Abstract
Rationale: Bronchopulmonary dysplasia (BPD) is the chronic lung disease of infancy that occurs in premature infants after oxygen and ventilator therapy for acute respiratory disease at birth. Despite improvement in current therapies, the clinical course of infants with BPD is often characterized by marked hypoxemia that can become refractory to therapy. Preacinar anatomic and functional communications between systemic and pulmonary vascular systems has been established in fetal lungs, but whether increased intrapulmonary anastomotic vessels or their failure to regress after birth contributes to hypoxemia in preterm infants with BPD is unknown.
Objectives: We sought to find histologic evidence of intrapulmonary anastomotic vessels in lungs of patients who died of severe BPD.
Methods: We collected lung tissues from fatal BPD cases and performed histology, immunohistochemistry, and high-precision three-dimensional reconstruction techniques.
Measurements and Main Results: We report histologic evidence of intrapulmonary vessels that bridge pulmonary arteries and veins in the distal lungs of infants dying with severe BPD. These prominent vessels appear similar to “misaligned pulmonary veins” described in the lethal form of congenital lung disorder, alveolar capillary dysplasia.
Conclusions: We found striking histological evidence of precapillary arteriovenous anastomotic vessels in the lungs of infants with severe bronchopulmonary dysplasia. We propose that persistence or expansion of these vessels after premature birth provides the anatomic basis for intrapulmonary shunt and hypoxemia in neonates with severe bronchopulmonary dysplasia and may play a significant role in the morbidity and mortality of BPD.
Keywords: intrapulmonary shunt, bronchopulmonary dysplasia, pulmonary circulation, vascular development
Bronchopulmonary dysplasia (BPD) is the chronic lung disease of infancy that occurs in premature infants after oxygen and ventilator therapy for acute respiratory disease at birth (1). Despite marked advances in perinatal care, including antenatal steroids, surfactant therapy, improved respiratory care, and others, BPD remains one of the major sequelae of prematurity, leading to prolonged oxygen or ventilator dependency, recurrent respiratory exacerbations, and related problems (2). With premature birth and related lung injury, the normal sequence of lung development is disrupted, resulting in the histologic pattern of alveolar simplification (larger but fewer alveoli with decreased septation) and impaired or “dysmorphic” vascular growth. The distal pulmonary arterioles often appear reduced in number and abnormally distributed within the lung parenchyma (3).
Changes in vascular growth and structure contribute to pulmonary hypertension (PH), which is associated with high morbidity and mortality in infants with BPD (4). In addition to PH, the reduced alveolar-capillary surface area with decreased arterial number further contributes to the need for prolonged oxygen and ventilator therapy due to abnormal gas exchange with marked hypoxemia (5). Decreased lung vascular growth also leads to late cardiopulmonary abnormalities, such as hypoxemia with acute respiratory infections, exercise intolerance, recurrent edema, and progression of pulmonary hypertension. Although several mechanisms contribute to abnormal lung function and gas exchange, an increased susceptibility for profound hypoxemia with infections, stress, or activity may also be related to impaired lung surface area due to decreased vascular and alveolar growth (6–9).
Although PH is often associated with severe BPD (10), many late signs of disease and hypoxemia cannot be adequately explained on a physiologic basis by elevated pulmonary artery pressure alone. Studies in infants have shown decreased lung diffusion capacity for carbon monoxide (DlCO) even in patients with relatively mild BPD (5), and decreased DlCO can persist into adulthood (5). In addition, physiological shunt studies with infusions of bubbles during echocardiography suggest the presence of prominent intrapulmonary shunting during exercise in older subjects, including survivors of BPD (11). Although precapillary arteriovenous anastomoses have been identified in the fetal lung (13–15), histologic evidence of these vessels has not been demonstrated in older infants or patients with BPD. Whether persistence or expansion of intrapulmonary arteriovenous anastomotic vessels (IAAV) contributes to late pulmonary disease and whether these vascular channels contribute to hypoxemia in the setting of premature infants with severe BPD has been controversial. Importantly, the existence of anastomotic vessels in the setting of BPD has not been previously shown.
Based on past physiologic reports in older patients with BPD and to address this controversy, we hypothesized that intrapulmonary anastomotic vessels can be identified in lung tissue from preterm infants dying with severe BPD. We further hypothesized that the normal lung vascular growth patterns and spatial relationships between pulmonary arteries and veins and bronchial vessels are markedly altered in severe BPD. To study this question, we carefully examined lung histology at autopsy from infants who died with severe BPD and used histologic methods, immunohistochemistry, and three-dimensional reconstruction techniques to examine the relationships of these vascular channels.
Methods
BPD Study Population and Age-matched Control Subjects
Lung histology was obtained at the time of autopsy from nine preterm infants who died with severe BPD (Table 1). The patients’ gestational ages and birth weights ranged between 26 to 32 weeks and 600 to 960 g, respectively. Age at death was between 3 weeks and 7 months, and all of the patients required prolonged mechanical ventilator support. Seven patients had histologic evidence of pulmonary hypertension at autopsy, including increased pulmonary arterial medial thickening. Lungs from two age-matched control patients were used as non-BPD control subjects. Both infants died of acute cardiovascular arrest of unknown cause; one died at 2 weeks (born at term gestation) and the other died at 7 months (born at 36 weeks of gestation). We defined PH by standard clinical echocardiographic criteria, including estimated right ventricular systolic pressure above 0.5 systemic values as determined by tricuspid regurgitant jet velocities, or in the absence of a consistent and detectable tricuspid regurgitant jet, findings of marked septal flattening, right ventricular dilation, and right ventricular hypertrophy (4).
