Abstract
Background:
Chronic obstructive pulmonary disease (COPD) is associated with clinically relevant extra pulmonary manifestations; one of them is weight loss. However, there are very few studies from North India available in relation to body mass index (BMI) and Oxygen saturation (SpO2) with COPD.
Aims:
To study the prevalence of undernutrition among stable COPD patients and correlation of COPD severity with SpO2 and BMI.
Settings and Design:
A prospective study was carried out at a tertiary care hospital.
Subjects and Methods:
COPD patients were diagnosed and staged as per global initiative for chronic obstructive lung disease (GOLD) guidelines. SpO2 was measured using pulse oxymeter and BMI categorization was done as per new classification for Asian Indians (2009). Statistical analysis was done using Statistical Package for Social Sciences Version 15.0.
Results:
Out of 147 COPD patients, 85 (57.8%) were undernourished. The prevalence of undernourished BMI was 25%, 50.8%, 61.7%, and 80% in stage I, II, III and IV respectively; statistically significant (P < 0.050). The mean SpO2 was 95.50 ± 1.41, 95.05 ± 2.42, 94.37 ± 2.28 and 93.05 ± 1.39 in stage I, II, III and IV respectively; statistically significant (F = 4.723; P = 0.004).
Conclusions:
The overall prevalence of under nutrition among COPD patients was 57.8%. With increasing COPD stage the BMI and median SpO2 value decreased in progressive manner. Association of SpO2 and COPD stages could be explored further in order to suggest an additional marker of disease severity that would add a new dimension in the management of COPD.
KEY WORDS: BMI, COPD, prevalence, SpO2, undernutrition
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease state, characterised by airflow limitation that is not fully reversible. The airflow limitation is usually progressive and is associated with an abnormal inflammatory response of the lungs to noxious particles or gases, primarily caused by cigarette smoking. Although COPD affects the lungs, it also produces significant systemic consequences like nutritional abnormality, weight loss, and peripheral muscle dysfunction.[1]
Chronic obstructive pulmonary disease (COPD) has been redefined[1] to indicate that apart from the deleterious effects on the lungs, it is associated with clinically relevant extra pulmonary manifestations.[1,2,3,4] Systemic consequences now recognised as important features of the disease contribute to exercise intolerance, decreased health status, and increased mortality.[1,2,3,4,5,6] The present study was carried out with an aim to assess the nutritional status and to find out correlation between severity of COPD with SpO2 and BMI.
SUBJECTS AND METHODS
All adult consecutive patients of COPD diagnosed on the basis of history, clinical examination and investigations, attending outpatient department of pulmonary medicine were included in the study. The mean age of the patients was 55.66 ± 9.73 years. We excluded subjects with associated recent myocardial infarction, active pulmonary tuberculosis, malignancies, and HIV. The study was approved by institutional ethical committee.
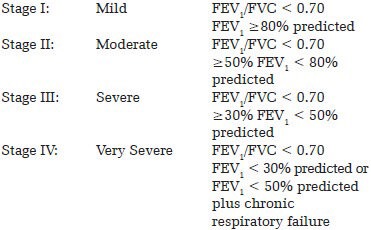
The spirometry was done by Medikro Spirostar USB M9479 (Finland) machine. Diagnosis of COPD was based on GOLD guidelines i.e. cough, sputum production, or dyspnea with history of exposure to risk factors e.g. tobacco smoking and spirometry showing forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) less than 0.7. ‘Smoking history of one pack year’ is smoking 20 cigarettes or bidis per day for one year. Bidi smoke is considered as injurious as cigarette smoke because it has been shown that bidi smoked at 2 puffs/minute produces similar amounts of steam-volatile phenols, hydrogen cyanide, and benzopyrene as unfiltered cigarette at 1 puff/minute.[7] Body mass index (BMI) of the patients was calculated by measuring the weight in kgs divided by [height (in meter)2]. Based on their BMI, the patients were assigned to the following four groups (As per new classification for Asian Indians):[8]
Undernourished: <18.0 kg/m2
Normal weight: 18-22.9 kg/m2
Overweight: 23-24.9 kg/m2
Obese: >25 kg/m2
Smoking history was obtained from the subjects and individual pack year was calculated.
