Abstract
The 21 amino acid peptide endothelin-1 (ET-1) regulates a diverse array of physiological processes, including vasoconstriction, angiogenesis, nociception, and cell proliferation. Most of the effects of ET-1 are associated with an increase in intracellular calcium concentration. The calcium influx and mobilization pathways activated by ET-1, however, vary immensely. This review will begin with the basics of calcium signaling, and investigate the different ways intracellular calcium concentration can increase in response to a stimulus. The focus will then shift to ET-1, and discuss how ET receptors mobilize calcium. We will also examine how disease alters calcium-dependent responses to ET-1 by discussing changes to ET-1-mediated calcium signaling in hypertension, since there is significant interest in the role of ET-1 in this important disease. A list of unanswered questions regarding ET-mediated calcium signals are also presented, as well as perspectives for future research of calcium mobilization by ET-1.
Keywords: endothelin, calcium signaling, G protein-coupled receptors, ion channels, vasculature
Introduction
Endothelin-1 (ET-1) is a 21-amino acid peptide, originally characterized as an endothelium-derived constricting factor in the vasculature [1, 2]. ET-1 also affects a host of non-vascular tissues, including brain, kidney, intestine and adrenal gland [3]. Further experimentation discovered two G protein-coupled receptors (GPCR’s) to which ET-1 binds: the ETA and ETB receptor. Physiological responses to ET-1 can be attributed to ETA receptors, ETB receptors, or both. ET-1 is not solely a vasoconstrictor; ET-1 stimulates angiogenesis, induces astrocyte proliferation, activates nociceptive neurons, constricts bronchi, and stimulates the production of several inflammatory mediators in neutrophils and macrophages [4-7]. Dysfunction or dysregulation in the endothelin (ET) system is present in chronic pain, acute renal failure, asthma, colorectal cancer, and stroke, but dysfunction is most apparent in vascular diseases like hypertension [8-11].
The role of ET-1 in the vasculature extends beyond its vasoconstricting properties. Plasma ET-1 levels are elevated in humans with salt-sensitive essential hypertension, and vascular ET-1 expression is increased in severe hypertension [12]. In types of human hypertension where plasma ET-1 remains steady, vascular tissues from hypertensive humans exhibit exaggerated reactivity to ET-1 [13]. In the DOCA-salt model of hypertension, vascular contraction to ET-1 is decreased even though plasma ET-1 concentrations are increased [14]. This implies that the tissue responses to ET-1 are altered due to dysfunctional ET receptor signaling and not concentration-dependent activation of ET-1-mediated pathways [15].
As the number of biological responses affected by ET-1 grows, one tenet remains unchanged: many, if not most, of the responses to ET-1 are calcium-dependent. Whether ET receptors increase intracellular calcium concentration ([Ca2+]i) by activating extracellular calcium influx or intracellular calcium release depends on the tissue, ET receptor type and the response being measured [16-18]. These increases in [Ca2+]i can be due to voltage-dependent calcium influx, store-operated calcium entry, voltage-independent calcium influx, release of one of several intracellular calcium stores, or any combination therein [19-21]. The calcium increases in some cells are transient; in others, ET-1 causes a slow and prolonged increase in intracellular calcium. Subtle changes to these calcium currents can cause major alterations in cellular function, ultimately leading to the pathogenesis of disease. As such, the complex mechanisms by which ET-1 can modulate intracellular calcium to alter cellular function remain a novel and intriguing area of investigation, and are the focus of this review. While this review will discuss mechanisms common to ET-1-dependent responses in many tissue types and diseases, the effects of ET-1 in the vasculature during hypertension are highlighted.
This review will begin with a primer on calcium signaling, regulation of calcium influx, and mobilization of calcium form intracellular calcium stores. We will then explore how calcium influx and mobilization are activated by ET-1, and how the interactions between calcium and ET-1 are altered during hypertension. Finally, we will present a list of unanswered questions regarding ET-1-mediated calcium signaling, and offer our perspectives for future research of calcium mobilization by ET-1.
The Basics of Calcium Signaling
Responses regulated by ET-1 have been associated with increases in [Ca2+]i, either by influx of calcium or release of intracellular calcium stores. [Ca2+]i is tightly regulated by a multitude of ion channels and exchangers that control influx, efflux, sequestration, and release of calcium [22-24]. Table 1 outlines the different types of plasma membrane calcium channels, and the receptors that modulate intracellular calcium release. Included is a description of their characteristics, known pharmacological activators, and known pharmacological inhibitors.[19, 25-31]
Table 1.
Calcium channels, their characteristics, and pharmacological agents used to understand their function. Included are both voltage-dependent, voltage-independent, and endoplasmic reticular calcium channels. Abbreviations: V0.5, voltage of half-maximal activation; NSCC, non-selective cation channel; TRP, transient receptor potential channel; P2X, ATP-sensitive purinergic ion channel; 5-HT3, serotonin receptor subfamily 3.
| Voltage-Gated Calcium Channels (VGCC’s) | |||||
|---|---|---|---|---|---|
| Common Name |
Official Name |
Characteristics | Specific Activators |
Specific Inhibitors |
Ref. |
| L-type | CaV1.2 | Cardiac and smooth muscle Ca2+ Channel. Regulates contraction. Moderate activation threshold (V0.5= −10 mV). Relatively slow inactivation rate. |
BAYK-8644 | nifedipine, verapamil |
[25] |
| N-type | CaV2.2 | Neuronal Ca2+ Channel. Regulates neurotransmitter release. High activation threshold (V0.5= +10 mV). Moderate inactivation rate (100-800 msec). |
-- | ω-conotoxin CVIA, ω- grammatoxin SIA |
[25] |
| P/Q-type | CaV2.1 | Neruonal Ca2+ Channel. Regulates neurotransmitter release. Moderate activation threshold (V0.5= −10 mV). Inactivation rate varies by β subunit (0.09-1000 msec). |
-- | ω-conotoxin MVIIC, ω- agatoxin IIIA |
[25] |
| R-type | CaV2.3 | Neuronal Ca2+ Channel. Regulates Ca2+-dependent gene expression and enzyme activity. High activation threshold (V0.5= +5 mV). Fast inactivation rate (2.1-2.4 msec). |
-- | SNX-482, ω- PnTx3-3 |
[25] |
| T-type | CaV3.1 | Neuronal/dendritic Ca2+ Channel. Regulates action potentials and subthreshold potential oscillations. Low activation threshold (V0.5= −45 mV). Moderate inactivation rate (20-50 msec). |
-- | kurtoxin, mibefradil |
[25] |
| Voltage-Independent Calcium Channels (VICC’s) | |||||
|---|---|---|---|---|---|
| Abbr. | Full Name | Characteristics | Activators | Inhibitors | Ref. |
| NSCC’s | Non-selective cation channels |
Ion channels that lack specificity for a specific cation. Examples: NSCC-1 and NSCC-2, most TRP channels. |
maitotoxin | LOE-908 | [19] |
| LGCC’s | Ligand-gated calcium channels |
Ion channels activated by binding of a ligand to the channel. Examples: P2×, 5-HT3, and NACh receptors. |
Varies by type |
Varies by type |
[26-28] |
| SOCC’s | Store-operated calcium channels |
Ca2+ channel activated by depletion of sarcoplasmic Ca2+ stores. Examples: STIM1/Orai complexes and TRPC channels. |
SR Ca2+ depletion |
SKF-96365, 2-APB |
[29] |
| Receptors Mediating Intracellular Calcium Release | |||||
|---|---|---|---|---|---|
| Abbr. | Full Name | Characteristics | Activators | Inhibitors | Ref. |
| IP3R | Inositol 1,4,5- trisphosphate Receptor |
Tetrameric receptor in the endoplasmic reticular membrane that functions as a low-conductance cation channel; activated by IP3. |
IP3 | Xesto- spongin C |
[30] |
| RyR | Ryanadine Receptor |
Tetrameric receptor in the endoplasmic reticular membrane that functions as a high-conductance cation channel; activated by increased intracellular calcium. |
Caffeine | Iberiotoxin | [31] |
Increases in [Ca2+]i can be due to influx only, stores release only, or a portion of both – and the contribution of each source of calcium varies between receptors. This complex regulatory mechanism exists to control [Ca2+]i because small changes in amplitude, duration and location of calcium influx are sufficient to cause a wide variation of physiological responses [32]. The pathways for calcium influx and calcium stores release are multi-faceted and tightly controlled, since small changes in intracellular calcium can be the difference between cell survival and cell death [33]. Before examining how ET-1 can increase [Ca2+]i, we will briefly discuss the mechanisms utilized by many GPCR’s to increase calcium influx and cause [Ca2+]i release.
