Abstract
Rickettsia parkeri
is a recently recognized human pathogen primarily associated with the Gulf Coast tick Amblyomma maculatum, with immature stages of this tick reported from wild vertebrates. To better understand the role of vertebrates in the natural history of this bacterium, we evaluated small mammals and ground-dwelling birds for evidence of infection with R. parkeri or exposure to the organism. We sampled small mammals (n=39) and passerines (n=47) in both north-central and southeast Mississippi, while northern bobwhite (Colinus virginianus) samples (n=31) were obtained from farms in central Mississippi. Blood from all sampled animals was tested using polymerase chain reaction (PCR) for spotted fever group rickettsiae (SFGR), and for antibodies to SFGR using R. parkeri antigen. Ectoparasite samples were removed from animals and included mites, lice, fleas, and immature ticks. Of 39 small mammal samples collected, 7 were positive for antibodies to SFGR; none tested positive by PCR for DNA of SFGR. Of 47 passerine blood samples collected, none were positive for DNA of SFGR by PCR, nor did any show serological evidence of exposure. Finally, none of 31 northern bobwhite samples tested were positive for SFGR DNA, while 7 were seropositive for rickettsial antibodies. Detection of seropositive rodents and quail suggests a role for these host species in the natural history of SFGR, possibly including R. parkeri, but the extent of their role has not yet been elucidated.
Key Words: Birds, Ectoparasites, Gulf Coast tick, Rickettsiae, Small mammals
Introduction
Several tick-borne diseases have recently increased in incidence and geographic distribution, warranting classification as emerging infectious diseases (Gratz 1999; Fritz 2009). Rickettsiosis caused by Rickettsia parkeri, a member of the spotted fever group rickettsiae (SFGR), is a recently recognized tick-borne disease first reported in a human in 2004 (Paddock et al. 2004). Although these diseases mainly affect humans, wildlife may play an important role in their persistence in nature. This role may include serving as a reservoir or amplifier of infection, or as a primary host in the tick life cycle and as a dead-end for the pathogen.
Vertebrate hosts play a role in the natural maintenance of some rickettsial organisms. The causative agent of Rocky Mountain spotted fever (RMSF), Rickettsia rickettsii, has been reported to circulate at high enough levels in Colombian and golden-mantled ground squirrels, meadow mice, and snowshoe hares, to infect naïve feeding ticks (Burgdorfer et al. 1966). In South America, capybaras and opossums (Didelphis spp.) have been implicated as amplifiers of this organism as well (Labruna 2009). Rickettsia typhi is maintained by a rat-flea life cycle (Azad 1990), and has also been found in spleen tissue samples from opossums (Didelphis marsupialis; Williams et al. 1992), suggesting that this opossum is a good host for proliferation of the bacterium. In Mississippi, mammals such as raccoons, opossums, cottontail rabbits, and white-tailed deer, have been shown to have antibodies to SFGR (Norment et al. 1985; Castellaw et al. 2011). Identifying vertebrates that are part of tick-borne agent life cycles provides important information about the ecology and epidemiology of such pathogens that is relevant to the veterinary and public health sectors.
Rickettsia parkeri is a member of the Alpha-proteobacteria, in the family Rickettsiaceae, and is phylogenetically closest to R. africae (Fournier et al. 1998; Roux and Raoult 2000). This organism, like other SFGR, requires a vertebrate or invertebrate host for both proliferation and survival, as it uses energy from its host's cells (Weiss 1973). Because R. parkeri was only recently recognized as a pathogen after almost 70 years of being considered non-pathogenic (Parker 1939; Paddock et al. 2004), our understanding of its natural history is minimal. Although the main tick vector is Amblyomma maculatum (Parker 1939; Philip et al. 1978), the role played by vertebrate hosts of this tick in the cycle of R. parkeri is not known. Transovarial and transstadial forms of transmission are important in perpetuating the life cycle for many rickettsiae (Azad and Beard 1998), thus immature stages of the ticks may be more important than adults in spreading infection to other ticks and naïve vertebrate hosts. Transovarially-infected larvae have the potential to spread the infection to the vertebrate hosts they feed on, and transstadially to nymphal stages, which again have the ability to transmit the pathogen to their vertebrate hosts. Larval and nymphal Gulf Coast ticks are generally found on ground-dwelling animals (Bishopp and Hixson 1936; Hixson 1940), thus our objective was to evaluate wild birds and small mammals for exposure to and infection with SFGR such as R. parkeri. Ultimately, these data should contribute to an understanding of the natural history of SFGR in vertebrate hosts.