Table 1.
Study population
| Patient | Birth Weight (g) | Gestational Age (wk) | Sex | Age at Death | Cause of Death | Perinatal History | Pulmonary Hypertension | Ventilation at Death; FiO2 |
|---|---|---|---|---|---|---|---|---|
| 1 | 886 | 26 | F | 3 wk | Severe BPD | PPROM | Y | Y; 1.00 |
| 2 | 680 | 28 | F | 17 mo | Severe BPD | Y | Y; 0.80–1.00 | |
| 3 | 960 | 26 | M | 11 wk | Severe BPD + sepsis | N | Y; 1.00 | |
| 4 | 723 | 25 | M | 12 mo | Acute respiratory arrest | Tracheoesophageal fistula | Y | Y; 0.40–1.00 |
| 5 | 600 | 32 | M | 4 mo | Severe BPD | IUGR, TTTS, oligohydramnios | Y | Y; 0.80–1.00 |
| 6 | N/A | 26 | F | 3 wk | CNS injury + severe BPD | N | Y; 0.60–1.00 | |
| 7 | N/A | 27 | M | 2 mo | Endocarditis | Y | Y; 0.40–1.00 | |
| 8 | 815 | 26 | M | 8 mo | Severe BPD | Y | Y; 1.00 | |
| 9 | N/A | 29 | M | 9 mo | Sudden arrest at home | Y | N; nasal canula O2 |
Definition of abbreviations: BPD = bronchopulmonary dysplasia; F = female; IUGR = intrauterine growth retardation; M = male; N = no; PPROM = preterm premature rupture of membranes; TTTS = twin-twin transfusion syndrome; Y = yes.
See text for details.
Immunohistochemistry
Immunohistochemistry was performed with the routine streptavidin-biotin peroxidase method using the Ventana Benchmark automatic slide stainer. Primary antibodies (CD34; Ventana Inc, Tucson, AZ; D2-40, Dako, Carpinteria, CA) used were commercially available.
Three-Dimensional Reconstruction
Tissue samples were obtained from post mortem lungs of control subjects, patients with BPD, and patients with alveolar capillary dysplasia from multiple regions, including central and peripheral cuts of each lobe, which were serially sectioned at 5-μm intervals. Serial stacks were subsequently stained with hematoxylin and eosin stains. To elucidate the endothelial cells and the lymphatics, one section out of every 12 was stained with pan-endothelial marker CD34, and subsequent section were stained with lymphatic-specific marker D2-40; altogether, 36 sections were used in two patients with BPD and two age-matched control patients. Sections were then placed onto a microscope that was equipped to trace and three-dimensionally reconstruct (Stereo Investigator; Microbrightfield, Williston, VT), as we have previously described (16, 17). Each section was then traced, and each vascular and bronchial element was given a distinct color: veins (blue), anastomotic vessels (yellow), arteries (red), arterial muscle (light blue), lymphatics (pink), and airways (green). The adjacent section was aligned and traced, and this process was repeated until the entire stack was completed. The stack of images was then three-dimensionally reconstructed, and images and videos were captured.
Immunostaining
Although no reliable immunomarkers for human tissue are currently available to readily differentiate between vessels of systemic (bronchial) or pulmonary origin, we performed immunostaining with fatty acid binding protein-4 (FABP4) and tissue-plasminogen activator (t-PA) in an attempt to determine the origin of IAAV. These markers have been previously shown to preferentially label airway vasa vasorum of large-sized pulmonary vessels or bronchial vessels, respectively (18, 19). FABP4 stain identified capillary-sized endothelium adjacent to large bronchi and pulmonary arteries as well as lymphatic vessels; however, larger systemic vessels (i.e., bronchial arteries and veins) were not marked with FABP4 in either BPD or age-matched control lungs. Immunostaining for t-PA did not consistently identify human vascular endothelium (data not shown). In addition, we used coup-TF2, a specific marker for veins that has been used in animal models (20), in an attempt to confirm venous origin of the anastomotic vessels; however, we failed to find consistent labeling in our human autopsy lung tissue samples.
Results
BPD Study Population and Age-matched Control Subjects
Lung histology was studied in nine preterm infants who died with severe BPD (Table 1). The patients’ gestational ages and birth weights ranged between 26 to 32 weeks and 600 to 960 g, respectively. Age at death was between 3 weeks and 7 months, and all of the patients required prolonged mechanical ventilator support.
Eight of the nine patients died from severe hypoxic respiratory failure with sustained hypoxemia and recurrent cyanotic episodes despite high FiO2 (ranging from 0.40–1.00) and despite aggressive mechanical ventilation (Table 1). Two patients required tracheostomy for chronic ventilator care. One infant died suddenly at home while receiving low-flow supplemental oxygen (1.00) by nasal canula.
