Systemic Disease
3.9.1. Diabetes
Several structural changes in the cornea of diabetes patients have been reported [149, 150] and an influence on the biomechanical properties of the cornea could also be hypothesized. Several studies have investigated the impact of diabetes on corneal biomechanical parameters; however, the results are rather controversial among different studies [151–156]. Goldich et al. [154] found that CH, CRF, and CCT were significantly higher in diabetic eyes compared to healthy eyes. Hager et al. [155] reported a significantly higher CH in diabetic eyes than in nondiabetic eyes after correcting for age, IOP, and CCT. By contrast, Şahin et al. [156] reported that CH was significantly lower in diabetic patients, whereas CRF was not significantly different from that of control subjects. The authors hypothesized that lower CH in diabetic patients may be explained by a decrease in the dampening effects of the cornea as a result of an alteration in the collagenous components in diabetic eyes due to collagen cross-linking. The reasons for such contradictory results among different studies lie in the differences in age range and CCT and diversity of diabetes types and severity enrolled. In some studies most patients presented type 2 diabetes, while in others there were a similar number of patients with type 1 and type 2 of diabetes. In fact, as recently shown by Scheler et al. [152], biomechanical properties of the cornea seem to be altered depending on the glucose control. In their study, Scheler et al. found that in diabetes, CH and CRF were significantly correlated to glycated haemoglobin (HbA1c); diabetic patients with elevated HbA1c showed an increased CH indicating an increase in the viscosity of the ground substance that is associated with higher corneal shearing strength and increased damping most likely due to a nonenzymatic glycosylation of proteoglycans and glycosaminoglycan that affects the corneal damping behaviour [152].
4. New Imaging Techniques to Measure the Corneal Biomechanical Properties
Given the promising nature of the possibility of measuring corneal biomechanics in vivo, there has been an increasing interest in the development of methods that allow minimally invasive mechanical test of the cornea which may permit a better understanding of the differences in corneal properties between a wide range of ocular conditions and healthy eyes as well as an improvement in the early detection of potential problematic corneas. Until now, many studies covering measurement of corneal biomechanical properties in a wide range of topics have been performed and published using the ORA device as previously described, but other new in vivo techniques of corneal biomechanical measurements are under development. However, with the exception of the Corvis ST, most of these new noninvasive or minimally invasive techniques are experimental prototypes that despite being promising still have many drawbacks such as not being commercially available, being of high costs, and lacking evidence of accuracy and availability for clinical purposes that need to be overcome.
One technique is the Corneal Visualization Scheimpflug Technology (Corvis ST; Oculus, Wetzlar, Germany) which is commercially available since 2011. This device is based on a noncontact air puff tonometer combined with an ultrahigh speed Scheimpflug camera. The Scheimpflug camera records 4330 images per second along an 8 mm horizontal corneal coverage during corneal deformation under an air puff indentation [157]. This camera allows a dynamic inspection of the deformation process of the cornea and provides further detailed information of biomechanical characterization of the cornea. The Corvis ST output parameters include time and length of the flattened cornea in the first applanation; corneal velocity during the first applanation moment; time from start until the second applanation; length and corneal velocity during the second applanation moment; time from start until the highest concavity of cornea is reached; and maximum deformation amplitude (from start to the highest concavity) at the corneal apex, among others. However, the machine is still under development and new parameters are being continuously added to the output and only available for research purposes. A definition of the parameters currently available in the commercial version of the instrument is provided in Appendix B. Clinical outcomes are limited and preliminary results have found significant differences of corneal deformation response among normal and keratoconic corneas for many parameters such as corneal speed during deformation, corneal applanation length, and deformation amplitude. All of them seem to be relevant parameters to define the corneal stiffness and corneal viscoelastic properties and are promising in the evaluation of several corneal conditions and the outcomes of different surgical procedures [158–161].
Another prototype device is the Dynamic Corneal Surface Topography [162] that involves surface topographic corneal imaging, with a Dynamic Rasterstereographic Corneal Topography (d.RCT) with off-axis geometry, during an air puff indentation by an NCT [163]. This device includes an imaging arm, a calibrated grid arm, and a digital camera. When fluorescein is instilled into the cornea and the fluorescent emissions are excited by the projected grid, an image of which is then captured that contains the three-dimensional information from the corneal surface. After approximately 12 ms from the beginning of the air puff, when the air puff pressure is maximum, another image is taken, which corresponds with the largest corneal deformation. From the two images that are acquired (predeformation and middeformation), biomechanical properties can then be determined using a model of corneal viscoelasticity, based on the applied force and the stress-strain relationship of discrete surface segments across the cornea by measuring corneal shape and displacement between the predeformation state and the middeformation state [164].
Another novel method is based on high speed Swept Source Ocular Coherence Tomography (ssOCT) combined with an air puff NCT [165]. The cornea is deformed by the air puff, and during the 20 ms of applanation time, the ssOCT acquires multiple A-scans at the center of the air puff, allowing observation of the dynamics of the anterior and posterior corneal surfaces. From the analysis of the scan, one can obtain information about the biomechanical behaviour of the cornea during the applanation process. Pilot results in normal subjects showed the validity of the technique in IOP measurement [165]. However, the system needs improvements particularly in a faster acquisition system and a large clinical study is required to fully understand the potential of the system in the clinical setting.
Brillouin Optical Microscopy is another noncontact technique that uses the combination of a confocal microscope with an ultrahigh resolution spectrometer to perform Brillouin imaging of the cornea [166]. It has the ability to visualize corneal elasticity and measure the depth-dependent variation of elastic modulus within the cornea noninvasively with three-dimensional resolution. This device was firstly used in bovine corneas and is currently in development for use in human eyes [166].
Shear wave propagation velocity has been used to measure corneal biomechanical properties in vivo, through the use of linear elastic model approximation, in which the Young's modulus and Poisson's ratio can be estimated from the shear wave speed [18, 167, 168]. However, corneal strain and corneal hydration strongly affects the wave speed by attenuating high-frequency shear wave and do not reproduce the nonlinear properties of the cornea. Recently, a new method has been developed: the Quantitative Ultrasonic Spectroscopy (QUSi) [169]. The QUSi has improvements in the form of wave propagation that are not available in clinical ultrasound and derives more information of the reflected full-wave forms. Once corneal acoustic and elastic properties have been shown to correlate [170], this method is currently being developed to map corneal elastic properties and so to determine an elastic constant of the cornea called the aggregate modulus, which provides a measure of its stiffness [169].
Corneal Transient Elastography (CTE) is another technique that is under development for ophthalmologic use and was adapted from a technology in current use for the analysis of breast tissue imaging [171]. It combines the generation of a remote palpation in the cornea and ultrafast (20 000 frames/s) ultrasonic images of the resulting corneal displacements that evolve into a shear wave propagation whose local speed was directly linked to local elasticity. The mainly improvements was at the level of the echographic probe that was specifically designed to couple a homogenous transverse compression wave to the tissue (supersonic mode) and an ultrafast echographic acquisition mode, allowing high resolution and quantitative maps of the whole corneal elasticity [172].
Optical interferometric techniques were also used to measure corneal biomechanical properties because they are noncontact, highly sensitive, and capable of simultaneously recording information from across the whole surface. Holographic interferometry has been used to assess qualitatively keratoplasty wound integrity in vivo [173]. Electronic speckle pattern interferometry (ESPI) was used to quantify the effect of microkeratome flap creation on the displacement response of the sheep cornea [174]; however, these techniques are extremely sensitive to environmental disturbances such heat and vibration that may influence its accuracy. Radial shearing speckle pattern interferometry (RSSPI) [175] is an interferometric technique where the two images contain information on the topography of the surface location which changes as applied pressure is altered and is much more resistant to physical disturbances. The differential magnification between the two images allows a mathematical analysis to detect changes in radial strain. It has been used to describe the progressive increase in corneal Young's modulus as a function of aging in human corneas [49] and to quantify the magnitude of the stiffening effect of corneal cross-linking [175].
Another technique uses a physical probe to indent the central cornea with an electronically controlled microprecision motor coupled with simultaneous video-topography imaging of the cornea. It is called Dynamic Corneal Imaging (DCI) and measures the change in curvature of the cornea as it bends [176]. in this technique, greater difference flexing curves have been demonstrated with lower IOP, thinner corneas, and in keratoconic versus normal corneas as well, which is consistent with more easily deformable corneas [176].
