Abstract
Rationale: Although asthma is usually considered to originate in childhood, adult-onset disease is being increasingly reported.
Objectives: To contrast the proportion and natural history of adult-onset versus pediatric-onset asthma in a community-based cohort. We hypothesized that asthma in women is predominantly of adult onset rather than of pediatric onset.
Methods: This study used data from the Coronary Artery Risk Development in Young Adults (CARDIA) cohort in the United States over a 25-year period. Adult- and pediatric-onset asthma phenotypes were studied, as defined by age at onset of 18 years or older. Subjects with asthma were categorized by sex, obesity, atopy, smoking, and race by mean age/examination year, using a three-way analysis of covariance model. Natural history of disease was examined using probabilities derived from a Markov chain model.
Measurements and Main Results: Asthma of adult onset became the dominant (i.e., exceeded 50%) phenotype in women by age 40 years. The age by which adult-onset asthma became the dominant phenotype was further lowered for obese, nonatopic, ever-smoking, or white women. The prevalence trend with increasing time for adult-onset disease was greater among subjects with nonatopic than atopic asthma among both sexes. Furthermore, adult-onset asthma had remarkable sex-related differences in risk factors. In both sexes, the quiescent state for adult-onset asthma was less frequent and also “less stable” over time than for pediatric-onset asthma.
Conclusions: Using a large national cohort, this study challenges the dictum that most asthma in adults originates in childhood. Studies of the differences between pediatric- and adult-onset asthma may provide greater insight into the phenotypic heterogeneity of asthma.
Keywords: adult-onset, pediatric-onset, obesity, nonatopic, recrudescent
Asthma, a chronic inflammatory airway disease, is usually considered a disease of childhood onset. However, a large number of adults are reporting the onset of asthma past childhood (1). Unfortunately, longitudinal data on asthma in adults are sparse (1, 2). This constitutes a significant gap in the current literature, because an estimated 18.7 million adults reported current asthma, as compared with 7 million children, in the United States in 2010 (3). The annual U.S. medical expenditure attributable to asthma in adults in 2005 was $18 billion (4), estimated at more than twofold higher than that attributable to asthma in children (5). Death rates during the period 2001 to 2010 were seven times higher for adults with asthma than for children (6). Despite its overall significance, asthma in adults, and particularly adult-onset asthma, remains understudied. The literature in this field primarily relies on knowledge of either childhood asthma followed into early adulthood (7) or occupational asthma (8). Our objective was to evaluate the natural history of disease in a community-based cohort of young and middle-aged adults with asthma followed for 25 years. We hypothesized that asthma in middle-aged women is predominantly of adult onset rather than of pediatric onset.
Methods
Study Design
This study used data from the Coronary Artery Risk Development in Young Adults (CARDIA) cohort in the United States over a 25-year period. During 1985 to 1986, the investigators recruited 5,115 subjects, equally divided between blacks and whites and men and women, aged 18 to 30 years, from the general population of Birmingham, Alabama; Chicago, Illinois; and Minneapolis, Minnesota; and from the membership of the Oakland Kaiser-Permanente Health Plan in Oakland, California. Follow-up examinations were completed 2, 5, 7, 10, 15, 20, and 25 years later (Y2, Y5, Y7, Y10, Y15, Y20, and Y25, respectively). Detailed methods, instruments, and quality control procedures are described at the CARDIA website (http://www.cardia.dopm.uab.edu) and in other published reports (9, 10). Retention of CARDIA participants has been excellent. For example, 3,499 persons were followed at Y25 examination visit, constituting 72% of the survivors from the baseline cohort. We excluded two subjects who underwent sex change and one subject who withdrew consent.
Predictor and Outcome Variables
Self-reported information was obtained from all participants using standardized questionnaires. Prevalent (i.e., ever-life) asthma was defined by a self-reported provider diagnosis at that or any prior examination visit. By mean age of 50 years (i.e., Y25 examination visit), 1,112 subjects (21.8% of the initial cohort) reported prevalent asthma. After excluding 142 subjects (12.8% of those with prevalent asthma) who did not provide their age of asthma onset, our study population sample size was 970 at the Y25 visit. After similar exclusions, 484, 590, 665, 731, 824, and 900 subjects with prevalent asthma by mean ages of 25 (Y0), 27 (Y2), 32 (Y7), 35 (Y10), 40 (Y15), and 45 (Y20) years, respectively, were identified; the increase in prevalence was explained by the incident disease at each examination (asthma was not queried at CARDIA Y5 examination, mean age 30 yr). Adult-onset and pediatric-onset asthma phenotypes were studied; adult onset was defined by a self-report of age of onset of 18 years or older. To minimize recall bias, age of onset was determined from the first examination visit at or after the onset of disease.
All subjects with asthma were classified into four discrete categories at each evaluation period (i.e., incident, persistent, quiescent, and recrudescent state), using identical questions at each examination (time i) and each subsequent examination (time i+1;). As defined in Table 1, incident asthma was defined by the new occurrence of asthma among subjects who did not have prior asthma. Among subjects with asthma, persistent disease at subsequent visit was defined by report of asthma symptoms or use of asthma medications at two consecutive examination visits. On the other hand, if these items were absent on initial visit (at time i) but present on subsequent visit (at time i+1), the disease was defined as recrudescent at the subsequent visit. If these items were both absent on subsequent visit (at time i+1), the disease was defined as quiescent at the subsequent visit. These categories were first defined at Y2 examination (mean age, 27 yr), using information about change in status between Y0 and Y2 examinations and then changed per the data at each subsequent examination. In the event of a missing visit, we classified subjects as though there was no change in status until the subsequent visit. In our study of the natural history of asthma, we focused on quiescent, recrudescent, and persistent asthma states and the between-examination transitions between states, using a Markov chain model (11). A Markov chain is a stochastic system that transits from one state to another in a chain-like manner, as shown in Figure 1 (11). The transitions between states are random, and the probabilities associated with various state changes are called transition probabilities and are computed as summary percentages across time (11). Transition probabilities between asthma states applied over time are then used to compute stationary probabilities for each asthma state.
Table 1.
