Abstract
Rationale: Plastic bronchitis (PB) is a rare and deadly condition that is characterized by the formation of airway casts. It most frequently occurs in children with underlying congenital heart disease that has been surgically palliated by the Fontan procedure. The Fontan circulation results in above-normal central venous pressure, and it has been hypothesized that the formation of airway casts is due to lymph leak. Knowledge of plastic bronchitis pathogenesis is poor and stems mostly from published case reports.
Objectives: To garner information about cast pathogenesis by characterizing inflammatory cell phenotypes in existing formalin-preserved, paraffin-embedded samples and generating protein and cytokine–chemokine profiles of airway cast homogenates.
Methods: We used immunofluorescence confocal microscopy, state-of-the-science proteomics, and a cytokine array assay to immunophenotype cellular content and to generate protein and cytokine profiles of plastic bronchitis airway casts, respectively.
Measurements and Main Results: Neutrophils, eosinophils, macrophages, and B lymphocytes were identified in cast samples; there were notably fewer T lymphocytes. Fibrin(ogen) was an abundant protein in the cast proteome. Histone H4 was also abundant, and immunofluorescence microscopy demonstrated it to be mostly extracellular. The cytokine profile of plastic bronchitis casts was proinflammatory.
Conclusions: Plastic bronchitis airway casts from children with Fontan physiology are composed of fibrin and are cellular and inflammatory in nature, providing evidence that their formation cannot be explained simply by lymph leak into the airways. Consequences of cellular necrosis including extracellular histones and the apparent low number of T cells indicate that a derangement in inflammation resolution likely contributes to cast formation.
Keywords: inflammation, proteomics, fibrin, histones, cytokines
Plastic bronchitis (PB) is considered a rare, most often pediatric disease that occurs in association with congenital heart disease (CHD) after surgical palliation by the Fontan procedure (1, 2). It is characterized by the formation of obstructive airway plugs or casts reported to be composed of mucin and/or fibrin (3–6). Because the Fontan circuit often results in above-normal central venous pressure, it has been postulated that PB casts form because of lymph leak into the airways or are secondary to lymphatic dysfunction or lymphatic trauma during surgery (7, 8). Abnormal hemodynamics inherent to Fontan physiology may also contribute to injury of the alveolar–capillary barrier (1).
Alternatively, a classification scheme that used existing cast pathology reports categorized PB airway casts from children with surgically repaired CHD as acellular, noninflammatory, and mucinous (referred to as type II) (4, 5). Given the apparent rarity of PB, prospective study of the illness is challenging. As such, knowledge of the natural history and pathogenesis of the disease is extremely limited. This has hindered our ability to identify and provide effective treatment to these children, which is particularly troublesome because PB in children with Fontan physiology is associated with a high mortality rate, repeated hospitalizations and procedures, and enhanced health care costs, and takes a high emotional toll on these children and their families (9).
We have previously shown in a longitudinal study that PB airway casts from children with CHD are composed primarily of fibrin with little mucin (3). We also found areas of cellularity and notable acellular regions by hematoxylin and eosin (H&E) staining of cast sections. In addition, our clinical observation has been that airway cast production and expectoration is sporadic, suggesting an inflammatory trigger (9). Although there is no evidence of an infectious etiology (7, 8), infection or inflammation seems to precipitate disease exacerbations (9). Collectively, these findings and observations suggest that cast pathogenesis may be more complex than presently appreciated and may involve a fibrin–inflammatory process in the airways. To this end, we immunophenotyped existing pathological samples of airway casts from children with Fontan physiology and conducted protein profiling experiments to identify mechanisms related to airway cast formation. These results may be used to direct future studies aimed at preventive strategies and effective treatments for this life-threatening pediatric disease. Some of the results of these studies have been previously reported in the form of an abstract (10).
Methods
Study Population and Sample Collection
Existing formalin-fixed, paraffin-embedded airway casts and freshly frozen, spontaneously expectorated airway casts from four children with underlying CHD and Fontan physiology were used. Existing bronchoalveolar lavage (BAL) fluid samples that were acquired from healthy subjects and patients enrolled in the acute lung injury (ALI) Specialized Center of Clinically Oriented Research (SCCOR) randomized trial of granulocyte-macrophage colony-stimulating factor in the treatment of ALI were also used (11). All samples used in this study were acquired under protocols approved by the University of Michigan (Ann Arbor, MI) Institutional Review Board. The details of cast sample acquisition and handling have been previously reported (3).
Immunophenotyping
Existing formalin-fixed, paraffin-embedded airway casts were sectioned and some sections were H&E stained whereas others were stained for myeloperoxidase (neutrophils), CD14 and CD11b (macrophages), CD20 (B lymphocytes), CD4 (T cells and eosinophils), eosinophilic peroxidase, and CD8 (T cells), using fluorescent antibodies. Immunohistochemistry (IHC) was conducted with antibodies for CD3 and CD20 to validate the immunofluorescence (IF) staining for T and B cells, respectively. More details about the IF and IHC staining methods are available in the online supplement.
Global Protein Profiling
Unbiased global protein profiles were generated from whole airway casts from three patients (12). Additional details about the global protein profiling methods are available in the online supplement.
Cytokine Protein Array
The cytokine profile of PB casts was determined by simultaneously measuring the signal intensities of 36 analytes in cast homogenates, using a protein array assay (human cytokine array, panel A; cat. no. ARY005; R&D Systems, Minneapolis, MN). Homogenates were prepared in the same manner as those used for protein profiling. Existing BAL samples from patients with another fibrin–inflammatory lung disease, ALI, and healthy control subjects were also assayed to benchmark the cytokine profile of PB airway casts. More details about these samples and the cytokine array assay are available in the online supplement.
Data Analysis
Global Protein Profiling
The normalized spectral abundance factor for each protein in each of the three assayed casts was calculated; these details can be found in the online supplement. False discovery rates were calculated according to a reverse decoy database search strategy (http://www.matrixscience.com/help/decoy_help.html).
Cytokine Protein Array
The mean normalized density value (±SEM) of each analyte from cast homogenates and from ALI and healthy control BAL samples was determined. These mean signal intensity data were used to construct a heat map of analytes, using R software (version 2.15.1; http://www.r-project.org/).
