Abstract
Background
To estimate the clinical benefit of HAART initiation versus deferral in a given month among patients with CD4 counts <800 cells/µL.
Methods
In this observational cohort study of HIV-1 seroconverters from CASCADE, we constructed monthly sequential nested subcohorts from 1/1996 to 5/2009 including all eligible HAART-naïve, AIDS-free individuals with a CD4 count <800 cells/uL. The primary outcome was time to AIDS or death among those who initiated HAART in the baseline month compared to those who did not, pooled across subcohorts and stratified by CD4. Using inverse-probability-of-treatment-weighted survival curves and Cox proportional hazards models, we estimated the absolute and relative effect of treatment with robust 95% confidence intervals (in parentheses).
Results
Of 9,455 patients with 52,268 person-years of follow-up, 812 (8.6%) developed AIDS and 544 (5.8%) died. Within CD4 strata of 200–349, 350–499, and 500–799 cells/µL, HAART initiation was associated with adjusted hazard ratios for AIDS/death of 0.59 (0.43,0.81), 0.75 (0.49,1.14), and 1.10 (0.67,1.79), respectively; and with adjusted 3-year cumulative risk differences of −4.8% (−7.0%,−2.6%), −2.9% (−5.0%,−0.9%), and 0.3% (−3.7%,4.2%), respectively. In the analysis of all-cause mortality, HAART initiation was associated with adjusted hazard ratios of 0.71 (0.44,1.15), 0.51 (0.33,0.80) and 1.02 (0.49,2.12), respectively. Numbers needed to treat to prevent one AIDS event or death within 3 years were 21 (14,38) and 34 (20,115) in CD4 strata of 200–349 and 350–499 cells/µL, respectively.
Conclusions
Compared to deferring in a given month, HAART initiation at CD4 counts <500 (but not 500–799) cells/µL was associated with slower disease progression.
Introduction
Introduction of highly active antiretroviral therapy (HAART) in 1996 reduced morbidity and mortality in HIV-1 infected individuals.1 Randomized controlled trials conducted in immunocompromised patients (e.g. those with a CD4 count ≤200 cells/µL) demonstrated that rates of AIDS or death were halved in patients starting HAART compared with rates in patients treated with drugs from only one class over approximately a one-year period.2, 3
A central, unresolved issue in the care of HIV-1 infected patients is when HAART should be initiated. Randomized evidence is not likely to be available before 2015.4 Observational studies of three large multicenter seroprevalent cohorts have suggested clinical benefit to initiating therapy at CD4 counts above 350 cells/µL, however the magnitude and thresholds for benefit were quite different.5–7
Our objective was to provide clinically-relevant information about the relative and absolute benefits of HAART initiation at different CD4 counts to support treatment decisions for AIDS-free, HAART-naive individuals living with HIV. We applied a novel approach to a cohort of 9,455 HIV-1 seroconverters to estimate the benefit of initiating versus deferring HAART on long-term disease progression and death.
Methods
Study population
Patients included in this analysis were enrolled in one of 23 clinical cohorts in Europe, Australia and Canada participating in the CASCADE Collaboration which pools data on individuals with a well-estimated date of seroconversion (<2 years between last negative and first positive HIV tests).8 Individuals ≥13 years old at seroconversion were included in this analysis.
Study design
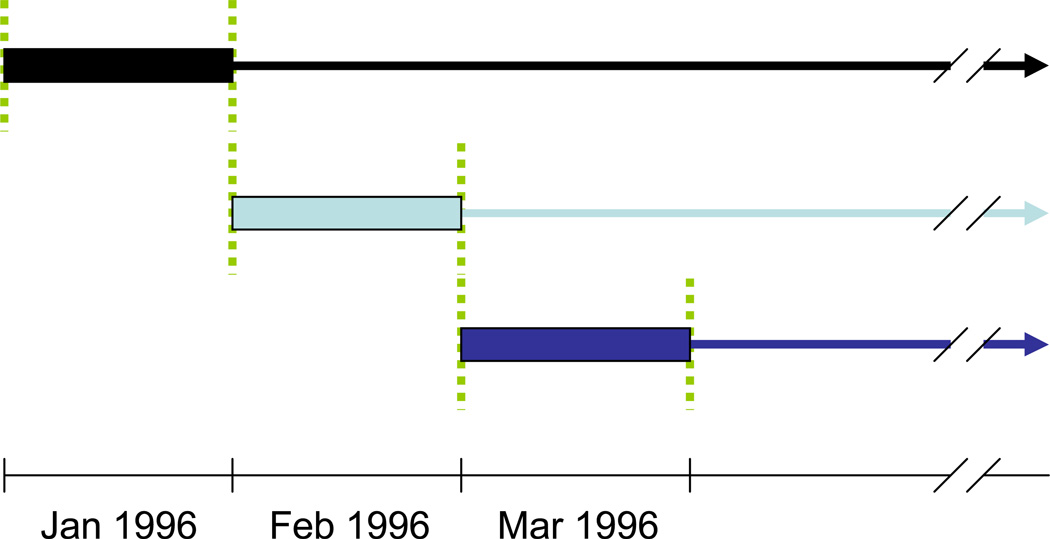
We created a set of sequential nested subcohorts (a special case of a nested structural model9–10), rather than a marginal structural model as used in a recent analysis.5 We first considered all individuals who were eligible as of 1 January 1996 and imagined a cohort study in which the subsequent disease progression of those who initiated HAART during this month was compared to that of patients who did not initiate HAART during this month (Figure 1). Among those patients who remained HAART-naive and otherwise eligible at the end of January 1996, we defined a new cohort for February 1996 to compare individuals who first initiated HAART in this month to those who did not. We created a new subcohort with all eligible individuals for each month between January 1996 and May 2009, classified each treatment naïve individual in the subcohort according to whether or not they initiated HAART in the index month, pooled the data across all 161 subcohorts, stratified the data into separate analyses based on CD4 count at baseline, and finally estimated the absolute and relative measures of association with HAART initiation. We used a robust variance11 to account for the fact that the same individual could contribute to more than one subcohort. To emulate the clinical scenario in which treatment decisions are made, we did not select a single alternative treatment strategy. Rather, we allowed the comparison group to encompass the range of treatment strategies present in this population. Thus, the survival times of patients who deferred HAART in the index month were used to represent the average population prognosis of individuals who were AIDS-free and HAART naïve with a CD4 count in the specified stratum but did not start HAART immediately, weighted by the number of trials each individual contributed in the CD4 stratum.
Figure 1.
Construction of sequential nested subcohorts. 1. Identify all eligible patients, assess covariates, and determine exposure group during January 1996 to create the first subcohort. 2. Measure days from February 1, 1996 to the date of first AIDS diagnosis, death or censoring for each patient. 3. Repeat Steps 1 and 2 for each month between Feb 1996 and May 2009 resulting in 161 subcohorts.
HAART was defined as any regimen containing ≥3 antiretrovirals. Patients were eligible if they 1) were HAART-naïve as of the first of the month, 2) had not experienced the endpoint of interest (i.e., AIDS or death) as of the last of the month, 3) had ≤21 days (cumulative) of mono or dual therapy, and 4) had a qualifying CD4 count (<800 cells/µL, ≥180 days post-seroconversion and within the prior 365 days). Eligibility criteria were time varying. A patient who did not have a qualifying CD4 count available at the time of the first subcohort for which he/she was otherwise eligible could still be included in a subsequent subcohort as soon as a qualifying CD4 count was recorded.
