Abstract
The uteroplacental vasculature is refractory to α-adrenergic stimulation, and large conductance Ca2+-activated K+ channels (BKCa) may contribute. We examined the effects of uterine artery (UA) BKCa inhibition with tetraethylammonium (TEA) on hemodynamic responses to phenylephrine (PE) at 101 to 117 days and 135 to 147 days of ovine gestation, obtaining dose responses for mean arterial pressure (MAP), heart rate (HR), and uteroplacental blood flow (UPBF) and vascular resistance (UPVR) before and during UA TEA infusions. The UA α1-adrenergic receptors (α1-ARs) were assessed. The PE increased MAP and UPVR and decreased HR and UPBF dose dependently at both gestations (P < .001, analysis of variance). The %▵MAP was less at 135 to 147 days before and during TEA infusions (P ≤ .008); however, responses during TEA were greater (P ≤ .002). The PE increased %▵UPVR>>%▵MAP, thus %▵UPBF fell. The TEA enhanced PE-mediated increases in %▵UPVR at 135 to 147 days (P ≤ .03). The UA α1-AR expression was unchanged in pregnancy. Uterine vascular responses to PE exceed systemic vascular responses throughout pregnancy and are attenuated by BKCa activation, suggesting BKCa protect UPBF.
Keywords: vascular sensitivity, pressor responses, α1-adrenergic receptors, uteroplacental blood flow
Introduction
Pregnancy is associated with numerous cardiovascular changes directed toward ensuring fetal growth and well-being and maternal adaptation, for example, cardiac output increases >50% and is redistributed in order to ensure the growth of the uteroplacental vascular bed and thus uterine oxygen and nutrient delivery while maintaining increases in maternal organ blood flows.1–3 Pregnant women and other species also develop attenuated pressor responses to infused vasoconstrictors, including α-adrenergic agents and angiotensin II (ANG-II).4–7 This appears to protect against the elevated circulating levels of catecholamines and ANG-II and increased sympathetic outflow associated with normal pregnancy.3,8–10 The mechanisms responsible for the attenuated vascular sensitivity remain unclear but may include altered endothelial and/or vascular smooth muscle (VSM) function, receptor expression, prostaglandin or nitric oxide (NO) synthesis, or changes in receptor signaling. Notably, this refractoriness is absent in women with preeclampsia or those destined to develop gestational hypertension, resulting in enhanced pressor responses.7,11,12
The uterine vascular bed in intact pregnant sheep is also refractory to α-adrenergic agents and ANG-II in pregnancy.13,14 However, it is more sensitive to α-adrenergic stimulation and refractory to ANG-II compared to the systemic vasculature.13,15–20 The attenuated uterine responses to ANG-II are explained by predominant expression of type 2 ANG-II receptors (AT2Rs) in uterine VSM versus type 1 (AT1Rs) in peripheral VSM.20–23 The explanation for the increased uteroplacental sensitivity to α-adrenergic agents is less clear but may include differences in α-adrenergic receptors, increases in VSM Ca2+ sensitivity and contractile proteins, and/or remodeling.24–28 However, the mechanisms that contribute to the generalized uterine refractoriness to these agonists in pregnancy are unknown.
Potassium channels regulate vascular reactivity in numerous vascular beds.20,29 Large conductance Ca2+-activated K+ channels (BKCa) are expressed in VSM, can modulate vasoconstrictor and vasodilator responses, and this function varies in different vascular beds.29–32 The BKCa consist of 4 α-subunits derived from a single gene product that form a homotetramer and undergo posttranslational modification, resulting in varied function.30,33 They are associated with up to 4 regulatory β-subunits derived from separate genes, which vary in the β:α stoichiometry and channel function.30,34–37 The β1-subunit predominates in uterine artery (UA) VSM, and its C-terminal end senses increases in intracellular Ca2+, resulting in BKCa activation, closure of voltage-gated Ca2+ channels, decreases in intracellular Ca2+, and repolarization of cell membranes in order to maintain vascular tone.30,34,36,37 Notably, UA β1-subunit expression increases in the last third of ovine pregnancy,33 increasing β1:α subunit which may modify UA Ca2+ sensitivity,31 contributing to the attenuated uterine vasoconstrictor responses seen in normotensive pregnancy.
In the present studies, we determined whether BKCa contribute to attenuated uteroplacental sensitivity to α1-adrenergic stimulation in pregnant sheep in the last third of gestation and whether α1-adrenergic receptor (α1-AR) expression is altered within the uterine vascular bed or differs from the systemic vasculature. We also examined simultaneous pressor responses to systemic phenylephrine (PE) infusions before and during UA infusions of tetraethylammonium (TEA), an inhibitor of BKCa function.30,38
Methods
Animal Model
Time-dated pregnant Western sheep (n = 8; 6 singletons and 2 twins) were used in these studies. The surgical procedures have been reported6,39 and are briefly described. Animals delivered to UT Southwestern Medical Center were allowed 1 week to recover. At 95 to 104 days of gestation (term ∼150 days), a percutaneous jugular venous catheter was placed for infusion of preoperative sedatives and intraoperative fluids, and the animals were fasted overnight. The following morning they received intramuscular (im) atropine (0.088 mg/kg), intravenous (iv) sodium pentobarbital and ketamine hydrochloride, were intubated, and the abdomen prepared for sterile surgery. During surgery, the animals received inhalation anesthesia with 1.5% isoflurane (Mallinckroft Veterinary Inc, Mundelein, Illinois) and oxygen via a rebreathing anesthesia machine. The gravid uterus was isolated through a midline abdominal incision, and electromagnetic flow probes (6.0 or 7.0 mm internal diameter; Carolina Medical, King, North Carolina) were placed on the main UA proximal to the first bifurcation in both uterine horns. Polyvinyl catheters containing heparinized saline (250 Units/mL) were inserted retrograde into a distal branch of each UA for intra-arterial infusions of tetraethylammonium chloride (TEA). The abdominal incision was closed as previously described.6,39 Catheters were placed in the femoral artery to monitor mean arterial pressure (MAP) and heart rate (HR) and femoral vein to infuse systemic drugs. Catheters and flow probe leads were brought to the flank via a subcutaneous tunnel, exited via a puncture wound in the skin, and stored in a canvas pouch attached to the skin with sterile stainless steel pins. Animals received 10% dextrose in 0.9% saline during surgery and postoperatively, as well as penicillin (600 000 units iv), gentamycin (150 mg im), and flunixin meglumine (100 mg iv) for 2 days after surgery. Catheters were flushed daily with heparinized saline (250 Units/mL) to maintain patency. Studies were terminated with iv phenobarbital, 200 mg/kg. The studies described were approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center.
