Abstract
Cardiovascular disease (CVD) is one of the leading causes of death in the Western world. The replacement of damaged vessels and valves has been practiced since the 1950’s. Synthetic grafts, usually made of bio-inert materials, are long-lasting and mechanically relevant, but fail when it comes to “biointegration”. Decellularized matrices, instead, can be considered biological grafts capable of stimulating in vivo migration and proliferation of endothelial cells (ECs), recruitment and differentiation of mural cells, finally, culminating in the formation of a biointegrated tissue. Decellularization protocols employ osmotic shock, ionic and non-ionic detergents, proteolitic digestions and DNase/RNase treatments; most of them effectively eliminate the cellular component, but show limitations in preserving the native structure of the extracellular matrix (ECM). In this review, we examine the current state of the art relative to decellularization techniques and biological performance of decellularized heart, valves and big vessels. Furthermore, we focus on the relevance of ECM components, native and resulting from decellularization, in mediating in vivo host response and determining repair and regeneration, as opposed to graft corruption.
Keywords: Cardiovascular, decellularization, matrix, progenitors, tissue engineering
Introduction
Organ transplantation is still the ultimate treatment for end-stage organ failure. Even if donor organs were not in short supply, the transplant recipient would still be at risk of chronic immune rejection and lifelong immunosuppression treatment. The change of paradigm that tissue engineering, combined to regenerative medicine, has introduced is in providing exogenously fabricated “biological supports”, i.e. cells, biomaterials, growth factors, or combination of them, which could boost the “endogenous biofabrication” of new tissues. To date, although numerous modern technologies, such as the use of bioprinters, bioreactors and induced pluripotent stem cells, have been employed to fabricate tissues, the generation of a functional whole organ has not yet been accomplished. This is due, in part, to a lacking knowledge of mechanisms of organ development, and also to logistic issues.
The approach of combining biomaterials with cells and growth factors is not sufficient to recapitulate the complexity of tissue regeneration. The use of decellularized matrices, at least, would overcome the need for the tissue engineer to artificially recreate the conditions for ECM deposition [1,2]. Decellularized matrices, if properly prepared, would offer a microenvironment naturally dense of molecular cues able to drive endogenous biofabrication of a new patent tissue. Several drawbacks might be encountered when a native matrix is processed. Alterations in the ECM composition could result in mis-repopulation of the decellularized matrix once implanted in vivo.
Decellularized matrix for heart engineering
Heart failure (HF) is defined as an abnormality of cardiac structure or function leading to failure to deliver oxygen at a rate commensurate to metabolic needs of tissues. It can be caused by several conditions affecting the heart, such as ischemic heart disease, valvular heart disease, hypertension or cardiomyopathies [3].
An estimated 83.6 million American adults (>1 in 3) have one or more types of cardiovascular disease (CVD), 5.1 million Americans ≥20 years of age suffer from HF. HF incidence approaches 10 per 1000 population after 65 years of age. The 2009 overall any-mention death rate for HF was 82.3 [4].
Despite the advances in clinical evaluation and management, heart transplantation is still the mainstay for end-stage HF [5,6]. The gap between the supply and the demand for donor organs [7], as well as the consequences for the patient of lifelong immunosuppression and chronic rejection, make the implantation of a bioartificial heart highly desirable alternative to allo-transplantation. While the regeneration of a functional organ has not been accomplished yet, tissue engineering and regenerative medicine research have obtained promising results for heart regeneration. The cardiac tissue engineering traditional approach relies mainly on the use of synthetic or biological matrix materials and heart cells. Usually scaffold materials such as gelatin, collagen, alginate, or synthetic polymers are seeded in vitro with cardiac cells to reconstitute contractile cardiac muscle-like patches. Tissue coherent contractions, low diastolic tension, and syncytial propagation of action potentials are then tested in vivo once the patch is implanted [8,9]. Insufficient cell migration into the scaffold and an inflammatory reaction due to scaffold biodegradation are often encountered in vivo and can be remedied by using Okano’s cell sheet technology, which layers cell sheets to construct 3-D functional tissues without any artificial scaffold [10]. However, the effective support of a severely compromised heart requires the fabrication of hearts or heart patches with proper size, proven contractile features and vascular provision. Parallel channels and artificial oxygen carriers have been investigated to provide appropriate metabolic exchange to engineered heart patches [11-13].
Biocompatible three dimensional ECM-based scaffolds with preserved geometry and vascular tree can be generated from the decellularization of cadaveric hearts [14]. Decellularized hearts might be suitable to engineer or regenerate the entire organ and can be used for whole-organ transplantation or as a source of myocardial tissue parts. Xenogeneic ECMs have already been used successfully to replace/repair numerous tissues and organs in both preclinical animal studies and human clinical applications. In particular, the ECM derived from the porcine small intestinal submucosa (SIS) and urinary bladder submucosa (UBS), have been employed as a vascular graft [15-18]. Ott et al. first described a method to decellularize hearts by coronary perfusion [19]. In this procedure, the aorta of a rat heart was cannulated for retrograde heart perfusion with ionic detergents. The decellularization preserved the underlying extracellular matrix and produced an acellular, perfusable vascular architecture, competent acellular valves and intact chamber geometry. The constructs were then reseeded with cardiac and endothelial cells and maintained for up to 28 days in a bioreactor simulating coronary perfusion, physiological load and electrical stimulation. The cultured organoid was able to generate contractions. The perfusion-decellularization approach is particularly efficient for whole organ decellularization since it reduces the diffusion distance required for decellularizing agents to reach cells. It also takes advantage of convective forces to facilitate tissue removal of cellular material [20]. Several protocols have been employed for the generation of acellular cardiac scaffolds from whole hearts or myocardial tissue (see Table 1). The quality of each resulting cardiac ECM can be subjected to variables like the age and the pathological conditions of the donor. Moreover, unless the cardiac ECM is solubilized in a hydrogel or used as a small cardiac patch, the evaluation of its performance in vivo in models of whole heart transplantation is not yet practically feasible.
Table 1.
Some of the most commonly used protocols of decellularization and recellularization
| tissue/organ | Decell. method | Recell. method | Notes | Ref |
|---|---|---|---|---|
| Human pericardium from cadaveric donors | Hypotonic buffer, SDS in hypotonic buffer, and nuclease solution | In vitro seeding of human dermal fibroblasts and A549 cells | No difference in glycosaminoglycan content and tensile strength | [160] |
| Porcine ventricular myocardial tissue | SDS and Triton X-100 detergents. Pepsin-solubilization of the myocardial matrix | In vitro seeding of neonatal rat cardiomyocytes and in vivo injection in left ventricle of rats | Maintained glycosaminoglycan content. Good cell-conductivity | [161-163] |
| Intact adult porcine heart | Pulsatile retrograde aortic perfusion. Serial perfusion of enzymatic, non-ionic and ionic detergent, hypotonic and hypertonic solutions | In vitro seeding of chicken cardiomyocyte | ECM retained collagen, elastin, and glycosaminoglycans, and mechanical integrity | [164] |
| Porcine whole heart | Langendorff decellularization model: perfusion of Trypsin/EDTA and TritonX 100/deoxycholic acid (DCA). | none | Retained collagen, proteoglycan and elastin | [165] |
| Adult rat heart | Comparison of different solutions: 1) SDS/TritonX100-based v/s 2) Trypsin plus Triton/DCA-based v/s 3) SDS/DCA/saponin-based | In all groups successful reseeding with C2C12 myoblasts in vitro. | Laminin detected in all groups. Collagen IV removed in group 2, elastin not detected in the last group | [166] |
| Human Left ventricular myocardium tissue | Comparison between SDS-based, Triton X-100-based, DCA-based, hypo/hypertonic solution-based decellularization protocols | In vitro culture with mesenchymal stem cells, iPS-derived cardiomyocytes and native neonatal mouse cardiomyocytes | All the protocols support cell viability and growth. Best cell removal and ECM architecture maintenance with SDS-based protocol | [167] |
Decellularized matrix for heart valve engineering
Heart valves are responsible for unidirectional blood flow from atria to ventricles and from ventricles to cardiac arteries. Several pathologies, such as rheumatic fever or infective endocarditis, can lead to alteration of heart valve function. Congenital heart defects, including tetralogy of Fallot and Patent Ductus Arteriosus, can also affect valves. Valvular heart diseases are common in the general population and can lead to HF and arrhythmias [21]. About 2.5% of US population [21] is affected, and prevalence increases with age, reaching over 13% for those 75 and older [22]. Currently, optimal treatment for valvular heart diseases is either surgical repair or replacement [23]. Mechanical valves, the most commonly used prosthesis, have excellent durability but carry lifetime risks of thromboembolic and hemorrhagic events [24,25]. Bioprosthetic valves are often porcine aortic valves mounted on a stent or a Dacron support, sometimes they are made of bovine pericardium. Pericardium is usually bovine in origin, and pericardial valves are almost invariably stented. These valves are fixed in glutaraldehyde which crosslinks collagen fibers and reduces tissue antigenicity, and anti-mineralization treatments applied to the last generation valves reduces the risk of calcification [26,27]. However, the still unavoidable in vivo structural degeneration of xeno-bioprosthesis accounts for the higher risk of reoperation when compared to mechanical valve replacement [28]. In the future, tissue engineering is expected to provide enduring and non- immunogenic heart valves, possibly able to grow and remodel as the age of the patient advances [29,30]. In a traditional tissue engineering approach, a fundamental requirement for heart valve engineering is a three dimensional scaffold with appropriate mechanical properties which is seeded with appropriate cell types [31]. Examples of decellularized grafts include aortic homografts [32] and porcine valves and pericardium [33]. ECM can be obtained by using different protocols, all involving the processing of the tissue in a decellularization solution, containing alternatively ionic and/or non-ionic detergents or enzymatic digestion buffers, with hypotonic or hypertonic washings (see Table 2).
Table 2.
