Abstract
Since mutations in mitochondrial DNA (MtDNA) have been shown to be a cause of many mitochondrial diseases as well as aging, it is important to understand the origin of these mutations and how replication proteins modulate this process. DNA polymerase γ (pol γ) is the polymease that is responsible for replication and repair of mtDNA. Pol γ has three main roles in mtDNA maintanence and mutagenesis. As the only known DNA polymerase in mitochondria, pol γ is required for all replication and repair functions and is the main source of errors produced in our mtDNA. Pol γ is also sensitive to a host of antiviral nucleoside analogs used to treat HIV-1 infections, which can cause an induced mitochondrial toxicity. Finally, the gene for pol γ, POLG, is a genetic locus for several mitochondrial disease with over 150 genetic mutations currently identified.
Introduction
Mutations accumulate in mtDNA with age, and mutation of mtDNA has been shown to promote premature aging in mice (Dimauro and Davidzon, 2005). Point mutations, deletions and depletion of mtDNA have been observed in many human diseases (Wallace, 1999). Therefore is is essential to understand the origins of mutations in our mtDNA. Mitochondrial DNA is replicated and repaired by DNA polymerase γ (Pol γ). Pol γ, encoded by the POLG gene, is the only polymerase found in animal cell mitochondrial and is involved in replication, mutagenesis, and repair of mtDNA as well is a target of antiviral nucleoside analogs that cause mitochondrial toxicity. DNA polymerase gamma has three main roles in health and disease:
Spontaneous mutagenesis - as the only polymerase involved in mtDNA replication and repair, the origin of most spontaneous mutation is due to errors produced by pol γ.
NRTI induced mitochondrial toxicity - Pol γ is the only replicative DNA polymerase sensitive to a host of nucleoside analogs used to treat HIV infection and as a consequence patients being treated with antiviral therapies such as AZT, ddNs, D4T, 3TC and others, may develop an induced mitochondrial toxicity.
Mutations in the gene for Pol γ - The POLG gene is a locus for several mitochondrial diseases and more than 150 disease mutations have been identified in the POLG gene from patients with mitochondrial disease.
The relevance of pol γ in each of these the health topics is discussed in this chapter.
Pol γ in mtDNA replication
Of the 16 DNA polymerases in the eukaryotic cell, only pol γ is known to function in the mitochondria (Bebenek and Kunkel, 2004, Ropp and Copeland, 1996, Sweasy et al., 2006). Thus, pol γ is absolutely essential for mtDNA replication and repair. The holoenzyme of pol γ consists of a catalytic subunit (encoded by POLG at chromosomal locus 15q25) and a dimeric form of its accessory subunit (encoded by POLG2 at chromosomal locus 17q24.1). The catalytic subunit is a 140 kDa enzyme (p140) that has DNA polymerase, 3′-5′ exonuclease and 5′ dRP lyase activities (Graziewicz et al., 2006). The accessory subunit is a 55 kDa protein (p55) required for tight DNA binding and processive DNA synthesis (Lim et al., 1999). The pol γ holoenzyme functions in conjunction with the mitochondrial DNA helicase, Twinkle, and the mtSSB to form the minimal replication apparatus (Korhonen et al., 2004) (Table 1). Other factors required for initation of mtDNA replication and repair are listed in Table 1.
Table 1.
Gene products required for mtDNA replication and repair
| Function | Gene | Protien | Size | Chromosome |
|---|---|---|---|---|
| Core replication | POLG | DNA polymerase | 140 kDa | 15q25 |
| POLG2 | DNA polymerase accessory sutunit | 55 kDa | 17q23-24 | |
| SSB | single stranded DNA binding protein | 15 kDa | 7q34 | |
| PEO1 (Twinkle) | helicase | 77 kDa | 10q24 | |
| Replication and repair accessory factors | DNA ligase III | ligase | 96kDa | 17q11.2-12 |
| RNase H1 | RNase | 32 kDa | 19p13.2 | |
| Topo I | topoisomerase | 67 kDa | 8q24.3 | |
| Topo IIIα | topoisomerase | 112 kDa | 17p12-11.2 | |
| Fen-1 | 5′-3′ Flap endonuclease | 43 kDa | 11q12 | |
| DNA2 | 5′-3′ DNA/RNA endonuclease/exonulclease | 130 kDa | 10q21.3-q22.1 | |
| ExoG | 5′-3′ Exonuclease | 41 kDa | 3p21.3 | |
| DNA repair proteins | UDG | Uracil DNA glycosylase | 27.5 kDa | 12q23-q24.1 |
| OGG1 | 8-oxo-dG glycosylase | 38 kDa | 3p26.2 | |
| NTH1 | Thymine glycol glycosylase | 34 kDa | 16p13.3 | |
| MUTYH | glycosylase | 60 kDa | 1p34.3-p32.1 | |
| NEIL1 | Fapy glycosylase | 44 kDa | 15q4.2 | |
| APE1 | Ap endonuclease | 35 kDa | 14q11.2-q12 | |
| APE2 | Ap endonuclease | 57 kDa | Xp11.22 | |
| Transcription | mtRNA Pol | Core RNA polymerase | 150 kDa | 19q13.3 |
| mtTFA | Transcription factor | 24 kDa | 10q21 | |
| mtTFB1 | Transcription factor | 39 kDa | 6q25.1-q25.3 | |
| mtTFB2 | Transcription factor | 45 kDa | 1q44 |
Mutations in mitochondrial DNA can arise from DNA damage from exogenous sources or from endogenous oxidative stress, which are believed to arise mostly from electron leakage in the electron transport chain during oxidative phosphorylation. Mutations can also arise as spontaneous errors of replication during either DNA replication or repair events. As the only DNA polymerase known to exist in mammalian mitochondrial, pol γ is likely to produce these spontaneous errors. Comparison of mutation spectrum from in vivo sources with in vitro copied DNA by the highly purified human pol γ reveals that over 85% of mutation detected in vivo could be recapitulated in vitro by pol γ (Zheng et al., 2006). This indicates that spontaneous errors by pol γ account for the majority of base pair substitution mutations. Thus, understanding the fidelity of pol γ is critical.