Ascertainment of AIDS and Death
The primary outcome of interest was the combined endpoint of time to first AIDS diagnosis or death from any cause. Analyses were repeated using death from all causes as the sole outcome. For each subcohort, follow-up began on the first day of the next month. Patients who did not experience an outcome of interest during follow-up were censored when they were last known alive.
Assessment of Covariates
We considered the following potential confounders: female sex, injection drug use as likely mode of transmission, documented seroconversion illness, hepatitis B and hepatitis C co-infection. Time-varying covariates included age, duration of infection, calendar year, CD4 measures (most recent, nadir, number of tests, days since last test), viral load (VL) measures (availability of ≥1 test, most recent [log10 copies per mL], peak [log10 copies per mL], number of tests, days since last test). All time varying characteristics were measured before the first day of follow-up.
Statistical analysis
Kaplan-Meier curves were used to visualize the crude (unadjusted) effect of initiating HAART compared to not initiating HAART in the index month, pooling across subcohorts. We estimated the hazard ratios for initiating HAART compared to deferring HAART during the index month separately for five CD4 strata (0–49, 50–199, 200–349, 350–499, and 500–799 cells/µL) using Cox proportional hazards models. All analyses followed an intent-to-treat approach and did not take into account treatment changes (i.e., interruptions, discontinuation or later initiations).
To account for potential differences in the baseline prognosis of those who initiated HAART compared to those who deferred HAART during the index month, we estimated inverse-probability-of-treatment weights as a function of baseline covariate values. We used these weights to create adjusted Kaplan-Meier survival curves,12 to estimate adjusted hazard ratios using weighted Cox proportional hazards models, and to estimate the adjusted absolute effect of HAART initiation on the cumulative risk of AIDS and death.13 Weights were truncated at the 0.05th and 99.95th percentile to reduce their variability and improve the stability of the final effect estimates.14 Confidence intervals on risk differences were obtained by bootstrap with 1000 complete resamples with replacement from the data.15, 16 We assumed a normal approximation of the parameter distribution and used the empirical standard error.
Sensitivity & Subgroup Analyses
We assessed the sensitivity of our results to alternative ways of conducting the analysis. These included a) shortening the period during which CD4 counts were considered eligible from 365 days to 45 days, which decreases the number of subcohorts in which an individual participates when their CD4 count has not been obtained immediately before or during the subcohort month; b) beginning follow-up in January 1998 rather than January 1996 to assess the influence of early, suboptimal HAART regimens; and c) requiring a baseline VL for subcohort eligibility. We examined the impact of non-standard treatment in the comparison group by censoring follow-up at the earliest of the 22nd day of cumulative mono- or dual-therapy, or six months after the patient’s first CD4 count <200 cells/µL if they remained HAART-naïve at this point. We also conducted a second version of this sensitivity analysis censoring individuals at six months after their first CD4 count <350 cells/µL if they remained HAART-naïve. Because the effect of HAART may differ in patients with a history of injection drug use (IDU), we conducted subgroup analyses of patients with (IDU+) or without a history of injection drug use (IDU−).
Results
Of 18,347 patients in the CASCADE Collaboration as of May 2009, 9455 were included in this analysis. The majority of patients excluded from the analysis were no longer alive, AIDS-free, ART-naïve, or in active follow-up at the beginning of the study period (January 1,1996) or at 6 months post-seroconversion. Many were no longer AIDS-free and ART-naïve at enrollment or at the time of their first eligible CD4 count. Thus, we analyzed data from 9,455 HIV-1 seroconverters who were eligible for ≥1 subcohort after 1 January 1996 with a total of 52,268 person-years of follow-up (median=4.7 years, IQR=2.0–9.1). The majority were male (n=7367, 78%) and infected through sex between men (n=5341, 56%) or sex between men and women (n=2363, 25%). The median age at seroconversion was 30.3 years (IQR=25.4–36.8) and the median duration of infection was 1.3 years (IQR=0.8–3.4) at the time of entry into the first subcohort. During follow-up, 812 (8.6%) patients developed AIDS and 544 (5.8%) died. On average, each individual contributed to 12 (IQR=4–26) subcohorts (eTable 1).
At baseline, those who initiated HAART had a poorer prognosis in some respects (higher VLs, shorter duration of infection, and slightly lower CD4 counts) compared to those who deferred HAART in a given month (Table 1). In other respects, they had a better prognosis (less likely to have a history of IDU and less likely to be co-infected with hepatitis). Across all CD4 strata, CD4 counts were more recent in those initiating therapy and these patients were more likely to have available VL measures. The majority of deferring patients eventually went on to HAART therapy, generally in the same CD4 stratum or the next lower stratum (eTable 2). The only exception was the 500–799 stratum in which nearly half of patients remained HAART naïve at last follow up. Of these naïve patients, most had CD4 counts above 350 cells/mL at last follow up. The use of all-NRTI regimens containing abacavir in the first HAART regimen was similar between those who initiated and those who deferred (eTable 3).
Table 1.
Description of participants† comparing those who initiated HAART (I) to those who deferred HAART (D) at baseline.