Calcium Influx
Generally, calcium enters a cell by passing through a calcium channel that opens in response to any number of stimuli. The calcium concentration within a cell is much lower than the calcium concentration in the extracellular fluid (100 nM vs. 2.5 mM, respectively) [34]. This calcium concentration gradient allows calcium ions to move through the channels and into a cell by passive diffusion. Membrane depolarization, ligand binding, and release of intracellular stores are all capable of causing plasma membrane calcium channels to open [35]. Those that open due to membrane depolarization are the voltage-gated calcium channels (VGCC’s) and any others are considered voltage-independent calcium channels (VICC’s). The VICC’s can be further broken down into store-operated calcium channels (SOCC’s), ligand-gated calcium channels (LGCC’s) and non-selective cation channels (NSCC’s).
Release of Calcium Stores
The major store of intracellular calcium is the endoplasmic reticulum, or the sarcoplasmic reticulum in muscle cells [23]. Calcium is liberated from sarcoplasmic/endoplasmic reticulum (SER) stores through two calcium channels: inositol 1,4,5-trisphosphate (IP3) receptors and ryanodine receptors [36, 37].
IP3 is produced when phospholipase C (PLC) hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2), generating both IP3 and diacylglycerol (DAG) [38]. DAG affects calcium stores release indirectly, while IP3 does so directly. DAG activates protein kinase-C (PKC), which then can inhibit IP3 production by PLC [39]. PKC also phosphorylates VGCC’s, which alters their function to either inhibit or sustain calcium influx [21]. IP3 activates IP3 receptors on the SER membrane, which then open and allow Ca2+ to leave the SER and enter the cytoplasm [40].
Ryanodine receptors, when activated by local increases in intracellular calcium, cause additional calcium release from SER stores [41]. As such, ryanodine receptors amplify small calcium signals caused by voltage-dependent calcium influx or IP3-mediated calcium release [42]. In addition to amplification of calcium signals, ryanodine receptors are involved in the termination of calcium influx across the plasma membrane. Ryanodine receptors are on the SER membrane closest to the plasma membrane, whereby a “spark” of calcium from ryanodine receptors will activate calcium-sensitive potassium channels and close VGCC’s as the membrane hyperpolarizes [43]. Ryanodine receptors serve to amplify calcium signals rapidly, but then to also terminate voltage-dependent calcium influx.
Thus, IP3 receptors and ryanodine receptors activate pathways that tightly regulate Ca2+ release from SER stores, and regulate voltage-dependent Ca2+ entry both spatially and temporally. The interplay between PLC, IP3, DAG, and PKC also keeps intracellular Ca2+ concentration precisely controlled, while still allowing for rapid release of minute amounts of Ca2+ in response to a stimulus.
The Relationship between ET-1 and Calcium
The interdependence between ET-1 and calcium is apparent when examining both the physiological effects of ET receptor activation and ET-1 synthesis. Although multiple cell types synthesize ET-1, the predominant sources of ET-1 are vascular endothelial cells [44]. Molecules that increase endothelial cell [Ca+2]i augment expression of preproendothelin-1 (ppET-1) mRNA via a calcium/calmodulin/calmodulin kinase (Ca2+/CaM/CaM-K) pathway [45, 46]. The physiological responses elicited by ET-1 can be both calcium-dependent and calcium-independent [16, 47, 48]. Some examples of calcium-dependent processes regulated by ET-1 can be found in Table 2. Table 2 also separates ETA receptor-dependent responses from ETB receptor responses, and notes the specific calcium sources activated by each receptor. In some responses, ETA receptors regulate calcium store release and ETB receptors regulate calcium influx (e.g. bronchoconstriction). In others, ETA or ETB receptors regulate both calcium influx and stores release. No correlation exists between ET receptor subtype and the source of calcium governing the response. Thus, calcium influx and mobilization by ET-1 is cell type-specific, with regard to which ET receptor subtypes are involved. [49-64]
Table 2.
Examples of physiological processes mediated by ET receptors that are dependent upon calcium. (A): ETA receptor-dependent responses. (B): and ETB receptor-dependent responses. These examples highlight the diverse responses caused by ET-1, and dependent on Ca2+. Not only do the responses differ between cell type and tissue type, but between receptor subtype as well. No correlation exists between ET receptor subtype and either extracellular calcium influx or intracellular calcium release.
| A: Calcium-Dependent Physiological Responses Mediated by ETA Receptors | ||||
|---|---|---|---|---|
| Tissue/Cell Type: | Response(s): | Calcium Influx |
[Ca2+]i Release |
Ref. |
| Neutrophils | Activation and degranulation | ✓ | [47,48] | |
| Cardiac myocytes | Inhibition of C-type Natriuretic Peptide (CNP) signaling | ✓ | ✓ | [49] |
| Human bronchus | Release of intracellular Ca2+ stores, causing bronchoconstriction | ✓ | [50] | |
| Thin limb, loop of Henle | Unknown; thought to regulate sodium and water reabsorption | ✓ | ✓ | [51] |
| Aortic smooth muscle | Vasoconstriction | ✓ | ✓ | [52] |
| Venous smooth muscle | Wave-like Ca2+ currents, ultimately causing venoconstriction | ✓ | ✓ | [53] |
| Human optic nerve head | Ca2+-dependent proliferation | ✓ | ✓ | [54] |
| Mouse osteoblasts | Induces bone formation | ✓ | ✓ | [55] |
| Rat carotid body | Hypoxia up-regulates ETA receptors, which increases mitogenesis | ✓ | [56] | |
| Olfactory mucosa non-neuronal cells |
Unknown; both transient and sustained Ca2+ entry | ✓ | ✓ | [57] |
| B: Calcium-Dependent Physiological Responses Mediated by ETB Receptors | ||||
|---|---|---|---|---|
| Tissue/Cell Type: | Response(s): | Calcium Influx |
[Ca2+]i Release |
Ref. |
| Neutrophils | Chemotactic neutrophil migration | ✓ | [47] | |
| Human bronchus | Ca2+ influx followed by Ca2+ stores release, causing bronchoconstriction | ✓ | ✓ | [50] |
| Collecting duct | Inhibition of water reabsorption and Na+-K+-ATPase activity | ✓ | ✓ | [58,59] |
| Guinea pig gall bladder | Constriction | ✓ | [60] | |
| Human umbilical vein | Ca2+ influx, causing venoconstriction | ✓ | [61] | |
| Endothelial cells | Increased NO production and vasodilatation | ✓ | ✓ | [62] |
| Olfactory mucosa sensory neurons |
Unknown; both transient and sustained Ca2+ entry | ✓ | ✓ | [57] |
Some of the most-studied effects of ET-1 are those in the vasculature, where ET-1 acts as a potent vasoconstrictor [65]. ET-1-induced increases in [Ca+2]i are similar in pattern to those caused by other calcium-dependent vasoconstrictors, where calcium increases in two stages. First, there is an initial increase in [Ca+2]i from intracellular calcium stores, which is followed by a sustained increase in [Ca+2]i due to the influx of calcium from the extracellular space [20]. This pattern of initial release/sustained influx is evident in non-vascular cells as well, whereby ET-1 can induce neutrophil migration, attenuate cGMP formation in astrocytes, stimulate diuresis and natriuresis, and cause vasorelaxation [56, 66-68]. What differs between cells and tissues are the specific mechanisms that regulate the initial extracellular calcium influx and the sustained intracellular calcium mobilization in response to ET receptor activation.
Cytoplasmic Calcium Changes Evoked by ET-1
Voltage-Dependent Calcium Influx
Mechanism
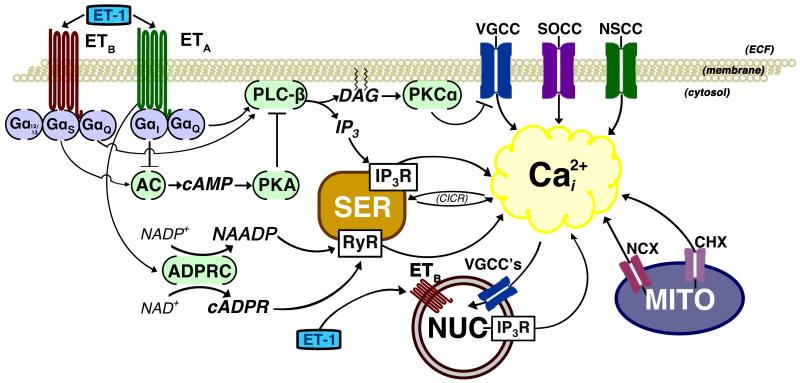
The specific VGCC’s implicated in ET-1-induced calcium entry vary, which is not surprising due to the range of responses ET-1 influences (see Figure 1). L-type, T-type, and R-type calcium channels have all been associated with voltage-dependent calcium influx caused by ET-1, and the relative involvement of each channel type depends on the species and cell type being studied [69, 70]. The activation of multiple calcium channels during voltage-dependent calcium influx is not unique to ET-1; what is interesting is that ET-1 may regulate VGCC’s directly as well as indirectly. The idea that ET-1 can act as a calcium channel opener was proposed as early as 1988, but it is also possible that ET receptor activation alters the voltage-gating properties of VGCC’s indirectly, to increase calcium influx through them [71, 72]. Several researchers published compelling evidence against the theory that ET-1 was a direct agonist of L-type VGCC’s, instead postulating that ET-1 altered VGCC function through second messengers like PLC and protein kinase C (PKC) [73-75].