The human catalytic subunit of pol γ has high base substitution fidelity that results from high nucleotide selectivity and exonucleolytic proofreading (Longley et al., 2001). Pol γ is also relatively accurate for base incorporation in non-iterated and short repetitive sequences where a misinsertion event occurs, on average, once per 500,000 nucleotides synthesized (Longley et al., 2001). However, when copying homopolymeric sequences longer than four nucleotides, pol γ has lower frameshift fidelity, suggesting that homopolymeric runs in mtDNA may be particularly prone to frameshift mutation in vivo due to replication errors by pol γ. Inclusion of the p55 accessory subunit, which is important for processivity of pol γ, decreases frameshift and base substitution fidelity. Kinetic analyses indicate that p55 lowers fidelity of replication by promoting extension of mismatched termini (Longley et al., 2001). Pol γ contains an intrinsic 3′ to 5′ exonuclease activity that contributes to replication fidelity. In human pol γ, substitution of Asp198 and Glu200 with alanine in the ExoI motif eliminated detectable 3′-5′ exonuclease function in vitro (Longley et al., 1998b). Comparing the in vitro rates of base substitution errors for the exonuclease deficient and proficient forms of human pol γ indicated that the proofreading function contributes at least 20-fold to the fidelity of base selection (Longley et al., 2001).
Pol γ in mitochondrial DNA repair
DNA repair in mitochondrial is limited to base excision repair which is well suited with a host of glycosylases (Table 1) to recognize base damage, as would occur during oxidative stress. Mitochondrial base excision repair can proceed via two pathways, single-nucleotide-BER (SN-BER) or long-patch BER (LP-BER) (Copeland and Longley, 2008). With either repair pathway, an oxidized or damaged base is recognized and cleaved by a specific glycosylase, leaving an abasic site that is cleaved on the 5′ end by AP endonuclease to generated a nick with a 5′deoxyribose phosphate (dRP) flap. During single nucleotide BER, the mitochondrial DNA polymerase, pol γ, fills the gap and cleaves the 5′dRP moiety prior to ligation (Longley et al., 1998a).
LP-BER activity in mitochondrial extracts and identification of proteins required for LP-BER in mitochondria has recently been described (Akbari et al., 2008, Liu et al., 2008, Szczesny et al., 2008). LP-BER requires an activity to remove the displaced 5′DNA commonly known as a 5′-flap structure and Liu et al found FEN-1 in their mitochondrial preparations that could carry out this activity (Liu et al., 2008). Furthermore, DNA2, originally identified as a yeast nuclear DNA helicase with endonuclease activity, has also been implicated in mitochondrial LP-BER, as well as having a possible role in mtDNA replication (Zheng et al., 2008). In this capacity, DNA2 functions with FEN-1 to process 5′ protruding flaps due to strand displacement synthesis during LP-BER prior to ligation by ligase III.