Schematic view of different protocols of decell/recell for valves
| Tissue | Decell. Method | Recell. Method | Notes | Ref |
|---|---|---|---|---|
| Porcine and sheep pulmonary valve conduits | Trypsin/EDTA digestion | In vivo after orthotopic implantation in sheep | reconstitution of surface endothelial cell monolayer and interstitial myofibroblasts. Calcifications | [168] |
| Porcine aortic valves | Comparison Triton X-100 v/s Trypsin | In vitro EC seeding | Changes in the extracellular matrix constitution in both cases, EC-mediated ECM deposition. | [169] |
| Porcine pulmonary heart valves | Deoxycholic acid | In vivo | Efficient cell-lysis without integrity loss of the interstitial proteoglycans | [170,171] |
| Porcine aortic and pulmonary roots | Tert-octylphenyl-polyoxyethylene plus sodium deoxycolate (TOPOE+DOC) compared to trypsin and SDS | In vitro seeding with human ECs and myofibroblasts | Toxic influence of SDS on EC viability. TOPOE+DOC completely remove porcine cells and enable recellularization | [172] |
| Porcin aortic and pulmonary valve conduits | Triton X-100 and hypotonic washings | None | Differential distribution of elastin and glycosaminoglycan | [173] |
| Porcine aortic valves | Polyethylene glycol and gamma irradiation | In vivo, subcutis of rats and descending aorta of dogs | Mechanical strength and collagen content not different from native porcine tissues. Good recellularization, few calcifications. | [174] |
| Porcine mitral heart valve | Deoxycholic acid | Othotopically in pigs | Deposition of fibrin and platelet material. limited ingrowth of both endothelial and myofibroblast-like cells | [175] |
| Aortic homograft leaflets | Trypsin | In vitro seeding with cardiac mesenchymal stromal cell | Rescuing up to the 90% of the original cell density and differentiation towards endothelial lineage. | [32] |
| Porcine pulmonary valve | Triton X-100 and ammonium hydroxide. | CD133 Ab-conjugation (self-seeding valves) and transplantation into the pulmonary position of sheep | Endothelialization. No calcification or thrombi. Good Young’s modulus and tensile strength. | [176] |
Results from the few clinical studies that have been performed are conflicting. The SynerGraft valve, which was developed as an acellular (nonglutaraldehyde-fixed) porcine aortic prosthetic valve [34], is the prototype of the decellularized valves used in the Ross procedure. CryoValve SynerGraft, as named after CryoLife patented the decellularization technology, has been tested in many clinical studies. In 2003, three children implanted with decellularized porcine heart valve SynerGraft died because of valve rupture or early severe degeneration, followed by the post-mortem observation that the xenogenic collagen matrix of the Synergraft valve elicits a strong inflammatory response. Also the grafts showed poor cellularization and fibrosis [35]. A subsequent report, comparing a new CryoValve SynerGraft decellularized pulmonary allograft to a standard cryopreserved allograft (SCA) in patients aged in a range of 4 months to 58 years, showed a similar rate of reoperation in patients undergoing the Ross procedure, while the quantification of valve regurgitation was in favour of Synergraft [36]. In conclusion, the early clinical and hemodynamic results were encouraging although not significantly different from the SCA. Another decellularized porcine pulmonary heart valve, Matrix P (AutoTissue GmbH), has been tested for the reconstruction of the right ventricular outflow tract (RVOT) during repair of congenital or acquired heart disease or to replace the pulmonary valve during the Ross procedure. Recently, in a study involving 93 pediatric patients undergoing RVOT reconstruction using Matrix P and Matrix P Plus valves, conduit failure was reported in 35.5% of the patients and conduit disfunction in 29% [37]. Failure occurred for either dilation or stenosis of the graft, and histological analysis showed inflammation and poor cellularization. Another study, where the Matrix P valves were implanted in 61 patients (range: 9 days to 50 years) with congenital heart disease, showed favourable intermediate-term performance [38]. Other studies reported conflicting results [39], calling for further testing.
Decellularized matrix for vessel engineering
Almost 20% of procedures performed in males and 11% of those performed in females yearly in the United States involve the cardiovascular system. Of those, a considerable number, consisting of over 500,000 procedures a year, are arterial bypass operations [40]. Arterial bypasses are needed to restore blood flow downstream an arterial occlusion, most commonly due to atherosclerosis. Generally, autologous arteries, such as radial or internal mammary artery, or veins, i.e. the saphenous vein, are used as bypass graft material. However, Almost 40% of patients needing bypass surgery may not have autologous vessels of the appropriate quality or length [41] and even if appropriate venous tissue is available, in vivo remodelling, including intima hyperplasia, and mechanical injuries frequently lead to graft occlusion [42,43]. Synthetic grafts, used as a standard alternative to autologous vessels, are also not immune to occlusive graft failure in the long term [44]. Procedures involving the replacement of large conductance vessels, or part of them, are less frequent, but conditions such as aortic aneurysm and dissection, for instance, are life-threatening and often requiring emergency surgery. The damaged section of aorta is often replaced with synthetic grafts such as Dacron and ePTFE, which function exceptionally well under high flow, low-resistance conditions and maintain a 90% patency rate after five years, but show a 20% decreased patency rate over a five year period when applied to small caliber arteries because of thrombotic complications [45].
Taking advantage of natural and synthetic biopolymers and different cell seeding technologies, tissue engineering has developed vascular conduits showing proper structural and functional features, such as swelling and stretching properties, suture-retention and cell conductivity [46-52]. However, the limited proliferative rate of adult smooth muscle cells [53], as well as the senescence they undergo in culture, accounts for the poor in vivo mechanical performance of tissue engineered blood vessels. Inter alia, the biofabrication of blood vessels is still costly and time consuming [54].
The pioneering research of Malone et al. [55] and Lalka et al. [56] first reported that implanted cell-free arterial allografts do not undergo immunologic alterations. The simple treatment with SDS resulted in the formation of an ECM tube with morphologically intact elastin and collagen network, that was easy to suture and immediately blood perfused after in vivo grafting. Decellularization technologies were not advanced yet, when these scientists introduced a simple and powerful concept: reducing allograft/xenograft antigenicity as opposed to immunosuppressing the graft host!
Similarly to synthetic grafts, decellularized matrices would be readily available. Unlike synthetic grafts, they would provide the proper microenvironment for supporting cell invasion, growth and differentiation. A future goal for the tissue engineering is to identify decellularization techniques that can provide vascular grafts with both mechanical properties of native vessels and immuno-privileged characteristics of autologous vessels. In a recent study by Fitzpatrick et al., different protocols were applied to decellularize segments of porcine aorta and it was shown that the TritonX-100/sodium-deoxycholate treatment is a more effective option than TritonX-100/EDTA and SDS treatments since it effectively lyses VSMCs and results in less variability in mechanical behavior at in vivo stretch ratios [44]. In another report both SDS and Triton X-100 treatments were able to remove cells effectively from porcine aorta and the major ECM structure was preserved, while trypsin treatment disrupted the cross-linked network of collagen and elastin fibers [57]. Dimuzio’s group has decellularized the human saphenous vein by using SDS, showing that decellularized veins have a burst and suture-holding strength similar to fresh veins, as well as unchanged collagen morphology [58]. The same group reported a canine model of bilateral carotid interposition of a decellularized jugular vein allograft: the decellularized allograft exhibited satisfactory strength, reduced antigenicity compared to fresh allograft, and supported cellular repopulation [59].
To the best of our knowledge, the clinical application of the decellularization technology has been restricted to a single patient case [60]. A 49-year-old woman underwent surgery for a large malignant pelvic tumour causing the occlusion of the iliac vein. The iliac vein was reconstructed by using a tissue-engineered neo-vein, previously developed from a decellularized vein allograft that was reseeded in a bioreactor with recipient-derived endothelial cells. The interposition graft was patent for 24 months, before the progression of the malignancy lead to graft occlusion. Humacyte, Inc., has conducted the first-in-human pilot study to assess safety and efficacy of its innovative bioengineered blood vessel in end-stage renal disease patients (Figure 1). Human vascular cells were isolated and used to grow bioengineered vessels in bioreactors. After decellularization, the bioengineered vessels (6 mm in diameter and 40 cm in length) were tested for suture retention strength and burst pressure, respectively comparable to human saphenous vein and human mammary artery. The Humacyte’s study is open label and single arm. It was initiated in December 2012 and it has enrolled 28 patients thus far. All the implanted bioengineered vessels have been demonstrated to be patent and only 8/28 patients were assisted with interventions to restore or maintain patency. No indications of infections, immune response, dilatation and aneurysms have been observed (Abstracts from the American Heart Association’s Emerging Science Series April 24, 2013).
Figure 1.
First-in-man evaluation of an investigational bioengineered blood vessel. Kindly provided by Prof. Laura Niklason, Yale University and Humacyte, Inc.
Surgeons have been using cryopreserved vascular allografts successfully for many years to treat arterial occlusive disease and to repair arterial aneurysms. Vascular allografts demonstrate high patency rates but contain viable cells, which may evoke an immune rejection. Decellularization techniques efficiently remove cells and can be optimized to guarantee the maintenance of the microarchitecture and the biomechanical properties of native vessels (see Table 3).
Table 3.
The most commonly used decellularization/repopulation techniques for big vessels, as well as some of the basic milestones that have driven present research on decellularized matrices in vascular biology
| Tissue | Decell. Method | Recell. Method | Notes | Ref |
|---|---|---|---|---|
| Swine arteries | Sodium deoxycholate 4% | None | Young’s modulus, compliance, burst pressure, and suture retention strength were unchanged, while ultimate strain and stress relaxation were altered | [177] |
| Human umbilical vein | Comparison between detergent treatment (Triton X-100, sodium deoxycholate, IGEPAL-CA630), osmotic lysis (3 m NaCl, distilled water) and peroxyacetic acid treatmentTriton X-100 or Trypsin | In vitro seeding of ECs with endothelial cells | Seeded ECs did not remain viable. Partial loss of fibronectin, laminin and elastic fibers | [178] |
| Human umbilical artery | CHAPS and sodium dodecyl sulfate | In vitro seeding of ECs and in vivo implant in nude rats | Preserved ECM, supported endothelialisation and retained function in for up to 8 weeks. | [179] |
| Human common femoral arteries | Single freeze-thaw cycle followed by incubation in hypotonic tris buffer and low concentration SDS | In vitro seeding with mouse 3T3 cells or baby kidney cells | Retention of burst pressure, compliance, and tensile properties. No cell toxicity detected | [180] |
| Porcine carotid arteries and tissue-engineered arteries | Comparison between non ionic detergent treatment (1% Triton X-100), hypo-hypertonic shock treatment and ionic detergent treatment (CHAPS) | Seeding of porcine carotid artery SMCs | CHAPS did not appear to compromise the ECM. Vessels were dilated. | [181] |
| Porcine abdominal aorta | Mechanical shaking device | None | Preliminary mechanical tests | [182] |
| Porcin Tissue-engineered vessels | CHAPS and SDS treatment | Seeding of recipient endothelial progenitor cells (EPC) or endothelial cell (EC). Implantation in the porcine carotid artery | Resistance to clotting and intimal hyperplasia. | [183] |
| Porcine descending aorta | Sonication | None | Good Decellularization efficiency and short treatment time. | [184] |
ECM components of cardiovascular tissues
The extracellular matrix (ECM) mediates the interaction between the cell and the surrounding microenvironment in a model of dynamic reciprocity, in which cells secrete ECM components and ECM proteins regulate cell proliferation and differentiation to finally determine tissue morphogenesis and homeostasis in development and disease [61]. Far from being a merely structural component of any tissue, the ECM represents a huge reservoir of biophysical stimuli and signalling molecules. The regulation of cell fate mediated by ECM is also essential during tissue repair, wherein a very delicate balance in the amount, composition and spatial organization of the newly produced matrix marks the border between a regeneration process and scar formation [62,63]. A profound knowledge of the structure and the signalling mediated by the ECM of cardiovascular tissues is needed in order to rationalize the use of decellularized vessels, valves and even entire hearts. Modifications in matrix proteins, depending on decellularization techniques, might account for in vivo fibrosis, calcification, poor endothelialisation, and ultimately for the failure of the implanted patches.
