Summary
In the 1970s and 1980s, it was observed that rodents could offset excess calories ingested when they were fed a human-like `cafeteria diet'. Although it was erroneously concluded that this so-called diet-induced thermogenesis was because of brown adipose tissue (BAT), it led to efforts to test whether variations in brown fat in humans may explain the susceptibility to obesity. However, from evidence on the inability of ephedrine or beta-3 adrenergic agonists to induce BAT thermogenesis, it was concluded that the thermogenic role of BAT was unimportant in adult humans largely because humans had low numbers of brown adipocytes. Solid evidence on the actual numbers of brown adipocytes in humans was not available. We are now re-evaluating the role of BAT for the treatment of obesity given the following recent observations (i) studies in nuclear medicine by using PET/CT scanning reveal the presence of BAT in adult humans; and (ii) recent data suggest that a new transcription factor called PDRM16 may control the induction of BAT. These recent discoveries should revamp our effort to target the molecular development of brown adipogenesis in the treatment of obesity.
Keywords: Brown adipose tissue, obesity, PRDM16
Weight gain results from a sustained imbalance between energy intake and energy expenditure favouring positive energy balance. However, such a simple statement belies the complex, multifactorial nature of obesity and the numerous biological and behavioural factors that can affect both sides of the energy expenditure equation. Ironically, as scientists try to unravel the complex biological causes of obesity, it is clear that our societal environment is marked by an over-abundant accessibility of food rich in energy coupled with a strong trend to reduced physical activity. In this `obesogenic environment', not only the prevalence of obesity has risen to unthinkable levels (1), but it now also seems that babies born at the beginning of the 21st century may have shorter life expectancies than their parents (2). However, not everyone becomes obese in our new detrimental environment, not only because of conscious behavioural efforts to eat less and stay more active but also because of potential biological factors such as the presence of active brown adipose tissue (BAT), a system that can dissipate energy surpluses in rodents when treated with adrenergic agonists. However, the application of this method to enhance metabolic inefficiency requires the presence of adequate levels of BAT. A recent study by Spiegelman's group suggests now that a new transcription factor called PDRM16 may control a bidirectional switch between skeletal muscle and BAT (3). Therefore, like skeletal muscle, BAT may be up-regulated in humans by such a regulator and may be efficacious at dissipating some of the excess energy sneaking in our body as a result of passive over-consumption of energy and lack of physical activity.
Brown adipose tissue is an exquisitely designed tissue/organ system evolved for the maintenance of body temperature. At the metabolic, protein and transcription levels, the BAT is up-regulated principally by the sympathetic nervous system when production of heat is needed to maintain body temperature. Furthermore, the long-term need to provide heat can induce proliferation of brown adipocytes in specific deposits that are highly innervated and vascularized for both rapid and reversible activation of thermogenesis. Years ago, the groups of Nicholls and Ricquier described a mechanism for heat production based upon a specific highly abundant protein in the inner mitochondria of the brown adipocytes that uncouples the production of chemical energy as ATP from oxidative phosphorylation and instead produces heat (4,5). Until quite recently, BAT has been thought to be mostly important in small mammals and infants to maintain body temperature, but a function in the physiology of adult humans was dismissed because of low numbers of brown adipocytes (6,7). However, unrelated pursuits in nuclear medicine using PET/CT scanning techniques have revealed the presence of BAT in adult humans, especially after cold exposure (8,9). The question now is how we can use PDRM16 up-regulation to induce this amazing organ not only to generate heat but also to enhance fat oxidation and therefore reduce obesity.