| Initiate | Defer | ||||
|---|---|---|---|---|---|
| CD4 strata | n median |
% (IQR) |
n median |
% (IQR) |
|
| 0–49 cells/µL (Nu=183) | |||||
| Subcohort observations, ns | 107 | 527 | |||
| Person-years of follow-up | 3.3 | (1.4–7.1) | 1.5 | (0.4–6.7) | |
| Female | 24 | 22.4% | 75 | 14.2% | |
| Injection drug use | 20 | 18.7% | 350 | 66.4% | |
| Hepatitis C co-infection | 21 | 19.6% | 270 | 51.2% | |
| Hepatitis B co-infection | 30 | 28.0% | 207 | 39.3% | |
| Year of seroconversion | 1996 | (1992–1999) | 1992 | (1988–1995) | |
| Age at seroconversion, years | 28 | (25–36) | 29 | (25–33) | |
| Duration of infection, years | 5.3 | (1.3–8.9) | 7.2 | (3.6–10.5) | |
| CD4 | count, cells/µL | 25 | (15–38) | 28 | (15–37) |
| nadir, cells/µL | 25 | (15–37) | 28 | (15–37) | |
| age, days | 19 | (10–34) | 76 | (27–161) | |
| Viral load | none available | 15 | 14.0% | 204 | 38.7% |
| most recent, log copies/mL‡ | 5.1 | (4.5–5.7) | 3.6 | (0.0–4.9) | |
| peak, log copies/mL‡ | 5.4 | (4.6–5.7) | 4.5 | (0.0–5.1) | |
| age, days‡ | 18 | (4–35) | 18 | (0–105) | |
| 50–199 cells/µL (Nu=1521) | |||||
| Subcohort observations, ns | 832 | 5,259 | |||
| Person-years of follow-up | 3.4 | (1.4–6.2) | 3.7 | (1.3–8.0) | |
| Female | 197 | 23.7% | 1,165 | 22.2% | |
| Injection drug use | 117 | 14.1% | 1,612 | 30.7% | |
| Hepatitis C co-infection | 151 | 18.1% | 1,680 | 31.9% | |
| Hepatitis B co-infection | 188 | 22.6% | 1,743 | 33.1% | |
| Year of seroconversion | 1999 | (1994–2002) | 1993 | (1990–1998) | |
| Age at seroconversion, years | 31 | (26–38) | 29 | (25–35) | |
| Duration of infection, years | 3.2 | (1.4–6.4) | 5.3 | (2.9–9.1) | |
| CD4 | count, cells/µL | 149 | (110–176) | 157 | (119–180) |
| nadir, cells/µL | 140 | (104–170) | 147 | (108–173) | |
| age, days | 23 | (13–39) | 53 | (19–117) | |
| Viral load | none available | 45 | 5.4% | 1203 | 22.9% |
| most recent, log copies/mL‡ | 5.0 | (4.4–5.4) | 4.4 | (2.2–5.0) | |
| peak, log copies/mL‡ | 5.1 | (4.7–5.6) | 4.7 | (2.9–5.3) | |
| age, days‡ | 24 | (13–43) | 34 | (2–100) | |
| 200–349 cells/µL (Nu=4459) | |||||
| Subcohort observations, ns | 1,792 | 33,824 | |||
| Person-years of follow-up | 3.0 | (1.3–6.1) | 3.6 | (1.6–6.9) | |
| Female | 371 | 20.7% | 6,755 | 20.0% | |
| Injection drug use | 175 | 9.8% | 5,546 | 16.4% | |
| Hepatitis C co-infection | 226 | 12.6% | 6,788 | 20.1% | |
| Hepatitis B co-infection | 361 | 20.1% | 8,652 | 25.6% | |
| Year of seroconversion | 2000 | (1995–2003) | 1997 | (1992–2001) | |
| Age at seroconversion, years | 31 | (25–38) | 30 | (25–36) | |
| Duration of infection, years | 2.8 | (1.5–5.6) | 4.0 | (2.1–7.2) | |
| CD4 | count, cells/µL | 270 | (238–309) | 297 | (260–324) |
| nadir, cells/µL | 251 | (219–288) | 270 | (228–306) | |
| age, days | 24 | (13–44) | 57 | (24–111) | |
| Viral load | none available | 66 | 3.7% | 4971 | 14.7% |
| most recent, log copies/mL‡ | 4.7 | (4.1–5.1) | 4.3 | (3.4–4.8) | |
| peak, log copies/mL‡ | 5.0 | (4.5–5.4) | 4.6 | (3.9–5.1) | |
| age, days‡ | 26 | (14–49) | 47 | (12–102) | |
| 350–499 cells/µL (Nu=5527) | |||||
| Subcohort observations, ns | 1,005 | 62,734 | |||
| Person-years of follow-up | 4.6 | (1.8–8.0) | 3.7 | (1.6–7.3) | |
| Female | 222 | 22.1% | 12,457 | 19.9% | |
| Injection drug use | 119 | 11.8% | 8,651 | 13.8% | |
| Hepatitis C co-infection | 172 | 17.1% | 11,017 | 17.6% | |
| Hepatitis B co-infection | 201 | 20.0% | 15,040 | 24.0% | |
| Year of seroconversion | 1998 | (1994–2001) | 1997 | (1993–2002) | |
| Age at seroconversion, years | 30 | (25–37) | 30 | (25–36) | |
| Duration of infection, years | 2.4 | (1.2–4.7) | 3.5 | (1.9–6.4) | |
| CD4 | count, cells/µL | 408 | (375–447) | 425 | (390–460) |
| nadir, cells/µL | 369 | (320–413) | 376 | (326–423) | |
| age, days | 23 | (13–42) | 70 | (32–128) | |
| Viral load | none available | 63 | 6.3% | 8371 | 13.3% |
| most recent, logcopies/mL‡ | 4.6 | (3.7–5.1) | 4.1 | (3.3–4.7) | |
| peak, log copies/mL‡ | 4.9 | (4.2–5.3) | 4.5 | (3.7–5.0) | |
| age, days‡ | 25 | (14–47) | 58 | (17–117) | |
| 500–799 cells/µL (Nu=5162) | |||||
| Subcohort observations, ns | 615 | 78,483 | |||
| Person-years of follow-up | 5.8 | (2.4–8.5) | 4.1 | (1.8–7.6) | |
| Female | 144 | 23.4% | 16,824 | 21.4% | |
| Injection drug use | 79 | 12.8% | 10,689 | 13.6% | |
| Hepatitis C co-infection | 100 | 16.3% | 13,383 | 17.1% | |
| Hepatitis B co-infection | 137 | 22.3% | 18,901 | 24.1% | |
| Year of seroconversion | 1997 | (1994–2000) | 1997 | (1993–2001) | |
| Age at seroconversion, years | 29 | (25–35) | 29 | (25–36) | |
| Duration of infection, years | 2.2 | (1.2–4.8) | 3.4 | (1.8–6.2) | |
| CD4 | count, cells/µL | 588 | (538–660) | 611 | (550–690) |
| nadir, cells/µL | 513 | (411–594) | 517 | (429–597) | |
| age, days | 19 | (11–32) | 78 | (38–141) | |
| Viral load | none available | 38 | 6.2% | 12,201 | 15.5% |
| most recent, log copies/mL‡ | 4.4 | (2.8–5.0) | 3.8 | (2.7–4.4) | |
| peak, log copies/mL‡ | 4.7 | (3.8–5.1) | 4.1 | (3.2–4.7) | |
| age, days‡ | 21 | (10–38) | 63 | (16–128) | |
Nu = unique individuals; ns = number of subcohort observations.
Number of participants is not unique within a given CD4 stratum due to an individual participant potentially contributing to multiple subcohorts. CD4 age defined as days between last CD4 count and start of follow-up. Viral load age defined as days between last viral load measure and start of follow-up.
All descriptive statistics based on 185,178 subcohort observations (ns) with the exception of rows marked by ‡ for which the denominator is only those subcohort observations with a viral load measure available.
Unadjusted incidence rates and adjusted hazard ratios (aHRs) stratified by CD4 count are presented in Table 2. Considering first the combined endpoint of AIDS or death, the effect of initiating rather than deferring HAART in a given month was protective at CD4 counts <350 cells/µL. At CD4 counts of 350–499 cells/µL, there was a 25% reduction in the hazard of AIDS or death (aHR=0.75, 95%CI: 0.49, 1.14). At CD4 counts of 500–799 cells/µL, AIDS-free survival was not different in the two groups after adjusting for covariates (aHR=1.10, 95%CI: 0.67, 1.79). In the analysis of all-cause mortality, HAART initiation appeared to have a stronger effect on death than on the combined endpoint at CD4 counts of 350–499 cells/µL (aHR=0.51, 95%CI: 0.33, 0.80). We observed no benefit at CD4 counts of 500–799 cells/µL (aHR=1.02, 95%CI: 0.49, 2.12).
Table 2.