Materials and Methods
Small mammal samples
Sites in Mississippi for trapping small mammals were chosen based on the presence of A. maculatum as identified by previous studies (Goddard and Norment 1983; Goddard and Paddock 2005), or property owners. Trapping was performed at two locations (Fig. 1) in winter 2009 and spring 2010, using Sherman live-traps. The traps were baited using peanut butter and oats and placed in a grid with 10-m spacing between traps. The traps were checked starting at 0700–0800 h the following morning, and every 2 h for the remainder of the day. Trapped small mammals were processed on site. Blood (0.3 mL maximum) was collected from the saphenous vein using heparin-coated capillary tubes. The animal was then combed for ectoparasites as thoroughly as possible without causing undue stress, which were collected and placed in 70% ethanol. All mammals were then released at their original capture sites.
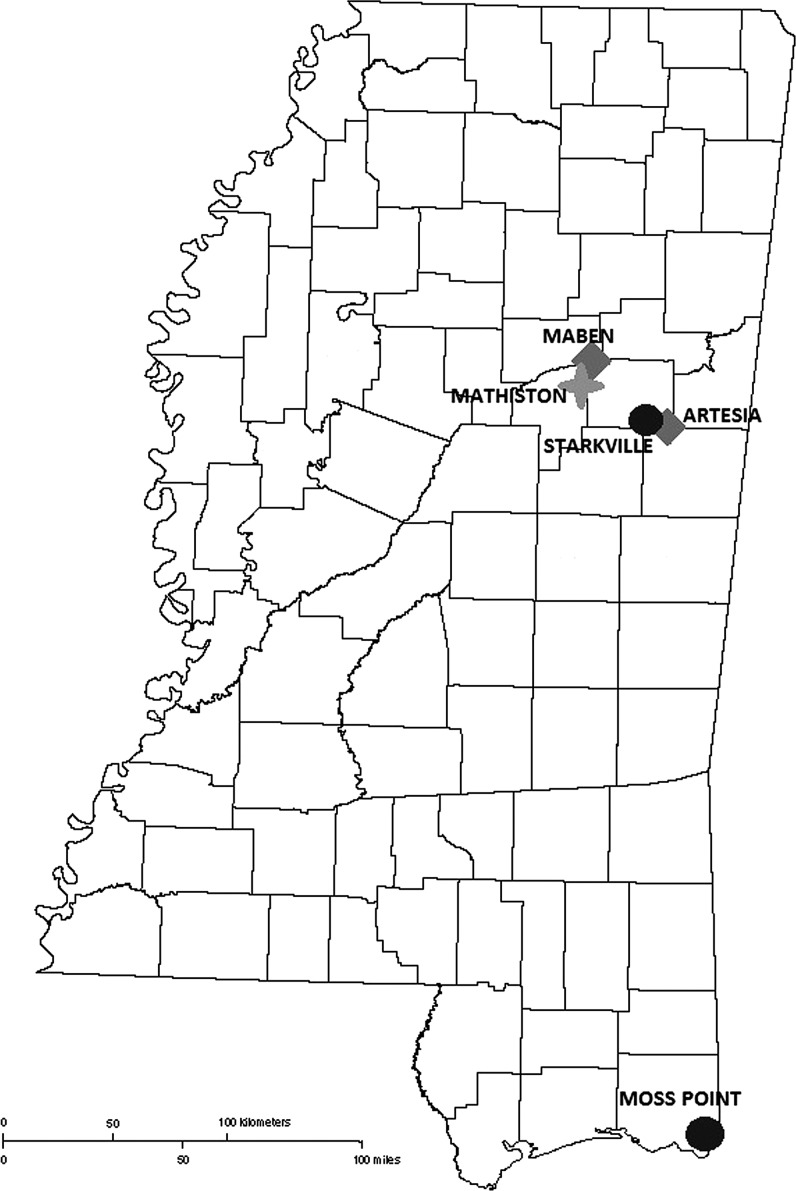
FIG. 1.