Questions used to assess asthma states at each examination
| Has a doctor or nurse ever said that you have asthma? | If yes, at what age were you first told this? | Have you had this (asthma) in the past year? OR Are you taking medications for asthma or any breathing problem? | |||
|---|---|---|---|---|---|
| Time of question administration | Time i | Time i+1 | Time i | Time i+1 | |
| Never asthma (time i+1) | No | No | N/A | No | No |
| Incident asthma (time i+1) | No | Yes | Self-reported year of incidence to determine adult-onset asthma | N/A | N/A |
| Persistent asthma (time i+1) | Yes | Yes | — | Yes | Yes |
| Recrudescent asthma (time i+1) | Yes | Yes | — | No | Yes |
| Quiescent asthma (time i+1) | Yes | Yes | — | Yes/No | No |
Definition of abbreviation: N/A = not applicable.
The medication-related component of the definition in the last column is limited by our equating the “need” for the medication with “report of taking” the medication under a prescription.
Figure 1.
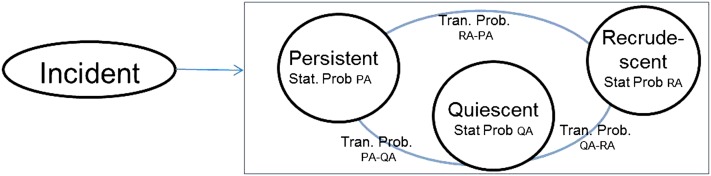
Schematic representing various states of asthma (based on a Markov chain model). Stationary probabilities (Stat. Prob.) were calculated for persistent asthma (PA), quiescent asthma (QA), and recrudescent asthma (RA) states. Transition probabilities (Tran. Prob.) were calculated for the transitions between persistent asthma to quiescent asthma (PA-QA), quiescent asthma to recrudescent asthma (QA-RA), and recrudescent asthma to persistent asthma (RA-PA) states.
Height and weight were measured by certified technicians using standardized equipment with participants wearing light clothing and no shoes. Obesity was defined by a body mass index greater than or equal to 30 kg/m2 at each examination. Atopy was defined by the self-report of hay fever at Y0 examination. Sex, race, smoking status, weekly hours of cumulative environmental tobacco smoke exposure, and menopause were also self-reported. Obesity, current and ever-smoking status, environmental tobacco smoke exposure, and menopausal status (in women) at each visit were analyzed as time-varying predictors.
Statistical Analysis
For simplicity of discussion, adult-onset asthma was summarized as a percentage of subjects with asthma and was divided into several categories (i.e., men and women; obese and nonobese; atopic and nonatopic; ever-smokers and never-smokers; current and not current smokers; those exposed and not exposed to environmental tobacco smoke; blacks and whites; and, among women, premenopausal and postmenopausal) by mean age associated with the examination year. Prevalence trends were analyzed by a three-way analysis of covariance model; the mean age was considered a linear factor.
To study the natural history of adult-onset asthma and to contrast that with pediatric-onset asthma, a Markov chain model was used (12). This allowed the calculation of the stationary and transition probabilities between the various asthma states after stratifying by pediatric versus adult-onset asthma category. Factors affecting the transitions from quiescent to recrudescent asthma state over 25 years were studied using logistic models (Proc Genmod function in SAS) in univariate and multivariable analyses. A two-sided P-value of less than 0.05 was considered statistically significant for all tests. All statistical analyses were done using the SAS package version 9.3 (Cary, NC).
All subjects gave informed consent for their participation in the study. This study was approved by the Institutional Review Boards at University of New Mexico, Albuquerque, New Mexico and at each of the CARDIA study sites.
Results
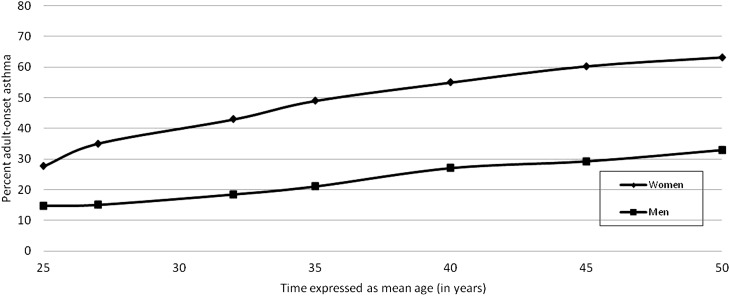
At a mean age of 25 years, sex was equally distributed among those with asthma (i.e., 48.6 and 51.4% were men and women, respectively; P = 0.58). Yet, the proportion of adult-onset disease was higher among women than men with asthma at that time (27.6 vs. 14.7%, P = 0.001; Figure 2). The prevalence trend with increasing time for adult-onset asthma was greater among women than men (P for sex interaction in longitudinal analysis using analysis of covariance < 0.001; Figure 2). This was explained by a higher annual percent incident rate of adult-onset asthma among women than men in our study (1.39 vs. 0.77%). Not surprisingly, therefore, the proportion of adult-onset disease remained greater among women than men at each age studied (all P ≤ 0.001, Chi-square; Figure 2).
Figure 2.
Age-related increase in percent adult-onset asthma by sex. Adult-onset asthma became the dominant phenotype (i.e., exceeded 50%) by age 40 years in women with asthma. On the contrary, pediatric-onset asthma remained the dominant phenotype in men aged 50 years or younger.