Figure 1.
This cartoon illustrates mechanisms that are linked to ET receptor-dependent increases in [Ca2+]i. In addition to the plasma membrane receptors, ETB receptors in the nuclear membrane may mobilize calcium when activated by cytosolic ET-1. While the ETA receptor is shown only in the plasma membrane, ETB receptors are found in both the plasma and nuclear membranes. It is unknown if ETA receptors are in the membrane of other organelles. Many of the same mechanisms are attributed to both ETA- and ETB-dependent increases in [Ca2+]i. Arrows represent activation; teed lines represent inhibition. Abbreviations: AC, adenylate cyclase; ADPRC, ADP-ribosyl cyclase; cADPR, cyclic ADP ribose; cAMP, cyclic adenosine monophosphate; CHX, calcium/hydrogen antiporter; CICR, calcium-induced calcium release; DAG, diacylglycerol; ECF, extracellular fluid; ET-1, endothelin-1; IP3, inositol trisphosphate; IP3R, inositol trisphosphate receptor; MITO, mitochondria; NAADP, nicotinic acid adenine dinucleotide phosphate; NAD+, nicotinamide adenine dinucleotide; NADP, nicotinamide adenine dinucleotide phosphate; NCX, sodium/calcium exchanger; NSCC, non-selective cation channel; NUC, nucleus; PKA, protein kinase A; PKCα, protein kinase C-α; PLC-β, phospholipase C-β; RyR, ryanodine receptor; SER, smooth endoplasmic reticulum; SOCC, store-operated calcium channel; VGCC, voltage-gated calcium channel.
ET Receptor Dependence
Voltage-dependent calcium entry is regulated by neither the ETA receptor nor the ETB receptor exclusively. Depending on the cell, tissue, and experimental conditions, activation of either or both ET receptors can regulate voltage-dependent calcium influx. In cardiac myocytes, for example, ETA receptors as well as ETB receptors regulate specific voltage-dependent calcium currents [76]. ETA receptors mediate ET-1-dependent inhibition of voltage-dependent calcium currents caused by isoproterenol. In the same cells, ET-1-dependent stimulation of calcium currents after exposure to atrial natriuretic peptide (ANP) is mediated by ETB receptors. Cell, tissue, and conditional variability has made it difficult to characterize the exact mechanisms by which each ET receptor causes voltage-dependent calcium influx, as well as the relative contribution and importance of voltage-dependent calcium influx to ET-1-mediated responses. Nevertheless, ET receptor-mediated membrane depolarization and voltage-dependent calcium influx is an important mechanism by which ET-1 can increase [Ca+2]i [77-79].
Changes in Hypertension
Pharmacological inhibition of voltage-dependent calcium influx is a well-established and often-used treatment for hypertension, as calcium channel blockers (e.g. nifedipine) decrease blood pressure by inhibiting calcium influx and reducing vasoconstriction [80]. These drugs have cardio-protective benefits as well; prolonged treatment with nifedipine not only lowers blood pressure, but it also improves endothelium-dependent vasorelaxation and reduces ET-1-dependent contraction [81]. Another calcium channel blocker, lacidipine, decreases ventricular hypertrophy and prepro-ET-1 expression in spontaneously-hypertensive rats [82]. These findings reinforce the importance of calcium mobilization in the vasculature, and show that the relationship between ET-1 and calcium is not a one-way street: ET-1 mobilizes calcium, but increases in calcium also augment the synthesis of ET-1. So, inhibition of voltage-dependent calcium influx decreases ET-1’s deleterious effects on vascular function during hypertension, while simultaneously decreasing transcription of ET-1 precursors and ultimately reducing ET-1 production.
Voltage-Independent Calcium Influx
Mechanism
Regardless of the type of VGCC’s associated with ET-1-induced calcium influx or the direct/indirect activation of VGCC’s by ET-1, inhibition of all voltage-dependent calcium channels does not abolish the inward calcium currents caused by ET-1 [83]. In some excitable tissues, VGCC’s are not activated in response to ET-1 [84]. Therefore, the remaining ET-1-induced calcium influx is through any of several voltage-independent calcium entry pathways (see Figure 1). As previously defined, voltage-independent calcium channels (VICC’s) include calcium channels that are activated by a ligand directly (LGCC’s), activated by intracellular calcium release (SOCC’s), or by indirect activation through G-protein-dependent signaling pathways (NSCC’s) (see Table 1). Although no LGCC activated by ET-1 is known currently, pharmacological investigation shows SOCC’s and NSCC’s are important influx pathways in ET-1-induced smooth muscle contraction, MAP Kinase phosphorylation, and arachidonic acid release [85-87]. The relative contribution of SOCC’s and NSCC’s to ET-1-mediated calcium influx is dependent on the concentrations of ET-1 used. Inhibition of NSCC’s or SOCC’s by SKF-96365 or LOE-908, respectively, abolished the calcium currents caused by low concentrations of ET-1 (≤ 0.1 nM) [88]. In the same study, however, calcium currents caused by higher concentrations of ET-1 were only abolished by a combination of SKF-96365 and LOE-908. Since the opening of both SOCC’s and NSCC’s could be stimulated by release of intracellular calcium stores, differentiating ET-1-induced extracellular calcium influx from calcium-induced calcium influx has proven difficult [29].
ET Receptor Dependence
Similar to voltage-dependent calcium entry, voltage-independent calcium entry can be regulated by either ETA receptors or ETB receptors, depending on the cell or tissue type [85]. Neither ET receptor is associated with calcium influx through only one type of VICC in all cell types.
Changes in Hypertension
Changes to voltage-independent calcium entry in hypertension are not well described, but calcium influx through VICC’s appears to have little affect on systemic blood pressure. Treatment with Ginoside-Rd, a purported VICC inhibitor, did not lower systemic blood pressure in hypertensive rats [89, 90]. In the same experiment, however, VICC inhibition decreased vascular remodeling and ET-1-induced smooth muscle cell proliferation [90]. So, while there is little evidence that voltage-independent calcium influx is involved in the pathogenesis of hypertension, both ET-1 and VICC’s are implicated in the progression of hypertension-induced vascular hypertrophy.
Release of Intracellular Calcium Stores
Mechanism
ET receptors cause intracellular calcium stores release by activating PLC and increasing IP3 production [20, 91]. Similar to ET-1’s effects on calcium influx pathways, the calcium released from IP3-sensitive stores does not account for the increase in [Ca+2]i entirely [21]. Intracellular calcium must also come from other reticular stores (e.g. ryanodine-sensitive stores) or an atypical intracellular calcium store (e.g. mitochondrial stores and lysosomal stores) that ET-1 can mobilize. In peritubular smooth muscle cells and renal afferent arterioles, ET-1 alters cyclic-ADP ribose production to sensitize ryanodine-activated SER stores [92-94]. Neither IP3-sensitive stores nor atypical calcium stores, however, account for ET-1-induced increases in intracellular calcium entirely, consistently, and across cell types.
ET Receptor Dependence
While many studies confirm that ET-1 causes intracellular calcium release, none provide evidence that ETA receptors and ETB receptors mobilize different intracellular calcium stores. The intracellular calcium stores mobilized by ET-1 depend upon the cell type, and not the ET receptor subtype.
Changes in Hypertension
Smooth muscle cells from hypertensive rats maintain increased [Ca2+]i after depolarization, which implies intracellular calcium storage and mobilization are altered during hypertension [95]. However, IP3-mediated calcium released by ET-1 stimulation is blunted in DOCA-salt hypertension and unchanged in spontaneously-hypertensive rats [96]. Thus, even though basal [Ca2+]i is increased in hypertension, the ability of ET-1 to mobilize calcium directly is impaired.