Another technique uses Optical Coherence Tomography Elastography [177] to generate in vivo 2D maps of corneal deformation as it is indented by a concave curved lens to preserve the curvature of the cornea as it deforms. It has the potential to measure local and depth variations in the mechanical properties of the cornea owing to its ability to measure strain throughout all the stroma, providing measures of local viscoelastic properties such as elastic modulus, shear modulus, and hysteresis [177]. Current efforts include the development of 3D analysis routines and stress sequences for in vivo use.
5. Conclusions
The published literature sheds light on the potential utility of the biomechanical corneal properties to a better comprehension of the mechanical behaviour of this complex tissue. However, it also shows some to some controversial results in relevant areas such as their impact on intraocular pressure measurement, preoperative refractive surgery assessment, and surgical treatment of keratoconus. New parameters derived from a more detailed analysis of the outcomes as well as new technologies are promising in consolidating the utility of the biomechanical corneal properties as a clinical tool and a very relevant field for the future improvement of safety and efficacy of different eye health care strategies.
Appendices
A.
Parameters obtained from signal analysis of the Ocular Response Analyzer:
P1 area and P2 area: areas under the curves of Peaks 1 and 2, measuring 75% of peaks height.
P1 area1 and P2 area1: areas under the curves of Peaks 1 and 2, measuring 50% of peaks height.
h1 and h2: height of the signal Peaks 1 and 2, measuring 75% of Peaks height.
h11 and h21: height of the signal Peaks 1 and 2, measuring 50% of Peaks height.
w1 and w2: full width of signal Peaks 1 and 2 at 25% of the maximum of the infrared signal peaks.
w11 and w21: width of signal Peaks 1 and 2 at half of the maximum of the infrared signal peaks. These two parameters are also called by other authors FWHM1 and FWHM2 [25].
aspect1 and aspect2: ratio between width (w) and height (h) of Peaks 1 and 2, measuring 75% of peaks height.
aspect11 and aspect21: ratio between width (w) and height (h) of Peaks 1 and 2, measuring 50% of peaks height.
uslope1 and uslope2: rate of increase from base (at 25% of maximum of the infrared signal peaks) to Peaks 1 and 2.
dslope1 and dslope2: rate of decrease from Peaks 1 and 2 (at 25% of maximum of the infrared signal peaks) to base.
uslope11 and uslope21: rate of increase from base (at 50% of maximum of the infrared signal peaks) to Peaks 1 and 2.
dslope11 and dslope21: rate of decrease from Peaks 1 and 2 (at 50% of maximum of the infrared signal peaks) to base.
dive1 and dive2: distance from the first spike of Peaks 1 and 2 to the top of the graph, measuring 75% of peaks height.
slew1 and slew2: ratio between dive and with (w) of Peaks 1 and 2, measuring 75% of peaks height.
mslew1 and mslew2: the longest continuous line in peaks without a break, measuring 75% of peaks height.
path1 and path2: absolute value of path length around the peaks, measuring 75% of peaks height.
path11 and path21: absolute value of path length around the peaks, measuring 50% of peaks height.
Aindex and bindex: number of times that the peaks change their direction, measuring 75% of peaks height.
aplhf: high frequency “noise” in regions between peaks (normalized by product of average of peak heights × width of region), measuring 75% of peaks height.
Another parameters that have been also analysed include [28]
Peak 1 and Peak 2: maximum heights of the corresponding infrared signal peaks,
PIT: time between Time in and Time out,
Pmax: maximum value of the air pressure curve,
TPmax: Time al in which the maximum air pressure occurs,
DID: damping-induced delay is the time between Time2 and the time corresponding to the symmetrical position of P 1 on Peak 2.
B.
Parameters obtained from image analysis of the Corneal Visualization Scheimpflug Technology are as follows:
IOP: an ordinary NCT measurement that is based on the first applanation.
Pachy: measurement of central corneal thickness (CCT) with optical pachymetry.
Time of Appl 1 (1st A-time): time from start until the first applanation.
Length of Appl 1 (1st A length): length of the flattened cornea in the first applanation.
Velocity of Appl 1 (Vin): corneal velocity during the first applanation moment.
Time of Appl 2 (2nd A-time): time from start until the second applanation.
Length of Appl 2 (2nd A length): length of the flattened cornea in the second applanation.
Velocity of Appl 2 (Vout): corneal velocity during the second applanation moment.
Time of Hi Con (HC time): time from start until the highest concavity of cornea is reached.
Deformation amplitude (DA): maximum deformation amplitude (from start to the highest concavity) at the corneal apex.
Disclosure
The authors do not have any financial or commercial interest in the devices mentioned in the present study.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Hay JG. The Biomechanics of Sports Techniques. 4th edition. Englewood Cliffs, NJ, USA: Prentice-Hall; 1993. [Google Scholar]
- 2.Ambrosio L, Netti PA, Nicolais L. Soft Tissue. New York, NY, USA: Springer; 2002. [Google Scholar]
- 3.Luce DA. Determining in vivo biomechanical properties of the cornea with an Ocular Response Analyzer. Journal of Cataract and Refractive Surgery. 2005;31(1):156–162. doi: 10.1016/j.jcrs.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 4.Ortiz D, Piñero D, Shabayek MH, Arnalich-Montiel F, Alió JL. Corneal biomechanical properties in normal, post-laser in situ keratomileusis, and keratoconic eyes. Journal of Cataract and Refractive Surgery. 2007;33(8):1371–1375. doi: 10.1016/j.jcrs.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 5.González-Méijome JM, Villa-Collar C, Queirós A, Jorge J, Parafita MA. Pilot study on the influence of corneal biomechanical properties over the short term in response to corneal refractive therapy for myopia. Cornea. 2008;27(4):421–426. doi: 10.1097/ICO.0b013e318164e49d. [DOI] [PubMed] [Google Scholar]
- 6.Kirwan C, O’Keefe M. Corneal hysteresis using the Reichert ocular response analyser: findings pre- and post-LASIK and LASEK. Acta Ophthalmologica. 2008;86(2):215–218. doi: 10.1111/j.1600-0420.2007.01023.x. [DOI] [PubMed] [Google Scholar]
- 7.Grise-Dulac A, Saad A, Abitbol O, et al. Assessment of corneal biomechanical properties in normal tension glaucoma and comparison with open-angle glaucoma, ocular hypertension, and normal eyes. Journal of Glaucoma. 2012;21(7):486–489. doi: 10.1097/IJG.0b013e318220daf0. [DOI] [PubMed] [Google Scholar]
- 8.Hoeltzel DA, Altman P, Buzard K, Choe K-I. Strip extensiometry for comparison of the mechanical response of bovine, rabbit, and human corneas. Journal of Biomechanical Engineering. 1992;114(2):202–215. doi: 10.1115/1.2891373. [DOI] [PubMed] [Google Scholar]
- 9.Dowling NE. Mechanical Behavior of Materials, Engineering Methods for Deformation Fracture and Fatigue. 3rd edition. Upper Saddle River, NJ, USA: 2007. [Google Scholar]
- 10.Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. Journal of Cataract and Refractive Surgery. 2005;31(1):146–155. doi: 10.1016/j.jcrs.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz NJ, Mackay RS, Sackman JL. A theoretical and experimental study of the mechanical behavior of the cornea with application to the measurement of intraocular pressure. The Bulletin of Mathematical Biophysics. 1966;28(4):585–643. [Google Scholar]
