Abstract
For patients with persistent asthma, inhaled corticosteroids (ICS) are a mainstay of controller therapy. These medications are usually prescribed to be taken daily and have been shown to be associated with decreased asthma morbidity. Adherence to daily treatment is very low in many populations in the United States. The purpose of this study is to evaluate the seasonal use of ICS prescription filling as reactive behavior primarily after an asthma exacerbation in a pediatric population. The study population is a subgroup of the Tennessee Asthma and Bronchiolitis Study. The children in this study were enrolled in Tennessee Medicaid (TennCare). The subjects had asthma and were 6 to 9 years of age during the years 2005 to 2010. Prescription filling was determined using claims data, and asthma exacerbations were defined by use of systemic rescue corticosteroids (RCS). In this cohort of 13,114 children with asthma, ICS and RCS filling were highly seasonal and trended with fall and winter peaks in asthma exacerbations. Prescription refilling was very low, with an average of three ICS fills per child who filled at least one during the study period. Among these children, 54.1% (7,096) had an asthma exacerbation during the study period. Among ICS users, 68.5% (3,441/5,020) had a disease exacerbation. ICS filling occurred overwhelmingly on the same day as RCS fills. The seasonal filling patterns of ICS coincide with asthma exacerbations. ICS adherence is low and inconsistent in this population of children with asthma. Increased adherence to ICS, particularly before the seasonal virus epidemics, could greatly reduce asthma morbidity.
Keywords: asthma, prevention, adherence, seasonality
Pediatric asthma exacerbations are among the most common causes of childhood hospitalizations despite the widespread availability and known effectiveness of controller medications (1). Adherence to controller medications such as inhaled corticosteroids (ICSs) is known to prevent morbidity and mortality from asthma (2, 3), with an estimated 21% reduced risk of death from an asthma attack for every prescription of ICS used in the previous year (4). Rates of ICS use are low in many populations, particularly in poor and minority groups. The reasons for disparities in adherence among different subpopulations have been attributed to a number of factors, including variation in attitudes toward prescriptions, level of education, and access to health care (5–8).
Adherence is not only variable across different populations but also at different times of the year. Asthma exacerbations are highly seasonal, with low rates in the summer and increasing rates in the fall and throughout the winter (9). The fall increase, coined the “September asthma epidemic,” is largely theorized to be due to increased circulation of human rhinovirus, perhaps in conjunction with aeroallergen season (10). In a study by Sears and Johnston (11), it was suggested that asthma controller medication use largely followed the same temporal pattern as asthma exacerbation rates, suggesting, though not proving, that prescription filling often follows an increase in symptoms rather than being used to prevent them. If these patterns are persisting in large populations, they could be attributed to hundreds of thousands of preventable hospitalizations and doctors visits every year.
An ecological study of ICS use was undertaken among a Medicaid population over a 5-year period to study seasonal patterns in ICS filling compared with seasonal patterns of asthma exacerbations. A goal of this research was to determine if there was evidence of large proportions of ICS prescriptions occurring immediately after asthma exacerbations.
Methods
The Tennessee Asthma and Bronchiolitis Study is a retrospective birth cohort of over 90,000 children born between 1995 and 2001, enrolled in Tennessee Medicaid (TennCare), and followed longitudinally for the outcome of asthma (12). All children were enrolled continuously, with no more than 90 consecutive days not covered in TennCare, to be eligible for inclusion. A subset of eligible children (n = 51,724), from birth years 1999 to 2001, was followed from ages 6 to 9. Each subject had equal follow-up time. For the purposes of this study, rescue corticosteroid (RCS) filling, which could follow a phone contact, outpatient visit, ED visit, or hospitalization, was used as a marker of an asthma exacerbation.
