Abstract
Background: Active smokers are prevalent in hospitalized and critically ill patients. Cigarette smoking and nicotine withdrawal may increase delirium in these populations. This systematic review aims to determine whether active cigarette smoking increases the risk for delirium in hospitalized and intensive care unit (ICU) patients.
Methods: A systematic search of English-, Spanish-, and French-language articles published from 1966 to April 2013 was performed. Studies were included if they measured cigarette smoking as a risk factor and delirium as an outcome in adult hospitalized or ICU patients. Methodologic quality of studies was assessed using both the validated Newcastle Ottawa Scale and an additional evidence-based quality rating scale.
Results: A total of 14 cohort studies of surgical and ICU populations were included in the review. No studies in non-ICU inpatients were identified. The incidence of delirium ranged from 9 to 52%, and the prevalence of active smokers ranged from 9 to 44%. The quality of assessment for active smoking varied widely. None of the studies used biochemical measures to determine cigarette smoke exposure. Of the six studies restricting the smoking group to active smokers only, active smoking was independently associated with delirium in one study, trended toward an association in one study, and showed a dose response in one study. Quantitative summary measures were not calculated due to study heterogeneity and missing data.
Conclusions: There is currently insufficient evidence to determine if cigarette smoking is a risk factor for delirium. Future studies should consider using biochemical measures of cigarette smoke exposure to objectively quantify smoking behavior.
Keywords: delirium, smoking, risk factors, critical illness, hospitalization
Delirium is a clinical syndrome characterized by an acute alteration in attention and cognition. Delirium occurs in up to 56 and 80% of hospitalized (1) and ICU patients (2), respectively, and is associated with prolonged hospital stay (3), subsequent functional and cognitive decline (4, 5), and even death (6, 7). Despite its profound impact on patients, we still have an incomplete understanding of the etiology and risk factors for delirium in hospitalized patients. Although many of the known predisposing factors are nonmodifiable (e.g., advanced age, dementia, comorbidities), factors that are modifiable in the acute setting are potential targets for delirium prevention (e.g., sedation-induced coma, immobilization, pain, disorientation, sleep deprivation).
Cigarette smoking may represent a potentially modifiable risk factor for delirium in hospitalized and ICU patients. The primary mechanism through which cigarette smoking is postulated to contribute to delirium is through nicotine withdrawal in the setting of abrupt smoking cessation due to acute illness and hospitalization (8–10). Studies suggest that delirium is caused by imbalances in neurotransmitters due to factors such as systemic inflammation, metabolic derangements, acute stress responses, and exposure to psychoactive medications (11). A leading hypothesized mechanism for delirium is deficiency of acetylcholine in the central nervous system (12). A relative deficiency in acetylcholine also plays a central role in the pathophysiology of nicotine withdrawal. Specifically, the up-regulation and desensitization of nicotinic acetylcholine receptors in the brain in the setting of chronic nicotine exposure and their subsequent unoccupied state during periods of abstinence are believed to be associated with withdrawal symptoms (13). Indeed, nicotine withdrawal and delirium share common features, such as confusion, restlessness, and irritability. Furthermore, the time course of nicotine withdrawal symptoms, which peak in the first week of abstinence and can last up to 2–4 weeks after cessation (14, 15), overlaps with the onset of delirium, which is commonly diagnosed at the time of presentation to the hospital or ICU, or several days postoperatively. If an association between active smoking and delirium is identified, nicotine replacement therapy should be investigated as a potential preventive intervention for hospitalized smokers at risk for delirium. Additional postulated mechanisms for how cigarette smoking increases delirium risk include microvascular changes and increasing atherosclerotic burden. However, these additional effects are likely due to chronic exposure rather than acute smoking cessation; hence, they are less likely to be modifiable in the setting of hospitalization, and are not feasible targets for preventative strategies.
Several studies have tested whether cigarette smoking is a risk factor for delirium. Thus far, these studies have provided conflicting results. Accordingly, we sought to determine whether smokers who are admitted to the hospital or ICU are at a higher risk for delirium compared with nonsmokers by systematically reviewing the evidence. We hypothesize that active cigarette smoking is an independent risk factor for the development of delirium in these patient populations. Given the high prevalence of active smokers in both hospitalized and ICU populations (40–57%) (16–19), determining whether active smoking contributes to delirium is clinically relevant and important, especially because this represents a potentially modifiable risk factor through the use of nicotine replacement therapy. The results of this study have previously been published in abstract form (20).