In addition, each of the patients had echocardiographic evidence of moderate to severe PH, as defined by standard criteria, including estimated right ventricular systolic pressure above 0.5 systemic values by measured tricuspid regurgitant jet velocities, or in the absence of a consistent and detectable tricuspid regurgitant jet, findings of marked septal flattening, right ventricular dilation, and right ventricular hypertrophy (4). These infants had no echocardiographic evidence of right-to-left intracardiac or extrapulmonary shunt by echocardiogram. All patients had histologic evidence of pulmonary vascular remodeling at autopsy, including increased pulmonary arterial medial thickening. Lungs from two age-matched control patients were used as non-BPD control subjects, including two infants who died of acute cardiovascular arrest of unknown cause; one died at 2 weeks (born at term gestation), and the other died at 7 months (born at 36 wk of gestation).
Histology Reveals the Presence of Abnormal Centrally Located Vascular Channels in BPD Lung Tissues
Each of these study cases showed typical features of severe BPD, which included enlarged distal airspaces with “simplified” alveolar structures, decreased numbers of interstitial capillaries, and muscularization of the small pulmonary arteries. The large pulmonary veins appeared nonobstructive, as there were no structural vessel changes suggestive of pulmonary venous hypertension, medial thickening (or “arterialization”), diffuse capillary congestion, or venous malformations.
Dilated and thin-walled vascular channels were prominent and frequently blood-filled within the bronchoarterial bundles in all of the lung tissue samples from each of the patients with BPD (Figures 1c, 2a, and 2b; see Video E1 in the online supplement) Although varied in number, these large vascular channels were readily present in all sections. Some variability existed regarding the location of vascular channels within the bronchoarterial bundle (Figure 2). These vascular channels were more commonly present between the pulmonary arteries and airways, but vascular channels were also noted branching laterally toward the pulmonary artery (Figure 2c), the airway (Figure 2d), or, rarely, focally encircling small airways (Figures 2e and 2f). Vascular channels were noted alongside different-sized airways, including bronchi and bronchioles. In most cases, congested small microvascular plexuses surrounding the airways and pulmonary arteries are also noted adjacent to vascular channels (Figures 2a and 2b). This histologic appearance has previously been referred to as chain-type arteriovenous malformations in patients who developed intrapulmonary shunting after cavopulmonary shunt operation (21).
Figure 1.
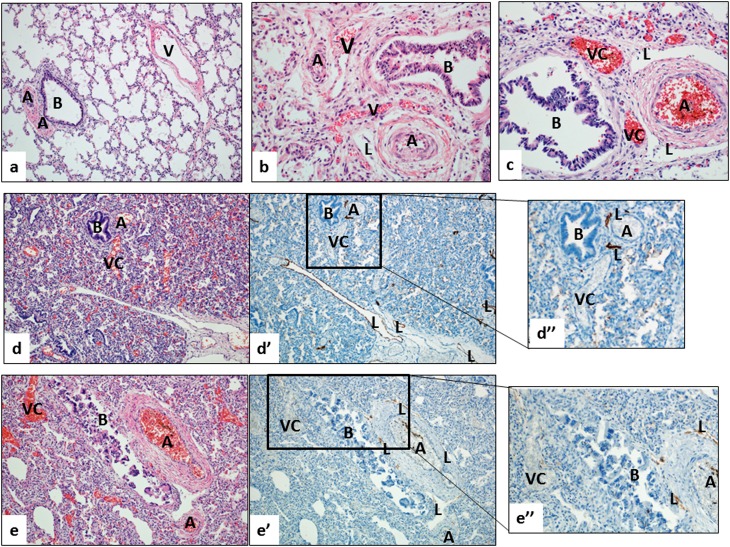
(a–c) Lung histology from young infants without respiratory disease (normal; a), with alveolar capillary dysplasia and misalignment of pulmonary veins (ACD/MPV; b), and bronchopulmonary dysplasia (BPD; c). (a) In the age-matched control lung, the pulmonary vein (V) is separated from the neighboring bronchoarterial bundle (B = bronchiole; A = pulmonary artery). Note the lack of prominent vessels other than the pulmonary arteries (A) near the airway (B). (b) In ACD/MPV, this normal vascular anatomy is disturbed, showing thin-walled and blood-filled vessels (“misaligned pulmonary veins,” V) that are abnormally located near pulmonary arteries (A) and bronchiole (B). (c) In severe BPD, prominent, thin-walled vascular channels with no elastic lamina (VC) are located near pulmonary arteries and airways, similar to the pattern seen in ACD (B = bronchiole; A = pulmonary artery; L = lymphatic vessel). Note that small and congested capillary network adjacent to VCs and surrounding airways and pulmonary arteries are seen in ACD and BPD lungs, but not in the lung of age-matched control. (d–e) The prominent blood-filled vascular channels (VC) in BPD lungs are not lymphatic channels. Serial step sections stained with hematoxylin and eosin (d, e) and D2-40 (d′, e′) show that the prominent vessels in the bronchoarterial bundles are not labeled with the lymphatic marker D2-40; this finding is further highlighted with a higher magnification (d′′, e′′). In contrast, typical lymphatic channels (L) show strong immunoreactivity with D2-40.
Figure 2.