The Possibilities: Beyond Calcium Channels and Reticular Stores
Increases in intracellular calcium caused by ET-1 are due to a mixture of voltage-dependent calcium influx, voltage-independent calcium influx, and mobilization of calcium from multiple intracellular stores. These mechanisms vary in their predominance as a calcium source at different times, in different cell types, and at different concentrations of ET-1. No calcium source is linked to ETA or ETB receptors with any specificity across multiple cell types or tissues, since either ET receptor can cause calcium influx through identical pathways in different tissues. ET-1-mediated responses utilize a combination of all of these calcium sources, and no one source dominates across multiple cell types. While many of these individual pathways have been investigated, it is still unclear how ET-1 modulates both influx and release of calcium simultaneously, and these mechanisms do not account for all the calcium that is mobilized in response to ET-1.
Recent exploration into ET-1’s calcium signaling pathways has offered some interesting alternatives to the traditional influx/release pathways attributed to ET-1-induced calcium mobilization, which will be discussed next. While these novel findings are not able to integrate and explain the entirety of ET-1-induced calcium mobilization, they present new and different insights into the function of ET-1 and ET receptors, as well as novel mechanisms for maintaining intracellular calcium concentrations.
Calcium Extrusion
In addition to calcium influx mechanisms that increase [Ca2+]i, there are calcium efflux mechanisms that lower the concentration of intracellular calcium back to basal levels. Due to the large inward concentration gradient, calcium efflux requires the use of energy (in the form of ATP) to push calcium out of the cell and back into the extracellular space [34]. If these mechanisms were inhibited by ET-1, the net result would be prolonged elevations of intracellular calcium concentration due to retention of calcium [97]. Thus, inhibition of calcium efflux mechanisms represents another means by which ET-1 can modify [Ca2+]i. ET-1 suppresses plasma membrane Calcium-ATPase function and expression in hepatic sinusoidal endothelial fenestrae, leading to contraction [98]. ET-1 also causes the sodium/calcium exchanger (NCX) in the plasma membrane of ventricular myocytes to operate in reverse mode, whereby NCX become calcium influx pumps instead of acting as calcium efflux pumps [99]. Future research is needed to elucidate the physiological effects which can be attributed to prolonged increases in [Ca2+]i by ET-1.
ET-1 Signaling in the Nucleus
Traditionally, ET receptors are thought to be plasma membrane receptors, where they can be activated by extracellular ET-1 to initiate a G-protein-dependent intracellular signaling cascade. Recent work by Bkaily et al, shows the presence of ETB receptors and R-type VGCC’s in the nuclear membrane (see Figure 1). They further postulate that internalization of plasma-membrane ET receptors frees ET-1 from the receptor, and this cytosolic ET-1 activates ETB receptors in the nuclear membrane [100]. The activation of nuclear ETB receptors causes an increase in nuclear calcium by opening R-type calcium channels and Na+/Ca2+ exchangers (NCX) on the nuclear membrane, as well as indirect activation of IP3 receptors and ryanodine receptors located in the nucleoplasmic reticulum [101]. The sequestration of calcium within the nucleus may serve as a regulatory element for maintaining calcium homeostasis within the cell, much like sarcoplasmic reticular or mitochondrial uptake of calcium. It also may regulate the expression of calcium-sensitive genes, or act as a protective mechanism to buffer the nucleus against calcium depletion or overload [102, 103]. If calcium can enter the nucleus, it is likely that it can leave the nucleus as well. What remains to be seen is if nuclear ET receptors signal differently than membrane ET receptors, and how intracellular ET-1 can regulate both intra-nuclear and intracellular calcium mobilization.
Understanding of the function of nuclear GPCR’s is a work-in-progress. Much of this research has focused on the angiotensin (AT) AT1 receptor. Binding sites for angiotensin-II on the nuclei of rat hepatocytes turned out to be AT1 receptors that regulate reactive oxygen species (ROS) production [104, 105]. Further investigation will explain the function of nuclear ET receptors and their role in maintaining calcium homeostasis.
ET Receptor Dimerization
GPCR’s are capable of forming homodimers (e.g. 2 β2 adrenergic receptors together) and heterodimers (e.g. M2 muscarinic receptor with an M3 muscarinic receptor) [106, 107]. Research into GPCR dimerization is increasing as the physiological relevance of GPCR dimers becomes more apparent [108]. HEK-293 cells transfected with human ETA receptors, human ETB receptors, or both ETA and ETB receptors show that these receptors can form both homodimers (ETA/ETA; ETB/ETB) and heterodimers (ETA/ETB) [109]. The physiological and pharmacological evidence of ET receptor dimers continues to grow as well, as ETA and ETB receptors functionally interact in human bronchi, saphenous vein, and C6 glioma cells [110-114]. ET receptors have the N- and C-terminal binding motifs for covalent interaction, and can be successfully co-immunoprecipitated from transfected cells [115]. While co-immunoprecipitation of ETA and ETB receptors from many native tissues has proven difficult, the ET receptors have been co-precipitated successfully rat pulmonary resistance arteries [116].
When the transfected HEK-293 cells were used to investigate the effects of ET receptor dimers on ET-1-induced calcium signaling, ETA and ETB homodimers mediated transient increases in [Ca+2]i (approximately 1 minute), while ETA/B heterodimers caused a sustained increase in [Ca+2]i that lasted over 10 minutes [117, 118]. Thus, the presence of different ET receptor homo- and heterodimers changes the profile of the calcium response. These studies did not examine the mechanisms by which dimers alter calcium signaling, but the fact that dimers can modify calcium signaling is fascinating. Exploring how ET receptor dimers can change calcium influx, alter release of intracellular calcium, or decrease calcium extrusion and sequestration is worth further investigation.
Perspectives
Where do we go from here, as we continue to disassemble the mechanisms by which ET-1 increases intracellular calcium? Below are thoughts on this question, where we consider mechanisms that may become relevant to ET-1-dependent calcium signaling as research progresses.
ET Receptors on other Organelles
The presence of receptors and ion channels on intracellular organelles is not limited to the nucleus, as functional TRPV1 channels were discovered on the endoplasmic reticular membrane of dorsal root ganglion neurons [119]. If ET receptors can be found in the membrane of calcium-storing organelles, such as mitochondria or endoplasmic reticulae, they may mobilize calcium and alter cellular function in new and novel ways. If this were true, such a discovery would change the way we think about both calcium signaling and GPCR function.
Receptor/Channel Interaction
The ability of ET receptors to alter the activity of other receptors, and vice versa, is already noted in the kidney, where ETB receptors in the renal proximal tubule can interact with dopamine D3 receptors (another GPCR) to alter natriuresis [120]. Interactions between different GPCR’s can influence the type and function of ion channels associated with them, possibly by recruiting different scaffolding and accessory proteins to form a multi-meric signaling complex [121]. For example: TRPC1 receptors and BK channels can associate in vascular smooth muscle, and blockade of either TRPC1 or BK channels enhances ET-1 contraction of small mesenteric arteries [122]. Understanding if ET-1 can influence receptor and channel interactions remains relatively unexplored.
The Future: Calcium and Beyond
As seen in Figure 1, the current model of ET-1-dependent calcium mobilization is extremely complex for a single peptide with only two officially recognized receptors [3]. Equally numerous are the tools employed to elucidate the mechanisms, from in vivo experiments and in vitro functional assays to cell culture and cell transfection. As each piece of the puzzle of how ET-1 changes intracellular calcium is put into place, we see that it is part of a larger puzzle that challenges how we think about receptor-dependent calcium signaling mechanisms.
Acknowledgements
The formulation of this manuscript would not have been possible without numerous insights from my mentors and fellow scientists, especially Dr. Bill Jackson and Erika M. Boerman.