Map of Mississippi showing vertebrate blood sampling sites for small mammals and passerines (circles; Starkville and Moss Point), and quail (diamonds; Maben and Artesia). The four-point star shows a site at which only passerine samples were obtained (Mathiston).
Avian samples
Passerines were caught in spring and summer 2009 using mist nets in two north-central sites (Starkville: +33° 29’ 6.85”, −88° 46’ 41.13”; Mathiston: +33° 31’ 44.21”, −89° 7’ 54.97”), and at one coastal site (Moss Point: +30° 23’ 56.58”, −88° 27’ 28.99”; Fig. 1). Blood samples were collected from captured birds via jugular venipuncture (0.3 mL maximum) with syringes interiorly coated with heparin. The birds' heads were then examined for any attached ticks before being released at their capture sites. Sampling passerines was done with a scientific collection permit from the Mississippi Department of Wildlife, Fisheries, and Parks (U.S. Fish and Wildlife Service permit MB027041-1).
We collected samples from two northern bobwhite farms in north-central Mississippi that housed quail in conditions amenable to ticks. Specifically, the birds were housed in runs on the ground with wire fencing. One farm was in Maben (+33° 34’ 37.64”, −89° 3’ 7.82”), and the other was near Starkville (+33° 22’ 34.05”, −88° 41’ 30.98”). Blood was collected from the jugular vein as was done for passerines. Quail were checked for ectoparasites around their heads and then released.
DNA extractions
Small mammal blood samples were extracted using GE Healthcare's Illustra blood genomicPrep Mini Spin kit (GE Healthcare, Piscataway, NJ). Avian blood was extracted using the QIAamp DNA Blood Midi kit (Qiagen, Valencia, CA). In all cases, 50 μL of blood was extracted following the manufacturer's protocols.
Polymerase chain reaction (PCR)
A nested PCR assay targeting the rickettsial outer membrane protein A (rompA) gene specific for SFGR was used with primers 190-70 and 190-701 for the primary reaction, and primers 190-FN1 and 190-RN1 for the secondary reaction (Sumner et al. 2007). Rickettsia parkeri DNA extracts (Tate's Hell strain) and non-template water controls were included in the nested PCR assay.
Indirect fluorescent antibody (IFA) test
Plasma from heparinized blood samples was tested to determine if SFGR-specific antibodies were present. Samples were screened at a 1:64 dilution using fluorescein isothiocyanate (FITC) anti-rat (KPL, Gaithersburg, MD) and FITC anti-mouse (KPL) as secondary antibodies for rat samples and for other small mammal samples, respectively. FITC anti-bird (KPL) was used for passerine samples, and FITC anti-chicken (KPL) was used for northern bobwhite samples. End-point titrations were determined on positive samples using twofold serial dilutions ranging from 1:64 to 1:1024. Diluted samples were placed on R. parkeri antigen-coated 12-well slides, incubated for 35 min at 37°C, then washed in PBS followed by water. The appropriate FITCs were added to the wells, and the slides were again incubated at 37°C for 35 min. Finally, the slides were washed, counterstained with Eriochrome Black T, and air-dried before applying Vectashield® (Vector Laboratories, Inc., Burlingame, CA) and a cover-slip.
Ectoparasite identification
Samples were first sorted by order (or subclass Acari for mites), and then identified to species level by specialists: Dr. Lance A. Durden at Georgia Southern University; Dr. Jerome Goddard at Mississippi State University; Dr. Hans Klompen at Ohio State University; and Dr. Richard Robbins of the Armed Forces Pest Management Board. Vouchers were deposited in the Mississippi Entomological Museum, Mississippi State University.