Crude incidence rates (IR), crude hazard ratios (cHR) and adjusted hazard ratios (aHR) with 95% confidence intervals (CI) for the effect of initiating HAART (I) compared to deferring HAART (D) at baseline on time to first AIDS event or death and death alone stratified by CD4 count.
| AIDS or Death | Death | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 cells/µL |
PY FUu | IR/1000 PY* | IR/1000 PY* | ||||||||
| Eventsu | D | I | cHR (95%CI) | aHR (95%CI) | Eventsu | D | I | cHR (95%CI) | aHR (95%CI) | ||
| 0–49 (Nu =183) | 664 | 102 | 193.3 | 55.0 | 0.30 (0.19, 0.48) | 0.32 (0.17, 0.59) | 44 | 88.8 | 21.2 | 0.23 (0.12, 0.46) | 0.37 (0.14, 0.95) |
| 50–199 (Nu =1,521) | 6934 | 353 | 56.6 | 22.0 | 0.36 (0.28, 0.47) | 0.48 (0.31, 0.74) | 144 | 27.8 | 9.7 | 0.34 (0.24, 0.50) | 0.55 (0.28, 1.07) |
| 200–349 (Nu =4,459) | 22106 | 732 | 29.4 | 18.7 | 0.62 (0.51, 0.75) | 0.59 (0.43, 0.81) | 261 | 14.1 | 8.9 | 0.64 (0.50, 0.83) | 0.71 (0.44, 1.15) |
| 350–499 (Nu =5,527) | 29653 | 815 | 20.8 | 17.2 | 0.82 (0.66, 1.03) | 0.75 (0.49, 1.14) | 277 | 9.1 | 7.5 | 0.81 (0.57, 1.14) | 0.51 (0.33, 0.80) |
| 500–799 (Nu =5,162) | 28631 | 696 | 18.5 | 14.9 | 0.79 (0.59, 1.05) | 1.10 (0.67, 1.79) | 237 | 8.5 | 6.8 | 0.78 (0.51, 1.20) | 1.02 (0.49, 2.12) |
Nu=unique individuals within the CD4 stratum; PY FUu=unique person-years of follow-up within the CD4 stratum. Eventsu=unique events within the CD4 stratum. aHR adjusted via weighting for injection drug use, HIV test interval <30 days (indicator of seroconversion illness), female sex, time since seroconversion, age, calendar year, HCV co-infection, HBV co-infection, CD4 cell count, days between last CD4 count and start of follow-up, CD4 nadir, number of prior CD4 measures, most recent