List of Abbreviations
- AT
angiotensin
- AT1
angiotensin II receptor type 1
- BK channel
large-conductance calcium-activated potassium channel
- Ca2+/CaM/CaMK
calcium/calmodulin/calmodulin kinase
- cGMP
cyclic guanosine monophosphate
- DAG
diacylglycerol
- ET-1
endothelin-1
- GPCR
G protein-coupled receptor
- IP3
inositol trisphosphate
- LGCC
ligand-gated calcium channel
- MAPK
mitogen-activated protein kinase
- NSCC
nonselective cation channel
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PKC
protein kinase C
- PLC
phospholipase-C
- ppET-1
preproendothelin-1
- ROS
reactive oxygen species
- RyR
ryanodine receptor
- SER
sarcoplasmic/endoplasmic reticulum
- SOCC
store-operated calcium channel
- TRP
transient receptor potential cation channel
- VGCC
voltage-gated calcium channel
- VICC
voltage-independent calcium channel
References
- 1.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 2.Cai B-X, Li X-Y, Chen J-H, Tang Y-B, Wang G-L, Zhou J-G, Qui Q-Y, Guan Y-Y. Ginsenoside-Rd, a new voltage-independent Ca2+ entry blocker, reverses basilar hypertrophic remodeling in stroke-prone renovascular hypertensive rats. Eur J Pharmacol. 2009;606:142–149. doi: 10.1016/j.ejphar.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 3.Sokolovsky M. Endothelin receptor subtypes and their role in transmembrane signaling mechanisms. Pharmacol Ther. 1995;68:435–471. doi: 10.1016/0163-7258(95)02015-2. [DOI] [PubMed] [Google Scholar]
- 4.Davenport AP. International Union of Pharmacology. XXIX. Update on endothelin receptor nomenclature. Pharmacol Rev. 2002;54:219–226. doi: 10.1124/pr.54.2.219. [DOI] [PubMed] [Google Scholar]
- 5.Mencarelli M, Pecorelli A, Carbotti P, Valacchi G, Grasso G, Muscettola M. Endothelin receptor A expression in human inflammatory cells. Regul Pept. 2009;158:1–5. doi: 10.1016/j.regpep.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Motta EM, Chichorro JG, Rae GA. Role of ETA and ETB endothelin receptors on endothelin-1-induced potentiation of nociceptive and thermal hyperalgesic responses evoked by capsaicin in rats. Neurosci Lett. 2009;457:146–150. doi: 10.1016/j.neulet.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 7.Xu D-Y, Wu B, Li Z-Q, Wang Q-P, Zhang Y, Xue F, Ji J-F. Expression of endothelin receptor subtypes in the spiral ganglion neurons of the guinea pig. Int J Pediatr Otorhinolaryngol. 2009;74:164–167. doi: 10.1016/j.ijporl.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Ali H, Loizidou M, Dashwood M, Savage F, Sheard C, Taylor I. Stimulation of colorectal cancer cell line growth by ET-1 and its inhibition by ETA antagonists. Gut. 2000;47:685–688. doi: 10.1136/gut.47.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fagan KA, McMurtry IF, Rodman DM. Role of endothelin-1 in lung disease. Respir Res. 2001;2:90–101. doi: 10.1186/rr44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khodorova A, Montmayeur J-P, Strichartz G. Endothelin receptors and pain. The journal of pain: official journal of the American Pain Society. 2009;10:4–28. doi: 10.1016/j.jpain.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortega Mateo A, de Artiñano AA. Highlights on endothelins: a review. Pharmacol Res. 1997;36:339–351. doi: 10.1006/phrs.1997.0246. [DOI] [PubMed] [Google Scholar]
- 12.Pinto-Sietsma SJ, Paul M. A role for endothelin in the pathogenesis of hypertension: fact or fiction? Kidney Int Suppl. 1998;67:S115–121. doi: 10.1046/j.1523-1755.1998.06722.x. [DOI] [PubMed] [Google Scholar]
- 13.Cardillo C, Kilcoyne CM, Waclawiw M, Cannon RO, 3rd, Panza JA. Role of endothelin in the increased vascular tone of patients with essential hypertension. Hypertension. 1999;33:753–758. doi: 10.1161/01.hyp.33.2.753. [DOI] [PubMed] [Google Scholar]
- 14.Perez-Rivera AA, Fink GD, Galligan JJ. Vascular reactivity of mesenteric arteries and veins to endothelin-1 in a murine model of high blood pressure. Vascul Pharmacol. 2005;43:1–10. doi: 10.1016/j.vph.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Elijovich F, Laffer CL, Amador E, Gavras H, Bresnahan MR, Schiffrin EL. Regulation of plasma endothelin by salt in salt-sensitive hypertension. Circulation. 2001;103:263–268. doi: 10.1161/01.cir.103.2.263. [DOI] [PubMed] [Google Scholar]
- 16.Jouneaux C, Mallat A, Serradeil-Le Gal C, Goldsmith P, Hanoune J, Lotersztajn S. Coupling of endothelin B receptors to the calcium pump and phospholipase C via Gs and GQ in rat liver. J Biol Chem. 1994;269:1845–1851. [PubMed] [Google Scholar]
- 17.Luo G, Jamali R, Cao Y-X, Edvinsson L, Xu C-B. Vascular endothelin ETB receptor-mediated contraction requires phosphorylation of ERK1/2 proteins. Eur J Pharmacol. 2006;538:124–131. doi: 10.1016/j.ejphar.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 18.Zeng Q, Li X, Zhong G, Zhang W, Sun C. Endothelin-1 induces intracellular [Ca2+] increase via Ca2+ influx through the L-type Ca2+ channel, Ca2+-induced Ca2+ release and a pathway involving ETA receptors, PKC, PKA and AT1 receptors in cardiomyocytes. Sci China, C, Life Sci. 2009;52:360–370. doi: 10.1007/s11427-009-0046-z. [DOI] [PubMed] [Google Scholar]
- 19.Kawanabe Y, Nauli SM. Involvement of extracellular Ca2+ influx through voltage-independent Ca2+ channels in endothelin-1 function. Cell Signal. 2005;17:911–916. doi: 10.1016/j.cellsig.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Neylon CB. Vascular biology of endothelin signal transduction. Clin Exp Pharmacol Physiol. 1999;26:149–153. doi: 10.1046/j.1440-1681.1999.03013.x. [DOI] [PubMed] [Google Scholar]
- 21.Pollock DM, Keith TL, Highsmith RF. Endothelin receptors and calcium signaling. FASEB J. 1995;9:1196–1204. doi: 10.1096/fasebj.9.12.7672512. [DOI] [PubMed] [Google Scholar]
- 22.Parekh AB. Ca2+ microdomains near plasma membrane Ca2+ channels: impact on cell function. J Physiol. 2008;586:3043–3054. doi: 10.1113/jphysiol.2008.153460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung FP, Yung LM, Yao X, Laher I, Huang Y. Store-operated calcium entry in vascular smooth muscle. Br J Pharmacol. 2008;153:846–857. doi: 10.1038/sj.bjp.0707455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dolphin AC. A short history of voltage-gated calcium channels. Br J Pharmacol. 2006;147(Suppl 1):S56–62. doi: 10.1038/sj.bjp.0706442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 26.Derkach V, Surprenant A, North RA. 5-HT3 receptors are membrane ion channels. Nature. 1989;339:706–709. doi: 10.1038/339706a0. [DOI] [PubMed] [Google Scholar]
- 27.Noda M, Furutani Y, Takahashi H, Toyosato M, Tanabe T, Shimizu S, Kikyotani S, Kayano T, Hirose T, Inayama S, et al. Cloning and sequence analysis of calf cDNA and human genomic DNA encoding alpha-subunit precursor of muscle acetylcholine receptor. Nature. 1983;305:818–823. doi: 10.1038/305818a0. [DOI] [PubMed] [Google Scholar]
- 28.Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G. A new class of ligand-gated ion channel defined by P2× receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- 29.Putney JW. Capacitative calcium entry: from concept to molecules. Immunol Rev. 2009;231:10–22. doi: 10.1111/j.1600-065X.2009.00810.x. [DOI] [PubMed] [Google Scholar]
- 30.Taylor CW, Rahman T, Tovey SC, Dedos SG, Taylor EJ, Velamakanni S. IP3 receptors: some lessons from DT40 cells. Immunol Rev. 2009;231:23–44. doi: 10.1111/j.1600-065X.2009.00807.x. [DOI] [PubMed] [Google Scholar]
- 31.Mackrill JJ. Ryanodine receptor calcium channels and their partners as drug targets. Biochem Pharmacol. 2010;79:1535–1543. doi: 10.1016/j.bcp.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Bootman MD, Collins TJ, Peppiatt CM, Prothero LS, MacKenzie L, De Smet P, Travers M, Tovey SC, Seo JT, Berridge MJ, Ciccolini F, Lipp P. Calcium signalling--an overview. Semin Cell Dev Biol. 2001;12:3–10. doi: 10.1006/scdb.2000.0211. [DOI] [PubMed] [Google Scholar]