Results
Immune Cells Are Evident in Airway Casts
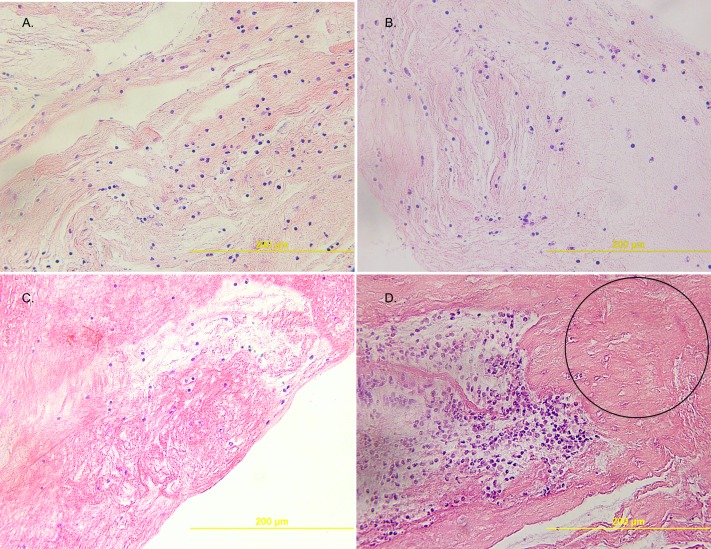
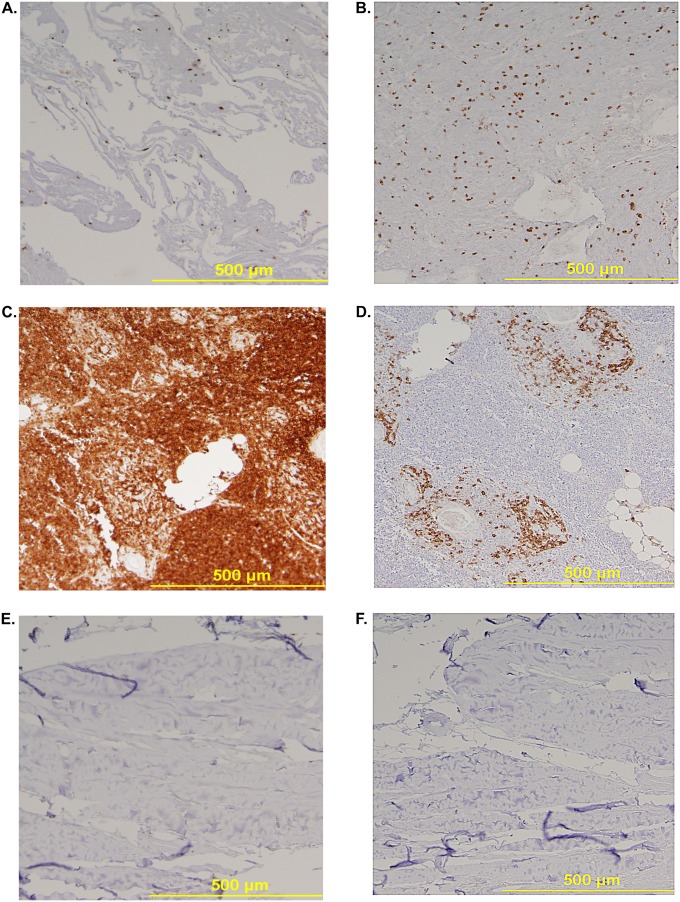
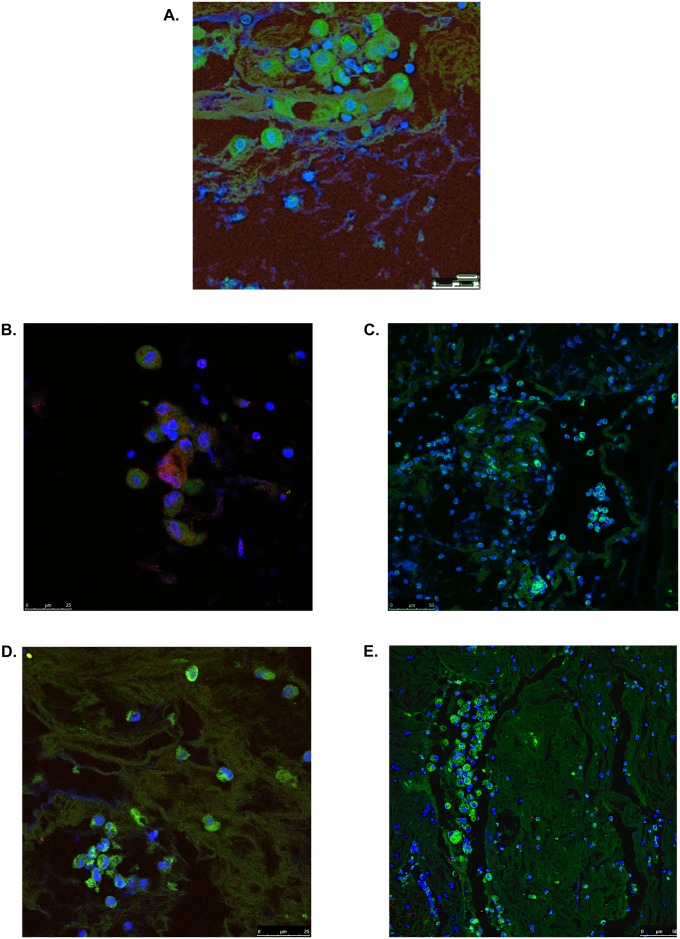
Airway cast samples from four children were studied. All patients had underlying single ventricle physiology (three with hypoplastic left heart syndrome and one with double inlet left ventricle) surgically palliated by the Fontan procedure. Overall, by H&E staining, the cellular content of PB airway casts appeared diffuse with an underlying uniform distribution of mononuclear cells (Figures 1A–1C). Some samples had clusters of cells alternated with paucicellular and variably conspicuous acellular zones (Figure 1D). The diffuse uniform distribution of mononuclear cells was further evidenced by IHC, which showed it to be attributable to lymphocytes most of which were B cells (CD20) rather than T cells (CD3) (Figures 2A–2F). Immunofluorescence microscopy revealed the presence of neutrophils, eosinophils, macrophages, and CD20+ B lymphocytes in PB airway casts (Figures 3A–3E). All cast sections positively stained for CD4, but there was a notable paucity of CD8-positive staining. In addition, 4′,6-diamidino-2-phenylindole staining showed extracellular DNA dispersed throughout the cast samples. More details about the immunophenotyping results can be found in the online supplement.
Figure 1.
(A–C) Representative hematoxylin–eosin (H&E)-stained sections (original magnification, ×40) that show the diffuse uniform distribution of mononuclear cells of plastic bronchitis (PB) airway casts and one (D) that shows a region of cell clusters and an acellular zone (circle). Each image is from one of the four studied patients.
Figure 2.
Representative sections of plastic bronchitis (PB) airway casts stained for (A) CD3 (T) cells and (B) CD20 (B) cells, showing that the uniform distribution of mononuclear cells is attributable primarily to B cells and that there are fewer T cells. CD3 and CD20 staining was confirmed by the staining of sections of (C and D) thymus and (E and F) sternum, respectively.
Figure 3.