Mitochondrial toxicity from antiviral inhibition of Pol γ
Nucleoside reverse transcriptase inhibitor (NRTI) therapy in HIV infected patients has been beneficial in extending life and slowing the progression of AIDS, but treatment with nucleoside analogs is accompanied by certain side effects. The most pronounced side effects from NRTI therapy are damage to the mitochondria and loss of mitochondrial function. Mitochondrial myopathies in patients on AZT (3′-azido-3′-deoxythmidine) therapy was first reported in 1990 (Dalakas et al., 1990). These patients had induced myopathies with ragged red fibers and reduced amounts of mitochondrial DNA (Arnaudo et al., 1991). Early investigations into the observed mitochondrial toxicity implicated pol γ in the process of toxicity. Pol γ is unique among the cellular replicative DNA polymerases in that it is highly sensitive to inhibition by anti-HIV nucleotide analogs such as AZT-TP, dideoxynucleotides, and other antiviral nucleotide analogs (Kaguni, 1988, Longley and Mosbaugh, 1991, Martin et al., 1994, Hart et al., 1992, Parker et al., 1991, Lewis et al., 1994, Copeland et al., 1992, Eriksson et al., 1995, Nickel et al., 1992, Huang et al., 1990, Huang et al., 1992, Lim and Copeland, 2001, Johnson et al., 2001). The general inhibitory effect of NRTIs on polymerases is: HIV-RT ≫ pol γ > pol β > pol α = pol ε (Kakuda, 2000). Mitochondrial toxicity may be caused by (1) direct inhibition of pol γ activity without incorporation; (2) termination of the growing nascent DNA strand by incorporation of these chain-terminating analogs into mitochondrial DNA; (3) alteration of the fidelity of DNA synthesis of pol γ; (4) the persistence of these analogs in mtDNA due to inefficient excision; or (5) a combination of any of these effects. Kinetic studies indicate that the apparent in vitro hierarchy of mitochondrial toxicity for the approved NRTIs is: ddC (dideoxycitidine, zalcitabine) ≥ ddI (dideoxyinosine, didanosine) ≥ D4T (2′,3′-didehydro-2′,3′-dideoxythymidine, stavudine) ≫3TC (2′,3′-dideoxy-3′-thiacytidine, lamivudine) >PMPA (9-(R)-2-(phosphonomethoxypropyl)adenine, tenofovir)> AZT (zidovudine) > CBV(guanine analog, abacavir) (Lim and Copeland, 2001, Johnson et al., 2001). During in vitro chain elongation by pol γ, dideoxynucleotides and D4T-TP are utilized nearly as efficiently as natural deoxynucleotides, whereas AZT-TP, 3TC-TP, PMPA and CBV-TP are only moderate inhibitors of DNA chain elongation (Lim and Copeland, 2001, Johnson et al., 2001). Once incorporated the polymerase may remove the terminal NRTI with the exonuclease activity intrinsic to pol γ. We previously found that pol γ is inefficient in removing terminally incorporated dideoxynucleotides, D4T, AZT, and CBV from DNA (Lim and Copeland, 2001). This finding predicts persistence of these analogs in vivo following successful incorporation. In contrast, removal of 3′-terminal 3TC residues is 50% as efficient as natural 3′-termini, predicting reduced persistence and lower toxicity for this analog. In addition to the triphosphate form of these analogs, metabolic intermediates have the potential to inhibit pol γ or other cellular targets. The cellular conversion of AZT to AZT-TP has been shown to accumulate the monophosphate intermediate in vivo at millimolar concentration (Furman et al., 1986, Frick et al., 1988). We have shown that the pol γ exonuclease activity is inhibited by AZT-monophosphate at concentrations known to occur in cells (Lim and Copeland, 2001). Thus, although their greatest inhibitory effects are through incorporation and chain termination, persistence of these analogs in DNA and inhibition of exonucleolytic proofreading are also likely to contribute to mitochondrial toxicity. 3TC-TP is one of the analogs least likely to be incorporated and yet is one of those most efficiently removed. This may explain the low mitochondrial toxicity induced by 3TC in vivo. Although AZT-TP is one of the analogs least likely to be incorporated into DNA by pol γ, once incorporated it is not efficiently removed from DNA by the pol γ exonuclease function. The inefficiency of pol γ to remove AZT from DNA may help to explain some of the AZT-induced mtDNA depletion observed in vivo.
Based on sequence alignment of the bacterial DNA polymerases within family A, mutagenesis studies, and available three-dimensional structures, three amino acids, Tyr951, Tyr955 and Glu895, in human DNA pol γ were studied for their role in NRTI selection (Lim et al., 2003). The function of these three residues accounts for the majority of the selection of incoming dNTPs. The cause of dideoxynucleoside and D4T sensitivity is mainly attributed to a single tyrosine in motif B, Tyr951, of human pol γ (Longley et al., 1998b, Lim et al., 2003). Substitution of this tyrosine residue with phenylalanine in the human enzyme reduces inhibition by dideoxynucleotide or D4T-TP by several thousand fold with only minor effects on overall polymerase function (Longley et al., 1998b, Lim et al., 2003).