Results
Sample collection
Twenty-four samples were obtained from rodents trapped in Moss Point in winter 2009 (n=12) and spring 2010 (n=12; Table 1). The small mammal samples obtained in Starkville were from animals trapped in association with a Mississippi State University course in winter 2009 (n=14), and independently in spring 2010 (n=1). Forty-seven passerines were caught by mist net and sampled (May through July 2009), from Starkville (n=31), Moss Point (n=8), and Mathiston (n=8). Twenty northern bobwhite samples were obtained from the farm near Starkville (May 2010), and 11 were collected from the farm in Maben (November 2010).
Table 1.
List of Vertebrate Species and Number Collected by Capture Site
| Starkville | Moss Point | Mathiston | Maben | |
|---|---|---|---|---|
| Small | Cotton Rat (4) | Cotton Rat (22) | N/A | N/A |
| Mammals | Woodland Vole (1) | Peromyscus sp. (2) | ||
| House mouse (2) | ||||
| Southern Short-tailed Shrew (6) | ||||
| Eastern Woodrat (1) | ||||
| Peromyscus sp. (1) | ||||
| Passerines | Northern Cardinal (12) | Northern Cardinal (4) | Northern Cardinal (5) | N/A |
| Northern Mockingbird (1) | Northern Mockingbird (2) | Eastern Wood Pewee (1) | ||
| Carolina Wren (3) | Carolina Wren (1) | Carolina Wren (1) | ||
| Brown Thrasher (1) | Gray Catbird (1) | Brown Thrasher (1) | ||
| Summer Tanager (1) | ||||
| Prothonotary Warbler (1) | ||||
| House Sparrow (5) | ||||
| Tufted Titmouse (1) | ||||
| Eastern Bluebird (2) | ||||
| Red-bellied Woodpecker (1) | ||||
| Unknown (2) | ||||
| Blue Grosbeak (1) | ||||
| Quail | Northern Bobwhite (20) | N/A | N/A | Northern Bobwhite (11) |
PCR results
No small mammal, passerine, or northern bobwhite samples tested positive for SFGR DNA by PCR. Positive controls showed bands of appropriate size on agarose gels.
IFA results
Five rodent samples were seropositive. Two hispid cotton rats (Sigmodon hispidus) from Moss Point tested positive in winter 2009. Also at Moss Point, two cotton rats and one Peromyscus species tested positive in spring 2010. No passerines tested positive for SFGR antibodies; however, seven northern bobwhites from Starkville were seropositive. No northern bobwhites from the farm in Maben were seropositive. End-point titrations of positive samples are shown in Table 2.
Table 2.
Plasma Titrations of Rodents and Bobwhite Quail Analyzed by Immunofluorescence Antibody Test
| No. positive (total sampled) | Titer Range | |
|---|---|---|
| Sigmodon hispidus | 4 (24) | 1:64–1:512 |
| Peromyscus sp. | 1 (2) | 1:512 |
| Colinus virginianus | 7 (20) | 1:64–1:256 |
Rodents listed were trapped at Moss Point in Mississippi. Quail samples were obtained from a farm outside of Starkville in Mississippi.
Ectoparasite samples
Four orders of ectoparasites were found on rodents, including two species of ticks, three genera of mites, one sucking louse species, and two flea species (Table 3). All specimens were mounted for identification. No ectoparasites were found on birds or shrews.
Table 3.