Adult-Onset Asthma Became the Dominant Phenotype among Women by Age 40 Years
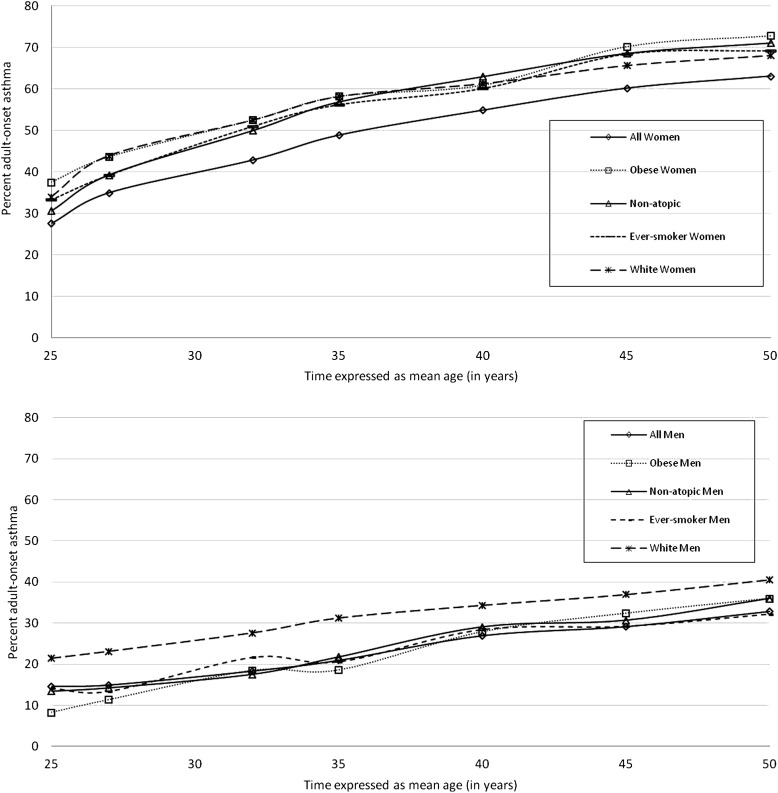
The minimum age by which the proportion of adult-onset disease became dominant (i.e., exceeded 50%) among women with asthma was approximately 40 years (Figure 2). The minimum age by which this proportion became dominant was, however, lowered to approximately 35 years among women with asthma who were either obese or nonatopic and further lowered to 32 years among those who were either ever-smokers or white (Figure 3). Among women with asthma, the first age point at which the difference in proportion of adult-onset disease became statistically significant was 45 years between obese and nonobese (P = 0.01; see Figure E1 in the online supplement), 32 years between atopic and nonatopic (P = 0.02 for age ≥ 32 yr; Figure E2), 25 years between ever-smokers and never-smokers (P = 0.04; Figure E3), and 27 years between blacks and whites (P = 0.008; Figure E6). Menopausal status was not associated with proportion of adult-onset disease. Unlike women, pediatric-onset asthma remained the dominant phenotype among men with asthma 50 years of age or younger (Figure 3).
Figure 3.
Age-related increase in percent adult-onset disease among women (top panel) and men with asthma (bottom panel), including those who were obese, nonatopic, ever-smokers, or white. The minimum age by which the proportion of adult-onset disease became dominant (i.e., exceeded 50%) was lowered to approximately 35 years among women with asthma who were either obese or nonatopic and further lowered to 32 years among women with asthma who were either ever-smokers or white.
Main Effects and Interaction Effects of Demographic Variables on the Proportion of Adult-Onset Disease among Men and Women with Asthma—A Longitudinal Analysis over a 25-Year Period
Independent of time, obesity increased the proportion of adult-onset disease among women with asthma but decreased it among men
Overall, the prevalence trend with increasing time for adult-onset disease was similar between obese and nonobese subjects with asthma, irrespective of whether women or men were studied (P for time–obesity interaction = 0.12 and 0.96, respectively; Table 2, Figure E1). Yet, obesity was associated with differing overall effects between men and women independent of time; lower proportions of adult-onset disease were seen among obese men but higher proportions among obese women with asthma, as compared with their nonobese counterparts (P < 0.001 for sex–obesity interaction; Table 2, Figure E1).
Table 2.
Main effects and interaction effects of various demographic variables on the proportion of adult-onset disease among men and women with asthma—A longitudinal analysis over a 25-year period
| Variable | Sex | Main Effect, Independent of Time: Estimate Expressed as % (P Value) | P Value for Interaction between Time and Variable | P Value for Interaction between Sex and Variable, Independent of Time |
|---|---|---|---|---|
| Obesity | Women | 10.0 (<0.001) | 0.12 | <0.001 |
| Men | −4.8 (<0.001) | 0.96 | ||
| Nonatopic state | Women | −13.0 (<0.001)* | 0.04† | <0.001 |
| Men | −1.2 (.25)* | 0.001† | ||
| Ever smoking | Women | 7.6 (0.003) | 0.69 | 0.001 |
| Men | −4.9 (0.002)* | 0.03† | ||
| Current smoking | Women | 3.4 (0.12) | 0.52 | <0.001 |
| Men | −11.6 (<0.001)* | 0.003† | ||
| Environmental tobacco smoke exposure | Women | 3.7 (0.15) | 0.54 | 0.006 |
| Men | −8.6 (0.008) | 0.47 | ||
| White race | Women | 12.6 (<0.001) | 0.35 | 0.21 |
| Men | 15.3 (<0.001) | 0.99 | ||
| Menopausal state | Women | 7.8 (0.08) | 0.18 | N/A |
| Men | N/A | N/A |
Definition of abbreviation: N/A = not applicable.
Obesity, smoking variables and menopausal state were analyzed as time-varying covariates.
Main effect was calculated without the interaction effect in the statistical model.
Overall, the prevalence trend with increasing time for adult-onset disease was steeper among nonatopic than atopic subjects with asthma for both women and men (Figure E2). On the other hand, the prevalence trend with increasing time for adult-onset disease was less steep among ever-smoking men with asthma compared with never-smoking men with asthma (Figure E3) as well as among currently smoking men with asthma as compared with not currently smoking men with asthma (Figure E4).
Independent of time, nonatopic state increased the proportion of adult-onset disease to a greater extent among women than men with asthma
Overall, the prevalence trend with increasing time for adult-onset disease was steeper among nonatopic than atopic subjects with asthma, irrespective of whether women or men were studied (P for time–atopy interaction = 0.04 and 0.001, respectively; Table 2, Figure E2). Yet, independent of time, nonatopic state was associated with a greater effect on the proportion of adult-onset disease among women than men with asthma (P < 0.001 for sex–atopy interaction; Table 2, Figure E2).
Independent of time, ever smoking increased the proportion of adult-onset disease among women with asthma but decreased it among men
Overall, the prevalence trend with increasing time for adult-onset disease was similar between ever-smoking and never-smoking women with asthma, but not so for men (P for time–smoking interaction = 0.69 and 0.03, respectively; Table 2, Figure E3; ever-smokers had a less steep prevalence trend than never-smokers among men). In addition, ever-smoking was associated with differing overall effects between men and women independent of time; higher proportions of adult-onset disease were seen among ever-smoking women with asthma but lower proportions among ever-smoking men, as compared with their never-smoking counterparts (P = 0.001 for sex–smoking interaction; Table 2, Figure E3).
