Abstract
Rationale: Factors associated with long-term brain dysfunction (LTBD) in survivors of chronic critical illness (CCI) have not been explored but may be important for clinical practice and planning by patients, families, and providers.
Objectives: To identify risk factors for LTBD after treatment for CCI and to explore the association between acute delirium and coma during hospital treatment and LTBD.
Methods: A prospective cohort study of adults admitted to a respiratory care unit for treatment of CCI.
Measurements and Main Results: Using the Confusion Assessment Method for ICU and the Richmond Agitation and Sedation Scale, we evaluated patients for delirium and coma during their hospital treatment for CCI. We collected data on other potential risk factors for LTBD by prospectively reviewing the patients’ medical records and interviewing surrogates. We contacted survivors by telephone at 6 months after discharge to assess brain function using the telephone Confusion Assessment Method. Among 385 patients treated for CCI, 213 (56.1%) were dead at 6 months, and 108 of 167 (64.7%) of survivors were impaired. We used multinomial logistic regression in which the outcomes were (1) death, (2) brain dysfunction, and (3) survival without LTBD. Older patients, patients with higher Acute Physiology Score, and those with multiple complications during treatment for CCI were more likely to have LTBD. Acute brain dysfunction during hospital treatment was also associated with an increased risk of LTBD (odds ratio, 2.14; 95% confidence interval, 1.02–4.52).
Conclusions: LTBD after treatment for CCI is associated with brain dysfunction during such treatment as well as with older age and higher severity of illness of the patients.
Keywords: critical illness, prolonged mechanical ventilation, cognition disorders, delirium
Chronic critical illness (CCI) is now understood to be a distinct syndrome in which persistent ventilator dependence is accompanied by characteristic derangements of other systems and organs (1, 2). This syndrome has been defined for administrative, research, and clinical purposes by the placement of tracheostomy after at least 10 days of difficulty weaning from mechanical ventilation (2). In a cohort of more than 200 such patients assessed prospectively and longitudinally, we found in a previous study that one-third were comatose throughout weeks to months of hospital treatment for CCI, one-half of those without coma were delirious, and one-half of the patients who survived were comatose or delirious at hospital discharge (3).
Prior research has shown that delirium is common during acute critical illness and is associated with unfavorable outcomes, including longer duration of mechanical ventilation, longer length of stay in the ICU and hospital, and higher mortality (4, 5). In addition, although delirium during the acute phase of critical illness usually lasts for a relatively short time (median, 2 d), survivors of acute critical illness have impairments in attention, memory, and executive function at long-term follow-up (6, 7). Girard and colleagues found that among patients with acute critical illness who were mechanically ventilated for more than 12 hours but less than 2 weeks, the duration of delirium in the ICU was an independent predictor of this type of cognitive impairment at 3-month and 12-month follow-up (8). However, these study findings are difficult to generalize to patients with CCI because patients receiving mechanical ventilation for more than 2 weeks were excluded from the study (8), and long-term brain dysfunction (LTBD) after treatment for CCI appears to be far more severe than the cognitive impairment documented in survivors of acute critical illness (3).
No prior research has investigated factors influencing the risk of LTBD in the chronically critically ill. Yet, such factors are relevant for communication and planning by clinicians, patients, and families about continuation of mechanical ventilation and other life-supporting therapies when critical illness enters a chronic phase, and some factors may be modifiable, now or in the future. In this prospective study, we followed patients during treatment for CCI in the respiratory care unit (RCU) of our acute care hospital and up to 6 months after discharge, performing longitudinal assessments for brain dysfunction. Patients were transferred to the RCU and included in this analysis after care in an adult acute care ICU; all were mechanically ventilated through a tracheostomy. Our specific aim was to evaluate the significance of brain dysfunction (delirium and coma) during treatment for CCI along with other factors as predictors of LTBD. Some of the results of this study have been previously reported in the form of an abstract (35).
Methods
Study Design, Setting, and Enrollment
Between January 2003 and December 2007, we enrolled consecutive chronically critically ill patients who had undergone elective tracheotomy for ICU weaning failure (3) and were admitted from adult ICUs in our tertiary care urban hospital to the hospital’s RCU. Patients enrolled during the early part of this study period were the subject of a prior report on prevalence and duration of brain dysfunction during CCI treatment (3). The RCU is a 14-bed, inpatient unit for chronically critically ill patients from all adult ICUs (medical, surgical, cardiothoracic surgical, cardiac, and neurosurgical) in the hospital. Patients are accepted into the RCU if they no longer need acute ICU care, are mechanically ventilated via tracheotomy, and, in the opinion of the referring ICU physician, are likely to be liberated from the ventilator. Patients cared for in this unit are comparable to those treated elsewhere in long-term acute care hospitals. We excluded patients who had a previous episode of ventilator dependence or tracheotomy and those lacking English proficiency. The structure and process of care in our RCU, which are highly standardized, have been described previously (3). The Institutional Review Board of Mount Sinai School of Medicine in New York approved this study, and we obtained informed consent from all subjects or appropriate surrogates.
