Abstract
Saffron is the most expensive spice in the world and consists of the dried stigmas of Crocus sativus L. It is used as food coloring and flavoring in food industry and traditional cooking and also in folk medicine as antispasmodic, carminative, stomachic, expectorant, aphrodisiac and cardiotonic. The present study has evaluated the diuretic activity of aqueous extract of dried saffron (stigma of Crocussativus) in rat. Aqueous extracts of saffron were administered to experimental rats orally as doses of 60, 120 and 240 mg/kg body weight (BW) and compared with hydrochlorothiazide (10 mg/kg B.W., intraperitoneally), a potent diuretic as positive control and normal saline solution as placebo for control group. The measured parameters for diuretic activity were total urine volume, urine electrolytes concentration such as sodium and potassium, creatinine and urea concentration. The treated rats with aqueous extract of saffron as doses of 120 and 240 mg/kg BW showed higher urine output when compared to the control group. Also, it has shown a significant dose-dependent increase in the excretion of electrolytes when compared to the control group. Our findings proved the diuretic activity of saffron which is used in traditional medicine, it can be an effective and safe strategy for related dysfunction. Also further studies are needed to identify the mechanisms of action, probably other effects and interactions with other medicines.
Keywords: Aqueous extract, diuretic activity, hydrochlorothiazide, saffron
INTRODUCTION
Saffron is the most expensive spice in the world and consists of the dried stigmas of Crocus sativus commonly known as saffron, belonging to Iridaceae family, is a perennial plant widely cultivated in different parts of the world, particularly in Iran.[1] Although the source of saffron is obscure, it is apparently originated from Asia Minor and Iran. The name of saffron is derived from Arabic word of za-faran meaning “be yellow.” Iran is the major producer of saffron in the world market, but its quality is reported not to be as well as saffron of other main supplier, like Spain.[2,3] Although the most usage of saffron is done as a food coloring and flavoring agent in the food industry and traditional cooking, but it is used in folk medicine as antispasmodic, carminative, stomachic, expectorant, aphrodisiac and cardiotonic. Recent pharmacological studies have reported that saffron extract have antitumor, anticonvulsant, antidepressant, anti-inflammatory, anti-hyperlipidemic, free radical scavenging and antioxidant properties.[4,5] Moreover, chemopreventive and protective effects of saffron extract on genotoxin-induced oxidative stress in animals have been reported.[5]
These properties are basically related to its contents of picrocrocin, safranal and crocins.[6,7] Picrocrocin is a monoterpene glycoside precursor of safranal (2,6,6-trimethyl-1,3-cyclohexadiene-1-carboxaldehyde), which is in turn the most abundant of the volatile compounds responsible for the aroma of this spice.[8] Crocin, a water soluble carotenoid, is found in the stigmas of saffron. Crocins are crocetin esters with glucose, gentiobiose, neapolitanose or triglucose sugar moieties where trans- or cis-configuration is found. Crocin is the chemical ingredient primarily responsible for saffron's yellowish color.[9]
As the plant is reported to have various medicinal uses, researchers have attempted to study the pharmacognostic effects of saffron. Importance of diuretic activity in traditional medicine is frequently mentioned, for example in ayurverdic medical system, some plants such as Cycleapeltata is evaluated by Hullatti et al.[10] Considering the various uses of Crocus sativus L in Iran's folk medicine as a diuretic, this study has aimed to evaluate the diuretic activity of the aqueous extract of saffron.
MATERIALS AND METHODS
Chemicals
All solvents (gradient grade) and chemicals (analytical grade) were obtained from Merck and had certificate of analysis (Darmstadt, Germany).
Plant material
Stigmas were collected from Fakhrabad, Bajestan city, Khorasan Rezavi province, Iran in November 2011 which is the time of collecting from the farm. The stigmas were air-dried in shadow and grounded into fine powder by a laboratory mill. The C. sativus L. was properly identified by Dr. Amir Hossein Jamshidi, academic member of Food and Drug Organization, Ministry of Health, Tehran, Iran. Voucher samples were preserved for references in the herbarium of the school of pharmacy (143-0339-1).
