Abstract
Auditory temporal processing deficits have been suggested to play a causal role in language learning impairments, and evidence of cortical developmental anomalies (microgyria (MG), ectopia) has been reported for language-impaired populations. Rodent models have linked these features, by showing deficits in auditory temporal discrimination for rats with neuronal migration anomalies (MG, ectopia). Since evidence from human studies suggests that training with both speech and non-speech acoustic stimuli may improve language performance in developmentally language-disabled populations, we were interested in whether/how maturation and early experience might influence auditory processing deficits seen in male rats with induced focal cortical MG.
Results showed that for both simple (Normal single tone), as well as increasingly complex auditory discrimination tasks (Silent gap in white noise and FM sweep), prior experience significantly improved acoustic discrimination performance -- in fact, beyond improvements seen with maturation only. Further, we replicated evidence that young adult rats with MG were significantly impaired at discriminating FM sweeps compared to shams. However, these MG effects were no longer seen when experienced subjects were retested in adulthood (even though deficits in short duration FM sweep detection were seen for adult MG rats with no early experience). Thus while some improvements in auditory processing were seen with normal maturation, the effects of early experience were even more profound, in fact resulting in amelioration of MG effects seen at earlier ages.
These findings support the clinical view that early training intervention with appropriate acoustic stimuli could similarly ameliorate long-term processing impairments seen in some language-impaired children.
Keywords: Auditory temporal processing, Learning impairment, Plasticity, Cortical developmental pathology, Dyslexia
1
Language related learning disabilities such as dyslexia and specific language impairment (SLI) represent two of the most common forms of developmental learning disabilities, affecting approximately 5-15% of the population (Bishop, 1997). Children with specific language impairment (delay in language acquisition/production) and dyslexia (specific reading impairment) show deficits in processing rapid changes in acoustic stimuli, and these deficits have been suggested to contribute to a failure in grapheme to phoneme representation during language learning (Miller-Shaul, 2005; Tallal, 2004). Further, studies show that early temporal processing indices are a strong predictor of subsequent language performance in both normal and at risk populations (Walker et al., 2006; Benasich et al., 2002, 2006; Choudhury et al., 2007). In parallel, research focused on identifying specific neuropathological substrates of language-related disabilities has revealed the presence of cortical developmental disruptions (microgyria, characterized by abnormal neocortical enfolding and lamination, and ectopic collections of improperly migrated neurons) in adult dyslexic brains examined postmortem (Galaburda et al., 1985), as well as in individuals with SLI using MRI (Guerreiro et al., 2002; Hage et al., 2006). The two lines of work presented above outline the progress that has been made in identifying factors that may contribute to developmental language impairments. However, significant limitations in technology and tools for clinical evaluation make it difficult to investigate the complex interactions between neurodevelopmental anomalies and profiles of language pathology in humans (Fitch and Tallal, 2003). Therefore, rodent models of cortical developmental pathology, comparable to anomalies seen in the brains of language-impaired individuals, have been used to address this issue. Specifically, rodent models of cortical developmental disruption (microgyria, ectopia, or heterotopia in neocortex) have consistently revealed rapid auditory processing impairments in male subjects, similar to those identified in language-impaired populations (Peiffer et al., 2004b, 2002a). That is, studies have repeatedly shown specific deficits in processing rapid and/or complex acoustic stimuli as a consequence of perinatal disruption to neuronal migration in male rats and mice (Threlkeld et al. 2006, 2007; Peiffer et al., 2004, 2002a; Clark et al., 2000b; Fitch et al., 1994) and these results parallel a higher incidence of neurodevelopmental disabilities in human males as compared to females (Rutter et al., 2003; Liederman et al., 2005). Convergent data thus indicate a link between perinatal cortical pathology (particularly involving neuronal migration), and subsequent auditory processing impairments (which may in turn contribute to language disruption in humans). As such, rodent models provide a valuable springboard to address complex questions involving the developmental trajectory and possible remediation of auditory processing deficits, which may help identify deficient processes and unique remediation strategies for humans with developmental language disabilities. Importantly, the tasks used to evaluate temporal processing in language impaired populations, and tasks used in rodent models of cortical developmental pathology, include key commonalities such as the presentation of variable duration silent gaps in white noise (a basic temporal processing task), as well as tone pair and FM sweep discrimination tasks (more spectrally and temporally demanding; Tallal et al., 2004; Fitch et al., 2008a). In both human and rodent paradigms, these tasks allow for the manipulation and detailed assessment of auditory temporal and spectral processing capabilities.
One question that is difficult to address in human studies of developmental language disability involves the relationship between auditory developmental maturation and auditory training, and the relative benefits of each to performance in the auditory temporal processing domain. Human studies evaluating language-impaired individuals have shown age related improvements in auditory temporal processing. For example, Hautus and associates (2003) found that children ages six to nine with reading impairments had significantly worse auditory temporal processing thresholds as compared to controls. However, individuals ages 10 to adult did not show the same impairments. Further, studies evaluating children and adults with SLI have revealed impairments in frequency discrimination in young but not older SLI individuals (McArthur and Bishop, 2004). Further, parallel findings have been observed in normal rodents and rodent models of cortical developmental pathology (e.g., postnatal day 1 (P1) induced MG). In particular, normal rats have been shown to significantly improve their detection of silent gaps in white noise as a function of age (ranging from P15 to P100), using modified acoustic startle procedures (Freedman et al., 2004; Dean et al., 1990). Moreover, the same profile of improvement in temporal processing seen in humans with language impairment has also been observed in juvenile and adult rats with cortical developmental pathology. Specifically, Peiffer and colleagues (2004) showed that juvenile rats (P24) with MG were impaired relative to shams at detecting short silent gaps imbedded in broadband white noise (7-10 ms). However, assessment of inexperienced adult sham and MG littermates showed no impairments in silent gap detection, suggesting that gap detection deficits may resolve with normal maturation. However, additional studies evaluating rodents with cortical developmental pathology have shown that more complex acoustic tasks presented in adulthood are still able to elicit temporal processing deficits, suggesting that maturation may shift deficits from easier to more difficult tasks (Clark et al., 2000a; Peiffer et al., 2002; 2004; Threlkeld et al., 2006; Tallal, 2004).