Additionally, the associations between current smoking and environmental tobacco smoke exposure with sex on adult-onset asthma were separately examined. Surprisingly, current smoking in men with asthma was associated with a lower prevalence trend and lower proportion of adult-onset asthma (P values of < 0.001, 0.003, and < 0.001 for main effect, time–smoking interaction, and sex–smoking interaction, respectively; Table 2, Figure E4). Findings similar to current smoking were also observed when high versus low levels of environmental tobacco smoke exposure (≥ 10 h weekly vs. < 10) were instead studied in relation to adult-onset asthma (details in online supplement, Figure E5).
Independent of time, whites had greater proportion of adult-onset disease than blacks among both men and women with asthma
Overall, the prevalence trend with increasing time for adult-onset disease was similar between white and black subjects with asthma, irrespective of whether women or men were studied (P for time–race interaction = 0.35 and 0.99, respectively; Table 2, Figure E6). Independent of time, the proportion of adult-onset disease was, however, greater among white men and women, as compared with their black counterparts, with no sex differences (P = 0.21 for sex–race interaction; Table 2, Figure E6).
Adult-Onset Disease Differed from Pediatric-Onset Disease among Adults with Respect to Key Clinical Variables
As shown in Table 3, adult-onset disease was more common than pediatric-onset disease among women as well as among older, obese, or nonatopic subjects at mean age of 45 years. Furthermore, adult-onset disease was associated with more respiratory symptoms and asthma medications (including inhaled corticosteroids) despite a higher prebronchodilator FEV1/FVC ratio (P ≤ 0.03 for all above analyses; Table 3).
Table 3.
Phenotypic differences between adult-onset and pediatric-onset asthma at Coronary Artery Risk Development in Young Adults Year 20 examination (mean age, 45 Yr), shown as an example
| Characteristic at CARDIA Y20 Examination | Subjects with Ever-Asthma of Adult Onset (n = 348) | Subjects with Ever-Asthma of Pediatric Onset (n = 306) | P Value |
|---|---|---|---|
| Mean ± SD or % | Mean ± SD or % | ||
| Female sex, % | 76.7 | 51.3 | <0.001 |
| White race, % | 48.0 | 44.8 | 0.41 |
| Age, yr | 45.4 ± 3.7 | 44.8 ± 3.6 | 0.03 |
| Age of onset of disease, yr | 33.3 ± 9.8 | 6.9 ± 4.9 | — |
| Obesity, % | 49.0 | 36.8 | 0.002 |
| BMI, kg/m2 | 31.5 ± 8.6 | 29.7 ± 7.5 | 0.005 |
| Atopy, % | 41.1 | 57.4 | <0.001 |
| Ever-smoking status,* % | 44.7 | 39.5 | 0.18 |
| Respiratory symptom score† | 2.9 ± 2.1 | 2.2 ± 1.9 | <0.001 |
| Number of asthma medications used | 0.7 ± 1.1 | 0.4 ± 0.9 | 0.002 |
| Inhaled corticosteroid use, % | 17.0 | 10.5 | 0.02 |
| Incident disease, % | 21.2 | 0 | — |
| Persistent disease, % | 36.9 | 35.6 | 0.84 |
| Quiescent disease, % | 33.6 | 55.0 | <0.001 |
| Recrudescent disease, % | 8.4 | 9.4 | 0.74 |
| Absolute prebronchodilator FEV1/FVC ratio at CARDIA Y20 examination | 76.7 ± 9.0 | 75.2 ± 8.5 | 0.03 |
| Change in CARDIA Y10-Y20 FEV1/FVC ratio‡ | −0.8 ± 5.1 | −1.5 ± 5.1 | 0.15 |
Definition of abbreviations: BMI = body mass index; CARDIA = Coronary Artery Risk Development in Young Adults study; Y10 = Year 10; Y20 = Year 20.
Of 900 subjects with prevalent (i.e., ever life) asthma at CARDIA Y20, data are available for 654 subjects. Spirometry at CARDIA Y20 examination was additionally available for 621 subjects.
Data were limited to those who had both established asthma diagnosis by CARDIA Y10 examination and had complete spirometry data at both Y10 and Y20 examinations (n = 426).
The number of respiratory symptoms was obtained from coding the following seven questions with binary responses: (1) Does your chest ever sound wheezy or whistling when you have a cold? (2) Does your chest sound wheezy or whistling occasionally apart from a cold? (3) Have you ever had an attack of wheezing that made you feel short of breath? (4) Have you had two or more episodes of attacks of wheezing that made you feel short of breath? (5) Are you troubled by shortness of breath when hurrying on the level or walking up a slight hill? (6) When walking on level ground, do you have to walk slower than people your age because of breathlessness? (7) Do you ever have to stop for breath when walking at your own pace on level ground?
Multivariable analysis examined the difference between the two groups related to sex, age, nonatopic status, and obesity, factors significant in the univariate analyses. After adjusting for the effect of remaining three covariates, sex and nonatopic status remained significant (P < 0.001 for both), whereas age and obesity trended toward significance (P = 0.07 and 0.10, respectively). Substituting obesity with BMI in the multivariable analyses did not change the results.
The Quiescent Asthma State Was Less Frequent and Also More Likely to Recrudesce over Time for Adult-Onset Disease than for Pediatric-Onset Disease among Adults
Adult-onset disease was less likely to be quiescent than pediatric-onset disease among adults with asthma across all examination visits (34.9 vs. 57.1%; P < 0.001). In the Markov theoretical model for steady state between various disease transitions, the percent of subjects in the quiescent state had calculated stationary probabilities of 60% for adult-onset and 81% for pediatric-onset disease. This also means that on any study visit, 40% of adult-onset and 19% of pediatric-onset patients were expected to have active (i.e., recrudescent or persistent) asthma.
Furthermore, transition probabilities over time for various asthma states were compared between adult-onset and pediatric-onset disease (Table 4). The probability of transition from quiescent to recrudescent state over time was twice as high (i.e., less stable) for adult-onset disease than for pediatric-onset disease (Fisher exact test: P < 0.005 for all, men, or women). On the other hand, transition probabilities over time for either recrudescent or persistent asthma states were similar between adult-onset and pediatric-onset disease.
Table 4.