- 33.Giorgi C, Romagnoli A, Pinton P, Rizzuto R. Ca2+ signaling, mitochondria and cell death. Curr Mol Med. 2008;8:119–130. doi: 10.2174/156652408783769571. [DOI] [PubMed] [Google Scholar]
- 34.Guerini D. The Ca2+ pumps and the Na+/Ca2+ exchangers. Biometals. 1998;11:319–330. doi: 10.1023/a:1009210001608. [DOI] [PubMed] [Google Scholar]
- 35.Wray S, Burdyga T, Noble K. Calcium signalling in smooth muscle. Cell Calcium. 2005;38:397–407. doi: 10.1016/j.ceca.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 36.Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- 37.Essin K, Gollasch M. Role of ryanodine receptor subtypes in initiation and formation of calcium sparks in arterial smooth muscle: comparison with striated muscle. J Biomed Biotechnol. 2009;2009:135249. doi: 10.1155/2009/135249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochocka AM, Pawelczyk T. Isozymes delta of phosphoinositide-specific phospholipase C and their role in signal transduction in the cell. Acta Biochim Pol. 2003;50:1097–1110. [PubMed] [Google Scholar]
- 39.Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev. 2000;80:1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- 40.Sureshan KM, Riley AM, Rossi AM, Tovey SC, Dedos SG, Taylor CW, Potter BVL. Activation of IP3 receptors by synthetic bisphosphate ligands. Chem. Commun. 2009:1204. doi: 10.1039/b819328b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Endo M. Calcium ion as a second messenger with special reference to excitation-contraction coupling. J Pharmacol Sci. 2006;100:519–524. doi: 10.1254/jphs.cpj06004x. [DOI] [PubMed] [Google Scholar]
- 42.del Valle-Rodriguez A, Lopez-Barneo J, Urena J. Ca2+ channel-sarcoplasmic reticulum coupling: a mechanism of arterial myocyte contraction without Ca2+ influx. EMBO J. 2003;22:4337–4345. doi: 10.1093/emboj/cdg432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bolton TB. Calcium events in smooth muscles and their interstitial cells; physiological roles of sparks. J Physiol. 2006;570:5–11. doi: 10.1113/jphysiol.2005.095604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Brien RF, Robbins RJ, McMurtry IF. Endothelial cells in culture produce a vasoconstrictor substance. J Cell Physiol. 1987;132:263–270. doi: 10.1002/jcp.1041320210. [DOI] [PubMed] [Google Scholar]
- 45.Strait KA, Stricklett PK, Kohan JL, Miller MB, Kohan DE. Calcium regulation of endothelin-1 synthesis in rat inner medullary collecting duct. Am J Physiol Renal Physiol. 2007;293:F601–606. doi: 10.1152/ajprenal.00085.2007. [DOI] [PubMed] [Google Scholar]
- 46.Marsen TA, Simonson MS, Dunn MJ. Roles of calcium and kinases in regulation of thrombin-stimulated preproendothelin-1 transcription. Am J Physiol. 1996;271:H1918–1925. doi: 10.1152/ajpheart.1996.271.5.H1918. [DOI] [PubMed] [Google Scholar]
- 47.Dallas A, Khalil RA. Ca2+ antagonist-insensitive coronary smooth muscle contraction involves activation of epsilon-protein kinase C-dependent pathway. Am J Physiol, Cell Physiol. 2003;285:C1454–1463. doi: 10.1152/ajpcell.00066.2003. [DOI] [PubMed] [Google Scholar]
- 48.Itoh H, Higuchi H, Hiraoka N, Ito M, Konishi T, Nakano T, Lederis K. Contraction of rat thoracic aorta strips by endothelin-1 in the absence of extracellular Ca2+ Br J Pharmacol. 1991;104:847–852. doi: 10.1111/j.1476-5381.1991.tb12516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bailey MA, Haton C, Orea V, Sassard J, Bailly C, Unwin RJ, Imbert-Teboul M. ETA receptor-mediated Ca2+ signaling in thin descending limbs of Henle’s loop: impairment in genetic hypertension. Kidney Int. 2003;63:1276–1284. doi: 10.1046/j.1523-1755.2003.00880.x. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y, Tipoe GL, Liong E, Leung S, Lam S-Y, Iwase R, Tjong Y-W, Fung M-L. Chronic hypoxia enhances endothelin-1-induced intracellular calcium elevation in rat carotid body chemoreceptors and up-regulates ETA receptor expression. Pflugers Arch. 2002;443:565–573. doi: 10.1007/s00424-001-0728-2. [DOI] [PubMed] [Google Scholar]
- 51.Dai J, Lee CH, Poburko D, Szado T, Kuo KH, van Breemen C. Endothelin-1-mediated wave-like [Ca2+]i oscillations in intact rabbit inferior vena cava. J Vasc Res. 2007;44:495–503. doi: 10.1159/000106553. [DOI] [PubMed] [Google Scholar]
- 52.Elferink JG, de Koster BM. Endothelin-induced activation of neutrophil migration. Biochem Pharmacol. 1994;48:865–871. doi: 10.1016/0006-2952(94)90356-5. [DOI] [PubMed] [Google Scholar]
- 53.Ge Y, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, Kohan DE. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol Renal Physiol. 2006;291:F1274–1280. doi: 10.1152/ajprenal.00190.2006. [DOI] [PubMed] [Google Scholar]
- 54.Ge Y, Bagnall A, Stricklett PK, Webb D, Kotelevtsev Y, Kohan DE. Combined knockout of collecting duct endothelin A and B receptors causes hypertension and sodium retention. AJP: Renal Physiology. 2008;295:F1635–F1640. doi: 10.1152/ajprenal.90279.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gouadon E, Meunier N, Grebert D, Durieux D, Baly C, Salesse R, Caillol M, Congar P. Endothelin evokes distinct calcium transients in neuronal and non-neuronal cells of rat olfactory mucosa primary cultures. Neuroscience. 2010;165:584–600. doi: 10.1016/j.neuroscience.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 56.Hay DW, Luttmann MA, Muccitelli RM, Goldie RG. Endothelin receptors and calcium translocation pathways in human airways. Naunyn-Schmied Arch Pharmacol. 1999;359:404–410. doi: 10.1007/pl00005368. [DOI] [PubMed] [Google Scholar]
- 57.Prasanna G, Krishnamoorthy R, Clark AF, Wordinger RJ, Yorio T. Human optic nerve head astrocytes as a target for endothelin-1. Invest Ophthalmol Vis Sci. 2002;43:2704–2713. [PubMed] [Google Scholar]
- 58.Someya A, Yuyama H, Fujimori A, Ukai M, Fukushima S, Sasamata M. Effect of YM598, a selective endothelin ETA receptor antagonist, on endothelin-1-induced bone formation. Eur J Pharmacol. 2006;543:14–20. doi: 10.1016/j.ejphar.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 59.Toffoli MC, Gabra BH, Teixeira CFP, Sirois P, Jancar S. Endothelins mediate neutrophil activation, ProMMP-9 release and endothelial cell detachment. Inflammation. 2007;30:28–37. doi: 10.1007/s10753-006-9018-7. [DOI] [PubMed] [Google Scholar]
- 60.Tokudome T, Horio T, Soeki T, Mori K, Kishimoto I, Suga S.-i., Yoshihara F, Kawano Y, Kohno M, Kangawa K. Inhibitory effect of C-type natriuretic peptide (CNP) on cultured cardiac myocyte hypertrophy: interference between CNP and endothelin-1 signaling pathways. Endocrinology. 2004;145:2131–2140. doi: 10.1210/en.2003-1260. [DOI] [PubMed] [Google Scholar]
- 61.Tsukahara H, Ende H, Magazine HI, Bahou WF, Goligorsky MS. Molecular and functional characterization of the non-isopeptide-selective ETB receptor in endothelial cells. Receptor coupling to nitric oxide synthase. J Biol Chem. 1994;269:21778–21785. [PubMed] [Google Scholar]
- 62.Zhang XF, Iwamuro Y, Enoki T, Okazawa M, Lee K, Komuro T, Minowa T, Okamoto Y, Hasegawa H, Furutani H, Miwa S, Masaki T. Pharmacological characterization of Ca2+ entry channels in endothelin-1-induced contraction of rat aorta using LOE 908 and SK&F 96365. Br J Pharmacol. 1999;127:1388–1398. doi: 10.1038/sj.bjp.0702661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bogoni G, Rizzi A, Calo G, Campobasso C, D’Orleans-Juste P, Regoli D. Characterization of endothelin receptors in the human umbilical artery and vein. Br J Pharmacol. 1996;119:1600–1604. doi: 10.1111/j.1476-5381.1996.tb16078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cardozo AM, D’Orléans-Juste P, Bkaily G, Rae GA. Simultaneous changes in intracellular calcium and tension induced by endothelin-1 and sarafotoxin S6c in guinea pig isolated gallbladder: influence of indomethacin. Can J Physiol Pharmacol. 2002;80:458–463. doi: 10.1139/y02-057. [DOI] [PubMed] [Google Scholar]