To describe the seasonal patterns, the rates of ICS and RCS prescription filling were assessed among 6- to 9-year-old children with asthma. Because ICS prescriptions covered by TennCare last 30 days, each ICS fill was considered to cover 30 days or the period until their next fill if one occurred in less than 30 days. In addition, there is no automated mail-order prescription filling in TennCare. Only one course of RCS was allowed to be counted within a 7-day period. Prescription fill data for both RCS and ICS is solely indicated in the database by billing to TennCare. Therefore, any outside sources of prescriptions or self-pay are not captured in the database, although these types of fills are unlikely in this low-income population. The denominator was calculated as the total number of children who were 6 to 9 years of age for each week between 2005 and 2010 within the cohort. A moving average smoothing technique was applied to make long-term trends clearer.
For the purposes of this study, a “reactive” ICS fill was one that occurred concurrent with the timing of the RCS event. Fills of ICS occurring on the same day as an RCS fill or within the next 2 days were therefore considered reactive. To investigate whether the seasonal patterns of inhaled corticosteroid use were due to reactive filling, an individual-level analysis of timing of ICS in relation to RCS fills was performed. This included calculating the distance in days between an RCS fill and the most recent ICS fill. This analysis included only children with asthma who filled both an RCS and an ICS. Only one RCS counted per child, which was defined as their first RCS fill occurring at least 45 days after their sixth birthday and at least 45 days before their ninth birthday (n = 3,339). Among children with ICS fills before and after an RCS event, ICS fills in distance days from RCS were compared using the Wilcoxon signed rank test. For a final analysis, children who filled an ICS both before and after their RCS (n = 1,053) were compared to assess if the distance in days between ICS filling and RCS filling was different.
Results
Each population subgroup used in various parts of the analysis, including eligibility criteria, are presented in the flow chart provided in Figure 1. Among the 13,114 6- to 9-year–old subjects defined as having asthma according to a previously validated algorithm incorporating data on medication usage and health care visits for asthma (13), 43.1% were female, 34% were African American, and 64% were white. Descriptive statistics for this cohort are given in Table 1. During the 3-year follow-up, 42.7% of African Americans and 34.4% of whites filled an ICS at least once. Among the 13,114 children with asthma, there were a total of 17,159 asthma exacerbations, as determined by the filling of a course of RCS. There were 7,096 individual children who received the 17,159 courses of RCS and 28,684 fills of ICS, representing 5,020 individual children. Among those who filled an ICS at least once, the median number of fills was three over the 3-year follow-up. The number of patients with asthma experiencing more than one exacerbation between the ages of 6 and 9 was 3,808 (29.0%).
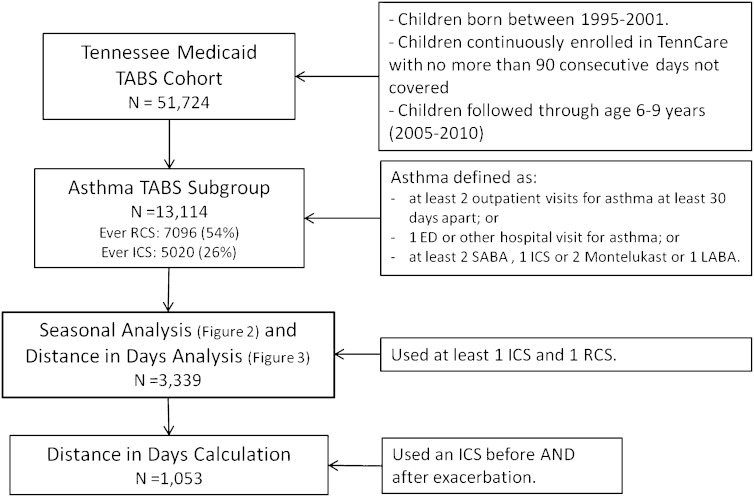
Figure 1.
Flow chart of each population subgroup used in the different analysis presented. The subgroup with 3,339 children 6 to 9 years of age was selected based on the use of at least one inhaled corticosteroid (ICS) and one rescue corticosteroid (RCS) in that 3-year period, indicating a cohort with a level of severity that benefits taking an ICS on a monthly basis. ED = emergency department; LABA = long-acting β-agonist; SABA = short-acting β-agonist; TABS = Tennessee Asthma and Bronchiolitis Study.