ECM in the heart
ECM is crucial for heart development [64]; the cardiac jelly, an ECM-rich acellular space between the endocardium and myocardium, is particularly important for the proper formation of the endocardial cushions at the atrioventricular (AV) junction. In adult life, the coupling of vessel endothelium and cardiomyocytes, as well as the coordination of cardiomyocyte contraction and relaxation is largely dependent on ECM [65]. In myocardial fibrosis, for instance, an altered secretion of ECM components by myofibroblasts causes a “mis-remodelling” of the tissue leading to cardiac muscle stiffness and contractile dysfunction [66,67]. Main components of heart ECM [64,66] include GAGs and proteoglycans as important structural molecules for creating loose and hydrated matrices during key events in development and disease. Some of them are:
- Hyaluronan (HA), a GAG which is synthesized at the plasma membrane and does not become linked to a core protein. It promotes cellular proliferation and motility in the cardiac jelly of developing heart [68].
- Chondroitin sulfate proteoglycans, such as versican, which is essential for the formation of endocardial cushion mesenchyme by epithelial– mesenchymal transformation (EMT), heart chamber specification and valvulogenesis [69,70].
- Heparan Sulfate Proteoglycans, such as perlecan which is important in the formation of the cardiomyocyte basement membrane and in maintaining the integrity of the ventricular wall [71].
Many different collagens are expressed in the heart, both in ventricular myocardium (type I, III, V) and heart valves (I, II, IV, XI, and XIII) [64]. Collagens provide elasticity and structural integrity to cardiac tissue. Fibronectin interacts with integrins, proteoglycans and collagens to mediate cellular adhesion. Fibronectin null embryos do not survive beyond embryonic day 10 (E10) due to cardiovascular (failure of heart tube formation in the most severely affected mutants) and vascular defects [72]. The remodelling of the ECM, important for the release of mediators (growth factors, cytokines, small peptides), is mainly carried out by matrix metalloproteases (MMPs) [65]. Disease states such as hypertension, excessive activation of the angiotensin aldosterone system, diabetes and hypoxia can lead to an over-activation of MMPs and consequent ECM degradation, impaired angiogenesis, myocardial hypertrophy and progression to heart failure [65,73-76]. Among MMPs, MMP9 appears to be the most involved in the pathological remodelling of ECM in heart failure [65,77], being associated with increasing endostatin and angiostatin (anti-angiogenic activity ) and vascular rarefaction [74,78].
ECM in heart valves
Mature heart valves are composed of highly organized ECM and valve interstitial cells (VICs), all surrounded by endothelial cells [79]. Valvular ECM is stratified into different layers and is responsible for biomechanical properties of the valves [79,80]. The ventricularis, facing the ventricle, is enriched in elastin and is responsible for valve extension and recoil [81]. Proteoglycans are interposed between the ventricularis and the fibrosa, and constitute the spongiosa. They provide cohesiveness between the layers and contribute to tissue viscoelasticity [82]. The fibrosa is close to the outflow surface and is mainly composed of collagens, that are responsible for tissue strength and durability [80,83]. Many disease conditions affecting heart valves involve degenerative changes of ECM. In calcific stenosis, a common disease of the elderly, mainly affecting the aortic valve, noxious stimuli such as hypertension, high serum cholesterol levels and smoking can induce differentiation of VICs to an osteoblastic phenotype [84]. Such cell types express osteogenic and chondrogenic markers and promote tissue calcification and degeneration [85]. Mitral prolapse consists in the displacement of a valvular leaflet of the mitral valve in the left atrium during systole. It has potential serious complications such as bacterial endocarditis, thromboemboli and atrial fibrillation. The pathogenic process underlying this condition is myxomatous degeneration, which is characterized histologically by a focal thickening of the spongiosa with an increase in proteoglycans content [86,87] together with an abnormal fibrillar organization, and an attenuation of the fibrosa [88]. Activated VICs secrete catabolic enzymes, including MMPs (MMP-1, MMP-13, MMP-2, and MMP-9), and are believed to play a major role in myxomatous degeneration [89].
ECM in blood vessels
Big blood vessels are constituted by three concentric layers, which are, progressing radially from the lumen; the intima, the media and the adventitia. Each layer is constituted by a structural ECM meshwork supporting resident cells. Vascular ECM not only provides the scaffold for attachment of the resident cells, but is also able to absorb and transduce shear and strain forces exerted by blood flow [90]. Endothelial cells produce and attach to a basal lamina (laminin, type IV collagen, entactin and perlecan are the main components) and contribute to the formation of the internal elastic lamina, which is very thin in veins and venules [91]. In pathological conditions such as atherosclerosis and hypertension, or after mechanical distension and disruption of the endothelial layer, such as after percutaneous coronary intervention; the tunica intima appears as a thick layer of sparse smooth muscle cells and myofibroblasts in a proteoglycan-rich stroma [92-94].
Tunica media consists of an ensemble of radially-arranged fenestrated sheets (lamellae) rich in elastin, immersed in collagen fibers, thin layers of proteoglycans, and smooth muscle cells. It is important to distinguish between elastin itself and the elastic fibers, which contain elastin and microfibrils. Microfibrils act as a scaffold for elastin assembly and elastic fiber overall growth [95]. The functional importance of the elastic components of the blood vessel walls is underlined by the fact that the genetic inactivation of its constituents leads to major health issues [96]. Elastin haploinsufficiency for instance causes supravalvular aortic stenosis [97] which can lead to hypertension, cerebrovascular disease and obstructive cardiomyopathy. Vascular lesions show irregular elastic fibers, excess of medial smooth muscle cells and intimal thickening and fibrosis. Homozygous loss of function in FBN1 gene, coding for fibrillin 1, causes Marfan syndrome with mitral valve prolapse and aortic root dilation as main cardiovascular affections [98,99]. Other biomechanically important constituents of tunica media ECM are fibulins, located either in the elastin core or its surrounding microfibrils, collagens (I, III, V, VI) and proteoglycans (versican, lumican, etc.).
The adventitial layer contains sparse fibroblasts surrounded by ECM, mainly composed of fibrillar collagens and proteoglycans, as well as vasa vasorum, providing nourishment to the vessel, and nervi vasorum (unmyelinated nerve fibers). Adventitia is the primary source of tensile strength in blood vessels, but also participates in the regulation of blood vessels tone through the activity of nervous fibers. Fibrillar collagens, in particular Collagen I and Collagen III, are responsible for blood vessels resistance to mechanical stress [100]. Autosomal dominant mutations in type III collagen results in Ehlers-Danlos syndrome type IV, which is characterized by spontaneous rupture of the bowel, uterus and blood vessels [101]. Adventitia undergoes remodelling in a number of pathological conditions such as hypertension or atherosclerosis. Adventitia fibroblasts are the main players of the remodelling process, which can be adaptive (positive) to vasoactive substances and hemodynamic stimuli, or constrictive (negative), leading to lumen reduction and stenosis [102,103]. Since the activation of proliferative and differentiative mechanisms in adventitia fibroblasts may shape the vessel wall and tone, caution should be used when decellularizing vessels, to avoid mis-recellularization in vivo from overactivated progenitors. Nevertheless, the integrity of elastic fibers in the media has to be maintained after decellularization. Indeed, it has been demonstrated that partial degradation of elastic fibers caused by NaOH and trypsin treatment of aortic xenografts significantly increases elastin-oriented calcification [104]. Finally, an intact Collagen IV (basement membrane) mediates migration and adhesion of endothelial cells [105], while the carboxy terminal globular domain is less active at promoting those events. Thus, decellularizing protocols, especially enzymatic ones, should take into account that a degraded Collagen IV might have repercussions for the in vivo re-endothelialization of decellularized grafts.
Immune response to ECM
One of the main causes for biological implant failure is the immune rejection of the graft itself. In case of allogenic implants (transplantation to a recipient from a genetically non-identical donor of the same species), a cell-mediated immune response will be activated by antigen presenting cells (APCs) presenting MHC-alloantigen to T Lymphocytes through direct (donor’s APCs are presenting graft antigens) or indirect (recipient’s infiltrating APCs process and present foreign graft proteins) allorecognition pathways [106]. Beyond the cellular response, involving CD4+ and CD8+ T-lymphocytes, NK cells and other phagocytes, also B-lymphocytes (humoral or antibody-mediated rejection) and cytokines (IL-12, IFN-γ, IL-6, IL-17, etc.) [107] play an important role in allograft rejection. Moreover, if the balance between proinflammatory (Th1, Th17 lymphocytes, IFN-γ, IL-17, etc) and anti-inflammatory (Th2, regulatory T cells, IL-4, IL-10, TGF-β etc.) players is not correctly established, a sustained chronic rejection of the graft can lead to vascular endothelium damage, blood supply deficiency, scar formation and ultimately functional loss of the implant [108]. On the other hand, xenogenic transplantation of organs and tissues ensues hyperacute rejection, which entails complement-activation, neutrophil infiltration and NK cell activation in response to natural xenoreactive antibodies (for instance the natural antibodies to Galα1,3Gal). Xenorejection has in the microvasculature a main target, thus, in addition to intragraft rejection events, systemic complications, such as thrombotic microangiopathy, can follow to the rapid graft destruction soon after implantation [109,110]. One of the most relevant antigens involved in hyperacute rejection following xenotransplantation is the saccharide α-Gal. This epitope is found as a cell surface molecule in most species, with the notable exception of humans and Old World monkeys [111,112]. Although the Gal epitope is considered the main obstacle to xenotransplantation, organs harvested from pigs that were knocked out for this antigen were rejected after a short time due to immune response towards non-Gal porcine antigens [113,114]. Furthermore, the presence of the Gal epitope has been demonstrated in biologic scaffolds composed of xenogenic ECM, such as porcine bioprosthetic heart valves [115], porcine cruciate ligaments [116] and porcine cartilage [117].
ECM proteins are among the most conserved proteins in evolution [118] but, it would be naive to think that the removal of xenogenic or allogenic cellular material would abolish graft immunogenicity [119,120]. In fact, ECM proteins have been shown to provide costimulatory signals to immune cells [121], For instance, neutrophils exhibit chemotaxis toward fragments of type IV collagen, laminins, and elastin [122]; a laminin-derived peptide (SIKVAV), isolated from human abdominal aortic aneurysm tissue can recruit neutrophils within 1 day and macrophages by 3 days when instilled in mouse lungs [123]; instillation of in vitro-generated bovine elastin fragments into the lungs of mice induce macrophage accumulation, which is prevented when BA4 (a monoclonal antibody raised against the bovine tropoelastin epitope VGVAPG) is given [124]. The fact that ECM fragments can promote immune cell recruitment must bring us to rethink, and maybe reinvent, the decellularization techniques currently in use, especially those involving enzymatic reactions that could easily unmask crypted ECM peptides with pro-inflammatory activity.