- 12.Jue B, Maurice DM. The mechanical properties of the rabbit and human cornea. Journal of Biomechanics. 1986;19(10):847–853. doi: 10.1016/0021-9290(86)90135-1. [DOI] [PubMed] [Google Scholar]
- 13.Hjortdal JO. On the biomechanical properties of the cornea with particular reference to refractive surgery. Acta Ophthalmologica Scandinavica. 1998;76(225):1–23. [PubMed] [Google Scholar]
- 14.Woo SL-Y, Kobayashi AS, Schlegel WA, Lawrence C. Nonlinear material properties of intact cornea and sclera. Experimental Eye Research. 1972;14(1):29–39. doi: 10.1016/0014-4835(72)90139-x. [DOI] [PubMed] [Google Scholar]
- 15.Andreassen TT, Simonsen AH, Oxlund H. Biomechanical properties of keratoconus and normal corneas. Experimental Eye Research. 1980;31(4):435–441. doi: 10.1016/s0014-4835(80)80027-3. [DOI] [PubMed] [Google Scholar]
- 16.Nash IS, Greene PR, Foster CS. Comparison of mechanical properties of keratoconus and normal corneas. Experimental Eye Research. 1982;35(5):413–424. doi: 10.1016/0014-4835(82)90040-9. [DOI] [PubMed] [Google Scholar]
- 17.Hjortdal JO. Extensibility of the normo-hydrated human cornea. Acta Ophthalmologica Scandinavica. 1995;73(1):12–17. doi: 10.1111/j.1600-0420.1995.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Prendiville PL, McDonnell PJ, Chang WV. An ultrasonic technique for the measurement of the elastic moduli of human cornea. Journal of Biomechanics. 1996;29(12):1633–1636. [PubMed] [Google Scholar]
- 19.Bryant MR, McDonnell PJ. Constitutive laws for biomechanical modeling of refractive surgery. Journal of Biomechanical Engineering. 1996;118(4):473–481. doi: 10.1115/1.2796033. [DOI] [PubMed] [Google Scholar]
- 20.Elsheikh A, Wang D, Brown M, Rama P, Campanelli M, Pye D. Assessment of corneal biomechanical properties and their variation with age. Current Eye Research. 2007;32(1):11–19. doi: 10.1080/02713680601077145. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton KE, Pye DC. Young’s modulus in normal corneas and the effect on applanation tonometry. Optometry and Vision Science. 2008;85(6):445–450. doi: 10.1097/OPX.0b013e3181783a70. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Qi H. Dissipated energy function, hysteresis and precondition of a viscoelastic solid model. Nonlinear Analysis: Real World Applications. 2010;11(2):907–912. [Google Scholar]
- 23.Feizi S, Jadidi K, Soheilian M. Possible protection of the posterior segment by a phakic intraocular lens. Journal of Cataract and Refractive Surgery. 2007;33(12):2144–2146. doi: 10.1016/j.jcrs.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Lam A, Chen D, Chiu R, Chui WS. Comparison of IOP measurements between ORA and GAT in normal Chinese. Optometry and Vision Science. 2007;84(9):909–914. doi: 10.1097/OPX.0b013e3181559db2. [DOI] [PubMed] [Google Scholar]
- 25.Pepose JS, Feigenbaum SK, Qazi MA, Sanderson JP, Roberts CJ. Changes in corneal biomechanics and intraocular pressure following LASIK using static, dynamic, and noncontact tonometry. The American Journal of Ophthalmology. 2007;143(1):39–47. doi: 10.1016/j.ajo.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 26.Kotecha A, Elsheikh A, Roberts CR, Zhu H, Garway-Heath DF. Corneal thickness- and age-related biomechanical properties of the cornea measured with the Ocular Response Analyzer. Investigative Ophthalmology and Visual Science. 2006;47(12):5337–5347. doi: 10.1167/iovs.06-0557. [DOI] [PubMed] [Google Scholar]
- 27.Lau W, Pye D. A clinical description of Ocular Response Analyzer measurements. Investigative Ophthalmology and Visual Science. 2011;52(6):2911–2916. doi: 10.1167/iovs.10-6763. [DOI] [PubMed] [Google Scholar]
- 28.Touboul D, Bénard A, Mahmoud AM, Gallois A, Colin J, Roberts CJ. Early biomechanical keratoconus pattern measured with an Ocular Response Analyzer: curve analysis. Journal of Cataract and Refractive Surgery. 2011;37(12):2144–2150. doi: 10.1016/j.jcrs.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 29.Shin JY, Choi JS, Oh JY, Kim MK, Lee JH, Wee WR. Evaluation of corneal biomechanical properties following penetrating keratoplasty using the Ocular Response Analyzer. Korean Journal of Ophthalmology. 2010;24(3):139–142. doi: 10.3341/kjo.2010.24.3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franco S, Lira M. Biomechanical properties of the cornea measured by the Ocular Response Analyzer and their association with intraocular pressure and the central corneal curvature. Clinical and Experimental Optometry. 2009;92(6):469–475. doi: 10.1111/j.1444-0938.2009.00414.x. [DOI] [PubMed] [Google Scholar]
- 31.Medeiros FA, Weinreb RN. Evaluation of the influence of corneal biomechanical properties on intraocular pressure measurements using the Ocular Response Analyzer. Journal of Glaucoma. 2006;15(5):364–370. doi: 10.1097/01.ijg.0000212268.42606.97. [DOI] [PubMed] [Google Scholar]
- 32.Lam AKC, Chen D, Tse J. The usefulness of waveform score from the Ocular Response Analyzer. Optometry and Vision Science. 2010;87(3):195–199. doi: 10.1097/OPX.0b013e3181d1d940. [DOI] [PubMed] [Google Scholar]
- 33.Kerautret J, Colin J, Touboul D, Roberts C. Biomechanical characteristics of the ectatic cornea. Journal of Cataract and Refractive Surgery. 2008;34(3):510–513. doi: 10.1016/j.jcrs.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 34.Spoerl E, Terai N, Scholz F, Raiskup F, Pillunat LE. Detection of biomechanical changes after corneal cross-linking using Ocular Response Analyzer software. Journal of Refractive Surgery. 2011;27(6):452–457. doi: 10.3928/1081597X-20110106-01. [DOI] [PubMed] [Google Scholar]
- 35.Zarei-Ghanavati S, Ramirez-Miranda A, Yu F, Hamilton DR. Corneal deformation signal waveform analysis in keratoconic versus post-femtosecond laser in situ keratomileusis eyes after statistical correction for potentially confounding factors. Journal of Cataract and Refractive Surgery. 2012;38(4):607–614. doi: 10.1016/j.jcrs.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 36.Sedaghat M, Naderi M, Zarei-Ghanavati M. Biomechanical parameters of the cornea after collagen crosslinking measured by waveform analysis. Journal of Cataract and Refractive Surgery. 2010;36(10):1728–1731. doi: 10.1016/j.jcrs.2010.06.056. [DOI] [PubMed] [Google Scholar]
- 37.Edmund C. Corneal elasticity and ocular rigidity in normal and keratoconic eyes. Acta Ophthalmologica. 1988;66(2):134–140. doi: 10.1111/j.1755-3768.1988.tb04000.x. [DOI] [PubMed] [Google Scholar]
- 38.Kirwan C, O’keefe M, Lanigan B. Corneal hysteresis and intraocular pressure measurement in children using the reichert Ocular Response Analyzer. The American Journal of Ophthalmology. 2006;142(6):990–992. doi: 10.1016/j.ajo.2006.07.058. [DOI] [PubMed] [Google Scholar]
- 39.Shah S, Laiquzzaman M, Bhojwani R, Mantry S, Cunliffe I. Assessment of the biomechanical properties of the cornea with the Ocular Response Analyzer in normal and keratoconic eyes. Investigative Ophthalmology and Visual Science. 2007;48(7):3026–3031. doi: 10.1167/iovs.04-0694. [DOI] [PubMed] [Google Scholar]