In addition to studying the effects of normal maturation on auditory processing in the presence or absence of cortical developmental pathology and language disorders, rodent models and human studies have sought to identify the influence of acoustic training on temporal processing, with one long-term application being the identification of auditory based remediation strategies for populations with language pathology. For example, Friedman and associates (2004) and Dean and associates (1990) both showed that, across five and three days of testing respectively on a silent gap procedure, intact rats significantly improved their gap detection performance across sessions - suggesting that repeated exposure to temporal processing tasks can improve performance. Additional work evaluating the effects of long-term exposure to temporal processing tasks in rodent models of developmental pathology is sparse (Peiffer et al., 2002a; Threlkeld et al., 2006). However, work evaluating the effects of auditory-based training has revealed changes in brain activation patterns, as well as improvements in speech processing in language impaired individuals (Tallal et al., 1996; Merzenich et al., 1996; Temple et al., 2003, 2000; Gaab et al., 2007). It thus appears that developmental maturation and auditory training both influence temporal processing and long-term language outcome in humans, and similar influences seem to act on auditory temporal processing in animal models of cortical developmental pathology (Habib, 2003; Fitch et al., 2008a; Hautus et al., 2003; Peiffer et al., 2004a; Freedman et al., 2004). However, while maturation and auditory experience (or training) may be central factors in the influence of auditory temporal processing and language outcome in human learning impaired populations, it is difficult to parse out the degree of influence each has on long-term processing outcome. Therefore, we set out to determine whether and how variables of normal maturation as well as early auditory processing experience would influence RAP deficits previously reported for rats with induced focal MG (similar to anomalies seen in human language impaired populations). We predicted that both increased age and early experience would lead to better processing outcomes in both microgyric and sham animals, and that the greatest improvements would be seen with early experience across maturation as compared to maturation alone.
2. Experimental procedures
2.1. Subjects
Subjects were 43 male Wistar rats born to time-mated dams (Charles River Laboratory, Wilmington, MA) at the University of Connecticut. Male subjects were assessed given prior evidence of auditory temporal processing deficits in male but not female rodents with neuronal migration anomalies (Peiffer et al., 2002a; 2004b), findings that parallel higher diagnostic rates of neurodevelopmental disorders (including dyslexia, epilepsy, autism and mental retardation) in males as compared to females (Rutter et al., 2003; Liederman et al., 2005). Subsets of pups received either a cortical plate focal freezing lesion (producing microgyria; MG) or sham procedure (see below) on postnatal day one (P1) and were culled into litters of 10 (8 males and 2 females, for details see Threlkeld et al., 2006). Male subjects (N= 43) were right or left ear marked and housed into pairs at P21 using a 12:12 light/dark cycle with food and water available ad libitum. Prior to behavioral testing, two different groups were assigned each including subjects from the sham and MG conditions. We sought to control for litter effects by assigning pups from each litter to each study condition. Thus at culling (P1), male subjects within each litter were randomly selected to receive either bilateral freezing lesion, or sham surgery. Subsequently at weaning (P21), approximately half of the subjects from each litter were selected to receive behavior testing in Juvenile/Young adulthood (G1A) and adulthood (G1B), while the remainder of the pups from a given litter were selected for adult testing only (G2). Group one (G1; sham n=10, MG n=11) received testing during both Juvenile/Young adulthood (P23-101, G1A) and also in the Adult (P+111, G1B) period (early experience). Group Two (G2; sham n=12, MG n=10) only received auditory testing as adults (adult without early experience, to assess maturation effects (see figure 1)). All procedures were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, including adequate measures to minimize pain and discomfort. The Institutional Animal Care and Use committee (IACUC) at the University of Connecticut approved all procedures.
Fig. 1.
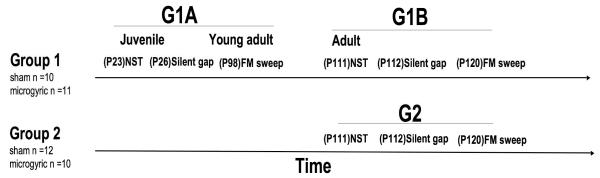
Timeline for behavior testing showing all testing procedures, test groups and age of testing (normal single tone (NST), silent gap detection, FM sweep detection, and juvenile/young adult (G1A), adult with early experience (G1B), adult without early experience (G2) and postnatal day (P)).
2.2. Induction of P1 freezing lesion (microgyria)
On the day of surgery (P1), litters were culled to 10 pups (8 male, 2 female), with male pups randomly assigned to receive double-pair freezing lesion (MG) or sham surgery. Females were retained to equalize litter size and avoid all-male litters. Prior to surgery, subjects were cryogenically anesthetized using crushed ice. Surgeries involved a 1 mm incision, followed by the placement of a 2 mm stainless steel probe cooled to −70°C on the skull-cap. Focal lesions were induced as bilateral pairs (two to each hemisphere) as previously described (Peiffer et al., 2004). This procedure has been shown to lead to the formation of cortical MG (Humphreys et al., 1990, Dvorak and Feit, 1977). Sham subjects received similar treatment, but with a room temperature probe. After surgery, pups were individually marked with footpad ink injections, warmed under a lamp and placed back with the mother until weaning at P21.
2.3. Behavioral testing: startle reduction
Auditory testing for group one (G1) began on P23 (G1A, juvenile/young adult period) and lasted until P101 (sham n=10, MG n=11). Starting on P111, G1 received an additional battery of auditory testing (G1B, adults with early experience), along with group two (G2), which was tested for the first time as adults (adults without early experience, sham n=12, microgyric n=10). Auditory testing involved a startle response paradigm that has been discussed extensively elsewhere (also called pre-pulse inhibition (PPI), see Fitch et al., 2008a; Peiffer et al., 2002b, 2004). Briefly, the startle modification paradigm involves the presentation of an auditory cue prior to a startle-eliciting stimulus (SES). The SES elicits an acoustic startle reflex (ASR) and if the preceding auditory cue is detected, the intensity of the ASR is reduced accordingly. In the current experiments, the SES was a 105 dB, 50 ms white noise burst. The most basic version of this task used a 75 db, 7 ms, 2300 Hz tone pre-stimulus. Relative comparison between the ASR amplitude in the presence (cued trial) and absence (uncued trial) of a pre-stimulus represents an objective measure of sensory detection (Marsh et al., 1975). The duration of each trial between each SES varied between 16-24 s to eliminate subjects’ ability to predict stimulus onset.