Observed transition probabilities among various asthma states between an examination visit and following examination visit—A univariate longitudinal analysis accumulative over a 25-year period
| Persistent Asthma After | Quiescent Asthma After | Recrudescent Asthma After | ||
|---|---|---|---|---|
| Persistent Asthma Before | ||||
| All | Adult-onset type | 83.0 | 17.0 | |
| Pediatric-onset type | 79.5 | 20.5 | ||
| Quiescent Asthma Before | ||||
| All | Adult-onset type | 59.9 | 40.1 | |
| Pediatric-onset type | 80.7 | 19.3 | ||
| Recrudescent Asthma Before | ||||
| All | Adult-onset type | 64.1 | 35.9 | |
| Pediatric-onset type | 60.2 | 39.8 |
Data are given as %. For adult-onset asthma, the transition probabilities over time between various asthma states are similar for men and women (Fisher exact test, all P ≥ 0.55). Similar findings were noted for pediatric-onset asthma as well (all P ≥ 0.06). The quiescent asthma state for adult-onset disease is significantly “less stable” over time than for pediatric-onset disease (Fisher exact test, P ≤ 0.005 for all, men and women). For recrudescent or persistent asthma states, the transition probabilities over time are, however, similar for adult-onset and pediatric-onset disease.
Factors associated with increased odds for recrudescence of quiescent asthma were then compared between adult-onset and pediatric-onset disease (Table 5). Women and ever-obese subjects with quiescent pediatric-onset asthma were more likely to recrudesce than men and never-obese subjects, respectively (P values in best multivariable model of 0.04 and 0.06, respectively). On the other hand, ever-smokers were more likely to recrudesce than never-smokers among those with adult-onset (P = 0.004) but not pediatric-onset quiescent asthma state (interaction P = 0.01).
Table 5.
Factors affecting the odds of transitioning from quiescent to recrudescent asthma state, stratified by adult versus pediatric-onset disease—Univariate and multivariable longitudinal analyses accumulative over a 25-year period
| Characteristic | Asthma Type | Univariate Model |
Full Multivariable Model |
Best Multivariable Model* | |||
|---|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | ||
| Female sex | Adult onset | 1.43 (0.67, 3.04) | 0.35 | 1.29 (0.64, 2.63) | 0.48 | ||
| Pediatric onset | 1.69 (1.07, 2.67) | 0.03 | 1.48 (0.92, 2.40) | 0.11 | 1.62 (1.02, 2.57) | 0.04 | |
| White race | Adult onset | 0.60 (0.31, 1.17) | 0.13 | 0.59 (0.29, 1.18) | 0.13 | ||
| Pediatric onset | 0.70 (0.44, 1.12) | 0.13 | 0.86 (0.52, 1.41) | 0.55 | |||
| Non-atopic status | Adult onset | 1.55 (0.82, 2.95) | 0.18 | 1.26 (0.64. 2.48) | 0.50 | ||
| Pediatric onset | 0.70 (0.43, 1.12) | 0.14 | 0.74 (0.45, 1.22) | 0.24 | |||
| Ever-smoker | Adult onset | 2.59 (1.35, 5.00) | 0.004 | 2.50 (1.29, 4.82) | 0.006 | 2.59 (1.35, 5.00) | 0.004 |
| Pediatric onset | 1.02 (0.64, 1.61) | 0.94 | 1.05 (0.67, 1.67) | 0.83 | |||
| Ever-obese | Adult onset | 1.63 (0.83, 3.18) | 0.15 | 1.17 (0.59, 2.31) | 0.66 | ||
| Pediatric onset | 1.64 (1.04, 2.59) | 0.03 | 1.52 (0.93, 2.47) | 0.09 | 1.57 (0.99, 2.49) | 0.06 | |
Definition of abbreviation: CI = confidence interval.
There were 580 transition events from quiescent to recrudescent state among those with pediatric-onset asthma and 182 similar events among those with adult-onset asthma. Women were more likely than men to transition over time from quiescent to recrudescent state among all subjects with asthma (29.5 vs. 18.0%; P = 0.01) as well as among those with pediatric-onset (23.5 vs. 15.4%; P = 0.03) or adult-onset disease (42.4 vs. 34.0%; P = 0.35). Ever-smokers were more likely than never-smokers to transition over time from quiescent to recrudescent state only among those with adult-onset disease (50.5 vs. 28.2%; P = 0.004). The interaction between ever-smoking and age-of-onset asthma category on transition to recrudescence was significant (P = 0.01) The best multivariable model was obtained using stepwise logistic regression using Proc Genmod function in SAS and is presented in the columns on the extreme right. We prefer to use the best multivariable model over the full multivariable model because of the observed correlation between the studied variables resulting in overadjustment in the full multivariable model.
Ever-obese subjects were more likely than never-obese subjects to transition over time from quiescent to recrudescent state among all (30.4 vs. 19.2%; P = 0.008) as well as among those with pediatric-onset (24.0 vs. 16.1%; P = 0.03) or adult-onset disease (P = 0.15).
Discussion
Adult-onset disease becomes the dominant phenotype by an age of 40 years among women with asthma and by even younger ages among obese, nonatopic, ever-smoking, or white women with asthma. Furthermore, adult-onset asthma has remarkable sex-related differences in risk factors. Thus, obesity, nonatopic state, and ever-smoking each increase the proportion of adult-onset disease among women with asthma but not men. Finally, in both sexes, the quiescent state for adult-onset asthma is less frequent and also “less stable” over time than for pediatric-onset asthma. Factors associated with recrudescence of quiescent asthma state differ between adult- and pediatric-onset diseases.
Using a large U.S.-based cohort of adults in the reproductive age group with a 25-year follow-up, our study challenges the commonly held belief among clinicians that most asthma in adults originates in childhood (13–16). Although this dictum may still hold true among middle-aged men, adult-onset disease becomes the dominant phenotype among women with asthma by 40 years of age and at even lower ages among specific subsets of women. Interestingly, this issue has been inadequately examined in the literature. Pediatric longitudinal asthma cohorts are often limited by insufficient follow-up into late adulthood (17). On the other hand, adult longitudinal cohorts often exclude the study of early-onset asthma (18–25). In a clinic-based population, Barnes suggested that the severe asthma subset in adults may more frequently start in adult life than in childhood (13). Our findings expand on this observation by demonstrating that not-so-severe asthma in middle-aged women also more frequently starts in adult life; less severity is suggested by the lower number of asthma medications used by our community-based cohort of subjects with asthma (as shown in Table 3).