Thus, the complete removal of cellular material, including DNA, RNAs and proteins, is necessary but not sufficient: special attention has to be given to the “new products” derived from the decellularization processing of the tissue. To date, very few reports have dealt with the issue of the immune response towards biological scaffolds composed of ECM. The role of adaptive immunity has been investigated most extensively for porcine small intestine submucosa ECM [125,126]: after its implantation in mice, it elicits mainly a Th2 type of response, which is associated with tissue remodelling and graft acceptance [127,128]. Innate immunity, which naturally provide immediate defense against foreign bodies, is obviously primarily involved in mediating the host response to biomaterials, and the monocyte-derived macrophages are indeed important “sentinels and soldiers” in this context [129]. Macrophages are a heterogeneous cell population, displaying a variety of phenotypes. In particular, they have been classified on the basis of functional properties in M1 and M2, in analogy with Th1 and Th2 cells [130,131]. M1 are the classically activated, proinflammatory macrophages, inducers and effectors of Th1 response [130]. While M2 macrophages are involved in Th2 response which is required for tissue regeneration and remodelling [132]. Badylak et al. have used implantation of biologic scaffolds (with and without cross-linking) derived from porcine small intestinal submucosa (SIS) to characterize the role of macrophage phenotype in the remodelling of the scaffold [133,134]. They found that the SIS scaffold elicited a CD163+ response (M2 profile) and showed constructive remodelling at 16 weeks, while the cross-linked-SIS device showed a predominately CD80+ and CCR7+ response (M1 profile), and at 16 weeks was characterized by chronic inflammation and fibrosis. The conclusion is that future strategies aimed at polarizing macrophages from an M1 to an M2 phenotype will be needed for a successful long term outcome of decellularized matrix implantation. Recent in vitro studies have showed that decellularized xeno-ECM, such as decellularized bovine pericardium can favour the polarization of macrophages towards an M2 phenotype [135,136] as compared to other materials commonly employed for medical devices, such as polydimethylsiloxane. Scaffolds composed of ECM showed to promote the switch M1-M2 also in vivo, after 7-14 days post-implantation [137,138], but mechanisms by which ECM based scaffold promote the M1 to M2 transition remain unknown. All these results are encouraging but more comparative (allo- v/s xeno-, biologic v/s synthetic, different decell. techniques) in vivo studies will be necessary for a better understanding of macrophage polarity and context dependent polarization profiles, especially in order to design strategies for tuning macrophage plasticity and reach the perfect biocompatibility of decellularized matrices.
In vivo recellularization
Obtaining a functional tissue or organ from an implanted decellularized matrix requires in vivo cell repopulation of the matrix. Recruitment of cells and progenitors from the neighbour tissues and circulation is the first step to get “biointegration” of the decellularized matrix. To date, we do not have a complete knowledge of what cell types, what chemoattracting and signalling molecules are needed, whether there is a precise chronological sequence of events and how we can modulate each and single event to achieve engraftment. The in vivo recruitment of the whole spectrum of parenchymal and stromal cells towards the ECM of complex organs, such as the heart, is not feasible yet. On the other hand, the study of recellularization events occurring in simpler structures, including heart valves and vascular conduits, might be the right start towards comprehension. Recellularization may be carried out, or simply started, in vitro, mainly by the use of bioreactors mimicking physiological organ conditions, such as 3D growth and controlled changes of specific environmental factors [139]. One of the most successful examples of the clinical application of decellularized matrices is the tissue-engineered airway by Macchiarini et al [140]. Here, the authors decellularized a human donor trachea and subsequently cultured the graft in a bioreactor with recipient epithelial cells and mesenchymal stem-cell-derived chondrocytes. The graft was then used to replace the left main bronchus of a 30-year old woman with end-stage bronchomalacia. There is no doubt that the scaffold per se is not able to be integrated and functional in vivo. Another study from Macchiarini’s group reports that decellularized not pre-seeded tracheas implanted in pigs collapse because of obstruction and infections, while cell-seeded tracheas are functional, suggesting a role for epithelial and mesenchymal cells in mediating perfusion and immune-tolerance of the graft [141]. It is not clear whether the implanted cells are directly contributing to the in vivo fabrication of the tissue or rather acting as reservoir of molecules activating micro- and macro-environment pathways of tissue regeneration. Unless the implanted cells are immortal or able to initiate a controlled and regulated program of cell renewal and apoptosis, the contribution of endogenous progenitor cells cannot be excluded from the whole regeneration process. It appears clear that tissue engineering and regenerative medicine have to “hold hands” in order to provide complementary solutions and treatment options. Several techniques, aforementioned in this review, have been used to recellularize vascular patches in vitro, before implantation. Re-endothelialization, in particular, seems to be crucial in order to decrease calcification and thrombus formation. Walles et al. showed that decellularized carotid artery and aorta undergo progressive calcification, while calcification is less pronounced in cellular native arteries [142]. Kasimir et al. decellularized heart valve matrix and reported platelet adhesion (CD41+ cells recruitment) and aggregate formation only on the surface of the non-seeded or partially denuded matrix, whereas after seeding with endothelial cells no platelet activation was detected [143]. Strategies to enhance in situ re-endothelialization have comprised the coating of decellularized heart valves with fibronectin (FN) [144]. FN is used to increase cell adhesion; cell adhesion is meant to provide not only physical support, but also pro survival signals to cells [145]. FN in combination with hepatocyte growth factor (HGF), can synergize re-endothelialization by both stabilizing cell-matrix adhesion and stimulating EC proliferation [146,147]. Heparin, and vascular endothelial growth factor (VEGF) have been used as bioactive coating to improve recellularization, as well [148], although full consideration should be given to VEGF-mediated hyperplasia of neointima. Other than adhesion and proliferation, blood perfusion of the implanted graft has to be achieved, not only to provide oxygen and nourishment to the graft, but also progenitor cells. Endothelial progenitor cells (EPCs), which are thought to be originated in the bone marrow, can contribute to vascular repair [149]. According to some authors, peripheral blood endothelial progenitor cells can be derived from monocyte/macrophages [150]. Also, myeloid angiogenic cells (MACs), although not able to become endothelial cells or be directly incorporated into a microvascular network as EPCs, have been described as an alternative population of activated M2 macrophages, able to induce vascular repair in vivo in a paracrine fashion [151]. Even progenitors derived from injured neointima could be exploited as an inner cell source to promote vascular repair. Tsai et al. developed a mouse model of restenosis by grafting a decellularized vessel to the carotid artery. Cells retrieved in the neointimal lesions were endothelial and smooth muscle cells, monocytes, and stem/progenitor cells expressing c-kit, Sca-1 and CD34 [152]. Ex-vivo cultured progenitors displayed the ability to differentiate into both endothelial and smooth muscle cells. This suggests that the ECM per se is able to recruit vessel progenitors which, if impeded from turning on restenosis, can be a useful source of endothelial and mural cells.
Future perspectives
In future, it will be mandatory to set up decellularization techniques that leave an intact ECM and to learn more about ECM biology to exploit native and bioengineered ECM molecules that allow a better recruitment of cells in vivo. The concept that ECM degradation can result in products with chemoattractive properties [153] needs to be further developed (Figure 2).
Figure 2.
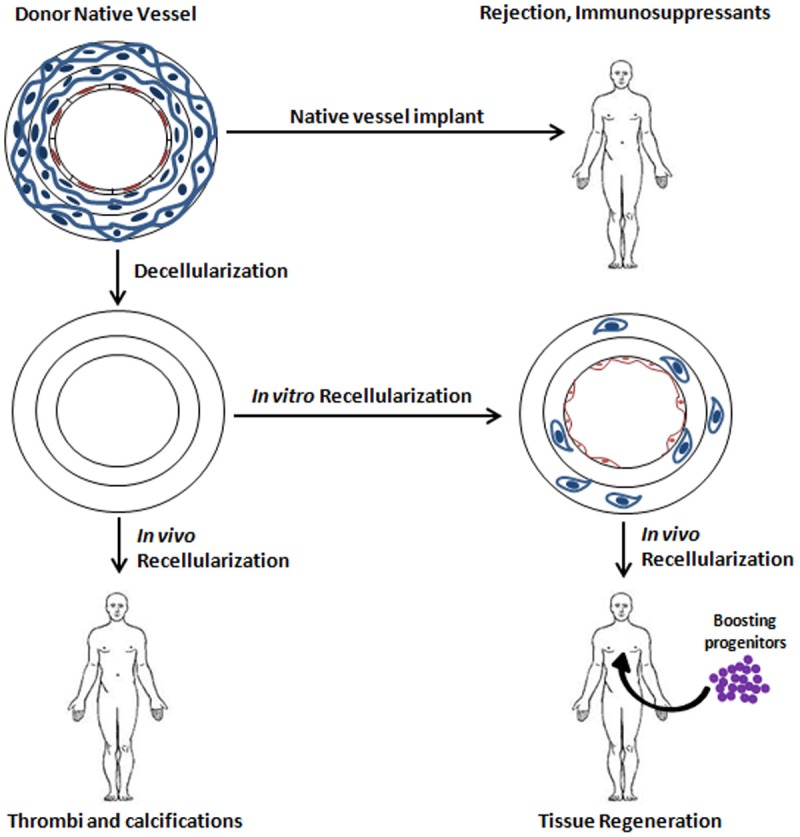
Scheme of different transplantation (TXP) approaches of vessels. Decellularization of the vessel reduces the risk of immune rejection. In vitro cell seeding of the decellularized vessel prior to implantation reduces the risk of thrombus formation and calcifications and induces progenitor recruitment and regeneration in vivo.
The enhancement of blood perfusion of decellularized grafts through the peripheral anastomosis could be achieved by providing an immediately active angiogenic boost. Many stem cells, including the promising amniotic fluid stem cells, are endowed with a reservoir of soluble factors that can exert paracrine effects on capillary ingrowth [154-156]. The in vitro pre-seeding of decellularized ECM can trigger a better recellularization in vivo. Human embryonic stem cells (ESCs) and human induced pluripotent stem cells (iPSCs) can be cultured on decellularized matrices and be reprogrammed into cells capable of angiogenesis and re-endothelialization as well as into parenchymal cells with positive implications for cell colonization of big organs, such as the heart [157-159]. However, caution must be used when such undifferentiated cells are used, since the safety profile is not completely investigated yet.
Moreover, the networking between different fields, including but not limited to stem cells, biomaterials, cell and matrix biology, will be the key for a successful application of decellularized matrices in the treatment of cardiovascular disease.
Acknowledgements
We thank Dr. John Rhodes, Cardiovascular Research Center, Yale Medical School, for thecritical reading of the manuscript. We have been honored to present in our review some of the unpublished interim results of Humacyte clinical trial, with the kind permission of Prof. Laura Niklason, Yale University. We are grateful to Laura for her terrific input.
Disclosure of conflict of interest
None to declare.