Preparation of extract
For maceration, 5 g of powdered stigma was macerated in 100 mL water for 24 h. The mixture was centrifuged, freeze dried and lyophilized powder was stored at -20 °C. The yield of the extract was 25% (w/w).
Pharmacological tests
Animals
Fortymale rat (in housed bred) with weight range of 200-220 g were used in all experiments and randomly divided into five groups. They were kept eight per cage in the animal house of Gonabad University of Medical Sciences (GUMS), Iran, with 12 h light dark cycle. The animals were fed with a balanced pellet diet and fresh tap water was continuously accessible. All experiments were conducted in accordance with the internationally accepted principles for laboratory animal use and care in animal ethics committee of GUMS (K/A/389/9).
Acute toxicity study
Acute toxicity study was done in biochemical laboratory, school of medicine Baghiyatallah University, Tehran, Iran according to OECD (Organization for Economic Co-operation and Development) Guideline 420[11] fixed dose method; with starting dose of 2000 mg/kg body weight adopted. Starting dose of 2000 mg/kg (per oral) of each 50% v/v aqueous extract was given to five animals for observation of behavioral change and death up to 72 h.
Diuretic test
The method of Lipschitz was applied for the assessment of diuretic activity. Five groups (each containing eight rats) were fasted and deprived of water for 18 h prior to the experiments. On experiment day, saffron aqueous extract was orally administrated to experimental rats at doses of 60, 120 and 240 mg/kg B.W. Control group animals received normal saline solution only. Immediately after dosing, the rats were placed at the metabolic cages (8 in each cage) specially designed to separate urine and faeces. Animals were kept at room temperature of 24 ± 2°C throughout the experiment. The urine was collected in measuring cylinder up to 5 h after dosing. During this period, food or water was not provided to the animals. The total volume of collected urine was measured for both control and treatment groups. The taken parameters for individual rat were body weight (before and after test period), total urine volume, urine sodium and potassium concentrations which were measured by SEAC flame photometer and creatinine. Urea concentration was measured by Selectera (ParsAzmoonKit®). Diuretic activity of different concentration of aqueous extract was compared with a potent diuretic agent like hydrochlorothiazide as positive control (10 mg/kg B.W.).[12]
Statistical analysis
Results are reported as mean ± SD, the test of significance (P < 0.03) was statistically analyzed using Student's t-test and ANOVA by SPSS software 15.
RESULTS
Urinary volume
The urinary volume (UV) during 5 h period collection in the eight control animals was 8.23 ± 1.2 mL. In the positive control group, which was treated with 10 mg/kg BW of hydrochlorothiazide, there was a significant increase in the UV as 35.67 ± 3.73 mL (P < 0.001) and also in 240 mg/kg BW tested group was 28.39 ± 1.9 mL. In the 60 and 120 mg/kg BW tested group, though the UV was significantly greater than that in the control group, but it was lesser than the UV in the positive control group. The UV for the recent groups was found to be 18.63 ± 1.23 mL and 20.04 ± 1.75, respectively (P < 0.03) as shown in Figure 1.
Figure 1.
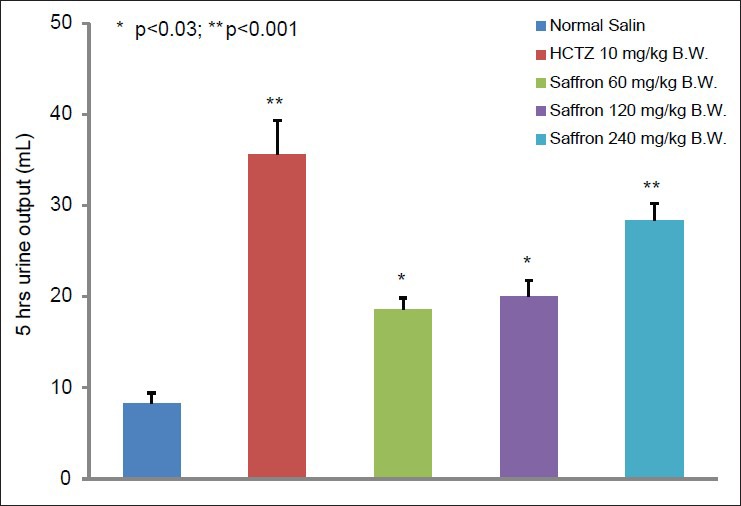
Effect of Crocus sativus L aqueous extract on urine volume in the rats (mean±S.E.M.) in comparison with hydrochlorothiazide and the control group (n=8)
Urinary sodium
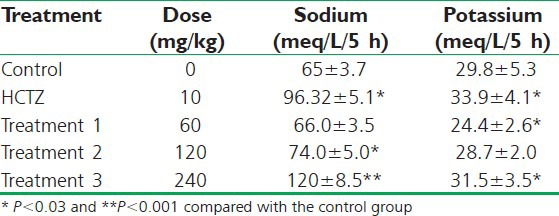
Urinary sodium (Na+) during the period of the 5 h collection in the control animals was 65 ± 3.7 meq/kg. In the positive control group, there was a significant increase in the Na+ excretion as 96.32 ± 5.1 meq/kg (P < 0.001) and in the test group (240 mg/kg) was significantly greater than the control group, and also the positive control group which is 120 ± 8.5 meq/kg (P < 0.001) as shown in Table 1.