Brain Dysfunction during CCI Treatment
As detailed elsewhere (3), a single trained research nurse performed assessments of brain dysfunction at multiple time points: study entry, biweekly in the RCU, and RCU discharge. These assessments included administration of the Richmond Agitation and Sedation Scale (RASS), which measures levels of consciousness, and evaluation of delirium by the Confusion Assessment Method for the ICU (CAM-ICU) (9, 10). As prescribed by the CAM-ICU, delirium assessments were deferred for stuporous or comatose patients (RASS level −4 or −5), for whom a second attempt to administer this instrument was made later the same day (9).
We used the multiple cognitive assessments during the RCU treatment period to create four categories of brain dysfunction: (1) “coma,” for patients found to be in RASS level −4 or −5 for greater than or equal to 75% of the assessments; (2) “delirium,” for patients with at least one episode of delirium and less than 75% of assessments in RASS level −4 or −5; (3) “not delirious or comatose,” for patients with no episode of delirium, less than 75% of assessments finding coma, and at least one assessment in which the patient was found not delirious by the CAM-ICU; and (4) “other,” for patients who did not fit into any of the first three categories (primarily comprising patients who refused to respond, were not available, or could not be evaluated for other technical reasons, at a preponderance of the prescribed assessments). As a complementary approach to describing the severity of brain dysfunction during treatment, we also determined duration of brain dysfunction by summing the number of days spent in coma or delirium in the RCU, assuming that each condition spanned from the day of the assessment in which coma or delirium was found to the day of the next biweekly assessment (11). We calculated the percentage of total RCU days with brain dysfunction as the total number of days in delirium or coma divided by the total RCU length of stay multiplied by 100.
Other Risk Factors and Potential Confounders
We collected data about factors that we determined a priori to be potential predictors of LTBD after treatment for CCI, as suggested by review of the literature on cognitive impairment after acute critical illness, clinical experience, and biological plausibility. Baseline patient characteristics, such as age, prior history of dementia, other chronic conditions comprising the Charlson Comorbidity Index Score (12), and preadmission living situation (private home vs. other), were obtained from in-person interviews of the patient’s surrogate or from the medical records. As our measure of prehospital functional ability, we used the motor scale of the Functional Independence Measure (13) and the Activities of Daily Living (ADL) (14). The Functional Independence Measure motor score includes 13 items (e.g., bathing, eating, walking) that are scored from 1 (needs total assistance) to 7 (completely independent), with total scores ranging from 13 (completely dependent) to 91 (completely independent). We reviewed medical records to determine hospital and ICU admitting diagnoses. We calculated the Acute Physiology Score component of the Acute Physiology and Chronic Health Evaluation II score (15), using laboratory and clinical data at the time of transfer to the RCU. We reviewed medical records for documentation of 16 complications (specified a priori) occurring during the period of RCU treatment, such as shock requiring vasopressors, cardiac arrest, myocardial infarction, pneumonia, bacteremia, skin ulcers, and new renal failure requiring dialysis.
Main Outcome Variable
Six months after discharge from the RCU, our research nurse telephoned survivors to administer the validated telephone version of the Confusion Assessment Method (CAM) after a preliminary assessment of the patient’s level of consciousness and capacity to respond (16). If a family member or caregiver reported that the patient was too impaired to respond, the nurse used a formal, written protocol to clarify whether the impairment was cognitive and/or physical by asking systematically and in detail whether the patient was (1) too physically weak or otherwise physically impaired to move to, hold, or speak into a phone; or whether (2) the patient was unable to respond to the telephone interview because of an alteration of consciousness (other than normal sleep or drowsiness), confusion, dementia, or other cognitive impairment. Patients meeting the second set of criteria were classified as having brain dysfunction, together with those who were able to respond to the telephone CAM interview and found to be delirious by that measure. Based on these assessments (and vital status) at the 6-month follow-up, we created as our main outcome a dependent variable with three possible values: dead, alive with brain dysfunction, and alive without brain dysfunction.
Statistical Analysis
We used descriptive statistics (means, medians, and percentages) to characterize the study cohort and summarize their outcomes. To compare outcomes of patients according to our brain dysfunction categories during the period of RCU treatment, we used Chi-square, Student t tests, or, where appropriate, their nonparametric equivalent. We used an indicator variable (earlier vs. later enrollment, defining “earlier” to include the first 203 patients we enrolled, as previously described [3]) to explore potential differences in case mix or clinical practice over the duration of the study. For exploration of predictors of 6-month outcomes, we used a multinomial logistic regression to describe the relationship between the potential predictor variables and the nominal outcome variable; survival without brain dysfunction was the reference category (17). On bivariable testing, any predictor variable with a P value of less than 0.25 for the two degrees-of-freedom test of the association of the outcome with that predictor was a candidate for the multivariable model, along with all other variables of probable clinical importance (17). We examined the relationship between independent variables using descriptive statistics, supplemented by the STATA command, collin, to assess for potential collinearity. Variables were retained in the final model based on the two degrees-of-freedom likelihood ratio test or their associated P value (< 0.05) for the nominal outcome variable or if they changed the effect estimate for another potential predictor variable by more than 20%. Sensitivity analyses explored whether the independent predictors would be changed by excluding patients with cognitive impairment at baseline or neurologic primary ICU diagnosis. For patients enrolled earlier in the study (3), we also explored whether exposure to opioids or benzodiazepines in the RCU was a significant confounder.