2.3.1. Apparatus
For testing, subjects were placed on a load cell platform (Med Associates, Georgia, VT, USA), which measured the subject’s ballistic motor response to the SES in mV. Signals were acquired and passed through a linear load cell amplifier (PHM-250-60) into a Biopac MP100WS acquisition system (Biopac Systems, Santa Barbra, CA) connected to two Macintosh computers, which recorded the subject’s movement and ASR as a mV signal. The maximum peak value defining the ASR for each trial was extracted by algorithm from the 200 ms following the onset of the SES, and this ASR represents a dependent variable. Auditory stimuli were generated using a Pentium 4 Dell PC with custom programmed software and a Tucker Davis Technologies (RP2) real time processor. Stimulus files were played through a Niles SI-1260 amplifier (Niles Audio Corporation, Miami, FL) connected to nine Cambridge Sound Works speakers (MC105), with sound levels calibrated by sound-level meter (Peiffer et al., 2002b). Each pair of platforms had one speaker centered and mounted 50 cm above. Attenuated response scores (ATT) were calculated from the peak ASR using the formula ([mean cued response/mean uncued response] × 100). In this formula, absolute response scores (as measured by load-cell displacement for each subject’s startle response) for cued and uncued trials are expressed as a ratio and multiplied by 100, thus ATT scores represent a percentage. ATT scores were analyzed as a second dependent variable for all tasks.
2.3.2. Single tone procedure
Pre-pulse inhibition or normal single tone startle paradigms are commonly used to assess sensory-motor gating (Fitch et al., 2008). In the current paradigm the single tone task was used to assess basic auditory acuity and pre-pulse inhibition prior to the evaluation of more complex temporal processing (e.g., Silent gap and FM sweep discrimination; analogous to auditory temporal tasks used to test language learning impaired populations; Tallal et al., 2004). The normal single tone test (NST) session was comprised of 104 trials (cued or uncued), presented in a pseudo-random order. Uncued trials consisted of a silent background followed by the 105 dB, 50 ms SES. On cued trials a 75 dB, 7 ms, 2300 Hz tone was presented 50 ms prior to the SES. Trials were variable in duration (16 – 24 sec, 20 sec on average). The G1A group received NST as the initial (1 day) auditory test on P23 and again on the first day of testing in adulthood (G1B, 1 day of testing on P111), to assess baseline PPI in the juvenile period and experience effects in adulthood. The G2 group also received the NST task (1 day) when tested for the first time as adults (P111). The first NST test session was used as a covariate for all additional acoustic analysis, to account for any variation in baseline startle between subjects. This is important, since our primary interest was to ascertain effects of age and experience on the discrimination of the pre-pulse cue, not differences in PPI per se.
2.3.3. Silent gap procedure
A silent gap procedure (similar to single tone) was utilized to assess simple auditory temporal processing (a commonly used tool for this purpose in human populations and rodent models; Threlkeld et al., 2006, Smith et al., 2006). Juvenile/Young adult subjects (G1A) were test for a total of 13 days on silent gap detection tasks. The first day of juvenile (G1A, P26) silent gap testing was used for analysis. Adults (G1B and G2, P112) only received one day of silent gap testing. Each session included 300 trials, each consisting of the presentation of a variable duration silent gap (0, 2, 5, 10, 20, 30, 40, 50, 75, or 100 ms) embedded in continuous 75 dB broadband white noise. The gap was presented 50 ms prior to a 105 dB burst of white noise. The uncued trials used a “gap” of 0 ms. The cue-burst interval for each task was maintained at 50 ms (Friedman et al., 2004; Peiffer et al., 2004). As before, G1 was tested on this task in the juvenile/young adult period (G1A; 3 days of testing) and again in the adult period (G1B), whereas G2 was only tested in adulthood (one day after the NST procedure).
2.3.4 FM sweep procedure
An FM sweep discrimination task was used to assess complex auditory temporal processing capabilities. The FM sweep discrimination task provides increasing processing demand beyond that of more basic silent gap detection and shares similarities to frequency shifts seen in phonemic sweeps (Fitch et al., 2008; Tallal, 2004). FM sweep sessions comprised of 104 trials, and a total of four sessions (one per day across 4 days) was also presented. G1 was exposed to this task in young adulthood (G1A; four days of testing starting on P98) and retested in the adult (G1B) period (four days of testing starting on P120). Conversely, G2 was presented with the FM sweep battery for the first time in adulthood, following silent gap testing (four days of testing starting on P120). The procedure involved the repeated presentation of a background 75 dB, downward FM sweep (2300-1900 Hz) separated by a within-stimulus inter-stimulus interval (ISI) of variable duration (125, 75, 50, 25 ms; one interval used per session). Each sequence was separated by a between sequence ISI, which was always 200 ms greater than the sweep duration. On uncued trials, the last FM sweep was followed by 50 ms of silence, followed by the 105 dB, 50 ms SES. On cued trials, an upward FM sweep (the reversal of the standard sweep, 1900-2300 Hz), was followed by 50 ms of silence and then the SES.
2.5 Histology
At the end of behavioral testing, subjects were weighed, anesthetized with ketamine/xylazine (100/15 mg/kg), and transcardially perfused with saline followed by 10% phosphate buffered formalin. The brains were removed, lesions were visually confirmed (appearing as indentations (microgyria) on the surface of the neocortex), and the location verified. All the subjects identified as microgyric showed evidence of focal cortical malformations, and no such malformations were seen in sham brains.
2.6 Statistics
For auditory testing procedures, ATT scores – the dependent variable – were compared using repeated measures ANOVAs between the following three Groups: G1A (juvenile/young adult); G1B (adult with early experience); and G2 (adult without early experience). Effects of Treatment (MG, sham) were analyzed within and between each testing condition (G1A, G1B & G2). For auditory processing measures, ATT scores from the first test experience on NST (G1A and G2) were used as a covariate, to account for individual differences in baseline pre-pulse inhibition.
3. Results
3.1. Neuromorphology results
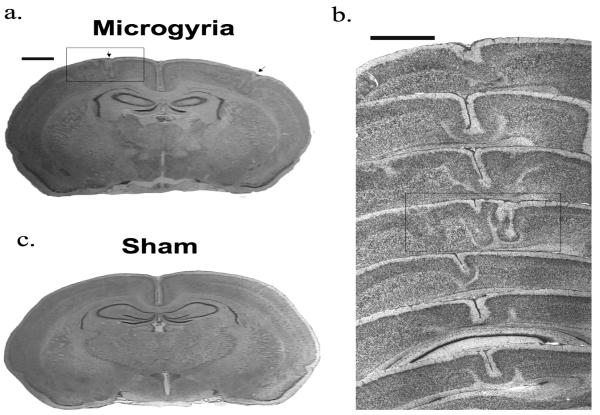
Analysis of post mortem brains revealed consistent location and size of microgyric malformations for all of the subjects that received P1 freezing lesion treatment. Malformations were primarily observed in sensorimotor cortex (SM-1), with some extension into frontal, temporal, and occipital cortices (Paxinos, 2004). None of the sham subjects showed any cortical malformations (see figure 2).
Fig. 2.
Photomicrographs of (a) typical bilateral microgyric malformations; (b) overlaid sections showing the extent of microgyria in one hemisphere (box shows section from whole brain, a., scale bars 500 μm); and (c) sham section.