Table 1.
Descriptive statistics for the population of 13,114 children 6–9 years of age with asthma from the Tennessee Asthma and Bronchiolitis Study cohort between 2005 and 2010
| Characteristic (N with variable) | n | Percent |
|---|---|---|
| Marital status (13,113) | ||
| Single | 8,198 | 63% |
| Married | 4,915 | 37% |
| Maternal age (13,084) | 22 (19–26) | |
| Maternal education (13,093) | ||
| Less than high school | 5,343 | 41% |
| High school | 6,184 | 47% |
| More than high school | 1,566 | 12% |
| Maternal smoking (13,093) | ||
| Smoking | 4,016 | 31% |
| Nonsmoking | 9,077 | 69% |
| Maternal asthma (7,935) | ||
| Asthma | 538 | 7% |
| No asthma | 7,397 | 93% |
| Maternal race (12,937) | ||
| White | 8,238 | 64% |
| Black | 4,354 | 34% |
| Hispanic | 345 | 3% |
| Infant race (12,182) | ||
| White | 7,545 | 62% |
| Black | 4,260 | 35% |
| Hispanic | 377 | 3% |
| Gestational age, d (13,114) | 274 (265–281) | |
| Birth weight, g (13,114) | 3,175 (2,778–3,515) |
* Percentages are given for categorical variables, and medians with interquartile ranges are given for continuous variables.
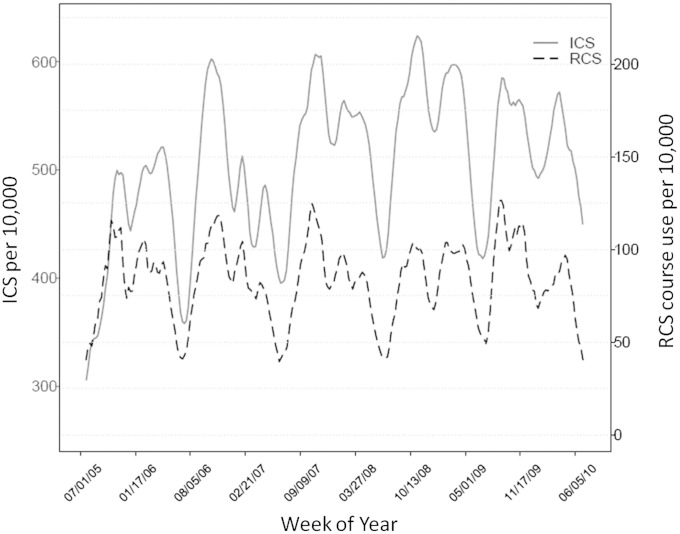
Seasonal trends of asthma exacerbations were evident in the cohort. The ICS and RCS medication use data are figuratively presented over the span of the five study years (Figure 2). Due to lower numbers at the tails, the figure is presented as beginning and ending in July of the first and last years. Increasing trends of asthma exacerbation rates began in late summer or early autumn and peaked during late autumn or early winter to approximately 1.5 times summer levels. Consistent decreasing trends in both ICS and RCS were apparent in the summer months and around school holidays, such as winter holiday break.
Figure 2.
Graph of the weekly rates per 10,000 of inhaled corticosteroid (ICS) prescription filling (left, solid line) and rescue corticosteroid RCS courses prescribed (right, dashed line) for children 6 to 9 years of age. The time period covered is July 2005 to July 2010.
There were 3,339 children who met the criteria (as described above) of inclusion in the cohort in having at least one ICS and one RCS within the allowed time frame and for whom patterns of ICS use in relation to one RCS use were compared. Among these children, there were 20,037 total ICS fills and 10,533 total RCS fills. In addition, 55% (1,833/3,339) of RCS fills were not preceded by an ICS fill 2 to 30 days prior. Among those who did not fill an ICS before their RCS fill, 1,384 (83%) did fill an ICS sometime thereafter (excluding same-day fills). On average, these fills occurred 180 days after the RCS (interquartile range [IQR], 53–398 d).