References
- 1.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 3.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology; Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banner NR, Bonser RS, Clark AL, Clark S, Cowburn PJ, Gardner RS, Kalra PR, McDonagh T, Rogers CA, Swan L, Parameshwar J, Thomas HL, Williams SG. UK guidelines for referral and assessment of adults for heart transplantation. Heart. 2011;97:1520–1527. doi: 10.1136/heartjnl-2011-300048. [DOI] [PubMed] [Google Scholar]
- 6.Mehra MR, Kobashigawa J, Starling R, Russell S, Uber PA, Parameshwar J, Mohacsi P, Augustine S, Aaronson K, Barr M. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates--2006. J Heart Lung Transplant. 2006;25:1024–1042. doi: 10.1016/j.healun.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Vega JD, Moore J, Murray S, Chen JM, Johnson MR, Dyke DB. Heart transplantation in the United States, 1998-2007. Am J Transplant. 2009;9:932–941. doi: 10.1111/j.1600-6143.2009.02568.x. [DOI] [PubMed] [Google Scholar]
- 8.Zimmermann WH, Melnychenko I, Eschenhagen T. Engineered heart tissue for regeneration of diseased hearts. Biomaterials. 2004;25:1639–1647. doi: 10.1016/s0142-9612(03)00521-0. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu T, Yamato M, Kikuchi A, Okano T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials. 2003;24:2309–2316. doi: 10.1016/s0142-9612(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 11.Radisic M, Deen W, Langer R, Vunjak-Novakovic G. Mathematical model of oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium containing oxygen carriers. Am J Physiol Heart Circ Physiol. 2005;288:H1278–1289. doi: 10.1152/ajpheart.00787.2004. [DOI] [PubMed] [Google Scholar]
- 12.Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12:2077–2091. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- 13.Park H, Radisic M, Lim JO, Chang BH, Vunjak-Novakovic G. A novel composite scaffold for cardiac tissue engineering. In Vitro Cell Dev Biol Anim. 2005;41:188–196. doi: 10.1290/0411071.1. [DOI] [PubMed] [Google Scholar]
- 14.Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends Mol Med. 2011;17:424–432. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Badylak SF, Lantz GC, Coffey A, Geddes LA. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res. 1989;47:74–80. doi: 10.1016/0022-4804(89)90050-4. [DOI] [PubMed] [Google Scholar]
- 16.Lantz GC, Badylak SF, Coffey AC, Geddes LA, Blevins WE. Small intestinal submucosa as a small-diameter arterial graft in the dog. J Invest Surg. 1990;3:217–227. doi: 10.3109/08941939009140351. [DOI] [PubMed] [Google Scholar]
- 17.Lantz GC, Badylak SF, Coffey AC, Geddes LA, Sandusky GE. Small intestinal submucosa as a superior vena cava graft in the dog. J Surg Res. 1992;53:175–181. doi: 10.1016/0022-4804(92)90031-t. [DOI] [PubMed] [Google Scholar]
- 18.Robinson KA, Li J, Mathison M, Redkar A, Cui J, Chronos NA, Matheny RG, Badylak SF. Extracellular matrix scaffold for cardiac repair. Circulation. 2005;112:I135–143. doi: 10.1161/CIRCULATIONAHA.104.525436. [DOI] [PubMed] [Google Scholar]
- 19.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 20.Soto-Gutierrez A, Wertheim JA, Ott HC, Gilbert TW. Perspectives on whole-organ assembly: moving toward transplantation on demand. J Clin Invest. 2012;122:3817–3823. doi: 10.1172/JCI61974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinkley DM, Gelfand EV. Valvular heart disease: classic teaching and emerging paradigms. Am J Med. 2013;126:1035–1042. doi: 10.1016/j.amjmed.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 23.Bonow RO, Carabello BA, Chatterjee K, de Leon AC Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, O’Rourke RA, Otto CM, Shah PM, Shanewise JS American College of Cardiology/American Heart Association Task Force on Practice G. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e1–142. doi: 10.1016/j.jacc.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Brown JM, O’Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg. 2009;137:82–90. doi: 10.1016/j.jtcvs.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Cannegieter SC, Rosendaal FR, Wintzen AR, van der Meer FJ, Vandenbroucke JP, Briet E. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med. 1995;333:11–17. doi: 10.1056/NEJM199507063330103. [DOI] [PubMed] [Google Scholar]
- 26.Chikwe J, Filsoufi F, Carpentier AF. Prosthetic valve selection for middle-aged patients with aortic stenosis. Nat Rev Cardiol. 2010;7:711–719. doi: 10.1038/nrcardio.2010.164. [DOI] [PubMed] [Google Scholar]
- 27.Human P, Zilla P. Inflammatory and immune processes: the neglected villain of bioprosthetic degeneration? J Long Term Eff Med Implants. 2001;11:199–220. [PubMed] [Google Scholar]
- 28.Smedira NG, Blackstone EH, Roselli EE, Laffey CC, Cosgrove DM. Are allografts the biologic valve of choice for aortic valve replacement in nonelderly patients? Comparison of explantation for structural valve deterioration of allograft and pericardial prostheses. J Thorac Cardiovasc Surg. 2006;131:558–564. e4. doi: 10.1016/j.jtcvs.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Rippel RA, Ghanbari H, Seifalian AM. Tissue- engineered heart valve: future of cardiac surgery. World J Surg. 2012;36:1581–1591. doi: 10.1007/s00268-012-1535-y. [DOI] [PubMed] [Google Scholar]
- 30.Vesely I. Heart valve tissue engineering. Circ Res. 2005;97:743–755. doi: 10.1161/01.RES.0000185326.04010.9f. [DOI] [PubMed] [Google Scholar]
- 31.Lam MT, Wu JC. Biomaterial applications in cardiovascular tissue repair and regeneration. Expert Rev Cardiovasc Ther. 2012;10:1039–1049. doi: 10.1586/erc.12.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dainese L, Guarino A, Burba I, Esposito G, Pompilio G, Polvani G, Rossini A. Heart valve engineering: decellularized aortic homograft seeded with human cardiac stromal cells. J Heart Valve Dis. 2012;21:125–134. [PubMed] [Google Scholar]
- 33.Cigliano A, Gandaglia A, Lepedda AJ, Zinellu E, Naso F, Gastaldello A, Aguiari P, De Muro P, Gerosa G, Spina M, Formato M. Fine structure of glycosaminoglycans from fresh and decellularized porcine cardiac valves and pericardium. Biochem Res Int. 2012;2012:979351. doi: 10.1155/2012/979351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Brien MF, Goldstein S, Walsh S, Black KS, Elkins R, Clarke D. The SynerGraft valve: a new acellular (nonglutaraldehyde-fixed) tissue heart valve for autologous recellularization first experimental studies before clinical implantation. Semin Thorac Cardiovasc Surg. 1999;11:194–200. [PubMed] [Google Scholar]
- 35.Simon P, Kasimir MT, Seebacher G, Weigel G, Ullrich R, Salzer-Muhar U, Rieder E, Wolner E. Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur J Cardiothorac Surg. 2003;23:1002–1006. doi: 10.1016/s1010-7940(03)00094-0. discussion 1006. [DOI] [PubMed] [Google Scholar]
- 36.Brown JW, Ruzmetov M, Eltayeb O, Rodefeld MD, Turrentine MW. Performance of SynerGraft decellularized pulmonary homograft in patients undergoing a Ross procedure. Ann Thorac Surg. 2011;91:416–422. doi: 10.1016/j.athoracsur.2010.10.069. discussion 422-413. [DOI] [PubMed] [Google Scholar]
- 37.Perri G, Polito A, Esposito C, Albanese SB, Francalanci P, Pongiglione G, Carotti A. Early and late failure of tissue-engineered pulmonary valve conduits used for right ventricular outflow tract reconstruction in patients with congenital heart disease. Eur J Cardiothorac Surg. 2012;41:1320–1325. doi: 10.1093/ejcts/ezr221. [DOI] [PubMed] [Google Scholar]
- 38.Konertz W, Angeli E, Tarusinov G, Christ T, Kroll J, Dohmen PM, Krogmann O, Franzbach B, Pace Napoleone C, Gargiulo G. Right ventricular outflow tract reconstruction with decellularized porcine xenografts in patients with congenital heart disease. J Heart Valve Dis. 2011;20:341–347. [PubMed] [Google Scholar]
- 39.Dohmen PM. Clinical results of implanted tissue engineered heart valves. HSR Proc Intensive Care Cardiovasc Anesth. 2012;4:225–231. [PMC free article] [PubMed] [Google Scholar]
- 40.Hall MJ, DeFrances CJ, Williams SN, Golosinskiy A, Schwartzman A. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report. 2010:1–20. 24. [PubMed] [Google Scholar]
- 41.Salacinski HJ, Goldner S, Giudiceandrea A, Hamilton G, Seifalian AM, Edwards A, Carson RJ. The mechanical behavior of vascular grafts: a review. J Biomater Appl. 2001;15:241–278. doi: 10.1106/NA5T-J57A-JTDD-FD04. [DOI] [PubMed] [Google Scholar]
- 42.Liu SQ, Moore MM, Yap C. Prevention of mechanical stretch-induced endothelial and smooth muscle cell injury in experimental vein grafts. J Biomech Eng. 2000;122:31–38. doi: 10.1115/1.429625. [DOI] [PubMed] [Google Scholar]
- 43.Meng X, Mavromatis K, Galis ZS. Mechanical stretching of human saphenous vein grafts induces expression and activation of matrix-degrading enzymes associated with vascular tissue injury and repair. Exp Mol Pathol. 1999;66:227–237. doi: 10.1006/exmp.1999.2260. [DOI] [PubMed] [Google Scholar]
- 44.Fitzpatrick JC, Clark PM, Capaldi FM. Effect of decellularization protocol on the mechanical behavior of porcine descending aorta. Int J Biomater. 2010;2010 doi: 10.1155/2010/620503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klinkert P, Post PN, Breslau PJ, van Bockel JH. Saphenous vein versus PTFE for above-knee femoropopliteal bypass. A review of the literature. Eur J Vasc Endovasc Surg. 2004;27:357–362. doi: 10.1016/j.ejvs.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, Ao Q, Wang A, Lu G, Kong L, Gong Y, Zhao N, Zhang X. A sandwich tubular scaffold derived from chitosan for blood vessel tissue engineering. J Biomed Mater Res A. 2006;77:277–284. doi: 10.1002/jbm.a.30614. [DOI] [PubMed] [Google Scholar]
- 47.McClendon MT, Stupp SI. Tubular hydrogels of circumferentially aligned nanofibers to encapsulate and orient vascular cells. Biomaterials. 2012;33:5713–5722. doi: 10.1016/j.biomaterials.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsumura G, Nitta N, Matsuda S, Sakamoto Y, Isayama N, Yamazaki K, Ikada Y. Long-term results of cell-free biodegradable scaffolds for in situ tissue-engineering vasculature: in a canine inferior vena cava model. PLoS One. 2012;7:e35760. doi: 10.1371/journal.pone.0035760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsumura G, Isayama N, Matsuda S, Taki K, Sakamoto Y, Ikada Y, Yamazaki K. Long-term results of cell-free biodegradable scaffolds for in situ tissue engineering of pulmonary artery in a canine model. Biomaterials. 2013;34:6422–6428. doi: 10.1016/j.biomaterials.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 50.Pankajakshan D, Agrawal DK. Scaffolds in tissue engineering of blood vessels. Can J Physiol Pharmacol. 2010;88:855–873. doi: 10.1139/y10-073. [DOI] [PubMed] [Google Scholar]