Plastic bronchitis airway casts from children with Fontan physiology contain immune cells. Immunofluorescence (IF) confocal microscopy revealed positive staining for clusters of cells expressing (A) myeloperoxidase (green), indicative of neutrophils (original magnification, ×100; zoom factor 2.0); (B) eosinophilic peroxidase (red), which colocalized with CD4 (green) (original magnification, ×100; zoom factor 2.0); and (C) CD14 (green) and CD11b (red), indicative of macrophages (original magnification, ×40). (D) The presence of B lymphocytes was detected by CD20 (green) staining (original magnification, ×100; zoom factor 1.14). (E) CD4 staining (green) revealed clusters of eosinophils and lymphocytes and CD8 staining (red) was sparse. Samples were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; blue) to visualize nuclei. Images are representative of at least one cast sample from four patients. The presence of T cells in cast samples was confirmed by immunohistochemistry (IHC) for CD3 (see Figure 2 and Figure E1). Representative IF and IHC of control slides that demonstrate antibody specificity and performance can be found in Figure 2 and in Figure E1.
Immune Cell Proteins and Signs of Cellular Necrosis Are Evident in Protein Profiles of Plastic Bronchitis Casts
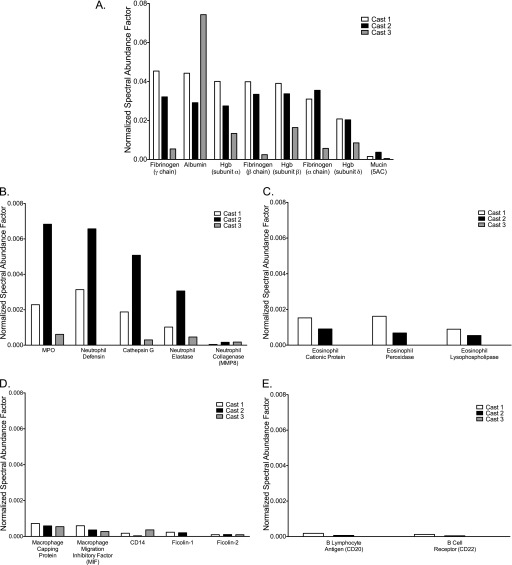
Using a state-of-the-science nano–liquid chromatography-tandem mass spectrometry protein profiling approach, whole spontaneously expectorated casts from three children were analyzed from which the total number of proteins assigned normalized spectral abundance factor values were 1,195, 843, and 924, respectively (see Table E1 in the online supplement). The false discovery rate for each was 0.6, 1.4, and 1.2%, respectively. Of these, there were 461 proteins common to all three casts (see Table E2). Proteins, including fibrin(ogen), known to be abundant in blood and lymph fluid, were present in all three cast samples (Figure 4A) and were more abundant than mucins (Figure E4). Lung epithelial proteins such as surfactant-associated proteins A2 and B were detected, but club cell (Clara cell) proteins and receptor for advanced glycation end products, known to be associated with epithelial cell injury, were not evident in any of the cast samples; only one cast had a detectable but low abundance of surfactant protein D (13).
Figure 4.
Fibrin(ogen) is abundant and proteins of immune cells are present in the proteome of plastic bronchitis airway casts. (A) Fibrin(ogen) is an abundant protein in the airway cast proteome. Mucin proteins were detected but at much lower levels relative to the most abundant proteins in the casts (also see Figure E4). Consistent with the results of the immunophenotyping studies, immune cell proteins were detected in the cast proteome. (B) Neutrophil proteins were the most abundant followed by (C) eosinophil, (D) monocyte/macrophage, and (E) lymphocyte proteins. Eosinophil proteins were not detected in one of the three cast samples and detected lymphocyte proteins were those associated with B cells; no T-cell proteins were detected. Data are the normalized spectral abundance factors of each respective protein in each of three plastic bronchitis airway casts.
The inflammatory nature of PB casts was further evidenced by the presence of immune cell proteins associated with neutrophils, eosinophils, monocyte/macrophages, and B lymphocytes (Figure 3). However, T-lymphocyte markers such as CD4, CD8, T-cell receptor, and perforin were not detected (14). There was also evidence of complement pathway activation because, in addition to the presence of complement proteins in the cast proteome (see Table E3), mannose-binding lectin serine protease-1, ficolins, and CD59 were detected (see Table E1) (15).
The presence of the three polypeptide chains (α, β, and γ) of fibrinogen and coagulation factor XIII provides convincing evidence of the fibrin composition (3, 16, 17) of these airway casts. However, there was a notable absence of thrombin, tissue factor, and factor VII (Table 1). Furthermore, the predominant fibrin composition of airway casts, the absence of plasminogen activators, and the low abundance of plasminogen activator inhibitor type-1 (PAI-1) suggests an absence of activation of the fibrinolytic system (18).
Table 1.
Coagulation proteins detected by liquid chromatography-tandem mass spectrometry protein profiling of plastic bronchitis airway casts
| Identified Proteins | Accession Number | Molecular Weight (kD) | Cast 1 | Cast 2 | Cast 3 |
|---|---|---|---|---|---|
| Fibrinogen γ chain | P02679 | 52 | 0.04537 | 0.03212 | 0.00541 |
| Fibrinogen β chain | P02675 | 56 | 0.03990 | 0.03343 | 0.00247 |
| Fibrinogen α chain | P02671 | 95 | 0.03099 | 0.03548 | 0.00570 |
| α1-Antitrypsin | P01009 | 47 | 0.01627 | 0.00825 | 0.03670 |
| α2-Macroglobulin | P01023 | 163 | 0.01088 | 0.00939 | 0.01328 |
| Plasminogen | P00747 | 91 | 0.00395 | 0.00287 | 0.00131 |
| α2-Antiplasmin | P08697 | 55 | 0.00356 | 0.00387 | 0.00119 |
| Fibronectin | P02751 | 263 | 0.00296 | 0.00722 | 0.00089 |
| Antithrombin-III | P01008 | 53 | 0.00296 | 0.00274 | 0.00205 |
| Prothrombin | P00734 | 70 | 0.00142 | 0.00097 | 0.00194 |
| Vitamin K–dependent protein S | P07225 | 75 | 0.00066 | 0.00044 | 0.00024 |
| Plasma kallikrein | P03952 | 71 | 0.00032 | 0.00000 | 0.00046 |
| Coagulation factor XIII A chain | P00488 | 83 | 0.00027 | 0.00187 | 0.00000 |
| Coagulation factor XIII B chain | P05160 | 76 | 0.00025 | 0.00003 | 0.00006 |
| Coagulation factor XII | P00748 | 68 | 0.00006 | 0.00000 | 0.00032 |
| Coagulation factor X | P00742 | 55 | 0.00004 | 0.00000 | 0.00011 |
| Plasminogen activator inhibitor-2 | P05120 | 47 | 0.00002 | 0.00000 | 0.00000 |
| von Willebrand factor | P04275 | 309 | 0.00002 | 0.00007 | 0.00011 |
| Coagulation factor V | P12259 | 252 | 0.00001 | 0.00000 | 0.00002 |
| Coagulation factor IX | P00740 | 52 | 0.00000 | 0.00000 | 0.00006 |
| Coagulation factor XI | P03951 | 70 | 0.00000 | 0.00000 | 0.00004 |
| Vitamin K–dependent protein C | P04070 | 52 | 0.00000 | 0.00000 | 0.00008 |
| Thrombospondin-1 | P07996 | 129 | 0.00000 | 0.00000 | 0.00023 |
| Plasminogen activator inhibitor-1 | P05121 | 45 | 0.00000 | 0.00000 | 0.00004 |
Data are the normalized spectral abundance factor (NSAF) rounded to five decimal places of each detected protein in each of three plastic bronchitis airway casts in order of abundance based on cast 1; plasmin cannot be discriminated from plasminogen and activated coagulation factors cannot be discriminated from the zymogen. The NSAF was calculated using the equation: NSAF = (SpC/MW)/Σ(SpC/MW)N, where SpC = spectral counts, MW = protein molecular weight (kD), and N = total number of proteins.