viral load (log10), days between last viral load and start of follow-up, peak viral load (log10), and number of prior viral load measures. Reference group for all estimates is comprised of subcohort observations during which HAART was not initiated during the index month.
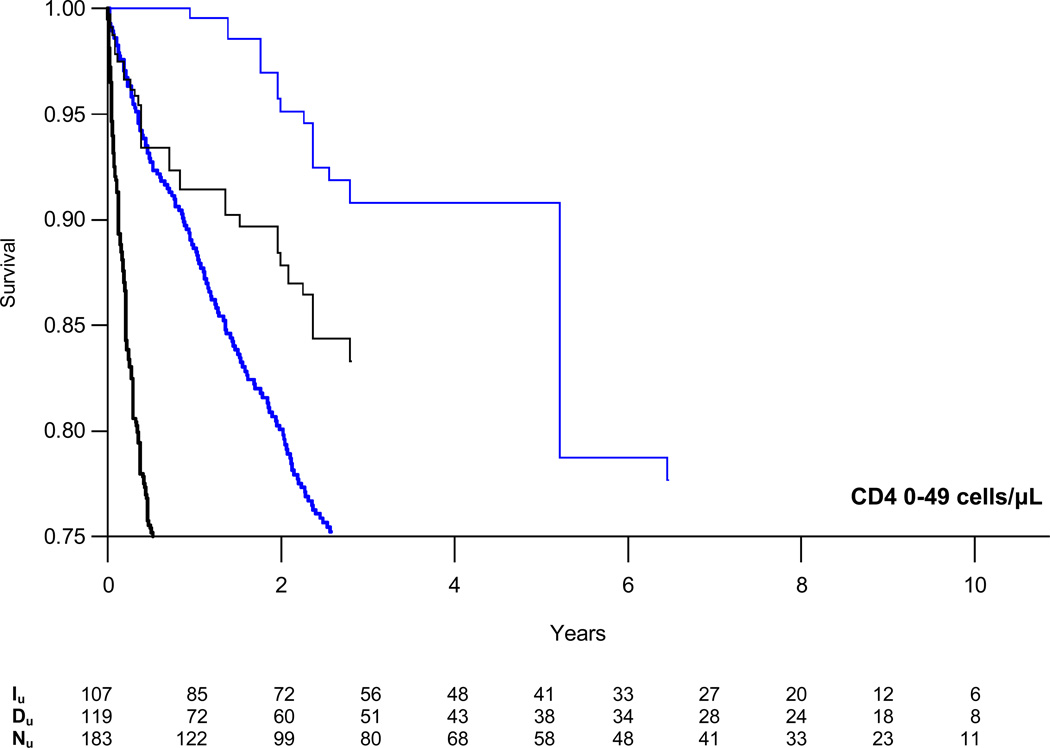
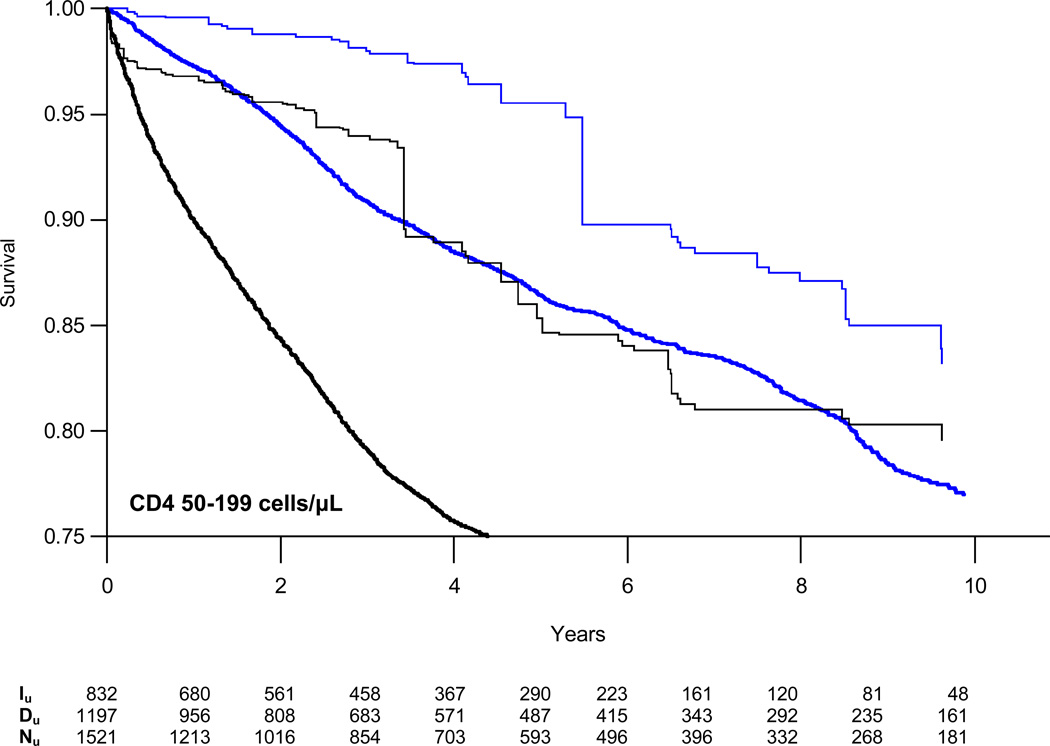
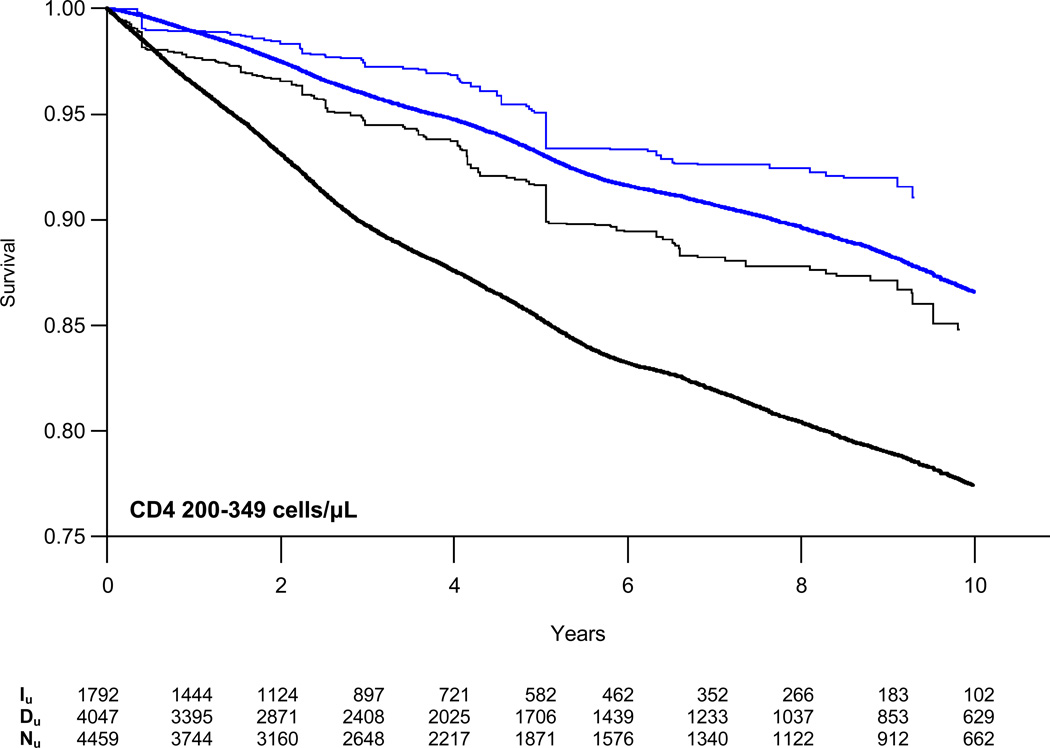
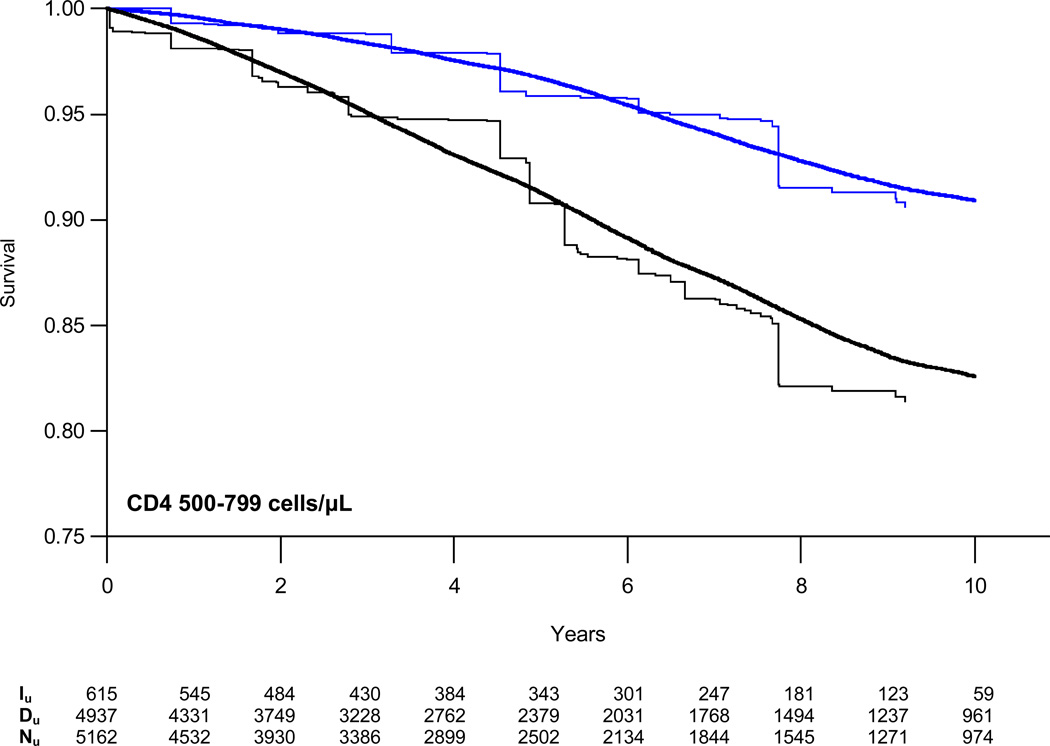
Weighted survival curves, stratified by CD4 count, are presented in Figure 2 with estimates of the absolute risk of AIDS/death or death alone at three years for those initiating and deferring therapy in Table 3. At CD4 counts of 200–349 cells/µL, the absolute difference in the proportion of patients who died or progressed to AIDS increased from −1.3% at one year to −6.4% at five years. The estimated number needed to treat (NNT) to prevent one event decreased from 79 to 16 at five years. Risk reduction was a third as large for patients with CD4 counts of 350–499 cells/µL with NNTs of 229 and 45 at one and five years, respectively. We found no reduction in the absolute risk of AIDS or death at CD4 counts of 500–799 cells/µL.
Figure 2.
Weighted semi-parametric survival curves for time to combined endpoint of first AIDS diagnosis or death from all causes (black lines) or death alone (blue lines) comparing patients who initiated (single weight lines) or deferred (doubly weight lines) HAART stratified by CD4 cell count. Iu = unique individuals in the HAART initiation group who remained in the risk set at time t; Du = unique individuals in the HAART deferral group who remained in the risk set at time t; Nu = unique individuals in the CD4 stratum overall who remained in the risk set at time t.
Table 3.