3.2. Auditory discrimination results
3.2.1 Single tone, Juvenile (G1A, P23) and adult (G1B & G2, P111)
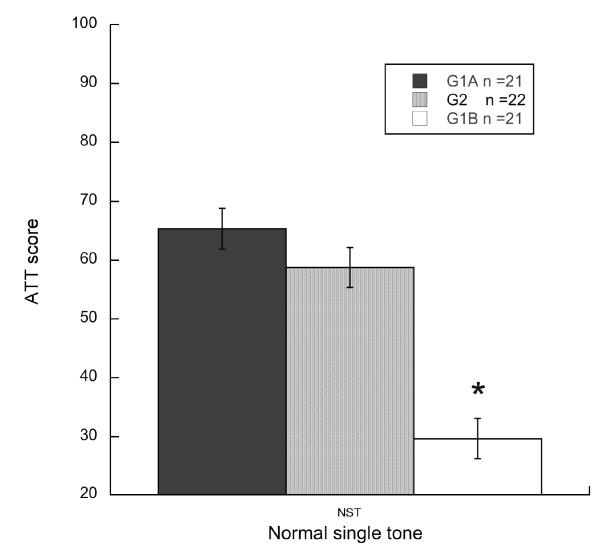
Significant differences were observed between cued and uncued absolute response amplitude scores for all groups as evidenced by paired samples t-test (p < .05), thus indicating significant discrimination of the single tone across all testing sessions. Results for one-way ANOVAs revealed a significant effect of Early Experience, (measured between juveniles, (G1A) and adults without early experience (G2) versus G1B (adults with early experience)), [F(1,40) = 67.04, p < .001] and [F(1,41) = 35.1, p < .001] respectively). Put another way, early experience (G1B) led to a 35% improvement in detection relative to naive juvenile subjects (G1A), and a 29% improvement compared to adults without early experience (G2). There was no significant effect of Age (G1A vs. G2, p > .05) suggesting that, unlike prior experience, normal maturation did not greatly influence baseline PPI for a simple tone (see figure 3).
Fig. 3.
Histograms showing normal single tone scores for the three test groups (juvenile (G1A), adult with early experience (G1B) & adult without early experience (G2). Significant differences were seen between G1B (adult group with early experience) and both juveniles (G1A) and adults without early experience (G2). Note: *p < .01.
3.2.2 Silent gap, juvenile (G1A, P26) and adult (G1B & G2, P112)
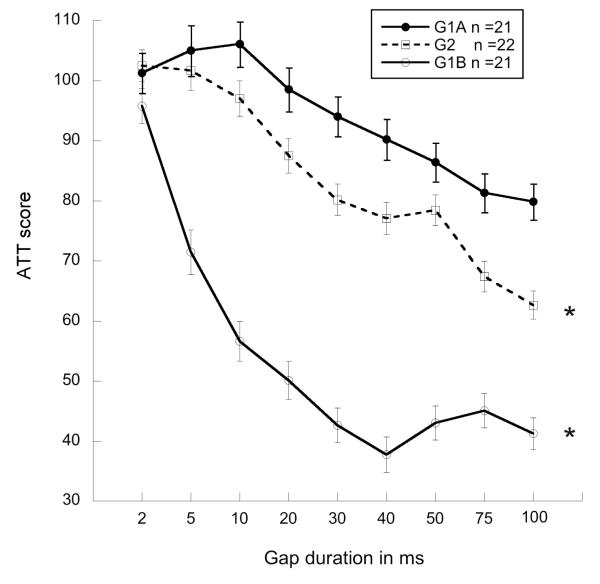
Results from repeated measures ANOVAs, for each comparison revealed a significant effect of age (G1A vs. G2, maturation without early experience) [F(1,40) = 85.1, p < .001], and an even greater effect of early experience (G1B vs. G2) [F(1,40) =10.5, p < .0001], indicating that prior acoustic experience improves gap detection beyond improvements of normal maturation (see figure 4). No treatment effects were seen on the silent gap detection task. Again, comparisons of percent improvement between each group revealed 30% better (lower) ATT scores in adults with early experience (G1B) as compared to adults without early experience (G2), while adults without early experience (G2) showed only a 10% improvement over juvenile subjects (G1A) for all gap durations. Thus, early experience led to approximately 3x greater improvement in performance relative to normal maturation alone, for this task.
Fig. 4.
Line graph showing attenuation scores on gap detection for the three test groups (G1A (juvenile), G1B (adult with early experience) & G2 (adult without early experience). Comparisons show an effect of Age between G1A and G2 (*p < .01), and a greater effect of Experience between G1B and G2 (*p < .001; Values reflect covariance to normal single tone (NST)).
3.2.3 FM sweep, young adult (G1A, P98-101) and adult (G1B & G2, P120-123)
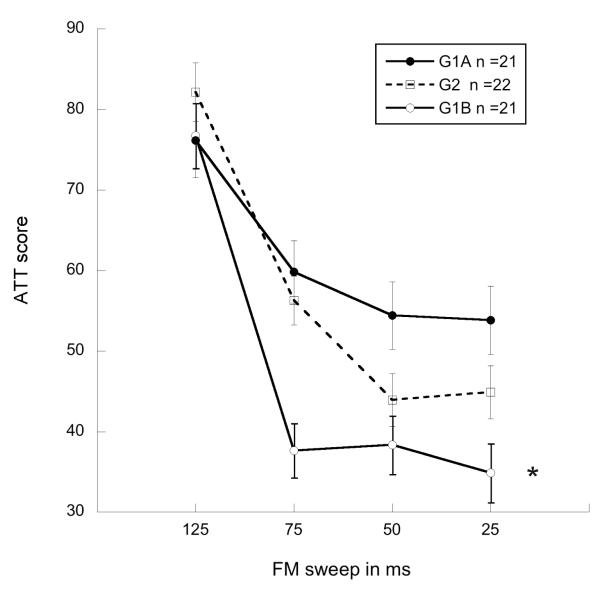
Results from a 2 (Treatment) × 4 (ISI) repeated measures ANOVA for G1A revealed a significant effect of treatment [F(1,18) = 8.3, p < .01], with young MG subjects performing significantly worse than young shams (see figure 5a). However, a 2 (Treatment) × 4 (ISI) repeated measures ANOVA for G1B (adults with early experience) no longer showed a significant effect of treatment [F(1,18) = .03, p = ns] (see figure 5b), indicating that treatment effects were ameliorated in experienced adults. In contrast, results from a Multivariate ANOVA for G2 revealed a near-significant effect of treatment at the shortest (25 ms) fm sweep duration [F(1, 21) = 2.43, p = .07 one-tail](microgyric worse than sham), suggesting persistent deficits in adults without early experience (see figure 5c). Further, examination of G1A and G2 performance combined on the FM task, using a 2 (Treatment) × 2 (Group) × 4 (ISI, 125, 75, 50, 25 ms) repeated measures ANOVA revealed an overall significant effect of treatment [F(1,38) =4.1, p < .05], again with MGs performing worse than shams. These results indicate that rapid auditory processing deficits were present in both adults without early experience (G2; Figure 5c) and in juvenile/young adults (G1A; Figure 5a), but not adults with early experience (G1B; Figure 5b). Further analysis using a 2 (Treatment) × 2 (Group; G1A & G2) × 2 (Short ISI; 50, 25 ms) repeated measures ANOVA again revealed a significant main effect of treatment [F(1,38) = 4.6, p < .05] (microgyric worse than sham), and also showed an effect of group [F(1,38) = 5.2, p < .05], with adults better than juveniles. These results indicate that, despite a failure to ameliorate MG deficits, maturation did improve performance overall for adult microgyric rats relative to younger microgyric subjects. An additional repeated measures ANOVA using 2 (Group; G1B, G2) × 4 (ISI) design, revealed a significant effect of early experience [F(1,38) = 7.4, p < .01] on FM sweep detection across four ISIs, indicating that prior auditory experience improved FM sweep detection, and appeared to ameliorate RAP deficits observed in young microgyrics beyond improvements seen with normal maturation (see figure 6). Again, differences in percent detection illustrated the significant advantages of early experience and maturation on rapid FM sweep (50-25 ms) detection, with early experienced (G1B) subjects having shown 16% lower (better) ATT scores as compared to juveniles (G1A), and adults without early experience (G2) having shown 9% better performance compared to juveniles (G1A) for 50 and 25 ms FM sweeps.