- 40.Lim L, Gazzard G, Chan YH, et al. Cornea biomechanical characteristics and their correlates with refractive error in Singaporean children. Investigative Ophthalmology and Visual Science. 2008;49(9):3852–3857. doi: 10.1167/iovs.07-1670. [DOI] [PubMed] [Google Scholar]
- 41.Shen M, Fan F, Xue A, Wang J, Zhou X, Lu F. Biomechanical properties of the cornea in high myopia. Vision Research. 2008;48(21):2167–2171. doi: 10.1016/j.visres.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 42.Abitbol O, Bouden J, Doan S, Hoang-Xuan T, Gatinel D. Corneal hysteresis measured with the Ocular Response Analyzer in normal and glaucomatous eyes. Acta Ophthalmologica. 2010;88(1):116–119. doi: 10.1111/j.1755-3768.2009.01554.x. [DOI] [PubMed] [Google Scholar]
- 43.Kamiya K, Shimizu K, Ohmoto F. Effect of aging on corneal biomechanical parameters using the Ocular Response Analyzer. Journal of Refractive Surgery. 2009;25(10):888–893. doi: 10.3928/1081597X-20090917-10. [DOI] [PubMed] [Google Scholar]
- 44.Kida T, Liu JHK, Weinreb RN. Effects of aging on corneal biomechanical properties and their impact on 24-hour measurement of intraocular pressure. The American Journal of Ophthalmology. 2008;146(4):567–572. doi: 10.1016/j.ajo.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mansouri K, Leite MT, Weinreb RN, Tafreshi A, Zangwill LM, Medeiros FA. Association between corneal biomechanical properties and glaucoma severity. The American Journal of Ophthalmology. 2012;153(3):419–427. doi: 10.1016/j.ajo.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foster PJ, Broadway DC, Garway-Heath DF, et al. Intraocular pressure and corneal biomechanics in an adult British population: the EPIC-Norfolk eye study. Investigative ophthalmology & visual science. 2011;52(11):8179–8185. doi: 10.1167/iovs.11-7853. [DOI] [PubMed] [Google Scholar]
- 47.Daxer A, Misof K, Grabner B, Ettl A, Fratzl P. Collagen fibrils in the human corneal stroma: structure and aging. Investigative Ophthalmology and Visual Science. 1998;39(3):644–648. [PubMed] [Google Scholar]
- 48.Elsheikh A, Geraghty B, Rama P, Campanelli M, Meek KM. Characterization of age-related variation in corneal biomechanical properties. Journal of the Royal Society Interface. 2010;7(51):1475–1485. doi: 10.1098/rsif.2010.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knox Cartwright NE, Tyrer JR, Marshall J. Age-related differences in the elasticity of the human cornea. Investigative Ophthalmology & Visual Science. 2011;52(7):4324–4329. doi: 10.1167/iovs.09-4798. [DOI] [PubMed] [Google Scholar]
- 50.Shah S, Laiquzzaman M, Cunliffe I, Mantry S. The use of the Reichert ocular response analyser to establish the relationship between ocular hysteresis, corneal resistance factor and central corneal thickness in normal eyes. Contact Lens and Anterior Eye. 2006;29(5):257–262. doi: 10.1016/j.clae.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 51.del Buey MA, Cristóbal JA, Ascaso FJ, Lavilla L, Lanchares E. Biomechanical properties of the cornea in fuchs’ corneal dystrophy. Investigative Ophthalmology and Visual Science. 2009;50(7):3199–3202. doi: 10.1167/iovs.08-3312. [DOI] [PubMed] [Google Scholar]
- 52.Leite MT, Alencar LM, Gore C, et al. Comparison of corneal biomechanical properties between healthy blacks and whites using the Ocular Response Analyzer. The American Journal of Ophthalmology. 2010;150(2):163–168. doi: 10.1016/j.ajo.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haseltine SJ, Pae J, Ehrlich JR, Shammas M, Radcliffe NM. Variation in corneal hysteresis and central corneal thickness among black, hispanic and white subjects. Acta Ophthalmologica. 2012;90(8):e626–e631. doi: 10.1111/j.1755-3768.2012.02509.x. [DOI] [PubMed] [Google Scholar]
- 54.Kida T, Liu JHK, Weinreb RN. Effect of 24-hour corneal biomechanical changes on intraocular pressure measurement. Investigative Ophthalmology and Visual Science. 2006;47(10):4422–4426. doi: 10.1167/iovs.06-0507. [DOI] [PubMed] [Google Scholar]
- 55.Chang SW, Tsai IL, Hu FR, Lin LLK, Shih YF. The cornea in young myopic adults. British Journal of Ophthalmology. 2001;85(8):916–920. doi: 10.1136/bjo.85.8.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang Z, Shen M, Mao G, et al. Association between corneal biomechanical properties and myopia in Chinese subjects. Eye. 2011;25(8):1083–1089. doi: 10.1038/eye.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plakitsi A, O’Donnell C, A Miranda M, Charman WN, Radhakrishnan H. Corneal biomechanical properties measured with the ocular response analyser in a myopic population. Ophthalmic and Physiological Optics. 2011;31(4):404–412. doi: 10.1111/j.1475-1313.2011.00852.x. [DOI] [PubMed] [Google Scholar]
- 58.Xu S, Xu A, Tao A, Wang J, Fan F, Lu F. Corneal biomechanical properties and intraocular pressure in high myopic anisometropia. Eye and Contact Lens. 2010;36(4):204–209. doi: 10.1097/ICL.0b013e3181e4a60a. [DOI] [PubMed] [Google Scholar]
- 59.Song Y, Congdon N, Li L, et al. Corneal hysteresis and axial length among Chinese secondary school children: the xichang pediatric refractive error study (X-PRES) report no. 4. The American Journal of Ophthalmology. 2008;145(5):819–826. doi: 10.1016/j.ajo.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 60.Radhakrishnan H, Miranda MA, O’Donnell C. Corneal biomechanical properties and their correlates with refractive error. Clinical and Experimental Optometry. 2012;95(1):12–18. doi: 10.1111/j.1444-0938.2011.00696.x. [DOI] [PubMed] [Google Scholar]
- 61.Goldmann H, Schmidt T. On applanation tonography. Ophthalmologica. 1965;150(1):65–75. doi: 10.1159/000304827. [DOI] [PubMed] [Google Scholar]
- 62.Davey PG, Elsheikh A, Garway-Heath DF. Clinical evaluation of multiparameter correction equations for Goldmann applanation tonometry. Eye. 2013;27(5):621–629. doi: 10.1038/eye.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ehrlich JR, Haseltine S, Shimmyo M, Radcliffe NM. Evaluation of agreement between intraocular pressure measurements using Goldmann applanation tonometry and Goldmann correlated intraocular pressure by Reichert’s ocular response analyser. Eye. 2010;24(10):1555–1560. doi: 10.1038/eye.2010.83. [DOI] [PubMed] [Google Scholar]
- 64.Bayoumi NHL, Bessa AS, El Massry AAK. Ocular Response Analyzer and Goldmann applanation tonometry: a comparative study of findings. Journal of Glaucoma. 2010;19(9):627–631. doi: 10.1097/IJG.0b013e3181ca7e01. [DOI] [PubMed] [Google Scholar]
- 65.Touboul D, Roberts C, Kérautret J, et al. Correlations between corneal hysteresis, intraocular pressure, and corneal central pachymetry. Journal of Cataract and Refractive Surgery. 2008;34(4):616–622. doi: 10.1016/j.jcrs.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 66.Kamiya K, Hagishima M, Fujimura F, Shimizu K. Factors affecting corneal hysteresis in normal eyes. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2008;246(10):1491–1494. doi: 10.1007/s00417-008-0864-x. [DOI] [PubMed] [Google Scholar]
- 67.Detry-Morel M, Jamart J, Hautenauven F, Pourjavan S. Comparison of the corneal biomechanical properties with the Ocular Response Analyzer (ORA) in African and Caucasian normal subjects and patients with glaucoma. Acta Ophthalmologica. 2012;90(2):e118–e124. doi: 10.1111/j.1755-3768.2011.02274.x. [DOI] [PubMed] [Google Scholar]