Classification of severity of airflow limitation in COPD
Severity Based on Post-Bronchodilator FEV1 as per GOLD guidelines[9]:
SpO2 of the patients was noted in OPD using Nonin Onyx Pure SAT® SpO2 Pulse Oxymeter.
RESULTS
A total of 147 patients of COPD were included in the study, the majority of subjects were males 139 (93.3%), and only 8 patients (6.7%) were female. Maximum number of subjects belonged to GOLD stage III 60 (40.8%), followed by Stage II 59 (40.1%), Stage IV 20 (13.6%) and minimum number of subjects belonged to stage I 8 (5.4%). It was observed that with increasing COPD stage, the SpO2 decreased in a progressive manner [Table 1].
Table 1.
Association between GOLD, COPD Stage and Gender, SpO2 and BMI
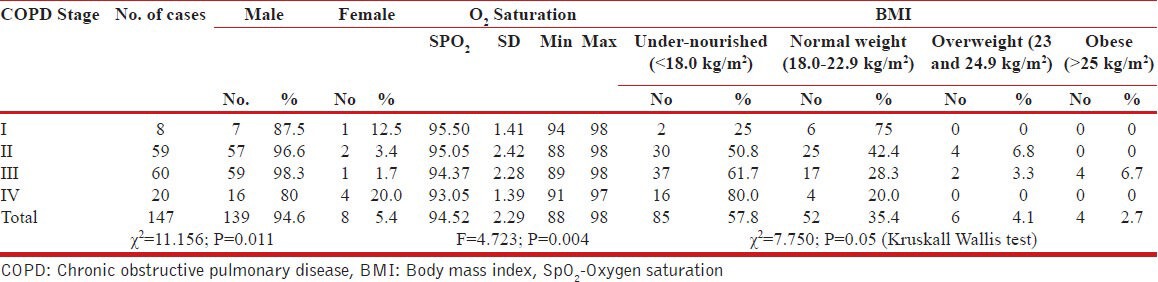
Out of 147 COPD patients, 85 (57.8%) patients were undernourished, 52 (35.4%) were of normal weight, 6 (4.1%) were of overweight and 4 (2.7%) were obese. The prevalence of undernourished BMI status was 25% in stage I, 50.8% in stage II, 61.7% in stage III and 80% in stage IV; statistically significant (P < 0.05) [Kruskall Wallis test].
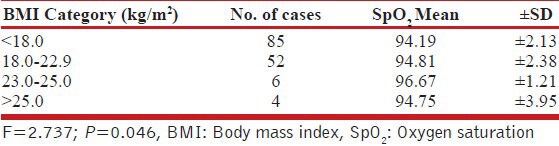
It was observed that mean O2 saturation was maximum in BMI category 23.0-25.0 kg/m2 and minimum in BMI category <18.0 kg/m2 [Table 2].
Table 2.
Association of SpO2 and BMI
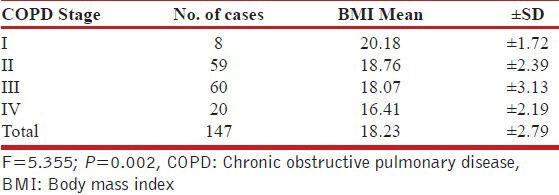
In relation to GOLD, COPD Staging, mean BMI decreased progressively as disease severity increased [Table 3].
Table 3.
Association between GOLD, COPD Stage, and Mean BMI
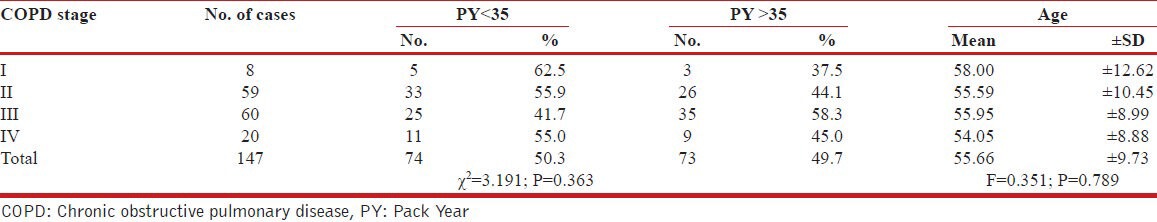
The association between GOLD, COPD stage and Pack year with age has been shown in Table 4.