Fig. 5.
Line graphs showing attenuation scores on FM sweep detection for sham and microgyric rats for: G1A (juvenile/young adult; 5a); G1B (adult with early experience; 5b); and G2 (adult with no early experience, 5c). Within-group comparisons revealed a significant Treatment effect in juvenile/young adults (G1A, *p < .01, microgyric worse than sham; 5a). No persistent Treatment effect was seen, however, when these subjects were tested again in adulthood (G1B; 5b). Comparably aged adults with no prior testing experience (G2) did, however, show a near-significant effect of Treatment at the shortest (25 ms) sweep duration (#p=.07, one-tailed, microgyric worse than sham; 5c). Moreover, an overall effect of Treatment was significant at the 50 and 25 ms sweeps (microgyric worse than sham) when naive juveniles (G1A) and naive adults (G2) were analyzed together (p<.01). Note that all values reflect covariance to NST.
Fig. 6.
Line graph showing attenuation scores on FM sweep detection for the three test groups (juvenile/young adult (G1A), adult with early experience (G1B) & adult without early experience (G2). Comparison shows a significant effect of Early Experience as compared to Age (*p < .01; values reflect covariance to NST).
4. Discussion
4.1.1 Auditory processing
It is difficult to dissociate the relative effects of maturation and experience when tracking clinical patients across extended periods of time, especially in those populations at risk for learning disabilities. However, using a rodent model of developmental learning impairment we were able to show a robust effect of early experience, over and above processing improvements resulting from a normal developmental trajectory (i.e., maturation). In the current series of experiments we showed that prior experience can improve performance by as much as three fold relative to improvements seen with normal maturation across simple (Normal single tone) and increasingly complex auditory discrimination tasks (Silent gap and FM sweep detection). Further, we have shown that auditory processing deficits observed in younger rats with developmental cortical malformations (MG) ameliorate following early auditory discrimination experience. We also observed that adult rats with no early auditory experience performed better than juveniles, presumably as a result of normal maturational trajectory. These results are similar to those reported by Peiffer and associates (2004), who found that while juvenile microgyric (MG) rats showed an impairment in the detection of short silent gaps as compared to shams, naïve adult microgyric subjects no longer showed impairments on this rapid but relatively simple task (Peiffer et al., 2004). In the current study, MG processing deficits were found to persist in the adult group with no early experience (G2) on the more complex rapid FM sweep task. Evidence for this derives from the analysis of combined juvenile/young adult (G1A), and adult (G2) subjects with no early experience, on short duration FM sweep detection, which in turn showed a significant effect of treatment (MG worse than sham) within both groups. Thus, while some improvements in rapid auditory processing were seen with age, presumably as a consequence of normal developmental maturation, the effects of experience were more profound, and appeared to ameliorate treatment effects seen between groups. These data support the notion that early intervention with acoustically driven temporal processing tasks might help to ameliorate RAP deficits seen in some humans with language learning disabilities.
It is important to consider the interpretation that testing experience in general altered behavioral performance in the early experience group, given that naive adults did not receive comparable handling during the early test period. However, several points counter this interpretation. First, the “non-auditory specific” experiences were limited, and were confined to tail-hold transfers into test cages. Non-tested counter-parts remained in their home tubs, but experienced the same disruptions in the housing room on test days (since all animals were housed in the same room). Moreover, all animals were pair housed and subject to cage cleaning and handling once a week, regardless of test schedule. Further, Although baseline PPI was altered in the early experience group relative to adults without early experience -- which may reflect effects of handling and/or general experience -- we specifically covaried for this measure, such that any general experience effects on PPI were covaried out of auditory temporal processing findings.
Finally, the current study utilized a double pair of lesions induced bilaterally in postnatal day one rat pups, leading to extensive cortical microgyria. It has been well documented that even single unilateral microgyric lesions produce spontaneous epileptiform activity around the lesion site, as well as changes in cortico-cortical and thalamo-cortical connectivity (Jacobs et al., 2000; Giannetti et al., 2000; Rosen et al., 2000). In addition, bilateral microgyria has been associated with more small and fewer large neurons in the medial geniculate nucleus of the thalamus in rats, and these findings parallel observed shifts in thalamic cell size (more small and fewer large cells in medial and lateral geniculate nuclei) in the brains of human dyslexics (Herman et al., 1997; Peiffer et al., 2002; Galaburda et al., 1985, 1994). While the current study did not directly address anatomical reorganization in MG subjects, our results do show that despite known changes in cortical circuits and physiology resulting from cortical developmental disruption, microgyric rodents with early auditory processing experience nevertheless were able to overcome temporal processing deficits relative to rats without early auditory processing experience. Future studies will hopefully seek to identify the relationship between potential cortical circuit reorganization after developmental disruption, and subsequent auditory processing impairments, as well as the intermediary effects of experience in ameliorating auditory temporal deficits.