Experimental Protocol
Animals were maintained in large holding pens within the laboratory. Studies were not performed until 5 days postoperative and scheduled to start at 104 days (n = 7) and 136 days (n = 7) of gestation, requiring 10 days to complete each time period. In all, 6 ewes were studied at both gestations, while 1 animal in each age group was studied at only 1 gestational age. This allowed us to determine whether age-dependent changes occurred in vascular responsiveness to PE and/or the BKCa channel inhibitor TEA (Sigma, St Louis, Missouri). On the day of study, baseline MAP, HR, and uteroplacental blood flow (UPBF) were recorded for 1 hour, after which a control cumulative dose–response curve for iv PE was generated at each gestational age using 1.29, 2.58, 12.9, 55.7 and 129 ug/min (Figure 1; Neosynephrine; Winthrop Laboratories, New York, New York). Each PE dose was infused 12 minutes to obtain steady-state responses, which occurred by 8 to 12 minutes, and was used for analyses. After completing the control PE dose responses, animals were monitored until all hemodynamic parameters returned to baseline and remained stable. At this time, TEA, a relatively selective BKCa inhibitor at concentrations <1.0 mmol/L,30,38 was continuously infused for 72 minutes via a UA catheter in the gravid horn to achieve the calculated concentrations of 0.005, 0.01, 0.02, 0.035, or 0.04 mmol/L. These concentrations are less than previously used32,39 and were chosen to minimize the effects on basal UPBF while providing partial BKCa blockade (Figure 1). These TEA levels are more specific for BKCa inhibition and are less likely to block most voltage-gated K+ channels (KV).29,30 Rates of TEA infusion were determined using the following equation and the measurement of UPBF in the infused uterine horn: Rate infusion (μg/min) = TEA level desired (μg/mL) × UPBF (mL/min). A cumulative PE dose response was repeated during the continuous TEA infusion. At completion, animals were monitored until hemodynamic parameters returned to baseline; 48 hours were allowed between doses of TEA. No more than 2 TEA doses were studied each week in the same order to prevent any cumulative effects on BKCa function. Measurements of MAP and HR were monitored via femoral arterial catheters using transducers (Gould, Houston, Texas); UPBF was monitored with flow meters (Carolina Medical Instruments Inc, East Bend, North Carolina). Hemodynamic parameters were collected and stored using “Pohnema” Data Acquisition software (Data Sciences International, Valley View, Ohio).
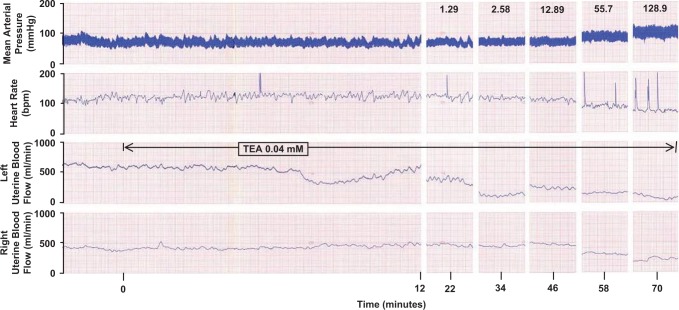
Figure 1.
Representative recording of the effects of a unilateral uterine arterial infusion of TEA (0.04 mmol/L) on mean arterial pressure, heart rate, and left and right uterine blood flow in a term pregnant ewe before and during the cumulative PE dose response. Responses shown are at 10 minutes of PE infusion ± 2 minutes. TEA indicates tetraethylammonium; PE, phenylephrine.
Immunoblot Analysis
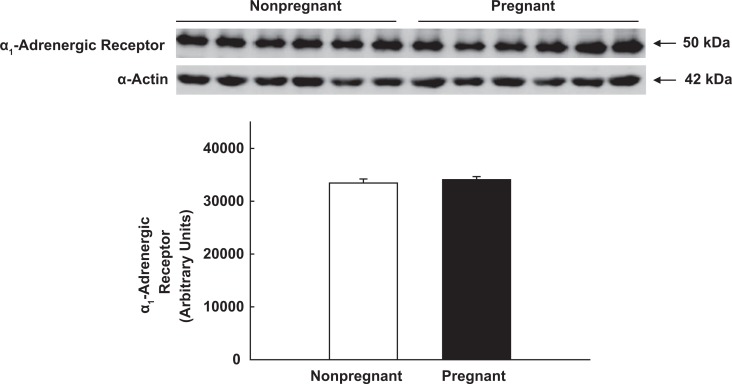
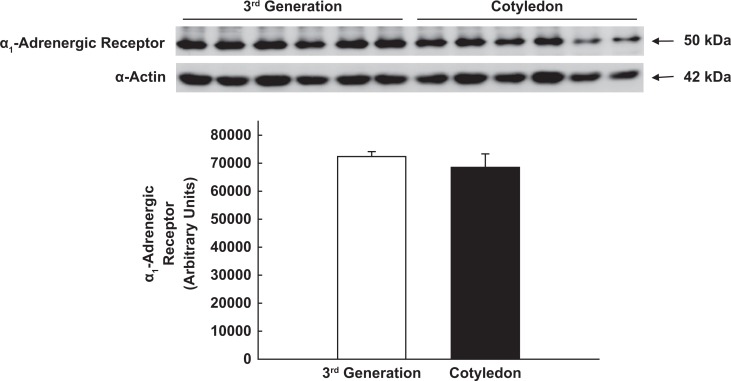
The α1-AR is the predominant adrenergic receptor in UA VSM40–42; but it is not known whether changes in UA sensitivity to α-adrenergic agonists in pregnancy or differences in uterine and systemic responses reflect alterations in receptor expression. We, therefore, collected proximal third- and fifth-generation UA from 6 nonpregnant ewes and third-generation (n = 6), precotyledonary or placental (n = 6) and mesenteric (n = 5) arteries from 6 near-term pregnant sheep. We examined precotyledonary arteries since placental blood flow accounts for >80% of total UPBF at term.1–3 Arteries were homogenized and 20 µg of soluble protein were loaded on 10% polyacrylamide gels, subjected to electrophoresis, and transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore, Billerica, Maryland). Immunoblots were blocked in buffer containing powdered milk (5% wt/vol) and incubated overnight at 4°C with antiserum against α1-ARs (1:500; ab54730; Abcam Inc, Cambridge, Massachusetts). Membranes were incubated with anti-mouse immunoglobulin G (IgG) at 1:1000. Regions containing receptor proteins were visualized by enhanced chemiluminescence. Densitometry was performed, and values expressed as arbitrary units. The loading protein was α-smooth muscle actin.
