Abstract
A conference, “Stem Cells and Cell Therapies in Lung Biology and Lung Diseases,” was held July 25 to 28, 2011 at the University of Vermont to review the current understanding of the role of stem and progenitor cells in lung repair after injury and to review the current status of cell therapy and ex vivo bioengineering approaches for lung diseases. These are rapidly expanding areas of study that provide further insight into and challenge traditional views of mechanisms of lung repair after injury and pathogenesis of several lung diseases. The goals of the conference were to summarize the current state of the field, to discuss and debate current controversies, and to identify future research directions and opportunities for basic and translational research in cell-based therapies for lung diseases. The goal of this article, which accompanies the formal conference report, is to provide a comprehensive review of the published literature in lung regenerative medicine from the last conference report through December 2012.
As a companion article to the conference report, “Stem Cells and Cell Therapies in Lung Biology and Diseases,” held at the University of Vermont in July 2011 (1), a comprehensive summary of relevant published literature in the rapidly growing fields of stem cells, cell therapies, and bioengineering in lung biology and diseases is presented. This review discusses developments in each of these areas with specific focus on advances and the published literature between 2010 and 2012. A comprehensive review of the previous literature in the field is summarized in reports from the previous workshops (2–4). Readers are also referred to a number of recent reviews published over the past approximately 2 years and to the published positions of the American Thoracic Society on embryonic stem cell research and stem cell medical tourism (5–25). Additional recent reviews published over the past approximately 2 years in each of the specific topics discussed below are included in each section. In accordance with recent guidelines from the American Thoracic Society and other Respiratory Disease organizations, the terms “Clara cell” and “Clara cell secretory protein” have been replaced by the terms “Club cell” and “Club cell secretory protein,” (CCSP) respectively (26, 27). Suggested nomenclature and overall conference recommendations are shown in Tables 1 and 2, respectively.
Table 1.
Glossary and definition of terminology
| Potency: Sum of developmental options available to cell. |
| Totipotent: Ability of a single cell to divide and produce all the differentiated cells in an organism, including extraembryonic tissues, and thus to (re)generate an organism in total. In mammals only the zygote and the first cleavage blastomeres are totipotent. |
| Pluripotent: Ability of a single cell to produce differentiated cell types representing all three embryonic germ layers and thus to form all lineages of a mature organism. Example: embryonic stem cells. |
| Multipotent: Ability of adult stem cells to form multiple cell types of one lineage. Example: hematopoietic stem cells. |
| Unipotent: Cells form one cell type. Example: spermatogonial stem cells (can only generate sperm) |
| Reprogramming: Change in epigenetics that can lead to an increase in potency, dedifferentiation. Can be induced by nuclear transfer, cell fusion, genetic manipulation. |
| Transdifferentiation: The capacity of a differentiated somatic cell to acquire the phenotype of a differentiated cell of the same or different lineage. An example is epithelial–mesenchymal transition, a process whereby fully differentiated epithelial cells undergo transition to a mesenchymal phenotype giving rise to fibroblasts and myofibroblasts. |
| Plasticity: Hypothesis that somatic stem cells have broadened potency and can generate cells of other lineages, a concept that is controversial in mammals. |
| Embryonic Stem Cell (ESC): Cell lines developed from the inner cell mass of early developing blastocysts. ESCs have the capacity for self-renewal and are pluripotent, having the ability to differentiate into cells of all embryologic lineages and all adult cell types. However, ESCs cannot form extraembryonic tissue such as trophectoderm. |
| Adult Stem Cell: Cells isolated from adult tissues including bone marrow, adipose tissue, nervous tissue, skin, umbilical cord blood, and placenta that have the capacity for self-renewal. In general, adult stem cells are multipotent, having the capacity to differentiate into mature cell types of the parent tissue. Some populations of adult stem cells, such as MSCs, exhibit a range of lineage differentiation that is not limited to a single tissue type. Whether adult stem cells exhibit plasticity and can differentiate into a wider variety of differentiated cells and tissues remains controversial. |
| Adult Tissue–Specific Stem Cell: Same as adult stem cells but with defined tissue specificity. A relatively undifferentiated cell within a given tissue that has the capacity for self-renewal through stable maintenance within a stem cell niche. Adult tissue-specific (endogenous) stem cells have a differentiation potential equivalent to the cellular diversity of the tissue in which they reside. The hematopoietic stem cell is a prototypical adult tissue stem cell. |
| Induced Pluripotent Stem Cell (iPSC): Reprogrammed adult somatic cells that have undergone dedifferentiation after the expression of reprogrammingtranscription factors such as Oct 3/4, Sox2, c-Myc, and Klf4. iPSCs are similar to ESCs in morphology, proliferation, gene expression, and in the ability to form teratomas. In vivo implantation of iPSCs results in formation of tissues from all three embryonic germ layers. iPSCs have been generated from both mouse and human cells. |
| Progenitor Cell: A collective term used to describe any proliferative cell that has the capacity to differentiate into different cell lineages within a given tissue. Unlike stem cells, progenitor cells have limited or no self-renewal capacity. The term “progenitor cell” is commonly used to indicate a cell can expand rapidly but undergoes senescence after multiple cell doublings. Terminology that takes into account the functional distinctions among progenitor cells is suggested below. |
| Transit-Amplifying Cell: The progeny of a endogenous tissue stem cell that retain relatively undifferentiated character, although more differentiated than the parent stem cell, and have a finite capacity for proliferation. The sole function of transit-amplifying cells is generation of a sufficient number of specialized progeny for tissue maintenance. |
| Obligate Progenitor Cell: A cell that loses its ability to proliferate once it commits to a differentiation pathway. Intestinal transit-amplifying cells are obligate progenitor cells. |
| Facultative Progenitor Cell: A cell that exhibits differentiated features when in the quiescent state yet has the capacity to proliferate for normal tissue maintenance and in response to injury. Bronchiolar Club cells are an example of this cell type. |
| Classical Stem Cell Hierarchy: A stem cell hierarchy in which the adult tissue stem cell actively participates in normal tissue maintenance and gives rise to a transit-amplifying cell. Within this type of hierarchy, renewal potential resides in cells at the top of the hierarchy (i.e., the stem and transit-amplifying cell), and cells at each successive stage of proliferation become progressively more differentiated. |
| Nonclassical Stem Cell Hierarchy: A stem cell hierarchy in which the adult tissue stem cell does not typically participate in normal tissue maintenance but can be activated to participate in repair after progenitor cell depletion. |
| Rapidly Renewing Tissue: Tissue in which homeostasis is dependent on maintenance of an active mitotic compartment. Rapid turnover of differentiated cell types requires continuous proliferation of stem and/or transit-amplifying cells. A prototypical rapidly renewing tissue is the intestinal epithelium. |
| Slowly Renewing Tissue: Tissues in which the steady-state mitotic index is low. Specialized cell types are broadly distributed, long-lived, and a subset of these cells, the facultative progenitor cell, retain the ability to enter the cell cycle. The relative stability of the differentiated cell pool is paralleled by infrequent proliferation of stem and/or transit amplifying cells. The lung is an example of a slowly renewing tissue. |
| Hematopoietic Stem Cell: Cell that has the capacity for self-renewal and whose progeny differentiate into all of the different blood cell lineages including mature leukocytes, erythrocytes, and platelets. |
| Endothelial Progenitor Cell: Circulating cells that have the potential to proliferate and differentiate into mature endothelial cells. Studies of EPCs have been complicated by the use of the same terminology to define at least two different cell populations that have different cell surface markers, different cell sources, and different abilities to differentiate into mature endothelial cells in vitro and in vivo. There is a critical need to develop a consensus definition of EPCs with particular emphasis on the functional capabilities of these cells. |
| Mesenchymal Stromal (Stem) Cell (MSC): Cells of stromal origin that can self-renew and have the ability to differentiate into a variety of cell lineages. Initially described in a population of bone marrow stromal cells, they were first described as fibroblastic colony forming units subsequently as marrow stromal cells, then as mesenchymal stem cells, and most recently as multipotent mesenchymal stromal cells or MSCs. MSCs have been isolated from a wide variety of tissues, including umbilical cord blood, Wharton’s jelly, placenta, adipose tissue, and lung. The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy has recently publisheded the minimal criteria for defining (human) MSCs. MSCs have been described to differentiate into a variety of mature cells types and may also have immunomodulatory properties. |
| Fibrocyte: A cell in the subset of circulating leukocytes that produce collagen and home to sites of inflammation. The identity and phenotypic characterization of circulating fibrocytes is more firmly established than that for EPCs. These cells express the cell surface markers CD34, CD45, CD13, MHC II and also express type 1 collagen and fibronectin. |
| Bronchiolar Stem Cell: A term applied to a rare population of toxin (i.e., naphthalene)-resistant Club cell secretory protein (CCSP)-expressing cells that localize to neuroepithelial bodies and the bronchoalveolar duct junction of the rodent lung. These cells proliferate infrequently in the steady-state but increase their proliferative rate after depletion of transit-amplifying (Club) cells. Lineage tracing studies indicate that these cells have the differentiation potential to replenish specialized cell types of the bronchiolar epithelium. Human correlates have not been identified. |
| Bronchioalveolar Stem Cell: A term applied to a small population of cells located at the bronchoalveolar duct junction in mice identified in vivo by dual labeling with CCSP and surfactant protein C (SPC) and by resistance to destruction with toxins (i.e., naphthalene). In culture, some of the dual-labeled cells also express Sca1 and CD34, self-renew, and give rise to progeny that express CCSP, pro-SPC, or aquaporin 5, leading to speculation that a single cell type has the capacity to differentiate into bronchiolar (Club cells) and alveolar (type 1 and 2 pneumocytes) lineages. The relationship of the cells studied in vitro to those observed by dual labeling in vivo is unclear. Human correlates have not been identified. |
Adapted with permission from Reference 4.
Table 2.
Overall conference summary recommendations
| Basic |
|---|
| • For studies evaluating putative engraftment, advanced histologic imaging techniques (e.g., confocal microscopy, deconvolution microscopy, electron microscopy, laser capture dissection, etc.) must be used to avoid being misled by inadequate photomicroscopy and immunohistochemical approaches. Imaging techniques must be used in combination with appropriate statistical and other analyses to maximize detection of rare events. |
| • Continue to elucidate mechanisms of recruitment, mobilization, and homing of circulating or therapeutically administered cells to lung epithelial, interstitial, and pulmonary vascular compartments for purposes of either engraftment or of immunomodulation. |
| • Encourage new research to elucidate molecular programs for development of lung cell phenotypes |
| • Continue to refine the nomenclature used in study of endogenous and exogenous lung stem cells |
| • Comparatively identify and study endogenous stem/progenitor cell populations between different lung compartments and between species. |
| • Increase focus on study of endogenous pulmonary vascular and interstitial progenitor populations |
| • Develop robust and consistent methodologies for the study of endogenous lung stem and progenitor cell populations. |
| • Develop more sophisticated tools to identify, mimic, and study ex vivo the relevant microenvironments (niches) for study of endogenous lung progenitor/stem cells. |
| • Continue to develop functional outcome assessments for endogenous progenitor/stem cells. |
| • Elucidate how endogenous lung stem and progenitor cells are regulated in normal development and in diseases. |
| • Identify and characterize putative lung cancer stem cells and regulatory mechanisms guiding their behavior. |
| • Continue to elucidate mechanisms by which embryonic and induced pluripotent stem cells develop into lung cells/tissue. |
| • Develop disease-specific populations of ESCs and iPS, for example for CF and α1-antitrypsin deficiency with the recognition that no strategy has yet been devised to overcome the propensity of ESCs and iPS cells to produce tumors. |
| • Continue to explore lung tissue bioengineering approaches such as artificial matrices and three-dimensional culture systems for generating lung ex vivo and in vivo from stem cells, including systems that facilitate vascular development. |
| • Evaluate effect of environmental influences including oxygen tension and mechanical forces including stretch and compression pressure on development of lung from stem and progenitor cells. |
| • Identify additional cell surface markers which characterize lung cell populations for use in visualization and sorting techniques. |
| • Strong focus must be placed on understanding immunomodulatory and other mechanisms of cell therapy approaches in different specific preclinical lung disease models. |
| • Improved preclinical models of lung diseases are necessary. |
| • Disseminate information about and encourage use of existing core services, facilities, and weblinks. |
| • Actively foster inter-institutional, multidisciplinary research collaborations and consortiums as well as clinical/basic partnerships. Include a program of education on lung diseases and stem cell biology. A partial list includes NHLBI Production Assistance for Cellular Therapies (PACT), NCRR stem cell facilities, GMP Vector Cores, small animal mechanics and CT scanner facilities at several pulmonary centers. |
| Translational |
| • Support high-quality translational studies focused on cell-based therapy for human lung diseases. Preclinical models will provide proof of concept; however, these must be relevant to the corresponding human lung disease. Disease-specific models, including large animal models where feasible, should be used and/or developed for lung diseases. |
| • Basic/translational/preclinical studies should include rigorous comparisons of different cell preparations with respect to both outcome and toxicological/safety endpoints. For example, it is not clear which MSC or EPC preparation (species and tissue source, laboratory source, processing, route of administration, dosing, vehicle, etc.) is optimal for clinical trials in different lung diseases. |
| • Incorporate rigorous techniques to unambiguously identify outcome measures in cell therapy studies. Preclinical models require clinically relevant functional outcome measures (e.g. pulmonary physiology/mechanics, electrophysiology, and other techniques). |
| Clinical |
| • Proceed with design and implementation of initial exploratory safety investigations in patients with lung diseases where appropriate such as ARDS/ALI, asthma, and others. This includes full consideration of ethical issues involved, particularly which patients should be initially studied. |
| • Provide increased clinical support for cell therapy trials in lung diseases. This includes infrastructure, use of NIH resources such as the PACT program and the NCRR/NIH Center for Preparation and Distribution of Adult Stem Cells (MSCs; http://medicine.tamhsc.edu/irm/msc-distribution.html), coordination among multiple centers, and registry approaches to coordinate smaller clinical investigations. |
| • Clinical trials must include evaluations of potential mechanisms and this should include mechanistic studies as well as assessments of functional and safety outcomes. Trials should include whenever feasible, collection of biologic materials should as lung tissue, BAL fluid, blood, etc. for investigation of mechanisms as well as for toxicology and other safety endpoints. |
| • Partner with existing networks, such as ARDSNet or ACRC, nonprofit respiratory disease foundations, and/or industry as appropriate to maximize the scientific and clinical aspects of clinical investigations. |
| • Integrate with other ongoing or planned clinical trials in other disciplines in which relevant pulmonary information may be obtained. For example, inclusion of pulmonary function testing in trials of MSCs in graft–vs.-host disease will provide novel and invaluable information about potential MSC effects on development and the clinical course of bronchiolitis obliterans. |
| • Work with industry to have access to information from relevant clinical trials. |
Definition of abbreviations: ARDS/ALI = acute respiraory distress syndrome/acute lung injury; ESC = embryonic stem cell; MSC = mesenchymal stromal (stem) cells.
Structural Engraftment of Circulating or Exogenously Administered Stem or Progenitor Cells
A number of publications over the past approximately 10 years have explored the question of whether bone marrow (BM)-derived cells, including hematopoietic stem cells, mesenchymal stromal (stem) cells (MSCs), endothelial progenitor cells (EPCs), and other populations as well as stem and progenitor cells isolated from other tissues such as adipose, placenta, cord blood, and others, could structurally engraft as mature differentiated airway and alveolar epithelial cells or as pulmonary vascular or interstitial cells. This literature has been extensively discussed and reviewed in previous conferences and elsewhere, and a current consensus is that lung epithelial engraftment is a rare phenomenon of unlikely physiologic significance (reviewed in References 2–4, 28). There continues to be interest in structural engraftment, and more recent literature continues to examine whether other cell populations, such as lineage-depleted nonhematopoietic BM cells, unfractionated BM, cord blood–derived hematopoietic progenitor cells, or amniotic fluid–derived cells (further discussed below), could engraft more effectively (29–31). Recent reports have described a population of CCSP (Scgb1a1)-expressing adult marrow cells in mice that appear to more robustly lodge and engraft in lung after systemic or intratracheal administration (32, 33). A comparable population of cells isolated from pig BM expressed embryonic stem cell markers Oct4 and stage-specific embryonic antigen 1 as well as CCSP, cytokeratin 18, and occludin (34). When grown in a commercially available epithelial differentiation growth medium, these cells expressed aquaporin 5 and surfactant protein C (SPC) and were targets for infection with influenza virus (34, 35). These more recent studies tended to use more sophisticated microscopic and other analytical techniques than the earlier studies. Nonetheless, epithelial engraftment in general continues to be rare and remains of unclear physiologic significance. In parallel, studies have suggested rare apparent engraftment of pulmonary interstitium and vasculature after total marrow transplant in a variety of injury models (reviewed in References 2–4). Despite continuing interest in the possibilities of engraftment of exogenous cells in the lung, emphasis remains on other areas, notably the immumomodulatory effects of administered cells and ex vivo tissue engineering.
Endogenous Lung Stem and Progenitor Cells
Endogenous tissue stem cells are thought to contribute to tissue maintenance and repair. Best characterized in the intestine and in the skin, these cells are rare, undifferentiated, and localized to specialized niches within each tissue. In the lung, endogenous epithelial stem and progenitor cells are thought to contribute to epithelial maintenance and injury repair. There is a large literature in mouse models and a growing literature describing putative endogenous distal airway stem and progenitor cells in human lungs (reviewed in References 2–4; more recent reviews in References 36–39). Some of the published studies have generated controversy, and there is no uniform agreement on the identity and/or function of endogenous lung epithelial stem or progenitor cells in mouse or human lungs (40–47). A smaller but growing literature also describes endogenous stem or progenitor cells that function to replace damaged stroma or pulmonary vasculature, predominantly in mouse models (48–51). However, one persistent issue in the literature is the terminology and nomenclature used. The terms “stem” and “progenitor” are often used interchangeably and inconsistently. Endogenous adult tissue–specific stem cells can be best appreciated as cells that have the capacity for self-renewal and that can give rise to daughter cells, termed “transit amplifying cells,” which give rise to the more specialized or differentiated cells specific to that organ. In general, tissue-specific stem cells have a wide potential differentiation capacity. In contrast, adult endogenous progenitor cells are best appreciated as tissue-specific cells that do not self-renew but that can differentiate into more specialized cells. It has been proposed that some of the differentiated cell types can be induced to a mitotically active state. In this capacity, these cells have been termed “facultative progenitor cells.” Such facultative progenitor cells perform general tissue functions on a daily basis but can enter the mitotic cell pool for tissue injury repair. Thus, a facultative progenitor cell pool functions as a large, broadly distributed pool of reparative cells and can supplement the reparative capacity of the tissue stem cell. Alternatively, the facultative progenitor pool may serve for routine tissue homeostasis and regeneration, whereas the tissue stem cells only come to play in more extreme situations of injury. A proposed list of terminologies was included in the report of the 2007 conference (3) and is repeated here (Table 1). Although there is some degree of consensus with the proposed definitions, there is disagreement and ongoing debate and discussion. Nonetheless, analyses of lung stem and progenitor cells in animal models, particularly the mouse, have provided important advances over the past 5 years.
It remains unclear whether paradigms and hierarchies described for endogenous stem and progenitor cells in organs such as the intestine and skin also apply to the lung, particularly the lung epithelium (36–39). The lung is a complex organ containing many distinct epithelial cell types that are distributed in several different regional microenvironments along the pulmonary tract. This is depicted in schematic form in Figure 1. Consequently, although identification of cells that can proliferate under steady-state or injury conditions has been relatively straightforward, characterization and classification of mitotically active putative endogenous stem and progenitor epithelial cells into a hierarchy has been challenging. Remaining questions are 1) whether the cells should be arranged into a hierarchy and 2) if there is a hierarchy, how the cells should be arranged, and what are the defining characteristics of cells at different levels of the hierarchy: is it differentiation and proliferation potential as in the intestine? More advanced lineage-tracing models and techniques in mice have begun to provide additional information. However, further lineage-tracing approaches are needed, and there are no available methods to comparably assess lineage hierarchies in putative human lung epithelial stem or progenitor cells.
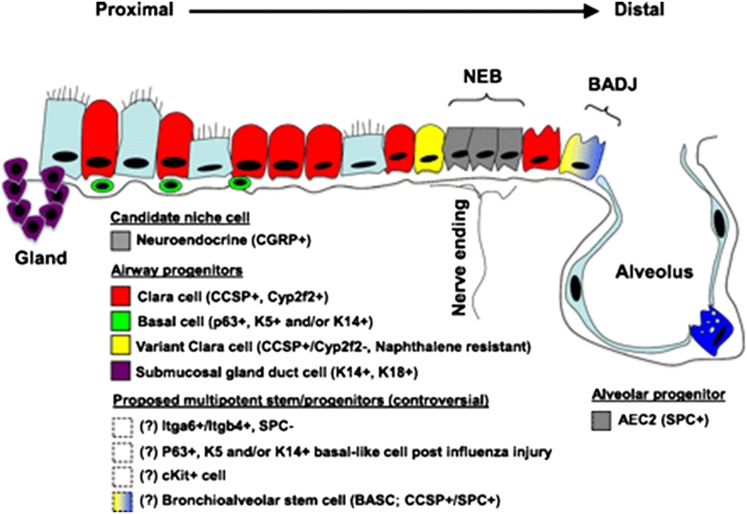
Figure 1.
Sschematic of proposed lung epithelial candidate stem or progenitor cells and their niches in the proximal conducting airways and distal alveoli. Cells whose localization or existence is not clear or accepted are indicated with dashed boxes and/or question marks. AEC2 = type 2 alveolar epithelial cell; BADJ = bronchoalveolar duct junction; Gland = submucosal gland duct; NEB = neuroepithelial body. Marker abbreviations used for each cell subtype include the following: CCSP = Club cell secretory protein; CGFP = calcitonin gene–related peptide; Itg = integrin; K = cytokeratin; SPC = surfactant protein C. Adapted by permission from Reference 20.
Other considerations specific to the lung include cell cycle time and frequency. Because the adult lung is a tissue that has a low constitutive epithelial turnover rate, lung injury models have been used in mice and other animal models to identify stem cells and progenitor cells by inducing cellular proliferation and repopulation of the lung epithelium. Some of the injury models are specific to particular regions of lung epithelium. For example, sulfur dioxide, ozone, and nitrogen dioxide inhalation, which damages the tracheal and large airways epithelium, have been used to study proximal airway stem cells and progenitor cells (reviewed in References 2–4, 36, 38, 52). Naphthalene administration, which specifically injures the Club cells in the bronchiolar epithelium, and bleomycin administration, which injures the alveolar epithelium, have been used to study more distal airway and alveolar regions (36, 38). These models have provided strong evidence for regional specificity of lung epithelial stem or progenitor populations in mouse lungs. For example, in the mouse, at least five populations of airway epithelial cells have been identified that have the ability to enter the cell cycle after injury to the lungs and can thus be considered as facultative progenitor cells: basal, Club-like, Club, pulmonary neuroendocrine, and type 2 (ATII) alveolar epithelial cells. The difference between Club cells and Club-like cells further highlights the difficulties in identifying facultative progenitors. A Club-like cell is a cell that expresses CCSP in the tracheobronchial epithelium; the original definition for the Club cell specified a terminal bronchiolar location (53). It is clear from ultrastructural analyses that there are epithelial secretory cells throughout the proximal to distal axis (54). However, they express a different repertoire of secreted proteins. Club and Club-like cells are also differentially sensitive to naphthalene and other toxic agents. Most importantly, the proximal Club-like cells are derived from a different progenitor than the distal airway Club cells (53).
Trachea and Large Airways
The trachea and large airway compartment contains two major epithelial cell lineages: basal and secretory/ciliated cells. Basal cells express high levels of the transcription factor Trp63, cytokeratins 5 and 14, and aquaporin 3 and can be isolated by fluorescence-activated cell sorting using expression of the nerve growth factor receptor. Lineage tracing studies in the mouse have revealed that basal cells can give rise to Club and ciliated cells in the proximal airways during homeostasis and after sulfur dioxide injury in mice (54, 55). Cell signaling pathways, including β-catenin, notch, and tissue factor, appear to regulate function and fate of the basal epithelial cells (56–60). Recent data suggest that, in mice, the facultative progenitor cell pool accounts for much of the airway epithelial cell replacement during normal homeostasis but can also significantly contribute to tissue repair after cellular injury (61). In contrast, older pulse-chase studies have demonstrated that the Club-like cell serves as a self-renewing cell type and as the progenitor for ciliated airway cells in rats (62). However, more recent lineage tracing of Club-like cells demonstrates that the Club-like cells do not replenish all cell types in the tracheal and proximal airways (63). This lack of consensus on whether a stem cell participates in repair of the upper airway epithelium reflects the need to use a comprehensive range of analytical methods in individual studies. This remains a significant limitation in the field. However, despite this, the use of multiple injury models in vivo, including exposure to SO2, detergent, and naphthalene, as well as in vitro analyses suggest that a subset of basal cells serve a role as tissue stem cells or facultative progenitor cells of the upper airway in mice. Similar conclusions have been derived using human proximal airway basal epithelial cells in ex vivo or in vitro culture systems (63, 64). Overall, the data for the trachea and larger airways, although limited, provide the strongest evidence to date for a lung tissue–specific stem cell. However, the situation is complex, and there may be subpopulations of basal epithelial cells that have more restricted lineages or specific roles. For example, a population of CD49fbright/Sca1+/ALDh+ cells can act as progenitor cells and repair naphthalene-injured tracheal epithelium (65). Recently, a stem/progenitor population of tracheal submucosal gland duct cells was identified that appears capable of regenerating submucosal gland tubules, ducts, and surface epithelium after hypoxic-ischemic injury (66, 67).
Distal Airways
In mice, the predominant epithelial cell of the smaller airways is the nonciliated Club cell, which exhibits characteristics of a facultative progenitor cell after injury to ciliated airway epithelial cells. However, unlike transit-amplifying cells in tissues with higher rates of epithelial turnover, such as intestine, Club cells exhibit a low proliferative index in the steady-state, are broadly distributed throughout the bronchiolar epithelium, and contribute to the specialized tissue function. Earlier pulse-chase studies identified the Club cell as a progenitor for ciliated cells. More sophisticated lineage tracing methods in subsequent studies demonstrated that, in mouse lungs, Club cells can self-renew and function as a progenitor for distal airway ciliated cells during homeostasis. However, available data suggest fundamental differences between Club-like and Club secretory cells in proximal versus distal airways, respectively (63), and thus the situation remains complicated.
In another widely used approach, the Club cell–specific toxin naphthalene has been used extensively to deplete the bronchiolar Club cell pool in mouse models. A population of naphthalene-resistant cells, termed “variant Club cells” (vCE), was identified in older studies as a bronchiolar stem cell (68). vCE are located within discrete microenvironments that include two different stem/progenitor cell niches: the neuroepithelial body and the bronchoalveolar duct junction. Another naphthalene-resistant progenitor cell population located at the bronchoalveolar duct junction, identified based on their coexpression of CCSP and the ATII cell marker SPC, have been termed bronchoalveolar stem cells (BASCs) (69). BASCs can be isolated using fluorescence-activated cell sorting to select for cell expression of the stem cell marker Sca-1 after elimination of hematopoietic cells and endothelial cells (CD45negCD31negSca-1pos). More recent studies have shown that the CD45negCD31negSca-1pos population is more heterogeneous than previously appreciated and that a more robust marker profile for enriching for BASCs uses CD45negCD31negEpCAMposSca-1lowCD24low flow cytometric separation (70–72). Several other overlapping stem/progenitor cell populations have recently been isolated in mouse lungs based on flow cytometric isolation of cells exhibiting the CD45negCD31negEpCAMhiCD49fposCD104posCD24low or the CD45negCD31negCD34negEpCAMposSca-1low autofluorescent low-population phenotypes (73). These cells form colonies expressing airway and/or alveolar lineage markers in a three-dimensional coculture matrigel assay with primary lung mesenchymal cells. The latter phenotype contains naphthalene-resistant bronchiolar progenitors. In contrast, CD45negCD31negCD34negEpCAMposSca-1low autofluorescent high-population cells contained the naphthalene-sensitive Club cells (73). Other recently described populations of putative mouse lung distal airway progenitor cell populations include populations of integrin α6β4pos SP-Cneg cells, CK5pos p63pos cells, and CK5pos p63pos cells (74, 75). These cells can have different localizations in the airway tree and may function differently in repair from experimentally induced lung injury (76).
A critical point these studies highlight is that careful and meticulous use of isolation and characterization techniques, including flow cytometry and immunohistochemistry, must be used. It is conceivable that several of the above-mentioned distal airway epithelial progenitor populations represent the same cells or phenotypic variants of the same cell population characterized in different ways in different laboratories. The diversity of interpretations highlights the widely recognized need for markers that are specific for the functionally distinct cell populations, more precise tools for lineage tracing, and further underscoring the importance of the in vivo microenvironment or niche on cell behavior (77). Increased collaboration and cross fertilization to share and compare methods is essential. However, phenotypic characterization is not enough, and functional assays, including lineage tracing and differentiation capacities, are critical to assess. Robust in vitro assays that recapitulate the in vivo environment (e.g., repopulation of decellularized lung scaffolds) will add further insight. Other in vivo assays (e.g., cell transplantation) are needed to assess the ability of putative lung stem/progenitor cells to reconstitute lung epithelial cell lineages within damaged or diseased tissue.
A further complication is that the putative airway progenitor cells may be quiescent during the response to some injuries (e.g., ozone depletion of the ciliated cell pool) and the fact that bronchiolar stem cells did not play a greater role in normal airway epithelial homeostasis and turnover than did the abundant pool of facultative progenitor Club cells (61). This study suggests that the neuroepithelial body–associated vCE likely function as a reserve population that can function in normal maintenance or, more relevantly, after depletion of the facultative progenitor pool of vCE. Additional studies are needed to further confirm and clarify this hypothesis in mouse and human lungs. Thus, although progress is being made in clarifying the identity and role of distal airway progenitor cells in mice, the role of these cell populations in normal homeostasis and in response to more severe injuries remains unclear.
Cell Signaling Pathways in Endogenous Distal Airway Progenitor Cells
Recent investigations have continued to explore cell signaling and other mechanisms regulating putative distal airway progenitor populations in mice. For example, manipulations of Kras, p27, MAPK, p18, protein kinase C iota, or Pten have been shown to induce an expansion of bronchiolar progenitor and BASC numbers and to enhance lung tumorigenesis (reviewed in References 2–4, 36, 38). Other cell signaling pathways, such as Wnt/β-catenin, Hedgehog, and Notch cell, are implicated in stem cell function in lung and other tissues (2–4, 36, 38). However, although stimulation of Wnt/β-catenin cell signaling appears to promote airway submucosal gland development, it inhibits differentiation of bronchiolar stem cells in lung and does not appear to play a key role in the maintenance or repair of the bronchiolar epithelium. The precise role of these and other pathways in endogenous lung stem and progenitor cells remains to be determined.