Table 4.
Association between GOLD, COPD Stage and PY, Age
A multivariate analysis was done to see any association between COPD stage as the dependent variable and age, gender, smoking packs/year and BMI as the independent variables. The outcome has been shown in Table 5.
Table 5.
Multivariate analysis to seek the association between COPD stage, age, sex and smoking
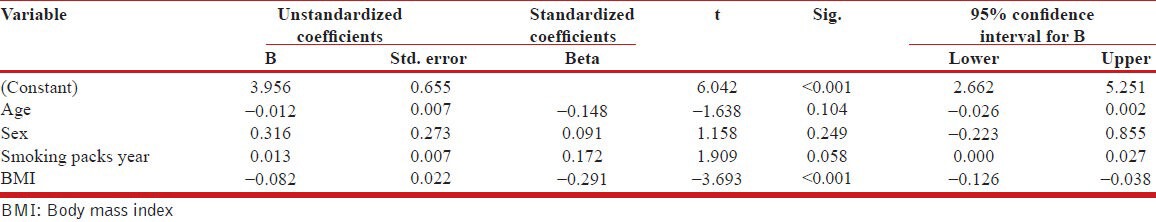
The multivariate model revealed only significant association between BMI and COPD stage (P < 0.001). None of the other variables were found to be significantly associated with the outcome of COPD stages.
DISCUSSION
In present study we have explored the correlation between COPD with BMI and SpO2. In this study the majority of subjects were males 139 (93.3%) and only 8 (6.7%) were females.
In the study, an inverse association between disease severity and SpO2 was seen and was observed that with increasing COPD stage the median SpO2 value decreased in a progressive manner [Figure 1].
Figure 1.
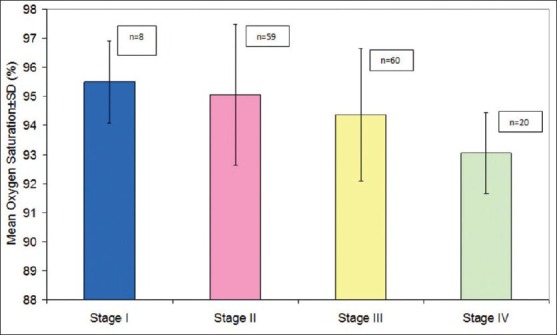
Association between GOLD, COPD stage and oxygen saturation. The mean SpO2 was 95.50 ± 1.41 in Stage I, 95.05 ± 2.42 in stage II, 94.37 ± 2.28 in stage III and 93.05 ± 1.39 in stage IV. Analysis of variance revealed a significant association between COPD stage and SpO2 (F = 4.723; P = 0.004)
In Stage I, the SpO2 was 95.50 ± 1.41 (range 94-98%) while in stage IV the mean SpO2 was 93.05 ± 1.39 (range 91-97%) [Table 1]. Analysis of variance revealed a significant association between COPD stage and SpO2. A study done by Rabe KF,[9] clearly showed that with deterioration of pulmonary function and disease progression the risk of alveolar hypoxia and consequent hypoxemia increases, which supports our result. Two large studies (UPLIFT and National Emphysema Treatment Trial) also revealed that proportion of patients with COPD were hypoxic and required oxygen therapy.[10,11] Hypoxemia associated with COPD results in poor quality of life, reduced exercise tolerance, poor neurocognitive function and finally increased risk of death.[12] A study done by Faganello MM et al.,[13] showed that lower peripheral SpO2 was risk factor for COPD exacerbation.