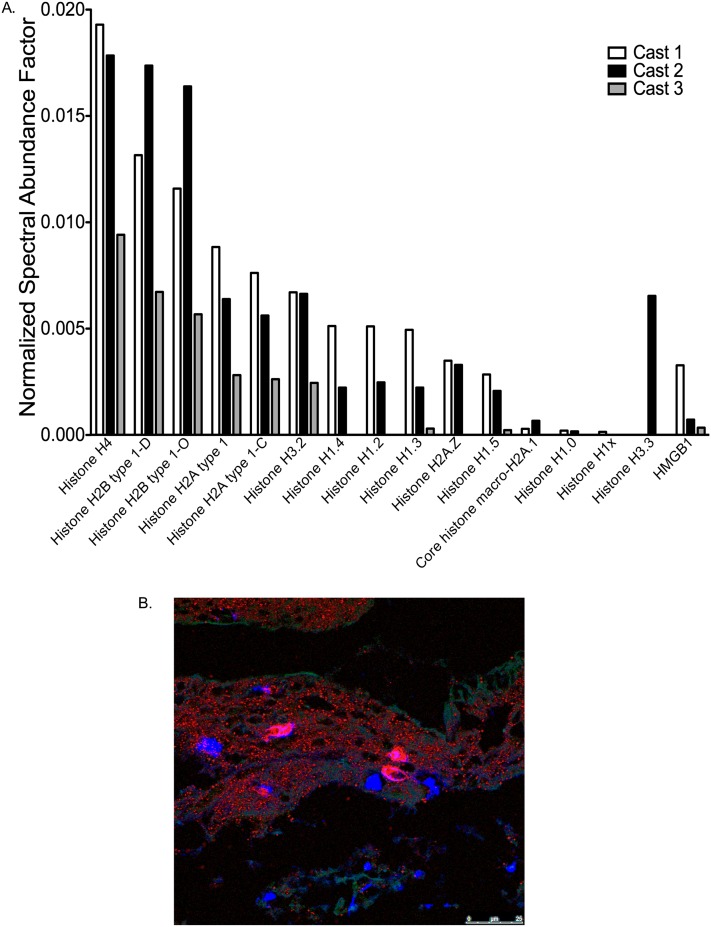
An unexpected finding in the cast proteome was the myriad of histones, which were the most abundant proteins in the cast samples exclusive of fibrin(ogen), albumin, and hemoglobin (Figure 4A). This result prompted us to stain cast sections for histone H4 and annexin A5, a nuclear and cytosolic protein, respectively (19, 20). The results of these experiments showed diffuse but widespread extracellular distribution of both proteins, suggesting the involvement of cellular necrosis in PB cast formation (Figure 5B).
Figure 5.
Histones are abundant in the plastic bronchitis airway cast proteome, and histone H4 is evident by immunofluorescence (IF) staining. (A) Histone H4 is the most abundant of the histones and is the eighth most abundant protein in the airway cast proteome. Another nuclear protein, high mobility group protein B1 (HMGB1), which is indicative of cell necrosis, had a similar abundance to histones in the cast proteome. (B) Representative IF micrograph (original magnification, ×100) of an airway cast section stained for histone H4 (red), DNA (blue), and annexin A5 (green) that illustrates the distribution of intracellular and nuclear material in plastic bronchitis airway casts. Representative IF of control slides that demonstrate antibody specificity and performance can be found in Figure E2. Data represented in (A) are the normalized spectral abundance factors of each respective protein in each of three plastic bronchitis airway casts.
The involvement of cellular necrosis was further evidenced by the presence of another nuclear protein in the proteome, high mobility group protein B1 (HMGB1), the abundance of which was in the range of that of histones (Figure 5A) (20, 21).
Cytokine Profile of Plastic Bronchitis Casts Is Proinflammatory
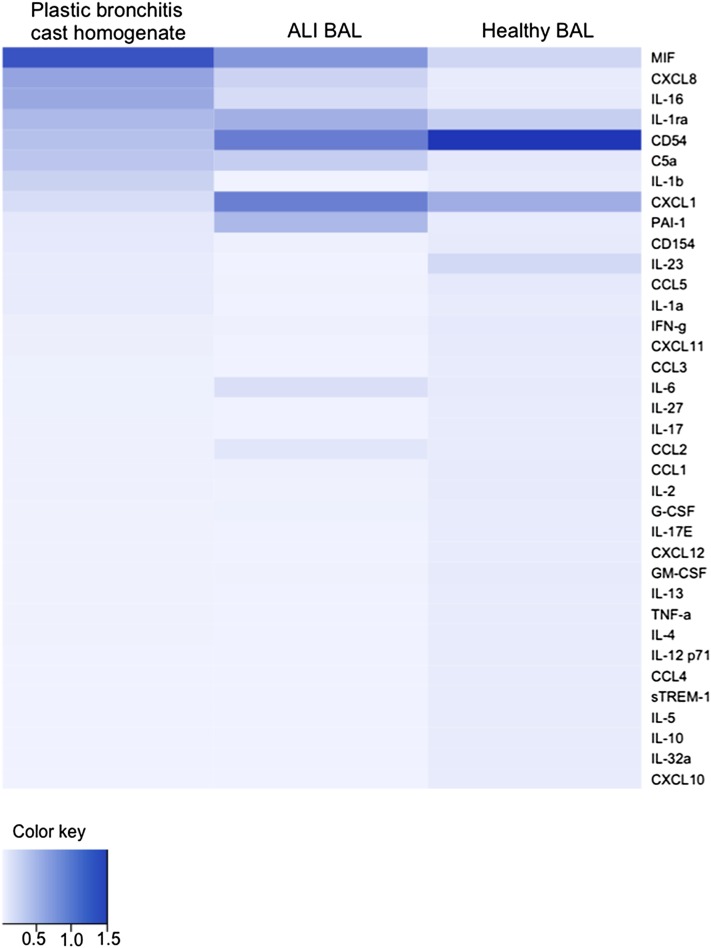
The most highly expressed of the measured cytokines in PB cast homogenates was macrophage migration inhibitory factor (MIF) followed by CXCL8 (IL-8), both of which are primary mediators of the inflammatory response (Figure 6). MIF was also detected in the proteome and is recognized as a potent initiator of inflammation that is elevated in a number of inflammatory diseases including asthma (22).