Statistical Analyses
Data were analyzed with SAS V9.01 using 3-way repeated measures analysis of variance (ANOVA) to examine interactions between PE dose, TEA, and gestational group. To determine differences in PE responses between treatment and within age groups at specific PE doses, we used 2-way ANOVA and all pairwise multiple comparison procedures using the Holm-Sidak method. Responses were examined to determine TEA dose effects, PE dose effects, differences between studies at 101 to 117 days and 135 to 147 days of gestation, and differences in simultaneous responses in MAP, HR, UPBF, and UPVR. Responses in the infused and noninfused uterine horns were compared. Uteroplacental vascular resistance (UPVR) was calculated using MAP (mm Hg) ÷ UBF (mL/min) during steady-state responses. Student independent samples t test was used where noted. Data are presented as means ± 1 standard error of the mean (SEM).
Results
Baseline Hemodynamic Data
Baseline hemodynamic variables did not differ before infusions of PE or different doses of TEA at either gestational age (P > 0.1); thus, baseline values were averaged for each animal and used to calculate baseline mean hemodynamic parameters at each gestational age. Although MAP and UPVR in each uterine horn decreased between study periods while HR and UPBF increased (Table 1), only the fall in TEA-treated uterine horn (UVRTx), that is, the gravid horn, achieved statistical significance. Similarly, total UPBF rose 28% from 943 ± 105 to 1207 ± 114 (P = .1), while the total UPVR fell 32% from 0.09 ± 0.01 to 0.06 ± 0.01 (P < .001); however, the rise in UPBF varied between animals, explaining the inability to detect a significant rise.
Table 1.
Baseline Hemodynamic Measurements in Mid- and Late-Gestation Sheep.
| MAP, mm Hg | HR, bpm | UPBFTX, mL/min | UPBFC, mL/min | UPVRTx, mm Hg·min/mL | UPVRC, mm Hg·min/mL | |
|---|---|---|---|---|---|---|
| Early (n = 7) | 80 ± 2.8 | 89 ± 5.9 | 611 ± 78 | 340 ± 49 | 0.15 ± 0.02 | 0.24 ± 0.03 |
| Late (n = 7) | 77 ± 5.4 | 94 ± 3.9 | 780 ± 95 | 476 ± 71 | 0.11 ± 0.01a | 0.20 ± 0.05 |
Abbreviations: TX, TEA-treated uterine horn; TC, control uterine horn.
a P = .047.
Blood Pressure Responses to PE
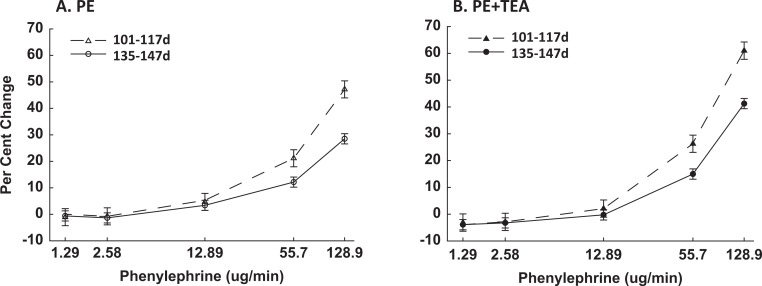
Since each hemodynamic parameter changed during the study period (Table 1), we used the relative responses or percentage change (%▵) from baseline to compare time periods and assess the responses to PE and TEA + PE. This also permits a comparison of changes in vascular sensitivity to PE in the absence and presence of UA BKCa blockade by each hemodynamic variable, which have different units of measurement.13,18 The PE alone increased MAP dose dependently at both gestational ages (Figure 2A; P < .0001, ANOVA); but responses were significantly less at 135 to 147 days (P ≤ .05, 2-way ANOVA), reflecting an interaction between gestational age and PE dose (P ≤ .008). The HR fell dose dependently (P < .0001, ANOVA), decreasing ∼30% at the highest PE dose (data not shown); but unlike MAP, there was no gestational age effect, P > .1.
Figure 2.
Comparison of PE-induced increases in mean arterial pressure in pregnant sheep at 101 to 117 days (▵, ▴) and 135 to 147 days (^, •) gestation in the absence (A, open symbols) and presence (B, solid symbols) of uterine artery TEA infusion. Data are the percentage change, analyzed by 2-way ANOVA and are shown as means ± SEM. The PE responses are dose dependent (P < .001, ANOVA), modified by gestational age (P ≤ .008, ANOVA), and increased in the presence of TEA. TEA indicates tetraethylammonium; PE, phenylephrine; SEM, standard error of mean; ANOVA, analysis of variance.