Adjusted estimates of the cumulative percentage of patients who would experience AIDS/death or death alone within 3 years of follow-up after deferring (D) or initiating (I) HAART at baseline, estimated risk differences (RD) and number needed to treat (NNT) with bootstrapped 95% confidence intervals (95%CI).
| CD4 cells/µL |
AIDS/Death | Death Alone | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | I | RD | (95%CI) | NNT | (95%CI) | D | I | RD | (95%CI) | NNT | (95%CI) | |
| 0–49 | 46.6 | 16.6 | −30.0 | (−45.1, −15.0) | 3 | (2, 7) | 26.8 | 8.6 | −18.2 | (−32.0, −4.4) | 6 | (3, 23) |
| 50–199 | 20.7 | 5.7 | −15.0 | (−19.7, −10.3) | 7 | (5, 10) | 9.1 | 1.9 | −7.2 | (−10.1, −4.4) | 14 | (10, 23) |
| 200–349 | 10.3 | 5.5 | −4.8 | (−7.0, −2.6) | 21 | (14, 38) | 4.1 | 2.7 | −1.4 | (−3.0, 0.3) | 74 | (33, ∞) |
| 350–499 | 6.3 | 3.4 | −2.9 | (−5.0, −0.9) | 34 | (20, 115) | 2.1 | 0.7 | −1.4 | (−2.2, −0.6) | 71 | (45, 165) |
| 500–799 | 4.9 | 5.2 | 0.3 | (−3.7, 4.2) | ∞ | n/a | 1.7 | 1.2 | −0.4 | (−2.0, 1.2) | 239 | (49, ∞) |
All estimates adjusted via weighting for injection drug use, HIV test interval <30 days (indicator of seroconversion illness), female sex, time since seroconversion, age, calendar year, HCV co-infection, HBV co-infection, CD4 cell count, days between last CD4 count and start of follow-up, CD4 nadir, number of prior CD4 measures, most recent viral load (log10), days between last viral load and start of follow-up, peak viral load (log10), and number of prior viral load measures.
When death from all causes was evaluated as the sole outcome, the absolute difference in the proportion of patients who died increased from essentially no difference at one year to −2.1% at five years for those with CD4 counts of 200–349 cells/µL. The NNT decreased from ~8000 to 49 over this time period. Similarly, the cumulative risk of death for patients with CD4 counts of 350–499 cells/µL differed by −0.3% at one year to −2.8% at five years with corresponding NNTs of 328 and 35, respectively. In patients with CD4 500–799, there was no reduction in the risk of death at one and five years, although there was a small difference at three years which favored HAART initiation.
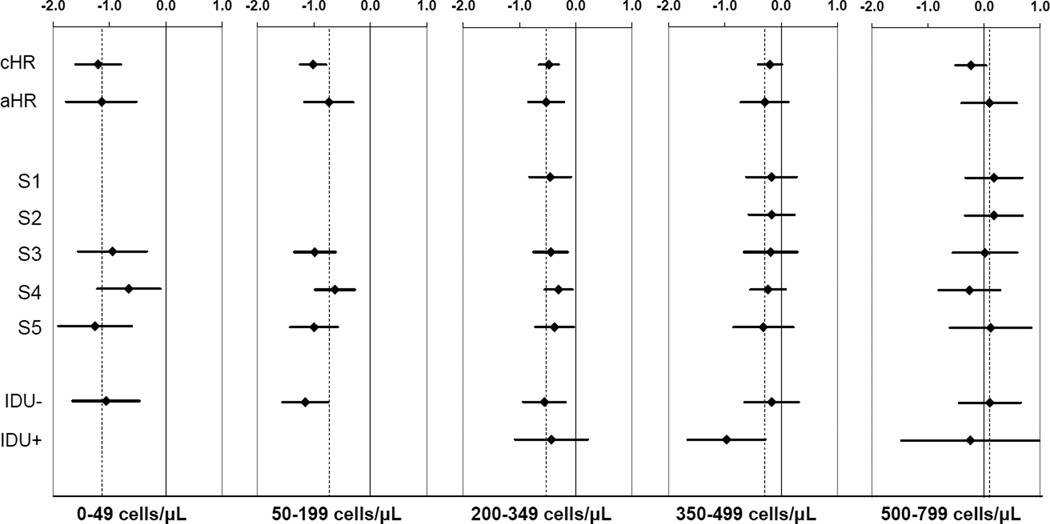
Results from sensitivity analyses suggest that these findings are robust to alternative ways of defining the eligible population and censoring outcomes of those who received non-standard treatment (Figure 3). We also found that excluding individuals with prior injection drug use did not have a meaningful effect on the magnitude of the association between HAART initiation and time to AIDS or death (eTable 4).
Figure 3.
Parts A–E. Assessing model sensitivity and results of subgroup analyses. Hazard ratios (on the natural log scale) and 95% confidence intervals for crude (cHR) and adjusted (aHR) analyses of time to first AIDS diagnosis or death from all causes. Sensitivity analyses include censoring outcomes of patients who initiated mono/dual therapy or failed to start HAART within six months after first CD4<200 (S1); censoring at mono/dual therapy for failure to initiate HAART within six months of first CD4 <350 (S2); requiring baseline viral load measure (S3), requiring CD4 cell count within last 45 days of baseline (S4), and beginning follow-up in January 1998 (S5). Subgroup analyses presented for those without (IDU−) and with (IDU+) known injection drug use history.
Discussion
This analysis of 9,455 HIV-1 seroconverters confirms the clinical benefit of initiating HAART with CD4 counts of 200–349 cells/µL. We estimated a 25% reduction in the relative hazard of AIDS or death and a 49% reduction in the relative hazard of death from all causes at CD4 counts of 350–499 cells/µL. The relatively low incidence of AIDS and death among those with CD4 counts of 350–499 indicates that patients and healthcare providers need to weigh the risks and benefits for each individual over an extended period of treatment.
While many studies have compared disease progression in patients starting HAART at different stages of disease with follow-up beginning at the time of treatment initiation, it is now appreciated that this study design is not ideally suited to inform the “when to start” question due to unobserved lead time and clinical events that occur during the time when patients are deferring therapy.17, 18 Kitahata et al5, Sterne et al6 and Cain et al7 report findings from observational analyses tailored to estimate the effect of early HAART initiation on clinical outcomes using data primarily from seroprevalent cohorts. Although the comparison groups differ and, thus, the effect estimates from these studies estimate different parameters, one can compare the conclusions of the studies in broad terms. Our findings agree with those of Kitahata, Sterne and Cain who found that deferring HAART to a CD4 count below 350 cells/µL is detrimental. Kitahata, but not Sterne, further conclude that deferring HAART to a CD4 count below 500 cells/µL is detrimental. (Cain et al began following patients at the first CD4 count <500 cells/µL, and thus, do not report effect estimates for treatment at CD4 counts >500 cells/µL.7) Unlike Kitahata et al, we did not observe a benefit at the population-level for initiation at 500–799 after adjusting for confounding.
The absolute risk of AIDS-related morbidity and mortality in the population can drive the degree to which HAART initiation is beneficial at a particular stage of disease. In our study, the weighted survival curves, absolute risks of disease progression, and NNT provide additional insight regarding the benefit that patients in resource rich settings can expect from HAART at different CD4 strata. At CD4 counts of 350–499 cells/µL, the benefits of treatment initiation only become evident beyond two years, suggesting that patients need to consider the long term course of treatment including the risk of adverse effects of HAART over an extended period of time.19
The decision to initiate therapy is a dynamic process, influenced by changes in the patient’s condition and readiness to adhere to the life-long regimens that are currently available to treat HIV. We reflected this dynamic process in our analysis by considering each month while a patient was AIDS-free and HAART-naïve as a point in time when therapy could have been initiated rather than representing patients at a single point in time such as the first measured CD4 count in a particular range. We then observed these individuals over an average of 4.7 years as they experienced the clinical consequences of initiating HAART (or not) at that point in time. By allowing individuals to contribute to multiple subcohorts as long as they remained eligible, we effectively estimated a weighted average of the benefit of initiating therapy at any time while an individual had a CD4 count in a given CD4 stratum compared to the prognosis that they would have experienced if they had not initiated HAART at that time. The resulting relative and absolute effect estimates can be used to help inform patient decisions about whether the benefit of therapy at this particular stage of disease is sufficient to outweigh the challenge of adhering to treatment, the risk of side effects, and the financial cost of medications over a longer period of treatment.