Disease mutations in the POLG gene
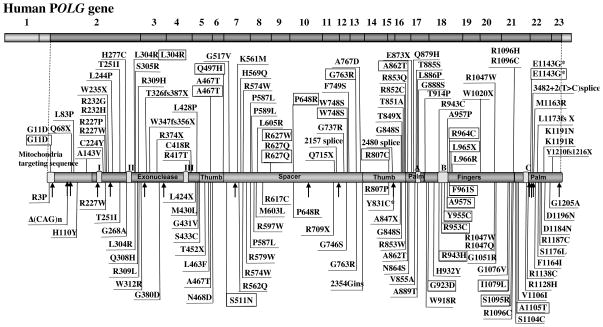
The POLG gene is one of several nuclear genes that is associated with mitochondrial DNA depletion or deletion disorders (Table 2). To date, more than 150 disease mutations have been identified in the POLG gene and an up-to-date mutation database can be found at http://tools.niehs.nih.gov/polg/ which shows these mutations to be equally distributed over the length of the protein (Figure 1). Disorders associated with POLG mutations have been defined to include the following, 1.) Myocerebrohepatopathy spectrum disorder (MCHS), 2) Alpers syndrome, 3) Ataxia neuropathy spectrum diorder (ANS), 4) Myoclonus epilepsy myopathy sensory ataxia (MEMSA), 5) Autosomal recessive progressive external ophthalmoplegia (arPEO), and 6) Autosomal dominant progressive ophthalmoplegia (adPEO) (Wong et al., 2008) (Figure 1). Also, alteration of the (CAG)10 repeat in the 2nd exon of POLG has been implicated in male infertility.
Table 2.
Nuclear loci that affect the stability of mitochondrial DNA
| Gene | Disorder | Chromosome locus | Function |
|---|---|---|---|
| POLG | PEO/Alpers/ataxia | 15q25 | Mitochondrial DNA polymerase |
| POLG2 | PEO | 17q23-24 | Pol γ accessory subunit |
| PEO1 (Twinkle) | PEO/ataxia | 10q24 | Mitochondrial DNA helicase |
| ANT1 | PEO | 4q34-35 | Adenine nucleotide translocator |
| TP | MNGIE | 22q13.32 | Thymidine phosphorylase |
| DGUOK | MtDNA depletion | 2p13 | Deoxyguanosine kinase |
| TK2 | MtDNA depletion | 16q22 | Mitochondrial thymidine kinase |
| MPV17 | MtDNA depletion | 2p21-23 | Mt inner membrane protein |
| SUCLA2 | MtDNA depletion | 13q12.2-q13.3 | Succinate-CoA ligase |
| RRM2B | MtDNA depletion | 8q23.1 | P53-Ribonucleotide reductase, small subunit |
| OPA1 | Dominant optic atrophy | 3q28-q29 | Dynamin related GTPase |
Figure 1.
Schematic diagram of human pol γ protein showing the location of amino acid substitutions resulting from disease and polymorphism mutations. Disease substitutions above the line that are not boxed are associated with Alpers and myocerebralhepatopathy syndrome, while boxed substations above the line are associated with ataxia-neuropathy syndromes. Mutations below the line are associated with various forms of progressive external ophthalmoplegia where boxed mutations are autosomal dominant PEO substitutions. The Δ(CAG)n repeat is associated with male infertility or idiopathic Parkinsons disease. Arrows and substitutions with an asteric depict the non-synonymous polymorphic amino acid changes.
MCHS includes myopathy or hypotonia, developmental delay, or dementia, and liver dysfunction (Wong et al., 2008). In addition, patients can have either a liver biopsy that excluded classical Alpers hepatopathy (Nguyen et al., 2006), or at least two of the following 8 findings: 1) neuropathy, 2) seizures, 3) elevated blood or cerebrospinal fluid lactic acid, 4) dicarboxylic aciduria, 5) renal tubular dysfunction with aminoaciduria, glucosuria, or bicarbonaturia, 6) hearing loss, 7) abnormal MRI with either cerebral volume loss, delayed myelination, or white matter disease, and 8) deficiency of either CIV (cytochrome c oxidase, COX) in isolation, or 2 or more electron transport complexes in skeletal muscle or liver biopsy (Wong et al., 2008). In some cases, patients came to diagnosis without, or before, the onset of liver dysfunction. In these cases, at least 3 of the 8 supportive diagnostic findings were required. Patients with POLG mutations meeting the diagnostic features for MCHS were first described in (Ferrari et al., 2005), and (de Vries et al., 2007).
Alpers syndrome or hepatocerebral degeneration is defined by the clinical triad of refractory, mixed-type seizures that often included a focal component, psychomotor regression that is episodic and triggered by intercurrent infection, and hepatopathy with or without acute liver failure (Wong et al., 2008). Although mtDNA deletion usually occurs in the very advanced stages in affected organs, mtDNA depletion analysis is not always a good predictor of the diagnosis or progression of the disease (Nguyen et al., 2006).