Human Endogenous Distal Airway Progenitor Cells
The identity of potential endogenous airway progenitor cells in human lungs remains less well understood. There are significant differences between the structure and cellular composition of mouse and human lungs. For example, submucosal glands and the pseudostratified epithelium containing basal cells, regions that are restricted to the trachea and upper airways in mice, extend from the trachea to the distal airways in humans. In addition, vCE or BASCs have not been identified in human lung tissue. Therefore, intensive investigation comparing contrasting putative endogenous airway stem and progenitor populations in mouse and human lungs are sorely needed. Several laboratories have recently described putative proximal and distal airway epithelial progenitor cells isolated from human lungs (78–84). These include a c-kitpos cell that appears to generate endodermal and mesodermal lineages in tissue culture (79). When injected into mouse lungs after cryoinjury, this cell population appears capable of stimulating or participating in repair of airway, pulmonary vasculature, and pulmonary interstitium. If true, this model would overturn the current concept that there is no single multipotent lung stem or progenitor cell capable of generating smooth muscle, vasculature, airways, and alveoli. These findings have generated intense discussion and require further functional characterization and validation in other laboratories (85).
Although it is attractive to speculate that lung diseases may in part be a consequence of endogenous lung stem or progenitor cell failure, more studies are needed to draw direct connections. In particular, little is known of progenitor cell function in chronic diseases such as emphysema or idiopathic pulmonary fibrosis. More suggestive information is available for the genetic lung disease cystic fibrosis (CF). The airway epithelium in patients with CF contains cuboidal cells that express primitive cell markers, including thyroid transcription factor (TTF-1, Nkx2.1) and cytokeratin 7 (reviewed in References 2–4). Neuroepithelial cells also express the CF transmembrane conductance regulator protein (CFTR), the defective protein in patients with CF, which appears to play a role in neuropeptide secretion. CFTR knockout (−/−) mice contain fewer pulmonary neuroendocrine cells during embryonic development but increased numbers of these cells after birth (2–4). These observations suggest that endogenous airway progenitor cell pathways in CF lungs may be altered, but this has not been extensively investigated or further clarified.
Endogenous Alveolar Progenitor Cells
Alveolar epithelial reparative potential remains centered on the ATII cell and the long-held concept that ATII cells are precursors for type 1 alveolar epithelial (ATI) cells (86–88). However, recent data suggest that several populations of distal airway epithelial and other progenitor cells in mice, including BASCs and CK5+/p63+ cells, can contribute to repair of damaged alveoli (69, 75, 89). In neonatal mice, a population of putative progenitor cells that expresses CCSP, stem cell antigen 1, stage-specific embryonic antigen 1, and Oct-4 have been identified (90, 91). These cells were able to form epithelial colonies and differentiate into ATI and ATII cells. However, the lineage relationship between ATII and ATI cells requires further clarification, particularly because ATI cells can be mitotic in vitro (89). Additional studies are needed to resolve these controversies.
Several studies suggest that endogenous lung epithelial progenitor cells may be targets for environmental agents, including pneumotrophic pathogens. Airway stem or progenitor-like cells in mice are susceptible to infection with severe acute respiratory syndrome and influenza viruses, raising the possibility that endogenous lung progenitor cells may be specific disease targets (75, 90). Comparably, the basal epithelial cells of the trachea and upper airways appear more susceptible to infection with the common cold rhinovirus (92). However, respiratory viruses target a wide range of respiratory epithelial cells in addition to progenitor cells. Endogenous progenitor cells may also be attractive candidates for targeting with gene transfer vectors that provide sustained expression. For example, intratracheally administered recombinant adeno-associated vectors may preferentially target vCE in adult mice, whereas recombinant lentivirus vectors administered into the amniotic fluid appear to preferentially target airway progenitors in fetal mouse lungs (93, 94).
Major challenges remain in the endogenous lung progenitor field. Continued areas for development include identification of adequate cell-specific markers, more sophisticated lineage-tracing tools, increased exploration of progenitors of the vascular and stromal lung compartments, and functional assays of putative endogenous progenitor cell populations. Existing cell type–specific markers in particular are in need of refinement as increasing knowledge is obtained about the inherent plasticity of lung cell types and as previously identified lineages are deconstructed. Adequate approaches must be developed for study of human lungs. Increased collaboration and cross fertilization must occur to reconcile differences in terminology and methodologies used in different laboratories. For example, disagreement or lack of consistent interpretation and application of seemingly straightforward terminology, such as “differentiated versus undifferentiated” and “specialized versus unspecialized,” has continued to impede progress. A list of suggested terminology is illustrated in Table 1 in the accompanying conference report, but this is likely to need revision in the near future.
Lung Cancer Stem Cells
There is intense interest in the connections between endogenous lung stem or progenitor cells and cancer stem cells. Cancer stem cells have been defined in transplantation assays as the cell subset that is capable of propagating disease. These cells are frequently termed “tumor initiating cells” and are hypothesized to be the cells that maintain tumor progression and disease resistance (95). Cancer stem cells are best described in leukemias, breast cancer, and brain cancers, but increasing evidence suggests lung cancers may contain rare populations of cancer stem cells (reviewed in References 96, 97). These studies suggest that the different types of lung cancer are initiated from distinct cell types and that the lung tumor–initiating cell may or may not have the same identity as the cancer stem cells that maintain established tumors. Given the diversity of lung cancer subtypes, this may not be surprising. Purification and characterization of the tumor-initiating cell and/or the cancer stem cell is an important aspect of studies designed to test the cancer stem cell hypothesis (96, 98, 99). Older studies (reviewed in References 2–4) have shown that CD45− side population cells have been identified in several human lung cancer cell lines and exhibit tumorigenic properties when subcutaneously implanted into nonobese diabetic SCID mice. Side population cells have also been identified in clinical lung cancer specimens. Dual-positive pro-SPC/CCSP positive cells, the BASCs discussed in the above section, have also been suggested as tumor-initiating cells. A number of recent reports implicate CD133+ cells as conferring resistance to chemotherapy and having tumor-initiating properties. Aldehyde dehydrogenase activity or expression of the oncofetal protein 5t4 have also been suggested as a marker for lung cancer stem cells (above studies reviewed in References 2–4). Recent studies have begun elucidating cell signaling and gene expression pathways, including PTEN, protein kinase C (iota), Wnt, hedgehog, c-kit, Akt, matrix metalloproteinases, and others, that may play roles in transformation of endogenous progenitor cells into lung cancer cells (2–4, 96–99). Despite growing data, further work is needed to clarify the connections between endogenous lung progenitor cells, their potential roles as lung cancer stem cells, and, most importantly, their potential role as therapeutic targets.
Embryonic Stem Cells and Induced Pluripotent Stem Cells
Studies evaluating embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs) for lung regeneration or repair have shown promising recent progress. Several laboratories had initially demonstrated that mouse and human ESCs could be induced in culture to acquire phenotypic markers of ATII cells, including expression of surfactant proteins and lamellar bodies, and even form pseudoglandular structures (reviewed in References 2–4, 20, 21). However, in general, this occurred at a low level unless the ESCs were transduced to select for cells expressing an antibiotic resistance cassette under regulatory control of a surfactant protein-C promoter fragment (100, 101). It has never been clear that the derived cells acquired other appropriate functions of alveolar cells. More recent protocols incorporating more sophisticated understanding and application of cell signaling pathways guiding embryologic lung development and development of definitive endoderm, as well as newly developed lineage tracing tools such as Nkx2.1-GFP expressing mice, have yielded more robust in vitro derivation of cells with phenotypic characteristics of type 2 and type 1 alveolar epithelial cells from murine and human ESCs and from iPSCs (Figure 2) (102–105). Investigation of other functional aspects of the ESC-derived lung cells, such as the ability to repopulate decellularized lung scaffolds, are being increasingly incorporated (Figure 2) (104). Recent culture protocols have also more robustly suggested the development of cells with phenotypic characteristics of airway cells, including those derived from iPSCs, obtained from patients with CF and will provide a solid basis for studying ESCs and iPSCs in genetic lung diseases such as CF (Figure 3) (105, 106). A growing number of studies suggest that the effects of matrix proteins, three-dimensional scaffolds, mechanical forces, and culture systems (e.g., the use of rotating bioreactors) will play critical roles in furthering our understanding of the means by which ESCs and iPSCs might be induced to acquire the phenotype of functional airway and alveolar epithelial cells (107–109). Little data are available on the development of lung pulmonary vasculature or stroma from cultured ESCs or iPSCs. The American Thoracic Society issued a statement in 2006 calling for expanded human embryonic stem cell research and a follow-up statement in 2010 after the temporary injunction on the use of human stem cells in the United States (5, 6). It is hoped that human embryonic and induced pluripotent stem cell research will continue to expand in the United States and that there will be further rapid advances in the study of ESCs for lung injury and repair. The awarding of the 2012 Nobel Prize in Medicine to Shinya Yamanaka, pioneer of the development of iPSC technology, underscores the importance attributed to this technology (110). The generation of disease-specific human iPSC lines from patients with genetic and acquired lung diseases, including CF, α1-antitrypsin disease, sickle cell, and scleroderma, provides further opportunity to use iPSCs for the study of lung diseases (111).
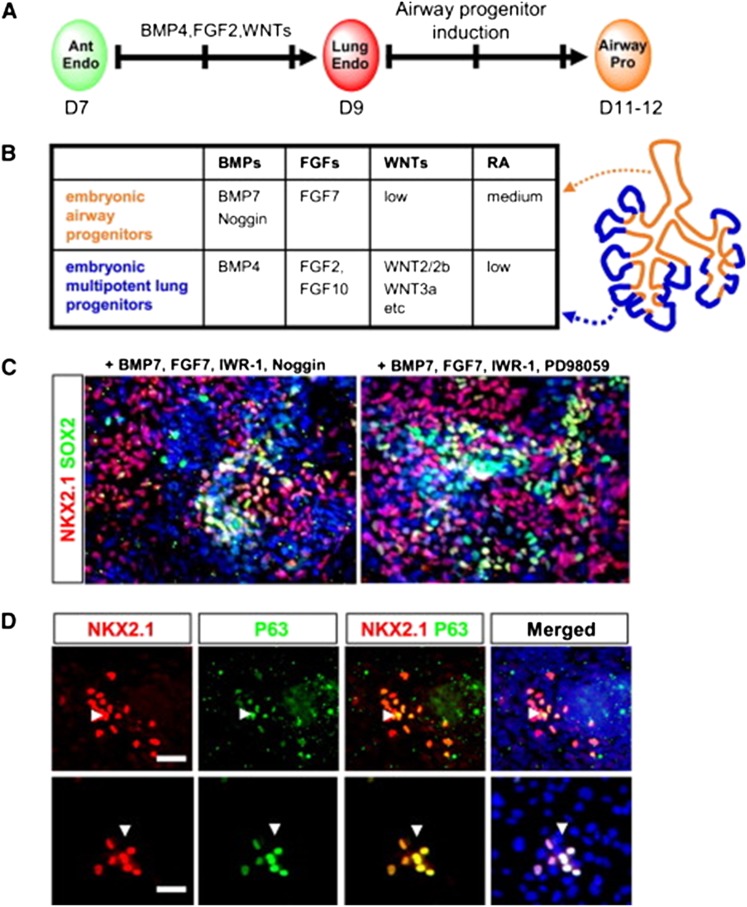
Figure 2.
Alveolar differentiation repertoire of embryonic stem cell (ESC)-derived Nkx2–1+ lung progenitors. (A and B) Immunostaining for alveolar epithelial markers T1α, pro-surfactant protein C (SPC), and Nkx2–1 on cells at the completion of the 25-day directed differentiation protocol. ESCs sorted on Day 15 based on Nkx2–1GFP+ expression gave rise to cells reminiscent of type 1 alveolar epithelial cells (AEC1) as they lost Nkx2–1 nuclear protein expression (green immunostain) while expressing T1α protein (A). (B) Other patches of cells appeared more reminiscent of distal SPC+ alveolar epithelial cells because they expressed punctate cytoplasmic pro-SPC protein and displayed SPC promoter activation while retaining Nkx2–1GFP expression. Arrow = SPC-dsRed and Nkx2–1GFP coexpressing cell (orange). Arrowhead = cell expressing only Nkx2–1GFP. (C) Schematic summarizing the decellularization-recellularization assay. (D) Hematoxylin and eosin stains of lung sections showing lung scaffold appearance with no recellularization (left panel) versus hypercellular sheets after reseeding with undifferentiated ESCs (middle panel) versus cells of alveolar structural morphologies after seeding with Nkx2–1GFP+ purified ESC-derived progenitors (right panel). Scale bars = 100 μm in three left panels. Zoom of the indicated boxed region is shown in far right panel with scale bar = 20 μm. (E and F) Nkx2–1+ nuclear protein (brown; arrowheads in E) immunostaining of engrafted cuboidal epithelial cells in the corners of alveoli derived 10 days after reseeding with Nkx2–1GFP+ sorted cells. Arrow = flattened nucleus of an Nkx2–1 negative cell (purple) lining the alveolar septum. Many of these flattened cells were T1α+ (F; arrowhead). Scale bars = 20 μm. (G) Control mouse lung without decellularization showing T1α apical membrane staining pattern (brown) of mature AEC1 (see Figure S7 in the online supplement). (H) Ciliated airway epithelial cell (arrow) 10 days after reseeding with differentiated/unsorted ESC-derived cells. Scale bar = 20 μm. All nuclei were counterstained with hematoxylin (purple). Adapted by permission from Reference 104.
Figure 3.
Stepwise differentiation of Nkx2.1+ lung progenitors from human induced pluripotent stem cells (iPSCs). (A) Schematic strategy and time line to generate Nkx2.1+ lung multipotent progenitors from human iPSCs. (B) High yield of definitive endoderm from CF1 RNA-induced pluripotent stem cells (RiPSCs) was obtained after treatment for 4 days in RPMI-1640 medium in the presence of 2% B27 supplement, Activin A (100 ng/ml), and 5 μM PI3 kinase inhibitor LY294002 with more than 90% of cells coexpressing transcription factors SOX17 and FOXA2. Scale bar = 200 μm. (C) Anteriorization of endoderm into foregut endoderm cells with SOX2 expression in Foxa2+ cells derived from CF1 RiPSCs after 4 days of treatment with 500 nM A-83–01 (TGF-β antagonist) and 100 ng/ml Noggin (bone morphogenetic protein [BMP]4 antagonist). Scale bar = 100 μm. (D) Confocal image after immunofluorescence staining showing that some Nkx2.1+ spheres contain basal cells positive for p63. Scale bar = 40 μm. Adapted by permission from Reference 105.
There are a growing number of publications assessing the effects of in vivo administration of ESCs or IPS cells or of several subpopulations of cells differentiated in vitro from human ESCs, including those expressing angiotensin-converting enzyme after mesoderm induction or those expressing CD166 after endodermal induction, in mouse models of silica-, sepsis-, or bleomycin-induced lung injuries (112–115). Whether the observed amelioration of lung injury resulted from structural engraftment of the administered cells reflects previously unsuspected paracrine effects of the ESCs- or iPSCs, including interactions with immune effector cells, or some other unknown mechanism of action is not clear. Further careful study, including elucidating a rational biologic role for ESCs or iPSCs cells in ameliorating lung injury, needs to be done (116).
EPCs
EPCs have been defined over the past decade as circulating BM-derived cells that express a variety of cell surface markers comparable to those expressed by vascular endothelial cells, adhere to endothelium at sites of hypoxia/ischemia, proliferate, and participate in new vessel formation (reviewed in References 2–4, 117, 118). Increasing evidence demonstrates that EPCs play a role in the pathogenesis of a wide variety of lung diseases, including pulmonary hypertension, pulmonary fibrosis, asthma, COPD, acute lung injury (ALI), lung cancer, bronchopulmonary dysplasia, and obstructive sleep apnea, in children (reviewed in References 2–4; recent articles reviewed in References 119–125). However, studies of EPCs in lung diseases have been hampered by a lack of consensus regarding identification of these cells (126–128). Although no specific marker for circulating EPCs has been identified, a panel of flow cytometry markers in conjunction with immunostaining has been used to identify and enumerate these cells in BM and in the circulation (128). However, even when similar techniques have been used in different reports, different groups of markers have been used, and differences in data acquisition and analysis can significantly alter study interpretations (128). The lack of unique cell surface markers to identify EPCs continues to complicate comparative assessments for pulmonary and other disease processes. As a result, a growing consensus in the field encourages the use of functional assays in vitro and in vivo in conjunction with the use of flow cytometry and immunohistochemistry not only to enumerate EPCs but also to better characterize their true ability to form functional endothelium. A critical area for future study remains to develop a consensus-based approach to the definition and use of EPCs, with particular emphasis on functional capabilities of these cells.
A number of hematopoietic and vascular endothelial cell subsets display similar cell surface epitopes, may be derived from each other, and can only be discriminated by an extensive gene expression analysis or with the use of a variety of functional assays. The interaction between hematopoietic stem cells and endothelial progenitor cells for generation, maintenance, or repair of the vasculature has become increasingly complex, with hematopoietic stem cells described as potentially contributing to hemogenic vascular endothelium and endothelium contributing to the derivation of hematopoietic lineages (129, 130). Dysregulation of these processes is increasingly being recognized as contributing to vascular diseases, including pulmonary hypertension, and to pathologic conditions, including asthma (121, 131). For example, immunodeficient mice transplanted with CD133+ BM-derived progenitor cells obtained from patients with pulmonary hypertension developed pathologic features of pulmonary hypertension (121). In contrast, CD34+ progenitor cells appear to contribute to vascular growth in postpneumonectomy lung regeneration in mice (132). In addition to circulating EPCs, several populations of vascular progenitor cells resident in different segments of the pulmonary vasculature have been recently identified (133). Characterization of these cells, their functional regulation, and their contribution to pulmonary and other vascular diseases is an area of intense current study.
Endothelial Progenitor Cells and Lung Diseases
The number of circulating EPCs has been correlated with several clinical variables in different lung diseases, demonstrating the potential utility of EPCs as biomarkers (reviewed in References 2–4; more recent articles are reviewed in References 120, 122, 123, 125). Increased circulating EPC numbers correlated with survival in ALI/acute respiratory distress syndrome (ARDS) and were associated with less residual lung damage in patients with pneumonia but were inversely associated with organ dysfunction in sepsis. An increase in the number of circulating EPCs in patients with COPD was associated with more abnormal spirometry, although different studies have shown that levels of circulating EPCs are inversely correlated with COPD disease severity, particularly in association with decreased body mass index but not necessarily with disease exacerbations (120, 123). Increased numbers of circulating EPCs also portended worse survival among those with non–small cell lung cancer (134–136). In subjects with asthma, numbers of circulating EPCs were increased compared with nonasthmatic control subjects, but this did not correlate with clinical outcomes (137). EPCs were recruited to the airways of patients with asthma after allergen challenge, and this correlated with increased number and diameter of blood vessels observed in lung tissue biopsies obtained 24 hours after challenge (124). Decreased numbers of circulating EPCs in cord blood have also been described in infants who later develop bronchopulmonary dysplasia (125).
Several clinical factors have been implicated in the mobilization of EPCs in lung diseases, and mechanisms for their effects have begun to be elucidated. Hypoxia appears to be a stimulus for EPC mobilization and recruitment, whereas hyperoxia is correlated with decreased circulating EPCs, particularly in preterm infants (138–140). These features may play a role in bronchopulmonary dysplasia and other disorders in premature infants and neonates exposed to high oxygen levels (140, 141). These observations also suggest that EPCs could contribute to lung repair after ALI. Defective lung development or defective lung repair in the setting of protracted inflammation and injury may result in part from inadequate contribution of local or circulating EPCs. Age has been reported to be inversely correlated with EPC number and with the ability of EPCs to home to ischemic tissues (2–4, 117). This may be mediated through the inability of aged tissues to normally activate hypoxia-inducible factor-1α–mediated hypoxia responses. The use of HMG-CoA reductase inhibitors has been demonstrated to have a beneficial effect on the mobilization of EPCs. This may be related to the effect of this class of drugs in the prevention of EPC apoptosis in response to noxious stimuli, including the effects of TNF-α and IL-1β, thereby enhancing EPC survival and differentiation (reviewed in Reference 4). Other pathways recently implicated in mobilization of EPCs include circulating VEGF, insulin-like growth factor 2, CXCL12, and CXCR2 chemokines (4, 117, 124, 142). However, increasing the number of circulating EPCs or developing methods to enhance their mobilization may not be appropriate for all diseases that affect the lung, particularly for lung cancers (135, 136, 143). EPCs may effect the development of lung tumor vasculature and can home to sites of lung metastases and in other cancers. Because neovascularization involves the recruitment of EPCs from the BM and potential contributions from endogenous endothelial cells in the pulmonary vasculature, these cells are a logical target for antiangiogenesis therapy. In addition, after systemic injection, EPCs localize to lung and appear to home to metastatic tumors in lung through as yet poorly understood mechanisms. This suggests that modification of EPCs to express suicide genes or other therapeutic molecules could be potentially used in cell-based therapy approaches for lung cancer (144, 145). Mechanisms controlling mobilization and homing of EPCs to lung remain poorly understood and are the subject for more intense investigation.
An increasing number of studies demonstrate that systemic administration of EPCs can mitigate experimentally induced lung injuries in preclinical rodent and dog models of pulmonary hypertension (reviewed in Reference 146), endotoxin-induced ALI (147–149), and bronchopulmonary dysplasia (150). Whether this includes structural contributions of the administered cells, paracrine stimulation of endogenous vascular progenitor cells, or other paracrine immunomodulatory actions remains unclear (146). A combination of all of these and other effects may occur in different disease states. EPCs can be transduced to express proangiogenic factors, such as endothelial nitric oxide synthetase (eNOS), or inhibitors of smooth muscle cell proliferation, such as calcitonin gene–related peptide, and appear to home to sites of endothelial damage and lung injury (146, 151). EPCs can also preferentially localize to areas of injured lung after systemic administration and may have paracrine effects to decrease inflammation (146, 152). These findings have generated several clinical investigations of EPC administration in pulmonary hypertension, further discussed below. Clarification of the specific cell types involved in the process of neoangiogenesis and better phenotypic markers and functional assays will result in less confusion surrounding the term EPC. Further elucidation of progenitor cells resident in the vasculature and the role of these and circulating vasculogenic cells in lung development and repair from injury are the focus of extensive current investigation. The potential role of exogenously administered vasculogenic cells, either unmodified or engineered to express angiogenic or other factors, shows increasing promise in several types of lung injury but must be considered cautiously given the potential deleterious roles in lung and other cancers.
Circulating Fibrocytes
Circulating fibrocytes were first described as a subset of circulating leukocytes that produced collagen and homed to sites of inflammation (153, 154). These cells are characterized by expression of the cell surface markers CD34, CD45, CD13, and MHC II and also express type 1 collagen and fibronectin. Circulating fibrocytes have been implicated in the pathogenesis of several lung diseases, including mouse and clinical models of pulmonary fibrosis, pulmonary hypertension, the subepithelial fibrosis that can develop in severe asthma, sickle cell lung disease, and clinical bronchiolitis obliterans in patients undergoing lung and BM transplant (older articles reviewed in References 2–4, 155–157; more recent articles reviewed in References 158–163). Recent articles continue to demonstrate that elevated levels of circulating fibrocytes have been suggested to indicate worse prognosis in idiopathic pulmonary fibrosis (164, 165) and pulmonary hypertension (163) and in the development of bronchiolitis obliterans after lung transplant (166). Numbers of circulating fibrocytes were highest in patients experiencing an acute IPF exacerbation, and the numbers returned to baseline with recovery (164). The content of fibrocytes in bronchoalveolar lavage fluid has also been recently suggested as a predictor of worse outcome in patients with ALI (167). Several cytokine and chemokine pathways, including stromal derived factor-1–CXCR4 axis, IL-10 actions through the CCL2/CCR2 axis, CCR5 and CCR7 axes, EGF receptor signaling, and TGF-β receptor signaling, have been implicated in recruitment to and subsequent proliferation of circulating fibrocytes in fibrotic lungs, but overall the mechanisms of fibrocyte recruitment to lung are poorly understood (157, 161, 168). Serum amyloid protein A, surfactant protein D, haptoglobin,cysteinyl leukotrienes, TGF-β receptor signaling, and hypoxia have also been implicated in fibrocyte recruitment and differentiation (155–157, 168–173). A recent study demonstrated that the prostacyclin analog treprostonil inhibited recruitment of circulating fibrocytes in a model of chronic hypoxia–induced pulmonary hypertension in mice (174). Matrix metalloproteinase expression may also be involved in recruiting fibrocytes to injured or posttransplant lungs (175). Viral infections have been suggested to increase recruitment or proliferation of fibrocytes in injured lungs through NF-κB–mediated MCP-1 and CXCL12 expression or through TGF-β or cysteinyl leukotriene–mediated pathways (176, 177). No studies have definitively proven that fibrocytes can differentiate into fibroblasts in vivo, and it is not clear whether their profibrotic actions are paracrine or via direct differentiation to fibroblasts/myofibroblasts. Regarding their potential paracrine actions, a recent study has demonstrated that fibrocytes from patients with IPF overexpress periostin, a matricellular protein that can help to activate lysyl oxidase to stiffen extracellular matrices (178).
Circulating fibrocytes may also be important in lung cancer development or metastasis. Circulating fibrocyte precursors found in blood of lung cancer patients contributed to tumor development when systemically administered to nonobese diabetic SCID mice engrafted with human lung cancer xenografts (179). Bone marrow–derived cells may also contribute to fibroblasts and myofibroblasts in tumor stromal tissue (180). These results suggest that specific inhibition of fibrocytes or their use as drug delivery vehicles may be important therapeutic targets in pulmonary vascular disease.
Amnion-derived Cells
Two different populations of cells isolated from human amniotic tissue, human amniotic epithelial cells (hAECs) and a heterogenous population of multipotent cells termed human amniotic fluid stem cells (hAFSCs), have been described to apparently engraft in limited amounts in mouse lungs and to have potential efficacy in lung injury (181). Before the amnion is derived from the embryonic epiblast before gastrulation, amniotic cells may retain the capacity to differentiate into the three embryonic lineages and then into cells derived from each lineage (182). In addition, a number of recent publications demonstrate that hAFSCs may have immunomodulatory properties comparable to those observed in MSCs obtained from other tissue sources. The hAFSCs are obtained from amniotic fluid aspirates and contain a mixed population of mesenchymal, stromal, and epithelial cells (181, 183). After microinjection into fetal mouse lungs, a c-kit+ subfraction of human hAFSCs localized to distal airway, where they expressed TTF1 and SPC (184). Systemic administration into hyperoxia- or naphthalene-injured adult mice resulted in localization and expression of characteristic alveolar or airway epithelial markers, respectively (184). Administration of c-kit+ hAFSCs also ameliorated experimentally induced kidney injury in immunodeficient mice (185). Other populations of hAFSCs appear to have features in common with MSCs isolated from other tissues. For example, systemic administration of hAFSCs ameliorated monocrotaline-induced pulmonary hypertension in rats (186). hAFSCs can also stimulate recruitment of host progenitor cells to function in tissue repair (187), and a recent report suggests that hAFSCs might be induced to express CFTR after coculture with CF airway epithelial cells (188).
Purified populations of hAECs express the capacity to differentiate into a number of lineages and also have immunomodulatory properties (189–191). hAECs also exhibit low immunogenicity, which, in combination with their antiinflammatory properties, has led to clinical use in burns, wound repair, and ophthalmalogic applications (181). Several recent papers have demonstrated the efficacy of purified amniotic epithelial cell populations or mixed populations of stromal and epithelial cells derived from digests of amnionic membranes in mouse and sheep models of endotoxin- or bleomycin-induced lung injury (190–195). Low-level engraftment and modulation of intrinsic inflammatory cells have been proposed as mechanisms (190–195). A recent report demonstrates that conditioned medium from amniotic fluid–derived MSCs can mitigate bleomycin-induced lung injury (196). Although administration of purified hAECs to bleomycin-injured immunocompetent mice resulted in abrogation of lung injury without any apparent host response to the cells (190), acute inflammatory responses were observed with the use of allogeneic (mouse) or xenogeneic (human) amniotic cells in bleomycin-injured mice (196). There are many other factors to consider for the use of amnion cells, including underlying medical conditions in the mother or a developing fetus and gestational age (183). Thus, although promising results have been obtained, further investigation is warranted into the mechanistic and potential translation applications of cells derived from the amnion and other placental tissues for use in lung repair and regeneration.
MSCs
MSCs were first described in 1968 as an adherent, clonogenic, nonphagocytic, and fibroblastic-like population of BM cells (reviewed in References 2–4, 197, 198). The nomenclature has changed over the years; MSCs were initially termed fibroblastic colony-forming units and subsequently as marrow stromal cells, mesenchymal stem cells, mesenchymal stromal cells, or multipotent mesenchymal stromal cells (197, 198). There is no consistency in the literature particularly with application of the more commonly currently used terms “mesenchymal stem cell” and “mesenchymal stromal cell.” In part, this depends on whether the MSCs are being used for their ability to differentiate into lineages potentially useful in regenerative medicine efforts and structural repair (differentiation into traditional osteoblast, chondrocyte, or adipocyte lineages or into other lineages including epithelial cells [199–205]) or for the immunomodulatory properties of the MSCs in the absence of structural engraftment (206–210). It is in this latter area that substantive progress continues to be made in understanding the mechanisms of MSC immunomodulatory actions and in increasing the possibility of using MSCs in treating lung diseases.
Definition and investigation of MSCs continues to be confounded by several issues. As detailed in previous conference reports and in a number of reviews, there can be significant differences in MSCs isolated from different species and from different strains within a given species, regardless of the actual tissue source of the MSCs, including properties such as cell surface epitopes, secretome, immunomodulatory actions, and genomic stability (2–4, 197, 211). Human MSCs derived from BM are the best characterized MSCs but continue to present some challenging features. Furthermore, although MSCs isolated from different tissue sources, including BM, adipose, cord blood, and placenta, generally express comparable cell surface markers and differentiate along recognized lineage pathways, differences in gene expression, lineage tendencies, and other properties have been described (197, 212). Investigations into the functional differences between MSCs isolated from different sources are an area of current intense investigation. It is not clear whether any one given source or origin of MSCs will prove superior for ameliorating specific diseases, including lung diseases. As with EPCs, many of the published studies have used different isolation and purification approaches. There is growing evidence that MSCs are heterogeneous and that different MSC subtypes exist (213). This is in part exemplified by observations that parallel preparations of MSCs from human BM aspirates isolated from the same normal donors in the same session can differ in features such as propagation rate and differentiation potential. This continues to complicate comparative assessments of published studies. The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy has defined minimal criteria for defining (human) MSCs (214). These criteria are being updated given the continued advances in understanding MSC biology with particular focus on developing potency assays applicable to clinical applications. A new framework for understanding and using the nomenclature, definition, and characterization of MSCs has been recently suggested (Table 3) (197).