The possible explanation of reduced SpO2, which is closely related to hypoxemia, is due to ventilation/perfusion (V/Q) mismatch. The low V/Q ratio in subject with significant degree of airway disease with heterogeneous alveolar hypoventilation results in substantial perfusion of unventilated lung and consequent physiological shunt.[14] The high V/Q ratio results when there is increased ventilation of poorly perfused lung and hence increased physiological dead space.[15] V/Q mismatch due to pulmonary emphysema and small airways disease is measurable even in subjects with mild COPD[16] but appears to increase with the progression of disease.[17] Altered ventilatory control is another important factor responsible to the occurrence of hypoxemia in COPD patients. Subjects with chronic airflow obstruction have blunted ventilatory responses to hypoxia,[18] and this is particularly the case, in those with chronic hypoxemia.[19]
It can be inferred that with increasing severity of COPD and decreasing lung functions, the SpO2 is affected. These findings are interesting and provide a basis for future research for use of SpO2 as a marker of COPD severity.
The relationship between BMI and COPD stage was explored multidimensionally. All of them yielded a significant inverse association. The association between COPD stage and BMI category revealed that with increasing COPD stage the proportion of subjects with undernourished BMI status increased significantly. The overall prevalence of undernourished BMI was 57.8% and was minimum in stage I (25%) and maximum in stage IV (80%). A study done by Cochrane[20] with 103 stable out patients, of whom 23% were malnourished, reported an association between reduced nutritional intake/malnourishment and severe lung disease. In our study, high percentage of undernourished subjects as compared to study done by Cochrane WJ was observed probably because of difference in geographical distribution, dietary intake pattern in the two countries, and also because of low socioeconomic status, late disease detection and intervention, in our setup. In another study done by Gupta et al.,[21] COPD in general was found to be associated with malnutrition (83% patients being BMI <20 kg/m2) also supported our result, as their patients were admitted and had more severe form of disease.
In our study we have found that 66.3% of patients with severe and very severe COPD (stage III and IV) are undernourished [Figure 2]. Our result is supported by study done by Schols et al.,[22] where he found that approximately 50% patients suffered from weight loss in severe COPD. As mentioned earlier, the possible explanation for the higher prevalence of undernutrition in our set up could be due to lower socioeconomic strata, poor health care facility, late diagnosis and, intervention. We would like to emphasize here for an urgent need to provide proper nutritional counselling to patients with severe COPD as it has been proved that low BMI is an independent risk factor for mortality in subjects with severe COPD.[23]
Figure 2.
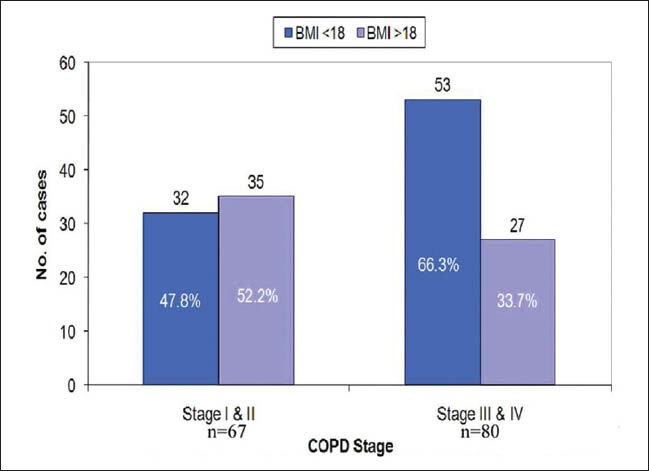
Association between GOLD, COPD stage and BMI. In stage (I+II) COPD, 47.8% of patients had BMI<18 and 52.2% had BMI>18 where as in stage (III +IV) COPD, 66.3 % of patients had BMI<18 and 33.7% had BMI>18
Similarly, the mean BMI was observed to be decreasing significantly with increasing age. The relationship with BMI was found to be holding similar trend with advancing COPD stages [Table 1]. All the associations were observed to be significant statistically too. Steuten et al.,[24] studied the prevalence of higher BMI in a large primary care population of patients with COPD in the Netherlands. The overall prevalence of high BMI in this population was 18%, with the highest prevalence in GOLD stages I and II (16% and 24%) and the lowest in GOLD stage IV (6%). Results in present study, followed the same trends. Rabinovich et al.,[25] too showed a positive association between BMI and lung functions, thereby indicating that patients with higher BMI had COPD of lower order. Similar observations were made in the Platino study by Montes de Oca et al.,[26] which focused on the problem of obesity and BMI but found that lung functions were more impaired in patients with low BMI as compared to those with higher BMI. Yang et al.,[27] too supported the similar findings and also found that low BMI was predicted as an indicator of mortality amongst the COPD patients, thus inferring that low BMI is an indicator of advanced stage of COPD. Harik-Khan et al.,[28] in their study showed that with increasing BMI the percentage of subjects with COPD progressively decreased, while the ratio of FEV1/FVC increased. All these findings are in consonance with the findings made in the present study.