Ataxia Neuropathy Spectrum (ANS) includes an overlapping clinical spectrum of disorders centered around ataxia and neuropathy in the absence of significant muscle weakness or myopathy. ANS includes mitochondrial recessive ataxia syndrome (MIRAS) (Hakonen et al., 2005), spinocerebellar ataxia and epilepsy (SCAE), and the ataxia neuropathy spectrum (Tzoulis et al., 2006). Myoclonus Epilepsy Myopathy Sensory Ataxia (MEMSA) is an overlapping spectrum of disorders of myopathy, epilepsy, and ataxia in the absence of ophthalmoplegia with or without ragged red fibers (Van Goethem et al., 2003b). ArPEO is the recessive form of progressive external ophthalmoplegia (Van Goethem et al., 2001). Most patients have additional symptoms that include sensory ataxia, neuropathy, dysarthria, and ophthalmoplegia (SANDO) (Van Goethem et al., 2003a). AdPEO is the dominant inherited form of progressive external ophthalmoplegia that can include parkinsonism (Van Goethem et al., 2001).
The A467T mutation is the most common POLG mutation and has been found to be associated with all of the disease symptoms mentioned above. Previous studies have shown that the A467T pol γ possesses only 4% of the wild-type DNA polymerase activity and is compromised for its ability to interact with the p55 accessory subunit (Chan et al., 2005). The W748S mutation which has nearly always been found to be in cis with E1143G mutation is a frequent cause of ataxia-neuropathy syndrome (Hakonen et al., 2005). The E1143G a single nucleotide polymorphism (SNP) which is found in 4% of European populations. The W748S mutations has intrinsic lower polymerase activity as well as a demonstrated lower affinity for DNA (Chan et al., 2006). We have found that the E1143G SNP can modulate the deleterious effect of the W748S mutation (Chan et al., 2006). This finding raises the possibility that other SNPs could potentially affect POLG enzymatic activity.
Four adPEO mutations, G923D, R943H, Y955C and A957S that are found in and around motif B in the active site of the polymerase were characterized biochemically (Graziewicz et al., 2004). Two of the substitutions, R943H and Y955C, change side chains that interact with the incoming dNTP and pol γ with these substiutions retained less than 1% of the wild-type polymerase activity and display a severe decrease in processivity. The significant stalling of DNA synthesis and extremely low catalytic activities of both enzymes are the two most likely causes of the severe clinical presentation in R943H and Y955C heterozygotes (Graziewicz et al., 2004). The substitution of Tyr955 to cysteine also increases nucleotide misinsertion replication errors 10–100 fold in the absence of exonucleolytic proofreading (Ponamarev et al., 2002). For the majority of the disease substitutions that have been studied in vitro, the biochemical defects correlate with the severity and age of onset found in patients (Chan and Copeland, 2009). Further analysis of disease substitutions as well as structural analysis should aid in the continued understanding of disease mutations in the POLG gene.
References
- AKBARI M, VISNES T, KROKAN HE, OTTERLEI M. Mitochondrial base excision repair of uracil and AP sites takes place by single-nucleotide insertion and long-patch DNA synthesis. DNA Repair (Amst) 2008;7:605–16. doi: 10.1016/j.dnarep.2008.01.002. [DOI] [PubMed] [Google Scholar]
- ARNAUDO E, DALAKAS M, SHANSKE S, MORAES CT, DIMAURO S, SCHON EA. Depletion of muscle mitochondrial DNA in AIDS patients with zidovudine- induced myopathy. Lancet. 1991;337:508–10. doi: 10.1016/0140-6736(91)91294-5. [DOI] [PubMed] [Google Scholar]
- BEBENEK K, KUNKEL TA. Functions of DNA polymerases. Adv Protein Chem. 2004;69:137–65. doi: 10.1016/S0065-3233(04)69005-X. [DOI] [PubMed] [Google Scholar]
- CHAN SS, COPELAND WC. DNA polymerase gamma and mitochondrial disease: Understanding the consequence of POLG mutations. Biochim Biophys Acta. 2009;1787:312–319. doi: 10.1016/j.bbabio.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAN SSL, LONGLEY MJ, COPELAND WC. The common A467T mutation in the human mitochondrial DNA polymerase (POLG) compromises catalytic efficiency and interaction with the accessory subunit. J Biol Chem. 2005;280:31341–31346. doi: 10.1074/jbc.M506762200. [DOI] [PubMed] [Google Scholar]