- 65.Thorin E, Webb DJ. Endothelium-derived endothelin-1. Pflugers Arch - Eur J Physiol. 2009:1–8. doi: 10.1007/s00424-009-0763-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elferink JG, de Koster BM. The effect of endothelin-2 (ET-2) on migration and changes in cytosolic free calcium of neutrophils. Naunyn-Schmied Arch Pharmacol. 1996;353:130–135. doi: 10.1007/BF00168749. [DOI] [PubMed] [Google Scholar]
- 67.Kohan DE. Biology of endothelin receptors in the collecting duct. Kidney Int. 2009;76:481–486. doi: 10.1038/ki.2009.203. [DOI] [PubMed] [Google Scholar]
- 68.Yeung VT, Ho SK, Tsang DS, Nicholls MG, Cockram CS. Endothelin-3 attenuates the cyclic GMP responses to C-type natriuretic peptide in cultured mouse astrocytes. J Neurosci Res. 1996;46:686–696. doi: 10.1002/(SICI)1097-4547(19961215)46:6<686::AID-JNR6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 69.Betts LC, Kozlowski RZ. Electrophysiological effects of endothelin-1 and their relationship to contraction in rat renal arterial smooth muscle. Br J Pharmacol. 2000;130:787–796. doi: 10.1038/sj.bjp.0703377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bkaily G, Naik R, D’Orléans-Juste P, Wang S, Fong CN. Endothelin-1 activates the R-type Ca2+ channel in vascular smooth-muscle cells. J Cardiovasc Pharmacol. 1995;26(Suppl 3):S303–306. [PubMed] [Google Scholar]
- 71.Hirata Y, Yoshimi H, Takata S, Watanabe TX, Kumagai S, Nakajima K, Sakakibara S. Cellular mechanism of action by a novel vasoconstrictor endothelin in cultured rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1988;154:868–875. doi: 10.1016/0006-291x(88)90220-3. [DOI] [PubMed] [Google Scholar]
- 72.Kasuya Y, Takuwa Y, Yanagisawa M, Masaki T, Goto K. A pertussis toxin-sensitive mechanism of endothelin action in porcine coronary artery smooth muscle. Br J Pharmacol. 1992;107:456–462. doi: 10.1111/j.1476-5381.1992.tb12767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Inoue Y, Oike M, Nakao K, Kitamura K, Kuriyama H. Endothelin augments unitary calcium channel currents on the smooth muscle cell membrane of guinea-pig portal vein. J Physiol (Lond) 1990;423:171–191. doi: 10.1113/jphysiol.1990.sp018017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Renterghem C, Vigne P, Barhanin J, Schmid-Alliana A, Frelin C, Lazdunski M. Molecular mechanism of action of the vasoconstrictor peptide endothelin. Biochem Biophys Res Commun. 1988;157:977–985. doi: 10.1016/s0006-291x(88)80970-7. [DOI] [PubMed] [Google Scholar]
- 75.Van Renterghem C, Vigne P, Barhanin J, Schmid-Alliana A, Frelin C, Lazdunski M. Molecular mechanism of endothelin-1 action on aortic cells. J Cardiovasc Pharmacol. 1989;13(Suppl 5):S186–187. doi: 10.1097/00005344-198900135-00051. [DOI] [PubMed] [Google Scholar]
- 76.Boixel C, Dinanian S, Lang-Lazdunski L, Mercadier JJ, Hatem SN. Characterization of effects of endothelin-1 on the L-type Ca2+ current in human atrial myocytes. Am J Physiol Heart Circ Physiol. 2001;281:H764–773. doi: 10.1152/ajpheart.2001.281.2.H764. [DOI] [PubMed] [Google Scholar]
- 77.Suzuki T. Endothelin-1-induced depolarization and hyperpolarization in submandibular ganglion neurons. Bull Tokyo Dent Coll. 2004;45:189–192. doi: 10.2209/tdcpublication.45.189. [DOI] [PubMed] [Google Scholar]
- 78.Hukovic N, Hadziselimovic R. Endothelin 1 action on isolated rat stomach and the role of calcium ions in ET 1 induced depolarization of smooth muscle cells BC3H1. Biochem Mol Biol Int. 1998;46:877–886. doi: 10.1080/15216549800204422. [DOI] [PubMed] [Google Scholar]
- 79.Lovenberg W, Miller RC. Endothelin: a review of its effects and possible mechanisms of action. Neurochem Res. 1990;15:407–417. doi: 10.1007/BF00969926. [DOI] [PubMed] [Google Scholar]
- 80.Richard S. Vascular effects of calcium channel antagonists: new evidence. Drugs. 2005;65(Suppl 2):1–10. doi: 10.2165/00003495-200565002-00002. [DOI] [PubMed] [Google Scholar]
- 81.Sudano I, Virdis A, Taddei S, Spieker L, Corti R, Noll G, Salvetti A, Luscher TF. Chronic treatment with long-acting nifedipine reduces vasoconstriction to endothelin-1 in essential hypertension. Hypertension. 2007;49:285–290. doi: 10.1161/01.HYP.0000254645.33321.a3. [DOI] [PubMed] [Google Scholar]
- 82.Feron O, Salomone S, Godfraind T. Action of the calcium channel blocker lacidipine on cardiac hypertrophy and endothelin-1 gene expression in stroke-prone hypertensive rats. Br J Pharmacol. 1996;118:659–664. doi: 10.1111/j.1476-5381.1996.tb15451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gardner JP, Tokudome G, Tomonari H, Maher E, Hollander D, Aviv A. Endothelin-induced calcium responses in human vascular smooth muscle cells. Am J Physiol. 1992;262:C148–155. doi: 10.1152/ajpcell.1992.262.1.C148. [DOI] [PubMed] [Google Scholar]
- 84.Pollock DM. L-type calcium channels in the renal microcirculatory response to endothelin. AJP: Renal Physiology. 2004;288:F771–F777. doi: 10.1152/ajprenal.00315.2004. [DOI] [PubMed] [Google Scholar]
- 85.Tanaka Y, Imai T, Igarashi T, Takayanagi K, Otsuka K, Yamaki F, Tanaka H, Shigenobu K. Comparison of the Ca2+ entry channels responsible for mechanical responses of guinea-pig aorta to noradrenaline and thapsigargin using SK&F 96365 and LOE 908. Naunyn-Schmied Arch Pharmacol. 2000;362:160–168. doi: 10.1007/s002100000272. [DOI] [PubMed] [Google Scholar]
- 86.Kawanabe Y, Nozaki K, Hashimoto N, Masaki T. Involvement of extracellular Ca2+ influx and epidermal growth factor receptor tyrosine kinase transactivation in endothelin-1-induced arachidonic acid release. Br J Pharmacol. 2003;139:1516–1522. doi: 10.1038/sj.bjp.0705386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ansari HR, Kaddour-Djebbar I, Abdel-Latif AA. Involvement of Ca2+ channels in endothelin-1-induced MAP kinase phosphorylation, myosin light chain phosphorylation and contraction in rabbit iris sphincter smooth muscle. Cell Signal. 2004;16:609–619. doi: 10.1016/j.cellsig.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 88.Miwa S, Iwamuro Y, Zhang XF, Inoki T, Okamoto Y, Okazawa M, Masaki T. Ca2+ entry channels in rat thoracic aortic smooth muscle cells activated by endothelin-1. Jpn J Pharmacol. 1999;80:281–288. doi: 10.1254/jjp.80.281. [DOI] [PubMed] [Google Scholar]
- 89.Guan YY, Kwan CY, He H, Sun JJ, Daniel EE. Effects of Panax notoginseng saponins on receptor-operated Ca2+ channels in vascular smooth muscle. Zhongguo Yao Li Xue Bao. 1994;15:392–398. [PubMed] [Google Scholar]
- 90.Cai BX, Li XY, Chen JH, Tang YB, Wang GL, Zhou JG, Qui QY, Guan YY. Ginsenoside-Rd, a new voltage-independent Ca2+ entry blocker, reverses basilar hypertrophic remodeling in stroke-prone renovascular hypertensive rats. Eur J Pharmacol. 2009;606:142–149. doi: 10.1016/j.ejphar.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 91.Perez-Zoghbi JF, Sanderson MJ. Endothelin-induced contraction of bronchiole and pulmonary arteriole s muscle cells Ca2+ mooth is by intracellular oscillations and Ca2+ regulated sensitization. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1000–1011. doi: 10.1152/ajplung.00184.2007. [DOI] [PubMed] [Google Scholar]
- 92.Barone F, Genazzani AA, Conti A, Churchill GC, Palombi F, Ziparo E, Sorrentino V, Galione A, Filippini A. A pivotal role for cADPR-mediated Ca2+ signaling: regulation of endothelin-induced contraction in peritubular smooth muscle cells. FASEB J. 2002;16:697–705. doi: 10.1096/fj.01-0749com. [DOI] [PubMed] [Google Scholar]