We acknowledge that if the ultimate treatment patterns of the deferrers had been different, the results of the study would have been different. In eTable 2, we describe the type and timing (relative to CD4 count) of antiretroviral therapy received by patients who comprised the deferred group for each CD4 strata. To evaluate the potential influence of individuals who were not treated consistent with current standard of care, we censored the outcomes of those who waited too long or used sub-optimal regimens, but the magnitude of our effect estimates was unaffected (Figure 3, S1 and S2). We also considered the possibility that the null effect in the 500–799 stratum was due to individuals who deferred HAART only briefly, but these patients comprised only ~5% of the deferred group. While the comparison groups did not follow standardized treatment algorithms, they do represent the real world experience of thousands of HIV-infected patients in care during the study period. We believe that these findings complement those from other recent studies which explicitly compared two specific, narrowly-defined treatment alternatives.
Patient well-being is adversely affected by many serious non-AIDS-defining conditions. For instance, immunodeficiency and uncontrolled viremia have been implicated in the development of cardiovascular disease20, 21 and non-AIDS-defining malignancies.22, 23 While CASCADE does not currently pool data on non-AIDS morbidity, this analysis does reflect the most serious outcome (death) due to non-AIDS conditions.
We have considered several alternative approaches to conducting this analysis in an effort to assess the robustness of our findings. We examined the effect of more restrictive inclusion criteria. To address confounding, we adjusted for a set of 20 covariates that we had a priori reason to suspect were associated with different rates of disease progression. We examined a wide range of possibilities for truncating the weights before deciding on a method that controlled for confounding without introducing instability in the estimates (eTable 5). Despite this, we cannot rule out the possibility that patients who initiated therapy had an inherently better or worse prognosis than those who deferred therapy related to unmeasured factors. We were reassured that in the 50–199 CD4 strata where we can compare to results from a randomized trial, our estimate is very similar to that from the trial.3
In the absence of results from well-conducted, long-term, randomized trials among patients with CD4 counts above 350 cells/µL, treatment decisions will need to be made based on the available evidence from observational cohorts. We used a novel approach applied to a unique cohort of seroconverters to reduce the potential for lead time bias. We found that treatment initiation at CD4 counts of 350–499 cells/µL was associated with slower disease progression. We did not observe any benefit to treatment initiated at 500–799 cells/µL.
Supplementary Material
Acknowledgements
We thank Bernadette Johnson, BS, MBA, The Blaze Group, for her SAS programming work on this project (paid using grant funds). We thank A. Sarah Walker, PhD, MSc, and Abdel Babiker, PhD, both salaried employees at the MRC Clinical Trials Unit, London, UK for advice regarding the study design and statistical analysis. We thank Til Stürmer, MD, MPH, University of North Carolina at Chapel Hill, for suggestions regarding implementation of the weighted models (no compensation). We thank Rosemary McKaig, MPH, PhD, NIAID / NIH, for her support as the study’s NIH Project Officer (salaried employee of NIH).
The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Dr Jonsson Funk had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This research was funded by a grant from NIH/NIAID (R01 AI 066920). Additional support provided by the University of North Carolina (UNC) Center for AIDS Research, funded by NIH/NIAID (P30 AI 50410). CASCADE has been funded through grants BMH4-CT97-2550, QLK2-2000-01431, QLRT-2001-01708 and LSHP-CT-2006-018949 from the European Union.
MJF has received salary support from GlaxoSmithKline (GSK) through a grant to the UNC Center for Excellence in Pharmacoepidemiology & Public Health, as well as travel grants from GSK to attend the International Observational HIV Cohorts Meetings in 2002, 2005 and 2006. JSF has been independent consultant for GSK (1998–2004) and salaried GSK employee (2004–2005), served a scientific advisory board for Tobira Therapeutics in 2006; and received unrestricted research grants from Merck & Co., Inc. and GSK in 2009. SRC received an honorarium of less than $2000 from GSK between 2005–2008, as well as consulting fees of less than $6000 per year from the Jaeb Center for Health Research between 2001–2009. KP has received a grant from GSK and an honorarium from Tibotec. ADW was a salaried employee of GSK until Dec 2010. JJE has been a consultant for Merck, GSK, Bristol-Myers Squibb, Tibotec, Chimerix, Avexa, Gilead Science, ViiV and Tobira and received grant support from Merck and GSK
CASCADE Collaboration
Steering Committee: Julia Del Amo (Chair), Laurence Meyer (Vice Chair), Heiner C. Bucher, Geneviève Chêne, Deenan Pillay, Maria Prins, Magda Rosinska, Caroline Sabin, Giota Touloumi.
Co-ordinating Centre: Kholoud Porter (Project Leader), Sara Lodi, Kate Coughlin, Sarah Walker, Abdel Babiker.
Clinical Advisory Board: Heiner C. Bucher, Andrea De Luca, Martin Fisher, Roberto Muga
Collaborators: Australia Sydney AIDS Prospective Study and Sydney Primary HIV Infection cohort (John Kaldor, Tony Kelleher, Tim Ramacciotti, Linda Gelgor, David Cooper, Don Smith); Canada South Alberta clinic (John Gill); Denmark Copenhagen HIV Seroconverter Cohort (Louise Bruun Jørgensen, Claus Nielsen, Court Pedersen); Estonia Tartu Ülikool (Irja Lutsar); France Aquitaine cohort (Geneviève Chêne, Francois Dabis, Rodolphe Thiebaut, Bernard Masquelier), French Hospital Database (Dominique Costagliola, Marguerite Guiguet), Lyon Primary Infection cohort (Philippe Vanhems), French PRIMO cohort (Marie-Laure Chaix, Jade Ghosn), SEROCO cohort (Laurence Meyer, Faroudy Boufassa); Germany German cohort (Osamah Hamouda, Claudia Kücherer, Barbara Bartmeyer); Greece Greek Haemophilia cohort (Giota Touloumi, Nikos Pantazis, Angelos Hatzakis, Dimitrios Paraskevis, Anastasia Karafoulidou); Italy Italian Seroconversion Study (Giovanni Rezza, Maria Dorrucci, Claudia Balotta), ICONA cohort (Antonella d’Arminio Monforte, Alessandro Cozzi-Lepri, Andrea De Luca.) Netherlands Amsterdam Cohort Studies among homosexual men and drug users (Maria Prins, Ronald Geskus, Jannie van der Helm, Hanneke Schuitemaker); Norway Oslo and Ulleval Hospital cohorts (Mette Sannes, Oddbjorn Brubakk, Anne-Marte Bakken Kran); Poland National Institute of Hygiene (Magdalena Rosinska, Joanna Gniewosz); Portugal Universidade Nova de Lisboa (Ricardo Camacho); Russia Pasteur Institute (Tatyana Smolskaya); Spain Badalona IDU hospital cohort (Roberto Muga, Jordi Tor), Barcelona IDU Cohort (Patricia Garcia de Olalla, Joan Cayla), Madrid cohort (Julia Del Amo, Jorge del Romero), Valencia IDU cohort (Santiago Pérez-Hoyos); Switzerland Swiss HIV Cohort Study (Heiner C. Bucher, Martin Rickenbach, Patrick Francioli); Ukraine Perinatal Prevention of AIDS Initiative (Ruslan Malyuta); United Kingdom Edinburgh Hospital cohort (Ray Brettle), Health Protection Agency (Valerie Delpech, Sam Lattimore, Gary Murphy), Royal Free haemophilia cohort (Caroline Sabin), UK Register of HIV Seroconverters (Kholoud Porter, Anne Johnson, Andrew Phillips, Abdel Babiker, Valerie Delpech), University College London (Deenan Pillay), University of Oxford (Harold Jaffe).