- 68.González-MIijome JM, Queirós A, Jorge J, Díaz-Rey A, Parafita MA. Intraoffice variability of corneal biomechanical parameters and Intraocular Pressure (IOP) Optometry and Vision Science. 2008;85(6):457–462. doi: 10.1097/OPX.0b013e3181783a5f. [DOI] [PubMed] [Google Scholar]
- 69.Poostchi A, Nicholas S, Wells AP. Recovery of corneal hysteresis after reduction of intraocular pressure in chronic primary angle-closure glaucoma. The American Journal of Ophthalmology. 2010;149(3):524–525. doi: 10.1016/j.ajo.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 70.Cankaya AB, Beyazyildiz E, Ileri D, Ozturk F. The effect of contact lens usage on corneal biomechanical parameters in myopic patients. Cornea. 2012;31(7):764–769. doi: 10.1097/ICO.0b013e3182248239. [DOI] [PubMed] [Google Scholar]
- 71.Hutchings N, Simpson TL, Hyun C, et al. Swelling of the human cornea revealed by high-speed, ultrahigh-resolution optical coherence tomography. Investigative Ophthalmology and Visual Science. 2010;51(9):4579–4584. doi: 10.1167/iovs.09-4676. [DOI] [PubMed] [Google Scholar]
- 72.Moezzi AM, Fonn D, Varikooty J, Richter D. Distribution of overnight corneal swelling across subjects with 4 different silicone hydrogel lenses. Eye and Contact Lens. 2011;37(2):61–65. doi: 10.1097/ICL.0b013e31820e0bc3. [DOI] [PubMed] [Google Scholar]
- 73.Lu F, Xu S, Qu J, et al. Central corneal thickness and corneal hysteresis during corneal swelling induced by contact lens wear with eye closure. The American Journal of Ophthalmology. 2007;143(4):616–622. doi: 10.1016/j.ajo.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 74.Lau W, Pye D. Changes in corneal biomechanics and applanation tonometry with induced corneal swelling. Investigative Ophthalmology & Visual Science. 2011;52(6):3207–3214. doi: 10.1167/iovs.10-6754. [DOI] [PubMed] [Google Scholar]
- 75.Alharbi A, Swarbrick HA. The effects of overnight orthokeratology lens wear on corneal thickness. Investigative Ophthalmology and Visual Science. 2003;44(6):2518–2523. doi: 10.1167/iovs.02-0680. [DOI] [PubMed] [Google Scholar]
- 76.Villa-Collar C, González-Méijome JM, Queirós A, Jorge J. Short-term corneal response to corneal refractive therapy for different refractive targets. Cornea. 2009;28(3):311–316. doi: 10.1097/ICO.0b013e31818a7d80. [DOI] [PubMed] [Google Scholar]
- 77.Chen D, Lam AKC, Cho P. A pilot study on the corneal biomechanical changes in short-term orthokeratology. Ophthalmic and Physiological Optics. 2009;29(4):464–471. doi: 10.1111/j.1475-1313.2008.00625.x. [DOI] [PubMed] [Google Scholar]
- 78.Mao XJ, Huang CC, Chen L, Lü F. A study on the effect of the corneal biomechanical properties undergoing overnight orthokeratology. Chinese Journal of Ophthalmology. 2010;46(3):209–213. [PubMed] [Google Scholar]
- 79.Nieto-Bona A, González-Mesa A, Villa-Collar C, Lorente-Velázquez A. Biomechanical properties in corneal refractive therapy during adaptation period and after treatment interruption: a pilot study. Journal of Optometry. 2012;5(4):164–1170. [Google Scholar]
- 80.Jayakumar J, Swarbrick HA. The effect of age on short-term orthokeratology. Optometry and Vision Science. 2005;82(6):505–511. doi: 10.1097/01.opx.0000168583.17327.6d. [DOI] [PubMed] [Google Scholar]
- 81.Hamilton DR, Johnson RD, Lee N, Bourla N. Differences in the corneal biomechanical effects of surface ablation compared with laser in situ keratomileusis using a microkeratome or femtosecond laser. Journal of Cataract and Refractive Surgery. 2008;34(12):2049–2056. doi: 10.1016/j.jcrs.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 82.Qazi MA, Sanderson JP, Mahmoud AM, Yoon EY, Roberts CJ, Pepose JS. Postoperative changes in intraocular pressure and corneal biomechanical metrics. Laser in situ keratomileusis versus laser-assisted subepithelial keratectomy. Journal of Cataract and Refractive Surgery. 2009;35(10):1774–1788. doi: 10.1016/j.jcrs.2009.05.041. [DOI] [PubMed] [Google Scholar]
- 83.Kamiya K, Shimizu K, Ohmoto F. Comparison of the changes in corneal biomechanical properties after photorefractive keratectomy and laser in situ keratomileusis. Cornea. 2009;28(7):765–769. doi: 10.1097/ICO.0b013e3181967082. [DOI] [PubMed] [Google Scholar]
- 84.Kamiya K, Shimizu K, Ohmoto F. Time course of corneal biomechanical parameters after laser in situ keratomileusis. Ophthalmic Research. 2009;42(3):167–171. doi: 10.1159/000230670. [DOI] [PubMed] [Google Scholar]
- 85.Shah S, Laiquzzaman M. Comparison of corneal biomechanics in pre and post-refractive surgery and keratoconic eyes by Ocular Response Analyser. Contact Lens and Anterior Eye. 2009;32(3):129–132. doi: 10.1016/j.clae.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 86.Chen S, Chen D, Wang J, Lu F, Wang Q, Qu J. Changes in Ocular Response Analyzer parameters after LASIK. Journal of Refractive Surgery. 2010;26(4):279–288. doi: 10.3928/1081597X-20100218-04. [DOI] [PubMed] [Google Scholar]
- 87.Ryan DS, Coe CD, Howard RS, Edwards JD, Bower KS. Corneal biomechanics following Epi-LASIK. Journal of Refractive Surgery. 2011;27(6):458–464. doi: 10.3928/1081597X-20110112-01. [DOI] [PubMed] [Google Scholar]
- 88.Gatinel D, Chaabouni S, Adam P-A, Munck J, Puech M, Hoang-Xuan T. Corneal hysteresis, resistance factor, topography, and pachymetry after corneal lamellar flap. Journal of Refractive Surgery. 2007;23(1):76–84. doi: 10.3928/1081-597X-20070101-12. [DOI] [PubMed] [Google Scholar]
- 89.Witzel de Medeiros F, Sinha-Roy A, Ruiz Alves M, Wilson SE, Dupps WJ. Differences in the early biomechanical effects of hyperopic and myopic laser in situ keratomileusis. Journal of Cataract and Refractive Surgery. 2010;36(6):947–953. doi: 10.1016/j.jcrs.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaushik S, Pandav SS, Banger A, Aggarwal K, Gupta A. Relationship between corneal biomechanical properties, central corneal thickness, and intraocular pressure across the spectrum of glaucoma. The American Journal of Ophthalmology. 2012;153(5):840–849. doi: 10.1016/j.ajo.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 91.Kirwan C, O’Malley D, O’Keefe M. Corneal hysteresis and corneal resistance factor in keratoectasia: findings using the Reichert Ocular Response Analyzer. Ophthalmologica. 2008;222(5):334–337. doi: 10.1159/000145333. [DOI] [PubMed] [Google Scholar]
- 92.Avetisov SE, Novikov IA, Bubnova IA, Antonov AA, Siplivyi VI. Determination of corneal elasticity coefficient using the ORA database. Journal of Refractive Surgery. 2010;26(7):520–524. doi: 10.3928/1081597X-20091030-01. [DOI] [PubMed] [Google Scholar]
- 93.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. Journal of Cataract and Refractive Surgery. 2003;29(9):1780–1785. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 94.El-Raggal TM. Riboflavin-ultraviolet A corneal cross-linking for keratoconus. Middle East African Journal of Ophthalmology. 2009;16(4):256–259. doi: 10.4103/0974-9233.58418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Poli M, Cornut PL, Balmitgere T, Aptel F, Janin H, Burillon C. Prospective study of corneal collagen cross-linking efficacy and tolerance in the treatment of keratoconus and Corneal ectasia: 3-year results. Cornea. 2013;32(5):583–590. doi: 10.1097/ICO.0b013e31825e8414. [DOI] [PubMed] [Google Scholar]
- 96.Bettis DI, Hsu M, Moshirfar M. Corneal collagen cross-linking for nonectatic disorders: a systematic review. Journal of Refractive Surgery. 2012;28(11):798–807. doi: 10.3928/1081597X-20121011-09. [DOI] [PubMed] [Google Scholar]