4.1.2 Implications for auditory plasticity and auditory training in language learning impaired populations and rodent models
The robust effects of early experience on auditory processing seen in the current study indicate a highly plastic auditory system. Further, the amelioration of complex (FM sweep) rapid auditory processing deficits in microgyric subjects with early experience is impressive, given the pervasive disruptions to cortical circuitry and physiology that accompany this pathology. These data draw important parallels to the benefits of early auditory training in human language learning impaired populations. For example, Tallal and associates (1996) showed that after four weeks of daily exposure to acoustically modified speech, children with language learning disabilities (up to three years behind their chronological age in language development) had significantly improved their speech discrimination and language comprehension scores by two years, bringing them within normal levels. Similarly, Merzenich and associates (1996) found that language impaired children with deficits in identifying rapidly changing auditory stimuli improved their auditory processing to normal levels after four weeks of training with an adaptive FM frequency discrimination task and a phonetic recognition “two-alternative forced-choice task.” In contrast, language impaired individuals who underwent “classical language training” and comparable periods of non-adaptive video game play did not show comparable improvements in their ability to recognize complex sequenced stimuli (Merzenich et al., 1996). More recently, Ortiz-Mantilla and associates (2007) showed that infants who were passively exposed to rapid auditory stimuli showed increased processing efficiency for fast repeating auditory signals, the discrimination of which has been suggested to play a critical role in language acquisition.
Importantly, the current battery of auditory testing was not adaptive per se, and did not require the active “learning” that is necessary for more complex operant-conditioning-based auditory discrimination tasks (Fitch et al., 1994; Benasich et al., 2002). However, while overt training was not present in our paradigm, evidence suggests that improvements in auditory discrimination on PPI tasks may yet be a product of associative learning. For example, Crofton and associates (1990) found strong support for an associative learning component related to the observed learning effects in PPI, specifically by showing learning effects in rats only after contingent (but not non-contingent) pairing of a pre-pulse cue with an SES. Given that subjects in the current study were presented with a variety of sweep durations across several trials and days, with successively shorter ISIs, it is possible that the gradual shift from long to shorter intervals could have provided a sort of “adaptive” task, and thus may have facilitated improvements in FM sweep acuity observed in the early experienced subjects. Similar evidence for the benefits of gradual reduction of ISI with a discrimination task on subsequent acuity can be seen in related studies (e.g., Peiffer et al., 2004; McClure et al., 2006), which showed a significant improvement in acuity for rats across multiple days of modified startle testing using incrementally more complex stimuli (see Fitch et al., 2008 for review). In theory, our order of cue and task presentation – from long to short, and simple to complex – may have mimicked the adaptive auditory training tasks successfully used by Tallal and associates (1996) and Merzenich and colleagues (1996) to gradually improve auditory temporal acuity and language outcome in language impaired children.
More recent studies using functional imaging techniques have shown that dyslexics who underwent four to eight weeks of auditory based training (using non-linguistic stimuli and acoustically modified speech) also improved their auditory temporal acuity relative to controls (results similar to those observed by Tallal et al., 1996 and Merzenich et al., 1996). In addition, these studies found increased functional activation in left temporo-parietal cortex and left prefrontal cortex to rapid auditory stimuli following training, both structures that have been implicated in language processing (Burton, 2001; Rumsey et al., 1992). These results strongly suggest that brain regions important for rapid auditory temporal processing have a diminished processing capacity in language-impaired individuals, and might be brought “back online” with extensive auditory training (Temple et al., 2003; Gaab et al., 2007; Habib, 2003). Importantly, recent physiological studies in rodents with cortical developmental disruption (microgyria in primary somatosensory cortex) also revealed reduced responses to “single pure-tones” and “periodic noise bursts” in primary auditory cortex and surrounding belt auditory cortical regions (ventral auditory fields (VAF)) as compared to shams (Escabi et al., 2007; Higgins et al., 2008). These studies suggest that belt auditory cortical areas (specifically VAF), which have neurons that are more frequency and intensity selective than neurons in primary auditory cortex, may show a specific loss in sound level tuning that are not seen in primary auditory cortex in microgyric rats as compared to shams. These regionally specific reductions in sound level tuning have been suggested to contribute to behaviorally observed auditory processing deficits in rats with induced MG, and may share parallels with regionally specific abnormalities in temporal and frontal cortical activation to auditory signals in humans with language impairment (Escabi et al., 2007; Higgins et al., 2008; Temple et al., 2003; Gaab et al., 2007; Habib, 2003). Future studies using rodent models of cortical developmental pathology and auditory temporal processing impairment should seek to investigate possible changes in physiological response properties of auditory cortical regions resulting from early auditory experience. These studies may someday help identify the mechanisms of plasticity that could be affected by intensive auditory training in humans, and thus lead to improved procedures for reducing the severity of language deficits related to abnormal auditory processing. Until then, it is clear from the present study that exposure to simple and complex discrimination tasks using a modified acoustic startle paradigm leads to the amelioration of processing deficits in microgyric rats and improves overall auditory discrimination, well beyond the subtle improvements resulting from normal maturation.
4.3 Conclusion
In closing, we found that early auditory discrimination experience utilizing a modified startle response paradigm improves auditory temporal processing beyond improvements seen with normal maturation. Further, we showed that early auditory processing experience ameliorates auditory temporal processing deficits seen in young microgyric rats as compared to shams and adults with no early acoustic experience.
Acknowledgements
This research was supported by NIH Grant HD20806.