Methods
Definition of Exposure and Outcome
This systematic review was performed according to Meta-analysis of Observational Studies in Epidemiology Guidelines (21). Studies were included if they met the following inclusion criteria: (1) subjects were adult inpatients (≥18 yr old); (2) a history of active cigarette smoking was assessed; and (3) data on delirium were provided. The exposure measure of cigarette smoking was defined as biochemical measurement of cigarette smoke biomarkers, or smoking history obtained through self-report, family or surrogate report, or medical chart. The outcome measure of delirium was defined as an acute alteration in attention and cognition, as assessed using validated diagnostic criteria.
Search Strategy
A PubMed search of English, Spanish, and French studies (1966 to April 2013), using the terms “delirium AND (habits OR smok* OR smoking cessation OR nicotine OR cigarette* OR tobacco)” was conducted (see Table E1 in the online supplement). Embase, CINAHL, PsycInfo, Web of Science, and the Centers for Disease Control and Prevention Tobacco Information and Prevention Databases were also searched. The search was supplemented by a manual search of bibliographies of retrieved articles and relevant published reviews. All articles that were deemed potentially relevant were located for full manuscript review.
Study Selection and Data Abstraction
Studies were excluded if they met one or more of the following criteria: diagnosis of delirium was not based on examination of study subjects; lack of concurrent control group; or study only published in abstract form, case report, or literature review. Two authors (S.J.H. and M.S., or S.J.H. and A.N.L.) independently reviewed titles and abstracts to identify articles for inclusion and exclusion using a priori criteria. A log of included and excluded citations, including justification for exclusion, was maintained. In the end, 14 publications were selected for a detailed and independent review by two investigators (S.J.H. and M.S., or S.J.H. and A.N.L.). A structured abstraction form was used to record the author and year of the study, study design, patient characteristics, method of measuring cigarette smoke exposure, prevalence of smoking, delirium assessment method, incidence of delirium, covariates in multivariate model, and univariate and multivariate odds ratios (ORs). Disagreements in the abstracted data were resolved by further review, discussion, and consensus between the two reviewing authors. Attempts were made to contact authors of studies with unreported data (n = 8). In the cases where contact was successful, the requested data were not available (n = 3). No assumptions were made for missing data. To determine whether meta-analytical pooling would be appropriate, studies were assessed for variability in population type, study design, and outcome reporting.
Quality Assessment
Two different methods were used to assess methodological quality of the studies. First, two physician reviewers independently assessed the studies using the Newcastle Ottawa Scale (NOS) (22), a validated scoring system that rates the selection, comparability, and assessment of outcome in cohort studies. However, the ability of the NOS to identify flaws in the definition and the assessment of cigarette smoking was limited (Table E2). This limitation could lead to misclassification of active cigarette smoking, and could bias the association between smoking and delirium. Therefore, we devised a scoring system a priori to critically concentrate on assessing this potential source of bias and to complement the NOS assessment (Quality rating scale for assessment of active cigarette smoking; Table 1). In this scale, the highest number of points was given to studies in which (1) the smoking group was restricted to active smokers only, and (2) the source of the smoking status assessment was objectively determined by biomarker measurement (as opposed to smoking history). The justification for the scoring system is as follows. Cigarette smoke biomarkers (e.g., cotinine) are sensitive and specific to smoking, and have been extensively used to establish causal relationships between smoking and disease in outpatient studies (23). Because well validated biomarker cutpoints accurately distinguish active smokers from second-hand smokers, biomarkers can be used to both identify active smokers and quantify the amount of cigarette smoking (24). These biomarkers have been shown to be superior to self-report, surrogate report, and medical records in the setting of inpatient and ICU populations (17, 19). Self-report can be limited by social desirability bias, recall bias, and, in the case of critically ill patients, may not be feasible due to respiratory failure and altered levels of consciousness. Compared with self-report, surrogates tend to underreport overall smoking status, and the accuracy of amount and duration smoked is even more limited (25, 26). Agreement between smoking history obtained from medical records compared with self-report is even poorer, and data on smoking history are often missing. Therefore, smoking history obtained through medical records was given the lowest credit (27, 28). Studies that restricted the smoking group to only active smokers were given credit, whereas studies that included both active and former smokers, or did not specify the smoking status of the subjects in the smoking group, were given no credit.
Table 1.