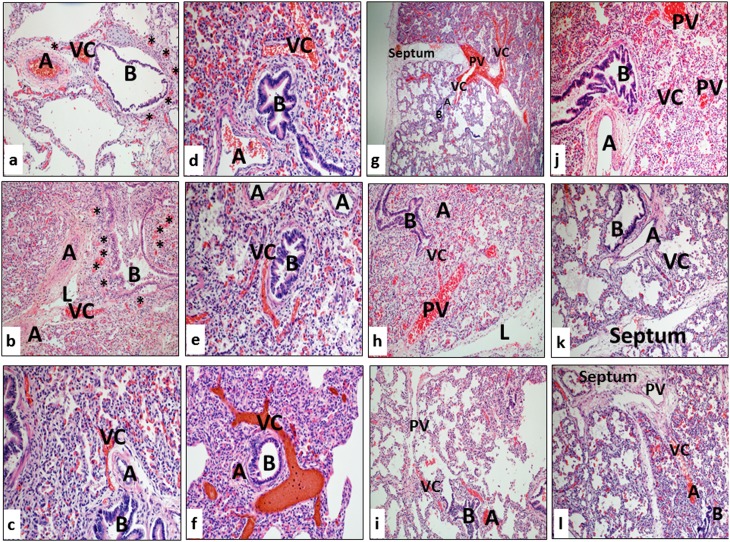
Histologic patterns illustrating numerous and prominent blood-filled vascular channels (VC) within and adjacent to the bronchoarterial bundles in each of the lung samples from patients dying with severe bronchopulmonary dysplasia (A = pulmonary artery; B = bronchiole; L = lymphatic channel). Asterisks in panels a and b indicate congested dilated microvascular plexuses adjacent to pulmonary arteries (A), airways (B), and vascular channels (VC). See details in text.
In many sections, vascular channels appeared to connect pulmonary veins located in the septum with the area surrounding the bronchoarterial bundle (Figures 2g–2i; Video E2). In several cases, a thin-walled vascular channel clearly branched from the pulmonary artery and was directed toward the primary septum (Figures 2j–2l; Video E2). This pattern of aberrant vascular growth, with vascular channels closely associated with airways and arteries, appeared strikingly similar to findings of “misalignment of pulmonary veins” in association with alveolar capillary dysplasia (Figure 1b), which is a rare, lethal congenital lung disorder for which the pattern of misalignment of pulmonary veins is considered pathognomonic (22).
The appearance of vascular channels in the BPD lungs is strikingly different from that of normal, age-matched lung (Figure 1, as examples). In contrast to the intimate association between airways and pulmonary arteries, the pulmonary veins lie remotely from bronchi and bronchioles in the normal lung (Figure 1). Small venules collecting the blood from the pulmonary capillaries merge into veins of increasing caliber and do not follow the bronchial tree. Rather, pulmonary veins typically travel within the collagenous, interlobular (or primary) septa along with lymphatic vessels (Figure 1a). Rarely, thin-walled, small-diameter vessels were noted within the bronchoarterial bundle of a control lung. Vascular channels within the BPD lungs had thin walls and lacked media or elastic lamina (Figure 1c). Immunostaining with D2-40, a specific marker for lymphatic endothelium, clearly distinguished these vascular channels from neighboring, and often dilated, lymphatic vessels (Figures 1d and 1e).
Three-Dimensional Reconstruction Confirms the Presence of Intrapulmonary Anastomotic Vessels in BPD Lungs
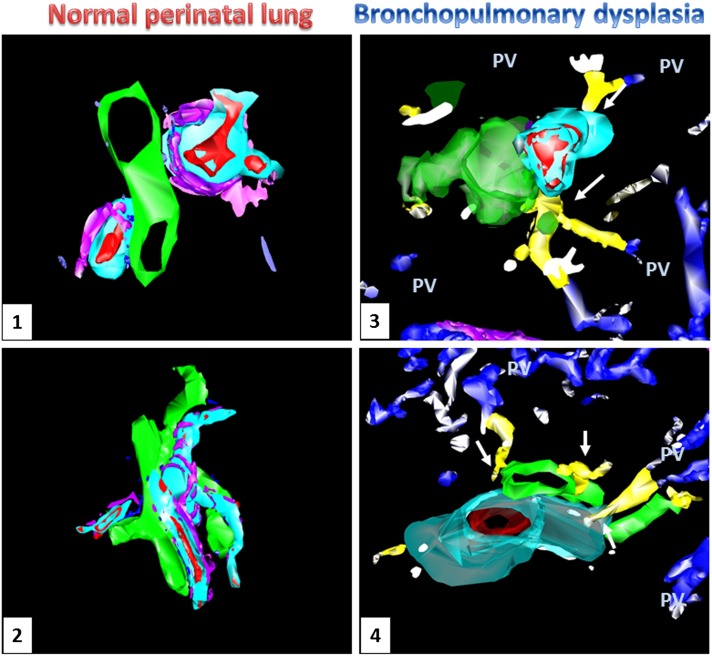
With the combination of serial sectioning, histology, immunohistochemistry (including anti-CD34 and D2-40 antibodies), and high-precision computerized three-dimensional reconstruction, we traced the course of the intrapulmonary vessels. We noted that vascular channels course in the lobular periphery, where pulmonary veins are typically located, and travel toward pulmonary arteries and appear to connect with microvascular plexuses surrounding pulmonary arteries (vasa vasorum) and airways (bronchial vascular plexus) (Figure 3, Panels 3 and 4; Video E2). In contrast, three-dimensional reconstruction of the control lungs failed to identify any vascular channels between pulmonary veins and the microvascular plexuses of pulmonary arteries and bronchi (Figure 3, Panels 1 and 2; Video E1).