References
- Benasich A, Tallal P. Infant discrimination of rapid auditory cues predicts later language impairment. Behav Brain Re. 2002;136:31–49. doi: 10.1016/s0166-4328(02)00098-0. [DOI] [PubMed] [Google Scholar]
- Benasich A, Choudhury N, Friedman J, Bealpe-Bonilla T, Chojnowska C, Gou Z. The infant as a prelinguistic model for language learning impairments: predicting from event-related potentials to behavior. Neuropsychologia. 2006;44(3):396–411. doi: 10.1016/j.neuropsychologia.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton MW. The role of the inferior frontal cortex in phonological processing. Cogn Sci. 2001;25:695–709. [Google Scholar]
- Bishop DV. Cognitive neuropsychology and developmental disorders: uncomfortable bedfellows. Q J Exp Psychol A. 1997;50(4):899–923. doi: 10.1080/713755740. [DOI] [PubMed] [Google Scholar]
- Chang B, Ly J, Appignani B, Bodell A, Apse K, Ravenscroft R, Sheen V, Doherty M, Hackney D, O’Connor M, Galaburda A, Walsh C. Reading impairment in the neuronal migration disorder of periventricular nodular heterotopia. Neurology. 2005;64(5):799–803. doi: 10.1212/01.WNL.0000152874.57180.AF. [DOI] [PubMed] [Google Scholar]
- Choudhury N, Leppanen PH, Leevers H, Benasich A. Infant information processing and family history of specific language impairment: converging evidence for RAP deficits from two paradigms. Dev. Sci. 2007;10(2):213–36. doi: 10.1111/j.1467-7687.2007.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M, Rosen G, Tallal P, Fitch RH. Impaired processing of complex auditory stimuli in rats with indiced cerebrocortical microgyria: An animal model of developmental language disabilities. J Cogn Neurosci. 2000a;12(5):828–39. doi: 10.1162/089892900562435. [DOI] [PubMed] [Google Scholar]
- Clark M, Rosen G, Tallal P, Fitch RH. Impaired two-tone processing ate repid rates in male rats with induced microgyria. Brain Res. 2000b;871(1):94–7. doi: 10.1016/s0006-8993(00)02447-1. [DOI] [PubMed] [Google Scholar]
- Crofton K, Dean K, Sheets L. Evidence for involvement of associative conditioning in reflex modification of the acoustic startle response with gaps in background noise. Psychobiology. 1990;18:467–474. [Google Scholar]
- Dean K, Sheets L. The effect of age and experience on inhibition of the acoustic startle response by gaps in background noise. Psychobiology. 1990;18(1):89–95. [Google Scholar]
- Dvorak K, Feit J, Jurankova Z. Experimentally Induced Focal Microgyria and Status Verrucosus Deformis in Rats - Pathogenesis and Interrelation Histological and Autoradiographical Study. Acta Neuropathol. 1978;44:121–129. doi: 10.1007/BF00691477. [DOI] [PubMed] [Google Scholar]
- Escabi M, Higgins N, Galaburda A, Rosen G, Read H. Early cortical damage in rat somatosensory cortex alters acoustic feature representation in primary auditory cortex. Neuroscience. 2007;150(4):970–83. doi: 10.1016/j.neuroscience.2007.07.054. [DOI] [PubMed] [Google Scholar]
- Farmer M, Klein R. The evidence for a temporal processing deficit-linked to dyslexia. Psychonom Bull Rev. 1995;2:460–493. doi: 10.3758/BF03210983. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Tallal P, Brown C, Galaburda A, Rosen G. Induced microgyria and auditory temporal processing in rats: a model for language impairment? Cereb Cortex. 1994;4(3):260–70. doi: 10.1093/cercor/4.3.260. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Tallal P. Neural mechanisms of language-based learning impairments: insights from human populations and animal models. Behav Cog Neurosci Rev. 2003;2(3):155–178. doi: 10.1177/1534582303258736. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Threlkeld S, McClure M, Peiffer A. Use of a modified prepulse inhibition paradigm to assess complex auditory discrimination in rodents. Brain Res. Bull. 2008a;76:1–7. doi: 10.1016/j.brainresbull.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch RH, Breslawski H, Rosen GD, Chrobak JJ. Persistent spatial working memory deficits in rats with bilateral cortical microgyria. Behav Brain Funct. 2008b;4(1):45. doi: 10.1186/1744-9081-4-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Peiffer A, Matthew C, Benasich A, Fitch R. Age and experience-related improvements in gap detection in the rat. Dev Brain Res. 2004;152:83–91. doi: 10.1016/j.devbrainres.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Gaab N, Gabrieli J, Deutsch G, Tallal P, Temple E. Neuronal correlates of rapid auditory processing are disrupted in children with developmental dyslexia and ameliorated with training: An fMRI study. Bestor Neurol Neurosci. 2007;25(3-4):295–310. [PubMed] [Google Scholar]
- Galaburda A, Sherman G, Rosen G, Aboitiz F, Geschwind N. Developmental Dyslexia: Four Consecutive Patients with Cortical Abnormalities. Ann Neurol. 1985;18(2):222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- Galaburda A, Menard M, Rosen G. Evidence for aberrant auditory anatomy in developmental dyslexia. Proc Natl Acad Sci USA. 1994;91:8010–8013. doi: 10.1073/pnas.91.17.8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannetti S, Gaglini P, Di Rocco F, Di Rocco C, Granato A. Organization of cortico-cortical associative projections in a rat model of microgyria. Neuroreport. 2000;11(10):2185–9. doi: 10.1097/00001756-200007140-00024. [DOI] [PubMed] [Google Scholar]
- Guerreiro M, Hage S, Guimaraes C, Abramides D, Fernandes W, Pacheco P, Piovesana A, Montenegro M, Cendes F. Developmental language disorder associated with polymicrogyria. Neurology. 2002;59:245–250. doi: 10.1212/wnl.59.2.245. [DOI] [PubMed] [Google Scholar]
- Habib M. The neurological basis of developmental dyslexia: An overview and working hypothesis. Brain. 2000;123:2373–2399. doi: 10.1093/brain/123.12.2373. [DOI] [PubMed] [Google Scholar]
- Hage S, Cendes F, Montenegro M, Abramides D, Guimaraes C, Guerreiro M. Specific Language Impairment: Linguistic and neurobiological aspects. Arq Neuropsiquiatr. 2006;64(2-A):173–180. doi: 10.1590/s0004-282x2006000200001. [DOI] [PubMed] [Google Scholar]
- Hautus M, Setchell G, Waldie K, Kirk I. Age related improvements in auditory temporal resolution in reading-impaired children. Dyslexia. 2003;9:37–45. doi: 10.1002/dys.234. [DOI] [PubMed] [Google Scholar]
- Higgins N, Escabi M, Rosen G, Galaburda A, Read H. Spectral processing deficits in belt auditory cortex following early postnatal lesions of somatosensory cortex. Neuroscience. 2008;153(2):535–49. doi: 10.1016/j.neuroscience.2008.01.073. [DOI] [PubMed] [Google Scholar]