Table 3.
Recent recommendations for investigation and use of mesenchymal stromal (stem) cells
| (1) The general population of mesenchymal stromal (stem) cells (MSCs) should continue to be identified as mesenchymal stromal cells, although this is not an ideal term. |
| (2) The term ‘‘mesenchymal stem cell’’ should be used to specifically describe a cell with documented self-renewal and differentiation characteristics. |
| (3) MSCs should be categorized as cultured or primary. This is an important distinction (see below) because the characteristics are likely to be different and should avoid confusion when comparisons are made between studies. |
| (4) The source of MSCs should be specified (e.g., adipose, bone marrow, cord blood, etc.); differences in cell characteristics are likely to be encountered. |
| (5) Species should be identified. This information is not always explicitly stated in the text of publications (except in the Methods section) and has led to confusion in the past. |
| (6) Minimum criteria for a surface marker profile need to be revisited and are likely to vary among species. |
| (7) The need to document the in vitro differentiation potential of the cells should be reexamined. |
| (8) The in vitro clonogenic capacity of MSCs should be enumerated. |
| (9) The reproducible representation of transcriptome, proteome, and secretome of MSCs should be evaluated, and the major factors influencing the signatures should be identified and specified. |
| (10) Consideration should be given to characterizing the cells according to tissue specificity (e.g., the differentiation potential of human umbilical cord perivascular cells is more extensive than for bone marrow MSCs). |
Adapted by permission from Reference 197.
It is becoming increasingly clear that the surrounding physical environment can profoundly affect MSC behavior. Culture variables, including culture surface composition and stiffness, mechanical forces, temperature, and culture density, can profoundly influence phenotype and behavior of MSCs (older articles reviewed in References 2–4; more recent articles reviewed in References 215–220). Stiffness and mechanical forces in particular are increasingly recognized as critical factors in directing MSC differentiation and other behaviors. The effect of the ambient oxygen environment has been recently further clarified, and it is becoming more apparent that hypoxic conditions may be preferable for culturing and manipulating MSCs (221–223).
To address some of the variations in properties of cultured MSCs, the National Center for Research Resources/National Institutes of Health have sponsored Center for Preparation and Distribution of Adult Stem Cells (MSCs) to serve as a preclinical resource for standardized preparations of mouse, rat, and human MSCs (http://medicine.tamhsc.edu/irm/msc-distribution.html). The National Heart, Lung and Blood Institute also sponsors the PACT (Production Assistance in Cellular Therapies) program, a training and good manufacturing practice resource that supports preclinical, investigational new drug preparation and clinical investigations with MSCs and other cell therapy (https://secure.emmes.com/pactweb/Facilities).
Endogenous lung MSCs
Cells with phenotypic characteristics of MSCs have been isolated from adult mouse lungs, from human nasal mucosa, and from lungs of human neonates and human lung transplant recipients (reviewed in References 2–4). The human lung MSCs (L-MSCs) share some similarities in gene expression and appear to have some immunomodulatory capabilities similar to those of BM-derived MSCs (224–226). The functional roles of L-MSCs are incompletely understood, but growing evidence suggests a role in fibrotic diseases and in the pathogenesis of bronchiolitis obliterans after lung transplantation (227–230). Ovalbumin sensitization and challenge increases the number of L-MSCs in mouse lungs, and L-MSCs may influence T-regulatory cell activity in bleomycin-induced lung fibrosis in mice (231). These observations suggest that lung MSCs may be involved in the regulation of local inflammatory immune responses. L-MSC homeostatic regulation and influences on migration, engraftment, and epithelial cell differentiation are also not well understood (232, 233). Other multipotent progenitor stromal cell populations have been described in lungs from other species, but the identity and role of these cells is not well understood (234).
Systemic MSC administration and the lung
A number of studies have demonstrated that, after systemic MSC administration, the cells initially localize in the lung vascular bed and that lung injury results in increased localization and/or retention of BM-derived cells in lung (reviewed in References 2–4). Whether this represents formation of cell emboli in the lung vasculature or specific adherence to pulmonary vascular adhesion or other molecules remains unclear. Furthermore, the source of the MSCs may influence retention in the lung. For example, MSCs derived from human umbilical cord blood are cleared more rapidly from the lungs than are human BM-derived MSCs (235). This reflects differences in the size of the MSCs from different sources and the differential expression of specific integrins and proteoglycan patterns. Retention in the lung may also trigger the MSCs to have functional effects. For example, embolization of systemically administered MSCs in lung was felt to result in secretion of an antiinflammatory protein, TSG-6 (236). Furthermore, although BM or adipose-derived MSCs can be induced in vitro to express phenotypic markers of alveolar or airway epithelial cells, engraftment with MSCs as lung epithelium, as with most other cell types investigated so far, is a rare event of uncertain physiologic significance in lung (199–204). However, some available data suggest that systemically administered MSCs can engraft as fibroblasts or myfibroblasts under certain injury conditions, further discussed below (237, 238).
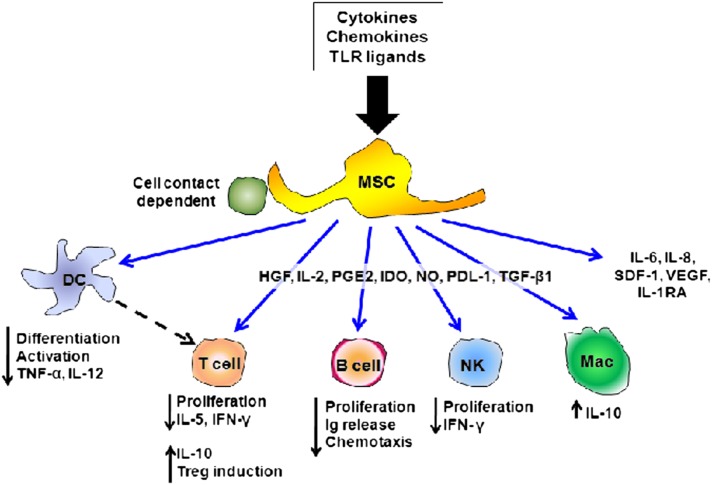
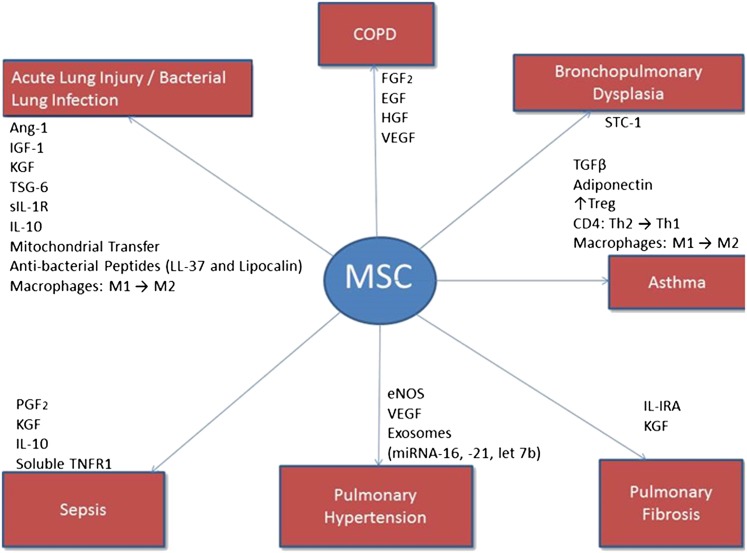
Mechanisms of MSC Immunomodulation of Lung Injury
Increasing focus is on the immunomodulatory and antiinflammatory properties of MSCs (Figure 4) (197, 206–210). MSCs can exist as pericytes lining vascular beds, where it is increasingly believed that they sample and accordingly modulate local environments to counter inflammatory or immune stimuli (209, 239). In BM, this serves to provide niches for hematopoietic stem cell differentiation. As such, MSCs can secrete multiple regulatory molecules such as growth factors and antiinflammatory cytokines, which modulate immune and inflammatory responses (reviewed in References 197, 206–210). MSCs also inhibit the proliferation and function of a broad range of immune cells, including T cells, B cells, natural killer cells, neutrophils, macrophages, and dendritic cells in in vitro model systems (reviewed in References 197, 206–210). MSCs inhibit T lymphocyte proliferation, activation, and cytokine release in response to alloantigens or to mitogenic stimuli through a dose-dependent direct suppressive effect on proliferation. MSCs also influence macrophage phenotype, in many cases promoting antiinflammatory M2 phenotype (240–242). The mechanisms of MSC actions on immune effector cell proliferation and function are only partly understood; direct cell–cell contact and release of soluble mediators have been proposed. MSCs can also act as antigen-presenting cells in in vitro model systems, a property influenced by exposure to IFN-γ and to TGF-β (243). A rapidly growing body of data suggests that release of episomal or microsomal particles by MSCs can influence behavior of the surrounding structural cells and the surrounding inflammatory cells (244–246). MSCs have also recently been demonstrated to form direct links with other cells, for example through connexin bridges, and to transfer mitochondria and likely other cytosolic components (247). Although the physiologic or repair role of these cell–cell links has not been elucidated, recent data demonstrate that this can result in replenishment of ATP-depleted recipient cells (247).
Figure 4.
Schematic illustrating the range of in vitro immune-modulating effects described for mesenchymal stromal (stem) cells (MSCs). DC = dendritic cell; HGF = hepatocyte growth factor; IDO = indoleamine 2,3-dioxygenase; IFN-γ = interferon γ; Ig = immunoglobulin; IL = interleukin; IL-1RA = interleukin-1 receptor antagonist; Mac = macrophage; NK = natural killer; PGE2 = prostaglandin E-2; SDF-1 = stem-cell–derived factor 1; TNF-α = tumor necrosis factor-α; TGF-β1 = transforming growth factor-β1; TLR = Toll-like receptor; VEGF = vascular endothelial growth factor. Adapted by permission from Reference 4.
It is also becoming increasingly clear that different inflammatory environments can profoundly influence MSC behavior (197, 206, 208, 247). This can result in significant changes in immunomodulatory properties and in potential immunogenicity of the MSCs with respect to lung diseases. For example, MSCs express a wide variety of chemokine and cytokine receptors, including those for TNF-α, IL-4, IL-17, and IFN-γ, cytokines increased in different lung injuries. Expression of MHC and costimulatory molecules can be altered by exposure to inflammatory mediators commonly found in vivo, such as IFN-γ and TGF-β (248). Chemotaxis and migration toward a wide variety of mediators, including stromal derived factor-1, TNF-α, macrophage migration inhibitory factor, TGF-β, hepatocyte growth factor (HGF), and others have been described (reviewed in References 2–4, 197, 206, 208). MSCs also express toll-like receptors, including the endotoxin receptor TLR4, the activation of which can produce a wide variety of effects, many of which are still being elucidated (197, 206, 208). Expression of TLRs by MSCs can be influenced by a number of factors, including exposure to bacterial toxins and to a variety of inflammatory mediators. Hypoxia, characteristic of many lung injuries and diseases, can also alter MSC expression of cell surface molecules and secretion of soluble mediators by MSCs (221–223). Overall, this rapidly growing body of evidence demonstrates that MSCs are malleable and can be significantly influenced by local inflammatory environments, including those found in lung injuries. This also offers a potential opportunity to manipulate MSCs ex vivo for optimizing potential clinical use. For example, conditioned media obtained from MSCs exposed ex vivo to hyperoxia was better able to ameliorate hyperoxia-induced lung injury in a rodent model, compared with conditioned media obtained from MSCs cultured in normoxia (249).
MSCs grown under routine tissue culture conditions exhibit low constitutive expression of major histocompatibility complex (MHC) class I and lack of constitutive expression of MHC class II and the costimulatory molecules CD80, CD86, and CD40 (reviewed in References 2–4, 197, 206, 208). In parallel, administration of non–HLA-matched allogeneic MSCs appears to be feasible and safe. However, the MSCs are likely influenced by the local inflammatory environment to express these or other cell surface epitopes that may trigger or be recognized by the host immune system. How this might affect MSC actions in vivo is not clear. Although the frequency of MSCs in the adult BM is low (< 0.1%), once isolated from BM or from other tissues, MSCs can be expanded ex vivo, which makes it possible to manufacture these cells for potential therapeutic purposes. Overall, these properties of MSCs make them an attractive potential therapeutic tool as vectors for delivery of disease-specific treatment substances or as immunomodulatory agents. However, careful consideration must be given to initial MSC heterogeneity, subsequent clonal heterogeneity, and the overall means of culture and expansion. Recent data suggest that freshly thawed cryopreserved MSCs may not be as effective as cells allowed to recover before administration (250). This may have significant ramifications for the design of clinical investigations using MSCs.
Development of Cell Therapy–based Approaches for Lung Diseases: Preclinical Models
A steadily increasing number of articles demonstrate the efficacy of systemic or intratracheal MSC administration in a growing spectrum of lung injury models in rodents and other animal models, in explanted human lungs, and in a slowly growing number of clinical investigations in lung diseases (reviewed in References 2–42, 51–258). This includes rodent and other animal models of ALI and bacterial lung infection, asthma, bronchiolitis obliterans, bronchopulmonary dysplasia, COPD, pulmonary ischemia reperfusion injury, obstructive sleep apnea, pulmonary fibrosis, pulmonary hypertension, radiation-induced lung injury, sepsis and burns, and other critical illness or autoimmune-related lung injuries including hemorrhagic shock, pancreatitis, silicosis, and ventilator-induced lung injury (articles published in 2011–2012 [259–297] are summarized in Table 4; older articles are summarized in the corresponding table in the previous conference report [4]). Systemically administered MSCs can also home to tumors through as yet unclear mechanisms and have been used for delivery of chemotherapeutic and other antitumor agents in mouse lung tumor models and may provide a viable therapy for lung cancers, particularly with MSCs engineered to express the tumor necrosis factor–related apoptosis inducing ligand or IFN-β (144, 145, 298–300). Administration of MSCs of BM or placental origin has also been demonstrated to decrease injury and inflammation in endotoxin or bacterially injured injured human lung explants (301–303). However, MSCs may not always ameliorate lung injury, and available preclinical data suggest that MSCs may contribute to lung fibrosis (237, 238). Although the mechanisms of the MSC effects are not completely understood, soluble mediators released by the MSCs appear to play important roles in the different models. Some of those implicated in the different model systems include angiopoietin 1, adiponectin, IL-1 receptor antagonist, IL-10, HGF, keratinocyte growth factor (KGF), and TGF-β (Figure 5). Transduction or transfection of the MSCs to overexpress secreted mediators, including angiopoietin-1 or KGF, or knockdown of TSG-6 using siRNA approaches further decreases endotoxin-mediated lung injury presumably through abrogation of endotoxin-mediated endothelial injury. Transduced MSCs overexpressing eNOS, IL-10, KGF, or a CCL2 inhibitor were found to be more effective in preventing monocrotaline-induced pulmonary hypertension, ischemia-reperfusion–induced lung injury, or bleomycin-induced pulmonary inflammation and subsequent fibrosis, respectively (2–4, 285, 286). MSCs appear also to act in part by decreasing the increased endothelial permeability found in ALI by secreting antibacterial peptides, by promoting an antiinflammatory M2 phenotype in alveolar macrophages, or by increasing monocyte phagocytic activity (255, 304–306). Mitochondrial transfer from MSCs to ATII cells has been demonstrated to replenish endotoxin-depleted ATP stores and to restore surfactant secretion (247). A growing number of reports suggest that administration of conditioned media obtained from MSCs may mimic many of the ameliorating effects resulting from MSC administration in different lung injury models (264, 272, 274, 278, 282, 290, 291). In part, this may reflect actions of exosomes or microsomal particles released by the MSCs (244–247, 291). However, the specific mediators, soluble protein, and exosome ahave not been clarified and are likely to be different for each lung injury model (Figure 5). A recent report suggests that MSCs may also promote repair through activation of endogenous distal lung airway progenitor cell populations in mouse models (277).
Table 4.
Preclinical studies of mesenchymal stromal (stem) cells in lung disease models
| Injury Model | Experimental Model, Route, and Timing of Treatment | MSC Source | MSCs Modified? | Syn Allo or Xeno | Outcome Compared with Injury Effects | Potential Mechanisms of MSC actions | Cell Controls? |
|---|---|---|---|---|---|---|---|
| Danchuk, 2011 (259) | Mouse: OP (IT) LPS | Mouse BM MSCs Texas (Tulane) MSC Core | siTSG-6 knockdown | Syn | • Dec histo injury 48 h | TSG-6 released by MSCs | IMR 90 lung fibroblasts |
| OP MSCs 4 h after LPS | • Dec lung edema 24/48 h | • Do not mimic effects and increase BAL total cell counts and PMNs | |||||
| P 1–2 | • Dec BAL protein 24 h | MSCs treated with siTSG-6 lose most effects | |||||
| 2.5 × 105 cells/mouse | • Dec in multiple inflamm cyto and chemokines at 24 h | ||||||
| Kim, 2011 (260) | Mouse: IT E. coli | Human umbilical cord MSCs | No | Xeno | • Inc survival | Nonspecified paracrine effects | Human fibroblasts MRC-5 |
| IT MSCs 3 h after E. coli | +CD73, v105; −CD 14, 34, 45; +HLA AB; −HLA DR | • Dec histo injury and lung edema Day 3 | • Do not mimic effects on survival, edema, cytokines, MPO and increase histologic inflammation | ||||
| P 5 | osteo/adipo/chrondo | • Dec BAL protein MPO Day 3 | |||||
| 105 cells/mouse | • Dec IL-1α, IL-1β, IL-6, TNF-α, MIP-2 in lung homogenates Bays 3 and 7 | ||||||
| • Dec bacterial CFU in BAL/blood Days 3 and 7 | |||||||
| Sun, 2011 (261) | Mouse: IT LPS | Primary human umbilical cord MSCs | No | Xeno | • Dec mortality | Nonspecified soluble mediators | Apoptotic MSCs (mitomycinC treated) |
| IT MSCs 4 h after LPS | +CD 29, 44, 73; −CD 34, 45, HLAII | • Dec histo injury (3 d) | • Did not mimic MSC results | ||||
| P 5–6 | osteo/adipo | • Dec BAL TNF-α, MIP-2, IFNγ (3 d) | |||||
| 106 cells/mouse | • Inc BAL IL-10 (3 d) | ||||||
| • Dec BAL Th1 CD4 T cells | |||||||
| • Inc BAL CD4/CD25/Foxp3 Tregs | |||||||
| Gupta, 2012 (262) | Mouse: IT E. coli | Mouse BM MSCs (Tulane) | No | Syn | • Inc survival 48 h | TNF-α released from alveolar macrophages stimulates lipocalin release from MSCs | 3T3 fibroblasts |
| IT MSCs 4 h after E. coli | • Dec histo injury 48 h | • Did not mimic any results | |||||
| P 5–10 | • Inc bacterial clearance | ||||||
| 7.5 × 105 cells/mouse | • Dec BAL MPO, TNF-α, MIP-2 at 8 h | ||||||
| • No change in BAL IL-10 | |||||||
| • No change in BAL defensins, collectins, SPD | |||||||
| Ionescu, 2012 (264) | Mouse: IT LPS | Mouse BM MSCs | No | Syn | • Dec BAL total cells, PMN | Conditioned media mimicked MSC effects | Primary lung fibroblasts or fibroblasgts conditioned media |
| MSCs or CM 4 h after LPS | +Sca1, CD29, 105; −CD11b, 154, 31; −CD 34,44,45; −ckit; −Flk1 | • Dec lung edema | Secretome analyses and neutralizing antibodies suggest IGF1 as a responsible mediator | • Did not mimic effects | |||
| P 2–8 | Osteo/adipo | • Dec histologic injury | |||||
| • Inc M2 phenotype | |||||||
| Islam, 2012 (247) | Mouse: IT LPS | Mouse BM MSCs | 1) MSCs from Cx43 mutant mice | Syn | • MSCs localize in alveolar spaces | Mitochondrial transfer from MSCs to type 2 cells | MSC from Connexin 43 mutants and those treated with siRISP |
| MSCs 4 h after LPS | 2) MSCs treatedwith SiRISP | • Form connexin 43 bridges to type 2 cells | • Did not mimic results 3T3 fibroblasts: down-regulated Cx43 expression | ||||
| P 2 | • Increased lung (type 2) ATP | ||||||
| 106 cells/mouse | • Improved surfactant production | ||||||
| • Dec histo injury, albumin leak, BAL inflamm cells | |||||||
| Krasnodembskaya, 2012 (292) | Mouse IT E. coli | Human BM MSCs | No | Xeno | • Dec BAL total cells, PMN, protein, MIP2 | LL37 release by MSCs | Human lung fibroblasts |
| IT MSCs 4 h after LPS | Texas (Tulane) MSC | • Dec bacterial growth in lung homogenates | Effects lost with use of neutralizing anti-LL37 antibody No release of hBD2 or 3, lipocalin, SPD | • Did not mimic effects | |||
| P 5 | Core | • Inc antibacterial activity of BAL fluid | |||||
| 10 × 106 cells/mouse | • Prestimulated MSC conditioned media decreased bacterial growth in in vitro culture | ||||||
| Nazarov, 2012 (302) | Explant human lung IT | Human BM MSCs | No | Allo | • Normalized alveolar fluid clearance | siRNAs to HGF, KGF, or FGF-β; each partly inhibited MSCs effects | Normal human lung fibroblasts did not mimic effects |
| LPS IT MSCs 1 h after LPS | Primary hCMSCs +CD73, 105, 117; +SSEA 3/4; −CD45,133,HLA-DR; +osteo/adipo | • Dec BAL fluid PMN, IL-1β, IL-8 | |||||
| P < 10 | • hCMSCs equivalent to BM-MSCs | ||||||
| 5 × 106 cells | • Partial restoration of fluid transport and permeability in primate AT2 monolayers | ||||||
| • cMSCs more effective than BM-MSCs | |||||||
| Lee, 2013 (303) | Explant human lung IT E. coli | Human BM MSCs Texas (Tulane) MSC Core | No | Allo | MSCs 1 h after E. coli | KGF mimicked effects | Normal human lung fibroblasts did not mimic effects |
| IT or IV MSCs or 1 h after LPS | • Improved fluid clearance | ||||||
| P < 10 | • Dec PMN, IL-1β, IL-8 | ||||||
| 5–10 × 106 cells | • Improved histologic injury | ||||||
| • Inc bacterial killing | |||||||
| • Dec bacteremia | |||||||
| • Labeled MSCs migrated to alveolar spaces in injured lungs | |||||||
| • IV or IT MSCs had equiv effects | |||||||
| • Inc macrophage bacterial phagocytosis | |||||||
| • No effect on macrophage phenotype | |||||||
| MSCs 2 h after higher E. coli dose | |||||||
| • Improved fluid clearance | |||||||
| • Dec PMN | |||||||
| • Dec bacteremeia | |||||||
| • Additive effects with antibiotic | |||||||
| Zhang, 2013 (265) | Mouse: IT LPS IT MSCs | Mouse or human adipose MSCs −CD11b, 31, 45; +CD29,106 +osteo/adipo | No | Syn and Xeno | 24 or 72 h after LPS Improved body weight | Soluble mediators | No |
| 4 h after LPS | Dec BAL fluid protein and albumin | ||||||
| P 3 | Dec BAL fluid total cells, PMNs, MPO activity | ||||||
| 7.5 × 105 cells/mouse | Dec histo injury | ||||||
| Dec mRNA (lung homogenates: IL-1α, IL-1β, MIP-1α, MIP-2, TNF-α | |||||||
| No change in mRNA for IL-10 | |||||||
| Dec lung homogenate IL-1β protein and inc in IL-10 (mMSC only) | |||||||
| In general mMSC more effective than hMSCs | |||||||
| Asthma | |||||||
| Firinci, 2011 (266) | Mouse: ovalbumin sensitization Days 0, 14, 21 | Mouse BM-adherent cells; +CD73, 105; −CD45; no diff info provided | No | Syn | • No engraftment | None (paracrine) | No |
| Challenge: ova aerosol 3 d/wk for 8 wk MSCs IV Day 75 | • Some dec in histo injury | ||||||
| P 2 | |||||||
| 1 × 106 cells/mouse | |||||||
| Harvest Day 82 (engraftment?) and Day 89 (lung assessment) | • Dec serum NO | ||||||
| Kapoor, 2011 (268) | In vitro study | Human BM Healthy donors P4–5 +CD29, 44, 105 -CD14, 45- | No | Allo | • Dec PMBC prolif (3[H]-Thymidine) | None (paracinre and cell–cell contact) | No |
| Peripheral blood monocyte cells from 7 patients with allergic asthma and four healthy control subjects | • Dec IFN-γ | ||||||
| Four dust mite sensitive/nonasthmatic | • Inc IL-10 | ||||||
| • Inc PMBC prolif in response to tetanus toxoid | |||||||
| • No induction of TReg cells | |||||||
| • Dec DM induced DC maturation | |||||||
| Precondition | |||||||
| • Dec response to dust mite antigen | |||||||
| • Required cell–cell contact | |||||||
| • Dec response to repeat challenge | |||||||
| Kavanaugh and Mahon, 2011 (269) | Mouse: ovalbumin-induced acute allergic airways inflammation; ovalbumin sensitization Days 0, 7, 14 MSCs IV Days 7/14 | Mouse (FVB) BM; +Sca1; low CD44, 106; −CD 11b, 11c, 34,35,117; osteo/adipo/Chondro | Allo | • Dec histo injury | None (paracrine) | PFA-fixed MSCs | |
| P 4–9 | • Dec BAL total cells, Eos, Macs | • No effect on histo, BAL except BAL IL-13 | |||||
| 5 × 106 cells/infusion | • Dec BAL IL-4, IL-13 | • Inc splenocyte IL-4, dec splenocyte IL-10, IL-13 | |||||
| ChallengeDays 25–27; harvest Day 28 | • Inc BAL IL-10 | ||||||
| • Dec splenocyte IL-4 | |||||||
| • Inc splenocyte IL-13, IL-10 | |||||||
| Lee, 2011 (270) | Mouse toluene disocyanate inhalation induced acute airways inflammation MSCs IV Day | Rat BM MSCs and adherent cells CD44+, CD45+ | No | Xeno | • Dec BAL total cells | None | No |
| P 8 | • Dec BAL macrophage, PMN, Eos | ||||||
| 105 cells/mouse | • Dec histologic injury | ||||||
| • Dec goblet cells | |||||||
| • Dec peribronchial collagen and SMA deposition | |||||||
| • Dec epithelial proliferation | |||||||
| • Dec airway hyper responsiveness (Penh) | |||||||
| Ou-Yang, 2011 (271) | Mouse: ovalbumin-induced acute allergic airways inflammation | Mouse BM; +CD90, Sca1; −CD44, 45 | AMD3100-treated MSCs | Syn | • Dec histo injury | None (paracrine) | AMD3100-treated MSCs retained less in lung |
| Ovalbumin sensitization Days 1, 8 | • Improved lung mechanics | ||||||
| MSCs IV Day 12 | • Dec BAL total cells, Eos | ||||||
| P 2–-4; | • Dec BAL fluid b-hexosaminidase | ||||||
| 2 × 106 cells/mouse | • Dec BAL IL-4, IL-5, IL-9 | ||||||
| • Inc BAL IFN-γ | |||||||
| • Lung homogenate: dec IL-4, inc IFN-γ | |||||||
| Ionescu, 2012 (272) | Mouse ovalbumin; acute model sensitization Days 1, 6; challenge Days 11, 13; harvest Day 15 | Mouse BM MSCs CD45+ vs. CD45− | Adiponectin KO MSCs | Syn | • Dec histo injury: acute and chronic | Adiponectin release by MSCs | Primary mouse lung fibroblasts |
| Chronic model sensitization Days 1, 8, 15, 29; first challenge Days 22, 24, 27; second challenge Days 31, 33, 36; harvest Day 38 | WT MSCs with neutralizing adiponectin Ab | • Dec BAL total cells, Eos, PMN, Macs: acute and chronic | • CM mimics decrease in BAL total cells and PMNs | ||||
| MSC-conditioned medium on all challenge days | • Dec airways resistance and elastance: acute dec elastance: chronic | • Does not mimic other effects | |||||
| P 2 | • Restored salbutamol responsiveness: chronic | APN KO MSCs and si knockdown of APN in wildtype MSCs lose MSC effects | |||||
| • Dec BAL IL-4, IL-13; inc IL-10: acute and chronic | |||||||
| • Promote alveolar mac M2 phenotype | |||||||
| • Inc Tregs in lung | |||||||
| Bronchopulmonary dysplasia | |||||||
| Chang, 2011 (273) | Neonatal rats: hyperoxia (95% FiO2) × 14 d | Human umbilical cord MSCs from a single donor | No | Xeno | Day 14 | Paracrine | No |
| IT MSCs postnatal Day 5 | • Inc survival | ||||||
| P 5 | • Dec alveolar injury | ||||||
| 103, 104, or 105 cells/rat | • Dec apoptosis | ||||||
| • Dec myeloperoxidase | |||||||
| • Dec mRNA TNF-α, IL-1β, IL-6, TGF-β | |||||||
| • Dose-dependent effect | |||||||
| Piero, 2012 (274) | Neonatal rats: hyperoxia (95% O2) post notes Day 4–14 | Human cord blood MSCs | No | Xeno | Prevention | Paracrine | Primary neonatal rat dermal fibroblast |
| IT MSCs or Conditioned media Day 4 or Day 14 | Human cord blood peri vascular cells | • MSC Day 4/lung harvest Day 22 | |||||
| P 3 | Conditioned media | • Partial preservation of alveolar growth | |||||
| 6 × 105 cells/rat | • Prevent decrease in lung compliance | ||||||
| Regeneration | |||||||
| • MSC Day 14/lung harvest Day 35 | |||||||
| • Restore normal alveolar architecture | |||||||
| Long term | |||||||
| • MSC Day 4/lung harvest 6 mo | |||||||
| • No obvious lesions on CT scans | |||||||
| • Improved exercise capacity | |||||||
| Conditioned media | |||||||
| • Daily, Day 4–Day 21 | |||||||
| • Improved alveolar architecture | |||||||
| • Improved lung function | |||||||
| • Attenuated decrease in pulm vasculature | |||||||
| • Prevented arterial wall remodeling | |||||||
| • Dec RV hypertrophy | |||||||
| Tropea, 2012 (277) | Neonatal mice: hyperoxia × 14 d | No | Syn | • Inc # of BASCs | Stimulation of endothelial activation? | Pulmonary artery smooth muscle cell and CM did not mimic effects | |
| IV MSCs on Day 4 | • Inc growth and differentiation of BASC | Effects mimicked by MSC conditioned medium | |||||
| 5 × 104 cells | |||||||
| Zhang H, 2012 (275) | Neonatal rat: hyperoxia × 7 d | Rat BM: +CD29, 44, 90; −CD11b, 34, 45; adipo/osteo | Lentiviral transduced to express GFP | Syn | Reversed weight loss | Paracrine | No |
| IV MSCs Day 7;harvest Days 1, 3, 7, 14 | Dec histologic injury | ||||||
| P | Dec TNF-α,TGF-β in lung homogenate | ||||||
| 105 cells/rat | Inc IL-10 in lung homogenate | ||||||
| Zhang X, 2012 (276) | Neonatal mice: hyperoxia × 45 d | Mouse BM: +CD105, 106; −CD34, 45; adipo/osteo | No | Syn | Inc survival | Paracrine | No |
| IP MSCs on Day 7; harvest on Day 45 | Dec histo injury | ||||||
| P | Dec TGF-β, Col-1α, TIMP-1 in lung homogenates | ||||||
| 105 cells/mouse | Dec serum IL-1, TNF-α | ||||||
| Wascak, 2012 (249) | Neonatal rats: Hyperoxia (95%) × 14 d | BM MSCs Adipo/Chond/Osteo | MSCs exposed to hyperoxia (95%) for 24 h | Syn | MSC-O2CM > MSC-CM | Soluble mediators STC-1 | Primary rat lung fibroblasts Did not mimic effects |
| IV MSC conditioned media for 21 d starting on Day 0 | Dec pulm HTN, RV hypertrophy | ||||||
| P | Dec histologic injury | ||||||
| Cells/rat | Preconditioning of MSCs | ||||||
| No change in antioxidant capacity | |||||||
| Inc STC-1 expression | |||||||
| No change in HGF, KGF, VEGF, IGF expression | |||||||
| Sustsko, 2013 (278) | Neonatal rat: hyperoxia (90%) Day 2–16 | BM MSCs from normal and GFP rats | No | Allo | D16 and D30 assessments | Paracrine | No |