Our data are consistent with the literature that women are more at risk for adult-onset asthma than men (19, 26–29). Although many reasons have been hypothesized, it is believed that reproductive hormones may partly explain these sex differences (30). Our study shows that ever having smoked is a risk factor for adult-onset asthma, but only for women. The literature regarding the risk of asthma among smokers, however, remains conflicting (18, 25, 26, 31, 32). In one longitudinal study, Jamrozik and colleagues hypothesized that smoking influences the probability of being diagnosed with asthma through the development of either impaired lung function or exacerbation of respiratory symptoms (18). Unlike ever-smoking, we did not find either current smoking or environmental tobacco smoke exposure to be risk factors for adult-onset asthma in women but found them to be seemingly protective among men. Our findings may be explained by selection bias from “healthy smoker effect” that is particularly strong among men with asthma. Furthermore, our data are also consistent with other studies that demonstrate obesity to be a risk factor for incident asthma primarily among women (33–38). Few longitudinal studies have examined the relationship of atopy (resulting from Th2 pathway of immunologic reactions [39]) to adult-onset asthma. Although atopy is a strong risk factor for pediatric-onset asthma (40), its association with adult-onset asthma is controversial. Thus, although atopy was a risk factor for adult-onset asthma in the Finnish twin cohort (21), other longitudinal studies did not demonstrate a predictive association (18, 24, 41). On the other hand, our data remarkably suggest that in women, a nonatopic state increases risk for adult-onset disease, suggesting immunological differences between the adult-onset and pediatric-onset asthma phenotypes. We were surprised to find that white, rather than black, subjects with asthma were at higher risk for adult-onset disease. It appears that most black subjects with asthma, despite their higher prevalence of obesity and ever-smoking state, develop asthma during childhood. This finding may be partly explained by genetic differences or racial differences in childhood allergen exposures related to differences in socioeconomic status.
The current literature, primarily derived from cross-sectional studies, further suggests that among adults, asthma of adult onset is more “difficult to control” than asthma of pediatric onset (13, 42, 43). Our longitudinal study supports this conclusion by demonstrating that adult-onset disease is more likely to be persistent and, conversely, less likely to be quiescent than pediatric-onset disease among adults with asthma. Furthermore, once quiescent, recrudescence into active state is more likely among adult-onset than pediatric-onset disease among adults with asthma. Interestingly, women and obese subjects are more likely to recrudesce than their respective counterparts, particularly with pediatric-onset asthma (13, 44). It is unclear why smoking is associated with recrudescence only in adult-onset disease.
The strengths of our study include its sex-specific stratified analysis, the well-defined study population set within a cohort structure, and our longitudinal analyses over 25 years. The study, however, has some limitations. Self-report of provider-diagnosed asthma is the standard definition used in epidemiological studies but has misclassification bias, based on the controversial “gold standard” of airway hyperresponsiveness (45). Our results could be explained by a greater erroneous overdiagnosis among women or underdiagnosis among men. This is unlikely, because Aaron and colleagues showed that men in fact were more likely than women to be overdiagnosed (45). Furthermore, obese subjects or smokers were not more likely to be overdiagnosed than their respective counterparts (45). Although Aaron and colleagues did not evaluate the effect of atopy on asthma misclassification, it is remarkable that season of testing did not affect misclassification rates (45). In addition, self-report may miss subjects with mild asthma severity. However, this is unlikely, given that most subjects with asthma in our study had asthma of intermittent or mild persistent severity (46). Similarly, asthma self-report may include early chronic obstructive pulmonary disease, particularly among smokers. Although this is possible, it seems less likely because most subjects with asthma in our study had normal prebronchodilator FEV1/FVC ratio at mean age 45 years (Table 3). It is also possible that asthma diagnosed in adulthood may in fact originate earlier but is missed during childhood (17, 47). Although possible, it appears that the two asthma phenotypes are indeed different with respect to their natural history, relationship to key risk factors, respiratory symptom severity, need for medications, and lung function in adulthood (Table 3). Although our study has divided all subjects with asthma into two phenotypes (adult onset vs. pediatric onset), others have described a greater number of phenotypes; our study does not consider these alternative phenotypes of asthma (Table E2 [43]). Recall bias may misclassify age of onset. However, 95.2% of all subjects with asthma consistently classified their disease onset status at subsequent examination visits. Recall bias related to medication component of the asthma definitions was minimized by trained investigators verifying pill containers and inhalers. Furthermore, self-report of atopy was obtained at Y0 examination and not collected at subsequent follow-up examinations or corroborated objectively. Additionally, occupational history in relation to onset of asthma in adults was not obtained. However, the twofold greater annual incidence of asthma among women than men in this cohort is not consistent with the reported epidemiology of occupational asthma (48–50). Finally, although the CARDIA cohort may not be representative of the overall U.S. population, our findings are relevant to urban and suburban populations.
In summary, this longitudinal study challenges the commonly held belief among clinicians that most asthma in adults originates in childhood. In fact, adult-onset asthma becomes the dominant phenotype in women by an age of 40 years and at an even lower age among obese, nonatopic, ever-smoker, or white women. Clinicians should be reminded that adult-onset disease is associated with different risk factors, greater symptoms and medication use, and a quiescent state that is both less frequent and “less stable” over time than pediatric-onset asthma. Studies of the differences between pediatric- and adult-onset asthma may provide additional insight into the phenotypic heterogeneity of asthma.
Acknowledgments
Acknowledgment
The authors thank Bharat Thyagarajan, M.D., Ph.D., at University of Minnesota, Minneapolis, MN, for his careful critique of the manuscript.
Footnotes
This study is supported by CARDIA contracts N01-HC-48047–50 and N01-HC-95095, and an ancillary study, R01 HL 53560; and by 1K23HL094531–01 (A.S.) and grant 5M01 RR00997 (A.S.) from the National Institutes of Health.