- Herman A, Galaburda A, Fitch RH, Carter A, Rosen G. Cerebral microgyria, thalamic cell size and auditory temporal processing in male and female rats. Cereb Cortex. 1997;7:453–464. doi: 10.1093/cercor/7.5.453. [DOI] [PubMed] [Google Scholar]
- Humphreys P, Rosen G, Press D, Sherman G, Galaburda A. Freezing Lesions of the Developing Rat Brain: A Model For Cerebrocortical Microgyria. J Neuropathol Exp Neurol. 1991;50:145–160. doi: 10.1097/00005072-199103000-00006. [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Graber KD, Kharazia VN, Parada I, Prince DA. Postlesional epilepsy: The ultimate brain plasticity. Epilepsia. 2000;41(6):S153–S161. doi: 10.1111/j.1528-1157.2000.tb01574.x. [DOI] [PubMed] [Google Scholar]
- Liederman J, Kantrowitz L, Flannery K. Male vulnerability to reading disability is not likely to be a myth: a call for new data. J Learn Disabil. 2005;38(2):109–129. doi: 10.1177/00222194050380020201. [DOI] [PubMed] [Google Scholar]
- Marsh R, et al. The role of small changes in the acoustic environment in modifying the startle reflex. J. Exp. Psychol. Anim. Behav. Process. 1975;1:235–244. doi: 10.1037//0097-7403.1.3.235. [DOI] [PubMed] [Google Scholar]
- McArthur D, Bishop G. Immature cortical responses to auditory stimuli in specific language impairment: evidence from ERPs to rapid tone sequences. Developmental Science. 2004;7(4):11–18. doi: 10.1111/j.1467-7687.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- McClure M, Threlkeld S, Rosen G, Fitch RH. Rapid audiroty processing and learning deficits in rats with P1 versus P7 neonatal hypoxic-ischemic injury. Behav Brain Res. 2006;172:114–121. doi: 10.1016/j.bbr.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure M, Threlkeld S, Fitch RH. Auditory processing and learning/memory following erythropoietin administration in neonatally hypoxic-ischemic injured rats. Brain Res. 2007;1132(1):203–9. doi: 10.1016/j.brainres.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Merzenich M, Jenkins W, Johnston P, Schreiner C, Miller S, Tallal P. Temporal processing deficit of language-learning impaired children ameliorated by training. Science. 1996;271:77–80. doi: 10.1126/science.271.5245.77. [DOI] [PubMed] [Google Scholar]
- Miller-Shaul S. The characteristics of young and adult dyslexic readers on reading and reading related cognitive tasks as compared to normal readers. Dyslexia. 2005;11:132–151. doi: 10.1002/dys.290. [DOI] [PubMed] [Google Scholar]
- Ortiz-Mantilla S, Chojnowska C, Choudhury N, Benasich AA. Latency effects on infant auditory cortical evoked response as a function of varying exposure to passive and active auditory discrimination paradigms. Society for Neuroscience. 2006 (772.14) [Google Scholar]
- Paxinos G, editor. The Rat Nervous System. Third Edition Elsevier; San Diego, CA: 2004. Ch. 2; pp. 27–73. [Google Scholar]
- Peiffer A, Rosen G, Fitch RH. Rapid auditory processing and MGN morphology in microgyric rats reared in varied acoustic environments. Brain Research. Dev Brain Res. 2002a;138(2):187–93. doi: 10.1016/s0165-3806(02)00472-8. [DOI] [PubMed] [Google Scholar]
- Peiffer A, Rosen G, Fitch RH. Sex differences in rapid auditory processing deficits in ectopic BXSB/MpJ mice. Neuroreport. 2002b;13(17):2277–80. doi: 10.1097/00001756-200212030-00021. [DOI] [PubMed] [Google Scholar]
- Peiffer A, Friedman J, Rosen G, Fitch RH. Impaired gap detection in juvenile microgyric rats. Dev Brain Res. 2004a;152(2):93–98. doi: 10.1016/j.devbrainres.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Peiffer A, Rosen GD, Fitch RH. Sex differences in rapid auditory processing deficits in microgyric rats. Brain Res Dev Brain Res. 2004b;148(1):53–7. doi: 10.1016/j.devbrainres.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Rosen G, Herman A, Galaburda A. Sex Differences in the Effects of Early Neocortical Injury on Neuronal Size Distribution of the Medial Geniculate Nucleus in the Rat are Mediated by Perinatal Gonadal Steroids. Cerebral Cortex. 1999;9:27–34. doi: 10.1093/cercor/9.1.27. [DOI] [PubMed] [Google Scholar]
- Rosen G, Burstein D, Galaburda A. Changes in Efferent and Afferent Connectivity in Rats With Induced Cerebrocortical Microgyria. J Comp Neurol. 2000;418:423–440. [PubMed] [Google Scholar]
- Rumsey JM, Andreason P, Zametkin AJ, Aquino T, King AC, Hamburger SD, et al. Failure to activate the left temporoparietal cortex in dyslexia. An oxygen 15 positron emission tomographic study. Arch Neurol. 1992;49(5):527–534. doi: 10.1001/archneur.1992.00530290115020. [DOI] [PubMed] [Google Scholar]
- Rutter M, Caspi A, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. 2003. [DOI] [PubMed]
- Smith N, Trainor L, Shore D. The development of temporal resolution: Between-channel gap detection in infants and adults. J. Speech Lang. Hear. Res. 2006;49:1104–1113. doi: 10.1044/1092-4388(2006/079). [DOI] [PubMed] [Google Scholar]
- Tallal P, Miller S, Bedi G, Byma G, Wang X, Nagarajan S, Schreiner C, Jenkings W, Merzenich M. Language comprehension in language-leraning impaired children improved with acoustically modified speech. Science. 1996;271:81–84. doi: 10.1126/science.271.5245.81. [DOI] [PubMed] [Google Scholar]
- Tallal P. Improving language and literacy is a matter of time. Nat Rev Neurosci. 2004;5(9):721–8. doi: 10.1038/nrn1499. [DOI] [PubMed] [Google Scholar]
- Temple E, Poldrack R, Protopapas A, Nagarajan S, Salz T, Tallal P, Merzenich M, Gabrieli J. Disruption of the neural response to rapid acoustic stimuli in dyslexia: Evidence from functional MRI. PNAS. 2000;97(25):13907–13912. doi: 10.1073/pnas.240461697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E, Deutsch G, Poldrack R, Miller S, Tallal P, Merzenich M, Gabrieli J. Neural deficits in children with dyslexia ameliorated by behavioral remediation: Evidence from functional MRI. PNAS. 2003;100(5):2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlkeld, et al. Developmental timeframes for induction of microgyria and rapid auditory processing deficits in the rat. Brain Res. 2006;1109(1):22–31. doi: 10.1016/j.brainres.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Threlkeld S, McClure M, Bai J, Wang Y, LoTruco J, Rosen G, Fitch RH. Developmetnal disruptions and behavioral impairments in rats following in utero RNAi of Dyx1c1. Brain Res Bull. 2007;71(5):508–14. doi: 10.1016/j.brainresbull.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K, Hall S, Klein R, Phillips D. Development of perceptual correlates of reading performance. Brain Res. 2006;1124:126–141. doi: 10.1016/j.brainres.2006.09.080. [DOI] [PubMed] [Google Scholar]