Figure 3.
Three-dimensional reconstruction of microscopic images from age-matched control (Panels 1 and 2) and bronchopulmonary dysplasia (BPD) (Panels 3 and 4) lungs. Prominent vascular channels (yellow) are present within the bronchoarterial bundles of BPD lungs (artery: red; connecting vessels: blue, pulmonary veins: blue; airway: green; lymphatic: pink; arterial muscular wall: aqua), but not in the control lungs. These vascular channels appear to course toward the vessels located within the interlobular septa (pulmonary veins, PV) and make contact with the microvessels surrounding the pulmonary arteries and airways (white arrows).
With our histologic and three-dimensional model, we provided tissue-based evidence for the presence of prominent and blood-filled intrapulmonary vessels in the distal lung of patients with severe BPD. Because of their location and histologic and immunophenotypic characteristics, these vascular channels are most likely veins. Vessels with such distinct appearance and course were not present in age-matched control lungs. In addition, we note that the histologic pattern of these vessels shares similar features with misalignment of pulmonary veins in alveolar capillary dysplasia (Figure 1b).
Discussion
Improved obstetrical and neonatal care over time has increased survival of even the smallest of immature newborns, but BPD persists as a major problem, occurring in an estimated 10,000 to 15,000 infants per year in the United States alone (2). Ongoing research continues to shed light on the pathobiology of BPD, but our current knowledge remains limited, especially regarding our understanding of the pathophysiology that causes progressive disease with poor responsiveness to therapy in severe BPD (8, 9). Insights into mechanisms through which disruption of lung development alters lung structure and function in severe BPD are of great importance. One of the exciting advances in the field of developing biology is the recognition of the crucial role blood flow plays in vessel development during embryogenesis (23). In fact, reduced pulmonary blood flow and altered endothelial signaling have been shown to contribute to poor lung growth and impaired alveolarization in experimental models (24). Mechanisms that coordinate growth of the highly complex, three dimensional organization of systemic (bronchial), pulmonary arterial, pulmonary venous, and lymphatic vessels within the lung are poorly understood, and little is known about how communications among these vascular systems impact the regulation of lung development, physiology, and pathology. The existence of IAAV that bridge pulmonary arteries and veins has been identified in the late-gestation fetus (13–15), but IAAV are believed to disappear during the late neonatal period (15), and their potential physiologic roles are controversial. Such vessels have been suggested as contributing to intrapulmonary shunt in adults during catecholamine infusion, with exercise, and in an older patient with BPD (11, 12, 25–29). Our findings support the hypothesis that IAAV are prominent and persist in the preterm infants with severe BPD and our speculation that such vessels contribute to hypoxemia from intrapulmonary anastomoses with sustained respiratory insufficiency in severe BPD.
Previous physiologic data suggest that right-to-left intrapulmonary shunt pathways exist in adults, as demonstrated by echocardiographic studies during exercise, with exposure to hypoxia and during infusions of catecholamines (12, 26–29). Furthermore, IAAV are present in pathologic conditions in adult lungs after cavopulmonary shunt operations (30) and with hepatopulmonary syndrome (31). Recruitment of IAAV leads to shunting of blood away from distal airspace, thereby decreasing gas exchange and causing systemic hypoxemia (as illustrated in Figure 4). Decreased perfusion may further cause local tissue hypoxia, further hindering lung growth and repair, altering vascular remodeling, increasing vasoconstriction, and worsening PH, which are key features of severe BPD. Prominent bronchial and other systemic-to-pulmonary collateral vessels are found in morphometric studies of infants with BPD and can be readily identified in many infants during cardiac catheterization; they could contribute to significant shunting of blood flow within the lung, causing edema and need for higher FiO2 (4). The vessels highlighted in this report, however, lack typical features of systemic vessels and did not stain for the systemic endothelial marker, FABP4 (18). It must be emphasized, however, that we have not determined whether the predominant direction of blood flow is primarily right-to-left or left–to-right through these pathways, and that it is possible that these pathways could contribute to persistent or recurrent pulmonary edema that often contributes to the pathophysiology of BPD.
Figure 4.