Figure 6.
Heat map of the 36 measured analytes, based on descending mean signal intensity in plastic bronchitis cast homogenates relative to the bronchoalveolar lavage (BAL) of patients with acute lung injury (ALI) and healthy control subjects. Data were generated from four plastic bronchitis homogenate samples, two of which were also used for the acquisition of proteomic data, and four BAL samples from patients with ALI and healthy control subjects. Statistical comparisons between analytes in plastic bronchitis cast homogenates compared with ALI BAL or BAL from healthy control subjects are shown in Figures E5 and E6.
IL-16 was also abundant in cast homogenates. Consistent with the proinflammatory profile of airway casts was increased IL-1β, a recruiter of macrophages, and the associated increase in IL-1 receptor antagonist (23–25). In the context of cell death, elevated levels of IL-1α (see Figures E5 and E6), a cytokine that has a functional role in the nucleus in addition to its receptor-mediated actions, have been shown to be associated with cell necrosis rather than apoptosis (26). Consistent with the proteome data, the level of PAI-1 was surprisingly low given the fibrin(ogen) content of the casts. On the basis of this initial survey, the cytokine profile of PB is proinflammatory, which substantiates the involvement of inflammatory processes in the airway cast formation. The cytokine profile of PB relative to that of BAL from another fibrin–inflammatory lung illness, ALI (27, 28), and healthy control subjects can be found in the online supplement.
Discussion
This study represents the first report of data that substantiate that airway casts produced by children with underlying single ventricle physiology and a Fontan circuit are cellular, inflammatory, and composed of fibrin (3). The lower abundance of mucins relative to fibrin makes the hypothesis that PB airway casts form as a result of mucin hypersecretion into the airways unlikely. In addition, the cellular content, proinflammatory cytokine profile, and cast proteome illustrate the complex biological processes involved in PB airway cast formation in these children.
The most notable finding from the cellular immunophenotyping of PB airway casts was the sparseness of T lymphocytes, in particular CD8+ cells. This is unexpected because the lymphocyte population of the airways is similar to that of the systemic circulation (29) and the immune response is not only important in inflammation but is essential for its resolution (30–33). Much attention has been paid to the role of neutrophils and macrophages, particularly regarding acute inflammation (34). However, lymphocytes have gained recognition for their role in regulating the magnitude of acute inflammatory events as well as in restorative physiology (30). The paucity of T cells in PB airway cast samples raises the possibility that an aberrancy in the T-cell population and function contributes to the apparent unchecked propagation of fibrinous exudates in the airways. This does not necessarily eliminate the possibility that lymph leak participates in cast pathogenesis, but it does suggest that lymph leak alone cannot account for airway cast formation. The involvement of other, additional processes was revealed by the proteomics and cytokine profiles of cast homogenates.
The profile of inflammatory cells found by IF microscopy was mirrored in the proteome of the airway casts. The most prominent immune cell proteins were from neutrophils, followed by eosinophils and monocytes/macrophages. Despite this cellular composition, none of the studied patients carried the diagnosis of asthma and none had bacterial respiratory infections, including acute bronchitis or pneumonia, at the time of cast expectoration. B-lymphocyte protein abundance was low relative to other immune cell proteins, and T-cell proteins were absent. This finding was substantiated by IHC for which the uniform mononuclear cellular content of the casts was attributed to B cells (CD20) with far fewer detected T cells (CD3) (Figure 2). On the basis of IF microscopy, most T cells in PB casts were CD4+ but in the scope of this study, we did not perform additional characterizations of the T-cell populations present in these samples. Collectively, these data provide supportive evidence of an unexpected and atypical T-cell repertoire in airway casts that warrants further study.
The proteome provided additional insight into potential mechanisms of airway cast formation because of the abundance of histones in the cast homogenate samples. This remarkable finding was elaborated by IF microscopy which showed that histone H4 was predominantly extracellular. This, in parallel with the pattern of 4′,6-diamidino-2-phenylindole and annexin 5A staining, unveiled a picture of cellular necrosis and incomplete resolution of inflammation. Extracellular histones can propagate inflammation via enhanced cytokine production (35) and may further airway cast formation via histone-induced aggregation of plasma proteins such as fibrinogen, which can occur concomitantly with fibrin formation (16, 17, 36, 37). We do not yet know the source of the histones or what, if any, post-translational modifications have occurred, but the physiological consequences of extracellular histones and other nuclear proteins released by necrotic cells, such as HMGB-1, include the promotion of cell toxicity and inflammation (20, 35–37). In the milieu of the cast microenvironment, extracellular histones in the presence of neutrophil proteins such as myeloperoxidase can also mediate cytotoxicity (38). In aggregate, these data suggest that cellular necrosis may contribute to a derangement in or incomplete resolution of extravascular inflammation that culminates in the formation of fibrin airway casts.
Our findings also introduce the possibility that either fibrinolytic resistance or even “fibrinolytic shutdown” may contribute to excessive fibrin formation in the airways of children with PB (39, 40). With the extent of fibrin deposition in the airway, it would be reasonable to expect to find evidence of activation of the fibrinolytic system in the cast proteome (41, 42). This was not the case because neither urokinase nor tissue plasminogen activator (tPA) was detected in the cast homogenate proteome, and PAI-1 was of low abundance in one sample and was not detected in the other two cast samples. This finding was substantiated by the cytokine array profile, in which the PAI-1 signal intensity of these samples was similar to that in the BAL from healthy control subjects. Notably, one of the most potent inducers of the fibrinolytic system, thrombin, was not detected in the proteome of the cast homogenates (43). However, we have previously found detectable levels of tPA in a PB airway cast by ELISA (3). In ex vivo experiments, PB airway cast mass decreased after incubation with phosphate-buffered saline. This reduction was enhanced when tPA was exogenously applied. These results suggest that both plasmin and plasminogen are present in airway casts, a notion substantiated by the proteomics data presented here, and it may be possible that urokinase plasminogen activator (uPA) and/or tPA is (are) present at levels below the limit of detection of our proteomics platform. Neutrophil elastase was also evident in the cast proteome. It, like plasmin, is also capable of activating the fibrinolytic system (39). Nevertheless, despite the presence of these proteases, these mechanisms seem insufficient to overcome the aberrant fibrin accumulation in the airway. This may be due, in part, to the possibility that histone-bound fibrinogen aggregates may be resistant to degradation by plasmin (44, 45).