- CHAN SSL, LONGLEY MJ, COPELAND WC. Modulation of the W748S mutation in DNA polymerase {gamma} by the E1143G polymorphism in mitochondrial disorders. Hum Mol Genet. 2006;15:3473–3483. doi: 10.1093/hmg/ddl424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COPELAND WC, CHEN MS, WANG TS. Human DNA polymerases alpha and beta are able to incorporate anti-HIV deoxynucleotides into DNA. J Biol Chem. 1992;267:21459–64. [PubMed] [Google Scholar]
- COPELAND WC, LONGLEY MJ. DNA2 resolves expanding flap in mitochondrial base excision repair. Mol Cell. 2008;32:457–8. doi: 10.1016/j.molcel.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALAKAS MC, ILLA I, PEZESHKPOUR GH, LAUKAITIS JP, COHEN B, GRIFFIN JL. Mitochondrial myopathy caused by long-term zidovudine therapy. N Engl J Med. 1990;322:1098–105. doi: 10.1056/NEJM199004193221602. [DOI] [PubMed] [Google Scholar]
- DE VRIES MC, RODENBURG RJ, MORAVA E, VAN KAAUWEN EP, TER LAAK H, MULLAART RA, SNOECK IN, VAN HASSELT PM, HARDING P, VAN DEN HEUVEL LP, SMEITINK JA. Multiple oxidative phosphorylation deficiencies in severe childhood multi-system disorders due to polymerase gamma (POLG1) mutations. Eur J Pediatr. 2007;166:229–234. doi: 10.1007/s00431-006-0234-9. [DOI] [PubMed] [Google Scholar]
- DIMAURO S, DAVIDZON G. Mitochondrial DNA and disease. Ann Med. 2005;37:222–32. doi: 10.1080/07853890510007368. [DOI] [PubMed] [Google Scholar]
- ERIKSSON S, XU B, CLAYTON DA. Efficient incorporation of anti-HIV deoxynucleotides by recombinant yeast mitochondrial DNA polymerase. J Biol Chem. 1995;270:18929–18934. doi: 10.1074/jbc.270.32.18929. [DOI] [PubMed] [Google Scholar]
- FERRARI G, LAMANTEA E, DONATI A, FILOSTO M, BRIEM E, CARRARA F, PARINI R, SIMONATI A, SANTER R, ZEVIANI M. Infantile hepatocerebral syndromes associated with mutations in the mitochondrial DNA polymerase-{gamma}A. Brain. 2005;128:723–731. doi: 10.1093/brain/awh410. [DOI] [PubMed] [Google Scholar]
- FRICK LW, NELSON DJ, ST CLAIR MH, FURMAN PA, KRENITSKY TA. Effects of 3′-azido-3′-deoxythymidine on the deoxynucleotide triphosphate pools of cultured human cells. Biochem Biophys Res Commun. 1988;154:124–9. doi: 10.1016/0006-291x(88)90659-6. [DOI] [PubMed] [Google Scholar]
- FURMAN PA, FYFE JA, ST CLAIR MH, WEINHOLD K, RIDEOUT JL, FREEMAN GA, LEHRMAN SN, BOLOGNESI DP, BRODER S, MITSUYA H, BARRY DW. Phosphorylation of 3′-azido-3′-deoxythymidine and selective interaction of the 5′-triphosphate with human immunodeficiency virus reverse transcriptase. Proc Natl Acad Sci U S A. 1986;83:8333–7. doi: 10.1073/pnas.83.21.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAZIEWICZ MA, LONGLEY MJ, BIENSTOCK RJ, ZEVIANI M, COPELAND WC. Structure-function defects of human mitochondrial DNA polymerase in autosomal dominant progressive external ophthalmoplegia. Nat Struct Mol Biol. 2004;11:770–776. doi: 10.1038/nsmb805. [DOI] [PubMed] [Google Scholar]
- GRAZIEWICZ MA, LONGLEY MJ, COPELAND WC. DNA polymerase gamma in Mitochondrial DNA Replication and Repair. Chemical Reviews. 2006;106:383–405. doi: 10.1021/cr040463d. [DOI] [PubMed] [Google Scholar]
- HAKONEN AH, HEISKANEN S, JUVONEN V, LAPPALAINEN I, LUOMA PT, RANTAMAKI M, GOETHEM GV, LOFGREN A, HACKMAN P, PAETAU A, KAAKKOLA S, MAJAMAA K, VARILO T, UDD B, KAARIAINEN H, BINDOFF LA, SUOMALAINEN A. Mitochondrial DNA polymerase W748S mutation: a common cause of autosomal recessive ataxia with ancient European origin. Am J Hum Genet. 2005;77:430–41. doi: 10.1086/444548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HART GJ, ORR DC, PENN CR, FIGUEIREDO HT, GRAY NM, BOEHME RE, CAMERON JM. Effects of (−)-2′-deoxy-3′-thiacytidine (3TC) 5′-triphosphate on human immunodeficiency virus reverse transcriptase and mammalian DNA polymerases alpha, beta, and gamma. Antimicrob Agents Chemother. 1992;36:1688–94. doi: 10.1128/aac.36.8.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG P, FARQUHAR D, PLUNKETT W. Selective action of 3′-azido-3′-deoxythymidine 5′-triphosphate on viral reverse transcriptases and human DNA polymerases. J Biol Chem. 1990;265:11914–8. [PubMed] [Google Scholar]