- 51.Kakisis JD, Liapis CD, Breuer C, Sumpio BE. Artificial blood vessel: the Holy Grail of peripheral vascular surgery. J Vasc Surg. 2005;41:349–354. doi: 10.1016/j.jvs.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 52.L’Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, Chronos NA, Kyles AE, Gregory CR, Hoyt G, Robbins RC, McAllister TN. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12:361–365. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poh M, Boyer M, Solan A, Dahl SL, Pedrotty D, Banik SS, McKee JA, Klinger RY, Counter CM, Niklason LE. Blood vessels engineered from human cells. Lancet. 2005;365:2122–2124. doi: 10.1016/S0140-6736(05)66735-9. [DOI] [PubMed] [Google Scholar]
- 54.Kelm JM, Lorber V, Snedeker JG, Schmidt D, Broggini-Tenzer A, Weisstanner M, Odermatt B, Mol A, Zund G, Hoerstrup SP. A novel concept for scaffold-free vessel tissue engineering: self-assembly of microtissue building blocks. J Biotechnol. 2010;148:46–55. doi: 10.1016/j.jbiotec.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Malone JM, Brendel K, Duhamel RC, Reinert RL. Detergent-extracted small-diameter vascular prostheses. J Vasc Surg. 1984;1:181–191. doi: 10.1067/mva.1984.avs0010181. [DOI] [PubMed] [Google Scholar]
- 56.Lalka SG, Oelker LM, Malone JM, Duhamel RC, Kevorkian MA, Raper BA, Nixon JC, Etchberger KJ, Dalsing MC, Cikrit DF, et al. Acellular vascular matrix: a natural endothelial cell substrate. Ann Vasc Surg. 1989;3:108–117. doi: 10.1016/S0890-5096(06)62002-5. [DOI] [PubMed] [Google Scholar]
- 57.Zou Y, Zhang Y. Mechanical evaluation of decellularized porcine thoracic aorta. J Surg Res. 2012;175:359–368. doi: 10.1016/j.jss.2011.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schaner PJ, Martin ND, Tulenko TN, Shapiro IM, Tarola NA, Leichter RF, Carabasi RA, Dimuzio PJ. Decellularized vein as a potential scaffold for vascular tissue engineering. J Vasc Surg. 2004;40:146–153. doi: 10.1016/j.jvs.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 59.Martin ND, Schaner PJ, Tulenko TN, Shapiro IM, Dimatteo CA, Williams TK, Hager ES, DiMuzio PJ. In vivo behavior of decellularized vein allograft. J Surg Res. 2005;129:17–23. doi: 10.1016/j.jss.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 60.Teebken OE, Puschmann C, Rohde B, Burgwitz K, Winkler M, Pichlmaier AM, Weidemann J, Haverich A. Human iliac vein replacement with a tissue-engineered graft. Vasa. 2009;38:60–65. doi: 10.1024/0301-1526.38.1.60. [DOI] [PubMed] [Google Scholar]
- 61.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol. 2000;15:290–301. doi: 10.1007/s004670000461. [DOI] [PubMed] [Google Scholar]
- 63.Ghosh AK, Quaggin SE, Vaughan DE. Molecular basis of organ fibrosis: potential therapeutic approaches. Exp Biol Med (Maywood) 2013;238:461–481. doi: 10.1177/1535370213489441. [DOI] [PubMed] [Google Scholar]
- 64.Lockhart M, Wirrig E, Phelps A, Wessels A. Extracellular matrix and heart development. Birth Defects Res A Clin Mol Teratol. 2011;91:535–550. doi: 10.1002/bdra.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mishra PK, Givvimani S, Chavali V, Tyagi SC. Cardiac matrix: A clue for future therapy. Biochim Biophys Acta. 2013;1832:2271–2276. doi: 10.1016/j.bbadis.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Howard CM, Baudino TA. Dynamic cell-cell and cell-ECM interactions in the heart. J Mol Cell Cardiol. 2013 doi: 10.1016/j.yjmcc.2013.10.006. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 67.Davis J, Molkentin JD. Myofibroblasts: Trust your heart and let fate decide. J Mol Cell Cardiol. 2013 doi: 10.1016/j.yjmcc.2013.10.019. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Toole BP. Hyaluronan in morphogenesis. Semin Cell Dev Biol. 2001;12:79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- 69.Henderson DJ, Copp AJ. Versican expression is associated with chamber specification, septation, and valvulogenesis in the developing mouse heart. Circ Res. 1998;83:523–532. doi: 10.1161/01.res.83.5.523. [DOI] [PubMed] [Google Scholar]
- 70.Kern CB, Twal WO, Mjaatvedt CH, Fairey SE, Toole BP, Iruela-Arispe ML, Argraves WS. Proteolytic cleavage of versican during cardiac cushion morphogenesis. Dev Dyn. 2006;235:2238–2247. doi: 10.1002/dvdy.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Costell M, Carmona R, Gustafsson E, Gonzalez- Iriarte M, Fassler R, Munoz-Chapuli R. Hyperplastic conotruncal endocardial cushions and transposition of great arteries in perlecan- null mice. Circ Res. 2002;91:158–164. doi: 10.1161/01.res.0000026056.81424.da. [DOI] [PubMed] [Google Scholar]
- 72.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 73.Moshal KS, Tyagi N, Moss V, Henderson B, Steed M, Ovechkin A, Aru GM, Tyagi SC. Early induction of matrix metalloproteinase-9 transduces signaling in human heart end stage failure. J Cell Mol Med. 2005;9:704–713. doi: 10.1111/j.1582-4934.2005.tb00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Givvimani S, Tyagi N, Sen U, Mishra PK, Qipshidze N, Munjal C, Vacek JC, Abe OA, Tyagi SC. MMP-2/TIMP-2/TIMP-4 versus MMP-9/TIMP-3 in transition from compensatory hypertrophy and angiogenesis to decompensatory heart failure. Arch Physiol Biochem. 2010;116:63–72. doi: 10.3109/13813451003652997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mishra PK, Tyagi N, Sen U, Joshua IG, Tyagi SC. Synergism in hyperhomocysteinemia and diabetes: role of PPAR gamma and tempol. Cardiovasc Diabetol. 2010;9:49. doi: 10.1186/1475-2840-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cleutjens JP. The role of matrix metalloproteinases in heart disease. Cardiovasc Res. 1996;32:816–821. [PubMed] [Google Scholar]
- 77.Tyagi SC. Proteinases and myocardial extracellular matrix turnover. Mol Cell Biochem. 1997;168:1–12. doi: 10.1023/a:1006850903242. [DOI] [PubMed] [Google Scholar]
- 78.Grant MA, Kalluri R. Structural basis for the functions of endogenous angiogenesis inhibitors. Cold Spring Harb Symp Quant Biol. 2005;70:399–410. doi: 10.1101/sqb.2005.70.017. [DOI] [PubMed] [Google Scholar]
- 79.Hinton RB, Yutzey KE. Heart valve structure and function in development and disease. Annu Rev Physiol. 2011;73:29–46. doi: 10.1146/annurev-physiol-012110-142145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schoen FJ. Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering. Circulation. 2008;118:1864–1880. doi: 10.1161/CIRCULATIONAHA.108.805911. [DOI] [PubMed] [Google Scholar]
- 81.Vesely I. The role of elastin in aortic valve mechanics. J Biomech. 1998;31:115–123. doi: 10.1016/s0021-9290(97)00122-x. [DOI] [PubMed] [Google Scholar]
- 82.Eckert CE, Fan R, Mikulis B, Barron M, Carruthers CA, Friebe VM, Vyavahare NR, Sacks MS. On the biomechanical role of glycosaminoglycans in the aortic heart valve leaflet. Acta Biomater. 2013;9:4653–4660. doi: 10.1016/j.actbio.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krishnamurthy VK, Opoka AM, Kern CB, Guilak F, Narmoneva DA, Hinton RB. Maladaptive matrix remodeling and regional biomechanical dysfunction in a mouse model of aortic valve disease. Matrix Biol. 2012;31:197–205. doi: 10.1016/j.matbio.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mohler ER 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 85.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grande-Allen KJ, Griffin BP, Ratliff NB, Cosgrove DM, Vesely I. Glycosaminoglycan profiles of myxomatous mitral leaflets and chordae parallel the severity of mechanical alterations. J Am Coll Cardiol. 2003;42:271–277. doi: 10.1016/s0735-1097(03)00626-0. [DOI] [PubMed] [Google Scholar]
- 87.Gupta V, Barzilla JE, Mendez JS, Stephens EH, Lee EL, Collard CD, Laucirica R, Weigel PH, Grande-Allen KJ. Abundance and location of proteoglycans and hyaluronan within normal and myxomatous mitral valves. Cardiovasc Pathol. 2009;18:191–197. doi: 10.1016/j.carpath.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tamura K, Fukuda Y, Ishizaki M, Masuda Y, Yamanaka N, Ferrans VJ. Abnormalities in elastic fibers and other connective-tissue components of floppy mitral valve. Am Heart J. 1995;129:1149–1158. doi: 10.1016/0002-8703(95)90397-6. [DOI] [PubMed] [Google Scholar]
- 89.Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104:2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- 90.Didangelos A, Yin X, Mandal K, Baumert M, Jahangiri M, Mayr M. Proteomics characterization of extracellular space components in the human aorta. Mol Cell Proteomics. 2010;9:2048–2062. doi: 10.1074/mcp.M110.001693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paulsson M. Basement membrane proteins: structure, assembly, and cellular interactions. Crit Rev Biochem Mol Biol. 1992;27:93–127. doi: 10.3109/10409239209082560. [DOI] [PubMed] [Google Scholar]
- 92.Glover C, Ma X, Chen YX, Miller H, Veinot J, Labinaz M, O’Brien E. Human in-stent restenosis tissue obtained by means of coronary atherectomy consists of an abundant proteoglycan matrix with a paucity of cell proliferation. Am Heart J. 2002;144:702–709. doi: 10.1067/mhj.2002.123577. [DOI] [PubMed] [Google Scholar]
- 93.ER OB, Ma X, Simard T, Pourdjabbar A, Hibbert B. Pathogenesis of neointima formation following vascular injury. Cardiovasc Hematol Disord Drug Targets. 2011;11:30–39. doi: 10.2174/187152911795945169. [DOI] [PubMed] [Google Scholar]
- 94.Glover C, O’Brien ER. Pathophysiological insights from studies of retrieved coronary atherectomy tissue. Semin Interv Cardiol. 2000;5:167–173. doi: 10.1053/siic.2000.0136. [DOI] [PubMed] [Google Scholar]
- 95.Rosenbloom J, Abrams WR, Mecham R. Extracellular matrix 4: the elastic fiber. FASEB J. 1993;7:1208–1218. [PubMed] [Google Scholar]
- 96.Arteaga-Solis E, Gayraud B, Ramirez F. Elastic and collagenous networks in vascular diseases. Cell Struct Funct. 2000;25:69–72. doi: 10.1247/csf.25.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morris CA. Genetic aspects of supravalvular aortic stenosis. Curr Opin Cardiol. 1998;13:214–219. [PubMed] [Google Scholar]
- 98.Gray JR, Davies SJ. A clinical severity grading scale for Marfan syndrome. J Med Genet. 1996;33:758–759. doi: 10.1136/jmg.33.9.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Segura AM, Luna RE, Horiba K, Stetler-Stevenson WG, McAllister HA Jr, Willerson JT, Ferrans VJ. Immunohistochemistry of matrix metalloproteinases and their inhibitors in thoracic aortic aneurysms and aortic valves of patients with Marfan’s syndrome. Circulation. 1998;98:II331–337. discussion II337-338. [PubMed] [Google Scholar]