Basal MAP, HR, and UPBF in both uterine horns were unchanged during the initial 10 minutes of the unilateral UA infusion of TEA (Figure 1). During the TEA infusions, PE dose dependently increased MAP at both gestational ages and the relative rise in MAP remained less at 135 to 147 days (Figure 2B; P ≤ .004, 2-way ANOVA). The HR also decreased dose dependently (P = .05, data not shown). Notably, PE-mediated increases in %▵MAP were greater during UA TEA infusions at both gestational ages (P ≤ .002, 2-way ANOVA), especially with the highest PE doses, which were always studied last. This suggests TEA may have entered and accumulated in the systemic circulation during the 72-minute infusion. This was also associated with a greater fall in HR (P = .05, data not shown), likely reflecting an enhanced baroresponse due to the increased pressor response. There was no TEA dose effect on PE-induced responses in MAP or HR at 101 to 117 days (P > .1, ANOVA); however, a modest dose effect occurred at term, P = .05, suggesting increased sensitivity.
Uterine Vascular Responses
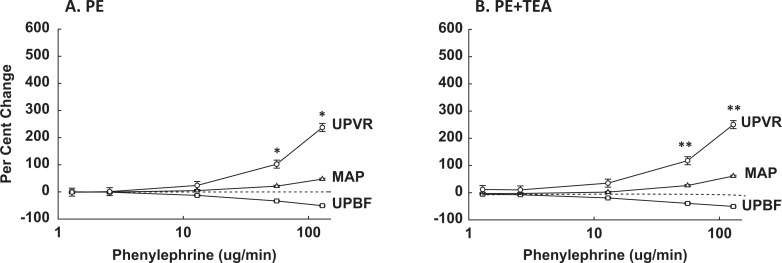
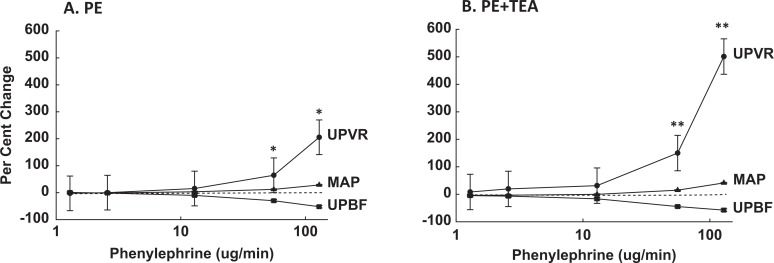
The best way to assess uterine responses to systemic infusions of vasoactive agents is to compare the relative changes (%▵) in UPVR, MAP, and UPBF, which takes into account simultaneous changes in perfusion pressure and vascular resistance.13,18 The PE alone increased UPVR and decreased UPBF dose dependently at both gestational ages (Figures 3A and 4A; P < .0001, ANOVA); however, the relative rise in UPVR was modestly less at 135 to 147 days, P = .08, while the fall in %▵UPBF did not differ, P > .1. Notably, the rise in %▵UPVR exceeded the rise in %▵MAP (P ≤ .003) at both gestational ages, thus %▵UPBF fell, demonstrating greater uterine versus systemic vascular sensitivity to PE throughout pregnancy (Figures 3A and 4A). The fall in %▵UPBF was not proportional to increases in %▵UPVR due to the simultaneous rise in perfusion pressure, that is, %▵MAP.
Figure 3.
Comparison of the simultaneous relative changes in uteroplacental vascular resistance (UPVR, ^), mean arterial pressure (MAP, ▵), and uteroplacental blood flow (UPBF, □) during systemic PE infusions in the absence (A) and presence (B) of UA infusions of TEA at 101 to 117 days of gestation. Data were analyzed by 2-way ANOVA and are shown as means ± SEM. The PE increased UPVR and MAP and decreased UPBF dose dependently without and with TEA (P < .0001, ANOVA), and %▵UPVR exceeded %▵MAP at the doses noted (*P ≤ .008, **P ≤ .01; Holm-Sidak method). TEA indicates tetraethylammonium; PE, phenylephrine; SEM, standard error of mean; ANOVA, analysis of variance; UA, uterine artery.
Figure 4.
Comparison of the simultaneous relative changes in uteroplacental vascular resistance (UPVR, •), mean arterial pressure (MAP, ▴), and uteroplacental blood flow (UPBF, ▪) during systemic PE infusions in the absence (A) and presence (B) of UA infusions of TEA at 135 to 147 days of gestation. Data were analyzed by 2-way ANOVA and are shown as means ± SEM. The PE increased UPVR and MAP and decreased UPBF dose dependently without and with TEA (P < .0001, ANOVA), and %▵UPVR exceeded %▵MAP at the doses noted (*P < .001; **P ≤ .002; Holm-Sidak method). TEA indicates tetraethylammonium; PE, phenylephrine; SEM, standard error of mean; ANOVA, analysis of variance; UA, uterine artery.
At 101 to 117 days, there was no TEA dose effect. However, PE in the presence of UA TEA increased %▵UPVR dose dependently (Figure 3B; P < .0001, ANOVA), which exceeded the simultaneous rise in %▵MAP (P < .01) and %▵UPBF fell dose dependently. Notably, the uterine responses to PE were greater in the presence of UA TEA, thus the fall in %▵UPBF increased (P = .018, 2-way ANOVA). Responses to PE in the untreated or contralateral uterine horn were not significantly affected (P > .1, ANOVA).
At 135 to 147 days, TEA had a modest dose effect on uterine responses to PE (data not shown, P = .03). In the presence of UA TEA, PE continued to dose dependently affect uterine hemodynamic parameters (Figure 4B; P < .0001, ANOVA), and the relative rise in UPVR exceeded simultaneous increases in %▵MAP (P < .002), thus %▵UPBF fell. Importantly, UA TEA was associated with greater PE-mediated increases in %▵UPVR and decreases in %▵UPBF at the highest doses of PE (P ≤ .03, 2-way ANOVA); that is, %▵UPVR increased 2-fold greater with TEA + PE at 128.9 μg/min. Additionally, the rise in %▵UPVR during UA BKCa blockade was ∼2-fold greater at 135 to 147 days versus 101 to 117 days of gestation, that is, 501% versus 250%, respectively. The PE-mediated responses in the contralateral uterine horn were unaffected by TEA infusions.