Author Contributions: A.S. was responsible for hypothesis generation, study design, data analysis, data interpretation, and manuscript preparation. C.Q. was responsible for data analysis, data interpretation, and manuscript preparation. M.S. was responsible for data interpretation and manuscript preparation. A.A. was responsible for data administration and manuscript preparation. J.H.A. was responsible for data analysis. L.J.S. was responsible for data interpretation and manuscript preparation. D.R.J. was responsible for hypothesis generation, study design, data collection, data analysis, and manuscript preparation.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Thomsen SF, Ulrik CS, Kyvik KO, Larsen K, Skadhauge LR, Steffensen I, Backer V. The incidence of asthma in young adults. Chest. 2005;127:1928–1934. doi: 10.1378/chest.127.6.1928. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein DI. The natural history of adult asthma: what do we know? Ann Allergy Asthma Immunol. 2000;84:469–470. doi: 10.1016/S1081-1206(10)62502-1. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and PreventionNational health interview survey (NHIS) data: 2010 lifetime and current asthma [accessed 2012 August 21]. Available from http://www.Cdc.Gov/asthma/nhis/2010/data.htm 2010
- 4.Sullivan PW, Ghushchyan VH, Slejko JF, Belozeroff V, Globe DR, Lin SL.The burden of adult asthma in the United States: evidence from the Medical Expenditure Panel Survey J Allergy Clin Immunol 2011127363–369.e1–3 [DOI] [PubMed] [Google Scholar]
- 5.Weiss KB, Sullivan SD, Lyttle CS. Trends in the cost of illness for asthma in the United States, 1985–1994. J Allergy Clin Immunol. 2000;106:493–499. doi: 10.1067/mai.2000.109426. [DOI] [PubMed] [Google Scholar]
- 6.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, Liu X.Trends in asthma prevalence, health care use, and mortality in the united states, 2001–2010NCHS data brief, no 94Hyattsville, MD: National Center for Health Statistics; 2012 [PubMed] [Google Scholar]
- 7.Martinez FD. Links between pediatric and adult asthma. J Allergy Clin Immunol. 2001;107:S449–S455. doi: 10.1067/mai.2001.114993. [DOI] [PubMed] [Google Scholar]
- 8.Maestrelli P. Natural history of adult-onset asthma: insights from model of occupational asthma. Am J Respir Crit Care Med. 2004;169:331–332. doi: 10.1164/rccm.2312012. [DOI] [PubMed] [Google Scholar]
- 9.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 10.Hughes GH, Cutter G, Donahue R, Friedman GD, Hulley S, Hunkeler E, Jacobs DR, Jr, Liu K, Orden S, Pirie P, et al. Recruitment in the coronary artery disease risk development in young adults (CARDIA) study. Control Clin Trials. 1987;8:68S–73S. doi: 10.1016/0197-2456(87)90008-0. [DOI] [PubMed] [Google Scholar]
- 11.Markov AA. Link Dt. Classical text in translation: an example of statistical investigation of the text Eugene Onegin concerning the connection of samples in chains. Science in Context. 2006;19:591–600. [Google Scholar]
- 12.National Institutes of Health National Heart, Lung, and Blood Institute. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007. Morbidity and mortality: 2007 chart book on cardiovascular, lung, and blood diseases. [Google Scholar]
- 13.Barnes N. Most difficult asthma originates primarily in adult life. Paediatr Respir Rev. 2006;7:141–144. doi: 10.1016/j.prrv.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Sherman CB, Tosteson TD, Tager IB, Speizer FE, Weiss ST. Early childhood predictors of asthma. Am J Epidemiol. 1990;132:83–95. doi: 10.1093/oxfordjournals.aje.a115646. [DOI] [PubMed] [Google Scholar]
- 15.Martin AJ, McLennan LA, Landau LI, Phelan PD. The natural history of childhood asthma to adult life. BMJ. 1980;280:1397–1400. doi: 10.1136/bmj.280.6229.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson HR, Pottier AC, Strachan DP. Asthma from birth to age 23: incidence and relation to prior and concurrent atopic disease. Thorax. 1992;47:537–542. doi: 10.1136/thx.47.7.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stern DA, Morgan WJ, Halonen M, Wright AL, Martinez FD. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372:1058–1064. doi: 10.1016/S0140-6736(08)61447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamrozik E, Knuiman MW, James A, Divitini M, Musk AW. Risk factors for adult-onset asthma: a 14-year longitudinal study. Respirology. 2009;14:814–821. doi: 10.1111/j.1440-1843.2009.01562.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Dales R, Tang M, Krewski D. Obesity may increase the incidence of asthma in women but not in men: longitudinal observations from the Canadian National Population Health Surveys. Am J Epidemiol. 2002;155:191–197. doi: 10.1093/aje/155.3.191. [DOI] [PubMed] [Google Scholar]
- 20.Ford ES, Mannino DM, Redd SC, Mokdad AH, Mott JA. Body mass index and asthma incidence among USA adults. Eur Respir J. 2004;24:740–744. doi: 10.1183/09031936.04.00088003. [DOI] [PubMed] [Google Scholar]
- 21.Huovinen E, Kaprio J, Koskenvuo M. Factors associated to lifestyle and risk of adult onset asthma. Respir Med. 2003;97:273–280. doi: 10.1053/rmed.2003.1419. [DOI] [PubMed] [Google Scholar]
- 22.Nystad W, Meyer HE, Nafstad P, Tverdal A, Engeland A. Body mass index in relation to adult asthma among 135,000 Norwegian men and women. Am J Epidemiol. 2004;160:969–976. doi: 10.1093/aje/kwh303. [DOI] [PubMed] [Google Scholar]
- 23.Gunnbjornsdottir MI, Omenaas E, Gislason T, Norrman E, Olin AC, Jogi R, Jensen EJ, Lindberg E, Bjornsson E, Franklin K, et al. Obesity and nocturnal gastro-oesophageal reflux are related to onset of asthma and respiratory symptoms. Eur Respir J. 2004;24:116–121. doi: 10.1183/09031936.04.00042603. [DOI] [PubMed] [Google Scholar]