| MSCs or conditioned media (CM) on D9 | Improved pulmonary hemodynamics | ||||||
| P | Improved oxygenation | ||||||
| 2 × 106 cells/rat | Improved lung function (PenH) | ||||||
| Dec histo injury/improved alveolarization | |||||||
| Inc capillary density | |||||||
| Dec mRNA for IL-1β, IL-6 | |||||||
| Inc mRNA for TTF-1 | |||||||
| MSC = CM | |||||||
| D100 assessment | |||||||
| Improved histo injury (MSC > CM) | |||||||
| Inc capillary density (MSC = CM) | |||||||
| Improved RVSP (MSC only) | |||||||
| No effect on RVH | |||||||
| COPD | |||||||
| Hoffman, 2011 (279) | Mouse: IT elastase | Mouse BM (Tulane) | No | Syn | • Higher retention of L-MSCs in lung | Nonpostulated | No |
| IV MSCs 6–7 wk after elastase | Lung MSCs from primary explant cultures | • Dec histo injury (MLI) at 22–28 d | |||||
| P 7 | Flow characterization? | ||||||
| 106 cells/mouse | Adipo/chondro/osteo | ||||||
| Huh, 2011 (417) | Rat: 6-mo cigarette smoke exposure | Total BMCs: freshly isolated and filtered | Male cells into female recipients | Syn | • 2 mo after BMCs | Soluble mediators | CM normal human lung fibroblasts and human pulmonary artery smooth muscle cells did not inc proliferation of the HPAECs |
| BM cells, MSCs, or conditioned media | Rat BM MSCs +CD73, 90; −CD34, 45 | • Improvement evident after 1 wk | |||||
| P 4 MSCs | +adipo/osteo | • Dec histo injury | |||||
| 6 × 106 BMCs vs. 6 × 105 MSCs/rat | CM from P3–5 MSCs | • Enhanced prolif and dec apoptosis (AT2 and vasc endo cells) | |||||
| • Inc aKT phosphorylation | |||||||
| • Inc KGF | |||||||
| • Inc number of small blood vessels | |||||||
| • Dec pulm HTN | |||||||
| • MSCs and MSC CM had similar effects on improving lung histo and number of small arteries | |||||||
| • MSCs but not MSC-CM improved pulm HTN | |||||||
| • MSC CM inc in vitro proliferation of human pulmonary artery endothelial cells | |||||||
| • No significant engraftment of either BMC or MSC | |||||||
| Schweitzer, 2011 (281) | Mouse: cigarette smoke exposure for 24 wk | Human ASCs Mouse BM MSCs | No | Syn and xeno | • Minimal cell retention in lung dec over time with CS exposure | Paracrine effects | No |
| NOD-SCID mouse (for xenogeneic) human ASCs 3 d after VEGFR blockade | Characterization? | • Dec histo injury (MLI, alveolar volume) and caspase activation | |||||
| MSCs IV ever other week during last 2 moof CS exposure | • Dec weight loss, inc subcutaneous fat | ||||||
| P < 3 | • Dec BAL mac/PMN | ||||||
| 5 × 105 cell/infusion | • Dec lung homogenate caspase 3, p38 MAPK phos, JNK, AKT | ||||||
| • Dec CS induced BM suppression | |||||||
| • Dec VEFGR blockade-induced air space enlargement | |||||||
| • Human ASCs decreased histologic injury and caspase 3 activation after VEGFR blockade | |||||||
| • Emboli if used > 5 × 105 MSCs or if > passage 3 | |||||||
| • hASC CM inc wound repair in cultured lung endothelial cells dec with water soluble CS extract | |||||||
| Ingenito, 2012 (234) | Sheep: IT elastase 5 doses over 20 wk | Sheep lung-derived MSCs; | No | Syn | • Inc lung tissue mass and perfusion | Paracrine effects Inc epithelial proliferation in in vitro coculture expts | No |
| L-MSCs in fibinogen/fibrin/poly-l-lysine scaffolds | +S100A4 −fibrillin 1; CD45, α-SMA, col I | • Inc histo cellularity, cell retention, ECM | |||||
| P 5 | Adipo/chrondo/osteo | • Improved lung mechanics | |||||
| 5–10 × 106 cells/scaff | |||||||
| Kim, 2012 (282) | Mouse: lung fibroblasts; in vitro CSE exposure | Rat BM Plastic adherent | Yes (ISCT) | Xeno Syn | • In vitro CSE exposure | FGF2 release by MSCs | Conditioned media from RF2-6 cells, HFASMCS, NHLFs |
| Rats: in vivo cigarette smoke 8-wk rat, 5 d/wk, 6 mo | CM P3–5 from 90% confluent cells | • Dec in CSE induced capsase 3, p53, p21, p27, Akt, p-Akt expression | • Did not mimic effects | ||||
| IV MSC or CM 2× wk IV for 5 wk starting at Week 8; killed 3 wk later | • Inc in CSE-induced dec ECM expression and collagen gel contraction | ||||||
| P 3–5 | • Dec in CSE-induced COX-2 and PGE synthase 2 expression | ||||||
| • PI3K inhibitor partially reversed the effects | |||||||
| • In vivo CS exposure restored fibroblast proliferation, inc Akt | |||||||
| Katsha, 2011 (280) | Mouse: IT elastase | Mouse BM MSCs; +CD 73, 90, 105; −CD 11b, 45; adipo/osteo | si EGF–treated MSCs | Syn | • Dec histo injury at Days 7,14,21 after MSCs | EGF production by MSCs induces SLPI in cultured MLE-12 cells | BLKCL4 lung fibroblasts |
| IT MSCs 14 d after injury | • Dec BAL IL-1β at Days 3 and 5 after MSCs | • Do not mimic effects | |||||
| 5 × 105 cells/mouse | • Dec IL-1β mRNA in lung homgenates at Days 1,3,5,7 after MSCs | ||||||
| P 5 | • Inc EGF, HGF, and SLPI mRNA at various time points after MSCs | ||||||
| Pulmonary fibrosis | |||||||
| Saito, 2011 (284) | Mouse: IT Bleo IV MSCs 24 h after bleo | Mouse BM | Lentiviral transduction to express CCL2 inhibitor (7ND) | Syn | • Improved survival | Soluble mediator activating CCL2 pathways | No |
| P 5–15 | • Dec histologic injury | ||||||
| 5 × 105 cells/mouse | • Dec lung collagen | ||||||
| • Dec BAL inflammatory cells | |||||||
| • Dec BAL IL-1β, IL-6 | |||||||
| • 7ND-expressing MSCs more effective than MSCs | |||||||
| Lim, 2012 (285) | Mouse: C56Bl/6, SCID IN bleo | Human BM MSCs (Tulane) | No | Xeno | SCID mice | None specified | No |
| MSCs IV 24 h after bleo | • Inc body weight | ||||||
| P 3 | • Inc tidal volume/lung mechanics | ||||||
| 1 × 106 cells/mouse | • Dec histo injury | ||||||
| • Dec lung collagen (hydroxyproline) | |||||||
| • Dec MIP-1α mRNA | |||||||
| • Inc TGF-β mRNA | |||||||
| • Dec CD45+ cells by histo | |||||||
| C57/BL/6 mice | |||||||
| • No effect on histo/lung function/collagen/CD45+ cells | |||||||
| • Dec MIFMIP-1α, TGF-β, TNF-α mRNA | |||||||
| Ischemia-reperfusion Injury | |||||||
| Sun, 2011 (283) | Rats: 30 min clamp of left mainstem bronchus and PA | Rat adipose adherent cells cultured for 14 d. | No | Syn | • Imp oxygenation 72 h | None specified (paracrine effect) | No cell controls |
| IV MSCs at 1,6, and 24 h | Likely a heterogenous mix containing MSCs and EPCs | • Dec histo injury 72 h | |||||
| P | +CD 29, 31, 34, 90, 271 others as listed in paper | • Lung homogenates PCR | |||||
| 1.5 × 106 cells/infusion | • Dec IL-1β, TNF-α, MMP-9, Bax, caspase 3, endothelin. Inc eNOS, IL-10, adiponection, heme oxygenase, glutathione reductase, gluthione peroxidase, BCL-2 | ||||||
| • Lung homogenate westerns: dec VCAM-1, ICAM-1, TNF-α, NK-kB, cytosolic cytochrome C, Cx43; Inc HO, mitochondrial cytochrome C | |||||||
| Pulmonary hypertension | |||||||
| Jungebluth, 2011 (288) | Rat: left pulmonary artery ligation | Rat BM | No | Syn | • 12 wk | Paracrine | No |
| IT MSCs immediately after ligation | • Improved pulmonary hemodynamics | ||||||
| P? | • Dec RVH | ||||||
| 6 × 106 cells/rat | • Improved oxygenation | ||||||
| • Improved lung mechanics (penH) | |||||||
| • Improved exercise capacity | |||||||
| • Decreased histo injury | |||||||
| • Dec liver damage | |||||||
| Liang, 2011 (289) | Mouse: chronic hypoxia 8–10% | Mouse: BM WT mice KO mice | No | Syn | • WT | Lung fibroblasts | |
| MSCs IV 5 wk to 2 wk | Heme oxygenase | • HO−/− | • Did nott mimic MSC effects | ||||
| P | SPC-HO mouse | • SPC HO reverse established disease | |||||
| Cells/mouse | Dox-inducible lung Ho-1 | Nl RV pressure | |||||
| Dec RV hypotrophy | |||||||
| Prevented RV infarction | |||||||
| • Dec hypoxic induced inc in MCP-1, IL-6 | |||||||
| • Dec hypoxic induced dec in IL-IR-N | |||||||
| • CM led to dec PASMC proliferation | |||||||
| Hansmann, 2012 (290) | Mice: hyperoxia × 2 wk | Mouse BM +CD73, 90, 105; +ckit, Sca-1; −CD11b, 14, 19, 31, 34; −CD45, 79α | No | Syn | • 4 wk | Soluble mediators | Primary mouse lung fibroblasts or fibroblasts conditioned medium |
| MSCs or CM IV at 2 wk followed by 2–4 wk normoxia | Dec alveolar injury, vascular remodeling | • Did not mimic MSC or MSC-CM effects | |||||
| P | Inc number pulm blood vessels | ||||||
| Cells/mouse | • 6 wk | ||||||
| Normalized lung function | |||||||
| Reversed pulm HTN, RV hypertrophy | |||||||
| Dec peripheral artery muscularization | |||||||
| Lee, 2012 (291) | Neonatal mice: Hypoxia-induced pulm HTN (8.5% O2 × 48 h) | Mouse BM Human cord blood (Wharton’s jelly) | No | Syn | • Mouse MSC CM or exosomes | miRNA-16, -21, let7b transfer via exosomes | Mouse primary dermal fibroblasts |
| MSCs, CM, or exosomes before hypoxia | Dec BAL macrophage, MCP-1, HIMF, FIZZ | • CM or exosomes partly decreased injuury | |||||
| P | • Mex-depleted media | ||||||
| Cells/mouse | Didnot mimic effects | ||||||
| • Two doses (before hypoxia and at 4 d) | |||||||
| Partial reduction in pulm HTN, RV hypertrophy, lung vascular remodeling | |||||||
| • Single high dose | |||||||
| More potent effect | |||||||
| Dec stat 3, miR-17 | |||||||
| • Human CB MSCs | |||||||
| Dec stat 3 in pulmonary artery endothelial cells | |||||||
| Sepsis | |||||||
| Krasnodembskaya, 2012 (292) | Mouse: IP P. aeruginosa | Human BM Texas (Tulane) MSC Core | CD13−, CD45− | Xeno | • Inc survival | 3T3 fibroblasts | |
| IV MSCs 18r after P. aeruginosa | • Dec blood CFU PA | • Did not replicate results | |||||
| P 3–10 | • No change in blood TNF-α, MIP-2, IL-10 | ||||||
| 106 cells/mouse | • Inc blood monocyte phagocytic activity | ||||||
| • Inc C5a | |||||||
| • Inc spleen M2 (CD163+, CD206+) | |||||||
| • Inc blood PAI-1 | |||||||
| • Inc platelets | |||||||
| Other critical illness or autoimmune-induced lung diseases: hemorrhagic shock, lupus, silicosis, ventilator-induced lung injury | |||||||
| Curley, 2011 (293) | Rat: injurious ventilation, insp pressure 35 cm, resp rate 18, Peep 0 goal: > 50% dec in static compliance | Primary rate BM MSCs | No | Syn | • Improved oxygenation at 48 h | Soluble mediators as conditioned media mimicked all effects | • Primary rat dermis fibroblasts |
| MSCs IV immediately after injury and then again at 24 h | • Improved lung compliance at 48 h | Parallel in vitro studies with human BM MSC CM CM improved A549 wound healing | • Neither fibroblasts or fibroblast CM mimicked effects in vivo | ||||
| P | • Dec edema at 48 h | Anti-KGF but not anti-HGFb neutralizing abs abrogated effect | |||||
| 2 × 106 cells/rat | • Dec histo injury at 48 h | ||||||
| • Dec BAL protein total cells, PMNs at 48 h | |||||||
| • Dec BAL TNF-α, inc BAL IL-10 at 48 h | |||||||
| Pati, 2011 (294) | Rats: controlled bleed (hemorrhagic shock) | Lonza primary human MSCs (BM?); +CD44, 105; −CD31 | No | Xeno | • Dec histo injury 4 d | MSCs and MSC CM inhibits VEGFA-induced perm in cultured human pulm endothelial cells | No cell controls |
| MSCs with fluid resuscitation IV 1 and 24 h after bleed | • Dec lung edema 4 d | Restores β-catenin and VE-cadherin expression MSCs but not MSC | |||||
| P 1 | • Preserved lung VE chadherin, claudin 1, occludin, and PDFG-β at 4 d | CM inhibit inflamm cell adherence to PECs Dec TNF-α stimulated inc in PEC VCAM-1 and ICAM-1 expression | |||||
| 2 × 106 cells/dose/rat | • No effect on MAP | Cell–cell contact required | |||||
| • Dec serum TNF-α, MCP-1, MIP-1a at 2 h | |||||||
| • Inc serum IL-10 at 2 h | |||||||
| • No change in lung or serum cytokines at 96 h | |||||||
| Chimenti, 2012 (295) | Rat (Sprague-Dawley): injurious ventilation, 80 breaths/min at 25 ml/kg Vt | Lewis Rat BM MSCs Texas (Tulane) MSC core | No | Allo | • 3-h analyses | Soluble mediators | No |
| IV or IT MSCs 30 min before injurious ventilation | • Dec histo injury | ||||||
| P 5 | • Improved fluid clearance | ||||||
| 106 cells/rat | • Dec BALF total protein, PMN, IL-1β, MIP-2 | ||||||
| • Dec VCAM1 expression on histo sections | |||||||
| • Inc IL-1-RA mRNA in lung homogenates | |||||||
| • No effect on HGF or KGF mRNA | |||||||
| • IT and IV MSCs produce comparable results | |||||||
| Wang, 2012 (297) | Rat: sodium taurocholate-induced acute pancreatitis related ALI | Sprague-Dawly rat BM MSCs | No | Syn | • Dec pulmonary edema | Nonspecified (soluble mediators) | No cell controls |
| • Dec histologic lung inflammation | |||||||
| P 3 | • Dec lung MPO activity | ||||||
| 106 cells/rat | • Dec TNF-α and substance P mRNA in lung homogenates |
Definition of abbreviations: ASC = adipose-derived mesenchymal stromal cell; ALI = acute lung injury; Ang 1 = angiopoietin 1; BAL = bronchoalveolar lavage; BALF = bronchoalveolar lavage fluid; BASC = bronchoalveolar stem cells; BM = bone marrow; CFU = colony forming units; CM = conditioned media; Dec = decreased; GFP = green fluorescent protein; hASC = human amniotic fluid–derived stem cell; hCMSC = human chorionic stromal cell; HGF = hepatocyte growth factor; histo = histological; HO = heme oxygenase; HTN = hypertension; IM = intramuscular; IN = intranasal; Inc = increaesd; iNOS = inducible nitric oxide synthase; IT = intratracheal; IV = intravenous; KGF = keratinocyte growth factor; KO = knockout; LPS = lipopolysaccharide; MSC = mesenchymal stromal (stem) cell; MIF = migration inhibitory factor; MPO = myeloperoxidase; NOD = nonobese diabetic; OP = oropharyngeal; P = passage; PA = pulmonary artery; PLF = pleural lavage fluid; PMBC = peripheral blood mononuclear cell; Pulm = pulmonary; RV = right ventricular; SPC = surfactant protein C; SSEA = stage-specific embryonic antigen 1; TNF-α = tumor necrosis factor-α; VEGFA = vascular endothelial growth factor A; WT = wild type.
Figure 5.
Schematic of known mesenchymal stromal (stem) cell (MSC) actions in different preclinical lung injury models. Ang-1 = angiopoietin 1; EGF = epidermal growth factor; FGF2 = fibroblast growth factor 2; HGF = hepatocyte growth factor; IGF-1 = insulin-like growth factor 1; IL-10 = interleukin 10; KGF = keratinocyte growth factor; PGE2 = prostaglandin E2; sIL-1R = soluble interleukin 1 receptor antagonist; STC-1 = stanniocalcin 1; Treg = T regulatory cell; TSG-6 = tumor necrosis factor–inducible gene 6 protein; TNF-α = tumor necrosis factor-α; TGF-β1 = transforming growth factor-β1; VEGF = vascular endothelial growth factor. Adapted by permission from Reference 416.
MSCs also exert effects on lung inflammation and injury through primary interactions with the immune system rather than through direct actions in the lung. For example, a growing body of evidence suggests that MSCs ameliorate allergic airways inflammation in mice by increasing T-regulatory cells or by promoting a Th1 phenotype in vivo in antigen-specific CD4 T cells and in circulating antigen-specific immunoglobulins as a means of abrogating Th2-mediated lung injury (4, 267, 272). As such, MSCs appear to be capable of a spectrum of effects in different lung injuries and critical illnesses. This is a critically important point because clinical use of MSCs must be tailored toward the specific disease process.
Many of the studies discussed above used different preparations of MSCs. As detailed for recent publications in Table 4, these ranged from populations of heterogenous plastic adherent adipose stromal cells to purified, well-characterized BM-derived MSCs obtained from core facilities such as the NCRR/NIH sponsored Texas (formerly Tulane) Center for Preparation and Distribution of Adult Stem Cells (MSCs) (http://medicine.tamhsc.edu/irm/msc-distribution.html). Few studies have directly compared different MSC preparations in lung disease models. In one of the few available comparison studies, human chorionic tissue–derived MSCs were more effective than human BM-derived MSCs in mitigating endotoxin-induced inflammation in explanted human lungs (302). In contrast, mouse adipose-derived MSCs were more effective than human adipose MSCs in ameliorating endotoxin-induced lung injury in mice (265). Differences between sources of MSCs have immediate clinical implications because several clinical investigations are planned or are underway in lung diseases using MSCs obtained from adipose and placental tissues as well as from BM. However, whether cells from different tissues sources behave differently in different clinical lung diseases remains poorly defined, and additional preclinical and clinical comparison studies are necessary. Differences between syngeneic, allogeneic, and xenogeneic MSC administration have been less well explored in preclinical lung injury models. A growing number of studies demonstrate the efficacy of human MSCs in lung injury models in immune-deficient (4, 281, 285) and immune-competent mice (4, 260, 261, 273, 274, 285, 294). However, a recent head-to-head comparison of human MSCs in ameliorating bleomycin-induced lung injury in immune-deficient versus immune-competent mice suggests that human MSCs may be less effective in immune-competent mice (285).
Although the overall consistency of studies demonstrating the ability of MSCs to ameliorate different types of lung injuries is encouraging, continued rigor must be applied to understand the mechanisms of MSC effects when comparing MSCs isolated by different protocols and obtained from different sources. Furthermore, the specificity of MSC effects as compared with the potential antiinflammatory or immunomodulatory effects of other stromal cells (e.g., fibroblasts) must be carefully considered (307, 308). For example, systemic administration of primary dermal fibroblasts similarly decreased ragweed pollen– or ovalbumin-induced allergic airways inflammation compared with MSC administration (267). Fibroblasts transduced to express angiopoietin-1 decreased acute endotoxin-induced lung injury (309). However, intratracheal administration of 3T3 fibroblasts did not mimic the effects of MSCs in ameliorating endotoxin-induced ALI (reviewed in Reference 4). These results suggest that MSCs and stromal cells (e.g., fibroblasts) may share similar antiinflammatory mechanisms. However, fibroblasts are also heterogeneous depending in part on tissue source, and thus potential antiinflammatory effects may differ depending on their origin. Furthermore, fibroblasts are less likely to have the same degree of low immunogenicity as do MSCs and can provoke lung inflammation (267). Nonetheless, comparison of MSCs with fibroblasts in different injury models will likely provide important insights into the antiinflammatory mechanisms of MSCs, and it is important to include appropriate cell controls (e.g., fibroblasts) when assessing MSC effects in different lung injury models.
Immunomodulation and Amelioration of Lung Injuries by Other Cell Types
Other cell populations are increasingly described as having ameliorating effects in mouse models of lung injury and in sepsis. These include human amniotic fluid or amnion-derived epithelial cells (181–196). A growing body of literature also describes the effectiveness of a heterogenous population of BM-derived mononuclear cells in ameliorating injury in mouse models of ALI, allergic airways inflammation, COPD, hemorrhagic shock, sepsis, and silicosis (310–319). The BM-derived mononuclear cell fraction does contain MSCs, and it is not clear whether it is the MSC fraction that is responsible for the observed effects. The use of BM-derived mononuclear fractions suggests a potential convenient means of autologous cell therapies for lung diseases. The BM cells are harvested, prepared, and administered even on the same day. This avoids the steps necessary for isolation, purification, and expansion involved in the use of MSCs as well as all of the other considerations for MSC use discussed above. However, it is unclear whether BM mononuclear cells, including MSCs, harvested from individuals with different lung diseases will be appropriately functional for use in mitigating a specific lung injury. The use of autologous BM cells is also potentially problematic in light of medical tourism issues, as further discussed below.
Several reports describe amelioration of ALI, bleomycin-induced fibrosis, sepsis-induced lung injury, or silicosis in mouse models by administration of embryonic stem cells, induced pluripotent cells, CD133+ epithelial progenitor cells, human cord blood–derived hematopoietic progenitor cells, and ATII cells (31, 112–115, 320, 321). Varying levels of engraftment of the different cell types have been described, but the different published reports suggest potential paracrine or immunomodulatory effects as the primary mechanisms of action. It is unclear as to why or how these cells would have immunomodulatory effects, and much further characterization and clarification is required.
Lung Tissue Bioengineering
A continued rapidly growing area of investigation is the use of three-dimensional matrices or other artificial scaffolding for growth of functional tracheal or lung tissue from stem, progenitor, or other cells ex vivo and in vivo. These approaches have been increasingly successfully used in regeneration of other tissues including skin, vasculature, cartilage, bone, and more recently in more complex organs, including heart and liver (322, 323). Significant progress continues to be made with trachea using synthetic scaffolds and decellularized cadaveric or donor tracheas. Each of these types of scaffolds can be seeded with stromal and/or epithelial cells derived from the eventual recipient, resulting in the growth of tissue resembling that of normal trachea (324–330). A number of different synthetic scaffold materials, cytokines, and growth factors have been investigated for use in promoting cell growth in the scaffolds, but the optimal components or combinations are not clear (331, 332). Growing investigation of patients treated with tracheal replacements using decellularized of artificial tracheas has shown promising yet mixed results (333–335). Comparable progress has occurred with the use of natural and synthetic scaffolds of larynx and diaphragm, and it is anticipated that initial clinical investigations will occur in the relatively near future (322, 336–340).
Given the complex threedimensional architecture and structure–function relationships of the lung, this is a more formidable task; nonetheless, there has been significant progress in several areas. Three-dimensional culture systems have been investigated for a number of years as matrices for ex vivo lung parenchymal development and for the study of growth factors and mechanical forces on lung remodeling (4, 322, 341). These studies have included implantation of various scaffolds impregnated with stem or other cells to produce functioning lung tissue (342–345). Comparable scaffold approaches have been used to study the creation of pulmonary vascular networks and to investigate the effects of vascular endothelial cells on the development of airway and alveolar epithelial tissues (346, 347)
These studies demonstrate the power of three-dimensional culture systems using synthetic scaffolds to evaluate lung development and repair ex vivo and in vivo. However, artificial scaffolds do not fully replicate the complexity of the lung architecture; nor do they contain all the matrix components essential for normal lung development and function. As such, a rapidly growing body of recent work has focused on use of more natural models, including nasal septa and decellularized whole lung as more physiologic scaffolds, in which the native structure of the lung and relevant extracellular matrix (ECM) components, including collagens, fibronectin, and laminin, are left intact after removal of cellular material with detergents and other agents (104, 348–364). The use of decellularized lungs for growing alveolar cells in culture was first described 25 years ago (349) and has been reinvigorated for testing in in vivo applications. The decellularized lungs can be mechanically ventilated and can undergo vascular perfusion to provide more physiologic study of ex vivo lung generation. Two pioneering studies demonstrated that inoculating decellularized rat lungs with different mixes of fetal rat lung homogenates, a human vascular endothelial cell line, and A549 carcinoma cells resulted in recellularization of the decellularized scaffolds (353, 354). Although the resulting cellular architecture did not necessarily resemble that of native lung, the recellularized lungs were able to be surgically implanted in rats that had previously undergone unilateral pneumonectomy with short-term survival of the rats, and some degree of gas exchange and vascular perfusion was achieved. A follow-up study demonstrated more prolonged survival of rats implanted with decellularized lungs recellularized with a mix of fetal lung and human vascular endothelial cells (355). However, after 2 weeks, the implanted lungs had developed extensive inflammatory and consolidative changes.
Although these important proof-of-concept studies have stimulated intense investigation, they have also illustrated a number of practical issues before the use of decellularized human lungs for clinical transplantation. These include the source of the lung, the decellularization process used, the type of cell(s) to be inoculated, the use of bioreactor culture systems and other environmental considerations, implantation, ethics, and the overall practicality of this approach (322, 363–366). Regarding decellularization, detergent-based methods and physical methods such as freeze-thaw have been used. However, the optimal approach has not been clarified for lung or for other tissues, and several different approaches have been used for lungs (322, 359, 361, 362, 367–370). It is also unclear whether age of prior injury of the lung affects subsequent decellularization or recellularization (361, 371). Recent data suggest that detergent-based decellularization maintains the injury patterns in emphysematous and fibrotic lungs (361, 372). Lungs obtained from older mice and those with experimentally induced fibrotic lung injury appear to support the growth and proliferation of intratracheally inoculated stromal and epithelial cells. However, the growth of epithelial cells is significantly diminished in decellularized lungs obtained from mice with experimentally induced emphysematous injury (361). Because extracellular matrix proteins significantly affect growth, differentiation, migration, and other properties of lung epithelial and other cell types (373–375), these findings illustrate that detailed consideration needs to be given to the ECM composition of the decellularized lung scaffold. Storage and sterilization conditions used for decellularized lungs may significantly affect the composition and architecture of the decellularized scaffold and the ability of inoculated cells to survive, proliferate, and differentiate (356). It is also unclear whether cadaveric lungs, as opposed to those obtained from organ donors, will also be useful. Delayed necropsy can significantly affect the composition and architecture of the decellularized scaffold and the ability to recellularize (356).
An underlying assumption for the clinical use of decellularized whole organ scaffolds, including lung, is that they will be relatively nonimmunogenic and minimize any detrimental host response in transplant recipients (321, 376, 377). However, ECM and other proteins remaining in decellularized scaffolds can provoke immune responses (376–378). Some of these may be beneficial because a growing literature suggests scaffold-induced polarization of macrophages to antiinflammatory M2 phenotype with subsequent permissive effects on implanted scaffolds (379, 380). Regarding lung, proteomic assessments using mass spectrometry and/or Western blotting demonstrate that a wide range of residual proteins, including intracellular, nuclear, and cytoskeletal proteins, can remain in the lungs despite apparent effective decellularization (356, 358, 361, 362, 372). Whether these residual proteins provoke immune responses is the focus of intense inquiry. Overall, the biological activity of the scaffold needs to be examined with care, particularly because it is altered by detergents and other reagents used to prepare the scaffold. This should include detailed proteomic assessments of decellularized scaffolds to fully appreciate the range of potentially immunogenic proteins remaining in scaffolds.
A further question for the use of decellularized whole lung scaffolds is clarifying optimal approaches to recellularization. This includes consideration of the types of cells inoculated into the lungs and of the environmental conditions used to promote growth of functional lung tissue. A growing range of cell types have been inoculated into decellularized lungs, including embryonic stem cells, adult BM-derived MSCs, lung progenitor cells, and fetal lung homogenates, as well as several different mouse and human epithelial or endothelial cell lines (Figure 2) (351–359, 361, 362). The combination of cells used, the order of inoculation, the route of inoculation, and subsequent environmental conditions for optimal growth and differentiation have yet to be worked out. The use of bioreactor systems that provide a closed sterile environment, vascular perfusion, and positive- or negative-pressure ventilation is one current approach (351, 353, 354, 381–383).