Another example of aberrant and excessive fibrin deposition in the respiratory system, in this case the nasal passages, was recently described (46). Albeit the underlying etiology of nasal polyps associated with chronic rhinosinusitis (NPCR) is likely unique to that of PB airway casts, the findings of abnormal and excessive fibrin formation and reduced fibrinolysis are surprisingly similar. In aggregate, these data suggest that this phenomenon may not be that uncommon.
The cytokine profile of PB in conjunction with the found neutrophils and eosinophils in cast samples is one that is proinflammatory. Although our assessment of cytokines and chemokines was not exhaustive, in aggregate, our data suggest that cast formation may represent the culmination of an inflammatory “perfect storm” that is not met with the needed inflammation resolution response. The PB cytokine profile was unique to that of ALI with the most abundant cytokines in airway cast homogenates being proinflammatory, including MIF, CXCL8, and IL-16. MIF has been recognized for its ability to amplify inflammation and, interestingly, MIF and CXCL8 are known inflammatory cytokines of cystic fibrosis and asthma, respectively (22, 23, 47, 48). Less is known about IL-16 but it, like CXCL8, has been associated with asthma (49). This proinflammatory cytokine is most often associated with lymphocytes and it signals via the CD4 receptor (49). Given the greater abundance of eosinophil proteins in the cast proteome, eosinophils are a likely source of IL-16 (50, 51). CXCL8 is produced primarily by macrophages and is a potent neutrophil chemokine that is recognized for its role in inflammatory airway disease (48). In the context of our findings, which implicate a disruption in inflammation resolution as a potential mechanism in PB airway cast formation, the elevation in IL-1α in conjunction with the abundance of HMGB-1 and histones may represent a “danger signal” or damage-associated molecular pattern that is associated with airway cast formation (26, 52).
Limitations of the Study
The findings presented here are from a small sample of children with PB but represent an exquisitely unique data set, which is the first to lend insight into the pathogenesis of airway cast formation. We acknowledge that there are limitations to our study. Because cast samples are submitted by express mail (3), sample degradation is possible; this is an inherent limitation of the study. Other variables that could influence differences across samples, particularly regarding the proteome, include differences in cast mass and the amount of insoluble fraction after homogenization. Although this is not a reflection on our proteomics technique, it could result in different protein abundance across samples even though the same amount of protein from each sample was analyzed. There was a difference in cast mass of the three samples subjected to proteomics (see Figure E3) in our study. The size of expectorated cast is unpredictable, is likely influenced by the duration of formation in the airway, and may change over time for any given patient as well as across patients. As such, each cast represents a unique “snapshot in time” of each patient’s illness at the point when the cast was expectorated. We recognize that additional studies will be required to elaborate and confirm our findings.
Cast composition may also be influenced by medications. These children are prescribed complex therapeutic regimens that typically consist of inhaled and oral medications including β-agonists, corticosteroids, azithromycin, aspirin, diuretics, and antihistamines. In some cases, dornase alfa, inhaled alteplase, and acetylcysteine are used to treat exacerbations. Given our small sample size, we cannot make confident conclusions about the impact drug therapy may have had on cast composition.
Inclusion in our study was limited to children with Fontan physiology. Plastic bronchitis is reported most often in this patient population, but it has also been described in patients with cystic fibrosis and asthma (5, 6). To date, the approach to characterizing and classifying PB has been from a disease-associated perspective rather than a histopathological one (4–6). The latter may be more informative because fibrin airway cast formation is not likely exclusive of the underlying disease, it can be more directly linked to molecular mechanisms associated with the processes involved in cast formation, and, to date, no associations between demographic or clinical variables have been identified (9).
For this first study, we elected to focus on characterizing the immune cell content of PB airway casts as determined by immunophenotyping of airway cast sections. We used these data in an iterative manner to direct proteomics data mining. We recognize that this approach is not comprehensive of all the potential mechanisms that may contribute to airway cast formation. We have therefore included the first proteomics data set of PB airway casts in this publication to foster the development of hypotheses by other investigators to drive this field of research and bring attention to this perplexing severe pulmonary ailment that most often affects children.
In conclusion, we put forth the hypothesis that PB airway cast formation in children with Fontan physiology is due to aberrant and excessive fibrin deposition that is driven by inadequate resolution of inflammation. The release of histones and other nuclear proteins secondary to cellular necrosis propagates inflammation and fibrinogen aggregation in the airway. These provocative findings provide a foundation for future investigation of the mechanisms that lead to these phenomena as well as studies directed at identifying the initiating event(s). Unraveling the complexity of these processes will advance understanding of PB, create opportunities for drug target discovery, and will have application in other fibrin–inflammatory airway illnesses including ALI, asthma, and NPCR.
Acknowledgments
Acknowledgment
The authors thank Drs. Daniel Lawrence, Richard Simon, and Thomas Sisson for help and insightful comments and suggestions.
Footnotes
Supported by grant R15HD065594 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the Daniel Foy Plastic Bronchitis Research Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health.
Author Contributions: All authors contributed to the preparation of the manuscript. J.R.: involved in and contributed to the conception and design of the study; responsible for cast processing and cataloging; designed and conducted immunophenotyping experiments. G.M.: assisted with immunophenotyping experiments; performed the cytokine array assays. M.F.: involved in the conception of the study and the acquisition, analysis, and interpretation of the proteomics data. L.S.: involved in the conception of the immunophenotyping experiments; participated in image interpretation. J.M.: involved in the conception of the immunophenotyping experiments; participated in image interpretation. T.J.S.: contributed BAL samples and was involved in the interpretation of the cytokine array data. K.R.S.: involved in the conception of the study and data interpretation. C.F.: involved in the conception of the study and data interpretation. M.W.R.: involved in the conception of the study and data interpretation. K.A.S.: responsible for the conception, design, and execution of the study; oversaw the acquisition and analysis of data and was involved in the data interpretation.