- HUANG P, FARQUHAR D, PLUNKETT W. Selective action of 2′,3′-didehydro-2′,3′-dideoxythymidine triphosphate on human immunodeficiency virus reverse transcriptase and human DNA polymerases. J Biol Chem. 1992;267:2817–22. [PubMed] [Google Scholar]
- JOHNSON AA, RAY AS, HANES J, SUO Z, COLACINO JM, ANDERSON KS, JOHNSON KA. Toxicity of antiviral nucleoside analogs and the human mitochondrial DNA polymerase. J Biol Chem. 2001;276:40847–57. doi: 10.1074/jbc.M106743200. [DOI] [PubMed] [Google Scholar]
- KAGUNI LS, WERNETTE CM, CONWAY MC, YANG-CASHMAN P. Eukaryotic DNA Replication. 6. Cold Spring Harbor Press; 1988. Structural and catalytic features of the mitochondrial DNA polymerase from Drosophila melanogaster embryos. [Google Scholar]
- KAKUDA TN. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin Ther. 2000;22:685–708. doi: 10.1016/S0149-2918(00)90004-3. [DOI] [PubMed] [Google Scholar]
- KORHONEN JA, PHAM XH, PELLEGRINI M, FALKENBERG M. Reconstitution of a minimal mtDNA replisome in vitro. Embo J. 2004;23:2423–9. doi: 10.1038/sj.emboj.7600257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS W, SIMPSON JF, MEYER RR. Cardiac mitochondrial DNA polymerase-gamma is inhibited competitively and noncompetitively by phosphorylated zidovudine. Circ Res. 1994;74:344–8. doi: 10.1161/01.res.74.2.344. [DOI] [PubMed] [Google Scholar]
- LIM SE, COPELAND WC. Differential incorporation and removal of antiviral deoxynucleotides by human DNA polymerase gamma. J Biol Chem. 2001;276:23616–23. doi: 10.1074/jbc.M101114200. [DOI] [PubMed] [Google Scholar]
- LIM SE, LONGLEY MJ, COPELAND WC. The mitochondrial p55 accessory subunit of human DNA polymerase gamma enhances DNA binding, promotes processive DNA synthesis, and confers N-ethylmaleimide resistance. J Biol Chem. 1999;274:38197–203. doi: 10.1074/jbc.274.53.38197. [DOI] [PubMed] [Google Scholar]
- LIM SE, PONAMAREV MV, LONGLEY MJ, COPELAND WC. Structural Determinants in Human DNA Polymerase gamma Account for Mitochondrial Toxicity from Nucleoside Analogs. J Mol Biol. 2003;329:45–57. doi: 10.1016/s0022-2836(03)00405-4. [DOI] [PubMed] [Google Scholar]
- LIU P, QIAN L, SUNG JS, DE SOUZA-PINTO NC, ZHENG L, BOGENHAGEN DF, BOHR VA, WILSON DM, SHENB, DEMPLE B. Removal of Oxidative DNA Damage via FEN1-Dependent Long-Patch Base Excision Repair in Human Cell Mitochondria. Mol Cell Biol. 2008;28:4975–4987. doi: 10.1128/MCB.00457-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONGLEY MJ, MOSBAUGH DW. Properties of the 3′ to 5′ exonuclease associated with porcine liver DNA polymerase gamma. Substrate specificity, product analysis, inhibition, and kinetics of terminal excision. J Biol Chem. 1991;266:24702–11. [PubMed] [Google Scholar]
- LONGLEY MJ, NGUYEN D, KUNKEL TA, COPELAND WC. The Fidelity of Human DNA Polymerase gamma with and without Exonucleolytic Proofreading and the p55 Accessory Subunit. J Biol Chem. 2001;276:38555–62. doi: 10.1074/jbc.M105230200. [DOI] [PubMed] [Google Scholar]
- LONGLEY MJ, PRASAD R, SRIVASTAVA DK, WILSON SH, COPELAND WC. Identification of 5′-deoxyribose phosphate lyase activity in human DNA polymerase gamma and its role in mitochondrial base excision repair in vitro. Proc Natl Acad Sci U S A. 1998a;95:12244–12248. doi: 10.1073/pnas.95.21.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONGLEY MJ, ROPP PA, LIM SE, COPELAND WC. Characterization of the native and recombinant catalytic subunit of human DNA polymerase gamma: identification of residues critical for exonuclease activity and dideoxynucleotide sensitivity. Biochemistry. 1998b;37:10529–39. doi: 10.1021/bi980772w. [DOI] [PubMed] [Google Scholar]
- MARTIN JL, BROWN CE, MATTHEWS-DAVIS N, REARDON JE. Effects of antiviral nucleoside analogs on human DNA polymerases and mitochondrial DNA synthesis. Antimicrob Agents Chemother. 1994;38:2743–9. doi: 10.1128/aac.38.12.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NGUYEN KV, SHARIEF F, CHAN SSL, COPELAND WC, NAVIAUX RK. Molecular Diagnosis of Alpers Syndrome. J Hepatology. 2006;45:108–116. doi: 10.1016/j.jhep.2005.12.026. [DOI] [PubMed] [Google Scholar]