The possible explanation of nutritional abnormality and weight loss is due to decreased caloric intake and increased basal metabolic rate.[22,29,30,31] Loss of muscle mass is main cause of weight loss in COPD patients, where loss of fat free mass contributes to lesser extent.[22] It has been seen that at a microscopic level, muscle fiber atrophy and alteration of fiber type can occur.[32] Plasma levels of certain pro-inflammatory cytokines like TNF-α are increased in COPD that can provoke muscle cell apoptosis and protein degradation via the ubiquitin/proteasome system leading to loss of muscle mass.[32,33] In most patients with COPD there is an imbalance between metabolic requirement and calorie intake leading to weight loss.[34]
Increased BMR may be due to use of β2 agonist.[35] Systemic inflammation could also play a significant role as shown by relationship between metabolic derangement and increased level of inflammatory mediators in COPD,[33] and tissue hypoxia may also make a contribution.[36] One reason for this association could be attributed to loss of metabolic functions owing to lack of SpO2. In a study by Rabinovich et al.,[25] it has been shown that there is a loss of mitochondrial function among the low BMI patients of COPD. They observed that in COPD patients with low BMI, the mitochondrial respiration was significantly lower as compared to those with normal BMI and their corresponding lung functions were also impaired. These findings indicate that due to unavailability of oxygen as a consequence of COPD the metabolic functions are impaired at cellular level thereby resulting in a subsequent loss in weight.
Considering the fact that BMI correlates with mortality in COPD patients as found in a study done by Gray et al.,[37] low BMI should be considered at greater risk of mortality. However, there are some studies including that of Labban et al.,[38] which has raised question mark on the use of BMI as a criterion for assessment of nutritional status on the premise that patients with COPD show a marked expansion of total body water that could mask the effects of malnutrition on body weight.
Although, a significant difference in mean SpO2 was observed with BMI category (P = 0.046), yet it was random in nature and did not show a linearity. It was observed that mean O2 saturation was maximum in BMI category 23.0-25.0 kg/m2 and minimum in BMI category <18.0 kg/m2 [Table 2].
In the multivariate analysis, we tried to explore the role of age, sex, smoking packs year and BMI with COPD stage but could get only significant association between BMI and COPD stage. One of the reasons of our inability to find any association with sex could be the disproportionate size of two genders in present study whereas smoking packs per year could not emerge as an associating factor as most of the patients were heavy smokers. However, emergence of BMI as an independent marker associated with COPD staging in both univariate as well as multivariate analysis was the most significant finding of the present study.