- 24.Basagana X, Sunyer J, Zock JP, Kogevinas M, Urrutia I, Maldonado JA, Almar E, Payo F, Anto JM. Incidence of asthma and its determinants among adults in Spain. Am J Respir Crit Care Med. 2001;164:1133–1137. doi: 10.1164/ajrccm.164.7.2012143. [DOI] [PubMed] [Google Scholar]
- 25.Ronmark E, Lundback B, Jonsson E, Jonsson AC, Lindstrom M, Sandstrom T. Incidence of asthma in adults–report from the obstructive lung disease in northern Sweden study. Allergy. 1997;52:1071–1078. doi: 10.1111/j.1398-9995.1997.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 26.King ME, Mannino DM, Holguin F. Risk factors for asthma incidence. A review of recent prospective evidence. Panminerva Med. 2004;46:97–110. [PubMed] [Google Scholar]
- 27.Guerra S, Sherrill DL, Bobadilla A, Martinez FD, Barbee RA. The relation of body mass index to asthma, chronic bronchitis, and emphysema. Chest. 2002;122:1256–1263. doi: 10.1378/chest.122.4.1256. [DOI] [PubMed] [Google Scholar]
- 28.Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol. 2005;115:897–909, quiz 910. doi: 10.1016/j.jaci.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 29.Eagan TM, Brogger JC, Eide GE, Bakke PS. The incidence of adult asthma: a review. Int J Tuberc Lung Dis. 2005;9:603–612. [PubMed] [Google Scholar]
- 30.Melgert BN, Ray A, Hylkema MN, Timens W, Postma DS. Are there reasons why adult asthma is more common in females? Curr Allergy Asthma Rep. 2007;7:143–150. doi: 10.1007/s11882-007-0012-4. [DOI] [PubMed] [Google Scholar]
- 31.Vesterinen E, Kaprio J, Koskenvuo M. Prospective study of asthma in relation to smoking habits among 14,729 adults. Thorax. 1988;43:534–539. doi: 10.1136/thx.43.7.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Troisi RJ, Speizer FE, Rosner B, Trichopoulos D, Willett WC. Cigarette smoking and incidence of chronic bronchitis and asthma in women. Chest. 1995;108:1557–1561. doi: 10.1378/chest.108.6.1557. [DOI] [PubMed] [Google Scholar]
- 33.Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159:2582–2588. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 34.Beckett WS, Jacobs DR, Jr, Yu X, Iribarren C, Williams OD. Asthma is associated with weight gain in females but not males, independent of physical activity. Am J Respir Crit Care Med. 2001;164:2045–2050. doi: 10.1164/ajrccm.164.11.2004235. [DOI] [PubMed] [Google Scholar]
- 35.Boulet LP. Influence of obesity on the prevalence and clinical features of asthma. Clin Invest Med. 2008;31:E386–E390. doi: 10.25011/cim.v31i6.4926. [DOI] [PubMed] [Google Scholar]
- 36.McLachlan CR, Poulton R, Car G, Cowan J, Filsell S, Greene JM, Taylor DR, Welch D, Williamson A, Sears MR, et al. Adiposity, asthma, and airway inflammation. J Allergy Clin Immunol. 2007;119:634–639. doi: 10.1016/j.jaci.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 37.Sood A, Qualls C, Li R, Schuyler M, Beckett WS, Smith LJ, Thyagarajan B, Lewis CE, Jacobs DR. Lean mass predicts asthma better than fat mass among females. Eur Respir J. 2011;37:65–71. doi: 10.1183/09031936.00193709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herrera-Trujillo M, Barraza-Villarreal A, Lazcano-Ponce E, Hernandez B, Sanin LH, Romieu I. Current wheezing, puberty, and obesity among Mexican adolescent females and young women. J Asthma. 2005;42:705–709. doi: 10.1080/02770900500265306. [DOI] [PubMed] [Google Scholar]
- 39.Settipane RJ, Settipane GA. IgE and the allergy-asthma connection in the 23-year follow-up of Brown University students. Allergy Asthma Proc. 2000;21:221–225. doi: 10.2500/108854100778248890. [DOI] [PubMed] [Google Scholar]
- 40.Illi S, von Mutius E, Lau S, Nickel R, Niggemann B, Sommerfeld C, Wahn U. The pattern of atopic sensitization is associated with the development of asthma in childhood. J Allergy Clin Immunol. 2001;108:709–714. doi: 10.1067/mai.2001.118786. [DOI] [PubMed] [Google Scholar]
- 41.Settipane GA, Greisner WA, III, Settipane RJ. Natural history of asthma: a 23-year followup of college students. Ann Allergy Asthma Immunol. 2000;84:499–503. doi: 10.1016/S1081-1206(10)62512-4. [DOI] [PubMed] [Google Scholar]
- 42.ten Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. Factors associated with persistent airflow limitation in severe asthma. Am J Respir Crit Care Med. 2001;164:744–748. doi: 10.1164/ajrccm.164.5.2011026. [DOI] [PubMed] [Google Scholar]
- 43.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bisgaard H, Bonnelykke K.Long-term studies of the natural history of asthma in childhood J Allergy Clin Immunol 2010126187–197.quiz 198–199 [DOI] [PubMed] [Google Scholar]
- 45.Aaron SD, Vandemheem KL, Boulet LP, Mclvor RA, Fitzgerald JM, Hernandez P, Lemiere C, Sharma S, Field SK, Alvarez GG, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121–1131. doi: 10.1503/cmaj.081332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 47.Bellanti JA, Settipane RA. The challenge of pediatric asthma: adult diseases begin in childhood. Allergy Asthma Proc. 2007;28:109–110. [PubMed] [Google Scholar]
- 48.Knoeller GE, Mazurek JM, Moorman JE. Work-related asthma–38 states and District of Columbia, 2006–2009. MMWR Morb Mortal Wkly Rep. 2012;61:375–378. [PubMed] [Google Scholar]
- 49.Paris C, Ngatchou-Wandji J, Luc A, McNamee R, Bensefa-Colas L, Larabi L, Telle-Lamberton M, Herin F, Bergeret A, Bonneterre V, et al. Work-related asthma in France: recent trends for the period 2001–2009. Occup Environ Med. 2012;69:391–397. doi: 10.1136/oemed-2011-100487. [DOI] [PubMed] [Google Scholar]
- 50.Knoeller GE, Mazurek JM, Moorman JE. Work-related asthma among adults with current asthma in 33 states and DC: evidence from the asthma call-back survey, 2006–2007. Public Health Rep. 2011;126:603–611. doi: 10.1177/003335491112600419. [DOI] [PMC free article] [PubMed] [Google Scholar]