Results
Among 633 patients who had undergone tracheotomy and were consecutively admitted to the RCU, 460 were eligible and 385 (83.7%) were enrolled in the study (Figure 1). Baseline characteristics of these patients are presented in Table 1. Most patients were older adults (60% were ≥ 65 yr old) who had been living at home without cognitive impairment before this hospitalization. The majority (63.9%) were admitted to the RCU from the medical ICU. Although stays in the RCU and in the hospital were long, averaging 30 and 64 days, respectively, one-half (200 of 385) of our study patients were never liberated from mechanical ventilation (Table 2). Most patients (263 of 379, 69.4%) developed at least one significant complication during their treatment for CCI (Table 2). The majority (210 of 264, 79.5%) of the hospital survivors required custodial care after discharge (Table 2). Complete follow-up data were obtained for 380 of 385 (98.7%) of patients at 6 months after discharge from the RCU. At this time point 213 (56.1%) were dead and 108 (28.4%) were living with LTBD.
Figure 1.
Flow diagram for subject enrollment and follow-up. Six-month follow-up assessments were performed by a single research nurse who telephoned survivors or their caregivers/surrogates. CAM = Confusion Assessment Method.
Table 1.
Characteristics of the study participants (N = 385)
| Characteristics | Value |
|---|---|
| Age, median (IQR), yr | 71 (58–80) |
| Male | 212 (55) |
| Race/ethnicity | |
| White, not Hispanic | 195 (50.7) |
| Black, not Hispanic | 98 (25.5) |
| Hispanic | 80 (20.8) |
| Cognitively impaired at hospital admission* | 63 (16.4) |
| Residence before hospital admission† | |
| Private home | 274 (71.4) |
| Nursing home or rehabilitation facility | 61 (15.9) |
| Other | 49 (12.8) |
| FIM Motor Score at hospital admission, median (IQR)‡ | 85 (25–91) |
| Independent in Activities of Daily Living at hospital admission‡ | 197 (51.7) |
| Primary diagnosis at ICU admission§ | |
| Cardiovascular | 94 (24.5) |
| Pulmonary | 128 (33.3) |
| Neurologic | 65 (16.9) |
| Surgical | 51 (13.3) |
| Other | 46 (12.0) |
| ICU transferring to RCU | |
| Medical | 246 (63.9) |
| Surgical | 44 (11.4) |
| Cardiothoracic | 6 (1.6) |
| Neurosurgical | 65 (16.9) |
| Cardiac care | 24 (6.2) |
| ICU length of stay, median (IQR), d | 15 (11–21) |
| APACHE II Score (at study entry), mean (SD) | 19.8 (5.3) |
| Acute Physiology Score (at study entry), mean (SD) | 10.5 (4.9) |
| Charlson Comorbidity Index score, median (IQR) | 3 (1–5) |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II; FIM = Functional Independence Measure; IQR = interquartile range; RCU = respiratory care unit.
Data are given as number (percentage) except where noted.
History of dementia, decreased level of consciousness, confusion, memory loss, or other cognitive impairment, as reported by family or evidenced in medical records.
N = 384.
N = 381.
N = 382.
Table 2.
Outcomes of chronically critically ill study patients
| Outcome | Not Delirious or Comatose* (N = 104) | Delirious or Comatose* (N = 281) | All (N = 385) | P Value† |
|---|---|---|---|---|
| Hospital length of stay, median (IQR), d | 57 (40–82) | 53 (42–73) | 54 (41–76) | 0.440‡ |
| Multiple RCU complications | 25.5 | 41.2 | 36.9 | 0.005 |
| Liberated from mechanical ventilation at RCU discharge | 62.5 | 42.7 | 48.1 | 0.001 |
| Hospital discharge destination | <0.001§ | |||
| Home | 28.2 | 8.9 | 14.1 | |
| Rehabilitation facility | 19.4 | 5.4 | 9.1 | |
| Nursing home or acute care hospital | 34.0 | 49.3 | 45.2 | |
| Died in hospital | 18.5 | 36.1 | 31.3 | |
| Cumulative mortality | ||||
| RCU mortality | 12.5 | 22.8 | 20.0 | 0.025 |
| Hospital mortality | 18.3 | 35.9 | 31.2 | 0.001 |
| 3-mo Mortality | 28.2 | 58.8 | 50.5 | <0.001 |
| 6- mo Postdischarge outcome|| | <0.001 | |||
| Dead | 37.9 | 62.8 | 56.1 | |
| Alive with LTBD | 30.1 | 27.8 | 28.4 | |
| Alive without LTBD | 32.0 | 9.4 | 15.5 | |
Definition of abbreviations: CAM-ICU = Confusion Assessment Method for the Intensive Care Unit; IQR = interquartile range; LTBD = long-term brain dysfunction; RCU = respiratory care unit.
Data presented as percentage except where noted.