Adrenergic Receptors
There was no difference in α1-AR expression in proximal third-generation UA VSM from nonpregnant and term pregnant ewes (Figure 5; P > .1) or in UA and mesenteric arteries from the pregnant sheep (P > .1; data not shown). Since >80% of total UPBF represents placental blood flow at term pregnancy,1–3 we also compared third-generation UA with precotyledonary or placental artery in pregnant ewes. There also was no difference within the uterine vascular bed (Figure 6; P > .1). Finally, α1-AR expression was not different between precotyledonary arteries in pregnant sheep and fifth-generation UA from nonpregnant ewes (P > .1; data not shown).
Figure 5.
Immunoblot analysis of α1-adrenergic receptor expression in proximal third generation uterine artery smooth muscle from nonpregnant and term pregnant ewes. α-smooth muscle actin was the loading protein. P = .5, independent samples t test.
Figure 6.
Immunoblot analysis of α1-adrenergic receptor expression in proximal third-generation uterine and cotyledon/placental arterial smooth muscle from term pregnant ewes. α-smooth muscle actin was the loading protein. P = .4, independent samples t test.
Discussion
Pregnancy is associated with the development of refractoriness to several pressor agents,4–7 and this is absent in women with and destined to develop gestational hypertension or preeclampsia.7,11,12 The uteroplacental vascular bed also becomes refractory to α-adrenergic agonists, and this also is absent in pregnant women with hypertension.13,15–18 Thus, the mechanisms modulating vascular sensitivity within the uterine and peripheral vascular beds may be similar, and understanding them might provide clues to the pathophysiology of gestational hypertension and related complications, for example, decreases in UPBF and fetal growth restriction. We determined whether VSM BKCa, which contribute to uterine vasodilation in pregnancy32,39 but are Ca2+ sensitive, also modulate uterine vascular responses to α1-adrenergic stimulation. The uteroplacental vascular responses to PE were greater than systemic during the last third of ovine pregnancy. However, uterine responses were attenuated at term compared to the earlier stage of gestation, and importantly, this was not due to changes in UA α1-AR expression. Notably, PE-mediated increases in %▵UPVR were enhanced during UA TEA infusions and greater at 135 to 147 days versus 101 to 117 days of gestation. Pressor responses to PE were also attenuated at term and were greater at both gestational ages during the continuous UA infusions of TEA. Thus, BKCa modulate α1-adrenergic sensitivity in the uteroplacental circulation and may also contribute to the attenuated systemic pressor responses in pregnancy. Thus, abnormal BKCa function or expression may contribute to the increased vascular sensitivity to vasoconstrictors seen in pregnant women with hypertension.8,9
The UPBF rises ∼30-fold in ovine pregnancy, optimizing fetal nutrient and oxygen delivery and the maintenance of fetal growth and well-being.1–3 This is paralleled by maternal systemic vasodilation and compensatory increases in the renin–angiotensin and sympathetic systems.2,8–10 Thus, maintenance of UPBF requires a balance between vasoconstrictors and vasodilators. The uterine vasculature is always refractory to ANG-II in women and sheep due to AT2R predominance in UA VSM throughout reproduction.18,20–22,43 In contrast, it is sensitive to α-adrenergic agonists and this increases in pregnancy20,23,24,27,28,44; thus, infusion of α-adrenergic agents increases %▵UPVR more than simultaneous increases in %▵MAP and %▵UPBF falls.13,19,45 This was seen at both gestational ages and was not due to differences in α1-AR expression in UA VSM during pregnancy. Annibale et al24,25 reported that this reflected UA remodeling in pregnant sheep with VSM hypertrophy and increased VSM actin–myosin contents and myosin light chain phosphorylation. This has been confirmed in several species and women20,23,26,27 and is associated with increased VSM Ca2+ sensitivity.28
The attenuated UA responses could reflect downregulation of α1-AR, which is predominant in UA VSM from several species.40–42 However, no one has compared nonpregnant and pregnant animals. Farley et al40 observed increased α1-AR binding in nonpregnant porcine UA versus mesenteric, which might explain in part the differences in uterine and systemic responses. However, pregnant UA were not examined. We23 recently reported that UA α1-AR expression did not differ in nonpregnant and pregnant women. In the present study, α1-AR expression in proximal UA VSM was also unchanged during ovine pregnancy. Furthermore, α1-AR expression did not differ in proximal third-generation and preplacental UA, which account for >85% of total UPBF at term.3 This parallels the enhanced α1-adrenergic responses seen throughout the ovine uteroplacental vascular bed.20 Additionally, there was no difference in UA and mesenteric artery α1-AR expression, as noted earlier, or between nonpregnant fifth and pregnant preplacental VSM. Thus, α1-AR expression is unchanged within the uterine vascular bed throughout reproduction. The enhanced UA sensitivity to adrenergic agonists could explain the flight or fight physiology that protects the mother by redirecting ∼25% of cardiac output from the uterus to essential organs and tissues in pregnancy.