The findings in present study suggest a possible association between severity of COPD and BMI, although it is accepted that the design of the study had limitations and measurement of Fat Free Mass Index (FFMI), C-reactive protein (CRP), total protein, serum albumin and inflammatory biomarkers, which could not be done because of limited resources. Despite these limitations, the results of the present study emphasize the relationship between COPD and BMI. A larger study, keeping the limitations as mentioned above under control, is recommended. Apart from finding the association between COPD and BMI, the study also found a significant association between SpO2 and COPD stage which could be explored further in order to suggest an additional marker of COPD severity that would add a new dimension to the traditional clinical tool in assessment and management of COPD.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Celli BR, MacNee W. ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 2.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease NHLBI/WHO Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) work-shop summary. Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 3.Satta A, Migliori GB, Spanevello A, Neri M, Bottinelli R, Canepari M, et al. Fiber types in skeletal muscles of chronic obstructive pulmonary disease patients related to respiratory function and exercise tolerance. Eur Respir J. 1997;10:2853–60. doi: 10.1183/09031936.97.10122853. [DOI] [PubMed] [Google Scholar]
- 4.Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1791–7. doi: 10.1164/ajrccm.157.6.9705017. [DOI] [PubMed] [Google Scholar]
- 5.Eid AA, Ionescu AA, Nixon LS, Lewis-Jenkins V, Matthews SB, Griffiths TL, et al. Inflammatory response and body composition in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1414–8. doi: 10.1164/ajrccm.164.8.2008109. [DOI] [PubMed] [Google Scholar]
- 6.Toral Marín J, Ortega F, Cejudo P, Elías T, Sánchez H, Montemayor T. Peripheral muscle strength in stable COPD patients: Correlation with respiratory function variables and quality of life. Arch Bronconeumol. 1999;35:117–21. [PubMed] [Google Scholar]
- 7.Pakhale SS, Jayant K, Bhide SV. Chemical analysis of smoke of Indian cigarettes, bidis and other indigenous forms of smoking–levels of phenol, hydrogen cyanide and benzo(a)pyrene. Indian J Chest Dis Allied Sci. 1990;32:75–81. [PubMed] [Google Scholar]
- 8.Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D, et al. Concensus Group. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–70. [PubMed] [Google Scholar]
- 9.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD Executive summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 10.Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–54. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 11.Martinez FJ, Foster G, Curtis JL, Criner G, Weinmann G, Fishman A, et al. NETT Research Group. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med. 2006;173:1326–34. doi: 10.1164/rccm.200510-1677OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim V, Benditt JO, Wise RA, Sharafkhaneh A. Oxygen therapy in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:513–8. doi: 10.1513/pats.200708-124ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faganello MM, Tanni SE, Sanchez FF, Pelegrino NR, Lucheta PA, Godoy I. BODE index and GOLD staging as predictors of 1-year exacerbation risk in chronic obstructive pulmonary disease. Am J Med Sci. 2010;339:10–4. doi: 10.1097/MAJ.0b013e3181bb8111. [DOI] [PubMed] [Google Scholar]
- 14.Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: Cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis. 2011;6:199–208. doi: 10.2147/COPD.S10611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner PD, Dantzker DR, Dueck R, Clausen JL, West JB. Ventilation perfusion inequality in chronic obstructive pulmonary disease. J Clin Invest. 1977;59:203–16. doi: 10.1172/JCI108630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbera JA, Ramirez J, Roca J, Wagner PD, Sanchez-Lloret J, Rodriguez-Roisin R. Lung structure and gas exchange in mild chronic obstructive pulmonary disease. Am Rev Respir Dis. 1990;141:895–901. doi: 10.1164/ajrccm/141.4_Pt_1.895. [DOI] [PubMed] [Google Scholar]
- 17.Sandek K, Bratel T, Hellström G, Lagerstrand L. Ventilation-perfusion inequality and carbon dioxide sensitivity in hypoxaemic chronic obstructive pulmonary disease (COPD) and effects of 6 months of long-term oxygen treatment (LTOT) Clin Physiol. 2001;21:584–93. doi: 10.1046/j.1365-2281.2001.00360.x. [DOI] [PubMed] [Google Scholar]