Categories result from multiple assessments of brain dysfunction during RCU treatment: “Comatose” = patient assessed by the Richmond Agitation-Sedation Scale (RASS) as level −4 or −5 for ≥75% of the assessments; “Delirious” = patient with at least one episode of delirium by the CAM-ICU and <75% of assessments finding coma; “Not Delirious or Comatose” = patient had no episode of delirium, <75% of assessments finding coma, and at least one assessment of “not delirious” by CAM-ICU; “Other” = Not meeting criteria for any other category (N = 14), grouped here within the “comatose” category.
Chi-square test used except where noted.
Wilcoxon rank-sum test.
Fisher exact test, N = 383.
N = 375.
A median of six (interquartile range four to nine) cognitive assessments were performed during the RCU treatment period. Overall, 22.1% (85 of 385) of patients were comatose, 47.5% (183 of 385) were delirious, and 27.0% (104 of 385) were not delirious or comatose, according to our study classifications. At the first assessment (study entry, on transfer from the ICU to the RCU), two-thirds of the patients had brain dysfunction: 126 (32.7%) were comatose, and 130 (33.8%) were delirious. Of those who were initially comatose, 70 of 126 (55.6%) remained comatose at all subsequent assessments in the RCU, and 20 of 112 (17.9%) of those without initial brain dysfunction went on to experience at least one episode of delirium during the RCU stay. Patients with brain dysfunction spent a median of 15 days (interquartile range, 6–25) in delirium or coma during the period of treatment for CCI, as measured by biweekly assessments.
Tables 3 and 4 show the factors associated with LTBD or death at 6-month follow-up (as compared with being alive without LTBD). Table 3 gives results based on the final predictor models including age, sex, race, hospital length of stay, Acute Physiology Score, Charlson Comorbidity Index Score, living arrangement before hospital admission, and multiple complications during the RCU stay. An additional model replaced living arrangement with dependence in Katz’s ADL scale. The two variables were not included in the same model because of collinearity: 80 of 108 (74.1%) of patients who were not living at home were also dependent in ADLs, whereas 104 of 273 (38.1%) of the patients who were living at home were dependent in ADLs. Factors associated with increased odds of LTBD included: increasing age (adjusted odds ratio [OR], 1.06; P < 0.001), functional impairment before the hospitalization as measured by dependence in ADLs (adjusted OR, 2.29; P = 0.070), higher Acute Physiology Score on admission to the RCU (adjusted OR, 1.33; P < 0.001), and the development of more than one complication during the RCU treatment period (adjusted OR, 3.18; P = 0.021) (Table 3). Odds of death were increased by: increasing age (adjusted OR, 1.08; P < 0.001), functional impairment before the hospitalization as measured by the patient not living at home (adjusted OR, 3.98; P = 0.01) or by dependence in ADLs (adjusted OR, 2.30; P = 0.056), higher Acute Physiology Score on admission to the RCU (adjusted OR, 1.35, P < 0.001), and the development of more than one complication during RCU treatment (adjusted OR, 4.22; P = 0.003).
Table 3.
Demographic and clinical factors associated with outcome at 6-month follow-up
| Factors | Dead* |
Alive with LTBD† |
||||
|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted OR (95% CI)‡ | P Value in Final Model§ | Unadjusted OR (95% CI) | Adjusted OR (95% CI) ‡ | P Value in Final Model§ | |
| Age (1 yr) | 1.07 (1.04–1.09) | 1.08 (1.05–1.10) | <0.001 | 1.06 (1.03–1.08) | 1.06 (1.04–1.09) | <0.001 |
| Prior cognitive impairment|| | 4.05 (1.20–13.63) | 0.66 (0.16–2.83) | — | 4.51 (1.28–15.81) | 1.35 (0.31–5.85) | — |
| Lived at home before hospital admission | 4.24 (1.83–9.79) | 3.98 (1.39–11.39) | 0.010 | 2.12 (0.85–5.27) | 2.37 (0.78–7.17) | 0.127 |
| Transferred to RCU from NSICU¶ | 1.22 (0.51–2.93) | 1.35 (0.38–4.73) | — | 2.48 (1.01–6.10) | 2.19 (0.64–7.54) | — |
| Charlson Comorbidity Index Score | 1.14 (1.02–1.27) | 1.07 (0.93–1.22) | 0.352 | 0.86 (0.75–0.99) | 0.81 (0.71–0.95) | 0.008 |
| Dependent in ADLs before admission** | 5.28 (2.65–10.54) | 2.30 (0.98–5.41)** | 0.056** | 3.50 (1.68–7.33) | 2.29 (0.94–5.62) | 0.070** |
| Acute Physiology Score of APACHE II | 1.32 (1.22–1.43) | 1.35 (1.23–1.49) | <0.001 | 1.28 (1.18–1.40) | 1.33 (1.20–1.47) | <0.001 |
| > 1 Complication during RCU stay†† | 3.06 (1.50–6.23) | 4.22 (1.63–10.90) | 0.003 | 2.42 (1.12–5.22) | 3.18 (1.19–8.51) | 0.021 |
Definition of abbreviations: ADL = Activities of Daily Living; APACHE II = Acute Physiology and Chronic Health Evaluation II; CI = confidence interval; LTBD = long-term brain dysfunction; NSICU = Neurosurgical Intensive Care Unit; OR = odds ratio; RCU = respiratory care unit.