African cohorts: Genital Shedding Study (US: Charles Morrison; Family Health International, Robert Salata, Case Western Reserve University, Uganda: Roy Mugerwa, Makerere University, Zimbabwe: Tsungai Chipato, University of Zimbabwe); Early Infection Cohorts (Kenya, Uganda, Rwanda, Zambia, South Africa: Pauli Amornkul, International AIDS Vaccine Initiative).
Footnotes
This work was previously presented, in part, at the Annual Meeting of the Society for Epidemiologic Research in Seattle, Washington on June 24, 2010 and as a late breaker at the XVIII International AIDS Conference in Vienna, Austria on July 22, 2010.
Financial Disclosures
The following authors have no conflicts of interest to report: KEH, JCT, JSK and MD.
Writing Committee: Michele Jonsson Funk, PhD; Jennifer S Fusco, BS; Stephen R Cole, PhD; James C Thomas, PhD; Kholoud Porter, PhD; Jay S Kaufman, PhD; Marie Davidian, PhD; Alice D White, PhD; Katherine E Hartmann, MD, PhD; Joseph J Eron, Jr, MD.
Author Contributions
Study conception & design: MJF, JSF, KP, MD, JJE; data analysis: MJF, SRC; acquisition of data and/or interpretation of data: MJF, JSF, SRC, JCT, KP, JSK, MD, ADW, KEH, JJE; drafting the manuscript: MJF, JSF; critical revision of the manuscript for important intellectual content: MJF, JSF, SRC, JCT, KP, JSK, MD, ADW, KEH, JJE; final manuscript approval: MJF, JSF, SRC, JCT, KP, JSK, MD, ADW, KEH, JJE.
Ethics Board Review
All clinical cohorts participating in the CASCADE Collaboration received approval from their individual ethics review boards except for the Danish cohort, which received approval from the National Data Registry Surveillance Agency because Danish law allowed collection and pooling of anonymized clinical data with approval from this agency alone. Two ethics review boards deemed their cohort participants exempt from providing signed informed consent. Signed informed consent was obtained from all others. Approval was also given by all ethics review boards to pool anonymized data for analyses and dissemination. This analysis was reviewed by the Institutional Review Board at the University of North Carolina and determined to be exempt from futher review.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998 Mar 26;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Cameron DW, Heath-Chiozzi M, Danner S, et al. Randomised placebo-controlled trial of ritonavir in advanced HIV-1 disease. The Advanced HIV Disease Ritonavir Study Group. Lancet. 1998 Feb 21;351(9102):543–549. doi: 10.1016/s0140-6736(97)04161-5. [DOI] [PubMed] [Google Scholar]
- 3.Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997 Sep 11;337(11):725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 4.Strategic Timing of Antiretroviral Treatment (START) - ClinicalTrials.gov. National Institutes of Health. 2010 Jan 30; Available from: http://clinicaltrials.gov/ct2/show/NCT00867048. [Google Scholar]
- 5.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009 Apr 30;360(18):1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sterne JA, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009 Apr 18;373(9672):1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cain LE, Logan R, Robins JM, et al. When to Initiate Combined Antiretroviral Therapy to Reduce Mortality and AIDS-Defining Illnesses in HIV-Infected Persons in Developed Countries. Annals of Internal Medicine. 2011;154:509–515. doi: 10.1059/0003-4819-154-8-201104190-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter K, Babiker A, Bhaskaran K, Darbyshire J, Pezzotti P, Walker AS. Determinants of survival following HIV-1 seroconversion after the introduction of HAART. Lancet. 2003 Oct 18;362(9392):1267–1274. doi: 10.1016/s0140-6736(03)14570-9. [DOI] [PubMed] [Google Scholar]
- 9.Hernan MA, Lanoy E, Costagliola D, Robins JM. Comparison of dynamic treatment regimes via inverse probability weighting. Basic Clin Pharmacol Toxicol. 2006 Mar;98(3):237–242. doi: 10.1111/j.1742-7843.2006.pto_329.x. [DOI] [PubMed] [Google Scholar]
- 10.Hernán MA, Robins JM, García Rodríguez LA. Discussion on "Statistical Issues Arising in the Women's Health Initiative". Biometrics. 2005;61(4):9. doi: 10.1111/j.0006-341X.2005.454_1.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee EW, Wei LJ, Amato DA. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. In: Klein JP, Goel PK, editors. Survival Analysis: State of the Art. Dordrecht: Kluwer Academic Publishers; 1992. pp. 237–247. [Google Scholar]
- 12.Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004 Jul;75(1):45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000 Sep;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008 Sep 15;168(6):656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 16.Mooney C, Duval R. Bootstrapping: A nonparametric approach to statistical inference. Newbury Park: Sage; 1993. [Google Scholar]
- 17.Cole SR, Li R, Anastos K, et al. Accounting for leadtime in cohort studies: evaluating when to initiate HIV therapies. Stat Med. 2004 Nov 15;23(21):3351–3363. doi: 10.1002/sim.1579. [DOI] [PubMed] [Google Scholar]
- 18.Sabin CA. Early antiretroviral therapy: the role of cohorts. Curr Opin HIV AIDS. 2009 May;4(3):200–205. doi: 10.1097/COH.0b013e32832c06ad. [DOI] [PubMed] [Google Scholar]
- 19.Sinclair JC, Cook RJ, Guyatt GH, Pauker SG, Cook DJ. When should an effective treatment be used? Derivation of the threshold number needed to treat and the minimum event rate for treatment. J Clin Epidemiol. 2001 Mar;54(3):253–262. doi: 10.1016/s0895-4356(01)00347-x. [DOI] [PubMed] [Google Scholar]
- 20.Calmy A, Gayet-Ageron A, Montecucco F, et al. HIV increases markers of cardiovascular risk: results from a randomized, treatment interruption trial. AIDS. 2009 May 15;23(8):929–939. doi: 10.1097/qad.0b013e32832995fa. [DOI] [PubMed] [Google Scholar]
- 21.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006 Nov 30;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 22.Bruyand M, Thiebaut R, Lawson-Ayayi S, et al. Role of uncontrolled HIV RNA level and immunodeficiency in the occurrence of malignancy in HIV-infected patients during the combination antiretroviral therapy era: Agence Nationale de Recherche sur le Sida (ANRS) CO3 Aquitaine Cohort. Clin Infect Dis. 2009 Oct 1;49(7):1109–1116. doi: 10.1086/605594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guiguet M, Boue F, Cadranel J, Lang JM, Rosenthal E, Costagliola D. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009 Dec;10(12):1152–1159. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.