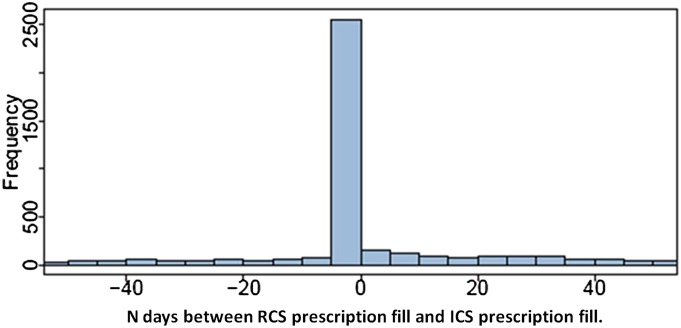
Among the 3,339 children, the frequency and distance in days of ICS filling from RCS filling was calculated and plotted as shown in Figure 3. Out of 6,678 total ICS fills, the largest proportion occurred on the same day as RCS fills (n = 2,446; 37%). For those who filled ICS before and after an exacerbation, the reactive filling days were closer to RCS use, although adherence remained very poor (median days of ICS fills before RCS, 53 [IQR, 21–131] vs. median days of ICS fills after RCS, 62 [IQR, 24–157]; P < 0.001).
Figure 3.
Among a cohort of patients with asthma between 6 to 9 years of age, the distribution of inhaled corticosteroid (ICS) fills (6,678 fills total) described as the number of days before or after their first rescue corticosteroid (RCS) fill (marker of asthma exacerbation). Negative values indicate number of days before the exacerbation.
Conclusions
Although there have been many investigations of ICS prescription filling, this study is novel in identifying reactive ICS filling patterns that closely coincide with seasonal disease exacerbations. The seasonal patterns of asthma exacerbations discerned here begin in the late summer/early fall in any given year and peak during the winter months. These patterns are consistent with previously reported trends described as the “September asthma epidemic.” Regular ICS prescription fills were extremely low in this population, and ICS filling trended closely with the patterns of exacerbations. ICS fills most often occurred just before or immediately after exacerbations (most often on the same day), with no significant increase in ICS filling after the first same-day fill. This is evidence of reactive, rather than proactive, filling of ICS prescriptions, suggesting that a large proportion of asthma exacerbations in this cohort would be prevented with use of ICS before the onset of seasonal epidemics. The lack of filling ICS within 50 days after an exacerbation indicates that adherence to controller treatment of asthma drops off sharply after treatment of exacerbations in this population.
The interpretation of this study is limited by its reliance on healthcare administrative data. Although there are clear advantages to having the very large sample size available through the TennCare records, we do not have direct clinical measurements on each child and must rely on the validated algorithm to diagnose asthma. The measures of prescription filling are also not a direct measure of adherence. There is no guarantee that prescriptions filled were used appropriately or for the recommended duration once received by the patient. It may be assumed, however, that because of the reimbursement structure within TennCare, there is not widespread ICS prescription filling not recorded within these administrative medical records. Recent studies have demonstrated that ICS could occasionally be prescribed on an as-needed basis for certain cases of mild persistent pediatric asthma rather than as a regular monthly prescription (14). However, studies suggesting this as a helpful regimen are relatively new and thus unlikely to have been practiced in Tennessee during our study years. In addition, the criteria of using at least one RCS and one ICS fill suggests a level of severity that would benefit from daily ICS use rather than intermittent use.
The vast majority of ICS were filled at the same time as an RCS fill; frequencies of ICS fills were very low outside of exacerbations regardless of whether filling preceded or followed the exacerbation. The low adherence rates to controller medications and the persistently high number of asthma exacerbations in the Tennessee Medicaid population suggest that focused interventions to increase the use of ICS prescription filling in late summer, perhaps coinciding with school beginning and continuing through the winter season, could have the potential effect of decreasing the number of asthma exacerbations resulting in patient morbidity and requiring costly treatment.