Odds of being dead at 6 months compared with being alive without LTBD.
Odds of being alive with LTBD compared with being alive without LTBD.
Except where indicated, adjusted for age, sex, race, hospital length of stay, Acute Physiology Score of APACHE II, Charlson Comorbidity Index score, living arrangement before hospital admission, multiple complications during RCU stay.
Final predictor model for 6-month outcomes included age, Acute Physiology Score, Charlson Comorbidity Index score, hospital length of stay, preadmission living arrangement, and more than one complication during RCU stay. We included sex and race despite a nonsignificant P value because of their clinical significance. Similar likelihood ratios can be obtained by substituting preadmission living arrangement with preadmission dependence in ADLs.
History of dementia, decreased level of consciousness, confusion, memory loss, or other cognitive impairment, as reported by family or evidenced in medical records.
Patients from NSICU versus those transferred from all other ICUs.
Because of collinearity, not adjusted for living arrangement before hospital admission.
Binary variable describing patients with one or zero complications (the median number in the population) versus those with more than one complication.
Table 4.
Association between brain dysfunction during treatment for chronic critical illness and 6-month outcomes
| Brain Dysfunction During RCU Treatment | Dead* |
Alive with LTBD† |
||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted OR (95% CI)‡ | Unadjusted OR (95% CI) | Adjusted OR (95% CI)‡ | |
| Delirium or coma§ | 5.66 (3.04–10.53) | 2.83 (1.39–5.76) | 3.15 (1.63–6.11) | 2.14 (1.02–4.52) |
| Coma§ | 23.43 (3.17–172.96) | 20.59 (2.67–158.75) | 15.88 (2.09–120.91) | 12.66 (1.61–99.49) |
| Percent of RCU days in delirium or coma|| | 1.03 (1.02–1.04) | 1.02 (1.01–1.04) | 1.03 (1.02–1.04) | 1.02 (1.01–1.03) |
Definition of abbreviations: CAM-ICU = Confusion Assessment Method for the Intensive Care Unit; CI = confidence interval; LTBD = long-term brain dysfunction; OR = odds ratio; RCU = respiratory care unit.
Odds of being dead at 6 months compared with being alive without LTBD.
Odds of being alive with LTBD compared with being alive without LTBD.
Adjusted for age, sex, race, Charlson Comorbidity Index score, hospital length of stay, preadmission living arrangement, multiple complications during RCU stay. Models that include brain dysfunction could not also be adjusted for Acute Physiology Score because Acute Physiology Score incorporates the Glasgow Coma Score.
“Coma” = assessed by the Richmond Agitation-Sedation Scale as level −4 or −5 for ≥75% of the assessments; “delirium” = at least one episode of delirium by the CAM-ICU and <75% of assessments finding coma; “not delirious or comatose” = no episode of delirium, <75% of assessments found coma, and at least one assessment of “not delirious” by CAM-ICU; “Other” = not meeting criteria for any other category. Patients in the Other category (N = 14) are grouped under “coma.”
Days spent in either delirium or coma divided by total RCU length of stay × 100.
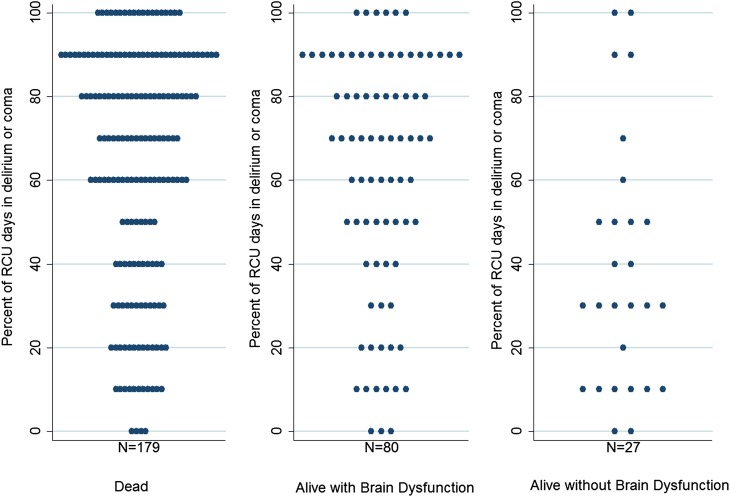
Table 4 shows the results of the models defining the relationship between the three distinct brain dysfunction variables and 6-month outcomes; all of these models were adjusted for age, sex, race, Charlson Comorbidity Index Score, living arrangement before hospital admission, and multiple complications during the RCU stay. We could not adjust these models for Acute Physiology Score because this score incorporates the Glasgow Coma Score, a measure of brain dysfunction. Brain dysfunction during treatment for CCI was associated with increased odds of brain dysfunction at 6-month follow-up, as shown in Table 4 and Figure 2. At 6 months, among the 85 patients who had been comatose in the RCU (i.e., patients in RASS level −4 or −5 for ≥75% of the assessments), 61 (71.8%) were dead and 23 (27.1%) were alive with LTBD; only one (1.2%) was alive without brain dysfunction. Figure 2 shows the percentage of total RCU days spent in delirium or coma in relation to the outcome at 6-month follow-up for all patients who had brain dysfunction in the RCU.