Although UA responses to PE increase during pregnancy,24,25 the uterine vasculature as a whole is refractory, protecting uterine oxygen and nutrient delivery.13 This was observed to increase in the last third of ovine gestation. Although UA synthesis of prostacyclin and/or nitric oxide may contribute, this is unclear.44,46–48 Alternatively, UA K+ channels may be involved. BKCa are expressed in human and ovine UA VSM and contribute to NO-induced relaxation, maintenance of basal UPBF and UA tone, estrogen-mediated increases in UPBF during pregnancy, and importantly, modulation of PE-induced contractions.32,38,39,49,50 The β1-regulatory subunit senses increases in intracellular Ca2+, predominates in UA VSM, and β1:α-subunit stoichiometry increases in the last third of ovine pregnancy.31,33,38 Thus, α1-AR-induced increases in intracellular Ca2+ would enhance BKCa open state, close voltage-gated Ca2+ channels, and attenuate vasoconstriction via a negative-feedback mechanism.30,34,36,37 We infused TEA doses at 1 order of magnitude less than prior studies to prevent a fall in basal UPBF while obtaining partial BKCa inhibition.32,39 Importantly, these doses also are less likely to inhibit KV within the uterine circulation.29,30 This is the first in vivo evidence that BKCa regulate UA sensitivity to α1-AR stimulation and this increases in late gestation, paralleling increases in β1:α stoichiometry and thus, enhanced sensitivity to changes in intracellular Ca2+.35,36 BKCa, therefore, modulate basal UPBF via membrane hyperpolarization and vasodilation via nitric oxide (NO)-cyclic guanosine monophosphate and estrogens and attenuate UA responses to increased circulating levels of catecholamines and increased sympathetic outflow in normal pregnancy via a negative-feedback mechanism. Abnormal UA BKCa expression and/or function may result in the loss of refractoriness to α-agonists and a fall in basal UPBF in hypertensive women.8,9 This is supported by studies showing increased channel activity in ovine UA in pregnancy.38
Pregnant women are also refractory to pressor agents, and those destined to develop gestational hypertension or preeclampsia have increased sensitivity to infused α-adrenergic agents and ANG-II.7,11,12 Defining the mechanisms involved would aid in delineating the pathophysiology of these hypertensive disorders and their consequences. Pregnant sheep also are refractory to these agents,6,10,13 which was confirmed in the present study, paralleling changes in women.11 Although vascular NO and/or prostaglandin synthesis may contribute to the attenuated pressor responses, this remains unclear.44,46,48,51 Animal models of hypertension in pregnancy may not recapitulate preeclampsia, which is unique to women. An alternative would be to define the mechanisms that regulate the development of refractoriness in pregnancy.
As noted, BKCa regulate basal tone and sensitivity to vasoconstrictors.29–31 When activated by increased intracellular Ca2+ and voltage, they attenuate VSM contractions by repolarizing VSM membranes, closing voltage-gated Ca2+ channels, and decreasing intracellular Ca2+.29,30,34,37 Decreases in either VSM α- or β1-regulatory subunits are associated with enhanced Ca2+ sensitivity, contractility, and hypertension.52–55 The role of BKCa in blood pressure regulation in pregnancy is unknown. Although the doses of intra-arterial TEA did not affect baseline MAP, pressor responses to systemic PE infusions were enhanced at the 2 highest PE doses infused. Thus, TEA appears to have entered the systemic circulation and altered peripheral VSM BKCa function. This was unanticipated and may reflect TEA accumulation in the systemic circulation due to a low TEA clearance rate during the 72-minute infusion. Unfortunately, we were unable to measure TEA levels. This, however, is consistent with the enhanced responses to PE observed in human UA rings after incubation with TEA.50 Alternatively, TEA may have evoked a central nervous system response; but this is unlikely since changes in MAP and HR were not seen with the higher doses previously infused into pregnant ewes.34,39 It is also possible that the BKCa sensitivity to TEA inhibition is greater than previously considered.32 Finally, it is unlikely this is due to TEA-induced release of a placental factor since the shorter infusions at higher concentrations had no effect on MAP, HR, or contralateral UPBF.34,39 Thus, BKCa may contribute to the regulation of pressor responses to α-adrenergic agents and maintenance of blood pressure in pregnancy. Future studies will need to characterize peripheral artery BKCa expression, activation, and function in pregnancy.
We have shown that uterine refractoriness to α1-adrenergic agonists occurs throughout ovine pregnancy and the uteroplacental vasculature is more sensitive to these agents than the peripheral vasculature at all times. We report for the first time that BKCa contribute to the attenuated uterine responses to α1-adrenergic agents seen in ovine pregnancy, suggesting a role in regulating UPBF in normal pregnancy. Surprisingly, pressor responses to PE were augmented during TEA infusions. It is unclear whether the attenuated pressor responses reflect changes in α-subunit density, β:α-subunit stoichiometry, and/or other mechanisms that modify BKCa activity. It also is unclear whether other TEA-sensitive K+ channels, for example, voltage-gated KV, are involved.56 Nonetheless, changes in α1-AR expression do not explain changes in UA sensitivity in pregnancy or difference between the uterine and systemic vasculature. Future studies should clarify the role of BKCa in blood pressure regulation and determine whether they contribute to increases in vascular sensitivity in the presence of hypertensive diseases, an area not yet explored.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by R01-HD008783-36 (CRR) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development and the George L. MacGregor Professorship in Pediatrics. The content is the responsibility of the authors and does not represent views of Eunice Kennedy Shriver National Institute of Child Health & Human Development and NIH.