- 100.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89:957–989. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK) Am J Med Genet. 1998;77:31–37. doi: 10.1002/(sici)1096-8628(19980428)77:1<31::aid-ajmg8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 102.Coen M, Gabbiani G, Bochaton-Piallat ML. Myofibroblast-mediated adventitial remodeling: an underestimated player in arterial pathology. Arterioscler Thromb Vasc Biol. 2011;31:2391–2396. doi: 10.1161/ATVBAHA.111.231548. [DOI] [PubMed] [Google Scholar]
- 103.Shi Y, Pieniek M, Fard A, O’Brien J, Mannion JD, Zalewski A. Adventitial remodeling after coronary arterial injury. Circulation. 1996;93:340–348. doi: 10.1161/01.cir.93.2.340. [DOI] [PubMed] [Google Scholar]
- 104.Bailey MT, Pillarisetti S, Xiao H, Vyavahare NR. Role of elastin in pathologic calcification of xenograft heart valves. J Biomed Mater Res A. 2003;66:93–102. doi: 10.1002/jbm.a.10543. [DOI] [PubMed] [Google Scholar]
- 105.Herbst TJ, McCarthy JB, Tsilibary EC, Furcht LT. Differential effects of laminin, intact type IV collagen, and specific domains of type IV collagen on endothelial cell adhesion and migration. J Cell Biol. 1988;106:1365–1373. doi: 10.1083/jcb.106.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gould DS, Auchincloss H Jr. Direct and indirect recognition: the role of MHC antigens in graft rejection. Immunol Today. 1999;20:77–82. doi: 10.1016/s0167-5699(98)01394-2. [DOI] [PubMed] [Google Scholar]
- 107.Sanchez-Fueyo A, Strom TB. Immunologic basis of graft rejection and tolerance following transplantation of liver or other solid organs. Gastroenterology. 2011;140:51–64. doi: 10.1053/j.gastro.2010.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seetharam A, Tiriveedhi V, Mohanakumar T. Alloimmunity and autoimmunity in chronic rejection. Curr Opin Organ Transplant. 2010;15:531–536. doi: 10.1097/MOT.0b013e32833b31f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Galvao FH, Soler W, Pompeu E, Waisberg DR, Mello ES, Costa AC, Teodoro W, Velosa AP, Capelozzi VL, Antonangelo L, Catanozi S, Martins A, Malbouisson LM, Cruz RJ Jr, Figueira ER, Filho JA, Chaib E, D’Albuquerque LA. Immunoglobulin G profile in hyperacute rejection after multivisceral xenotransplantation. Xenotransplantation. 2012;19:298–304. doi: 10.1111/xen.12002. [DOI] [PubMed] [Google Scholar]
- 110.Schuurman HJ, Cheng J, Lam T. Pathology of xenograft rejection: a commentary. Xenotransplantation. 2003;10:293–299. doi: 10.1034/j.1399-3089.2003.02092.x. [DOI] [PubMed] [Google Scholar]
- 111.Oriol R, Ye Y, Koren E, Cooper DK. Carbohydrate antigens of pig tissues reacting with human natural antibodies as potential targets for hyperacute vascular rejection in pig-to-man organ xenotransplantation. Transplantation. 1993;56:1433–1442. doi: 10.1097/00007890-199312000-00031. [DOI] [PubMed] [Google Scholar]
- 112.Cooper DK, Good AH, Koren E, Oriol R, Malcolm AJ, Ippolito RM, Neethling FA, Ye Y, Romano E, Zuhdi N. Identification of alpha-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: relevance to discordant xenografting in man. Transpl Immunol. 1993;1:198–205. doi: 10.1016/0966-3274(93)90047-c. [DOI] [PubMed] [Google Scholar]
- 113.Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, Lancos CJ, Prabharasuth DD, Cheng J, Moran K, Hisashi Y, Mueller N, Yamada K, Greenstein JL, Hawley RJ, Patience C, Awwad M, Fishman JA, Robson SC, Schuurman HJ, Sachs DH, Cooper DK. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 114.Chen G, Qian H, Starzl T, Sun H, Garcia B, Wang X, Wise Y, Liu Y, Xiang Y, Copeman L, Liu W, Jevnikar A, Wall W, Cooper DK, Murase N, Dai Y, Wang W, Xiong Y, White DJ, Zhong R. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005;11:1295–1298. doi: 10.1038/nm1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Konakci KZ, Bohle B, Blumer R, Hoetzenecker W, Roth G, Moser B, Boltz-Nitulescu G, Gorlitzer M, Klepetko W, Wolner E, Ankersmit HJ. Alpha-Gal on bioprostheses: xenograft immune response in cardiac surgery. Eur J Clin Invest. 2005;35:17–23. doi: 10.1111/j.1365-2362.2005.01441.x. [DOI] [PubMed] [Google Scholar]
- 116.Stone KR, Abdel-Motal UM, Walgenbach AW, Turek TJ, Galili U. Replacement of human anterior cruciate ligaments with pig ligaments: a model for anti-non-gal antibody response in long-term xenotransplantation. Transplantation. 2007;83:211–219. doi: 10.1097/01.tp.0000250598.29377.13. [DOI] [PubMed] [Google Scholar]
- 117.Stone KR, Ayala G, Goldstein J, Hurst R, Walgenbach A, Galili U. Porcine cartilage transplants in the cynomolgus monkey. III. Transplantation of alpha-galactosidase-treated porcine cartilage. Transplantation. 1998;65:1577–1583. doi: 10.1097/00007890-199806270-00007. [DOI] [PubMed] [Google Scholar]
- 118.Hutter H, Vogel BE, Plenefisch JD, Norris CR, Proenca RB, Spieth J, Guo C, Mastwal S, Zhu X, Scheel J, Hedgecock EM. Conservation and novelty in the evolution of cell adhesion and extracellular matrix genes. Science. 2000;287:989–994. doi: 10.1126/science.287.5455.989. [DOI] [PubMed] [Google Scholar]
- 119.Elkins RC, Lane MM, Capps SB, McCue C, Dawson PE. Humoral immune response to allograft valve tissue pretreated with an antigen reduction process. Semin Thorac Cardiovasc Surg. 2001;13:82–86. [PubMed] [Google Scholar]
- 120.Meyer SR, Nagendran J, Desai LS, Rayat GR, Churchill TA, Anderson CC, Rajotte RV, Lakey JR, Ross DB. Decellularization reduces the immune response to aortic valve allografts in the rat. J Thorac Cardiovasc Surg. 2005;130:469–476. doi: 10.1016/j.jtcvs.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 121.Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40:1101–1110. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Senior RM, Hinek A, Griffin GL, Pipoly DJ, Crouch EC, Mecham RP. Neutrophils show chemotaxis to type IV collagen and its 7S domain and contain a 67 kD type IV collagen binding protein with lectin properties. Am J Respir Cell Mol Biol. 1989;1:479–487. doi: 10.1165/ajrcmb/1.6.479. [DOI] [PubMed] [Google Scholar]
- 123.Adair-Kirk TL, Atkinson JJ, Broekelmann TJ, Doi M, Tryggvason K, Miner JH, Mecham RP, Senior RM. A site on laminin alpha 5, AQARSAASKVKVSMKF, induces inflammatory cell production of matrix metalloproteinase-9 and chemotaxis. J Immunol. 2003;171:398–406. doi: 10.4049/jimmunol.171.1.398. [DOI] [PubMed] [Google Scholar]
- 124.Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM, Shapiro SD. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest. 2006;116:753–759. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Allman AJ, McPherson TB, Badylak SF, Merrill LC, Kallakury B, Sheehan C, Raeder RH, Metzger DW. Xenogeneic extracellular matrix grafts elicit a TH2-restricted immune response. Transplantation. 2001;71:1631–1640. doi: 10.1097/00007890-200106150-00024. [DOI] [PubMed] [Google Scholar]
- 126.Allman AJ, McPherson TB, Merrill LC, Badylak SF, Metzger DW. The Th2-restricted immune response to xenogeneic small intestinal submucosa does not influence systemic protective immunity to viral and bacterial pathogens. Tissue Eng. 2002;8:53–62. doi: 10.1089/107632702753503054. [DOI] [PubMed] [Google Scholar]
- 127.Chen N, Field EH. Enhanced type 2 and diminished type 1 cytokines in neonatal tolerance. Transplantation. 1995;59:933–941. doi: 10.1097/00007890-199504150-00002. [DOI] [PubMed] [Google Scholar]
- 128.Chen Y, Chen J, Liu Z, Liang S, Luan X, Long F, Peng Y, Yan L, Gong J. Relationship between TH1/TH2 cytokines and immune tolerance in liver transplantation in rats. Transplant Proc. 2008;40:2691–2695. doi: 10.1016/j.transproceed.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 129.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:281–286. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- 130.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- 131.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 132.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 133.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109–116. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14:1835–1842. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 135.Ariganello MB, Simionescu DT, Labow RS, Lee JM. Macrophage differentiation and polarization on a decellularized pericardial biomaterial. Biomaterials. 2011;32:439–449. doi: 10.1016/j.biomaterials.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ariganello MB, Labow RS, Lee JM. In vitro response of monocyte-derived macrophages to a decellularized pericardial biomaterial. J Biomed Mater Res A. 2010;93:280–288. doi: 10.1002/jbm.a.32554. [DOI] [PubMed] [Google Scholar]
- 137.Brown BN, Ratner BD, Goodman SB, Amar S, Badylak SF. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33:3792–3802. doi: 10.1016/j.biomaterials.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brown BN, Londono R, Tottey S, Zhang L, Kukla KA, Wolf MT, Daly KA, Reing JE, Badylak SF. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012;8:978–987. doi: 10.1016/j.actbio.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004;22:80–86. doi: 10.1016/j.tibtech.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 140.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, Dickinson SC, Hollander AP, Mantero S, Conconi MT, Birchall MA. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 141.Go T, Jungebluth P, Baiguero S, Asnaghi A, Martorell J, Ostertag H, Mantero S, Birchall M, Bader A, Macchiarini P. Both epithelial cells and mesenchymal stem cell-derived chondrocytes contribute to the survival of tissue-engineered airway transplants in pigs. J Thorac Cardiovasc Surg. 2010;139:437–443. doi: 10.1016/j.jtcvs.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 142.Walles T, Puschmann C, Haverich A, Mertsching H. Acellular scaffold implantation--no alternative to tissue engineering. Int J Artif Organs. 2003;26:225–234. doi: 10.1177/039139880302600308. [DOI] [PubMed] [Google Scholar]
- 143.Kasimir MT, Weigel G, Sharma J, Rieder E, Seebacher G, Wolner E, Simon P. The decellularized porcine heart valve matrix in tissue engineering: platelet adhesion and activation. Thromb Haemost. 2005;94:562–567. doi: 10.1160/TH05-01-0025. [DOI] [PubMed] [Google Scholar]