This article has an online supplement, which is available from this issue’s table of contents at www.atsjournals.org
Portions of this work were presented at the American Thoracic Society Meeting, San Francisco; May 19–24, 2012.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Healy F, Hanna BD, Zinman R. Pulmonary complications of congenital heart disease. Paediatr Respir Rev. 2012;13:10–15. doi: 10.1016/j.prrv.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg DJ, Dodds K, Rychik J. Rare problems associated with the Fontan circulation. Cardiol Young. 2010;20:113–119. doi: 10.1017/S1047951110001162. [DOI] [PubMed] [Google Scholar]
- 3.Heath L, Ling S, Racz J, Mane G, Schmidt L, Myers JL, Tsai WC, Caruthers RL, Hirsch JC, Stringer KA. Prospective, longitudinal study of plastic bronchitis cast pathology and responsiveness to tissue plasminogen activator. Pediatr Cardiol. 2011;32:1182–1189. doi: 10.1007/s00246-011-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madsen P, Shah SA, Rubin BK. Plastic bronchitis: new insights and a classification scheme. Paediatr Respir Rev. 2005;6:292–300. doi: 10.1016/j.prrv.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Seear M, Hui H, Magee F, Bohn D, Cutz E. Bronchial casts in children: a proposed classification based on nine cases and a review of the literature. Am J Respir Crit Care Med. 1997;155:364–370. doi: 10.1164/ajrccm.155.1.9001337. [DOI] [PubMed] [Google Scholar]
- 6.Gibb E, Blount R, Lewis N, Nielson D, Church G, Jones K, Ly N. Management of plastic bronchitis with topical tissue-type plasminogen activator. Pediatrics. 2012;130:e446–e450. doi: 10.1542/peds.2011-2883. [DOI] [PubMed] [Google Scholar]
- 7.Fredenburg TB, Johnson TR, Cohen MD. The Fontan procedure: anatomy, complications, and manifestations of failure. Radiographics. 2011;31:453–463. doi: 10.1148/rg.312105027. [DOI] [PubMed] [Google Scholar]
- 8.Languepin J, Scheinmann P, Mahut B, Le Bourgeois M, Jaubert F, Brunelle F, Sidi D, de Blic J. Bronchial casts in children with cardiopathies: the role of pulmonary lymphatic abnormalities. Pediatr Pulmonol. 1999;28:329–336. doi: 10.1002/(sici)1099-0496(199911)28:5<329::aid-ppul4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 9.Caruthers RL, Kempa M, Loo A, Gulbransen E, Kelly E, Erickson SR, Hirsch JC, Schumacher KR, Stringer KA. Demographic characteristics and estimated prevalence of Fontan-associated plastic bronchitis. Pediatr Cardiol. 2013;34:256–261. doi: 10.1007/s00246-012-0430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Racz J, Mane G, Ford M, Schmidt L, Schumacher KR, Fifer C, Russell MW, Caruthers RL, Stringer KA. Complementary approaches of proteomics and immunophenotyping provide insight into the pathogenesis of plastic bronchitis [abstract] Am J Respir Crit Care Med. 2012;185:A2489. [Google Scholar]
- 11.Paine R, III, Standiford TJ, Dechert RE, Moss M, Martin GS, Rosenberg AL, Thannickal VJ, Burnham EL, Brown MB, Hyzy RC. A randomized trial of recombinant human granulocyte-macrophage colony stimulating factor for patients with acute lung injury. Crit Care Med. 2012;40:90–97. doi: 10.1097/CCM.0b013e31822d7bf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zybailov B, Mosley AL, Sardiu ME, Coleman MK, Florens L, Washburn MP. Statistical analysis of membrane proteome expression changes in Saccharomyces cerevisiae. J Proteome Res. 2006;5:2339–2347. doi: 10.1021/pr060161n. [DOI] [PubMed] [Google Scholar]
- 13.Ware LB, Koyama T, Billheimer DD, Wu W, Bernard GR, Thompson BT, Brower RG, Standiford TJ, Martin TR, Matthay MA. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest. 2010;137:288–296. doi: 10.1378/chest.09-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt H, Gelhaus C, Nebendahl M, Lettau M, Lucius R, Leippe M, Kabelitz D, Janssen O. Effector granules in human T lymphocytes: proteomic evidence for two distinct species of cytotoxic effector vesicles. J Proteome Res. 2011;10:1603–1620. doi: 10.1021/pr100967v. [DOI] [PubMed] [Google Scholar]
- 15.Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 16.Olman MA, Simmons WL, Pollman DJ, Loftis AY, Bini A, Miller EJ, Fuller GM, Rivera KE. Polymerization of fibrinogen in murine bleomycin-induced lung injury. Am J Physiol. 1996;271:L519–L526. doi: 10.1152/ajplung.1996.271.4.L519. [DOI] [PubMed] [Google Scholar]
- 17.Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 18.Sisson TH, Simon RH. The plasminogen activation system in lung disease. Curr Drug Targets. 2007;8:1016–1029. doi: 10.2174/138945007781662319. [DOI] [PubMed] [Google Scholar]
- 19.Cederholm A, Frostegard J. Annexin A5 as a novel player in prevention of atherothrombosis in SLE and in the general population. Ann N Y Acad Sci. 2007;1108:96–103. doi: 10.1196/annals.1422.011. [DOI] [PubMed] [Google Scholar]
- 20.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 21.Yang H, Rivera Z, Jube S, Nasu M, Bertino P, Goparaju C, Franzoso G, Lotze MT, Krausz T, Pass HI, et al. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc Natl Acad Sci USA. 2010;107:12611–12616. doi: 10.1073/pnas.1006542107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai F, Asojo OA, Cirillo P, Ciustea M, Ledizet M, Aristoff PA, Leng L, Koski RA, Powell TJ, Bucala R, et al. A novel allosteric inhibitor of macrophage migration inhibitory factor (MIF) J Biol Chem. 2012;287:30653–30663. doi: 10.1074/jbc.M112.385583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tillie-Leblond I, Pugin J, Marquette CH, Lamblin C, Saulnier F, Brichet A, Wallaert B, Tonnel AB, Gosset P. Balance between proinflammatory cytokines and their inhibitors in bronchial lavage from patients with status asthmaticus. Am J Respir Crit Care Med. 1999;159:487–494. doi: 10.1164/ajrccm.159.2.9805115. [DOI] [PubMed] [Google Scholar]
- 24.Herold S, Tabar TS, Janssen H, Hoegner K, Cabanski M, Lewe-Schlosser P, Albrecht J, Driever F, Vadasz I, Seeger W, et al. Exudate macrophages attenuate lung injury by the release of IL-1 receptor antagonist in gram-negative pneumonia. Am J Respir Crit Care Med. 2011;183:1380–1390. doi: 10.1164/rccm.201009-1431OC. [DOI] [PubMed] [Google Scholar]
- 25.Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E, White MR, Dinarello CA, Apte RN. IL-1α and IL-1β recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187:4835–4843. doi: 10.4049/jimmunol.1102048. [DOI] [PubMed] [Google Scholar]