Although the normal function of brown fat thermogenesis may be specific for the regulation of body temperature, many genetic and pharmacological studies in rodents have shown that abnormal constitutive over-expression of Ucp1 in white fat and skeletal muscle can drastically reduce both genetic and diet-induced obesity offering therefore a new safe molecular target for the treatment of obesity (10–12). Accordingly, bypassing normal cellular and molecular mechanisms of brown fat induction and Ucp1 activation has potentially provided the most effective strategies known for the reduction of obesity, and without serious side effects. This potential for brown fat adaptive thermogenesis as a drug target for obesity has not been ignored by the pharmaceutical industry. Unfortunately, many candidate agonists of the beta-3 adrenergic receptor have failed in human clinical trials, even though these drugs have been efficacious in rodent models of obesity. What is different in the human and mouse?
Most of the effects of genetic, pharmaceutical or cold-induced up-regulation of Ucp1 in the mouse models result in the emergence of new brown adipocytes in white fat depots with levels of Ucp1 up-regulated up to several hundred folds. Unfortunately, human white adipose tissue does not appear to be able to mount such transient induction of brown adipocytes. The failure of brown fat thermogenesis in humans appears to be based upon the lack of fundamental information on the mechanisms controlling the developmental origins of brown adipocytes in the discrete brown fat depots (e.g. interscapular brown fat) and in the diffusely localized brown adipocytes in various white fat depots. However, the recent paper by Spiegelman's group (3) elegantly describes a novel transcription factor, PRDM16, whose presence can promote the differentiation of preadipocytes and myoblasts into brown adipocytes and whose absence promotes the myogenic differentiation programme (Fig. 1). Importantly, the ability of PRDM16 to induce brown adipocyte lineage is restricted to the discrete brown fat depots line, such as that found in the interscapular region, but it does not participate in the induction of the diffuse brown adipocytes located in the white fat depots. The data support the genetic experiments indicating that interscapular BAT and brown adipocytes in white fat have separate independent developmental origins (13). PRDM16 is clearly an important player in brown adipogenesis, but it may not be sufficient as PRDM16 KO mice have significant levels of interscapular fat with Ucp1 expression (3). However, one does not know whether up-regulation of PRDM16 in humans can induce increased discrete BAT and/or diffuse brown adipocytes.
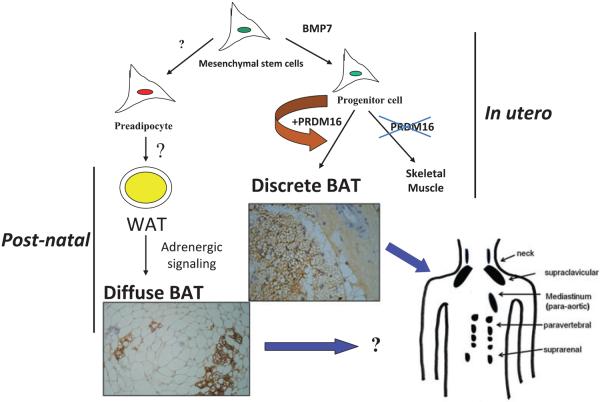
Figure 1.
Mesenchymal stem cells can have two major pathways, one into preadipocytes and another into progenitor cells of brown adipocytes or skeletal muscle cells. Bone morphogenetic protein 7 (BMP7) (15) activates a full programme of brown adipogenesis, including induction of early regulators of brown fat PRDM16 and PGC-1α. In the absence of PRDM16, these progenitor cells are transformed into skeletal muscle cells. Preadipocytes are differentiated into white adipocytes in subcutaneous and visceral adipose tissues. By stimulation of adrenergic signalling, some of these white adipocytes can be transformed into brown adipocytes diffused in the white adipose tissue (WAT). On the other hand, discrete brown adipocytes are concentrated in brown adipose tissue (BAT) depots such as in the neck, supraclavicular, paravertebral and suprarenal. The recently discovered brown adipocyte tissue regulator PRDM16 is responsible, at least in rodents, only for the generation of discrete BAT.