- 93.Guse AH, Lee HC. NAADP: a universal Ca2+ trigger. Science Signaling. 2008;1:1–7. doi: 10.1126/scisignal.144re10. [DOI] [PubMed] [Google Scholar]
- 94.Poburko D, Liao C-H, van Breemen C, Demaurex N. Mitochondrial regulation of sarcoplasmic reticulum Ca2+ content in vascular smooth muscle cells. Circulation Research. 2009;104:104–112. doi: 10.1161/CIRCRESAHA.108.180612. [DOI] [PubMed] [Google Scholar]
- 95.Tostes RC, Wilde DW, Bendhack LM, Webb RC. Calcium handling by vascular myocytes in hypertension. Braz J Med Biol Res. 1997;30:315–323. doi: 10.1590/s0100-879x1997000300004. [DOI] [PubMed] [Google Scholar]
- 96.Schiffrin EL. Intracellular signal transduction for vasoactive peptides in hypertension. Can J Physiol Pharmacol. 1994;72:954–962. doi: 10.1139/y94-133. [DOI] [PubMed] [Google Scholar]
- 97.Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89:1341–1378. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- 98.Yokomori H, Oda M, Ogi M, Yoshimura K, Nomura M, Fujimaki K, Kamegaya Y, Tsukada N, Ishii H. Endothelin-1 suppresses plasma membrane Ca2+-ATPase, concomitant with contraction of hepatic sinusoidal endothelial fenestrae. Am J Pathol. 2003;162:557–566. doi: 10.1016/S0002-9440(10)63849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aiello EA, Villa-Abrille MC, Dulce RA, Cingolani HE, Perez NG. Endothelin-1 stimulates the Na+/Ca2+ exchanger reverse mode through intracellular Na+ (Na+i)-dependent and Na+i-independent pathways. Hypertension. 2005;45:288–293. doi: 10.1161/01.HYP.0000152700.58940.b2. [DOI] [PubMed] [Google Scholar]
- 100.Bkaily G, Choufani S, Avedanian L, Ahmarani L, Nader M, Jacques D, D’Orléans-Juste P, Al Khoury J. Nonpeptidic antagonists of ETA and ETB receptors reverse the ET-1-induced sustained increase of cytosolic and nuclear calcium in human aortic vascular smooth muscle cells. Can J Physiol Pharmacol. 2008;86:546–556. doi: 10.1139/Y08-048. [DOI] [PubMed] [Google Scholar]
- 101.Bkaily G, Nader M, Avedanian L, Choufani S, Jacques D, D’Orléans-Juste P, Gobeil F, Chemtob S, Al-Khoury J. G-protein-coupled receptors, channels, and Na+-H+ exchanger in nuclear membranes of heart, hepatic, vascular endothelial, and smooth muscle cells. Can J Physiol Pharmacol. 2006;84:431–441. doi: 10.1139/y06-002. [DOI] [PubMed] [Google Scholar]
- 102.Bkaily G, Avedanian L, Jacques D. Nuclear membrane receptors and channels as targets for drug development in cardiovascular diseases. Can J Physiol Pharmacol. 2009;87:108–119. doi: 10.1139/Y08-115. [DOI] [PubMed] [Google Scholar]
- 103.Bkaily G, Pothier P, D’Orleans-Juste P, Simaan M, Jacques D, Jaalouk D, Belzile F, Hassan G, Boutin C, Haddad G, Neugebauer W. The use of confocal microscopy in the investigation of cell structure and function in the heart, vascular endothelium and smooth muscle cells. Mol Cell Biochem. 1997;172:171–194. [PubMed] [Google Scholar]
- 104.Booz GW, Conrad KM, Hess AL, Singer HA, Baker KM. Angiotensin-II-binding sites on hepatocyte nuclei. Endocrinology. 1992;130:3641–3649. doi: 10.1210/endo.130.6.1597161. [DOI] [PubMed] [Google Scholar]
- 105.Pendergrass KD, Gwathmey TM, Michalek RD, Grayson JM, Chappell MC. The angiotensin II-AT1 receptor stimulates reactive oxygen species within the cell nucleus. Biochem Biophys Res Commun. 2009;384:149–154. doi: 10.1016/j.bbrc.2009.04.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marinissen MJ, Gutkind JS. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol Sci. 2001;22:368–376. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- 107.Minneman KP. Heterodimerization and surface localization of G protein coupled receptors. Biochem Pharmacol. 2007;73:1043–1050. doi: 10.1016/j.bcp.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Panetta R, Greenwood MT. Physiological relevance of GPCR oligomerization and its impact on drug discovery. Drug Discov Today. 2008;13:1059–1066. doi: 10.1016/j.drudis.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 109.Dai X, Galligan JJ. Differential trafficking and desensitization of human ETA and ETB receptors expressed in HEK 293 cells. Exp Biol Med (Maywood) 2006;231:746–751. [PubMed] [Google Scholar]
- 110.Fukuroda T, Ozaki S, Ihara M, Ishikawa K, Yano M, Miyauchi T, Ishikawa S, Onizuka M, Goto K, Nishikibe M. Necessity of dual blockade of endothelin ETA and ETB receptor subtypes for antagonism of endothelin-1-induced contraction in human bronchi. Br J Pharmacol. 1996;117:995–999. doi: 10.1111/j.1476-5381.1996.tb16688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pate MA, Chester AH, Brown TJ, Roach AG, Yacoub MH. Atypical antagonism observed with BQ-123 in human saphenous vein. J Cardiovasc Pharmacol. 1998;31(Suppl 1):S172–174. doi: 10.1097/00005344-199800001-00049. [DOI] [PubMed] [Google Scholar]
- 112.Malik R, Vlasicova K, Sedo A. Functional cross-talk of Ca2+-mobilizing endothelin receptors in C6 glioma cells. Physiol Res. 2002;51:73–78. [PubMed] [Google Scholar]
- 113.Sexton PM, Morfis M, Tilakaratne N, Hay DL, Udawela M, Christopoulos G, Christopoulos A. Complexing receptor pharmacology: modulation of family B G protein-coupled receptor function by RAMPs. Ann N Y Acad Sci. 2006;1070:90–104. doi: 10.1196/annals.1317.076. [DOI] [PubMed] [Google Scholar]
- 114.Thakali K, Galligan JJ, Fink GD, Gariepy CE, Watts SW. Pharmacological endothelin receptor interaction does not occur in veins from ETB receptor deficient rats. Vascul Pharmacol. 2008;49:6–13. doi: 10.1016/j.vph.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gurevich VV, Gurevich EV. How and why do GPCRs dimerize? Trends Pharmacol Sci. 2008;29:234–240. doi: 10.1016/j.tips.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sauvageau S, Thorin E, Caron A, Dupuis J. Evaluation of endothelin-1-induced pulmonary vasoconstriction following myocardial infarction. Exp Biol Med (Maywood) 2006;231:840–846. [PubMed] [Google Scholar]
- 117.Evans NJ, Walker JW. Endothelin receptor dimers evaluated by FRET, ligand binding, and calcium mobilization. Biophys J. 2008;95:483–492. doi: 10.1529/biophysj.107.119206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Evans NJ, Walker JW. Sustained Ca2+ signaling and delayed internalization associated with endothelin receptor heterodimers linked through a PDZ finger. Can J Physiol Pharmacol. 2008;86:526–535. doi: 10.1139/Y08-050. [DOI] [PubMed] [Google Scholar]
- 119.Gallego-Sandín S, Rodríguez-García A, Alonso MT, García-Sancho J. The endoplasmic reticulum of dorsal root ganglion neurons contains functional TRPV1 channels. J Biol Chem. 2009;284:32591–32601. doi: 10.1074/jbc.M109.019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zeng C, Asico LD, Yu C, Villar VAM, Shi W, Luo Y, Wang Z, He D, Liu Y, Huang L, Yang C, Wang X, Hopfer U, Eisner GM, Jose PA. Renal D3 dopamine receptor stimulation induces natriuresis by endothelin B receptor interactions. Kidney Int. 2008;74:750–759. doi: 10.1038/ki.2008.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Franco R, Lluis C, Canela EI, Mallol J, Agnati L, Casado V, Ciruela F, Ferre S, Fuxe K. Receptor-receptor interactions involving adenosine A1 or dopamine D1 receptors and accessory proteins. J Neural Transm. 2007;114:93–104. doi: 10.1007/s00702-006-0566-7. [DOI] [PubMed] [Google Scholar]
- 122.Kwan H-Y, Shen B, Ma X, Kwok Y-C, Huang Y, Man Y-B, Yu S, Yao X. TRPC1 associates with BK(Ca) channel to form a signal complex in vascular smooth muscle cells. Circulation Research. 2009;104:670–678. doi: 10.1161/CIRCRESAHA.108.188748. [DOI] [PubMed] [Google Scholar]