Ectoparasites Found on Rodents Trapped in Moss Point, Mississippi
| Number of ectoparasites collected (no. hosts) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Acari | Anoplura | Ixodida | Siphonaptera | ||||||
| Host (total no.) | Androlaelaps | Androlaelaps fahrenholtzi | Gigantolaelaps | Ornithonyssus bacoti | Hoplopleura hirsuta | Amblyomma maculatum | Dermacentor variabilis | Orchopeas howardi | Polygenis gwyni |
| December 2009 | |||||||||
| Sigmodon hispidus (12) | 4 (3) | 0 | 0 | 0 | 4 (2) | 0 | 0 | 1 (1) | 0 |
| Peromyscus (1) | 0 | 0 | 6 (1) | 0 | 0 | 0 | 1a (1) | 0 | 0 |
| May 2010 | |||||||||
| Sigmodon hispidus (12) | 19 (5) | 0 | 0 | 2 (1) | 2 (2) | 2 (1) | 2(2) | 0 | 5 (5) |
| Peromyscus (1) | 0 | 2 (1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Specimen was larval stage; all other tick specimens were nymphal stages.
Orders (or subclass in the case of mites) are indicated.
Discussion
Cotton rats and northern bobwhite quail serve as important vertebrate hosts for immature stages of A. maculatum (Hixson 1937), and thus may play a role in the natural history of the A. maculatum-transmitted pathogen R. parkeri. However, our study provides evidence of exposure to SFGR as a group, which includes organisms such as R. rickettsii that uses Dermacentor variabilis as a primary vector. Therefore, these animals may have been exposed to different rickettsiae, particularly because both D. variabilis and A. maculatum were collected from rodents. On the other hand, a recent study reported 28% of unfed A. maculatum ticks from Florida and Mississippi to be infected with R. parkeri, representing a range of 11–40% in the individual counties sampled (Paddock et al. 2010). This is in stark contrast to a reported prevalence of generally less than 5% of Dermacentor ticks being infected with R. rickettsii (Burgdorfer et al. 1975; Linnemann et al. 1980; McDade and Newhouse 1986; Wikswo et al. 2008; Stromdahl et al. 2011).
While no rickettsial DNA was detected in blood samples, some animals showed strong antibody responses to R. parkeri antigen (specifically, two rodents from Moss Point had end-points of 1:512). This suggests that these animals were exposed to SFGR, likely sometime within the previous 2 months. Experimental infections using rabbits, guinea pigs, and mice, suggested that those animals generally show a peak antibody response between 10 and 20 dpi. The authors noted that a high dose (5.6×106 plaque-forming units) was needed to elicit a response from the mice (Anacker et al. 1979). Samples from animals in the current study that showed higher titers for SFGR antibodies (rodents at Moss Point) were collected during peak activity times for both larval and nymphal A. maculatum—late fall and early spring (Hixson 1937, 1940).
Results from PCR tests did not reflect those of IFA assays, but rickettsial DNA can be present in hosts without being in circulation. Rickettsiae are obligate intracellular parasites infecting endothelial cells (Pinkerton 1942). These cells are in general not found circulating throughout the body, although they can enter the bloodstream as a result of damage to the endothelium (Silverman 1984; Valbuena and Walker 2009). Other rickettsiae have been detected in wildlife by PCR, but at very low rates. For example, R. helvetica DNA was identified in 8 of 112 Sika deer samples in Japan (Inokuma et al. 2008). The PCR tests performed in this study did not detect rickettsial DNA in the blood; the organism may have been present in low numbers, or not circulating in the animals at the time samples were taken. Experimental infections performed in our laboratory resulted in isolation of R. parkeri from cotton rat tissues, including skin, blood, and spleen during acute infection, but not chronic infection (Moraru et al., unpublished data). The current study did not incorporate euthanasia and necropsy of field animals, and so did not allow for collection of such samples, which would be important to test whether R. parkeri occurs in tissues other than blood. It would also be of interest in future studies to perform methodical checks for ectoparasites such that some could be tested for rickettsial DNA.
Cotton rats and northern bobwhite have shown evidence of exposure to SFGR, and they are known hosts for A. maculatum, the primary vector of R. parkeri (Parker 1939; Sumner et al. 2007). If they do prove to play a role in the maintenance of R. parkeri, then this would have implications in the epidemiology of this pathogen. To gain a better understanding of the ecology of this system, future studies with these hosts should include serosurveys to detect R. parkeri-specific antibodies, testing of their ectoparasites for R. parkeri, and experimental infections with R. parkeri.