Figure 2.
Duration of brain dysfunction and 6-month outcomes. Turnip plots showing the percentage (rounded to the nearest 10%) of total respiratory care unit (RCU) days spent in delirium or coma according to 6-month outcome category for all patients who had brain dysfunction in the RCU (excluding the patients in each outcome category who did not have delirium or coma during the RCU treatment period). As described in the text, cognitive and vital status assessments were used to classify participants into three groups: dead, alive with long-term brain dysfunction, or alive without long-term brain dysfunction.
For the 203 patients for whom we had data on medication exposure, we found that the effect estimates for our three distinct brain dysfunction variables did not change significantly after adjusting for ever exposure to benzodiazepines or opioids, nor did it change significantly after adjusting for the cumulative doses of benzodiazepines or opioids during RCU treatment (see Table E1 on the online supplement). When we excluded the 63 patients with preexisting cognitive impairment from our study population, we found that the same factors were significantly associated with surviving with brain dysfunction and that the effect estimates were similar (Table E2).
Discussion
In this prospective study of 385 chronically critically ill patients, all of whom had undergone an elective tracheostomy for failing to wean from mechanical ventilation in an adult acute care ICU, we focused on factors associated with LTBD after hospital treatment for CCI. As we have previously shown in a smaller sample and confirmed in the present study of a much larger cohort, 84.5% of chronically critically ill patients will either die within 6 months of hospital discharge or live on with brain dysfunction (3). Here, we identified specific factors associated with LTBD. Older patients, those with higher illness severity at the onset of CCI, and those experiencing multiple complications during CCI treatment, were at significantly higher risk for LTBD. Brain dysfunction during the period of hospital treatment for CCI was independently associated with an increased risk for LTBD and death at 6 months. Although prior work has addressed associations between ICU delirium and milder forms of cognitive impairment in survivors of acute critical illness, our study contributes new information about predictors of severe brain dysfunction after treatment for CCI. These data are relevant for clinical care of the chronically critically ill and for planning by patients, families, and providers. They also highlight the importance of LTBD in future research addressing strategies for preventing and treating brain dysfunction in CCI.
As mortality rates from acute critical illness and injury decrease, there is increasing evidence of health-related sequelae for ICU survivors, including a variety of acquired psychological and cognitive disturbances (6, 18). Jackson and colleagues administered a battery of cognitive tests after discharge to survivors of acute critical illness (who had been mechanically ventilated for >12 hours but no more than 2 weeks) and found that cognitive impairment (defined by scoring ≥1.5 SDs below the mean on two or more of the nine cognitive tests) affected 79 and 71% of those who could undergo testing at 3- and 12-month follow-up, respectively (19). Among these patients, increasing duration of delirium during acute critical illness in the ICU was an independent predictor of worse cognitive performance at follow-up, after adjustment for age, education, preexisting cognitive function, severe sepsis, and exposure to sedatives in the ICU (8). Similar cognitive testing of 108 1-year survivors of acute critical illness after trauma (without intracranial hemorrhage) showed impairments in about one-half of the subjects, although nearly one-half of the study cohort had returned to either full-time or part-time work at the time of assessment (20). These studies and others indicate that patients returning to the community after treatment for acute critical illness often experience subclinical cognitive impairment and that the duration of delirium in the ICU is among factors influencing the risk of this long-term outcome. Our new findings with respect to CCI suggest that brain dysfunction (delirium and coma) in the hospital, which is typically of much longer duration than for acute critical illness (3), is associated with clinically apparent brain dysfunction at long-term follow-up.
The results of our study do not establish a causal relationship between brain dysfunction during CCI treatment and LTBD. However, unlike the other predictive factors we identified, brain dysfunction during treatment for CCI may be potentially modifiable (in the future, if not now) and thus merits particular attention. A growing number of studies involving patients with acute critical illness address risks of delirium and coma with different pharmacologic strategies for sedation during mechanical ventilation (21–24) and the use of various antipsychotic medications, such as haloperidol and atypical drugs, including ziprasidone (25–27). Recent clinical guidelines from the Society of Critical Care Medicine highlight the need for further data to support recommendations on pharmacologic and nonpharmacologic strategies to prevent and treat delirium in acute critical illness (28). Although some such data may emerge from ongoing trials, no research has yet been conducted on interventions to prevent or treat delirium or other manifestations of brain dysfunction in the chronically critically ill. Given the distinctive features of CCI, approaches for prevention and treatment of brain dysfunction in this specific context will need to include long-term follow-up to examine effects on brain dysfunction in the months after hospital discharge.