While the regulator PRDM16 has provided important insights into the developmental origins of discrete brown fat depots, the next important step will be to determine the origin(s) of diffusely localized brown adipocytes in white fat depots as, at least in rodents, this adipogenesis is more closely related to increased thermogenesis and reduced obesity. Enthusiasm for the promise of PRDM16 as a drug target needs to be tempered by the caveat that mice with an inactivated PRDM16 gene die at birth, suggesting that PRDM16 is a transcription factor with additional unknown functions in mammalian development. It has long been known that chronic increases in circulating catecholamines in patients with pheochromocytoma lead to large brown fat depots (14). This historical data, together with the recent findings of discrete brown fat depots uncovered by PET technologies (8), should stimulate a renewed effort to find strategies to induce more brown adipocytes and also to ask why the many previous studies with beta-3 agonists failed to significantly stimulate thermogenesis in humans. Maybe the lack of beta-3 adrenergic receptors in human white adipocytes is something that needs to be overcome to facilitate conversion of white to brown adipose tissue and to stimulate thermogenesis. It is also important to evaluate the effects of bone morphogenetic protein 7 on stimulating the enhanced expression of brown adipocytes as recently shown by Tseng's group (15). The discovery of previously uncovered BAT/cells in adult humans and its potential physiological significance in cold- and dietary-induced thermogenesis should revamp our effort to target the molecular development of brown adipogenesis in the treatment of obesity.
Footnotes
Conflict of Interest Statement No conflict of interest was declared.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 3.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heaton GM, Wagenvoord RJ, Kemp A, Jr, Nicholls DG. Brown-adipose-tissue mitochondria: photoaffinity labelling of the regulatory site of energy dissipation. Eur J Biochem. 1978;82:515–521. doi: 10.1111/j.1432-1033.1978.tb12045.x. [DOI] [PubMed] [Google Scholar]
- 5.Ricquier D, Kader JC. Mitochondrial protein alteration in active brown fat: a sodium dodecyl sulfate-polyacrylamide gel electrophoretic study. Biochem Biophys Res Commun. 1976;73:577–583. doi: 10.1016/0006-291x(76)90849-4. [DOI] [PubMed] [Google Scholar]
- 6.Astrup A, Bulow J, Christensen NJ, Madsen J. Ephedrine-induced thermogenesis in man: no role for interscapular brown adipose tissue. Clin Sci (Lond) 1984;66:179–186. doi: 10.1042/cs0660179. [DOI] [PubMed] [Google Scholar]
- 7.Astrup A, Bulow J, Madsen J, Christensen NJ. Contribution of BAT and skeletal muscle to thermogenesis induced by ephedrine in man. Am J Physiol. 1985;248:E507–515. doi: 10.1152/ajpendo.1985.248.5.E507. [DOI] [PubMed] [Google Scholar]
- 8.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 9.Tatsumi M, Engles JM, Ishimori T, Nicely O, Cohade C, Wahl RL. Intense (18)F-FDG uptake in brown fat can be reduced pharmacologically. J Nucl Med. 2004;45:1189–1193. [PubMed] [Google Scholar]
- 10.Ghorbani M, Himms-Hagen J. Appearance of brown adipocytes in white adipose tissue during CL 316,243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int J Obes Relat Metab Disord. 1997;21:465–475. doi: 10.1038/sj.ijo.0800432. [DOI] [PubMed] [Google Scholar]
- 11.Cummings DE, Brandon EP, Planas JV, Motamed K, Idzerda RL, McKnight GS. Genetically lean mice result from targeted disruption of the RII beta subunit of protein kinase A. Nature. 1996;382:622–626. doi: 10.1038/382622a0. [DOI] [PubMed] [Google Scholar]
- 12.Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest. 1995;96:2914–2923. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res. 2007;48:41–51. doi: 10.1194/jlr.M600287-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Ricquier D, Nechad M, Mory G. Ultrastructural and biochemical characterization of human brown adipose tissue in pheochromocytoma. J Clin Endocrinol Metab. 1982;54:803–807. doi: 10.1210/jcem-54-4-803. [DOI] [PubMed] [Google Scholar]
- 15.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]