Acknowledgments
This work was partially funded by a Morris Animal Foundation Veterinary Scholar grant and the Office of Research and Graduate Studies, College of Veterinary Medicine, Mississippi State University. We thank Susan Crittenden and Lance Crowley for allowing us to sample at their quail farms. Our appreciation to Cindy Evans for access to the site in Moss Point, and to Dr. Blake Layton for access to the site in Starkville.
Author Disclosure Statement
No competing financial interests exist.
References
- Anacker RL. Philip RN. Thomas LA, et al. Indirect hemagglutination test for detection of antibody to Rickettsia rickettsii in sera from humans and common laboratory animals. J Clin Microbiol. 1979;10:677–684. doi: 10.1128/jcm.10.5.677-684.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad FA. Beard CB. Rickettsial pathogens and their arthropod vectors. Emerg Infect Dis. 1998:4. doi: 10.3201/eid0402.980205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad AF. Epidemiology of murine typhus. Annu Rev Entomol. 1990;35:553–569. doi: 10.1146/annurev.en.35.010190.003005. [DOI] [PubMed] [Google Scholar]
- Bishopp FC. Hixson H. Biology and economic importance of the Gulf Coast tick. J Econ Entomol. 1936;29:1068–1076. [Google Scholar]
- Burgdorfer W. Adkins JTR. Priester LE. Rocky Mountain spotted fever (tick-borne typhus) in South Carolina: An educational program and tick/rickettsial survey in 1973 and 1974. Am J Trop Med Hyg. 1975;24:866–872. doi: 10.4269/ajtmh.1975.24.866. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W. Friedhoff KT., Jr Natural history of tick-borne spotted fever in the USA: Susceptibility of small mammals to virulent Rickettsia rickettsii. Bull World Health Org. 1966;35:149–153. [PMC free article] [PubMed] [Google Scholar]
- Castellaw AH. Chenney EF. Varela-Stokes AS. Tick-borne disease agents in various wildlife from Mississippi. Vector-Borne Zoonotic Dis. 2011;11:439–442. doi: 10.1089/vbz.2009.0221. [DOI] [PubMed] [Google Scholar]
- Fournier PE. Roux V. Raoult D. Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA. Int J Syst Bacteriol. 1998;48:839–849. doi: 10.1099/00207713-48-3-839. [DOI] [PubMed] [Google Scholar]
- Fritz CL. Emerging tick-borne diseases. Vet Clin North Am Small Anim Pract. 2009;39:265–278. doi: 10.1016/j.cvsm.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Goddard J. Norment BR. Notes on the geographical distribution of the Gulf Coast tick, Amblyomma maculatum (Koch) [Acari: Ixodidae] Entomol News. 1983;94:103–104. [Google Scholar]
- Goddard J. Paddock CD. Observations on distribution and seasonal activity of the Gulf Coast tick in Mississippi. J Med Entomol. 2005;42:176–179. doi: 10.1093/jmedent/42.2.176. [DOI] [PubMed] [Google Scholar]
- Gratz NG. Emerging and resurging vector-borne diseases. Annu Rev Entomol. 1999;44:51–75. doi: 10.1146/annurev.ento.44.1.51. [DOI] [PubMed] [Google Scholar]
- Hixson H. Environmental and host interrelationships and life history of the Gulf Coast tick (Amblyomma maculatum Koch) in southern Georgia. Entomology. Iowa State University. 1937:82. [Google Scholar]
- Hixson H. Field biology and environmental relationships of the Gulf Coast tick in Southern Georgia. J Econ Entomol. 1940;33:179–189. [Google Scholar]
- Inokuma H. Seino N. Suzuki M, et al. Detection of Rickettsia helvetica DNA from peripheral blood of Sika deer (Cervus nippon yesoensis) in Japan. J Wildl Dis. 2008;44:164–167. doi: 10.7589/0090-3558-44.1.164. [DOI] [PubMed] [Google Scholar]
- Labruna MB. Ecology of rickettsia in South America. Ann NY Acad Sci. 2009;1166:156–166. doi: 10.1111/j.1749-6632.2009.04516.x. [DOI] [PubMed] [Google Scholar]