Table 1.
Effect of orally administrated aqueous extract of saffron and HCTZ on sodium and potassium excreted in urine of rats (n=8)
Urinary Potassium
Urinary potassium excretion (K+) during the period of the 5 h collection in the control animals was found to be 29.8 ± 5.3 meq/kg. In the positive control group, there was a significant increase in the K+ excretion as 33.9 ± 4.1 meq/kg (P < 0.001), but there was a significant decrease in the K+ excretion in the test groups. 24.4 ± 2.6 meq/kg (P < 0.001) as shown in Table 1.
Urinary urea and creatinine
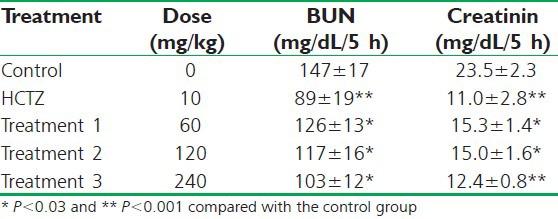
Urinary urea during the period of the 5 h collection in the control animals was 147 ± 17 mg/dL. In the positive group, there was a significant decrease in the urea excretion as 89 ±19 mg/dL (P < 0.001) and in the tested group, the urea excretion was significantly lesser than that of the control group, but greater than that of the positive group, i.e. 126 ±13 mg/dL (P < 0.03) for 60 mg/kg BW and 103 ± 12 for 240 mg/kg BW.
Also urinary creatinine in the control animals was 23.5 ± 2.3 mg/dL. In the control positive group, there was a significant decrease in the creatinine excretion as 11.0 ± 2.8 mg/dL (P < 0.001) and in the tested group, the creatinine excretion was significantly lesser than the control group, but greater than that of the positive group, i.e. 15.3 ± 1.4 mg/dL (P < 0.03) for 60 mg/kg BW and 12.4 ± 0.8 for 240 mg/kg BW. Table 2 shows the urinary urea and creatinine excretion.
Table 2.
Effect of orally administrated aqueous extract of saffron and HCTZ on the urea and creatinine excreted in urine of rats (n=8)
DISCUSSION
In the present study, we examined the diuretic activity of saffron, orally administered in rats for the first time. The results showed that saffron has a strong diuretic activity (in terms of cumulative urine output and diuretic action), hypernatremic (in terms of urinary Na+ level and sodium saliuretic index) and hyperkalemic (in terms of urinary K+ level and potassium saliuretic index) activities.
The existing animal studies do not support the aquaretic theory, however frequently diuretic herbs have been shown to influence renal electrolyte handling, particularly sodium and potassium, and thus have diuretic activity.[13,14,15,16]
Ying Zhang et al.,[17] compared diuretic activity of Rubusidaeus L. extracts with hydrochlorothiazide (synthetic diuretic) in rat. They showed that hydrochlorothiazide had a higher inhibitory effect on electrolyte (Na+, K+ and Cl-) in rats than the extracts, nevertheless their abilities for inhibiting the UV were similar with our results in the present study.