- 18.Flenley DC, Franklin DH, Millar JS. The hypoxic drive to breathing in chronic bronchitis and emphysema. Clin Sci. 1970;38:503–18. doi: 10.1042/cs0380503. [DOI] [PubMed] [Google Scholar]
- 19.Bradley CA, Fleetham JA, Anthonisen NR. Ventilatory control in patients with hypoxemia due to obstructive lung disease. Am Rev Respir Dis. 1979;120:21–30. doi: 10.1164/arrd.1979.120.1.21. [DOI] [PubMed] [Google Scholar]
- 20.Cochrane WJ, Afolabi OA. Investigation into the nutritional status, dietary intake and smoking habits of patients with chronic obstructive pulmonary disease. J Hum Nutr Diet. 2004;17:3–11. doi: 10.1046/j.1365-277x.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- 21.Gupta B, Kant S, Mishra R. Subjective global assessment of nutritional status of chronic obstructive pulmonary disease patients on admission. Int J Tuberc Lung Dis. 2010;14:500–5. [PubMed] [Google Scholar]
- 22.Schols AM, Soeters PB, Dingemans AM, Mostert R, Frantzen PJ, Wouters EF. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis. 1993;147:1151–6. doi: 10.1164/ajrccm/147.5.1151. [DOI] [PubMed] [Google Scholar]
- 23.Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1856–61. doi: 10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- 24.Steuten LM, Creutzberg EC, Vrijhoef HJ, Wouters EF. COPD as a multicomponent disease: Inventory of dyspnoea, underweight, obesity and fat free mass depletion in primary care. Prim Care Respir J. 2006;15:84–91. doi: 10.1016/j.pcrj.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabinovich RA, Bastos R, Ardite E, Llinàs L, Orozco-Levi M, Gea J, et al. Mitochondrial dysfunction in COPD patients with low body mass index. Eur Respir J. 2007;29:643–50. doi: 10.1183/09031936.00086306. [DOI] [PubMed] [Google Scholar]
- 26.Montes de Oca M, Tálamo C, Perez-Padilla R, Jardim JR, Muiño A, Lopez MV, et al. PLATINO Team. Chronic obstructive pulmonary disease and body mass index in five Latin America cities: The PLATINO study. Respir Med. 2008;102:642–50. doi: 10.1016/j.rmed.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 27.Yang L, Zhou M, Smith M, Yang G, Peto R, Wang J, et al. Body mass index and Chronic obstructive pulmonary disease-related mortality: A nationally Representative prospective study of 220,000 men in China. Int J Epidemiol. 2010;39:1027–36. doi: 10.1093/ije/dyq051. [DOI] [PubMed] [Google Scholar]
- 28.Harik-Khan RI, Fleg JL, Wise RA. Body mass index and the risk of COPD. Chest. 2002;121:370–6. doi: 10.1378/chest.121.2.370. [DOI] [PubMed] [Google Scholar]
- 29.Schols AM. Nutrition in chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2000;6:110–5. doi: 10.1097/00063198-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Engelen MP, Wouters EF, Deutz NE, Does JD, Schols AM. Effects of exercise on amino acid metabolism in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:859–64. doi: 10.1164/ajrccm.163.4.2006137. [DOI] [PubMed] [Google Scholar]
- 31.Engelen MP, Schols AM, Does JD, Gosker HR, Deutz NE, Wouters EF. Exercise-induced lactate increase in relation to muscle substrates in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:1697–704. doi: 10.1164/ajrccm.162.5.9910066. [DOI] [PubMed] [Google Scholar]
- 32.Kim HC, Mofarrahi M, Hussain SN. Skeletal muscle dysfunction in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3:637–58. doi: 10.2147/copd.s4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schols AM, Buurman WA, Staal van den Brekel AJ, Dentener MA, Wouters EF. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax. 1996;51:819–24. doi: 10.1136/thx.51.8.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schols AM, Wouters EF. Nutritional abnormalities and supplementation in chronic obstructive pulmonary disease. Clin Chest Med. 2000;21:753–62. doi: 10.1016/s0272-5231(05)70182-9. [DOI] [PubMed] [Google Scholar]
- 35.Amoroso P, Wilson SR, Moxham J, Ponte J. Acute effects of inhaled salbutamol on the metabolic rate of normal subjects. Thorax. 1993;48:882–5. doi: 10.1136/thx.48.9.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sridhar MK. Why do patients with emphysema lose weight? Lancet. 1995;345:1190–1. doi: 10.1016/s0140-6736(95)91984-8. [DOI] [PubMed] [Google Scholar]
- 37.Gray-Donald K, Gibbons L, Shapiro SH, Martin JG. Effect of nutritional status on exercise performance in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1989;140:1544–8. doi: 10.1164/ajrccm/140.6.1544. [DOI] [PubMed] [Google Scholar]
- 38.Laaban JP, Kouchakji B, Dore MF, Orvoen-Frija E, David P, Rochemaure J. Nutritional status of patients with chronic obstructive pulmonary disease and acute respiratory failure. Chest. 1993;103:1362–8. doi: 10.1378/chest.103.5.1362. [DOI] [PubMed] [Google Scholar]