Decellularized whole lung scaffolds also provide a powerful means to study the effects of ECM proteins and other factors on the growth and development of specific cell types. For example, the growth of fibroblasts on decellularized human lungs obtained from patients with idiopathic pulmonary fibrosis promoted a myofibroblast phenotype (372, 384). Mouse embryonic stem cells, differentiated in routine tissue culture dishes to endoderm and then to Nkx2.1-expressing cells, exhibited phenotypic markers consistent with ATII or ATI cells when cultured in decellularized mouse lung slices (104). Similarly, isolated mouse ATII cells expressed immunophenotypic markers consistent with ATI cell phenotype when cultured in decellularized mouse lung scaffolds (385). Another potential application of the decellularized lungs is for studying lung cancer biology (386).
In addition to genetic regulatory programs and culture approaches, including the use of three-dimensional scaffolds, mechanical forces also play a role in how differentiating tissues respond to gene instructions. During breathing, lung cells are subjected to complex mechanical loading, which includes shearing due to air movement, compression due to pressurization, and stretch due to the expansion of the lung tissue during normal breathing (reviewed in References 4, 386). The mechanical forces in utero are, in part, generated by fetal breathing–like movements produced by rhythmic contractions of the respiratory muscles with varying frequency and amplitude. This normal movement is essential in the development of fetal lung, differentiation of ATII cells, and synthesis of surfactant protein, and a number of in vitro studies have demonstrated that application of cyclic mechanical stretch to cultures of mixed fetal rodent lung cells increases SPC mRNA expression compared with cells cultured under static conditions (388; older articles reviewed in References 4, 387). Mechanical forces are also being increasingly recognized as playing significant roles in cellular and molecular mechanisms underlying lung injury and recovery from lung injury (388–390). Stretch and other mechanical influences, such as fluid shear, have been intensely investigated for their influence on the development of tissues such as cartilage, bone, and vascular structures from MSCs and EPCs as well as from ESCs. However, there remain relatively few studies evaluating the effects of physical forces on the development of lung epithelial cells or on the development of three-dimensional lung structures from endogenous lung progenitor cells or from embryonic or adult stem cells cultured in three-dimensional scaffolds (Figure 5) (107, 108, 352, 391, 392).
The challenges in developing complex three-dimensional functional lung tissues ex vivo will be in recapitulating the normal dynamic integrated three-dimensional network of epithelial cells, endothelial cells, fibroblasts, neuronal cells, lymphatic cells, and inflammatory cells in the appropriate environment and architecture with the correct effector molecules and mechanical forces, all of which are vital for proper function. A thorough understanding of the synergistic interactions between cells in physiologically relevant conditions is critical for the development of tissues that recapitulate the structure and function of the parental tissue in vivo. Recent studies demonstrated that human lung cells and human blood capillaries coated onto porous polydimethylsiloxane chips can mimic alveolar architecture and can be used to study pathophysiologic processes (393). This “lung-on-a-chip” device was further used to evaluate how nanoparticles and bacteria enter the lungs and may also be useful for high-throughput screening of drugs (394). The field is ripe for further comparable technologic advances in bioengineering approaches for lung regeneration.
Clinical Trials of Cell Therapies in Lung Diseases
The pros and cons of moving toward clinical trials with MSCs and other cell types (EPCs) have been vigorously debated at all four conferences to date and at a recent meeting held at the Nationa Heart, Lung and Blood Institute in November 2012 (395). Patient safety remains paramount, and a growing experience in clinical trials in other diseases, particularly with MSC-based cell therapies, continues to demonstrate short-term safety and lack of significant infusion-related toxicity, including signs or symptoms of clinically significant pulmonary emboli (396, 397).
Two pilot trials of autologous EPC administration for primary pulmonary hypertension conducted at Zhejiang University, Hangzhou, China in adult and pediatric patients demonstrated increased 6-minute walk capacity and improved hemodynamic variables, including mean pulmonary artery pressure, pulmonary vascular resistance, and cardiac output, 12 weeks after systemic administration of autologous EPCs with conventional therapy compared with patients receiving conventional therapy alone (398, 399). No adverse effects of EPC administration were noted, although long-term follow-up is pending. A therapeutic trial of administration of autologous early outgrowth EPCs transduced to express eNOS for patients with pulmonary hypertension, the Pulmonary Hypertension and Cell Therapy (PHaCeT) trial has been initiated at St. Michael’s Hospital in Toronto and the Jewish General Hospital in Montreal. Several other EPC-based trials are listed on the National Institutes of Health clinicaltrial.gov website.
MSCs have been used in clinical trials in a widening range of inflammatory and autoimmune diseases (e.g., graft-versus-host disease, autoimmune diabetes, and Crohn’s disease) and in acute myocardial infarction (197, 396, 397). One commercial preparation of MSCs (PROCHYMAL; Osiris Therapeutics Inc., Columbia MD) has recently been approved in Canada for use in refractory pediatric graft-versus-host disease (GVHD) (400). This is the first licensed MSC-based cell therapy product to be accessible for approved clinical use. One of the important findings to come out of the clinical trial experiences and published data is that administration of MSCs appears to be safe and well tolerated even in severely ill patients (397). There appear to be no significant infusion-related toxicities or short-term adverse effects. A recent autopsy evaluation of 18 patients with GVHD who had received systemic MSC infusions found no evidence of tumor or of ectopic growth and only minimal evidence of sustained presence of the up to 577 days after MSC administration (401). Although additional long-term evaluations are necessary, this study provides important safety data.
The first multicenter, double-blind, placebo-controlled phase II trial of PROCHYMAL (Osiris Therapeutics Inc., Columbia MD) for patients with moderate-severe COPD (FEV1/FVC < 0.70; 30% ≤ FEV1 ≤ 70%) has been completed and published (402). The primary goal of the trial, which was initiated in May 2008, was to determine the safety of MSC infusions in patients with lung disease. The secondary goal was initial estimation of the potential efficacy of MSCs for decreasing the chronic inflammation associated with COPD, thus improving pulmonary function and quality of life. The trial involved 62 patients in six participating sites in the United States. The trial demonstrated safety in an older population of significantly affected patients with COPD with a number of comorbidities. No infusional toxicity or clinical suggestion of significant pulmonary emboli was observed in multiple infusions of either study drug or of vehicle control (four infusions per patient for a total of 248 infusions). No mortality or serious adverse events attributable to the infusions were observed over a subsequent 2-year follow-up period, and no serious attributable changes were observed in a range of safety assessments including serum toxicologies, urinalyses, ECGs, or echocardiograms (pulmonary hypertension). However, no improvement in efficacy outcomes, including pulmonary functions, 6-minute-walk evaluation, quality of life questionnaires, or physician’s global assessment, were observed. Post hoc analysis demonstrated a significant initial decrease in the circulating C-reactive protein, commonly elevated in patients with COPD, in a subset of MSC-treated versus vehicle-treated patients who had elevated C-reactive protein levels at study onset (Figure 6). Although this trend lost significance over time, it continued for the duration of the 2-year observation period.
Figure 6.
Changes in FEV1% predicted, Borg dyspnea scale, and in circulating C-reactive protein (CRP) levels after systemic administration of mesenchymal stromal (stem) cell (MSCs) or vehicle control in patients with moderate-severe chronic obstructive pulmonary disease (COPD). Changes in circulating CRP levels are shown only for those who had elevated levels at screening (> 4.0 mg/L). *P < 0.05. Adapted by permission from Reference 402.
Although this trial failed to demonstrate efficacy, it provides a firm basis for safety of MSC use, including multiple infusions, in patients with chronic lung diseases and provides a potential mechanistic clue of in vivo MSC effects. However, as more is learned about the effects and mechanisms of MSC actions, disease choice becomes more important. Chronic persistent lung diseases with low-level or smoldering inflammation (e.g., COPD) or diseases in which currently available preclinical data suggest that MSCs may worsen the disease process (e.g., idiopathic pulmonary fibrosis) may not be the best therapeutic targets for MSC intervention (22, 24, 403–405). More acute inflammatory lung or systemic diseases (e.g., ARDS or sepsis/septic shock) or chronic immune-mediated lung diseases (e.g., severe asthma) may be better targets (251–258). To this end, clinical trials of MSCs for ARDS and for septic shock are in progress or in development in the United States and in Canada, respectively. These demonstrate growing efforts toward carefully conducted and closely regulated clinical trials of cell therapies for lung diseases taking place in Europe, Brazil, Australia, the United States, and Canada. Small case series have also been published detailing the effects of administering MSCs to patients with other diverse lung injuries, including lupus-associated diffuse alveolar hemorrhage or radiation-induced lung injury, or for mobilizing endogenous MSCs in patients with tuberculosis (297, 406, 407). More detailed investigation is required for these and any other lung disease that might be amenable to MSC-based cell therapies. In particular, currently available animal models of lung diseases are well recognized to not necessarily fully reflect actual clinical isease (406), and thus developing improved animal models in which to better assess potential effects of MSCs and other cell populations is a continuing ripe area for study.
Some additional considerations regarding systemic administration of MSCs, including the use of fetal calf or bovine serum versus heterologous species-specific serum, platelet lysate, or other alternatives, have been discussed in previous conference reports and more recently at the NHLBI Lung Cell Therapy meeting in December 2012 (2–4, 395). Other issues, such as potential pulmonary emboli after systemic cell administration, cell dose, dosing intervals, route of cell administration, and other additives, continue to undergo vigorous discussion and debate. In a recent review of autopsies of 18 patients with GVHD who had received systemic MSC administration, evidence of microemboli was found in only one patient (401). However, this patient was critically ill with ARDS and had disseminated intravascular coagulation at the time of death, each of which could have contributed to the development of microthrombi. No clinical signs or symptoms of pulmonary emboli were observed in the patients with COPD participating in the phase II trial discussed above (402). Another consideration for clinical use of MSCs is sample preparation. In many approaches, frozen bags of (non–HLA-matched allogeneic) MSCs are thawed at the bedside and directly infused. Although MSCs are a convenient direct way to administer MSCs, recent data suggest that freshly thawed MSCs may be “stunned” and may not regain full immunomodulatory properties for up to 24 hours after thawing (250). Although not yet directly tested head to head in animal models of lung diseases, the optimal conditions for using MSCs may not yet be established.
Whether MSCs undergo malignant transformation has been a topic of debate and concern and has been examined in detail in several recent articles (2–4, 409–411). The possibility of malignancy must be carefully considered in the clinical setting in evaluating the risks and benefits for a given patient. A recent review of autopsy findings in patients receiving allogeneic MSCs is reassuring because only minimal presence of MSCs was detected up to 577 days after administration (401). Nonetheless, continued intense scrutiny, long-term follow-up, and sensible caution seems advisable.
Therefore, in parallel with ongoing basic science investigations to better define the range and mechanisms of cell actions, as the community moves ahead toward further clinical trials, the following three questions must be addressed. (1) Is it safe? Short-term safety concerns are methodically being addressed and are so far being alleviated. (2) Can the treatment potentially cause harm? A case in point is idiopathic pulmonary fibrosis. Using admittedly imperfect preclinical rodent models of lung fibrosis, the available data suggest that MSC administration in the setting of established lung fibrosis will have no beneficial effect or contribute to the fibrotic process (22, 237, 238, 404, 405). (3) Will the proposed cell therapy approach have potential efficacy? This is where it is critically important to understand the mechanisms of MSC or other cell-based actions in specific lung disease pathogeneses. A robust preclinical literature supports the use of MSC-based therapeutic approaches in acute lung or critical illness inflammatory injuries, such as ARDS or sepsis, as well as bronchopulmonary dysplasia, and in more chronic immune mediated conditions such as bronchiolitis obliterans and asthma. There remains a sense of equipoise because preclinical lung disease models do not necessarily fully mimic human disease pathogeneses or predict clinical behaviors (408). Ongoing and planned clinical trials in a variety of lung diseases or critical illnesses (sepsis) in North America and in other parts of the world must proceed carefully and cautiously. As a community, we must be careful to not overestimate the potential and promise of cell-based therapies.
Medical Tourism
A growing number of websites and other venues offer unsubstantiated claims of cell therapy efficacy in a range of lung diseases. Significant harm and even death may result in patients who undergo these treatments (412–414). The FDA has recently begun working with other governmental agencies to attempt to regulate or in some cases close websites making unsubstantiated claims (415). As such, prominent nonprofit respiratory disease foundations, including the American Thoracic Society, American Lung Association, Pulmonary Hypertension Association, have joined with prominent stem cell societies, notably the International Society for Stem Cell Research, in issuing strong statements against stem cell medical tourism on their respective websites (7). The Stem Cell Working Group of the American Thoracic Society is coordinating an effort to have other leading respiratory disease foundations issue comparable statements and take comparable positions.
Acknowledgments
Acknowledgments
The author thanks Jason Bates, Daniel Chambers, Thomas Gilbert, Darrell Kotton, Conrad Liles, Carolyn Lutzko, Bethany Moore, Darwin Prockop, Jay Rajagopal, and Mervin Yoder for careful reading and suggestions for the review and Gwen Landis for administrative support.
Footnotes
The work was supported in part by National Heart, Lung and Blood Institute grant R13 HL097533.
This document has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Some of the text of this article was previously published in Weiss DJ, Bertoncello I, Borok Z, et al. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc 2011;8:223–272.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Weiss DJ, Bates JHT, Gilbert T, Liles WC, Lutzko C, Rajagopal J, Prockop DJ. Conference report: stem cells and cell therapies in lung biology and diseases. Ann Am Thorac Soc. In press doi: 10.1513/AnnalsATS.201304-089AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss DJ, Berberich MA, Borok Z, Gail DB, Kolls JK, Penland C, Prockop DJ. Adult stem cells, lung biology, and lung disease. NHLBI/Cystic Fibrosis Foundation Workshop. Proc Am Thorac Soc. 2006;3:193–207. doi: 10.1513/pats.200601-013MS. [DOI] [PubMed] [Google Scholar]
- 3.Weiss DJ, Kolls JK, Ortiz LA, Panoskaltis-Mortari A, Prockop DJ. Stem cells and cell therapy approaches for lung diseases: conference report. Proc Am Thorac Soc. 2008;5:637–667. doi: 10.1513/pats.200804-037DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss DJ, Bertoncello I, Borok Z, Kim C, Panoskaltsis-Mortari A, Reynolds S, Rojas M, Stripp B, Warburton D, Prockop DJ. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc. 2011;8:223–272. doi: 10.1513/pats.201012-071DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown JK, Hogan BLM, Randell SH, Stripp B, Weiss DJ. Human embryonic stem cell research: an official ATS research policy statement. Am J Respir Crit Care Med. 2006;173:1–3. [Google Scholar]
- 6.American Thoracic SocietyATS statement on embryonic stem cell research. Message to ATS members, September 9, 2010. Available from: http://www.thoracic.org/statements/resources/respiratory-disease-adults/stemcell.pdf
- 7.American Thoracic Society. Stem cells and cell therapies for lung diseases. Available from: http://patients.thoracic.org/materials/stem-cells.php [DOI] [PMC free article] [PubMed]
- 8.Abreu SC, Antunes MA, Pelosi P, Morales MM, Rocco PR. Mechanisms of cellular therapy in respiratory diseases. Intensive Care Med. 2011;37:1421–1431. doi: 10.1007/s00134-011-2268-3. [DOI] [PubMed] [Google Scholar]
- 9.Cribbs SK, Martin GS. Stem cells in sepsis and acute lung injury. Am J Med Sci. 2011;341:325–332. doi: 10.1097/MAJ.0b013e3181f30dee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones CP, Rankin SM. Bone marrow-derived stem cells and respiratory diseases. Chest. 2011;140:205–211. doi: 10.1378/chest.10-2348. [DOI] [PubMed] [Google Scholar]
- 11.Jungebluth P, Macchiarini P. Stem cell-based therapy and regenerative approaches to diseases of the respiratory system. Br Med Bull. 2011;99:169–187. doi: 10.1093/bmb/ldr028. [DOI] [PubMed] [Google Scholar]
- 12.Majka S, Burnham E, Stenmark KR. Cell-based therapies in pulmonary hypertension: who, what, and when? Am J Physiol Lung Cell Mol Physiol. 2011;301:L9–L11. doi: 10.1152/ajplung.00118.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moodley Y, Manuelpillai U, Weiss DJ. Cellular therapies for lung disease: a distant horizon. Respirology. 2011;16:223–237. doi: 10.1111/j.1440-1843.2010.01914.x. [DOI] [PubMed] [Google Scholar]
- 14.Alphonse RS, Rajabali S, Thebaud B. Lung injury in preterm neonates: the role and therapeutic potential of stem cells. Antioxid Redox Signal. 2012;17:1013–1040. doi: 10.1089/ars.2011.4267. [DOI] [PubMed] [Google Scholar]
- 15.Anversa P, Perrella MA, Kourembanas S, Choi AM, Loscalzo J. Regenerative pulmonary medicine: potential and promise, pitfalls and challenges. Eur J Clin Invest. 2012;42:900–913. doi: 10.1111/j.1365-2362.2012.02667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia O, Carraro G, Navarro S, Bertoncello I, McQualter J, Driscoll B, Jesudason E, Warburton D. Cell-based therapies for lung disease. Br Med Bull. 2012;101:147–161. doi: 10.1093/bmb/ldr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griesenbach U, Alton EW. Progress in gene and cell therapy for cystic fibrosis lung disease. Curr Pharm Des. 2012;18:642–662. doi: 10.2174/138161212799315993. [DOI] [PubMed] [Google Scholar]
- 18.Keeler AM, Flotte TR. Cell and gene therapy for genetic diseases: inherited disorders affecting the lung and those mimicking sudden infant death syndrome. Hum Gene Ther. 2012;23:548–556. doi: 10.1089/hum.2012.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiley JP, Senior RM. Pulmonary research in 2013 and beyond. A National Heart, Lung, and Blood Institute perspective. Am J Physiol Lung Cell Mol Physiol. 2012;303:L729–L732. doi: 10.1152/ajplung.00305.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotton DN. Next generation regeneration: the hope and hype of lung stem cell research. Am J Respir Crit Care Med. 2012;12:1255–1260. doi: 10.1164/rccm.201202-0228PP. [DOI] [PubMed] [Google Scholar]
- 21.Lau AN, Goodwin M, Kim CF, Weiss DJ. Stem cells and regenerative medicine in lung biology and diseases. Mol Ther. 2012;20:1116–1130. doi: 10.1038/mt.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNulty K, Janes SM. Stem cells and pulmonary fibrosis: cause or cure? Proc Am Thorac Soc. 2012;9:164–171. doi: 10.1513/pats.201201-010AW. [DOI] [PubMed] [Google Scholar]
- 23.O'Reilly M, Thebaud B. Cell-based strategies to reconstitute lung function in infants with severe bronchopulmonary dysplasia. Clin Perinatol. 2012;39:703–725. doi: 10.1016/j.clp.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzouvelekis A, Antoniadis A, Bouros D. Stem cell therapy in pulmonary fibrosis. Curr Opin Pulm Med. 2011;17:368–373. doi: 10.1097/MCP.0b013e328348744f. [DOI] [PubMed] [Google Scholar]
- 25.Vosdoganes P, Lim R, Moss TJ, Wallace EM. Cell therapy: a novel treatment approach for bronchopulmonary dysplasia. Pediatrics. 2012;130:727–737. doi: 10.1542/peds.2011-2576. [DOI] [PubMed] [Google Scholar]
- 26.Winkelmann A, Noack T. The Clara cell: a “Third Reich eponym”? Eur Respir J. 2010;36:722–727. doi: 10.1183/09031936.00146609. [DOI] [PubMed] [Google Scholar]
- 27.American Thoracic Society. 2013 nomenclature changes. Available from: http://www.atsjournals.org/page/nomenclature_2013
- 28.Kassmer SH, Krause DS. Detection of bone marrow-derived lung epithelial cells. Exp Hematol. 2010;38:564–573. doi: 10.1016/j.exphem.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kassmer SH, Bruscia EM, Zhang PX, Krause DS. Nonhematopoietic cells are the primary source of bone marrow-derived lung epithelial cells. Stem Cells. 2012;30:491–499. doi: 10.1002/stem.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fritzell JA, Jr, Mao Q, Gundavarapu S, Pasquariello T, Aliotta JM, Ayala A, Padbury JF, De Paepe ME. Fate and effects of adult bone marrow cells in lungs of normoxic and hyperoxic newborn mice. Am J Respir Cell Mol Biol. 2009;40:575–587. doi: 10.1165/rcmb.2008-0176OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Paepe ME, Mao Q, Ghanta S, Hovanesian V, Padbury JF. Alveolar epithelial cell therapy with human cord blood-derived hematopoietic progenitor cells. Am J Pathol. 2011;178:1329–1339. doi: 10.1016/j.ajpath.2010.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duchesneau P, Wong AP, Waddell TK. Optimization of targeted cell replacement therapy: a new approach for lung disease. Mol Ther. 2010;18:1830–1836. doi: 10.1038/mt.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong AP, Keating A, Lu WY, Duchesneau P, Wang X, Sacher A, Hu J, Waddell TK. Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium. J Clin Invest. 2009;119:336–348. doi: 10.1172/JCI36882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khatri M, Saif YM. Epithelial cells derived from swine bone marrow express stem cell markers and support influenza virus replication in vitro. PLoS ONE. 2011;6:e29567. doi: 10.1371/journal.pone.0029567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khatri M, Goyal SM, Saif YM. Oct4+ stem/progenitor swine lung epithelial cells are targets for influenza virus replication. J Virol. 2012;86:6427–6433. doi: 10.1128/JVI.00341-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rock JR, Hogan BL. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu Rev Cell Dev Biol. 2011;27:493–512. doi: 10.1146/annurev-cellbio-100109-104040. [DOI] [PubMed] [Google Scholar]
- 37.Pritchard S, Hoffman AM, Johnson KL, Bianchi DW. Pregnancy-associated progenitor cells: an under-recognized potential source of stem cells in maternal lung. Placenta. 2011;32:S298–S303. doi: 10.1016/j.placenta.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McQualter JL, Bertoncello I. Concise review: deconstructing the lung to reveal its regenerative potential. Stem Cells. 2012;30:811–816. doi: 10.1002/stem.1055. [DOI] [PubMed] [Google Scholar]
- 39.Xian W, McKeon F. Adult stem cells underlying lung regeneration. Cell Cycle. 2012;11:887–894. doi: 10.4161/cc.11.5.19328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hegab AE, Kubo H, Fujino N, Suzuki T, He M, Kato H, Yamaya M. Isolation and characterization of murine multipotent lung stem cells. Stem Cells Dev. 2010;19:523–536. doi: 10.1089/scd.2009.0287. [DOI] [PubMed] [Google Scholar]
- 41.Bertoncello I, McQualter J.Isolation and clonal assay of adult lung epithelial stem/progenitor cells. Curr Protocols Stem Cell Biol 2011;Chapter 2:Unit 2G.1. [DOI] [PubMed] [Google Scholar]
- 42.Borok Z, Whitsett JA, Bitterman PB, Thannickal VJ, Kotton DN, Reynolds SD, Krasnow MA, Bianchi DW, Morrisey EE, Hogan BL, et al. Cell plasticity in lung injury and repair: report from an NHLBI workshop, April 19–20, 2010. Proc Amer Thor Soc. 2011;8:215–22. doi: 10.1513/pats.201012-067CB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayes M, Curley GF, Laffey JG. Lung stem cells: from an evolving understanding to a paradigm shift? Stem Cell Res Ther. 2011;2:41. doi: 10.1186/scrt82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rackley CR, Stripp BR. Building and maintaining the epithelium of the lung. J Clin Invest. 2012;122:2724–2730. doi: 10.1172/JCI60519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Driscoll B, Kikuchi A, Lau AN, Lee J, Reddy R, Jesudason E, Kim CF, Warburton D. Isolation and characterization of distal lung progenitor cells. Methods Mol Biol. 2012;879:109–122. doi: 10.1007/978-1-61779-815-3_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reynolds SJ, Brechbul HM, Smith KS, Smith RW, Ghosh M. Lung epithelial healing: a modified seed and soil concept. Proc Am Thorac Soc. 2012;9:27–37. doi: 10.1513/pats.201201-008MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rock J, Konigshoff M. Endogenous lung regeneration: potential and limitations. Am J Respir Crit Care Med. 2012;186:1213–1219. doi: 10.1164/rccm.201207-1151PP. [DOI] [PubMed] [Google Scholar]
- 48.Murphy J, Summer R, Fine A. Stem cells in airway smooth muscle: state of the art. Proc Am Thorac Soc. 2008;5:11–14. doi: 10.1513/pats.200704-052VS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perl AK, Riethmacher D, Whitsett JA. Conditional depletion of airway progenitor cells induces peribronchiolar fibrosis. Am J Respir Crit Care Med. 2011;183:511–521. doi: 10.1164/rccm.201005-0744OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rinkevich Y, Mori T, Sahoo D, Xu PX, Bermingham JR, Jr, Weissman IL. Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nat Cell Biol. 2012;14:1251–1260. doi: 10.1038/ncb2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Volckaert T, Dill E, Campbell A, Tiozzo C, Majka S, Bellusci S, De Langhe SP. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J Clin Invest. 2011;121:4409–4419. doi: 10.1172/JCI58097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim JK, Vinarsky V, Wain J, Zhao R, Jung K, Choi J, Lam A, Pardo-Saganta A, Breton S, Rajagopal J, et al. In vivo imaging of tracheal epithelial cells in mice during airway regeneration. Am J Respir Cell Mol Biol. 2012;47:864–868. doi: 10.1165/rcmb.2012-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reynolds SD, Malkinson AM. Clara cell: progenitor for the bronchiolar epithelium. Int J Biochem Cell Biol. 2010;42:1–4. doi: 10.1016/j.biocel.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghosh M, Brechbuhl HM, Smith RW, Li B, Hicks DA, Titchner T, Runkle CM, Reynolds SD. Context-dependent differentiation of multipotential keratin 14-expressing tracheal basal cells. Am J Respir Cell Mol Biol. 2011;45:403–410. doi: 10.1165/rcmb.2010-0283OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghosh M, Smith MK, Smith RW, Li B, Hicks DA, Cole BB, Reynolds PR, Reynolds SD. β-Catenin dosage is a critical determinant of tracheal basal cell fate determination. Am J Pathol. 2011;179:367–379. doi: 10.1016/j.ajpath.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell. 2011;8:639–648. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith MK, Koch PJ, Reynolds SD. Direct and indirect roles for β-catenin in facultative basal progenitor cell differentiation. Am J Physiol Lung Cell Mol Physiol. 2012;302:L580–L594. doi: 10.1152/ajplung.00095.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suprynowicza FA, Upadhyay G, Krawczyk E, Kramer SC, Hebert JD, Liu X, Yuan H, Cheluvaraju C, Clapp PW, Boucher RC, Jr, et al. Conditionally reprogrammed cells represent a stem-like state of adult epithelial cells. Proc Natl Acad Sci USA. 2012;109:20035–20040. doi: 10.1073/pnas.1213241109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmad S, Ahmad A, Rancourt RC, Neeves KB, Loader JE, Hendry-Hofer T, Di Paola J, Reynolds SD, White CW. Tissue factor signals airway epithelial basal cell survival via coagulation and protease-activated receptor isoforms 1 and 2. Am J Respir Cell Mol Biol. 2013;48:94–104. doi: 10.1165/rcmb.2012-0189OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giangreco A, Arwert EN, Rosewell IR, Snyder J, Watt FM, Stripp BR. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc Natl Acad Sci USA. 2009;106:9286–9291. doi: 10.1073/pnas.0900668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Evans MJ, Johnson LV, Stephens RJ, Freeman G. Renewal of the terminal bronchiolar epithelium in the rat following exposure to NO2 or O3. Lab Invest. 1976;35:246–257. [PubMed] [Google Scholar]