- Linnemann CC. Schaeffer AE. Burgdorfer W, et al. Rocky Mountain spotted fever in Clermont County, Ohio. Am J Epi. 1980;111:31–36. doi: 10.1093/oxfordjournals.aje.a112872. [DOI] [PubMed] [Google Scholar]
- McDade JE. Newhouse VF. Natural history of Rickettsia rickettsii. Ann Rev Microbiol. 1986;40:287–309. doi: 10.1146/annurev.mi.40.100186.001443. [DOI] [PubMed] [Google Scholar]
- Norment BR. Stricklin LS. Burgdorger W. Rickettsia-like organisms in ticks and antibodies to spotted-fever group rickettsiae in mammals from Northern Mississippi. J Wild Dis. 1985;21:125–131. doi: 10.7589/0090-3558-21.2.125. [DOI] [PubMed] [Google Scholar]
- Paddock CD. Fournier PE. Sumner JW, et al. Isolation of Rickettsia parkeri and identification of a novel spotted fever group Rickettsia sp. from Gulf Coast ticks (Amblyomma maculatum) in the United States. Appl Environ Microbiol. 2010;76:2689–2696. doi: 10.1128/AEM.02737-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD. Sumner JW. Comer JA, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805–811. doi: 10.1086/381894. [DOI] [PubMed] [Google Scholar]
- Parker RR. Observations on an infectious agent from Amblyomma maculatum. Public Health Rep. 1939;54:1482–1484. [Google Scholar]
- Philip RN. Casper EA. Burgdorfer W, et al. Serologic typing of rickettsiae of the spotted fever group by microimmunofluorescence. J Immunol. 1978;121:1961–1968. [PubMed] [Google Scholar]
- Pinkerton H. The pathogenic rickettsiae with particular reference to their nature, biologic properties, and classification. Bacteriol Rev. 1942;6:37–78. doi: 10.1128/br.6.1.37-78.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux V. Raoult D. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB) Int J Syst Bacteriol. 2000;50:1449–1455. doi: 10.1099/00207713-50-4-1449. [DOI] [PubMed] [Google Scholar]
- Silverman DJ. Rickettsia rickettsii-induced cellular injury of human vascular endothelium in vitro. Infect Immun. 1984;44:545–553. doi: 10.1128/iai.44.3.545-553.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromdahl EY. Vince M. Jiang J, et al. Infrequency of Rickettsia rickettsii in Dermacentor variabilis removed from humans, with comments on the role of other human-biting ticks associated with spotted fever group rickettsiae in the United States. Vector-Borne Zoonotic Dis. 2011;11:969–977. doi: 10.1089/vbz.2010.0099. [DOI] [PubMed] [Google Scholar]
- Sumner JW. Durden LA. Goddard J, et al. Gulf Coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg Infect Dis. 2007;13:751–753. doi: 10.3201/eid1305.061468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valbuena G. Walker DH. Infection of the endothelium by members of the order Rickettsiales. Thromb Haemost. 2009;102:1071–1079. doi: 10.1160/TH09-03-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. Growth and physiology of rickettsiae. Bacteriol Rev. 1973;37:259–283. doi: 10.1128/br.37.3.259-283.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikswo ME. Hu R. Dasch GA, et al. Detection and identification of spotted fever group rickettsiae in Dermacentor species from Southern California. J Med Ent. 2008;45:509–516. doi: 10.1603/0022-2585(2008)45[509:daiosf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Williams SG. Sacci JB., Jr. Schriefer ME, et al. Typhus and typhuslike rickettsiae associated with opossums and their fleas in Los Angeles County, California. J Clin Microbiol. 1992;30:1758–1762. doi: 10.1128/jcm.30.7.1758-1762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]