- 97.Vinciguerra P, Albè E, Mahmoud AM, Trazza S, Hafezi F, Roberts CJ. Intra- and postoperative variation in Ocular Response Analyzer parameters in keratoconic eyes after corneal cross-linking. Journal of Refractive Surgery. 2010;26(9):669–676. doi: 10.3928/1081597X-20100331-01. [DOI] [PubMed] [Google Scholar]
- 98.Gutierrez R, Lopez I, Villa-Collar C, Gonzalez-Meijome JM. Corneal transparency after cross-linking for keratoconus: 1-year follow-up. Journal of Refractive Surgery. 2012;28(11):781–786. doi: 10.3928/1081597X-20121011-06. [DOI] [PubMed] [Google Scholar]
- 99.Goldich Y, Barkana Y, Morad Y, Hartstein M, Avni I, Zadok D. Can we measure corneal biomechanical changes after collagen cross-linking in eyes with keratoconus? A pilot study. Cornea. 2009;28(5):498–502. doi: 10.1097/ICO.0b013e318190734d. [DOI] [PubMed] [Google Scholar]
- 100.Piñero DP, Alio JL, Teus MA, Barraquer RI, Uceda-Montañés A. Modeling the intracorneal ring segment effect in keratoconus using refractive, Keratometric, and corneal aberrometric data. Investigative Ophthalmology and Visual Science. 2010;51(11):5583–5591. doi: 10.1167/iovs.09-5017. [DOI] [PubMed] [Google Scholar]
- 101.Piñero DP, Alio JL, Uceda-Montanes A, Kady BE, Pascual I. Intracorneal ring segment implantation in corneas with post-laser in situ keratomileusis keratectasia. Ophthalmology. 2009;116(9):1665–1674. doi: 10.1016/j.ophtha.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 102.Patel S, Marshall J, Fitzke FW., III Model for deriving the optical performance of the myopic eye corrected with an intracorneal ring. Journal of Refractive Surgery. 1995;11(4):248–252. doi: 10.3928/1081-597X-19950701-08. [DOI] [PubMed] [Google Scholar]
- 103.Piñero DP, Alio JL. Intracorneal ring segments in ectatic corneal disease—a review. Clinical and Experimental Ophthalmology. 2010;38(2):154–167. doi: 10.1111/j.1442-9071.2010.02197.x. [DOI] [PubMed] [Google Scholar]
- 104.Dauwe C, Touboul D, Roberts CJ, et al. Biomechanical and morphological corneal response to placement of intrastromal corneal ring segments for keratoconus. Journal of Cataract and Refractive Surgery. 2009;35(10):1761–1767. doi: 10.1016/j.jcrs.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 105.Gorgun E, Kucumen RB, Yenerel NM. Influence of intrastromal corneal ring segment implantation on corneal biomechanical parameters in keratoconic eyes. Japanese Journal of Ophthalmology. 2011;55(5):467–471. doi: 10.1007/s10384-011-0057-8. [DOI] [PubMed] [Google Scholar]
- 106.Alio JL, Piero DP, Daxer A. Clinical outcomes after complete ring implantation in corneal ectasia using the femtosecond technology: a pilot study. Ophthalmology. 2011;118(7):1282–1290. doi: 10.1016/j.ophtha.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 107.Salgado-Borges JM, Costa-Ferreira C, Monteiro M, et al. Refractive, tomographic and biomechanical outcomes after implantation of ferrara ICRS in keratoconus patients. International Journal of Keratoconus and Ectatic Corneal Diseases. 2012;1(1):16–21. [Google Scholar]
- 108.Piñero DP, Alio JL, Barraquer RI, Michael R. Corneal biomechanical changes after intracorneal ring segment implantation in Keratoconus. Cornea. 2012;31(5):491–499. doi: 10.1097/ICO.0b013e31821ee9f4. [DOI] [PubMed] [Google Scholar]
- 109.Pena-Garcia P, Vega-Estrada A, Barraquer RI, Burguera-Gimenez N, Alio JL. Intracorneal ring segment in keratoconus: a model to predict visual changes induced by the surgery. Investigative Ophthalmology & Visual Science. 2012;53(13):8447–8457. doi: 10.1167/iovs.12-10639. [DOI] [PubMed] [Google Scholar]
- 110.Mikielewicz M, Kotliar K, Barraquer RI, Michael R. Air-pulse corneal applanation signal curve parameters for the characterisation of keratoconus. The British Journal of Ophthalmology. 2011;95(6):793–798. doi: 10.1136/bjo.2010.188300. [DOI] [PubMed] [Google Scholar]
- 111.Ambrosio R, Borges JS, Costa-Ferreira C, et al. Intrastromal corneal ring segments for keratoconus: results and correlation with preoperative corneal biomechanics. The Brazilian Journal of Ophthalmology. 2012;71(2):89–99. [Google Scholar]
- 112.Yenerel NM, Kucumen RB, Gorgun E. Changes in corneal biomechanics in patients with keratoconus after penetrating keratoplasty. Cornea. 2010;29(11):1247–1251. doi: 10.1097/ICO.0b013e3181ca6383. [DOI] [PubMed] [Google Scholar]
- 113.Laiquzzaman M, Tambe K, Shah S. Comparison of biomechanical parameters in penetrating keratoplasty and normal eyes using the Ocular Response Analyser. Clinical and Experimental Ophthalmology. 2010;38(8):758–763. doi: 10.1111/j.1442-9071.2010.02353.x. [DOI] [PubMed] [Google Scholar]
- 114.Hosny M, Hassaballa MAM, Shalaby A. Changes in corneal biomechanics following different keratoplasty techniques. Clinical Ophthalmology. 2011;5(1):767–770. doi: 10.2147/OPTH.S21297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jafarinasab MR, Feizi S, Javadi MA, Hashemloo A. Graft biomechanical properties after penetrating keratoplasty versus deep anterior lamellar keratoplasty. Current Eye Research. 2011;36(5):417–421. doi: 10.3109/02713683.2011.556303. [DOI] [PubMed] [Google Scholar]
- 116.Feizi S, Einollahi B, Yazdani S, Hashemloo A. Graft biomechanical properties after penetrating keratoplasty in keratoconus. Cornea. 2012;31(8):855–858. doi: 10.1097/ICO.0b013e31823f8ce4. [DOI] [PubMed] [Google Scholar]
- 117.Jordan JF, Joergens S, Dinslage S, Dietlein TS, Krieglstein GK. Central and paracentral corneal pachymetry in patients with normal tension glaucoma and ocular hypertension. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2006;244(2):177–182. doi: 10.1007/s00417-005-0053-0. [DOI] [PubMed] [Google Scholar]
- 118.Pakravan M, Parsa A, Sanagou M, Parsa CF. Central corneal thickness and correlation to optic disc size: a potential link for susceptibility to glaucoma. The British Journal of Ophthalmology. 2007;91(1):26–28. doi: 10.1136/bjo.2006.106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lesk MR, Hafez AS, Descovich D. Relationship between central corneal thickness and changes of optic nerve head topography and blood flow after intraocular pressure reduction in open-angle glaucoma and ocular hypertension. Archives of Ophthalmology. 2006;124(11):1568–1572. doi: 10.1001/archopht.124.11.1568. [DOI] [PubMed] [Google Scholar]
- 120.Bochmann F, Ang GS, Azuara-Blanco A. Lower corneal hysteresis in glaucoma patients with acquired pit of the optic nerve (APON) Graefe’s Archive for Clinical and Experimental Ophthalmology. 2008;246(5):735–738. doi: 10.1007/s00417-007-0756-5. [DOI] [PubMed] [Google Scholar]
- 121.Morita T, Shoji N, Kamiya K, Fujimura F, Shimizu K. Corneal biomechanical properties in normal-tension glaucoma. Acta Ophthalmologica. 2012;90(1):e48–e53. doi: 10.1111/j.1755-3768.2011.02242.x. [DOI] [PubMed] [Google Scholar]
- 122.Congdon NG, Broman AT, Bandeen-Roche K, Grover D, Quigley HA. Central corneal thickness and corneal hysteresis associated with glaucoma damage. The American Journal of Ophthalmology. 2006;141(5):868–875. doi: 10.1016/j.ajo.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 123.Sullivan-Mee M, Billingsley SC, Patel AD, Halverson KD, Alldredge BR, Qualls C. Ocular Response Analyzer in subjects with and without glaucoma. Optometry and Vision Science. 2008;85(6):463–470. doi: 10.1097/OPX.0b013e3181784673. [DOI] [PubMed] [Google Scholar]