References
- 1. Rosenfeld CR, Morris FH, Jr, Makowski EL, Meschia G, Battaglia FC. Circulatory changes in the reproductive tissues of ewes during pregnancy. Gynecol Invest. 1974;5(5-6):252–268. [DOI] [PubMed] [Google Scholar]
- 2. Rosenfeld CR. Distribution of cardiac output in pregnancy. Am J Physiol. 1977;232(3):H231–H235. [DOI] [PubMed] [Google Scholar]
- 3. Rosenfeld CR. The Uterine Circulation. Ithaca, N.Y: Perinatology; 1989, 312 pages. [Google Scholar]
- 4. Chesley LC, Talledo E, Bohler CS, Zuspan FP. Vascular reactivity to angiotensin II and norepinephrine in pregnant and nonpregnant women. Am J Obstet Gynecol. 1965;91:837–842. [DOI] [PubMed] [Google Scholar]
- 5. Paller MS. Mechanism of decreased pressor responsiveness to ANG II, NE, and vasopressin in pregnant rats. Am J Physiol. 1984;247(1 pt 2):H100–H108. [DOI] [PubMed] [Google Scholar]
- 6. Rosenfeld CR, Gant NF., Jr The chronically instrumented ewe, a model for studying vascular reactivity to angiotensin II in pregnancy. J Clin Invest. 1981;67(2):486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tolledo OE, Rhodes K, Livingston E. Renin-angiotensin system in normal and toxemic pregnancies. I. Angiotensin infusion test. Am J Obstet Gynecol. 1966;96(1):141–143. [DOI] [PubMed] [Google Scholar]
- 8. Greenwood JP, Scott EM, Stoker JB, Walker JJ, David ASGM. Sympathetic neural mechanisms in normal and hypertensive pregnancy in humans. Circulation. 2001;104(18):2200–2204. [DOI] [PubMed] [Google Scholar]
- 9. Jarvis SS, Shibata S, Bivans TB, et al. Sympathetic activation during early pregnancy in humans. J Physiol. 2012;590(pt 15):3535–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosenfeld CR. Mechanisms regulating angiotensin II responsiveness by the uteroplacental circulation. Am J Physiol Regulatory Integrative Comp Physiol. 2001;281(4):R1025–R1040. [DOI] [PubMed] [Google Scholar]
- 11. Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest. 1973;52(11):2682–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tolledo OE, Chesley LC, Zuspan FP. Renin-angiotensin system in normal and toxemic pregnancies. III. differential sensitivity to angiotensin II and norepinephrine in toxemia of pregnancy. Am J Obstet Gynecol. 1968;100():218–221. [Google Scholar]
- 13. Magness RR, Rosenfeld CR. Systemic and uterine responses to α-adrenergic stimulation in pregnant and nonpregnant ewes. Am J Obstet Gynecol. 1986;155(4):897–904. [DOI] [PubMed] [Google Scholar]
- 14. Naden RP, Gant NF, Jr, Rosenfeld CR. The pressor response to angiotensin II: the role of peripheral and cardiac responses in pregnant and nonpregnant sheep. Am J Obstet Gynecol. 1984;148(4):450–457. [DOI] [PubMed] [Google Scholar]
- 15. Damron DP, Bernstein IM, Shapiro RE, Schonberg A. Uterine blood flow responses to alpha-adrenergic blockade in nulligravid women of reproductive age. J Soc Gynecol Invest. 2004;11(6):388–392. [DOI] [PubMed] [Google Scholar]
- 16. Erkkola RU, Pirhonen JP. Flow velocity waveforms in uterine and umbilical arteries during the angiotensin II sensitivity test. Am J Obstet Gynecol. 1990;162(5):1193–1197. [DOI] [PubMed] [Google Scholar]
- 17. Erkkola RU, Pirhonen JP. Uterine and umbilical flow velocity waveforms in normotensive and hypertensive subjects during the angiotensin II sensitivity test. Am J Obstet Gynecol. 1992;166(3):910–916. [DOI] [PubMed] [Google Scholar]
- 18. Naden RP, Rosenfeld CR. Effect of angiotensin II on uterine and systemic vasculature in pregnant sheep. J Clin Invest. 1981;68(2):468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosenfeld CR, Barton MD, Meschia G. Effects of epinephrine on distribution of blood flow in the pregnant ewe. Am J Obstet Gynecol. 1976;124(2):156–163. [DOI] [PubMed] [Google Scholar]
- 20. Rosenfeld CR, DeSpain K, Liu X-t. Defining the differential sensitivity to norepinephrine and angiotensin II in the ovine uterine vasculature. Am J Physiol Regul Integrat Compar Physiol. 2012;302(1):R59–R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cox BE, Rosenfeld CR, Kalinyak JE, Magness RR, Shaul PW. Tissue specific expression of vascular smooth muscle angiotensin II receptor subtypes during ovine pregnancy. Am J Physiol. 1996;271(1 pt 2):H212–H221. [DOI] [PubMed] [Google Scholar]
- 22. Cox BE, Word RA, Rosenfeld CR. Angiotensin II receptor characteristics and subtype expression in uterine arteries and myometrium during pregnancy. J Clin Endocrinol Metab. 1996;81(1):49–58. [DOI] [PubMed] [Google Scholar]
- 23. Rosenfeld CR, DeSpain K, Word RA, Liu X-t. Differential sensitivity to angiotensin II and norepinephrine in human uterine arteries. J Clin Endocrinol Metab. 2012;97(1):138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Annibale DJ, Rosenfeld CR, Kamm KE. Alterations in vascular smooth muscle contractility during ovine pregnancy. Am J Physiol Heart Circ Physiol. 1989;256(5 pt 2):H1282–H1288. [DOI] [PubMed] [Google Scholar]
- 25. Annibale DJ, Rosenfeld CR, Stull JT, Kamm KE. Protein content and myosin light chain phosphorylation in uterine arteries during pregnancy. Am J Physiol Cell. 1990;259(3 pt 1):C484–C489. [DOI] [PubMed] [Google Scholar]
- 26. Fuller R, Colton I, Gokina N, Mandala M, Osol G. Local versus systemic influences on uterine vascular reactivity during pregnancy in the single-horn gravid rat. Reprod Sci. 2011;18(8):723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology. 2009;24:58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xiao D, Zhang L. Calcium homeostasis and contraction of the uterine artery: effect of pregnancy and chronic hypoxia. Bio Reprod. 2004;70(4):1171–1177. [DOI] [PubMed] [Google Scholar]
- 29. Cox RH. Molecular determinants of voltage-gated potassium currents in vascular smooth muscle. Cell Biochem Biophysics. 2005;42(2):167–195. [DOI] [PubMed] [Google Scholar]