Srivastav et al.,[18] compared diuretic activities among methanolic extract of whole plant of Achyranthesaspera, normal saline and furosemide in rat by measuring both electrolytes (Na+, K+ and Cl-) and UV. They showed methanol extract of Achyranthesaspera significantly increased urinary output in comparison with the control group but the effect was much less than furosemide. They showed a higher inhibition for methanolic extract which was in agreement with our results in this study.
The diuretic efficacy of saffron was almost comparable (diuretic activity 0.86) to hydrochlorothiazide, widely used thiazide diuretic in clinical practice.[19] The onset of diuretic activity of saffron was extremely rapid, almost similar to oral synthetic diuretic such as furosemide, indicating quick absorption from the gastrointestinal tract. Further, the rapid onset of diuresis by saffron indicates that its diuretic action is unlikely to be mediated via a secondary organic metabolite. The diuretic activity of saffron lasted throughout the study period (up to 24 h) suggesting a slow clearance. Such an action profile is therapeutically desirable. Interestingly, the diuretic action of saffron was dose-dependent indicating that this effect is intrinsic, genuine and causal and possibly receptor mediated. However, even the receptors for clinically most important diuretics are yet unknown.[20]
Our study confirms that the use of saffron as diuretic by Iranian folk medicine, which is corroborated by other studies, that presenting renal alterations, confirming the diuretic effect in rats. There are several phytochemical compounds which could be responsible for diuretic effect such as flavonoids, saponins and other polar compounds. The preliminary pharmacognostic studies on phytochemical compounds have confirmed the presences of flavonoids, carbohydrates, tannins, anthocyanins, alkaloids and saponins in saffron.[21] Hence, the diuretic effects of aqueousextract of saffron may be due to the presence of flavonoids, saponins, anthocyanins and alkaloids which may act individually or in combination. The result of present study revealed that aqueous extract of saffron with dose of 240 mg/kg (the highest tested dose) has diuretic property equipotent to hydrochlorothiazide (a potent diuretic compound) at higher tested dose. The diuretic action of saffron aqueous extract maybe due to inhibition of Na+ and Cl- symport in the distal convoluted tubule or modulation of the activity of high affinity thiazides receptors in renal cortex.
CONCLUSION
This study confirms the proper diuretic activity of saffron which is used in Iranian folk medicine for diuresis. The main use of saffron is as a food additive in Iranian meal to improve taste and color, may be with its compounds in foods, the diuretic effect appears too. Although we do not yet totally understand which active compounds and mechanism of action cause these effects, use of animal models suggest the variety of possibilities. Some identified pharmacologic compounds, like crocin, picrocrocin and safranal need to be evaluated for therapeutic effects to know which is the active compound. As saffron is a safe and natural product, further study may pay attention to formulate dosage form from saffron and its compounds, for example in Iran saffron tablet is prepared from whole stigma to use in traditional and modern medicine. Also it needs some further studies for any probable effects and interactions with other related drugs in the treatment of patients. Our laboratory still investigates some renal mechanisms, for better understanding these renal and cardiovascular modifications.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Sanchez-Vioque R, Rodriguez–Conde MF, Reina-Urena JV, Escolano-Tercero MA, Herraiz-Penalvar D, Santana-Meridas O. In vitro antioxidant and metal chelating properties of corm, petal and leaf from saffron (Crocus sativus) Ind Crops Prod. 2012;39:149–53. [Google Scholar]