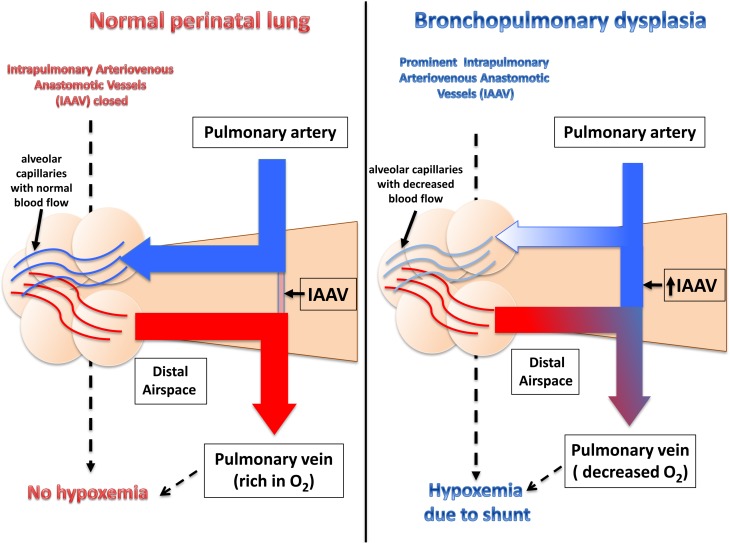
Schematic of blood flow redistribution via functional intrapulmonary arteriovenous anastomotic vessels (IAAV) in severe bronchopulmonary dysplasia (BPD) lung. In normal perinatal lung, pulmonary and bronchial arteries are the major contributor of the blood influx. Although present in the normal lung (13, 14), IAAV are generally not prominent or active. Thus, incoming blood flow is likely directed toward the distal lung parenchyma where the blood reaches the alveolar capillaries. In contrast, in the BPD lung precapillary IAAV are open and directly connect the pulmonary arterial and the venous circulation. Therefore, much of the incoming blood can bypass the distal lung without participation in gas exchange, resulting in significant shunt and hypoxemia.
Despite striking physiologic studies in older subjects (27), whether IAAV exist in BPD lungs and contribute to the pathology of BPD has been uncertain, and histologic evidence demonstrating IAAV in BPD lungs has previously been lacking. Our study is the first to demonstrate prominent IAAV in the lungs of infants with severe BPD. Serial sections and three-dimensional reconstruction identified blood-filled vessels bridging pulmonary veins and vascular networks surrounding pulmonary arteries and airways (Figures 1–3; Video E2). Because these channels have thin muscular walls, lack elastic lamina, have blood-filled lumens, and lack lymphatic endothelial marker expression, these vessels are likely veins (Figures 1 and 2). Prominent, centrally located veins have never been reported in BPD but are typical of the structural abnormalities of misalignment of pulmonary veins found in alveolar capillary dysplasia (15) (Figure 1b) and rarely in other neonatal lung disorders (e.g., severe congenital venous obstruction [32] and congenital pulmonary alveolar proteinosis [33]). Echocardiographic studies of patients with alveolar capillary dysplasia have led to speculation that these “misaligned pulmonary veins” are likely bronchial veins and that these vessels bridge the arterial and venous circulations of the lung. Bronchial veins drain lobular, segmental, and peripheral bronchiole arteries and return blood into pulmonary veins (34). In severe pulmonary venoocclusive disorders, these veins often appear congested and prominent, as observed in misalignment of pulmonary veins with alveolar capillary dysplasia, and are postulated to serve as “decompressing pathways” (35–37). Our three-dimensional reconstruction studies show that prominent veins within the BPD lung connect pulmonary veins within the primary septa with pulmonary arteries and vessels of bronchial and periarterial microvascular plexuses (vasa vasorum) (Figure 3; Video E2). It is possible that these vascular connections represent the previously described pulmonary precapillary anastomoses (38–40) that link pulmonary arteries and veins with systemic vascular networks that surround small airways and pulmonary arteries.
Mechanisms that alter IAAV size and function are unknown. We speculate that increased hemodynamic stress due to high pulmonary artery pressure or redistribution of pulmonary blood flow contribute to the persistence and hypertrophy of this vascular system in BPD, which has been observed with marked venous obstruction (32, 35–37). Whether or not the local production of vasoactive factors could contribute to the failure of IAAV to close after birth in preterm infants with severe BPD remains speculative. In addition, whether local hypoxia or hypoxemia leads to persistence of IAAV in BPD lung is unknown.
Our finding of prominent IAAV may provide an anatomic explanation for severe intrapulmonary shunt, as observed in patients with severe BPD who develop progressive disease and intractable hypoxemia with PH. Furthermore, by further increasing hypoxia in the already reduced gas-exchange area of peripheral lung, IAAV could induce additional lung injury in infants with BPD (Figure 4). Large systemic-to-pulmonary collateral vessels have been reported in studies of infants with BPD and can be identified in many infants during cardiac catheterization, which could also contribute to significant shunting of blood flow within the lung, causing edema and the need for higher FiO2 (4).
Limitations of this study include our inability to quantify flow across these vessels and their relative contribution to intrapulmonary anastomoses or to determine whether the predominant flow across these anastomoses vessels is right-to-left or left-to-right for each patient. Further physiologic studies are needed to assess their contribution to impaired gas exchange or the development of pulmonary edema in severe BPD. In addition, more work is needed to quantify and define the anatomic distribution of intrapulmonary arteriovenous anastomotic vessels throughout the lung in infants with severe BPD.
In summary, we demonstrate histologic evidence further characterizing the “dysmorphic” lung circulation in preterm infants that includes striking prominence of intrapulmonary arteriovenous anastomotic vessels in infants dying with severe BPD and PH. These structural changes suggest that altered distal vascular growth or abnormal physiologic regulation of intrapulmonary arteriovenous anastomotic vessels may contribute to the pathobiology of severe BPD, which appears similar to that observed in alveolar capillary dysplasia/misalignment of pulmonary veins. We speculate that persistence of intrapulmonary arteriovenous anastomotic vessels contributes to intrapulmonary shunt with resultant hypoxemia, pulmonary edema, and perhaps PH in severe BPD.