- 144.Assmann A, Delfs C, Munakata H, Schiffer F, Horstkotter K, Huynh K, Barth M, Stoldt VR, Kamiya H, Boeken U, Lichtenberg A, Akhyari P. Acceleration of autologous in vivo recellularization of decellularized aortic conduits by fibronectin surface coating. Biomaterials. 2013;34:6015–6026. doi: 10.1016/j.biomaterials.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 145.Lu Q, Rounds S. Focal adhesion kinase and endothelial cell apoptosis. Microvasc Res. 2012;83:56–63. doi: 10.1016/j.mvr.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ota T, Sawa Y, Iwai S, Kitajima T, Ueda Y, Coppin C, Matsuda H, Okita Y. Fibronectin-hepatocyte growth factor enhances reendothelialization in tissue-engineered heart valve. Ann Thorac Surg. 2005;80:1794–1801. doi: 10.1016/j.athoracsur.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 147.Huang SD, Liu XH, Bai CG, Lu FL, Yuan Y, Gong DJ, Xu ZY. Synergistic effect of fibronectin and hepatocyte growth factor on stable cell-matrix adhesion, re-endothelialization, and reconstitution in developing tissue-engineered heart valves. Heart Vessels. 2007;22:116–122. doi: 10.1007/s00380-006-0940-2. [DOI] [PubMed] [Google Scholar]
- 148.Zhou M, Liu Z, Wei Z, Liu C, Qiao T, Ran F, Bai Y, Jiang X, Ding Y. Development and validation of small-diameter vascular tissue from a decellularized scaffold coated with heparin and vascular endothelial growth factor. Artif Organs. 2009;33:230–239. doi: 10.1111/j.1525-1594.2009.00713.x. [DOI] [PubMed] [Google Scholar]
- 149.Miller-Kasprzak E, Jagodzinski PP. Endothelial progenitor cells as a new agent contributing to vascular repair. Arch Immunol Ther Exp (Warsz) 2007;55:247–259. doi: 10.1007/s00005-007-0027-5. [DOI] [PubMed] [Google Scholar]
- 150.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 151.Medina RJ, O’Neill CL, O’Doherty TM, Knott H, Guduric-Fuchs J, Gardiner TA, Stitt AW. Myeloid angiogenic cells act as alternative M2 macrophages and modulate angiogenesis through interleukin-8. Mol Med. 2011;17:1045–1055. doi: 10.2119/molmed.2011.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tsai TN, Kirton JP, Campagnolo P, Zhang L, Xiao Q, Zhang Z, Wang W, Hu Y, Xu Q. Contribution of stem cells to neointimal formation of decellularized vessel grafts in a novel mouse model. Am J Pathol. 2012;181:362–373. doi: 10.1016/j.ajpath.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 153.Reing JE, Zhang L, Myers-Irvin J, Cordero KE, Freytes DO, Heber-Katz E, Bedelbaeva K, McIntosh D, Dewilde A, Braunhut SJ, Badylak SF. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A. 2009;15:605–614. doi: 10.1089/ten.tea.2007.0425. [DOI] [PubMed] [Google Scholar]
- 154.Mirabella T, Cilli M, Carlone S, Cancedda R, Gentili C. Amniotic liquid derived stem cells as reservoir of secreted angiogenic factors capable of stimulating neo-arteriogenesis in an ischemic model. Biomaterials. 2011;32:3689–3699. doi: 10.1016/j.biomaterials.2011.01.071. [DOI] [PubMed] [Google Scholar]
- 155.Mirabella T, Hartinger J, Lorandi C, Gentili C, van Griensven M, Cancedda R. Proangiogenic soluble factors from amniotic fluid stem cells mediate the recruitment of endothelial progenitors in a model of ischemic fasciocutaneous flap. Stem Cells Dev. 2012;21:2179–2188. doi: 10.1089/scd.2011.0639. [DOI] [PubMed] [Google Scholar]
- 156.Mirabella T, Gentili C, Daga A, Cancedda R. Amniotic fluid stem cells in a bone microenvironment: driving host angiogenic response. Stem Cell Res. 2013;11:540–551. doi: 10.1016/j.scr.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 157.Ng SL, Narayanan K, Gao S, Wan AC. Lineage restricted progenitors for the repopulation of decellularized heart. Biomaterials. 2011;32:7571–7580. doi: 10.1016/j.biomaterials.2011.06.065. [DOI] [PubMed] [Google Scholar]
- 158.Lu TY, Lin B, Kim J, Sullivan M, Tobita K, Salama G, Yang L. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat Commun. 2013;4:2307. doi: 10.1038/ncomms3307. [DOI] [PubMed] [Google Scholar]
- 159.Margariti A, Winkler B, Karamariti E, Zampetaki A, Tsai TN, Baban D, Ragoussis J, Huang Y, Han JD, Zeng L, Hu Y, Xu Q. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. Proc Natl Acad Sci U S A. 2012;109:13793–13798. doi: 10.1073/pnas.1205526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mirsadraee S, Wilcox HE, Korossis SA, Kearney JN, Watterson KG, Fisher J, Ingham E. Development and characterization of an acellular human pericardial matrix for tissue engineering. Tissue Eng. 2006;12:763–773. doi: 10.1089/ten.2006.12.763. [DOI] [PubMed] [Google Scholar]
- 161.Singelyn JM, Sundaramurthy P, Johnson TD, Schup-Magoffin PJ, Hu DP, Faulk DM, Wang J, Mayle KM, Bartels K, Salvatore M, Kinsey AM, Demaria AN, Dib N, Christman KL. Catheter- deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J Am Coll Cardiol. 2012;59:751–763. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409–5416. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, Kwan OL, Strachan GM, Wong J, Schup-Magoffin PJ, Braden RL, Bartels K, DeQuach JA, Preul M, Kinsey AM, DeMaria AN, Dib N, Christman KL. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci Transl Med. 2013;5:173ra25. doi: 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wainwright JM, Czajka CA, Patel UB, Freytes DO, Tobita K, Gilbert TW, Badylak SF. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Part C Methods. 2010;16:525–532. doi: 10.1089/ten.tec.2009.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Weymann A, Loganathan S, Takahashi H, Schies C, Claus B, Hirschberg K, Soos P, Korkmaz S, Schmack B, Karck M, Szabo G. Development and evaluation of a perfusion decellularization porcine heart model--generation of 3-dimensional myocardial neoscaffolds. Circ J. 2011;75:852–860. doi: 10.1253/circj.cj-10-0717. [DOI] [PubMed] [Google Scholar]
- 166.Akhyari P, Aubin H, Gwanmesia P, Barth M, Hoffmann S, Huelsmann J, Preuss K, Lichtenberg A. The quest for an optimized protocol for whole-heart decellularization: a comparison of three popular and a novel decellularization technique and their diverse effects on crucial extracellular matrix qualities. Tissue Eng Part C Methods. 2011;17:915–926. doi: 10.1089/ten.TEC.2011.0210. [DOI] [PubMed] [Google Scholar]
- 167.Oberwallner B, Brodarac A, Choi YH, Saric T, Anic P, Morawietz L, Stamm C. Preparation of cardiac extracellular matrix scaffolds by decellularization of human myocardium. J Biomed Mater Res A. 2013 doi: 10.1002/jbma.35000. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 168.Leyh RG, Wilhelmi M, Rebe P, Fischer S, Kofidis T, Haverich A, Mertsching H. In vivo repopulation of xenogeneic and allogeneic acellular valve matrix conduits in the pulmonary circulation. Ann Thorac Surg. 2003;75:1457–1463. doi: 10.1016/s0003-4975(02)04845-2. discussion 1463. [DOI] [PubMed] [Google Scholar]
- 169.Grauss RW, Hazekamp MG, Oppenhuizen F, van Munsteren CJ, Gittenberger-de Groot AC, DeRuiter MC. Histological evaluation of decellularised porcine aortic valves: matrix changes due to different decellularisation methods. Eur J Cardiothorac Surg. 2005;27:566–571. doi: 10.1016/j.ejcts.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 170.Erdbrugger W, Konertz W, Dohmen PM, Posner S, Ellerbrok H, Brodde OE, Robenek H, Modersohn D, Pruss A, Holinski S, Stein-Konertz M, Pauli G. Decellularized xenogenic heart valves reveal remodeling and growth potential in vivo. Tissue Eng. 2006;12:2059–2068. doi: 10.1089/ten.2006.12.2059. [DOI] [PubMed] [Google Scholar]
- 171.Bloch O, Erdbrugger W, Volker W, Schenk A, Posner S, Konertz W, Dohmen PM. Extracellular matrix in deoxycholic acid decellularized aortic heart valves. Med Sci Monit. 2012;18:BR487–492. doi: 10.12659/MSM.883618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rieder E, Kasimir MT, Silberhumer G, Seebacher G, Wolner E, Simon P, Weigel G. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J Thorac Cardiovasc Surg. 2004;127:399–405. doi: 10.1016/j.jtcvs.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 173.Naso F, Gandaglia A, Formato M, Cigliano A, Lepedda AJ, Gerosa G, Spina M. Differential distribution of structural components and hydration in aortic and pulmonary heart valve conduits: Impact of detergent-based cell removal. Acta Biomater. 2010;6:4675–4688. doi: 10.1016/j.actbio.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 174.Ota T, Taketani S, Iwai S, Miyagawa S, Furuta M, Hara M, Uchimura E, Okita Y, Sawa Y. Novel method of decellularization of porcine valves using polyethylene glycol and gamma irradiation. Ann Thorac Surg. 2007;83:1501–1507. doi: 10.1016/j.athoracsur.2006.11.083. [DOI] [PubMed] [Google Scholar]
- 175.Honge JL, Funder JA, Jensen H, Dohmen PM, Konertz WF, Hasenkam JM. Recellularization of decellularized mitral heart valves in juvenile pigs. J Heart Valve Dis. 2010;19:584–592. [PubMed] [Google Scholar]
- 176.Jordan JE, Williams JK, Lee SJ, Raghavan D, Atala A, Yoo JJ. Bioengineered self-seeding heart valves. J Thorac Cardiovasc Surg. 2012;143:201–208. doi: 10.1016/j.jtcvs.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 177.Pellegata AF, Asnaghi MA, Stefani I, Maestroni A, Maestroni S, Dominioni T, Zonta S, Zerbini G, Mantero S. Detergent-enzymatic decellularization of swine blood vessels: insight on mechanical properties for vascular tissue engineering. Biomed Res Int. 2013;2013:918753. doi: 10.1155/2013/918753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mangold S, Schrammel S, Huber G, Niemeyer M, Schmid C, Stangassinger M, Hoenicka M. Evaluation of decellularized human umbilical vein (HUV) for vascular tissue engineering - comparison with endothelium-denuded HUV. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1603. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 179.Gui L, Muto A, Chan SA, Breuer CK, Niklason LE. Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Eng Part A. 2009;15:2665–2676. doi: 10.1089/ten.tea.2008.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wilshaw SP, Rooney P, Berry H, Kearney JN, Homer-Vanniasinkam S, Fisher J, Ingham E. Development and characterization of acellular allogeneic arterial matrices. Tissue Eng Part A. 2012;18:471–483. doi: 10.1089/ten.tea.2011.0287. [DOI] [PubMed] [Google Scholar]
- 181.Dahl SL, Koh J, Prabhakar V, Niklason LE. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant. 2003;12:659–666. [PubMed] [Google Scholar]
- 182.Pellegata AF, Asnaghi MA, Zonta S, Zerbini G, Mantero S. A novel device for the automatic decellularization of biological tissues. Int J Artif Organs. 2012;35:191–198. doi: 10.5301/ijao.5000079. [DOI] [PubMed] [Google Scholar]
- 183.Quint C, Kondo Y, Manson RJ, Lawson JH, Dardik A, Niklason LE. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci U S A. 2011;108:9214–9219. doi: 10.1073/pnas.1019506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Azhim A, Yamagami K, Muramatsu K, Morimoto Y, Tanaka M. The use of sonication treatment to completely decellularize blood arteries: a pilot study. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:2468–2471. doi: 10.1109/IEMBS.2011.6090685. [DOI] [PubMed] [Google Scholar]