- 2.Winterhalter P, Straubinger M. Saffron-renewed interest in an ancientspice. Food Rev Int. 2000;16:39–59. [Google Scholar]
- 3.Caballero-Ortega H, Pereda-Miranda R, Abdullaev FI. HPLC quantificationof major active components from 11 different saffron (Crocus sativusL.) sources. Food Chem. 2007;100:1126–31. [Google Scholar]
- 4.Asdaq SM, Inamdar MN. Potential of Crocus sativus (saffron) and its constituent, crocin, as hypolipideic and antioxidant in rats. Appl Biochem Biotechnol. 2010;162:358–72. doi: 10.1007/s12010-009-8740-7. [DOI] [PubMed] [Google Scholar]
- 5.Abdullaev FI. Biological effects of saffron. Biofactors. 1993;4:83–6. [PubMed] [Google Scholar]
- 6.Carmona M, Zalacain A, Salinas MR, Alonso GL. A new approach to saffron aroma. Crit Rev Food Sci Nutr. 2007;47:145–59. doi: 10.1080/10408390600626511. [DOI] [PubMed] [Google Scholar]
- 7.Carmona M, Zalacain A, Sanchez AM, Novella JL, Alonso GL. Crocetin esterspicrocrocin and its related compounds present in Crocus sativus stigmas and Gardenia jasminoides fruits. Tentative identification of seven new compounds by LC-ESI-MS. J Agric Food Chem. 2006;54:973–9. doi: 10.1021/jf052297w. [DOI] [PubMed] [Google Scholar]
- 8.Maggi L, Carmona M, Del Campo CP, Kanakis CD, Anastasaki EG, Tarantilis PA. Worldwide market screening of saffron volatile composition. J Sci Food Agric. 2009;89:1950–4. [Google Scholar]
- 9.Anastasaki E, Kanakis C, Pappas C, Maggi L, Zalacain A, Carmona M. Quantification of crocetin esters in saffron (Crocus sativus L.) using Raman spectroscopy and chemometrics. J Agric Food Chem. 2010;58:6011–7. doi: 10.1021/jf100143n. [DOI] [PubMed] [Google Scholar]
- 10.Hullatti KK, Gopikrishna UV, Kuppast IJ. Phytochemical investigation and diuretic activity of Cycleapeltataleaf extracts. J Adv Pharm Technol Res. 2011;2:241–4. doi: 10.4103/2231-4040.90880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.OECD Publishing; OECD (2002), Test No. 420: Acute Oral Toxicity-Fixed Dose Procedure, OECD Guidelines for the Testing of Chemicals, Section 4. [Google Scholar]
- 12.Lipschitz WL, Hadidian Z, Kerpcar A. Bioassay of Diuretics. J Pharmacol Exp Ther. 1943;79:97–110. [Google Scholar]
- 13.Pantoja CV, Martin NT, Norris BC, Contreras CM. Purification and bioassays of a diuretic and natriuretic fraction from garlic (Allium sativum) J Ethnopharmacol. 2000;70:35–40. doi: 10.1016/s0378-8741(99)00145-2. [DOI] [PubMed] [Google Scholar]
- 14.Galati EM, Tripodo MM, Trovato A, Miceli N, Monforte MT. Biologic effect of Opuntiafícusindica (L) Mill (Cactaceae) waste matter. Note I: Diuretic activity. J Ethnopharmacol. 2002;79:17–21. doi: 10.1016/s0378-8741(01)00337-3. [DOI] [PubMed] [Google Scholar]
- 15.Yarnell E. Botanical medicines for the urinary extract. World J Urol. 2002;20:285–93. doi: 10.1007/s00345-002-0293-0. [DOI] [PubMed] [Google Scholar]
- 16.Eduardo de Campos K, Coelho Balbi AP, José Queiroz de FreitasAlves M. Diuretic and hypotensive activity of aqueous extract of parsley seeds Petroselinumsativum in rats. Rev Bras Farmacogn. 2009;19:41–5. [Google Scholar]
- 17.Zhang Y, Zhang Z, Yang Y, Wang Y, Zu X, Guan D. Diuretic Activity of methanol extracts of Rubusidaeus L. Int J Biol. 2011;3:75–81. [Google Scholar]
- 18.Srivastav S, Singh P, Mishra G, Jha KK, Khosa RL. Achyranthes aspera-An important medicinal plant: A review, Scholars Research Library. J Nat Prod Plant Resour. 2011;1:1–14. [Google Scholar]
- 19.London: British Medical Association and the Royal Pharmacological Society of Great Britain; 2001. British National Formulary; pp. 63–9. [Google Scholar]
- 20.Odlind B. Site and mechanism of the action of diuretics. Acta Pharmacol Toxicol. 1984;54:5–15. doi: 10.1111/j.1600-0773.1984.tb03625.x. [DOI] [PubMed] [Google Scholar]
- 21.Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;2:7. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