- 124.Wells AP, Garway-Heath DF, Poostchi A, Wong T, Chan KCY, Sachdev N. Corneal hysteresis but not corneal thickness correlates with optic nerve surface compliance in glaucoma patients. Investigative Ophthalmology and Visual Science. 2008;49(8):3262–3268. doi: 10.1167/iovs.07-1556. [DOI] [PubMed] [Google Scholar]
- 125.Shah S, Laiquzzaman M, Mantry S, Cunliffe I. Ocular response analyser to assess hysteresis and corneal resistance factor in low tension, open angle glaucoma and ocular hypertension. Clinical and Experimental Ophthalmology. 2008;36(6):508–513. doi: 10.1111/j.1442-9071.2008.01828.x. [DOI] [PubMed] [Google Scholar]
- 126.Sun L, Shen M, Wang J, et al. Recovery of corneal hysteresis after reduction of intraocular pressure in chronic primary angle-closure glaucoma. The American Journal of Ophthalmology. 2009;147(6):1061–1066. doi: 10.1016/j.ajo.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 127.Mangouritsas G, Morphis G, Mourtzoukos S, Feretis E. Association between corneal hysteresis and central corneal thickness in glaucomatous and non-glaucomatous eyes. Acta Ophthalmologica. 2009;87(8):901–905. doi: 10.1111/j.1755-3768.2008.01370.x. [DOI] [PubMed] [Google Scholar]
- 128.Burgoyne CF, Crawford Downs J, Bellezza AJ, Francis Suh J-K, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Progress in Retinal and Eye Research. 2005;24(1):39–73. doi: 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 129.Ang GS, Bochmann F, Townend J, Azuara-Blanco A. Corneal biomechanical properties in primary open angle glaucoma and normal tension glaucoma. Journal of Glaucoma. 2008;17(4):259–262. doi: 10.1097/IJG.0b013e31815c3a93. [DOI] [PubMed] [Google Scholar]
- 130.Anand A, de Moraes CGV, Teng CC, Tello C, Liebmann JM, Ritch R. Corneal hysteresis and visual field asymmetry in open angle glaucoma. Investigative Ophthalmology and Visual Science. 2010;51(12):6514–6518. doi: 10.1167/iovs.10-5580. [DOI] [PubMed] [Google Scholar]
- 131.Prata TS, Lima VC, Guedes LM, et al. Association between corneal biomechanical properties and optic nerve head morphology in newly diagnosed glaucoma patients. Clinical & Experimental Ophthalmology. 2012;40(7):682–688. doi: 10.1111/j.1442-9071.2012.02790.x. [DOI] [PubMed] [Google Scholar]
- 132.Kotecha A. What biomechanical properties of the cornea are relevant for the clinician? Survey of Ophthalmology. 2007;52(6, supplement 2):S109–S114. doi: 10.1016/j.survophthal.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 133.Meek KM, Tuft SJ, Huang Y, et al. Changes in collagen orientation and distribution in keratoconus corneas. Investigative Ophthalmology and Visual Science. 2005;46(6):1948–1956. doi: 10.1167/iovs.04-1253. [DOI] [PubMed] [Google Scholar]
- 134.Hurmeric V, Sahin A, Ozge G, Bayer A. The relationship between corneal biomechanical properties and confocal microscopy findings in normal and keratoconic eyes. Cornea. 2010;29(6):641–649. doi: 10.1097/ICO.0b013e3181c11dc6. [DOI] [PubMed] [Google Scholar]
- 135.Mocan MC, Yilmaz PT, Irkec M, Orhan M. In vivo confocal microscopy for the evaluation of corneal microstructure in keratoconus. Current Eye Research. 2008;33(11-12):933–939. doi: 10.1080/02713680802439219. [DOI] [PubMed] [Google Scholar]
- 136.Fontes BM, Ambrósio R, Jr., Jardim D, Velarde GC, Nosé W. Corneal biomechanical metrics and anterior segment parameters in mild keratoconus. Ophthalmology. 2010;117(4):673–679. doi: 10.1016/j.ophtha.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 137.Saad A, Lteif Y, Azan E, Gatinel D. Biomechanical properties of keratoconus suspect eyes. Investigative Ophthalmology and Visual Science. 2010;51(6):2912–2916. doi: 10.1167/iovs.09-4304. [DOI] [PubMed] [Google Scholar]
- 138.Fontes BM, Ambrósio R, Jr., Velarde GC, Nosé W. Ocular Response Analyzer measurements in keratoconus with normal central corneal thickness compared with matched normal control eyes. Journal of Refractive Surgery. 2011;27(3):209–215. doi: 10.3928/1081597X-20100415-02. [DOI] [PubMed] [Google Scholar]
- 139.Cohen EJ, Myers JS. Keratoconus and normal-tension glaucoma: a study of the possible association with abnormal biomechanical properties as measured by corneal hysteresis. Cornea. 2010;29(9):955–970. doi: 10.1097/ICO.0b013e3181ca363c. [DOI] [PubMed] [Google Scholar]
- 140.Piñero DP, Alio JL, Barraquer RI, Michael R, Jiménez R. Corneal biomechanics, refraction, and corneal aberrometry in keratoconus: an integrated study. Investigative Ophthalmology and Visual Science. 2010;51(4):1948–1955. doi: 10.1167/iovs.09-4177. [DOI] [PubMed] [Google Scholar]
- 141.Johnson RD, Nguyen MT, Lee N, Hamilton DR. Corneal biomechanical properties in normal, forme fruste keratoconus, and manifest keratoconus after statistical correction for potentially confounding factors. Cornea. 2011;30(5):516–523. doi: 10.1097/ICO.0b013e3181f0579e. [DOI] [PubMed] [Google Scholar]
- 142.Galletti JG, Pförtner T, Bonthoux FF. Improved keratoconus detection by Ocular Response Analyzer testing after consideration of corneal thickness as a confounding factor. Journal of Refractive Surgery. 2012;28(3):202–208. doi: 10.3928/1081597X-20120103-03. [DOI] [PubMed] [Google Scholar]
- 143.Ambekar R, Toussaint KC, Wagoner Johnson A. The effect of keratoconus on the structural, mechanical, and optical properties of the cornea. Journal of the Mechanical Behavior of Biomedical Materials. 2011;4(3):223–236. doi: 10.1016/j.jmbbm.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 144.Fontes BM, Junior RA, Jardim D, Velarde GC, Nosé W. Ability of corneal biomechanical metrics and anterior segment data in the differentiation of keratoconus and healthy corneas. Arquivos Brasileiros de Oftalmologia. 2010;73(4):333–337. doi: 10.1590/s0004-27492010000400006. [DOI] [PubMed] [Google Scholar]
- 145.Schweitzer C, Roberts CJ, Mahmoud AM, Colin J, Maurice-Tison S, Kerautret J. Screening of forme fruste keratoconus with the Ocular Response Analyzer. Investigative Ophthalmology and Visual Science. 2010;51(5):2403–2410. doi: 10.1167/iovs.09-3689. [DOI] [PubMed] [Google Scholar]
- 146.Wolffsohn JS, Safeen S, Shah S, Laiquzzaman M. Changes of corneal biomechanics with keratoconus. Cornea. 2012;31(8):849–854. doi: 10.1097/ICO.0b013e318243e42d. [DOI] [PubMed] [Google Scholar]
- 147.Adamis AP, Filatov V, Tripathi BJ, Tripathi RC. Fuchs’ endothelial dystrophy of the cornea. Survey of Ophthalmology. 1993;38(2):149–168. doi: 10.1016/0039-6257(93)90099-s. [DOI] [PubMed] [Google Scholar]
- 148.Clemmensen K, Hjortdal J. Intraocular pressure and corneal biomechanics in Fuchs' endothelial dystrophy and after posterior lamellar keratoplasty. Acta Ophthalmologica. 2013 doi: 10.1111/aos.12137. [DOI] [PubMed] [Google Scholar]
- 149.Wiemer NGM, Dubbelman M, Kostense PJ, Ringens PJ, Polak BCP. The influence of chronic diabetes mellitus on the thickness and the shape of the anterior and posterior surface of the cornea. Cornea. 2007;26(10):1165–1170. doi: 10.1097/ICO.0b013e31814fa82f. [DOI] [PubMed] [Google Scholar]
- 150.Sudhir RR, Raman R, Sharma T. Changes in the corneal endothelial cell density and morphology in patients with type 2 diabetes mellitus: a population-based study, sankara nethralaya diabetic retinopathy and molecular genetics study (SN-DREAMS, report 23) Cornea. 2012;31(10):1119–1122. doi: 10.1097/ICO.0b013e31823f8e00. [DOI] [PubMed] [Google Scholar]