- 63.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hegab AE, Ha VL, Darmawan DO, Gilbert JL, Ooi AT, Attiga YS, Bisht B, Nickerson DW, Gomperts BN. Isolation and in vitro characterization of basal and submucosal gland duct stem/progenitor cells from human proximal airways. Stem Cells Trans Med. 2012;1:719–724. doi: 10.5966/sctm.2012-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghosh M, Helm KM, Smith RW, Giordanengo MS, Li B, Shen H, Reynolds SD. A single cell functions as a tissue-specific stem cell and the in vitro niche-forming cell. Am J Respir Cell Mol Biol. 2011;45:459–469. doi: 10.1165/rcmb.2010-0314OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hegab AE, Ha VL, Gilbert JL, Zhang KX, Malkoski SP, Chon AT, Darmawan DO, Bisht B, Ooi AT, Pellegrini M, et al. A novel stem/progenitor cell population from murine tracheal submucosal gland ducts with multipotent regenerative potential. Stem Cells. 2011;9:1283–1293. doi: 10.1002/stem.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hegab AE, Nickerson DW, Ha VL, Darmawan DO, Gomperts BN. Repair and regeneration of tracheal surface epithelium and submucosal glands in a mouse model of hypoxic-ischemic injury. Respirology. 2012;17:1101–1113. doi: 10.1111/j.1440-1843.2012.02204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol. 2002;161:173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 70.McQualter JL, Brouard N, Williams B, Baird BN, Sims-Lucas S, Yuen K, Nilsson SK, Simmons PJ, Bertoncello I. Endogenous fibroblastic progenitor cells in the adult mouse lung are highly enriched in the sca-1 positive cell fraction. Stem Cells. 2009;27:623–633. doi: 10.1634/stemcells.2008-0866. [DOI] [PubMed] [Google Scholar]
- 71.Raiser DM, Kim CF. Commentary: Sca-1 and cells of the lung: a matter of different sorts. Stem Cells. 2009;27:606–611. doi: 10.1002/stem.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zacharek SJ, Fillmore CM, Lau AN, Gludish DW, Chou A, Ho JW, Zamponi R, Gazit R, Bock C, Jager N, et al. Lung stem cell self-renewal relies on BMI1-dependent control of expression at imprinted loci. Cell Stem Cell. 2011;9:272–281. doi: 10.1016/j.stem.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Teisanu RM, Chen H, Matsumoto K, McQualter JL, Pott E, Foster WM, Bertoncello I, Stripp BR. Functional analysis of two distinct bronchiolar progenitors during lung injury and repair. Am J Respir Cell Mol Biol. 2011;44:794–803. doi: 10.1165/rcmb.2010-0098OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, Sonnenberg A, Wei Y, Vu TH. Integrin β6α4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121:2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;47:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen H, Matsumoto K, Brockway BL, Rackley CR, Liang J, Lee JH, Jiang D, Noble PW, Randell SH, Kim CF, et al. Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells. 2012;30:1948–1960. doi: 10.1002/stem.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zemke AC, Snyder JC, Brockway BL, Drake JA, Reynolds SD, Kaminski N, Stripp BR. Molecular staging of epithelial maturation using secretory cell-specific genes as markers. Am J Respir Cell Mol Biol. 2009;40:340–348. doi: 10.1165/rcmb.2007-0380OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tesei A, Zoli W, Arienti C, Storci G, Granato AM, Pasquinelli G, Valente S, Orrico C, Rosetti M, Vannini I, et al. Isolation of stem/progenitor cells from normal lung tissue of adult humans. Cell Prolif. 2009;42:298–308. doi: 10.1111/j.1365-2184.2009.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kajstura J, Rota M, Hall SR, Hosoda T, D'Amario D, Sanada F, Zheng H, Ogorek B, Rondon-Clavo C, Ferreira-Martins J, et al. Evidence for human lung stem cells. N Engl J Med. 2001;364:1795–1806. doi: 10.1056/NEJMoa1101324. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80.Fujino N, Kubo H, Suzuki T, Ota C, Hegab AE, He M, Suzuki S, Suzuki T, Yamada M, Kondo T, et al. Isolation of alveolar epithelial type II progenitor cells from adult human lungs. Lab Invest. 2011;91:363–378. doi: 10.1038/labinvest.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Turner J, Roger J, Fitau J, Combe D, Giddings J, Heeke GV, Jones CE. Goblet cells are derived from a FOXJ1-expressing progenitor in a human airway epithelium. Am J Respir Cell Mol Biol. 2011;44:276–284. doi: 10.1165/rcmb.2009-0304OC. [DOI] [PubMed] [Google Scholar]
- 82.Fujino N, Ota C, Suzuki T, Suzuki S, Hegab AE, Yamada M, Takahashi T, He M, Kondo T, Kato H, et al. Analysis of gene expression profiles in alveolar epithelial type II-like cells differentiated from human alveolar epithelial progenitor cells. Respir Investig. 2012;50:110–116. doi: 10.1016/j.resinv.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 83.Fujino N, Kubo H, Ota C, Suzuki T, Suzuki S, Yamada M, Takahashi T, He M, Suzuki T, Kondo T, et al. A novel method for isolating individual cellular components from the adult human distal lung. Am J Respir Cell Mol Biol. 2012;46:422–430. doi: 10.1165/rcmb.2011-0172OC. [DOI] [PubMed] [Google Scholar]
- 84.Oeztuerk-Winder F, Guinot A, Ochalek A, Ventura JJ. Regulation of human lung alveolar multipotent cells by a novel p38 MAPK/miR-17–92 axis. EMBO J. 2012;31:3431–3441. doi: 10.1038/emboj.2012.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anversa P, Kajstura J, Leri A, Loscalzo J. Tissue-specific adult stem cells in the human lung. Nat Med. 2011;17:1038–1039. doi: 10.1038/nm.2463. [DOI] [PubMed] [Google Scholar]
- 86.Hoffman AM, Ingenito EP. Alveolar epithelial stem and progenitor cells: emerging evidence for their role in lung regeneration. Curr Med Chem. 2012;19:6003–6008. doi: 10.2174/092986712804485872. [DOI] [PubMed] [Google Scholar]
- 87.Oeztuerk-Winder F, Ventura JJ. Isolation, culture, and potentiality assessment of lung alveolar stem cells. Methods Mol Biol. 2012;916:23–30. doi: 10.1007/978-1-61779-980-8_3. [DOI] [PubMed] [Google Scholar]
- 88.Dobbs LG, Johnson MD, Vanderbilt J, Allen L, Gonzalez R. The great big alveolar TI cell: evolving concepts and paradigms. Cell Physiol Biochem. 2010;25:55–62. doi: 10.1159/000272063. [DOI] [PubMed] [Google Scholar]
- 89.Buckley S, Shi W, Carraro G, Sedrakyan S, Da Sacco S, Driscoll BA, Perin L, De Filippo RE, Warburton D. The milieu of damaged alveolar epithelial type 2 cells stimulates alveolar wound repair by endogenous and exogenous progenitors. Am J Physiol Lung Cell Mol Physiol. 2011;45:1212–1221. doi: 10.1165/rcmb.2010-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ling TY, Kuo MD, Li CL, Yu AL, Huang YH, Wu TJ, Lin YC, Chen SH, Yu J. Identification of pulmonary Oct-4+ stem/progenitor cells and demonstration of their susceptibility to SARS coronavirus (SARS-CoV) infection in vitro. Proc Natl Acad Sci USA. 2006;103:9530–9535. doi: 10.1073/pnas.0510232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ratajczak MZ, Machalinski B, Wojakowski W, Ratajczak J, Kucia M. A hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissues. Leukemia. 2007;21:860–867. doi: 10.1038/sj.leu.2404630. [DOI] [PubMed] [Google Scholar]
- 92.Jakiela B, Brockman-Schneider R, Amineva S, et al. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am J Respir Cell Mol Biol. 2008;38:517–523. doi: 10.1165/rcmb.2007-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu X, Luo M, Guo C, et al. Analysis of adeno-associated virus progenitor cell transduction in mouse lung. Mol Ther. 2008;17:285–293. doi: 10.1038/mt.2008.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mishra S, Wang X, Smiley N, Xia P, Hong CM, Senadheera D, Bui KC, Lutzko C. Genetic modification of airway progenitors after lentiviral gene delivery to the amniotic fluid of murine fetuses. Am J Physiol Lung Cell Mol Physiol. 2011;44:562–570. doi: 10.1165/rcmb.2009-0235OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133–143. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- 96.Rivera C, Rivera S, Loriot Y, Vozenin MC, Deutsch E. Lung cancer stem cell: new insights on experimental models and preclinical data. J Oncol. 2011;2011:549181. doi: 10.1155/2011/549181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O'Flaherty JD, Barr M, Fennell D, Richard D, Reynolds J, O'Leary J, O'Byrne K. The cancer stem-cell hypothesis: its emerging role in lung cancer biology and its relevance for future therapy. J Thorac Oncol. 2012;7:1880–1890. doi: 10.1097/JTO.0b013e31826bfbc6. [DOI] [PubMed] [Google Scholar]
- 98.Xu X, Rock JR, Lu Y, Futtner C, Schwab B, Guinney J, Hogan BL, Onaitis MW. Evidence for type II cells as cells of origin of K-Ras-induced distal lung adenocarcinoma. Proc Natl Acad Sci USA. 2012;109:4910–4915. doi: 10.1073/pnas.1112499109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Justilien V, Regala RP, Tseng IC, Walsh MP, Batra J, Radisky ES, Murray NR, Fields AP. Matrix metalloproteinase-10 is required for lung cancer stem cell maintenance, tumor initiation and metastatic potential. PLoS ONE. 2012;7:e35040. doi: 10.1371/journal.pone.0035040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang D, Morales JE, Calame DG, Alcorn JL, Wetsel RA. Transplantation of human embryonic stem cell–derived alveolar epithelial type II cells abrogates acute lung injury in mice. Mol Ther. 2010;18:625–634. doi: 10.1038/mt.2009.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang D, Haviland DL, Burns AR, Zsigmond E, Wetsel RA. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:4449–4454. doi: 10.1073/pnas.0700052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roszell B, Mondrinos MJ, Seaton A, Simons DM, Koutzaki SH, Fong GH, Lelkes PI, Finck CM. Efficient derivation of alveolar type II cells from embryonic stem cells for in vivo application. Tissue Eng Part A. 2009;15:3351–3365. doi: 10.1089/ten.tea.2008.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Green MD, Chen A, Nostro MC, d'Souza SL, Schaniel C, Lemischka IR, Gouon-Evans V, Keller G, Snoeck HW. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotechnol. 2011;29:267–272. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Longmire TA, Ikonomou L, Hawkins F, Christodoulou C, Cao Y, Jean JC, Kwok LW, Mou H, Rajagopal J, Shen SS, et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10:398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mou H, Zhao R, Sherwood R, Ahfeldt T, Lapey A, Wain J, Sicilian L, Izvolsky K, Musunuru K, Cowan C, et al. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell. 2012;10:385–397. doi: 10.1016/j.stem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan LJ, Ratjen F, Ellis J, Rossant J. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol. 2012;30:876–882. doi: 10.1038/nbt.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lin YM, Zhang A, Rippon HJ, Bismarck A, Bishop AE. Tissue engineering of lung: the effect of extracellular matrix on the differentiation of embryonic stem cells to pneumocytes. Tissue Eng Part A. 2010;16:1515–1526. doi: 10.1089/ten.TEA.2009.0232. [DOI] [PubMed] [Google Scholar]
- 108.Wang Y, Wong LB, Mao H. Induction of ciliated cells from avian embryonic stem cells using three-dimensional matrix. Tissue Eng Part C Methods. 2010;16:929–936. doi: 10.1089/ten.tec.2009.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fluri DA, Tonge PD, Song H, Baptista RP, Shakiba N, Shukla S, Clarke G, Nagy A, Zandstra PW. Derivation, expansion and differentiation of induced pluripotent stem cells in continuous suspension cultures. Nat Methods. 2012;9:509–516. doi: 10.1038/nmeth.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kotton DN. The 2012 nobel prize in physiology or medicine: democratizing pluripotency for lung researchers. Am J Respir Crit Care Med. 2012;186:1080–1081. doi: 10.1164/rccm.201210-1857OE. [DOI] [PubMed] [Google Scholar]
- 111.Somers A, Jean JC, Sommer CA, Omari A, Ford CC, Mills JA, Ying L, Sommer AG, Jean JM, Smith BW, et al. Generation of transgene-free lung disease-specific human induced pluripotent stem cells using a single excisable lentiviral stem cell cassette. Stem Cells. 2010;28:1728–1740. doi: 10.1002/stem.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang KY, Shih HC, How CK, Chen CY, Hsu HS, Yang CW, Lee YC, Perng RP, Peng CH, Li HY, et al. IV delivery of induced pluripotent stem cells attenuates endotoxin-induced acute lung injury in mice. Chest. 2011;140:1243–1253. doi: 10.1378/chest.11-0539. [DOI] [PubMed] [Google Scholar]
- 113.Toya SP, Li F, Bonini MG, Gomez I, Mao M, Bachmaier KW, et al. Interaction of a specific population of human embryonic stem cell-derived progenitor cells with CD11b+ cells ameliorates sepsis-induced lung inflammatory injury. Am J Pathol. 2011;178:313–324. doi: 10.1016/j.ajpath.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Soh BS, Zheng D, Li Yeo JS, Yang HH, Ng SY, Wong LH, Zhang W, Li P, Nichane M, Asmat A, et al. CD166(pos) subpopulation from differentiated human ES and iPS cells support repair of acute lung injury. Mol Therapy. 2012;20:2335–2346. doi: 10.1038/mt.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Spitalieri P, Quitadamo MC, Orlandi A, Guerra L, Giardina E, Casavola V, Novelli G, Saltini C, Sangiuolo F. Rescue of murine silica-induced lung injury and fibrosis by human embryonic stem cells. Eur Respir J. 2012;39:446–457. doi: 10.1183/09031936.00005511. [DOI] [PubMed] [Google Scholar]
- 116.Fehrenbach F. Alveolar epithelial type II cells from embryonic stem cells: knights in shining armour? Eur Respir J. 2012;39:240–241. doi: 10.1183/09031936.00162111. [DOI] [PubMed] [Google Scholar]
- 117.Yoder MC. Progenitor cells in the pulmonary circulation. Proc Am Thorac Soc. 2011;8:466–470. doi: 10.1513/pats.201101-006MW. [DOI] [PubMed] [Google Scholar]
- 118.Yoder MC. Human endothelial progenitor cells. Cold Spring Harb Perspect Med. 2012;2:a006692. doi: 10.1101/cshperspect.a006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Berger S, Aronson D, Lavie P, Lavie L. Endothelial progenitor cells in acute myocardial infarction and sleep disordered breathing. Am J Respir Crit Care Med. 2013;187:90–98. doi: 10.1164/rccm.201206-1144OC. [DOI] [PubMed] [Google Scholar]
- 120.Barbera JA, Peinado VI. Vascular progenitor cells in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2011;8:528–534. doi: 10.1513/pats.201101-010MW. [DOI] [PubMed] [Google Scholar]
- 121.Asosingh K, Farha S, Lichtin A, Graham B, George D, Aldred M, Hazen SL, Loyd J, Tuder R, Erzurum SC. Pulmonary vascular disease in mice xenografted with human BM progenitors from patients with pulmonary arterial hypertension. Blood. 2012;120:1218–1227. doi: 10.1182/blood-2012-03-419275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cribbs SK, Sutcliffe DJ, Taylor WR, Rojas M, Easley KA, Tang L, Brigham KL, Martin GS. Circulating endothelial progenitor cells inversely associate with organ dysfunction in sepsis. Intensive Care Med. 2012;38:429–436. doi: 10.1007/s00134-012-2480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huertas A, Palange P. Circulating endothelial progenitor cells and chronic pulmonary diseases. Eur Respir J. 2011;37:426–431. doi: 10.1183/09031936.00034810. [DOI] [PubMed] [Google Scholar]
- 124.Imaoka H, Punia N, Irshad A, Ying S, Corrigan CJ, Howie K, O'Byrne PM, Gauvreau GM, Sehmi R. Lung homing of endothelial progenitor cells in humans with asthma after allergen challenge. Am J Respir Crit Care Med. 2011;184:771–778. doi: 10.1164/rccm.201102-0272OC. [DOI] [PubMed] [Google Scholar]
- 125.Baker CD, Balsubramanian V, Mourani PM, Sontag MK, Black CP, Ryan SL, Abman SH. Cord blood angiogenic progenitor cells are decreased in bronchopulmonary dysplasia. Eur Respir J. 2012;40:1516–1522. doi: 10.1183/09031936.00017312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yoder MC, Ingram DA. The definition of EPCs and other bone marrow cells contributing to neoangiogenesis and tumor growth: is there common ground for understanding the roles of numerous marrow-derived cells in the neoangiogenic process? Biochim Biophys Acta. 2009;1796:50–54. doi: 10.1016/j.bbcan.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yoder MC, Ingram DA. Endothelial progenitor cell: ongoing controversy for defining these cells and their role in neoangiogenesis in the murine system. Curr Opin Hematol. 2009;16:269–273. doi: 10.1097/MOH.0b013e32832bbcab. [DOI] [PubMed] [Google Scholar]
- 128.Mund JA, Estes ML, Yoder MC, Ingram DA, Jr, Case J. Flow cytometric identification and functional characterization of immature and mature circulating endothelial cells. Arterioscler Thromb Vasc Biol. 2012;2:1045–1053. doi: 10.1161/ATVBAHA.111.244210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yoder MC. Is endothelium the origin of endothelial progenitor cells? Arterioscler Thromb Vasc Biol. 2010;30:1094–1103. doi: 10.1161/ATVBAHA.109.191635. [DOI] [PubMed] [Google Scholar]
- 130.Bautch VL. Stem cells and the vasculature. Nat Med. 2011;17:1437–1443. doi: 10.1038/nm.2539. [DOI] [PubMed] [Google Scholar]
- 131.Duong HT, Erzurum SC, Asosingh K. Pro-angiogenic hematopoietic progenitor cells and endothelial colony-forming cells in pathological angiogenesis of bronchial and pulmonary circulation. Angiogenesis. 2011;14:411–422. doi: 10.1007/s10456-011-9228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chamoto K, Gibney BC, Lee GS, Lin M, Collings-Simpson D, Voswinckel R, Konerding MA, Tsuda A, Mentzer SJ. CD34+ progenitor to endothelial cell transition in post-pneumonectomy angiogenesis. Am J Respir Cell Mol Biol. 2012;46:283–289. doi: 10.1165/rcmb.2011-0249OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stevens T. Functional and molecular heterogeneity of pulmonary endothelial cells. Proc Am Thorac Soc. 2011;8:453–457. doi: 10.1513/pats.201101-004MW. [DOI] [PubMed] [Google Scholar]
- 134.Hilbe W, Dirnhofer S, Oberwasserlechner F, Schmid T, Gunsilius E, Hilbe G, Woll E, Kahler CM. CD133 positive endothelial progenitor cells contribute to the tumour vasculature in non-small cell lung cancer. J Clin Pathol. 2004;57:965–969. doi: 10.1136/jcp.2004.016444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dome B, Timar J, Dobos J, Meszaros L, Raso E, Paku S, Kenessey I, Ostoros G, Magyar M, Ladanyi A, et al. Identification and clinical significance of circulating endothelial progenitor cells in human non-small cell lung cancer. Cancer Res. 2006;66:7341–7347. doi: 10.1158/0008-5472.CAN-05-4654. [DOI] [PubMed] [Google Scholar]
- 136.Pircher A, Kahler CM, Skvortsov S, Dlaska M, Kawaguchi G, Schmid T, Gunsilius E, Hilbe W. Increased numbers of endothelial progenitor cells in peripheral blood and tumor specimens in non-small cell lung cancer: a methodological challenge and an ongoing debate on the clinical relevance. Oncol Rep. 2008;19:345–352. [PubMed] [Google Scholar]
- 137.Asosingh K, Swaidani S, Aronica M, Erzurum SC. Th1- and Th2-dependent endothelial progenitor cell recruitment and angiogenic switch in asthma. J Immunol. 2007;178:6482–6494. doi: 10.4049/jimmunol.178.10.6482. [DOI] [PubMed] [Google Scholar]
- 138.Satoh K, Kagaya Y, Nakano M, Ito Y, Ohta J, Tada H, Karibe A, Minegishi N, Suzuki N, Yamamoto M, et al. Important role of endogenous erythropoietin system in recruitment of endothelial progenitor cells in hypoxia-induced pulmonary hypertension in mice. Circulation. 2006;113:1442–1450. doi: 10.1161/CIRCULATIONAHA.105.583732. [DOI] [PubMed] [Google Scholar]
- 139.Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1073–L1084. doi: 10.1152/ajplung.00347.2006. [DOI] [PubMed] [Google Scholar]
- 140.Baker CD, Ryan SL, Ingram DA, Seedorf GJ, Abman SH, Balasubramaniam V. Endothelial colony-forming cells from preterm infants are increased and more susceptible to hyperoxia. Am J Respir Crit Care Med. 2009;180:454–461. doi: 10.1164/rccm.200901-0115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kheirandish-Gozal L, Bhattacharjee R, Kim J, Clair HB, Gozal D. Endothelial progenitor cells and vascular dysfunction in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2010;182:92–97. doi: 10.1164/rccm.200912-1845OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ormiston ML, Deng Y, Stewart DJ, Courtman DW. Innate immunity in the therapeutic actions of endothelial progenitor cells in pulmonary hypertension. Am J Respir Cell Mol Biol. 2010;43:546–554. doi: 10.1165/rcmb.2009-0152OC. [DOI] [PubMed] [Google Scholar]
- 143.Bogos K, Renyi-Vamos F, Dobos J, Kenessey I, Tovari J, Timar J, Strausz J, Ostoros G, Klepetko W, Ankersmit HJ, et al. High VEGFR-3-positive circulating lymphatic/vascular endothelial progenitor cell level is associated with poor prognosis in human small cell lung cancer. Clin Cancer Res. 2009;15:1741–1746. doi: 10.1158/1078-0432.CCR-08-1372. [DOI] [PubMed] [Google Scholar]
- 144.Arap W, Pasqualini R. Engineered embryonic endothelial progenitor cells as therapeutic Trojan horses. Cancer Cell. 2004;5:406–408. doi: 10.1016/s1535-6108(04)00121-7. [DOI] [PubMed] [Google Scholar]
- 145.Loebinger MR, Janes SM. Stem cells as vectors for antitumour therapy. Thorax. 2010;65:362–369. doi: 10.1136/thx.2009.128025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stewart DJ, Mei SH. Cell-based therapies for lung vascular diseases: lessons for the future. Proc Am Thorac Soc. 2011;8:535–540. doi: 10.1513/pats.201105-035MW. [DOI] [PubMed] [Google Scholar]
- 147.Mao M, Wang S-n, Lv X-j, Wang Y, Xu J-c. Intravenous delivery of bone marrow-derived endothelial progenitor cells improves survival and attenuates lipopolysaccharide-induced lung injury in rats. Shock. 2010;34:196–204. doi: 10.1097/SHK.0b013e3181d49457. [DOI] [PubMed] [Google Scholar]
- 148.Lam C-F, Roan J-N, Lee C-H, Chang P-J, Huang C-C, Liu Y-C, et al. Transplantation of endothelial progenitor cells improves pulmonary endothelial function and gas exchange in rabbits with endotoxin-induced acute lung injury. Anesth Analg. 2011;112:620–627. doi: 10.1213/ANE.0b013e3182075da4. [DOI] [PubMed] [Google Scholar]
- 149.Gao X, Chen W, Liang Z, Chen L. Autotransplantation of circulating endothelial progenitor cells protects against lipopolysaccharide-induced acute lung injury in rabbit. Int Immunopharmacol. 2011;11:1584–1590. doi: 10.1016/j.intimp.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 150.Balasubramaniam V, Ryan SL, Seedorf GJ, Roth EV, Heumann TR, Yoder MC, Ingram DA, Hogan CJ, Markham NE, Abman SH. Bone marrow-derived angiogenic cells restore lung alveolar and vascular structure after neonatal hyperoxia in infant mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L315–L323. doi: 10.1152/ajplung.00089.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lavoie JR, Stewart DJ. Genetically modified endothelial progenitor cells in the therapy of cardiovascular disease and pulmonary hypertension. Curr Vasc Pharmacol. 2012;10:289–299. doi: 10.2174/157016112799959413. [DOI] [PubMed] [Google Scholar]
- 152.Kahler CM, Wechselberger J, Hilbe W, Gschwendtner A, Colleselli D, Niederegger H, Boneberg EM, Spizzo G, Wendel A, Gunsilius E, et al. Peripheral infusion of rat bone marrow derived endothelial progenitor cells leads to homing in acute lung injury. Respir Res. 2007;8:50. doi: 10.1186/1465-9921-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 154.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 155.Herzog EL, Bucala R. Fibrocytes in health and disease. Exp Hematol. 2010;38:548–556. doi: 10.1016/j.exphem.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol. 2011;11:427–435. doi: 10.1038/nri2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dik WA. Acute lung injury: can the fibrocyte of today turn into the fibroguide of the future? Crit Care Med. 2010;40:300–301. doi: 10.1097/CCM.0b013e318236e7c8. [DOI] [PubMed] [Google Scholar]
- 158.Tourkina E, Bonner M, Oates J, Hofbauer A, Richard M, Znoyko S, Visconti RP, Zhang J, Hatfield CM, Silver RM, et al. Altered monocyte and fibrocyte phenotype and function in scleroderma interstitial lung disease: reversal by caveolin-1 scaffolding domain peptide. Fibrogenesis Tissue Repair. 2011;4:15. doi: 10.1186/1755-1536-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Field JJ, Burdick MD, DeBaun MR, Strieter BA, Liu L, Mehrad B, Rose CE, Jr, Linden J, Strieter RM. The role of fibrocytes in sickle cell lung disease. PLoS ONE. 2012;7:e33702. doi: 10.1371/journal.pone.0033702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gambaryan N, Cohen-Kaminsky S, Montani D, Girerd B, Huertas A, Seferian A, Humbert M, Perros F. Circulating fibrocytes and pulmonary arterial hypertension. Eur Respir J. 2012;39:210–212. doi: 10.1183/09031936.00039811. [DOI] [PubMed] [Google Scholar]
- 161.Peng H, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Curr Opin Pharmacol. 2012;12:491–496. doi: 10.1016/j.coph.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Toshner MR, Morrell NW. The fibrocyte in pulmonary hypertension: we seek him here, we seek him there. Eur Respir J. 2012;39:5–6. doi: 10.1183/09031936.00127111. [DOI] [PubMed] [Google Scholar]
- 163.Yeager ME, Nguyen CM, Belchenko DD, Colvin KL, Takatsuki S, Ivy DD, Stenmark KR. Circulating fibrocytes are increased in children and young adults with pulmonary hypertension. Eur Respir J. 2012;39:104–111. doi: 10.1183/09031936.00072311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, Margetts PJ, Farkas L, Dobranowski J, Boylan C, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–594. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 165.Fujiwara A, Kobayashi H, Masuya M, Maruyama M, Nakamura S, Ibata H, Fujimoto H, Ohnishi M, Urawa M, Naito M, et al. Correlation between circulating fibrocytes, and activity and progression of interstitial lung diseases. Respirology. 2012;17:693–698. doi: 10.1111/j.1440-1843.2012.02167.x. [DOI] [PubMed] [Google Scholar]
- 166.LaPar DJ, Burdick MD, Emaminia A, Harris DA, Strieter BA, Liu L, Robbins M, Kron IL, Strieter RM, Lau CL. Circulating fibrocytes correlate with bronchiolitis obliterans syndrome development after lung transplantation: a novel clinical biomarker. Ann Thorac Surg. 2011;92:470–477. doi: 10.1016/j.athoracsur.2011.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Quesnel C, Piednoir P, Gelly J, Nardelli L, Garnier M, Lecon V, Lasocki S, Bouadma L, Philip I, Elbim C, et al. Alveolar fibrocyte percentage is an independent predictor of poor outcome in patients with acute lung injury. Crit Care Med. 2012;40:21–28. doi: 10.1097/CCM.0b013e31822d718b. [DOI] [PubMed] [Google Scholar]
- 168.Sun L, Louie MC, Vannella KM, Wilke CA, LeVine AM, Moore BB, Shanley TP. New concepts of IL-10-induced lung fibrosis: fibrocyte recruitment and M2 activation in a CCL2/CCR2 axis. Am J Physiol Lung Cell Mol Physiol. 2011;300:L341–L353. doi: 10.1152/ajplung.00122.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gan Y, Reilkoff R, Peng X, Russell T, Chen Q, Mathai SK, Homer R, Gulati M, Siner J, Elias J, et al. Role of semaphorin 7a signaling in transforming growth factor beta1-induced lung fibrosis and scleroderma-related interstitial lung disease. Arthritis Rheum. 2011;63:2484–2494. doi: 10.1002/art.30386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hoyles RK, Derrett-Smith EC, Khan K, Shiwen X, Howat SL, Wells AU, Abraham DJ, Denton CP. An essential role for resident fibroblasts in experimental lung fibrosis is defined by lineage-specific deletion of high-affinity type II transforming growth factor beta receptor. Am J Respir Crit Care Med. 2011;183:249–261. doi: 10.1164/rccm.201002-0279OC. [DOI] [PubMed] [Google Scholar]
- 171.Wang CH, Huang CD, Lin HC, Huang TT, Lee KY, Lo YL, Lin SM, Chung KF, Kuo HP. Increased activation of fibrocytes in patients with chronic obstructive asthma through an epidermal growth factor receptor-dependent pathway. J Allergy Clin Immunol. 2012;129:1367–1376. doi: 10.1016/j.jaci.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 172.Aono Y, Ledford JG, Mukherjee S, Ogawa H, Nishioka Y, Sone S, Beers MF, Noble PW, Wright JR. Surfactant protein-D regulates effector cell function and fibrotic lung remodeling in response to bleomycin injury. Am J Respir Crit Care Med. 2012;185:525–536. doi: 10.1164/rccm.201103-0561OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Murray LA, Chen Q, Kramer MS, Hesson DP, Argentieri RL, Peng X, Gulati M, Homer RJ, Russell T, van Rooijen N, et al. TGF-beta driven lung fibrosis is macrophage dependent and blocked by serum amyloid P. Int J Biochem Cell Biol. 2011;43:154–162. doi: 10.1016/j.biocel.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 174.Nikam VS, Wecker G, Schermuly R, Rapp U, Szelepusa K, Seeger W, Voswinckel R. treprostinil inhibits the adhesion and differentiation of fibrocytes via the cyclic adenosine monophosphate-dependent and Ras-proximate protein-dependent inactivation of extracellular regulated kinase. Am J Respir Cell Mol Biol. 2011;45:692–703. doi: 10.1165/rcmb.2010-0240OC. [DOI] [PubMed] [Google Scholar]
- 175.Sato M, Hirayama S, Lara-Guerra H, Anraku M, Waddell TK, Liu M, Keshavjee S. MMP-dependent migration of extrapulmonary myofibroblast progenitors contributing to posttransplant airway fibrosis in the lung. Am J Transplant. 2009;9:1027–1036. doi: 10.1111/j.1600-6143.2009.02605.x. [DOI] [PubMed] [Google Scholar]