Acknowledgments
Acknowledgment
The authors thank the Bureau of TennCare and the Tennessee Department of Health for providing the data.
Footnotes
Supported by the National Institutes of Health/National Institute of Environmental Health Sciences K12 ES015855 (Vanderbilt VEHSS) program at Vanderbilt University Medical Center and National Institutes of Health grant K24 AI77930.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123:S131. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 2.Lasmar L, Camargos P, Champs NS, Fonseca MT, Fontes MJ, Ibiapina C, Alvim C, Moura JAR. Adherence Rate to Inhaled Corticosteroids and Their Impact on Asthma Control. Allergy. 2009;64:784–789. doi: 10.1111/j.1398-9995.2008.01877.x. [DOI] [PubMed] [Google Scholar]
- 3.Bauman LJ, Wright E, Leickly FE, Crain E, Kruszon-Moran D, Wade SL, Visness CM. Relationship of adherence to pediatric asthma morbidity among inner-city children. Pediatrics. 2002;110:e6. doi: 10.1542/peds.110.1.e6. [DOI] [PubMed] [Google Scholar]
- 4.Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332–336. doi: 10.1056/NEJM200008033430504. [DOI] [PubMed] [Google Scholar]
- 5.Van Dellen QM, Stronks K, Bindels PJE, Pattemore PK, van Aalderen WMC. Adherence to inhaled corticosteroids in children with asthma and their parents. Respir Med. 2008;102:755–763. doi: 10.1016/j.rmed.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Menckeberg TT, Bouvy ML, Bracke M, Kaptein AA, Leufkens HG, Raaijmakers JAM, Horne R. Beliefs about medicines predict refill adherence to inhaled corticosteroids. J Psychosom Res. 2008;64:47–54. doi: 10.1016/j.jpsychores.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Ponieman D, Wisnivesky JP, Leventhal H. Musumeci-Szabó TJ, Halm EA. Impact of positive and negative beliefs about inhaled corticosteroids on adherence in inner-city asthmatic patients. Ann Allergy Asthma Immunol. 2009;103:38–42. doi: 10.1016/S1081-1206(10)60141-X. [DOI] [PubMed] [Google Scholar]
- 8.Williams L, Joseph CL, Peterson EL, Moon C, Xi H, Krajenta R, Johnson R, Wells K, Booza JC, Tunceli K. Race-ethnicity, crime, and other factors associated with adherence to inhaled corticosteroids. J Allergy Clin Immunol. 2007;119:168–175. doi: 10.1016/j.jaci.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 9.Storr J, Lenney W. School holidays and admissions with asthma. Arch Dis Child. 1989;64:103. doi: 10.1136/adc.64.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston NW, Johnston SL, Norman GR, Dai J, Sears MR. The September epidemic of asthma hospitalization: school children as disease vectors. J Allergy Clin Immunol. 2006;117:557–562. doi: 10.1016/j.jaci.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 11.Johnston NW, Sears MR. Asthma exacerbations+ 1: epidemiology. Thorax. 2006;61:722. doi: 10.1136/thx.2005.045161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu P, Dupont WD, Griffin MR, Carroll KN, Mitchel EF, Gebretsadik T, Hartert TV. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178:1123–1129. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enriquez R, Griffin MR, Carroll KN, Wu P, Cooper WO, Gebretsadik T, Dupont WD, Mitchel EF, Hartert TV. Effect of maternal asthma and asthma control on pregnancy and perinatal outcomes. J Allergy Clin Immunol. 2007;120:625–630. doi: 10.1016/j.jaci.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 14.Martinez FD, Chinchilli VM, Morgan WJ, Boehmer SJ, Lemanske RF, Mauger DT, Strunk RC, Szefler SJ, Zeiger RS, Bacharier LB. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:650–657. doi: 10.1016/S0140-6736(10)62145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]