Acknowledgments
Acknowledgment
The authors thank Dr. Rubin Tuder, Department of Medicine, University of Colorado, and Dr. Ron Jaffe, Division of Pediatric Pathology, University of Pittsburgh Medical Center, for their advice and help with this manuscript.
Footnotes
Supported by National Institutes of Health grants HL085703 (S.H.A.) and HL68702 (S.H.A.).
Author Contributions: C.G.: designed experiments, analyzed data, wrote manuscript. S.S.-L.: generated and analyzed data, reviewed manuscript. S.H.A.: designed experiments, analyzed data, reviewed manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respiratory therapy of hyaline-membrane disease: bronchopulmonary dysplasia. N Engl J Med. 1967;276:357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 2.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 3.Coalson JJ. Pathology of bronchopulmonary dysplasia. Semin Perinatol. 2006;30:179–184. doi: 10.1053/j.semperi.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Mourani PM, Abman SH.Pulmonary vascular disease in BPD: physiology, diagnosis and treatment. In: Abman SH, editor. Bronchopulmonary dysplasia. NY: Informa; 2010. pp: 347–363. [Google Scholar]
- 5.Balinotti JE, Chakr VC, Tiller C, Kimmel R, Coates C, Kisling J, Yu Z, Nguyen J, Tepper RS. Growth of lung parenchyma in infants and toddlers with chronic lung disease of infancy. Am J Respir Crit Care Med. 2010;181:1093–1097. doi: 10.1164/rccm.200908-1190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L600–L609. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- 7.Abman SH. Bronchopulmonary dysplasia: a vascular hypothesis. Am J Respir Crit Care Med. 2001;164:1755–1756. doi: 10.1164/ajrccm.164.10.2109111c. [DOI] [PubMed] [Google Scholar]
- 8.Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol. 2005;67:623–661. doi: 10.1146/annurev.physiol.67.040403.102229. [DOI] [PubMed] [Google Scholar]
- 9.Thebaud B, Abman SH. Bronchopulmonary dysplasia—where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med. 2007;175:978–985. doi: 10.1164/rccm.200611-1660PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;164:1971–1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- 11.Lovering AT, Romer LM, Haverkamp HC, Hokanson JS, Eldridge MW. Excessive gas exchange impairment during exercise in a subject with a history of bronchopulmonary dysplasia and high altitude pulmonary edema. High Alt Med Biol. 2007;8:62–67. doi: 10.1089/ham.2006.0816. [DOI] [PubMed] [Google Scholar]
- 12.Laurie SS, Elliott JE, Goodman RD, Lovering AT. Catecholamine-induced opening of intrapulmonary arteriovenous anastomoses in healthy humans at rest. J Appl Physiol. 2012;113:1213–1222. doi: 10.1152/japplphysiol.00565.2012. [DOI] [PubMed] [Google Scholar]
- 13.Robertson B. Anastomoses in the human lung: postnatal formation and obliteration of arterial anastomoses in the human lung: a microangiographic and histologic study. pediatrics. 1969;43:971. [PubMed] [Google Scholar]
- 14.Wilkinson MJ, Fagan DG. Postmortem demonstration of intrapulmonary arteriovenous shunting. Arch Dis Child. 1990;65:435–437. doi: 10.1136/adc.65.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMullan DM, Hanley FL, Cohen GA, Portman MA, Riemer RK. Pulmonary arteriovenous shunting in the normal fetal lung. J Am Coll Cardiol. 2004;44:1497–1500. doi: 10.1016/j.jacc.2004.06.064. [DOI] [PubMed] [Google Scholar]
- 16.Sims-Lucas S. Analysis of 3D branching pattern: hematoxylin and eosin method. Methods Mol Biol. 2012;886:73–86. doi: 10.1007/978-1-61779-851-1_7. [DOI] [PubMed] [Google Scholar]
- 17.Sims-Lucas S, Argyropoulos C, Kish K, McHugh K, Bertram JF, Quigley R, Bates CM. Three-dimensional imaging reveals ureteric and mesenchymal defects in Fgfr2-mutant kidneys. J Am Soc Nephrol. 2009;20:2525–2533. doi: 10.1681/ASN.2009050532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghelfi E, Karaaslan C, Berkelhamer S, Akar S, Kozakewich H, Cataltepe S. Fatty acid-binding proteins and peribronchial angiogenesis in bronchopulmonary dysplasia. Am J Respir Cell Mol Biol. 2011;45:550–556. doi: 10.1165/rcmb.2010-0376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin EG, Santell L, Osborn KG. The expression of endothelial tissue plasminogen activator in vivo: a function defined by vessel size and anatomic location. J Cell Sci. 1997;110:139–148. doi: 10.1242/jcs.110.2.139. [DOI] [PubMed] [Google Scholar]
- 20.You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- 21.Duncan BW, Kneebone JM, Chi EY, Hraska V, Isik FF, Rosenthal GL, Jones TK, Starnes SL, Lupinetti FM. A detailed histologic analysis of pulmonary arteriovenous malformations in children with cyanotic congenital heart disease. J Thorac Cardiovasc Surg. 1999;117:931–938. doi: 10.1016/S0022-5223(99)70374-0. [DOI] [PubMed] [Google Scholar]