Several recent studies have documented important deficiencies in communication between clinicians and surrogates for patients with CCI, which contribute to discordant expectations for recovery of patients and interfere with informed decision making (29, 30). When patients became chronically critically ill, virtually all surrogates in one study affirmed the value of information about the patient’s expected cognitive function after the hospitalization, whereas two-thirds of these surrogates reported that they had not received information on this topic (29, 30). Data suggest that for many patients with CCI, survival with severe brain dysfunction may be less acceptable even than death (31). Thus, our study’s findings indicate that brain dysfunction during treatment for CCI should be included in a proactive and sensitive discussion with the patient’s surrogate about prognosis and care goals, acknowledging the limitations of group data and the importance of individual characteristics and preferences in determining these goals. Direct clinician counseling can be supplemented with a validated informational brochure for families about CCI, which was developed for this purpose by the Society of Critical Care Medicine’s Patient and Family Support Committee (32). An ongoing clinical trial supported by the National Institutes of Health is evaluating the effectiveness of proactive family meetings by a Supportive Information Team consisting of a palliative care physician and nurse, as compared with usual care for patients with CCI.
Our study has limitations. We did not test for long-term deficits in executive function and memory that have been the focus of research on cognitive impairment after acute critical illness. We used a telephone version of the CAM (16), which has been validated in a cohort of older patients with significant cognitive and functional impairment, but has not been used by others in a cohort with CCI; although we followed a systematic and scripted approach that we have previously used to evaluate patients who could not respond to the telephone CAM (3), this approach has not been formally validated. Future studies will be strengthened by face-to-face cognitive assessments at follow-up. In addition, we could not formally test the validity of the caregivers’ assessments of the patients’ baseline cognitive status. However, less than 20% of patients in this study had a history of cognitive impairment before hospital admission, suggesting that the high prevalence of LTBD in our hospital survivors is clinically meaningful. Because we did not measure level of education in our study cohort, our models could not explore cognitive reserve, measured in terms of education, as a potential predictor or effect modifier. It is possible that the mix of patients admitted to our RCU, and clinical practices for their care, may have evolved over the period of the study, although, within the scope of our data collection, we sought to address this possibility in our analyses. Although our protocol provided for frequent assessments, we did not assess for brain dysfunction daily. Thus, our measurements of duration of brain dysfunction are not exact. We explored a number of risk factors that cannot be modified. Rigorous prospective studies are needed to determine the modifiability of brain dysfunction for the chronically critically ill. Finally, this study was performed in one tertiary care center in which chronically critically ill patients are cared for in a hospital-based RCU. Our findings may not be generalizable to all patients with CCI, including those cared for in other settings, such as long-term acute care hospitals. However, characteristics and outcomes of chronically critically ill patients across care venues appear to be very similar (2, 33, 34), and our eligibility criteria were designed to encompass a broad group of patients requiring prolonged mechanical ventilation after failure to wean in an ICU.
Conclusions
The chronically critically ill are a large and growing population of patients at high risk for LTBD. As studies go forward seeking a better understanding of, and interventions to address, cognitive impairment after acute critical illness, research should also focus specifically on the distinct syndrome of CCI. Given the relationship we have identified between brain dysfunction during hospital treatment of CCI and months thereafter, it is important to consider both short-term and long-term effects of strategies to prevent and alleviate such dysfunction in the hospital. Timely and effective communication about expected outcomes and about appropriate goals of care in relation to an individual patient’s characteristics, values, and preferences will continue to be an essential component of clinical care.
Acknowledgments
Acknowledgment
The authors thank Alice F. Mercado, R.N., M.B.A., for her meticulous collection and verification of data and valuable assistance in all other aspects of study implementation, and Stefanie P. Weiss, M.A., for her expert assistance with preparation of this manuscript.
Footnotes
Supported by the National Institute on Aging (NIA) grant R01AG21172. J.E.N. is the recipient of a K07 Academic Career Leadership Award from the NIA (AG034234). R.S.M. is the recipient of a Mid-Career Investigator Award in Patient-Oriented Research AGK24AG022345 from the NIA.