- 176.Vannella KM, Luckhardt TR, Wilke CA, van Dyk LF, Toews GB, Moore BB. Latent herpesvirus infection augments experimental pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:465–477. doi: 10.1164/rccm.200905-0798OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Krug LT, Torres-Gonzalez E, Qin Q, Sorescu D, Rojas M, Stecenko A, Speck SH, Mora AL. Inhibition of NF-kappaB signaling reduces virus load and gammaherpesvirus-induced pulmonary fibrosis. Am J Pathol. 2010;177:608–621. doi: 10.2353/ajpath.2010.091122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Naik PK, Bozyk PD, Bentley JK, Popova AP, Birch CM, Wilke CA, Fry CD, White ES, Sisson TH, Tayob N, et al. Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303:L1046–L1056. doi: 10.1152/ajplung.00139.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ishii G, Ito TK, Aoyagi K, Fujimoto H, Chiba H, Hasebe T, Fujii S, Nagai K, Sasaki H, Ochiai A. Presence of human circulating progenitor cells for cancer stromal fibroblasts in the blood of lung cancer patients. Stem Cells. 2007;25:1469–1477. doi: 10.1634/stemcells.2006-0449. [DOI] [PubMed] [Google Scholar]
- 180.Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, Alison MR, Wright NA. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492–8495. doi: 10.1158/0008-5472.CAN-04-1708. [DOI] [PubMed] [Google Scholar]
- 181.Hodges RJ, Lim R, Jenkin G, Wallace EM. Amnion epithelial cells as a candidate therapy for acute and chronic lung injury. Stem Cells Int. 2012;2012:709–763. doi: 10.1155/2012/709763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 2007;77:577–588. doi: 10.1095/biolreprod.106.055244. [DOI] [PubMed] [Google Scholar]
- 183.Da Sacco S, Sedrakyan S, Boldrin F, Giuliani S, Parnigotto P, Habibian R, Warburton D, De Filippo RE, Perin L. Human amniotic fluid as a potential new source of organ specific precursor cells for future regenerative medicine applications. J Urol. 2010;183:1193–1200. doi: 10.1016/j.juro.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Carraro G, Perin L, Sedrakyan S, Giuliani S, Tiozzo C, Lee J, Turcatel G, De Langhe SP, Driscoll B, Bellusci S, et al. Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages. Stem Cells. 2008;26:2902–2911. doi: 10.1634/stemcells.2008-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Perin L, Sedrakyan S, Giuliani S, Da Sacco S, Carraro G, Shiri L, Lemley KV, Rosol M, Wu S, Atala A, et al. Protective effect of human amniotic fluid stem cells in an immunodeficient mouse model of acute tubular necrosis. PLoS ONE. 2010;5:e9357. doi: 10.1371/journal.pone.0009357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Angelini A, Castellani C, Ravara B, Franzin C, Pozzobon M, Tavano R, Libera LD, Papini E, Vettor R, De Coppi P, et al. Stem-cell therapy in an experimental model of pulmonary hypertension and right heart failure: role of paracrine and neurohormonal milieu in the remodeling process. J Heart Lung Transplant. 2011;30:1281–1293. doi: 10.1016/j.healun.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 187.Mirabella T, Poggi A, Scaranari M, Mogni M, Lituania M, Baldo C, Cancedda R, Gentili C. Recruitment of host's progenitor cells to sites of human amniotic fluid stem cells implantation. Biomaterials. 2011;32:4218–4227. doi: 10.1016/j.biomaterials.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 188.Paracchini V, Carbone A, Colombo F, Castellani S, Mazzucchelli S, Gioia SD, Degiorgio D, Seia M, Porretti L, Colombo C, et al. Amniotic mesenchymal stem cells: a new source for hepatocyte-like cells and induction of CFTR expression by coculture with cystic fibrosis airway epithelial cells. J Biomed Biotechnol. 2012;2012:575471. doi: 10.1155/2012/575471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hodges RJ, Jenkin G, Hooper SB, Allison B, Lim R, Dickinson H, Miller SL, Vosdoganes P, Wallace EM.Human amnion epithelial cells reduce ventilation-induced preterm lung injury in fetal sheep. Am J Obstet Gynecol 2012;206:448.e8–e15. [DOI] [PubMed]
- 190.Moodley Y, Ilancheran S, Samuel C, Vaghjiani V, Atienza D, Williams ED, Jenkin G, Wallace E, Trounson A, Manuelpillai U. Human amnion epithelial cell transplantation abrogates lung fibrosis and augments repair. Am J Respir Crit Care Med. 2010;182:643–651. doi: 10.1164/rccm.201001-0014OC. [DOI] [PubMed] [Google Scholar]
- 191.Murphy SV, Lim R, Heraud P, Cholewa M, Le Gros M, de Jonge MD, Howard DL, Paterson D, McDonald C, Atala A, et al. Human amnion epithelial cells induced to express functional cystic fibrosis transmembrane conductance regulator. PLoS ONE. 2012;7:e46533. doi: 10.1371/journal.pone.0046533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cargnoni A, Gibelli L, Tosini A, Signoroni PB, Nassuato C, Arienti D, Lombardi G, Albertini A, Wengler GS, Parolini O. Transplantation of allogeneic and xenogeneic placenta-derived cells reduces bleomycin-induced lung fibrosis. Cell Transplant. 2009;18:405–422. doi: 10.3727/096368909788809857. [DOI] [PubMed] [Google Scholar]
- 193.Murphy S, Lim R, Dickinson H, Acharya R, Rosli S, Jenkin G, Wallace E. Human amnion epithelial cells prevent bleomycin-induced lung injury and preserve lung function. Cell Transplant. 2011;20:909–923. doi: 10.3727/096368910X543385. [DOI] [PubMed] [Google Scholar]
- 194.Vosdoganes P, Hodges RJ, Lim R, Westover AJ, Acharya RY, Wallace EM, Moss TJ.Human amnion epithelial cells as a treatment for inflammation-induced fetal lung injury in sheep. Am J Obstet Gynecol 2011;205:156.e26–e33. [DOI] [PubMed]
- 195.Murphy SV, Shiyun SC, Tan JL, Chan S, Jenkin G, Wallace EM, Lim R. Human amnion epithelial cells do not abrogate pulmonary fibrosis in mice with impaired macrophage function. Cell Transplant. 2012;21:1477–1492. doi: 10.3727/096368911X601028. [DOI] [PubMed] [Google Scholar]
- 196.Cargnoni A, Ressel L, Rossi D, Poli A, Arienti D, Lombardi G, Parolini O. Conditioned medium from amniotic mesenchymal tissue cells reduces progression of bleomycin-induced lung fibrosis. Cytotherapy. 2012;14:153–161. doi: 10.3109/14653249.2011.613930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 198.Prockop DJ, Oh JY. Medical therapies with adult stem/progenitor cells (MSCs): a backward journey from dramatic results in vivo to the cellular and molecular explanations. J Cell Biochem. 2012;113:1460–1469. doi: 10.1002/jcb.24046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Loi R, Beckett T, Goncz KK, Suratt BT, Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med. 2006;173:171–179. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL, Weiss DJ. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med. 2008;177:701–711. doi: 10.1164/rccm.200706-859OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ma N, Gai H, Mei J, Ding FB, Bao CR, Nguyen DM, Zhong H. Bone marrow mesenchymal stem cells can differentiate into type II alveolar epithelial cells in vitro. Cell Biol Int. 2011;35:1261–1266. doi: 10.1042/CBI20110026. [DOI] [PubMed] [Google Scholar]
- 202.Maria OM, Tran SD. Human mesenchymal stem cells cultured with salivary gland biopsies adopt an epithelial phenotype. Stem Cells Dev. 2011;20:959–967. doi: 10.1089/scd.2010.0214. [DOI] [PubMed] [Google Scholar]
- 203.Li H, Xu Y, Fu Q, Li C. Effects of multiple agents on epithelial differentiation of rabbit adipose-derived stem cells in 3D culture. Tissue Eng Part A. 2012;18:1760–1770. doi: 10.1089/ten.tea.2011.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yan C, Qu P, Du H. Myeloid-specific expression of Stat3C results in conversion of bone marrow mesenchymal stem cells into alveolar type II epithelial cells in the lung. Sci China Life Sci. 2012;55:576–590. doi: 10.1007/s11427-012-4339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Baer PC. Adipose-derived stem cells and their potential to differentiate into the epithelial lineage. Stem Cells Dev. 2011;20:1805–1816. doi: 10.1089/scd.2011.0086. [DOI] [PubMed] [Google Scholar]
- 206.Le Blanc K, Mougiakakos D.Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol 2012;12:383–396. [DOI] [PubMed]
- 207.Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, Wang CY. The meaning, the sense, and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Francois M, Galipeau FM. New insights on translational development of mesenchymal stromal cells for suppressor therapy. J Cell Physiol. 2012;227:3535–3538. doi: 10.1002/jcp.24081. [DOI] [PubMed] [Google Scholar]
- 209.Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol. 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 210.Boregowda SV, Phinney DG. Therapeutic applications of mesenchymal stem cells: current outlook. BioDrugs. 2012;26:201–208. doi: 10.1007/BF03261879. [DOI] [PubMed] [Google Scholar]
- 211.Romieu-Mourez R, Coutu DL, Galipeau J. The immune plasticity of mesenchymal stromal cells from mice and men: concordances and discrepancies. Front Biosci. 2012;4:824–837. doi: 10.2741/E422. [DOI] [PubMed] [Google Scholar]
- 212.Baer PC, Geiger H.Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int 2012;2012:812693. [DOI] [PMC free article] [PubMed]
- 213.Phinney DG. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J Cell Biochem. 2012;113:2806–2812. doi: 10.1002/jcb.24166. [DOI] [PubMed] [Google Scholar]
- 214.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 215.Pek S, Wan ACA, Ying JY. The effect of matrix stiffness on mesenchymal stem cell differentiation in a 3D thixotropic gel. Biomaterials. 2010;31:385–391. doi: 10.1016/j.biomaterials.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 216.Lindner U, Kramer J, Behrends J, Driller B, Wendler NO, Boehrnsen F, Rohwedel J, Schlenke P. Improved proliferation and differentiation capacity of human mesenchymal stromal cells cultured with basement-membrane extracellular matrix proteins. Cytotherapy. 2010;12:992–1005. doi: 10.3109/14653249.2010.510503. [DOI] [PubMed] [Google Scholar]
- 217.Mathieu PS, Loboa EG. Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Eng Part B Rev. 2012;18:436–444. doi: 10.1089/ten.teb.2012.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kulangara K, Yang Y, Yang J, Leong KW. Nanotopography as modulator of human mesenchymal stem cell function. Biomaterials. 2012;33:4998–5003. doi: 10.1016/j.biomaterials.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yourek G, McCormick SM, Mao JJ, Reilly GC. Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regen Med. 2010;5:713–724. doi: 10.2217/rme.10.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Michalopoulos E, Knight RL, Korossis S, Kearney JN, Fisher J, Ingham E. Development of methods for studying the differentiation of human mesenchymal stem cells under cyclic compressive strain. Tissue Eng Part C Methods. 2012;18:252–262. doi: 10.1089/ten.tec.2011.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Das R, Jahr H, van Osch GJ, Farrell E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev. 2010;16:159–168. doi: 10.1089/ten.TEB.2009.0296. [DOI] [PubMed] [Google Scholar]
- 222.Boregowda SV, Krishnappa V, Chambers JW, Lograsso PV, Lai WT, Ortiz LA, Phinney DG. Atmospheric oxygen inhibits growth and differentiation of marrow-derived mouse mesenchymal stem cells via a p53-dependent mechanism: implications for long-term culture expansion. Stem Cells. 2012;30:975–987. doi: 10.1002/stem.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Prockop DJ. A long-awaited discovery: hypoxia prevents mouse cells from undergoing spontaneous p53-dependent transformation. Cytotherapy. 2012;14:1029–1031. doi: 10.3109/14653249.2012.718887. [DOI] [PubMed] [Google Scholar]
- 224.Jarvinen L, Badri L, Wettlaufer S, Ohtsuka T, Standiford TJ, Toews GB, Pinsky DJ, Peters-Golden M, Lama VN. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J Immun. 2008;181:4389–4396. doi: 10.4049/jimmunol.181.6.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Karoubi G, Cortes-Dericks L, Breyer I, et al. Identification of mesenchymal stromal cells in human lung parenchyma capable of differentiating into aquaporin 5-expressing cells. Lab Invest. 2009;89:1100–1114. doi: 10.1038/labinvest.2009.73. [DOI] [PubMed] [Google Scholar]
- 226.Bozyk PD, Popova AP, Bentley JK, Goldsmith AM, Linn MJ, Weiss DJ, Hershenson MB. Mesenchymal stromal cells from neonatal tracheal aspirates demonstrate a pattern of lung-specific gene expression. Stem Cells Dev. 2011;20:1995–2007. doi: 10.1089/scd.2010.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ricciardi M, Malpeli G, Bifari F, Bassi G, Pacelli L, Nwabo Kamdje AH, Chilosi M, Krampera M. Comparison of epithelial differentiation and immune regulatory properties of mesenchymal stromal cells derived from human lung and bone marrow. PLoS ONE. 2013;7:e35639. doi: 10.1371/journal.pone.0035639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Crestani B. Bronchoalveolar lavage brings mesenchymal stem cells to the light. Am J Respir Crit Care Med. 2012;185:7–8. doi: 10.1164/rccm.201110-1920ED. [DOI] [PubMed] [Google Scholar]
- 229.Walker N, Badri L, Wettlaufer S, Flint A, Sajjan U, Krebsbach PH, Keshamouni VG, Peters-Golden M, Lama VN. Resident tissue-specific mesenchymal progenitor cells contribute to fibrogenesis in human lung allografts. Am J Pathol. 2011;178:2461–2469. doi: 10.1016/j.ajpath.2011.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Badri L, Murray S, Liu LX, Walker NM, Flint A, Wadhwa A, Chan KM, Toews GB, Pinsky DJ, Martinez FJ, et al. Mesenchymal stromal cells in bronchoalveolar lavage as predictors of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2011;183:1062–1070. doi: 10.1164/rccm.201005-0742OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Jun D, Garat C, West J, Thorn N, Chow K, Cleaver T, Sullivan T, Torchia EC, Childs C, Shade T, et al. The pathology of bleomycin-induced fibrosis is associated with loss of resident lung mesenchymal stem cells that regulate effector T-cell proliferation. Stem Cells. 2011;29:725–735. doi: 10.1002/stem.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Badri L, Walker NM, Ohtsuka T, Wang Z, Delmar M, Flint A, Peters-Golden M, Toews GB, Pinsky DJ, Krebsbach PH, et al. Epithelial interactions and local engraftment of lung-resident mesenchymal stem cells. Am J Respir Cell Mol Biol. 2011;45:809–816. doi: 10.1165/rcmb.2010-0446OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Badri L, Lama VN. Lysophosphatidic acid induces migration of human lung-resident mesenchymal stem cells through the beta-catenin pathway. Stem Cells. 2012;30:2010–2019. doi: 10.1002/stem.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ingenito E, Tsai L, Murthy S, Tyagi S, Mazan M, Hoffman A. Autologous lung-derived mesenchymal stem cell transplantation in experimental emphysema. Cell Transplant. 2012;21:175–189. doi: 10.3727/096368910X550233. [DOI] [PubMed] [Google Scholar]
- 235.Nystedt J, Anderson H, Tikkanen J, Pietilä M, Hirvonen T, Takalo R, Heiskanen A, Satomaa T, Natunen S, Lehtonen S, et al. Cell surface structures influence lung clearance rate of systemically infused mesenchymal stromal cells. Stem Cells. 2013;31:317–326. doi: 10.1002/stem.1271. [DOI] [PubMed] [Google Scholar]
- 236.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Epperly MW, Guo H, Gretton JE, Greenberger JS. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29:213–224. doi: 10.1165/rcmb.2002-0069OC. [DOI] [PubMed] [Google Scholar]
- 238.Yan X, Liu Y, Han Q, Jia M, Liao L, Qi M, Zhao RC. Injured microenvironment directly guides the differentiation of engrafted Flk-1(+) mesenchymal stem cell in lung. Exp Hematol. 2007;35:1466–1475. doi: 10.1016/j.exphem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 239.Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12:126–131. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS ONE. 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 242.Ylostalo JH, Bartosh TJ, Coble K, Prockop DJ. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells. 2012;30:2283–2296. doi: 10.1002/stem.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Romieu-Mourez FM, Stock-Martineau S, Boivin MN, Bramson JL, Galipeau J. Mesenchymal stromal cells cross-present soluble exogenous antigens as part of their antigen-presenting cell properties. Blood. 2009;114:2632–2638. doi: 10.1182/blood-2009-02-207795. [DOI] [PubMed] [Google Scholar]
- 244.Aliotta JM, Lee D, Puente N, Faradyan S, Sears E, Amaral A, Goldberg L, Dooner MS, Pereira M, Quesenberry PJ. Progenitor/stem cell fate determination: interactive dynamics of cell cycle and microvesicles. Stem Cells Dev. 2012;21:1627–1638. doi: 10.1089/scd.2011.0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhang H, Liu X, Huang S, Bi X, Wang H, Xie L, Wang Y, Cao X, Xiao F, Yang Y, et al. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 2012;21:3289–3297. doi: 10.1089/scd.2012.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Thebaud B, Stewart DJ. Exosomes: cell garbage can, therapeutic carrier, or trojan horse? Circulation. 2012;126:2553–2555. doi: 10.1161/CIRCULATIONAHA.112.146738. [DOI] [PubMed] [Google Scholar]
- 247.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;8:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Hemeda H, Jakob M, Ludwig A, Giebel B, Lang S, Brandau S. Interferon-γ and tumor necrosis factor-a differentially affect cytokine expression and migration properties of mesenchymal stem cells. Stem Cells Dev. 2010;19:693–706. doi: 10.1089/scd.2009.0365. [DOI] [PubMed] [Google Scholar]
- 249.Waszak P, Alphonse R, Vadivel A, Ionescu L, Eaton F, Thebaud B. Preconditioning enhances the paracrine effect of mesenchymal stem cells in preventing oxygen-induced neonatal lung injury in rats. Stem Cells Dev. 2012;21:2789–2797. doi: 10.1089/scd.2010.0566. [DOI] [PubMed] [Google Scholar]
- 250.Francois M, Copland IB, Yuan S, Romieu-Mourez R, Waller EK, Galipeau J. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-gamma licensing. Cytotherapy. 2012;14:147–152. doi: 10.3109/14653249.2011.623691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Matthay MA, Taylor Thompson B, Read EJ, McKenna DH, Jr, Liu KD, Calfee CS, Lee JW. Therapeutic potential of mesenchymal stem cells for severe acute lung injury. Chest. 2010;138:965–972. doi: 10.1378/chest.10-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Matthay MA, Goolaerts A, Howard JP, Lee JW. Mesenchymal stem cells for acute lung injury: preclinical evidence. Crit Care Med. 2010;38:S569–S573. doi: 10.1097/CCM.0b013e3181f1ff1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Pierro M, Thebaud B. Mesenchymal stem cells in chronic lung disease: culprit or savior? Am J Physiol Lung Cell Mol Physiol. 2010;298:L732–L734. doi: 10.1152/ajplung.00099.2010. [DOI] [PubMed] [Google Scholar]
- 254.Gotts JE, Matthay MA. Mesenchymal stem cells and acute lung injury. Crit Care Clin. 2011;27:719–733. doi: 10.1016/j.ccc.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise review: mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011;29:913–919. doi: 10.1002/stem.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Gotts JE, Matthay MA. Mesenchymal stem cells and the stem cell niche: a new chapter. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1147–L1149. doi: 10.1152/ajplung.00122.2012. [DOI] [PubMed] [Google Scholar]
- 257.Lee JW, Zhu Y, Matthay MA. Cell-based therapy for acute lung injury: are we there yet? Anesthesiology. 2012;116:1189–1191. doi: 10.1097/ALN.0b013e3182567fc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Rankin S. Mesenchymal stem cells. Thorax. 2012;67:565–566. doi: 10.1136/thoraxjnl-2012-201923. [DOI] [PubMed] [Google Scholar]
- 259.Danchuk S, Ylostalo JH, Hossain F, Sorge R, Ramsey A, Bonvillain RW, Lasky JA, Bunnell BA, Welsh DA, Prockop DJ, et al. Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-alpha-induced protein 6. Stem Cell Res Ther. 2011;2:27. doi: 10.1186/scrt68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kim ES, Chang YS, Choi SJ, Kim JK, Yoo HS, Ahn SY, Sung DK, Kim SY, Park YR, Park WS. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells attenuates Escherichia coli-induced acute lung injury in mice. Respir Res. 2011;12:108. doi: 10.1186/1465-9921-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sun J, Han ZB, Liao W, Yang SG, Yang Z, Yu J, Meng L, Wu R, Han ZC. Intrapulmonary delivery of human umbilical cord mesenchymal stem cells attenuates acute lung injury by expanding CD4+CD25+ Forkhead Boxp3 (FOXP3)+ regulatory T cells and balancing anti- and pro-inflammatory factors. Cell Physiol Biochem. 2011;27:587–596. doi: 10.1159/000329980. [DOI] [PubMed] [Google Scholar]
- 262.Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, Serikov V, Matthay MA. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67:533–539. doi: 10.1136/thoraxjnl-2011-201176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Xu YL, Liu YL, Wang Q, Li G, Lu XD, Kong B. Intravenous transplantation of mesenchymal stem cells attenuates oleic acid induced acute lung injury in rats. Chin Med J (Engl) 2012;125:2012–2018. [PubMed] [Google Scholar]
- 264.Ionescu L, Byrne RN, van Haaftern T, Vadivel A, Alphonse RS, Rey-Parra GJ, Weissmann G, Hall A, Eaton F, Thebaud B. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012;303:L967–L977. doi: 10.1152/ajplung.00144.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Zhang S, et al. Comparison of the therapeutic effects of human and mouse adipose-derived stem cells in a murine model of lipopolysaccharide-induced acute lung injury. Stem Cell Res Ther. 2013;4:13. doi: 10.1186/scrt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Firinci F, Karaman M, Baran Y, Bagriyanik A, Ayyildiz ZA, Kiray M, Kozanoglu I, Yilmaz O, Uzuner N, Karaman O. Mesenchymal stem cells ameliorate the histopathological changes in a murine model of chronic asthma. Int Immunopharmacol. 2011;11:1120–1126. doi: 10.1016/j.intimp.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 267.Goodwin M, Sueblinvong V, Eisenhauer P, Ziats NP, Leclair L, Poynter ME, Steele C, Rincon M, Weiss DJ. Bone marrow derived mesenchymal stromal cells inhibit th2-mediated allergic airways inflammation in mice. Stem Cells. 2011;29:1137–1148. doi: 10.1002/stem.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kapoor S, Patel SA, Kartan S, Axelrod D, Capitle E, Rameshwar P. Tolerance-like mediated suppression by mesenchymal stem cells in patients with dust mite allergy-induced asthma. J Allergy Clin Immunol. 2011;129:1094–1101. doi: 10.1016/j.jaci.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 269.Kavanagh H, Mahon BP. Allogeneic mesenchymal stem cells prevent allergic airway inflammation by inducing murine regulatory T cells. Allergy. 2011;66:523–531. doi: 10.1111/j.1398-9995.2010.02509.x. [DOI] [PubMed] [Google Scholar]
- 270.Lee SH, Jang AS, Kwon JH, Park SK, Won JH, Park CS.Mesenchymal stem cell transfer suppresses airway remodeling in a toluene diisocyanate-induced murine asthma model. Allergy Asthma Immunol Res 2011;3:205–211. [DOI] [PMC free article] [PubMed]
- 271.Ou-Yang H-F, Huang Y, Hu X-B, Wu C-G. Suppression of allergic airway inflammation in a mouse model of asthma by exogenous mesenchymal stem cells. Exp Biol Med. 2011;236:1461–1467. doi: 10.1258/ebm.2011.011221. [DOI] [PubMed] [Google Scholar]
- 272.Ionescu LI, Alphonse RS, Arizmendi N, Morgan B, Abel M, Eaton F, Duszyk M, Vliagoftis H, Aprahamian TR, Walsh K, et al. Airway delivery of soluble factors from plastic-adherent bone marrow cells prevents murine asthma. Am J Respir Cell Mol Biol. 2012;46:207–216. doi: 10.1165/rcmb.2010-0391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Chang YS, Choi SJ, Sung DK, Kim SY, Oh W, Yang YS, Park WS. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells dose-dependently attenuates hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2011;20:1843–1854. doi: 10.3727/096368911X565038. [DOI] [PubMed] [Google Scholar]
- 274.Pierro M, Ionescu L, Montemurro T, Vadivel A, Weissmann G, Oudit G, Emery D, Bodiga S, Eaton F, Peault B, et al. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax. 2013;68:475–484. doi: 10.1136/thoraxjnl-2012-202323. [DOI] [PubMed] [Google Scholar]
- 275.Zhang H, Fang J, Su H, Yang M, Lai W, Mai Y, Wu Y. Bone marrow mesenchymal stem cells attenuate lung inflammation of hyperoxic newborn rats. Pediatr Transplant. 2010;16:589–598. doi: 10.1111/j.1399-3046.2012.01709.x. [DOI] [PubMed] [Google Scholar]
- 276.Zhang X, Wang H, Shi Y, Peng W, Zhang S, Zhang W, Xu J, Mei Y, Feng Z. Role of bone marrow-derived mesenchymal stem cells in the prevention of hyperoxia-induced lung injury in newborn mice. Cell Biol Int. 2012;36:589–594. doi: 10.1042/CBI20110447. [DOI] [PubMed] [Google Scholar]
- 277.Tropea KA, Leder E, Aslam M, Lau AN, Raiser DM, Lee JH, Balasubramaniam V, Fredenburgh LE, Mitsialis A, Kourembanas S, et al. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2012;302:L829–L837. doi: 10.1152/ajplung.00347.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Sutsko RP, Young KC, Ribeiro A, Torres E, Rodriguez M, Hehre D, Devia C, McNeice I, Suguihira C. Long-term reparative effects of mesenchymal stem cell therapy following neonatal hyperoxia-induced lung injury. Pediatr Res. 2013;73:46–53. doi: 10.1038/pr.2012.152. [DOI] [PubMed] [Google Scholar]
- 279.Hoffman AM, Paxson JA, Mazan MR, Davis AM, Tyagi S, Murthy S, Ingenito EP. Lung-derived mesenchymal stromal cell post-transplantation survival, persistence, paracrine expression, and repair of elastase-injured lung. Stem Cells Dev. 2011;20:1779–1792. doi: 10.1089/scd.2011.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Katsha AM, Ohkouchi S, Xin H, Kanehira M, Sun R, Nukiwa T, Saijo Y. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol Ther. 2011;19:196–203. doi: 10.1038/mt.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Schweitzer K, Johnstone BH, Garrison J, Rush N, Cooper S, Traktuev DO, Feng D, Adamowicz JJ, Van Demark M, Fisher AJ, et al. Adipose stem cell treatment in mice attenuates lung and systemic injury induced by cigarette smoking. Am J Respir Crit Care Med. 2011;183:215–225. doi: 10.1164/rccm.201001-0126OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Kim SY, Lee JH, Kim HJ, Park MK, Huh JW, Ro JY, Oh YM, Lee SD, Lee YS. Mesenchymal stem cell-conditioned media recovers lung fibroblasts from cigarette smoke-induced damage. Am J Physiol Lung Cell Mol Physiol. 2012;302:L891–L908. doi: 10.1152/ajplung.00288.2011. [DOI] [PubMed] [Google Scholar]
- 283.Sun CK, Yen CH, Lin YC, Tsai TH, Chang LT, Kao YH, Chua S, Fu M, Ko SF, Leu S, et al. Autologous transplantation of adipose-derived mesenchymal stem cells markedly reduced acute ischemia-reperfusion lung injury in a rodent model. J Transl Med. 2011;9:118. doi: 10.1186/1479-5876-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Saito S, Nakayama T, Hashimoto N, Miyata Y, Egashira K, Nakao N, Nishiwaki S, Hasegawa M, Hasegawa Y, Naoe T. Mesenchymal stem cells stably transduced with a dominant-negative inhibitor of CCL2 greatly attenuate bleomycin-induced lung damage. Am J Pathol. 2011;179:1088–1094. doi: 10.1016/j.ajpath.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Lim R, Miltonic P, Murphy S, Dickinson H, Chan ST, Jenkin G. Human mesenchymal stem cells reduce lung injury in immunocompromised mice but not in immunocompetent mice. Respiration. 2013;85:332–341. doi: 10.1159/000343078. [DOI] [PubMed] [Google Scholar]
- 286.Kanki-Horimoto S, Horimoto H, Mieno S, Kishida K, Watanabe F, Furuya E, Katsumata T. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation. 2006;114(Suppl):I181–I185. doi: 10.1161/CIRCULATIONAHA.105.001487. [DOI] [PubMed] [Google Scholar]
- 287.Umar S, de Visser YP, Steendijk P, et al. Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2009;297:H1606–H1616. doi: 10.1152/ajpheart.00590.2009. [DOI] [PubMed] [Google Scholar]
- 288.Jungebluth P, Luedde M, Ferrer E, Luedde T, Vucur M, Peinado VI, Go T, Schreiber C, von Richthofen M, Bader A, et al. Mesenchymal stem cells restore lung function by recruiting resident and nonresident proteins. Cell Transplant. 2011;20:1561–1574. doi: 10.3727/096368910X557254. [DOI] [PubMed] [Google Scholar]
- 289.Liang OD, Mitsialis SA, Chang MS, Vergadi E, Lee C, Aslam M, Fernandez-Gonzalez A, Liu X, Baveja R, Kourembanas S. Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells. 2011;29:99–107. doi: 10.1002/stem.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Hansmann G, Fernandez-Gonzalez A, Aslam M, Vitali SH, Martin T, Mitsialis SA, Kourembanas S. Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm Circ. 2012;2:170–181. doi: 10.4103/2045-8932.97603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee JW, Gupta N, Petrini M, Matthay MA. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1003–L1013. doi: 10.1152/ajplung.00180.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Curley GF, Hayes M, Ansari B, Shaw G, Ryan A, Barry F, O’Brien T, O’Toole D, Laffey JG. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax. 2012;67:496–501. doi: 10.1136/thoraxjnl-2011-201059. [DOI] [PubMed] [Google Scholar]
- 294.Pati S, Gerber M, Menge TD, et al. Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS One 2011;6:e25171. [DOI] [PMC free article] [PubMed]
- 295.Chimenti L, Luque T, Bonsignore MR, Ramirez J, Navajas D, Farre R. Pre-treatment with mesenchymal stem cells reduces ventilator-induced lung injury. Eur Respir J. 2012;40:939–948. doi: 10.1183/09031936.00153211. [DOI] [PubMed] [Google Scholar]
- 296.Shi D, Wang D, Li X, Zhang H, Che N, Lu Z, Sun L. Allogeneic transplantation of umbilical cord-derived mesenchymal stem cells for diffuse alveolar hemorrhage in systemic lupus erythematosus. Clin Rheumatol. 2012;31:841–846. doi: 10.1007/s10067-012-1943-2. [DOI] [PubMed] [Google Scholar]
- 297.Wang L, Tu XH, Zhao P, Song JX, Zou ZD. Protective effect of transplanted bone marrow-derived mesenchymal stem cells on pancreatitis-associated lung injury in rats. Mol Med Rep. 2012;6:287–292. doi: 10.3892/mmr.2012.922. [DOI] [PubMed] [Google Scholar]