Quality rating scale for assessment of active cigarette smoking
| Points | |
|---|---|
| Smoking group designation | |
| Active smoker only | 2 |
| Active and former smokers, or not reported | 0 |
| Source of smoking assessment | |
| Biomarker | 4 |
| Self-report only | 3 |
| Self-, surrogate, or chart report | 2 |
| Chart report only | 1 |
| Unclear | 0 |
Results
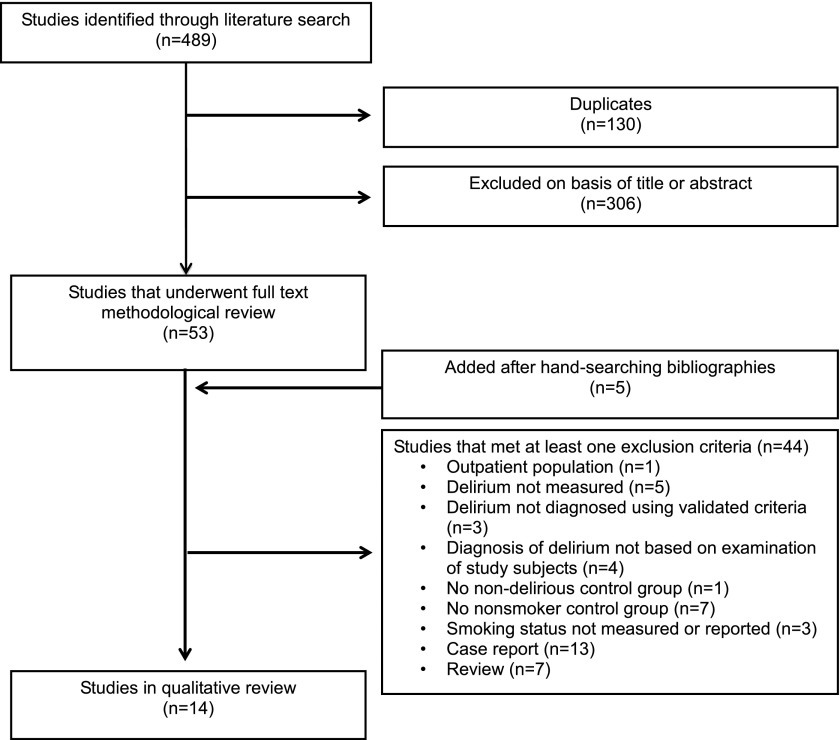
The comprehensive search yielded 489 unique articles (Figure 1). The 14 articles that were included in the qualitative review were published between 2001 and 2013 for 4,382 patients in 14 different countries (reviewer interrater κ = 0.92; Table 2) (29–42). All were cohort studies, and only one study was designed to test smoking as the primary risk factor for delirium (34). The 13 remaining studies were designed to assess for multiple risk factors associated with the development of delirium. Study populations in the reviewed literature were composed of three main groups: (1) critically ill patients; (2) cardiovascular surgery patients; and (3) other surgical patients. No studies of adult inpatients were identified. A total of 4 of the 14 studies focused on older adults, a population that is at particularly high risk for developing delirium (11). Quality rating scores using the NOS varied minimally between studies (Table 3).
Figure 1.
Literature search and selection. Modified by permission from Reference 20.
Table 2.