- 22.Langston C. Misalignment of pulmonary veins and alveolar capillary dysplasia. Pediatr Pathol. 1991;11:163–170. doi: 10.3109/15513819109064753. [DOI] [PubMed] [Google Scholar]
- 23.Le Noble F, Fleury V, Pries A, Corvol P, Eichmann A, Reneman RS. Control of arterial branching morphogenesis in embryogenesis: go with the flow. Cardiovasc Res. 2005;65:619–628. doi: 10.1016/j.cardiores.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Wallen LD, Perry SF, Alston JT, Maloney JE. Morphometric study of the role of pulmonary arterial flow in fetal lung growth in sheep. Pediatr Res. 1990;27:122–127. doi: 10.1203/00006450-199002000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Bryan TL, van Diepen S, Bhutani M, Shanks M, Welsh RC, Stickland MK. The effects of dobutamine and dopamine on intrapulmonary shunt and gas exchange in healthy humans. J Appl Physiol. 2012;113:541–548. doi: 10.1152/japplphysiol.00404.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stickland MK, Lovering AT. Exercise-induced intrapulmonary arteriovenous shunting and pulmonary gas exchange. Exerc Sport Sci Rev. 2006;34:96–106. doi: 10.1249/00003677-200607000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Eldridge MW, Dempsey JA, Haverkamp HC, Lovering AT, Hokanson JS. Exercise induced intrapulmonary arteriovenous shunting in healthy humans. J Appl Physiol. 2004;97:797–805. doi: 10.1152/japplphysiol.00137.2004. [DOI] [PubMed] [Google Scholar]
- 28.Lovering AT, Romer LM, Haverkamp HC, Pegelow DF, Hokanson JS, Eldridge MW. Intrapulmonary shunting and pulmonary gas exchange during normoxic and hypoxic exercise in healthy humans. J Appl Physiol. 2008;104:1418–1425. doi: 10.1152/japplphysiol.00208.2007. [DOI] [PubMed] [Google Scholar]
- 29.Lovering AT, Stickland MK, Amann M, Murphy JC, O'Brien MJ, Hokanson JS, Eldridge MW. Hyperoxia prevents exercise-induced intrapulmonary arteriovenous shunt in healthy humans. J Physiol. 2008;586:4559–4565. doi: 10.1113/jphysiol.2008.159350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freedom RM, Yoo SJ, Perrin D. The biological “scrabble” of pulmonary arteriovenous malformations: considerations in the setting of cavopulmonary surgery. Cardiol Young. 2004;14:417–437. doi: 10.1017/S1047951104004111. [DOI] [PubMed] [Google Scholar]
- 31.Vettukattil JJ. Pathogenesis of pulmonary arteriovenous malformations: role of hepatopulmonary interactions. Heart. 2002;88:561–563. doi: 10.1136/heart.88.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker AE, Becker MJ, Edwards JE. Dilated bronchial veins within pulmonary parenchyma. Observatins in congenital pulmonary venous obstruction. Arch Pathol. 1971;91:256–260. [PubMed] [Google Scholar]
- 33.Wallot M, Wagenvoort C, deMello D, Muller KM, Floros J, Roll C. Congenital alveolar proteinosis caused by a novel mutation of the surfactant protein B gene and misalignment of lung vessels in consanguineous kindred infants. Eur J Pediatr. 1999;158:513–518. doi: 10.1007/s004310051132. [DOI] [PubMed] [Google Scholar]
- 34.Matthews AW, Buchanan R. A case of pulmonary veno-occlusive disease and a new bronchoscopic sign. Respir Med. 1990;84:503–505. doi: 10.1016/s0954-6111(08)80117-5. [DOI] [PubMed] [Google Scholar]
- 35.Hintz SR, Vincent JA, Pitlick PT, Fineman JR, Steinhorn RH, Kim GE, Benitz WE. Alveolar capillary dysplasia: diagnostic potential for cardiac catheterization. J Perinatol. 1999;19:441–446. doi: 10.1038/sj.jp.7200243. [DOI] [PubMed] [Google Scholar]
- 36.Eulmesekian P, Cutz E, Parvez B, Bohn D, Adatia I. Alveolar capillary dysplasia: a six-year single center experience. J Perinat Med. 2005;33:347–352. doi: 10.1515/JPM.2005.067. [DOI] [PubMed] [Google Scholar]
- 37.Cullinane C, Cox PN, Silver MM. Persistent pulmonary hypertension of the newborn due to alveolar capillary dysplasia. Pediatr Pathol. 1992;12:499–514. doi: 10.3109/15513819209024200. [DOI] [PubMed] [Google Scholar]
- 38.Murata K, Itoh H, Todo G, Itoh T, Kanaoka M, Furuta M, Torizuka K. Bronchial venous plexus and its communication with pulmonary circulation. Invest Radiol. 1986;21:24–30. doi: 10.1097/00004424-198601000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Wagenvoort CA, Wagenvoort N. Arterial anastomoses, bronchopulmonary arteries, and pulmobronchial arteries in perinatal lungs. Lab Invest. 1967;16:13–24. [PubMed] [Google Scholar]
- 40.Deffebach ME, Charan NB, Lakshminarayan S, Butler J. The bronchial circulation. Small, but a vital attribute of the lung. Am Rev Respir Dis. 1987;135:463–481. doi: 10.1164/arrd.1987.135.2.463. [DOI] [PubMed] [Google Scholar]