Author Contributions: A.A.H. contributed to the study design, data analysis and interpretation, writing and review of the manuscript. R.S.M. contributed to the study design, data analysis and interpretation, writing and review of the manuscript. Q.D. contributed to the analysis and interpretation of the data, writing and review of the manuscript. S.W. contributed to the analysis and interpretation of the data, writing and review of the manuscript. J.E.N. acquired funding, supervised the data collection, and contributed to study design, data analysis and interpretation, writing and review of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Girard K, Raffin TA. The chronically critically ill: to save or let die? Respir Care. 1985;30:339–347. [PubMed] [Google Scholar]
- 2.Nelson JE, Cox CE, Hope AA, Carson SS. Chronic critical illness. Am J Respir Crit Care Med. 2010;182:446–454. doi: 10.1164/rccm.201002-0210CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson JE, Tandon N, Mercado AF, Camhi SL, Ely EW, Morrison RS. Brain dysfunction: another burden for the chronically critically ill. Arch Intern Med. 2006;166:1993–1999. doi: 10.1001/archinte.166.18.1993. [DOI] [PubMed] [Google Scholar]
- 4.Thomason JW, Shintani A, Peterson JF, Pun BT, Jackson JC, Ely EW. Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non-ventilated patients. Crit Care. 2005;9:R375–R381. doi: 10.1186/cc3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, Inouye SK, Bernard GR, Dittus RS. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins RO, Jackson JC, Hopkins RO, Jackson JC. Long-term neurocognitive function after critical illness. Chest. 2006;130:869–878. doi: 10.1378/chest.130.3.869. [DOI] [PubMed] [Google Scholar]
- 7.Wolters AE, Slooter AJ, van der Kooi AW, van Dijk D. Cognitive impairment after intensive care unit admission: a systematic review. Intensive Care Med. 2013;39:376–386. doi: 10.1007/s00134-012-2784-9. [DOI] [PubMed] [Google Scholar]
- 8.Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, Gordon SM, Canonico AE, Dittus RS, Bernard GR, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 10.Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, Francis J, Speroff T, Gautam S, Margolin R, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 11.Pisani MA, Kong SYJ, Kasl SV, Murphy TE, Araujo KLB, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180:1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Stineman MG, Shea JA, Jette A, Tassoni CJ, Ottenbacher KJ, Fiedler R, Granger CV. The Functional Independence Measure: tests of scaling assumptions, structure, and reliability across 20 diverse impairment categories. Arch Phys Med Rehabil. 1996;77:1101–1108. doi: 10.1016/s0003-9993(96)90130-6. [DOI] [PubMed] [Google Scholar]
- 14.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10:20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 15.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 16.Marcantonio ER, Michaels M, Resnick NM. Diagnosing delirium by telephone. J Gen Intern Med. 1998;13:621–623. doi: 10.1046/j.1525-1497.1998.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd edition. New York, NY: John Wiley & Sons; 2000. [Google Scholar]
- 18.Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, Zawistowski C, Bemis-Dougherty A, Berney SC, Bienvenu OJ, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 19.Jackson JC, Girard TD, Gordon SM, Thompson JL, Shintani AK, Thomason JW, Pun BT, Canonico AE, Dunn JG, Bernard GR, et al. Long-term cognitive and psychological outcomes in the awakening and breathing controlled trial. Am J Respir Crit Care Med. 2010;182:183–191. doi: 10.1164/rccm.200903-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson JC, Archer KR, Bauer R, Abraham CM, Song Y, Greevey R, Guillamondegui O, Ely EW, Obremskey W. A prospective investigation of long-term cognitive impairment and psychological distress in moderately versus severely injured trauma intensive care unit survivors without intracranial hemorrhage. J Trauma. 2011;71:860–866. doi: 10.1097/TA.0b013e3182151961. [DOI] [PubMed] [Google Scholar]
- 21.Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, Bernard GR, Ely EW. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Pisani MA, Murphy TE, Araujo KLB, Slattum P, Van Ness PH, Inouye SK. Benzodiazepine and opioid use and the duration of intensive care unit delirium in an older population. Crit Care Med. 2009;37:177–183. doi: 10.1097/CCM.0b013e318192fcf9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW, et al. SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 24.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 25.Girard TD, Pandharipande PP, Carson SS, Schmidt GA, Wright PE, Canonico AE, Pun BT, Thompson JL, Shintani AK, Meltzer HY, et al. MIND Trial Investigators. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Crit Care Med. 2010;38:428–437. doi: 10.1097/ccm.0b013e3181c58715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devlin JW, Roberts RJ, Fong JJ, Skrobik Y, Riker RR, Hill NS, Robbins T, Garpestad E. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38:419–427. doi: 10.1097/CCM.0b013e3181b9e302. [DOI] [PubMed] [Google Scholar]
- 27.Skrobik YK, Bergeron N, Dumont M, Gottfried SB. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30:444–449. doi: 10.1007/s00134-003-2117-0. [DOI] [PubMed] [Google Scholar]
- 28.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, et al. American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 29.Nelson JE, Mercado AF, Camhi SL, Tandon N, Wallenstein S, August GI, Morrison RS. Communication about chronic critical illness. Arch Intern Med. 2007;167:2509–2515. doi: 10.1001/archinte.167.22.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox CE, Martinu T, Sathy SJ, Clay AS, Chia J, Gray AL, Olsen MK, Govert JA, Carson SS, Tulsky JA.Expectations and outcomes of prolonged mechanical ventilation Crit Care Med 2009372888–2894.quiz 2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346:1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 32.Carson SS, Vu M, Danis M, Camhi SL, Scheunemann LP, Cox CE, Hanson LC, Nelson JE. Development and validation of a printed information brochure for families of chronically critically ill patients. Crit Care Med. 2012;40:73–78. doi: 10.1097/CCM.0b013e31822d7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carson SS. Outcomes of prolonged mechanical ventilation. Curr Opin Crit Care. 2006;12:405–411. doi: 10.1097/01.ccx.0000244118.08753.dc. [DOI] [PubMed] [Google Scholar]
- 34.Unroe M, Kahn JM, Carson SS, Govert JA, Martinu T, Sathy SJ, Clay AS, Chia J, Gray A, Tulsky JA, et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann Intern Med. 2010;153:167–175. doi: 10.1059/0003-4819-153-3-201008030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hope AA, Morrison RS, Du Q, Nelson J. Predictors of long-term brain dysfunction after chronic critical illness [abstract] Am J Respir Crit Care Med. 2010;181:A6713. [Google Scholar]