- NICKEL W, AUSTERMANN S, BIALEK G, GROSSE F. Interactions of azidothymidine triphosphate with the cellular DNA polymerases alpha, delta, and epsilon and with DNA primase. J Biol Chem. 1992;267:848–54. [PubMed] [Google Scholar]
- PARKER WB, WHITE EL, SHADDIX SC, ROSS LJ, BUCKHEIT RW, JR, GERMANY JM, SECRIST JAD, VINCE R, SHANNON WM. Mechanism of inhibition of human immunodeficiency virus type 1 reverse transcriptase and human DNA polymerases alpha, beta, and gamma by the 5′-triphosphates of carbovir, 3′-azido-3′-deoxythymidine, 2′,3′-dideoxyguanosine and 3′-deoxythymidine. A novel RNA template for the evaluation of antiretroviral drugs. J Biol Chem. 1991;266:1754–62. [PubMed] [Google Scholar]
- PONAMAREV MV, LONGLEY MJ, NGUYEN D, KUNKEL TA, COPELAND WC. Active Site Mutation in DNA Polymerase gamma Associated with Progressive External Ophthalmoplegia Causes Error-prone DNA Synthesis. J Biol Chem. 2002;277:15225–8. doi: 10.1074/jbc.C200100200. [DOI] [PubMed] [Google Scholar]
- ROPP PA, COPELAND WC. Cloning and characterization of the human mitochondrial DNA polymerase, DNA polymerase gamma. Genomics. 1996;36:449–58. doi: 10.1006/geno.1996.0490. [DOI] [PubMed] [Google Scholar]
- SWEASY JB, LAUPER JM, ECKERT KA. DNA polymerases and human diseases. Radiat Res. 2006;166:693–714. doi: 10.1667/RR0706.1. [DOI] [PubMed] [Google Scholar]
- SZCZESNY B, TANN AW, LONGLEY MJ, COPELAND WC, MITRA S. Long patch base excision repair in mammalian mitochondrial genomes. J Biol Chem. 2008;283:26349–26356. doi: 10.1074/jbc.M803491200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TZOULIS C, ENGELSEN BA, TELSTAD W, AASLY J, ZEVIANI M, WINTERTHUN S, FERRARI G, AARSETH JH, BINDOFF LA. The spectrum of clinical disease caused by the A467T and W748S POLG mutations: a study of 26 cases. Brain. 2006;129:1685–1692. doi: 10.1093/brain/awl097. [DOI] [PubMed] [Google Scholar]
- VAN GOETHEM G, DERMAUT B, LOFGREN A, MARTIN JJ, VAN BROECKHOVEN C. Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nat Genet. 2001;28:211–212. doi: 10.1038/90034. [DOI] [PubMed] [Google Scholar]
- VAN GOETHEM G, MARTIN JJ, DERMAUT B, LOFGREN A, WIBAIL A, VERVERKEN D, TACK P, DEHAENE I, VAN ZANDIJCKE M, MOONEN M, CEUTERICK C, DE JONGHE P, VAN BROECKHOVEN C. Recessive POLG mutations presenting with sensory and ataxic neuropathy in compound heterozygote patients with progressive external ophthalmoplegia. Neuromuscul Disord. 2003a;13:133–142. doi: 10.1016/s0960-8966(02)00216-x. [DOI] [PubMed] [Google Scholar]
- VAN GOETHEM G, MERCELIS R, LOFGREN A, SENECA S, CEUTERICK C, MARTIN JJ, VAN BROECKHOVEN C. Patient homozygous for a recessive POLG mutation presents with features of MERRF. Neurology. 2003b;61:1811–1813. doi: 10.1212/01.wnl.0000098997.23471.65. [DOI] [PubMed] [Google Scholar]
- WALLACE DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–8. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- WONG LJ, NAVIAUX RK, BRUNETTI-PIERRI N, ZHANG Q, SCHMITT ES, TRUONG C, MILONE M, COHEN BH, WICAL B, GANESH J, BASINGER AA, BURTON BK, SWOBODA K, GILBERT DL, VANDERVER A, SANETO RP, MARANDA B, ARNOLD G, ABDENUR JE, WATERS PJ, COPELAND WC. Molecular and clinical genetics of mitochondrial diseases due to POLG mutations. Hum Mutat. 2008;29:E150–E172. doi: 10.1002/humu.20824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHENG L, ZHOU M, GUO Z, LU H, QIAN L, DAI H, QIU J, YAKUBOVSKAYA E, BOGENHAGEN DF, DEMPLE B, SHEN B. Human DNA2 is a mitochondrial nuclease/helicase for efficient processing of DNA replication and repair intermediates. Mol Cell. 2008;32 doi: 10.1016/j.molcel.2008.09.024. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHENG W, KHRAPKO K, COLLER H, THILLY WG, COPELAND WC. Origins of human mitochondrial point mutations as DNA polymerase gamma-mediated errors. Mutation Research. 2006;599:11–20. doi: 10.1016/j.mrfmmm.2005.12.012. [DOI] [PubMed] [Google Scholar]