- 30. Nelson MT, Quayle JM. Physiologic roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol. 1995;268(4 pt 1):C799–C822. [DOI] [PubMed] [Google Scholar]
- 31. Hill MA, Yang Y, Ella SR, Davis MJ, Braun AP. Large conductance, Ca2+-activated K+ channels (BKCa) and arteriolar myogenic signaling. FEBS Letters. 2010;584(10):2033–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosenfeld CR, Cornfield DN, Roy T. Ca2+-activated K+ channels modulate basal and E2-induced rises in uterine blood flow in ovine pregnancy. Am J Physiol Heart Circ Physiol. 2001;281(1):H22–H31. [DOI] [PubMed] [Google Scholar]
- 33. Rosenfeld CR, Liu X-t, DeSpain K. Pregnancy modifies the large conductance Ca2+-activated K+ channel expression and cGMP-dependent signaling pathway in uterine vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2009;296(6):H1878–H1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brenner R, Perez GJ, Boner AD, et al. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature. 2000;407(6806):870–876. [DOI] [PubMed] [Google Scholar]
- 35. de Wet H, Allen M, Holmes C, Stobbart M, Lippiat JD, Callaghan R. Modulation of the BK channel by estrogens: examination at single channel level. Mol Membr Biol. 2006;23(5):420–429. [DOI] [PubMed] [Google Scholar]
- 36. Morrow JP, Zakharov SI, Liu G, Yang L, Sok AJ, Marx SO. Defining the BK channel domains required for β1-subunit modulation. Proc Natl Acad Sci USA. 2006;103(13):5096–5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Patterson AJ, Henrie-Olsen J, Brenner R. Vasoregulation at the molecular level: a role for the β1 subunit of the calcium-activated potassium (BK) channel. Trends Cardiovasc Med. 2002;12(2):78–82. [DOI] [PubMed] [Google Scholar]
- 38. Hu X-Q, Xiao D, Zhu R, et al. Pregnancy upregulates large-conductance Ca2+-activated K+ channel activity and attenuates myogenic tone in uterine arteries. Hypertension. 2011;58(6):1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosenfeld CR, Roy T, DeSpain K, Cox BE. Large-conductance Ca2+-dependent K+ channels regulate basal uteroplacental blood flow in ovine pregnancy. J Soc Gynecol Investig. 2005;12(6):402–408. [DOI] [PubMed] [Google Scholar]
- 40. Farley DB, Ford SP, Reynolds LP, Bhatnagar RK, Van Orden DE. Quantification of α1-adrenergic receptors in porcine uterine and mesenteric arteries. Am J Obstet Gynecol. 1984;150(5 pt 1):485–491. [DOI] [PubMed] [Google Scholar]
- 41. Isla M, Dyer DC. Characterization of α-adrenoreceptors in late pregnant ovine uterine artery. Eur J Pharmacol. 1990;178(3):321–331. [DOI] [PubMed] [Google Scholar]
- 42. Stjernquist M, Owman C. Adrenoreceptors mediating contraction in the human uterine artery. Human Reprod. 1990;5(1):19–24. [DOI] [PubMed] [Google Scholar]
- 43. Burrell JH, Lumbers ER. Angiotensin receptor subtypes in the uterine artery during ovine pregnancy. Eur J Pharmacol. 1997;330(2-3):257–267. [DOI] [PubMed] [Google Scholar]
- 44. Steele SC, Warren AY, Johnson IR. Effect of vascular endothelium on norepinephrine-induced contractions in uterine radial arteries from nonpregnant and pregnant human uterus. Am J Obstet Gynecol. 1993;168(5):1623–1628. [DOI] [PubMed] [Google Scholar]
- 45. Rosenfeld CR, West JA. Circulatory responses to systemic infusions of norepinephrine in the pregnant ewe. Am J Obstet Gynecol. 1977;127(4):376–383. [DOI] [PubMed] [Google Scholar]
- 46. Magness RR, Osei-Boaten K, Mitchell MD, Rosenfeld CR. In vitro prostacyclin production by ovine uterine and systemic arteries: Effects of angiotensin II. J Clin Invest. 1985;76(6):2206–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Magness RR, Sullivan JA, Li Y, Phernetton TM, Bird IM. Endothelial vasodilator production by uterine and systemic arteries. VI. Ovarian and pregnancy effects on eNOS and NO(x). Am J Physiol Heart Circ Physiol. 2001;280(4):H1692–H1698. [DOI] [PubMed] [Google Scholar]
- 48. Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol. 1997;272(2 pt 2):R441–R463. [DOI] [PubMed] [Google Scholar]
- 49. Rosenfeld CR, White RE, Roy T, Cox BE. Calcium-activated potassium channels and nitric oxide co-regulate estrogen-induced vasodilation. Am J Physiol Heart Circ Physiol. 2000;279(1):H319–H328. [DOI] [PubMed] [Google Scholar]
- 50. Rosenfeld CR, Word RA, DeSpain K, Liu XT. Large conductance Ca2+-activated K+ channels contribute to vascular function in nonpregnant human uterine arteries. Reprod Sci. 2008;15(7):651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Conrad KP, Colpys MC. Evidence against the hypothesis that prostaglandins are the vasopressor agents of pregnancy. Serial studies in chronically instrumented conscious rats. J Clin Invest. 1986;77(1):236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bratz IN, Dick GM, Partridge LD, Kanagy NL. Reduced molecular expression of K+ channel proteins in vascular smooth muscle from rats made hypertensive with Nω-nitro-L-arginine. Am J Physiol Heart Circ Physiol. 2005;289(3):H1277–H1283. [DOI] [PubMed] [Google Scholar]
- 53. Chang T, Wu L, Wang R. Altered expression of BK channel β1 subunit in vascular tissues from spontaneously hypertensive rats. Am J Hypertens. 2006;19(7):678–685. [DOI] [PubMed] [Google Scholar]
- 54. Faulhaber PS, Furstenau M, Lohn M, et al. Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca (2+) spark/STOC coupling and elevated bold pressure. Circ Res. 2000;87(11):E53–E60. [DOI] [PubMed] [Google Scholar]
- 55. Zhang Y, Gao Y, Zuo J, Lee KMKW, Janssen LJ. Alteration of arterial smooth muscle potassium channel composition and BKCa current modulation in hypertension. Eur J Pharmacol. 2005;514(2-3):111–119. [DOI] [PubMed] [Google Scholar]
- 56. Rosenfeld CR, Roy T. Large conductance Ca2+-activated and voltage-activated K+ channels contribute to the rise and maintenance of estrogen-induced uterine vasodilation and maintenance of blood pressure. Endocrinology. 2012;153(12):6012–6020. [DOI] [PMC free article] [PubMed] [Google Scholar]