- 298.Heo SC, Lee KO, Shin SH, Kwon YW, Kim YM, Lee CH, Kim YD, Lee MK, Yoon MS, Kim JH. Periostin mediates human adipose tissue-derived mesenchymal stem cell-stimulated tumor growth in a xenograft lung adenocarcinoma model. Biochim Biophys Acta. 2011;1813:2061–2070. doi: 10.1016/j.bbamcr.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 299.Hu YL, Huang B, Zhang TY, Miao PH, Tang GP, Tabata Y, Gao JQ. Mesenchymal stem cells as a novel carrier for targeted delivery of gene in cancer therapy based on nonviral transfection. Mol Pharm. 2012;9:2698–2709. doi: 10.1021/mp300254s. [DOI] [PubMed] [Google Scholar]
- 300.Chen Q, Cheng P, Yin T, He H, Yang L, Wei Y, Chen X. Therapeutic potential of bone marrow-derived mesenchymal stem cells producing pigment epithelium-derived factor in lung carcinoma. Int J Mol Med. 2012;30:527–534. doi: 10.3892/ijmm.2012.1015. [DOI] [PubMed] [Google Scholar]
- 301.Lee J, Fang X, Gupta N, et al. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Nazarov I, Lee JW, Soupene E, Etemad S, Knapik D, Green W, Bashkirova E, Fang X, Matthay MA, Kuypers FA, et al. Multipotent stromal stem cells from human placenta demonstrate high therapeutic potential. Stem Cells Transl Med. 2012;1:359–372. doi: 10.5966/sctm.2011-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA.Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med 2013;187:751–760. [DOI] [PMC free article] [PubMed]
- 304.Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biochem. 2010;285:26211–26222. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Pati S, Khakoo AY, Zhao J, Jimenez F, Gerber MH, Harting M, Redell JB, Grill R, Matsuo Y, Guha S, et al. Human mesenchymal stem cells inhibit vascular permeability by modulating vascular endothelial cadherin/beta-catenin signaling. Stem Cells Dev. 2011;20:89–101. doi: 10.1089/scd.2010.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, Matthay MA. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28:2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Haniffa MA, Wang XN, Holtick U, Rae M, Isaacs JD, Dickinson AM, Hilkens CM, Collin MP. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol. 2007;179:1595–1604. doi: 10.4049/jimmunol.179.3.1595. [DOI] [PubMed] [Google Scholar]
- 308.Sabatini F, Petecchia L, Tavian M, Jodon de Villeroche V, Rossi GA, Brouty-Boye D. Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Lab Invest. 2005;85:962–971. doi: 10.1038/labinvest.3700300. [DOI] [PubMed] [Google Scholar]
- 309.McCarter SD, Mei SH, Lai PF, Zhang QW, Parker CH, Suen RS, Hood RD, Zhao YD, Deng Y, Han RN, et al. Cell-based angiopoietin-1 gene therapy for acute lung injury. Am J Respir Crit Care Med. 2007;175:1014–1026. doi: 10.1164/rccm.200609-1370OC. [DOI] [PubMed] [Google Scholar]
- 310.Lassance RM, Prota LF, Maron-Gutierrez T, Garcia CS, Abreu SC, Passaro CP, Xisto DG, Castiglione RC, Carreira H, Jr, Ornellas DS, et al. Intratracheal instillation of bone marrow-derived cell in an experimental model of silicosis. Respir Physiol Neurobiol. 2009;169:227–233. doi: 10.1016/j.resp.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 311.Araujo IM, Abreu SC, Maron-Gutierrez T, Cruz F, Fujisaki L, Carreira H, Jr, Ornellas F, Ornellas D, Vieira-de-Abreu A, Castro-Faria-Neto HC, et al. Bone marrow-derived mononuclear cell therapy in experimental pulmonary and extrapulmonary acute lung injury. Crit Care Med. 2010;38:1733–1741. doi: 10.1097/CCM.0b013e3181e796d2. [DOI] [PubMed] [Google Scholar]
- 312.Prota LF, Lassance RM, Maron-Gutierrez T, Castiglione RC, Garcia CS, Santana MC, Souza-Menezes J, Abreu SC, Samoto V, Santiago MF, et al. Bone marrow mononuclear cell therapy led to alveolar-capillary membrane repair, improving lung mechanics in endotoxin-induced acute lung injury. Cell Transplant. 2010;19:965–971. doi: 10.3727/096368910X506845. [DOI] [PubMed] [Google Scholar]
- 313.Abreu SC, Antunes MA, Maron-Gutierrez T, Cruz FF, Carmo LG, Ornellas DS, Junior HC, Absaber AM, Parra ER, Capelozzi VL, et al. Effects of bone marrow-derived mononuclear cells on airway and lung parenchyma remodeling in a murine model of chronic allergic inflammation. Respir Physiol Neurobiol. 2011;175:153–163. doi: 10.1016/j.resp.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 314.Cabral RM, Branco E, Rizzo Mdos S, Ferreira GJ, Gregores GB, Samoto VY, Stopiglia AJ, Maiorka PC, Fioretto ET, Capelozzi VL, et al. Cell therapy for fibrotic interstitial pulmonary disease: experimental study. Microsc Res Tech. 2011;74:957–962. doi: 10.1002/jemt.20981. [DOI] [PubMed] [Google Scholar]
- 315.Maron-Gutierrez T, Castiglione RC, Xisto DG, Oliveira MG, Cruz FF, Pecanha R, Carreira-Junior H, Ornellas DS, Moraes MO, Takiya CM, et al. Bone marrow-derived mononuclear cell therapy attenuates silica-induced lung fibrosis. Eur Respir J. 2011;37:1217–1225. doi: 10.1183/09031936.00205009. [DOI] [PubMed] [Google Scholar]
- 316.Ornellas DS, Maron-Gutierrez T, Ornellas FM, Cruz FF, Oliveira GP, Lucas IH, Fujisaki L, Oliveira MG, Teodoro WR, Capelozzi VL, et al. Early and late effects of bone marrow-derived mononuclear cell therapy on lung and distal organs in experimental sepsis. Respir Physiol Neurobiol. 2011;178:304–314. doi: 10.1016/j.resp.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 317.Cruz FF, Antunes MA, Abreu SC, Fujisaki LC, Silva JD, Xisto DG, Maron-Gutierrez T, Ornellas DS, Sa VK, Rocha NN, et al. Protective effects of bone marrow mononuclear cell therapy on lung and heart in an elastase-induced emphysema model. Respir Physiol Neurobiol. 2012;182:26–36. doi: 10.1016/j.resp.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 318.Nandra KK, Takahashi K, Collino M, Benetti E, Wong WS, Goh FY, Suzuki K, Patel NS, Thiemermann C. Acute treatment with bone marrow-derived mononuclear cells attenuates the organ injury/dysfunction induced by hemorrhagic shock in the rat. Shock. 2012;37:592–598. doi: 10.1097/SHK.0b013e31824e4c0d. [DOI] [PubMed] [Google Scholar]
- 319.Abreu SC, Antunesa MA, Marion-Gutierrez T, Cruz FF, Ornella DS, Silva AL, Diaz BL, Ab’Saber AM, Capelozzi VL, Xisto DG, et al. Bone marrow mononuclear cell therapy in experimental allergic asthma: intratracheal versus intravenous administration. Respir Physiol Neurobiol. 2013;185:615–624. doi: 10.1016/j.resp.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 320.Serrano-Mollar A, Nacher M, Gay-Jordi G, Closa D, Xaubet A, Bulbena O. Intratracheal transplantation of alveolar type II cells reverses bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2007;176:1261–1268. doi: 10.1164/rccm.200610-1491OC. [DOI] [PubMed] [Google Scholar]
- 321.Germano D, Blyszczuk P, Valaperti A, Kania G, Dirnhofer S, Landmesser U, Lüscher TF, Hunziker L, Zulewski H, Eriksson U, et al. Prominin-1/CD133+ lung epithelial progenitors protect from bleomycin-induced pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:939–949. doi: 10.1164/rccm.200809-1390OC. [DOI] [PubMed] [Google Scholar]
- 322.Badylak SF, Weiss DJ, Caplan A, Macchiarini P. Engineered whole organs and complex tissues. Lancet. 2012;379:943–952. doi: 10.1016/S0140-6736(12)60073-7. [DOI] [PubMed] [Google Scholar]
- 323.Orlando G, Baptista P, Birchall M, De Coppi P, Farney A, Guimaraes-Souza NK, Opara E, Rogers J, Seliktar D, Shapira-Schweitzer K, et al. Regenerative medicine as applied to solid organ transplantation: current status and future challenges. Transpl Int. 2011;24:223–232. doi: 10.1111/j.1432-2277.2010.01182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Fishman JM, De Coppi P, Elliott MJ, Atala A, Birchall MA, Macchiarini P. Airway tissue engineering. Expert Opin Biol Ther. 2011;11:1623–1635. doi: 10.1517/14712598.2011.623696. [DOI] [PubMed] [Google Scholar]
- 325.Baiguera S, Del Gaudio C, Jaus MO, Polizzi L, Gonfiotti A, Comin CE, Bianco A, Ribatti D, Taylor DA, Macchiarini P. Long-term changes to in vitro preserved bioengineered human trachea and their implications for decellularized tissues. Biomaterials. 2012;33:3662–3672. doi: 10.1016/j.biomaterials.2012.01.064. [DOI] [PubMed] [Google Scholar]
- 326.Haag J, Baiguera S, Jungebluth P, Barale D, Del Gaudio C, Castiglione F, Bianco A, Comin CE, Ribatti D, Macchiarini P. Biomechanical and angiogenic properties of tissue-engineered rat trachea using genipin cross-linked decellularized tissue. Biomaterials. 2012;33:780–789. doi: 10.1016/j.biomaterials.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 327.Haykal S, Soleas JP, Salna M, Hofer SO, Waddell TK. Evaluation of the structural integrity and extracellular matrix components of tracheal allografts following cyclical decellularization techniques: comparison of three protocols. Tissue Eng Part C Methods. 2012;18:614–623. doi: 10.1089/ten.TEC.2011.0579. [DOI] [PubMed] [Google Scholar]
- 328.Hinderer S, Schesny M, Bayrak A, Ibold B, Hampel M, Walles T, Stock UA, Seifert M, Schenke-Layland K. Engineering of fibrillar decorin matrices for a tissue-engineered trachea. Biomaterials. 2012;33:5259–5266. doi: 10.1016/j.biomaterials.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 329.Jungebluth P, Bader A, Baiguera S, Moller S, Jaus M, Lim ML, Fried K, Kjartansdottir KR, Go T, Nave H, et al. The concept of in vivo airway tissue engineering. Biomaterials. 2012;33:4319–4326. doi: 10.1016/j.biomaterials.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 330.Jungebluth P, Moll G, Baiguera S, Macchiarini P. Tissue-engineered airway: a regenerative solution. Clin Pharmacol Ther. 2012;91:81–93. doi: 10.1038/clpt.2011.270. [DOI] [PubMed] [Google Scholar]
- 331.Go T, Jungebluth P, Baiguero S, Asnaghi A, Martorell J, Ostertag H, Mantero S, Birchall M, Bader A, Macchiarini P. Both epithelial cells and mesenchymal stem cell-derived chondrocytes contribute to the survival of tissue-engineered airway transplants in pigs. J Thorac Cardiovasc Surg. 2010;139:437–443. doi: 10.1016/j.jtcvs.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 332.de Mel A, Seifalian AM, Birchall MA. Orchestrating cell/material interactions for tissue engineering of surgical implants. Macromol Biosci. 2012;12:1010–1021. doi: 10.1002/mabi.201200039. [DOI] [PubMed] [Google Scholar]
- 333.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 334.Jungebluth P, Alici E, Baiguera S, Le Blanc K, Blomberg P, Bozoky B, Crowley C, Einarsson O, Grinnemo KH, Gudbjartsson T, et al. Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: a proof-of-concept study. Lancet. 2011;378:1997–2004. doi: 10.1016/S0140-6736(11)61715-7. [DOI] [PubMed] [Google Scholar]
- 335.Elliott MJ, De Coppi P, Speggiorin S, Roebuck D, Butler CR, Samuel E, Crowley C, McLaren C, Fierens A, Vondrys D, et al. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet. 2012;380:994–1000. doi: 10.1016/S0140-6736(12)60737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Turner CG, Klein JD, Steigman SA, Armant M, Nicksa GA, Zurakowski D, Ritz J, Fauza DO. Preclinical regulatory validation of an engineered diaphragmatic tendon made with amniotic mesenchymal stem cells. J Pediatr Surg. 2011;46:57–61. doi: 10.1016/j.jpedsurg.2010.09.063. [DOI] [PubMed] [Google Scholar]
- 337.Baiguera S, Gonfiotti A, Jaus M, Comin CE, Paglierani M, Del Gaudio C, Bianco A, Ribatti D, Macchiarini P. Development of bioengineered human larynx. Biomaterials. 2011;32:4433–4442. doi: 10.1016/j.biomaterials.2011.02.055. [DOI] [PubMed] [Google Scholar]
- 338.Birchall MA, Ayling SM, Harley R, Murison PJ, Burt R, Mitchard L, Jones A, Macchiarini P, Stokes CR, Bailey M. Laryngeal transplantation in minipigs: early immunological outcomes. Clin Exp Immunol. 2012;167:556–564. doi: 10.1111/j.1365-2249.2011.04531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Ma R, Lib M, Luo J, Yu H, Sun Y, Cheng S, Cui P. Structural integrity, ECM components and immunogenicity of decellularized laryngeal scaffold with preserved cartilage. Biomaterials. 2013;34:1790–1798. doi: 10.1016/j.biomaterials.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 340.Welham NV, Chang Z, Smith LM, Frey BL. Proteomic analysis of a decellularized human vocal fold mucosa scaffold using 2D electrophoresis and high-resolution mass spectrometry. Biomaterials. 2013;34:669–676. doi: 10.1016/j.biomaterials.2012.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Roomans GM. Tissue engineering and the use of stem/progenitor cells for airway epithelium repair. Eur Cell Mater. 2010;19:284–299. doi: 10.22203/ecm.v019a27. [DOI] [PubMed] [Google Scholar]
- 342.Andrade CF, Wong AP, Waddell TK, Keshavjee S, Liu M. Cell-based tissue engineering for lung regeneration. Am J Physiol Lung Cell Mol Physiol. 2007;292:L510–L518. doi: 10.1152/ajplung.00175.2006. [DOI] [PubMed] [Google Scholar]
- 343.Ingenito EP, Sen E, Tsai LW, Murthy S, Hoffman A. Design and testing of biological scaffolds for delivering reparative cells to target sites in the lung. J Tissue Eng Regen Med. 2010;4:259–272. doi: 10.1002/term.237. [DOI] [PubMed] [Google Scholar]
- 344.Miller C, George S, Niklason L. Developing a tissue-engineered model of the human bronchiole. J Tissue Eng Regen Med. 2010;4:619–627. doi: 10.1002/term.277. [DOI] [PubMed] [Google Scholar]
- 345.Tsunooka N, Hirayama S, Medin JA, Liles WC, Keshavjee S, Waddell TK.A novel tissue-engineered approach to problems of the postpneumonectomy space. Ann Thor Surg 2010;191:880–886. [DOI] [PubMed]
- 346.Hoganson DM, Pryor HI, II, Vacanti JP. Tissue engineering and organ structure: a vascularized approach to liver and lung. Pediatr Res. 2008;63:520–526. doi: 10.1203/01.pdr.0000305879.38476.0c. [DOI] [PubMed] [Google Scholar]
- 347.Mondrinos MJ, Koutzaki SH, Honesto M, Poblete M, Crisanti C, Lelkes PI, Finck CM. In vivo pulmonary tissue engineering: contribution of donor-derived endothelial cells to construct vascularization. Tissue Eng Part A. 2008;14:361–368. doi: 10.1089/tea.2007.0041. [DOI] [PubMed] [Google Scholar]
- 348.Panoskaltsis-Mortari A, Weiss DJ. Breathing new life Into lung transplantation therapy. Mol Ther. 2010;18:1581–1583. doi: 10.1038/mt.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Lwebuga-Mukasa JS, Ingbar DH, Madri JA. Repopulation of a human alveolar matrix by adult rat type II pneumocytes in vitro: a novel system for type II pneumocyte culture. Exp Cell Res. 1986;162:423–435. doi: 10.1016/0014-4827(86)90347-2. [DOI] [PubMed] [Google Scholar]
- 350.Antunes MB, Woodworth BJ, Bhargave G, Xiong G, Aguilar JL, Ratner AJ, Kreindler JL, Rubenstein RC, Cohen NA. Murine nasal septa for respiratory epithelial air-liquid interface cultures. Biotechniques. 2007;43:195–204. doi: 10.2144/000112531. [DOI] [PubMed] [Google Scholar]
- 351.Price AP, England KA, Matson AM, Blazar BR, Panoskaltsis-Mortari A. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A. 2010;16:2581–2591. doi: 10.1089/ten.tea.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G, Winston S, Wang J, Walls S, Nichols JE. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A. 2010;16:2565–2580. doi: 10.1089/ten.tea.2009.0730. [DOI] [PubMed] [Google Scholar]
- 353.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 355.Song JJ, Kim SS, Liu Z, Madsen JC, Mathisen DJ, Vacanti JP, Ott HC. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg. 2011;92:998–1005. doi: 10.1016/j.athoracsur.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 356.Bonenfant N, Sokocevic D, Wagner DE, Borg ZD, Lathrop M, Lam YW, Deng B, DeSarno M, Ashikaga T, Loi R, et al. The effects of storage and sterilization on de-cellularized and re-cellularized whole lung. Biomaterials. 2013;34:3231–3245. doi: 10.1016/j.biomaterials.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Bonvillain RW, Danchuk S, Sullivan DE, Betancourt AM, Semon JA, Eagle ME, Mayeux JP, Gregory AN, Wang G, Townley IK, et al. A nonhuman primate model of lung regeneration: detergent-mediated cecellularization and initial in vitro recellularization with mesenchymal stem cells. Tissue Eng Part A. 2012;18:2437–2452. doi: 10.1089/ten.tea.2011.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Daly AB, Wallis JM, Borg ZD, Bonvillain RW, Deng B, Ballif BA, Jaworski DM, Allen GB, Weiss DJ. Initial binding and re-cellularization of de-cellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Eng Part A. 2012;18:1–16. doi: 10.1089/ten.tea.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Jensen T, Roszell B, Zang F, Girard E, Matson A, Thrall R, Jaworski DM, Hatton C, Weiss DJ, Finck CM. A rapid lung de-cellularization protocol supports embryonic stem cell differentiation in vitro and following implantation. Tissue Eng Part C Methods. 2012;18:632–646. doi: 10.1089/ten.tec.2011.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Petersen TH, Calle EA, Colehour MB, Niklason LE. Matrix composition and mechanics of decellularized lung scaffolds. Cells Tissues Organs. 2012;195:222–231. doi: 10.1159/000324896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Sokocevic D, Bonenfant N, Wagner DE, Borg ZD, Lathrop M, Lam YW, Deng B, DeSarno M, Ashikaga T, Loi R, et al. The effect of age and emphysematous and fibrotic injury on the re-cellularization of de-cellularized lungs. Biomaterials. 2013;34:3256–3269. doi: 10.1016/j.biomaterials.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Wallis JM, Borg ZD, Daly AB, Deng B, Ballif BA, Allen GB, Jaworski DM, Weiss DJ. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng Part C Methods. 2012;18:420–432. doi: 10.1089/ten.tec.2011.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Nichols JE, Niles JA, Cortiella J. Production and utilization of acellular lung scaffolds in tissue engineering. J Cell Biochem. 2012;113:2185–2192. doi: 10.1002/jcb.24112. [DOI] [PubMed] [Google Scholar]
- 364.Song JJ, Ott HC. Bioartificial lung engineering. Am J Transplant. 2012;12:283–288. doi: 10.1111/j.1600-6143.2011.03808.x. [DOI] [PubMed] [Google Scholar]
- 365.Soto-Gutierrez A, Wertheim JA, Ott HC, Gilbert TW. Perspectives on whole-organ assembly: moving toward transplantation on demand. J Clin Invest. 2012;122:3817–3823. doi: 10.1172/JCI61974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Dolgin E. Artifical inspiration. Nature. 2012;489:s12–s14. doi: 10.1038/489S12a. [DOI] [PubMed] [Google Scholar]
- 367.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Akhyari P, Aubin H, Gwanmesia P, Barth M, Hoffmann S, Huelsmann J, Preuss K, Lichtenberg A. The quest for an optimized protocol for whole-heart decellularization: a comparison of three popular and a novel decellularization technique and their diverse effects on crucial extracellular matrix qualities. Tissue Eng Part C Methods. 2011;17:915–926. doi: 10.1089/ten.TEC.2011.0210. [DOI] [PubMed] [Google Scholar]
- 369.Boer U, Lohrenz A, Klingenberg M, Pich A, Haverich A, Wilhelm M. The effect of detergent-based decellularization procedures on cellular proteins and immunogenicity in equine carotid artery grafts. Biomaterials. 2011;32:9730–9737. doi: 10.1016/j.biomaterials.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 370.Brown BN, Freund JM, Han L, Rubin JP, Reing JE, Jeffries EM, Wolf MT, Tottey S, Barnes CA, Ratner BD, et al. Comparison of three methods for the derivation of a biologic scaffold composed of adipose tissue extracellular matrix. Tissue Eng Part C Methods. 2011;17:411–421. doi: 10.1089/ten.tec.2010.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Tottey S, Johnson SA, Crapo PM, Reing JE, Zhang L, Jiang H, Medberry CJ, Reines B, Badylak SF. The effect of source animal age upon extracellular matrix scaffold properties. Biomaterials. 2011;32:128–136. doi: 10.1016/j.biomaterials.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med. 2012;186:866–876. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Barker TH. The role of ECM proteins and protein fragments in guiding cell behavior in regenerative medicine. Biomaterials. 2011;32:4211–4214. doi: 10.1016/j.biomaterials.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 374.Pageau SC, Sazonova OV, Wong JY, Soto AM, Sonnenschein C. The effect of stromal components on the modulation of the phenotype of human bronchial epithelial cells in 3D culture. Biomaterials. 2011;32:7169–7180. doi: 10.1016/j.biomaterials.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 375.Lin H, Yang G, Tan J, Tuan RS. Influence of decellularized matrix derived from human mesenchymal stem cells on their proliferation, migration and multi-lineage differentiation potential. Biomaterials. 2012;33:4480–4489. doi: 10.1016/j.biomaterials.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 376.Franz S, Rammelt S, Scharnweber D, Simon JC. Immune responses to implants: a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32:6692–6709. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 377.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109–116. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Daly KA, Liu S, Agrawal V, Brown BN, Johnson SA, Medberry CJ, Badylak SF. Damage associated molecular patterns within xenogeneic biologic scaffolds and their effects on host remodeling. Biomaterials. 2012;33:91–101. doi: 10.1016/j.biomaterials.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 379.Brown BN, Ratner BD, Goodman SB, Amar S, Badylak SF. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33:3792–3802. doi: 10.1016/j.biomaterials.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Keane TJ, Londono R, Turner NJ, Badylak SF. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials. 2012;33:1771–1781. doi: 10.1016/j.biomaterials.2011.10.054. [DOI] [PubMed] [Google Scholar]
- 381.Petersen TH, Calle EA, Colehour MB, Niklason LE. Bioreactor for the long-term culture of lung tissue. Cell Transplant. 2011;20:1117–1126. doi: 10.3727/096368910X544933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Carterson AJ, Honer zu Bentrup K, Ott CM, Clarke MS, Pierson DL, Vanderburg CR, Buchanan KL, Nickerson CA, Schurr MJ. A549 lung epithelial cells grown as 3-D aggregates: alternative tissue culture model for Pseudomonas aeruginosa pathogenesis. Infect Immun. 2005;73:1129–1140. doi: 10.1128/IAI.73.2.1129-1140.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Crabbe A, De Boever P, Van Houdt R, Moors H, Mergeay M, Cornleius P. Use of the rotating wall vessel technology to study the effect of shear stress on growth behavior of Pseudomonas aeruginosa PA01. Environ Microbiol. 2008;10:2098–2110. doi: 10.1111/j.1462-2920.2008.01631.x. [DOI] [PubMed] [Google Scholar]
- 384.Tschumperlin DJ, Jones JC, Senior RM. The fibrotic matrix in control: does the extracellular matrix drive progression of idiopathic pulmonary fibrosis? Am J Respir Crit Care Med. 2012;186:814–816. doi: 10.1164/rccm.201208-1561ED. [DOI] [PubMed] [Google Scholar]
- 385.Shamis Y, Hasson E, Soroker A, Bassat E, Shimoni Y, Ziv T, Sionov RV, Mitrani E. Organ-specific scaffolds for in vitro expansion, differentiation, and organization of primary lung cells. Tissue Eng Part C Methods. 2011;17:861–870. doi: 10.1089/ten.tec.2010.0717. [DOI] [PubMed] [Google Scholar]
- 386.Mishra DK, Thrall MJ, Baird BN, Ott HC, Blackmon SH, Kurie JM, Kim MP. Human lung cancer cells grown on acellular rat lung matrix create perfusable tumor nodules. Ann Thorac Surg. 2012;93:1075–1081. doi: 10.1016/j.athoracsur.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Tschumperlin DJ, Boudreault F, Liu F. Recent advances and new opportunities in lung mechanobiology. J Biomech. 2010;43:99–107. doi: 10.1016/j.jbiomech.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Majumdar A, Arold SP, Bartolák-Suki E, Parameswaran H, Suki B. Jamming dynamics of stretch-induced surfactant release by alveolar type II cells. J Appl Physiol. 2012;112:824–831. doi: 10.1152/japplphysiol.00975.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Albaiceta GM, Blanch L.Beyond volutrauma in ARDS: the critical role of lung tissue deformation. Crit Care 2011;15:304. [DOI] [PMC free article] [PubMed]
- 390.Rocco PR, Dos Santos C, Pelosi P. Pathophysiology of ventilator-associated lung injury. Curr Opin Anaesthesiol. 2012;25:123–130. doi: 10.1097/ACO.0b013e32834f8c7f. [DOI] [PubMed] [Google Scholar]
- 391.Fridley KM, Fernandez I, Li MA, Kettlewell RB, Roy K. Unique differentiation profile of mouse embryonic stem cells in rotary and stirred tank bioreactors. Tissue Eng Part A. 2010;16:3285–3298. doi: 10.1089/ten.tea.2010.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Siti-Ismail N, Samadikuchaksaraei A, Bishop AE, Polak JM, Mantalaris A. Development of a novel three-dimensional, automatable and integrated bioprocess for the differentiation of embryonic stem cells into pulmonary alveolar cells in a rotating vessel bioreactor system. Tissue Eng Part C Methods. 2012;18:263–272. doi: 10.1089/ten.TEC.2011.0299. [DOI] [PubMed] [Google Scholar]
- 393.Huh D, et al. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Huh D, et al. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med. 2012;4:147. doi: 10.1126/scitranslmed.3004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Blaisdell C, et al. Cell therapy for lung diseases: report from an NIH-NHLBI Workshop November 13–14, 2012. Am J Respir Crit Care Med (In press) [DOI] [PMC free article] [PubMed]
- 396.Lalu MM, McIntyre LL, Stewart DJ. Mesenchymal stromal cells: cautious optimism for their potential role in the treatment of acute lung injury. Crit Care Med. 2012;40:1373–1375. doi: 10.1097/CCM.0b013e31824317f7. [DOI] [PubMed] [Google Scholar]
- 397.Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ Canadian Critical Care Trials Group. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS ONE. 2012;7:e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Wang XX, Zhang FR, Shang YP, Zhu JH, Xie XD, Tao QM, Zhu JH, Chen JZ. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a p54. Pilot randomized controlled trial. J Am Coll Cardiol. 2007;49:1566–1571. doi: 10.1016/j.jacc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 399.Zhu JH, Wang XX, Zhang FR, Shang YP, Tao QM, Zhu JH, Chen JZ. Safety and efficacy of autologous endothelial progenitor cells transplantation in children with idiopathic pulmonary arterial hypertension: open-label pilot study. Pediatr Transplant. 2008;12:650–655. doi: 10.1111/j.1399-3046.2007.00863.x. [DOI] [PubMed] [Google Scholar]
- 400.Cyranoski D. Canada approves stem cell product. Nat Biotechnol. 2012;30:571. [Google Scholar]
- 401.Von Bahr L, Batsis I, Moll G, Szako A, Sundberg B, Uzunel M, Ringden O, LeBlanc K. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30:1575–1578. doi: 10.1002/stem.1118. [DOI] [PubMed] [Google Scholar]
- 402.Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled randomized trial of mesenchymal stem cells in chronic obstructive pulmonary disease. Chest. 2013;143:1590–1598. doi: 10.1378/chest.12-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Tzouvelekis A, Koliakos G, Ntolios P, Baira I, Bouros E, Oikonomou A, Zissimopoulos A, Kolios G, Kakagia D, Paspaliaris V, et al. Stem cell therapy for idiopathic pulmonary fibrosis: a protocol proposal. J Transl Med. 2011;9:182. doi: 10.1186/1479-5876-9-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Toonkel RL, Hare JM, Matthay MA, Glassberg MK.Mesenchymal stem cells and idiopathic pulmonary fibrosis: potential for clinical testing. Am J Respir Crit Care Med (In press) [DOI] [PubMed]
- 405.Weiss DJ, Ortiz LA. Cell therapy trials for lung diseases: progress and cautions. Am J Respir Crit Care Med. In press doi: 10.1164/rccm.201302-0351ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Raghuvanshi S, Sharma P, Singh S, Van Kaer L, Das G. Mycobacterium tuberculosis evades host immunity by recruiting mesenchymal stem cells. Proc Natl Acad Sci USA. 2010;107:21653–21658. doi: 10.1073/pnas.1007967107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Kursova LV, Konoplyannikov AG, Pasov VV, Ivanova IN, Poluektova MV, Konoplyannikova OA. Possibilities for the use of autologous mesenchymal stem cells in the therapy of radiation-induced lung injuries. Bull Exp Biol Med. 2009;147:542–546. doi: 10.1007/s10517-009-0538-7. [DOI] [PubMed] [Google Scholar]
- 408.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM Acute Lung Injury in Animals Study Group. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44:725–738. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Prockop DJ, Brenner M, Fibbe WE, Horwitz E, Le Blanc K, Phinney DG, Simmons PJ, Sensebe L, Keating A. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy. 2010;12:576–578. doi: 10.3109/14653249.2010.507330. [DOI] [PubMed] [Google Scholar]
- 410.Prockop DJ. Defining the probability that a cell therapy will produce a malignancy. Mol Ther. 2010;18:1249–1250. doi: 10.1038/mt.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Prockop DJ, Keating A. Relearning the lessons of genomic stability of human cells during expansion in culture: implications for clinical research. Stem Cells. 2012;30:1051–1052. doi: 10.1002/stem.1103. [DOI] [PubMed] [Google Scholar]
- 412.Master Z, Resnik DB. Stem cell tourism and scientific responsibility. EMBO Rep. 2011;12:992–995. doi: 10.1038/embor.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Stem cell research: regulating translational application. Nat Cell Biol. 2012;14:557. doi: 10.1038/ncb2517. [DOI] [PubMed] [Google Scholar]
- 414.Qiu J. Trading on hope. Nat Biotechnol. 2010;27:790–792. doi: 10.1038/nbt0909-790. [DOI] [PubMed] [Google Scholar]
- 415.Zarzeczny A, Rachul C, Nisbet M, Caulfield T. Stem cell clinics in the news. Nat Biotechnol. 2012;28:1243–1246. doi: 10.1038/nbt1210-1243b. [DOI] [PubMed] [Google Scholar]
- 416.Weiss DJ, Rojas M.MSCs in chronic lung diseases: COPD and lung fibrosis in stem cell-dependent therapies-mesenchymal stem cells in chronic inflammatory disorders. Berlin, Germany: DeGruyter Press; (In press) [Google Scholar]
- 417.Huh JW, Kim SY, Lee JH, Lee JS, Ta QV, Kim M, Oh YM, Lee YS, Lee SD. Bone marrow cells repair cigarette smoke induced emphysema in rats. Am J Physiol Lung Cell Mol Physiol. 2011;301:L255–L266. doi: 10.1152/ajplung.00253.2010. [DOI] [PubMed] [Google Scholar]