- 151.Kotecha A, Oddone F, Sinapis C, et al. Corneal biomechanical characteristics in patients with diabetes mellitus. Journal of Cataract and Refractive Surgery. 2010;36(11):1822–1828. doi: 10.1016/j.jcrs.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 152.Scheler A, Spoerl E, Boehm AG. Effect of diabetes mellitus on corneal biomechanics and measurement of intraocular pressure. Acta Ophthalmologica. 2012;90(6):e447–e451. doi: 10.1111/j.1755-3768.2012.02437.x. [DOI] [PubMed] [Google Scholar]
- 153.Castro DPE, Prata TS, Lima VC, Biteli LG, de Moraes CGV, Paranhos A. Corneal viscoelasticity differences between diabetic and nondiabetic glaucomatous patients. Journal of Glaucoma. 2010;19(5):341–343. doi: 10.1097/IJG.0b013e3181b4caa1. [DOI] [PubMed] [Google Scholar]
- 154.Goldich Y, Barkana Y, Gerber Y, et al. Effect of diabetes mellitus on biomechanical parameters of the cornea. Journal of Cataract and Refractive Surgery. 2009;35(4):715–719. doi: 10.1016/j.jcrs.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 155.Hager A, Wegscheider K, Wiegand W. Changes of extracellular matrix of the cornea in diabetes mellitus. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2009;247(10):1369–1374. doi: 10.1007/s00417-009-1088-4. [DOI] [PubMed] [Google Scholar]
- 156.Şahin A, Bayer A, Özge G, Mumcuoğlu T. Corneal biomechanical changes in diabetes mellitus and their influence on intraocular pressure measurements. Investigative Ophthalmology and Visual Science. 2009;50(10):4597–4604. doi: 10.1167/iovs.08-2763. [DOI] [PubMed] [Google Scholar]
- 157.Ambrósio RJ, Jr., Caldas DL, Ramos IC, Santos RT, Pimentel LN, Roberts CJ. Corneal biomechanical assessment using dynamic ultra high-speed Scheimpflug technology noncontact tonometry (UHS-ST NCT): preliminary results. Proceedings of the American Society of Cataract and Refractive Surgery, the American Society of Ophthalmic Administrators (ASCRS-ASOA '11); March 2011; San Diego, Calif, USA. [Google Scholar]
- 158.Ambrósio R, Jr., Nogueira LP, Caldas DL, et al. Evaluation of corneal shape and biomechanics before LASIK. International Ophthalmology Clinics. 2011;51(2):11–38. doi: 10.1097/IIO.0b013e31820f1d2d. [DOI] [PubMed] [Google Scholar]
- 159.Hong J, Xu J, Wei A, et al. A new tonometer—the Corvis ST tonometer: clinical comparison with noncontact and Goldmann applanation tonometers. Investigative Ophthalmology and Visual Science. 2013;54(1):659–665. doi: 10.1167/iovs.12-10984. [DOI] [PubMed] [Google Scholar]
- 160.Hon Y, Lam AK. Corneal deformation measurement using Scheimpflug noncontact tonometry. Optometry and Vision Science. 2013;90(1):e1–e8. doi: 10.1097/OPX.0b013e318279eb87. [DOI] [PubMed] [Google Scholar]
- 161.Leung CK, Ye C, Weinreb RN. An ultra-high-speed Scheimpflug camera for evaluation of corneal deformation response and its impact on IOP measurement. Investigative Ophthalmology and Visual Science. 2013;54(4):2885–2892. doi: 10.1167/iovs.12-11563. [DOI] [PubMed] [Google Scholar]
- 162.Bonatti JA, Bechara SJ, Carricondo PC, Kara-José N. Proposal for a new approach to corneal biomechanics: dynamic corneal topography. Arquivos Brasileiros de Oftalmologia. 2009;72(2):264–267. doi: 10.1590/s0004-27492009000200028. [DOI] [PubMed] [Google Scholar]
- 163.Roberts C, Marous J, Mahmoud A. Dynamic corneal surface topography: in vivo measurement of biomechanical properties. Proceedings of the Oral Communication in 19th Biennial Meeting of the International Society for Eye Research; 2010; Montreal, Canada. [Google Scholar]
- 164.Glass DH, Roberts CJ, Litsky AS, Weber PA. A viscoelastic biomechanical model of the cornea describing the effect of viscosity and elasticity on hysteresis. Investigative Ophthalmology and Visual Science. 2008;49(9):3919–3926. doi: 10.1167/iovs.07-1321. [DOI] [PubMed] [Google Scholar]
- 165.Alonso-Caneiro D, Karnowski K, Kaluzny BJ, Kowalczyk A, Wojtkowski M. Assessment of corneal dynamics with high-speed swept source Optical Coherence Tomography combined with an air puff system. Optics Express. 2011;19(15):14188–14199. doi: 10.1364/OE.19.014188. [DOI] [PubMed] [Google Scholar]
- 166.Scarcelli G, Pineda R, Yun SH. Brillouin optical microscopy for corneal biomechanics. Investigative Ophthalmology and Visual Science. 2012;53(1):185–190. doi: 10.1167/iovs.11-8281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dupps WJ, Jr., Netto MV, Herekar S, Krueger RR. Surface wave elastometry of the cornea in porcine and human donor eyes. Journal of Refractive Surgery. 2007;23(1):66–75. doi: 10.3928/1081-597x-20070101-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liu J, He X, Pan X, Roberts CJ. Ultrasonic model and system for measurement of corneal biomechanical properties and validation on phantoms. Journal of Biomechanics. 2007;40(5):1177–1182. doi: 10.1016/j.jbiomech.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 169.He X, Liu J. A quantitative ultrasonic spectroscopy method for noninvasive determination of corneal biomechanical properties. Investigative Ophthalmology and Visual Science. 2009;50(11):5148–5154. doi: 10.1167/iovs.09-3439. [DOI] [PubMed] [Google Scholar]
- 170.Deffieux T, Montaldo G, Tanter M, Fink M. Shear wave spectroscopy for in vivo quantification of human soft tissues visco-elasticity. IEEE Transactions on Medical Imaging. 2009;28(3):313–322. doi: 10.1109/TMI.2008.925077. [DOI] [PubMed] [Google Scholar]
- 171.Tanter M, Bercoff J, Athanasiou A, et al. Quantitative assessment of breast lesion viscoelasticity: initial clinical results using supersonic shear imaging. Ultrasound in Medicine and Biology. 2008;34(9):1373–1386. doi: 10.1016/j.ultrasmedbio.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 172.Tanter M, Touboul D, Gennisson JL, Bercoff J, Fink M. High-resolution quantitative imaging of cornea elasticity using supersonic shear imaging. IEEE Transactions on Medical Imaging. 2009;28(12):1881–1893. doi: 10.1109/TMI.2009.2021471. [DOI] [PubMed] [Google Scholar]
- 173.Calkins JL, Hochheimer BF, Stark WJ. Corneal wound healing: holographic stress-test analysis. Investigative Ophthalmology and Visual Science. 1981;21(2):322–334. [PubMed] [Google Scholar]
- 174.Jaycock PD, Lobo L, Ibrahim J, Tyrer J, Marshall J. Interferometric technique to measure biomechanical changes in the cornea induced by refractive surgery. Journal of Cataract and Refractive Surgery. 2005;31(1):175–184. doi: 10.1016/j.jcrs.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 175.Cartwright NEK, Tyrer JR, Marshall J. In vitro quantification of the stiffening effect of corneal cross-linking in the human cornea using radial shearing speckle pattern interferometry. Journal of Refractive Surgery. 2012;28(7):503–508. doi: 10.3928/1081597X-20120613-01. [DOI] [PubMed] [Google Scholar]
- 176.Grabner G, Eilmsteiner R, Steindl C, Ruckhofer J, Mattioli R, Husinsky W. Dynamic corneal imaging. Journal of Cataract and Refractive Surgery. 2005;31(1):163–174. doi: 10.1016/j.jcrs.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 177.Ford MR, Dupps WJ, Jr., Rollins AM, Roy AS, Hu Z. Method for optical coherence elastography of the cornea. Journal of Biomedical Optics. 2011;16(1) doi: 10.1117/1.3526701.016005 [DOI] [PMC free article] [PubMed] [Google Scholar]