- 26.Cohen I, Rider P, Carmi Y, Braiman A, Dotan S, White MR, Voronov E, Martin MU, Dinarello CA, Apte RN. Differential release of chromatin-bound IL-1α discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci USA. 2010;107:2574–2579. doi: 10.1073/pnas.0915018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bastarache JA, Fremont RD, Kropski JA, Bossert FR, Ware LB. Procoagulant alveolar microparticles in the lungs of patients with acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1035–L1041. doi: 10.1152/ajplung.00214.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhandary YP, Shetty SK, Marudamuthu AS, Gyetko MR, Idell S, Gharaee-Kermani M, Shetty RS, Starcher BC, Shetty S. Regulation of alveolar epithelial cell apoptosis and pulmonary fibrosis by coordinate expression of components of the fibrinolytic system. Am J Physiol Lung Cell Mol Physiol. 2012;302:L463–L473. doi: 10.1152/ajplung.00099.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pabst R, Tschernig T. Lymphocytes in the lung: an often neglected cell. Numbers, characterization and compartmentalization. Anat Embryol (Berl) 1995;192:293–299. doi: 10.1007/BF00710098. [DOI] [PubMed] [Google Scholar]
- 30.Rajakariar R, Lawrence T, Bystrom J, Hilliard M, Colville-Nash P, Bellingan G, Fitzgerald D, Yaqoob MM, Gilroy DW. Novel biphasic role for lymphocytes revealed during resolving inflammation. Blood. 2008;111:4184–4192. doi: 10.1182/blood-2007-08-108936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 32.Workalemahu G, Foerster M, Kroegel C, Braun RK. Human γδ-T lymphocytes express and synthesize connective tissue growth factor: effect of IL-15 and TGF-β1 and comparison with αβ-T lymphocytes. J Immunol. 2003;170:153–157. doi: 10.4049/jimmunol.170.1.153. [DOI] [PubMed] [Google Scholar]
- 33.Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2005;31:674–686, discussion 686. doi: 10.1111/j.1524-4725.2005.31612. [DOI] [PubMed] [Google Scholar]
- 34.Liang J, Jung Y, Tighe RM, Xie T, Liu N, Leonard M, Gunn MD, Jiang D, Noble PW. A macrophage subpopulation recruited by CC chemokine ligand-2 clears apoptotic cells in noninfectious lung injury. Am J Physiol Lung Cell Mol Physiol. 2012;302:L933–L940. doi: 10.1152/ajplung.00256.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pemberton AD, Brown JK, Inglis NF. Proteomic identification of interactions between histones and plasma proteins: implications for cytoprotection. Proteomics. 2010;10:1484–1493. doi: 10.1002/pmic.200900818. [DOI] [PubMed] [Google Scholar]
- 37.Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011;118:3708–3714. doi: 10.1182/blood-2011-01-332676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayakawa M, Sawamura A, Gando S, Jesmin S, Naito S, Ieko M. A low TAFI activity and insufficient activation of fibrinolysis by both plasmin and neutrophil elastase promote organ dysfunction in disseminated intravascular coagulation associated with sepsis. Thromb Res. 2012;130:906–913. doi: 10.1016/j.thromres.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 40.Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis, thrombosis and organ dysfunction. Thromb Res. 2012;129:290–295. doi: 10.1016/j.thromres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 41.Nishiuma T, Sisson TH, Subbotina N, Simon RH. Localization of plasminogen activator activity within normal and injured lungs by in situ zymography. Am J Respir Cell Mol Biol. 2004;31:552–558. doi: 10.1165/rcmb.2004-0162OC. [DOI] [PubMed] [Google Scholar]
- 42.Hao Z, Guo C, Jiang X, Krueger S, Pietri T, Dufour S, Cone RE, O’Rourke J. New transgenic evidence for a system of sympathetic axons able to express tissue plasminogen activator (t-PA) within arterial/arteriolar walls. Blood. 2006;108:200–202. doi: 10.1182/blood-2005-12-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levin EG, Stern DM, Nawroth PP, Marlar RA, Fair DS, Fenton JW, II, Harker LA. Specificity of the thrombin-induced release of tissue plasminogen activator from cultured human endothelial cells. Thromb Haemost. 1986;56:115–119. [PubMed] [Google Scholar]
- 44.Campbell RA, Overmyer KA, Selzman CH, Sheridan BC, Wolberg AS. Contributions of extravascular and intravascular cells to fibrin network formation, structure, and stability. Blood. 2009;114:4886–4896. doi: 10.1182/blood-2009-06-228940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kppec M, Wegrzynowicz Z, Zajdel M, Sawecka J, Szumiel I. Effects of histones and dextran on some properties of fibrin, particularly on its susceptibility to plasmin. Thromb Res. 1974;5:359–374. doi: 10.1016/0049-3848(74)90173-x. [DOI] [PubMed] [Google Scholar]
- 46.Takabayashi T, Kato A, Peters AT, Hulse KE, Suh LA, Carter R, Norton J, Grammer LC, Cho SH, Tan BK. Excessive fibrin deposition in nasal polyps through reduction of tissue plasminogen activator expression. Am J Respir Cell Mol Biol. 2013;187:49–57. doi: 10.1164/rccm.201207-1292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adamali H, Armstrong ME, McLaughlin AM, Cooke G, McKone E, Costello CM, Gallagher CG, Leng L, Baugh JA, Fingerle-Rowson G, et al. Macrophage migration inhibitory factor enzymatic activity, lung inflammation, and cystic fibrosis. Am J Respir Crit Care Med. 2012;186:162–169. doi: 10.1164/rccm.201110-1864OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reeves EP, Williamson M, O’Neill SJ, Greally P, McElvaney NG. Nebulized hypertonic saline decreases IL-8 in sputum of patients with cystic fibrosis. Am J Respir Crit Care Med. 2011;183:1517–1523. doi: 10.1164/rccm.201101-0072OC. [DOI] [PubMed] [Google Scholar]
- 49.Cruikshank WW, Kornfeld H, Center DM. Interleukin-16. J Leukoc Biol. 2000;67:757–766. doi: 10.1002/jlb.67.6.757. [DOI] [PubMed] [Google Scholar]
- 50.Rand TH, Cruikshank WW, Center DM, Weller PF. CD4-mediated stimulation of human eosinophils: lymphocyte chemoattractant factor and other CD4-binding ligands elicit eosinophil migration. J Exp Med. 1991;173:1521–1528. doi: 10.1084/jem.173.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seegert D, Rosenstiel P, Pfahler H, Pfefferkorn P, Nikolaus S, Schreiber S. Increased expression of IL-16 in inflammatory bowel disease. Gut. 2001;48:326–332. doi: 10.1136/gut.48.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lefrancais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci USA. 2012;109:1673–1678. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]