Summary of included studies
| Study No. | First Author, Year, (Ref. No.) | Country, Location | Sample Size | Age(Yr) | Population | Incidence of Deliriumn (%) | Delirium Assessment Method | Delirium Assessment Frequency and Duration | Prevalence of Active Smoking(%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Dubois, 2001 (32) | Canada, single center | 198 | ≥18 | Medical and surgical ICU, intubated and nonintubated | 38 (19) | ICDSC ≥ 4 with psychiatric confirmation | q8 h × 5 d | NR |
| 2 | Lucidarme, 2010 (34) | France, multicenter | 144 | ≥18 | Medical and surgical ICU, intubated only | 40 (28) | ICDSC > 4 | Twice daily until ICU discharge | 31 |
| 3 | Ouimet, 2007 (36) | Canada, single center | 764 | ≥18 | Medical and surgical ICU, intubated and nonintubated | 243 (32) | ICDSC ≥ 4 | q1–8 h until ICU discharge | NR |
| 4 | Van Rompaey, 2009 (41) | Belgium, multicenter | 523 | ≥18 | Medical and surgical ICU, nonintubated only | 155 (30) | NEECHAM confusion scale ≤ 19 | NR | 25 |
| 5 | Benoit, 2005 (29) | Canada, single center | 102 | 41–88 | Elective aortic aneurysm repair | 34 (33) | DSM4 | Daily × 6 d postop | 28 |
| 6 | Bohner, 2003 (30) | Germany, single center | 153 | ≥18 | Elective arterial surgery | 60 (39) | DSM4 and DRS ≥ 12 | Daily × 7 d postop | 43 |
| 7 | Chang, 2008 (31) | Taiwan, single center | 288 | ≥20 | Elective and emergent cardiac surgery | 120 (42) | DSM4 | NR | NR |
| 8 | Koster, 2008 (33) | The Netherlands, single center | 112 | >45 | Elective cardiac surgery | 24 (21) | DOS ≥ 2 with DSM4 confirmation | 3 times daily × 5 d postop | 15 |
| 9 | Mardani, 2012 (35) | Iran, single center | 196 | ≥48–80 | Elective CABG | 34 (17) | DSM4 in patients with MMSE ≤ 23 | NR | NR |
| 10 | Rudolph, 2009 (37) | United States, multicenter | 122 | ≥60 | Elective cardiac surgery | 63 (52) | Delirium symptom interview and MDAS, or CAM–ICU | Preop; daily beginning 2 d postop | NR |
| 11 | Rudolph, 2007 (38) | International, multicenter | 1161 | ≥60 | Elective noncardiac surgery | 99 (9) | DSM3 | Daily × 7 d postop | 9 |
| 12 | Santos, 2004 (39) | Brazil, single center | 220 | >60 | Elective CABG | 74 (34) | DSM4 | Daily × 5 d postop | 15 |
| 13 | Yoshimura, 2004 (40) | Japan, single center | 100 | ≥18 | Elective liver resection for HCC | 17 (17) | CAM | Daily × 2 weeks postop | 44 |
| 14 | Zakriya, 2002 (42) | United States, single center | 168 | 50–98 | Surgical hip fracture repair | 47(28) | CAM | Daily postop until discharge | 16 |
Definition of abbreviations: CABG = coronary artery bypass graft surgery; CAM = Confusion Assessment Method; DOS = Delirium Observation Screening Scale; DRS = Delirium Rating Scale; DSM = Diagnostic and Statistical Manual of Mental Disorders; HCC = hepatocellular carcinoma; ICDSC = Intensive Care Delirium Screening Checklist; ICU = intensive care unit; MDAS = Memorial Delirium Assessment Scale; MMSE = Mini Mental Status Exam; NEECHAM = Neelon and Champagne Confusion Scale; NR = not reported; postop = postoperatively; preop = preoperatively.
All studies have prospective cohort design.
Table 3.
Results from individual studies arranged by quality of smoking history
| First Author, Year (Ref. No.) | Exposed | Unexposed | Smoking Assessment Method | Prevalence of Active Smoking(%) | Univariate OR for Delirium | Multivariate OR for Delirium* | Covariates in Multivariate Model | NOS Score(0–8) | Quality of Smoking Assessment (0–6) |
|---|---|---|---|---|---|---|---|---|---|
| Santos, 2004 (39) | Current smoker | Former + never smoker | Preoperative self-report | 15 | 1.96 (0.93–4.11) | 4.19 (1.35–13.05) | 1,3,6,8 | 6 | 5 |
| Benoit, 2005 (29) | Current smoker | Former + never smoker | Preoperative self-report | 28 | 1.16 (0.46–2.91) | NR† | 1,2,3,5,9 | 8 | 5 |
| Dubois, 2001 (32) | ≥20 cigarettes/d until admission | <20 cigarettes/d until admission | Self-, surrogate or chart report (in order of preference) | NR | 2.2 (1.07–4.51) | 2.2 (0.94–4.94) | 1,6,10,11 | 5 | 4 |
| Van Rompaey, 2009 (41) | >10 cigarettes/d | ≤10 cigarettes/d | Self- or surrogate report | 25 | 2.04 (1.05–3.95) | NR | 1,2,5,7,9,10,11 | 6 | 4 |
| Ouimet, 2007 (36) | Current smoker | Former + never smoker | Self-, surrogate, or chart report (in order of preference) | NR | NR‡, P = 0.0123 | NR | 1,2,3,6,7,9,10,11 | 7 | 4 |
| Chang, 2008 (31) | ≥20 cigarettes/d within 1 mo of surgery | <20 cigarettes/d within 1 mo of surgery | Chart report | NR | NR, P > 0.05 | — | 5 | 3 | |
| Rudolph, 2007 (38) | Current + former smoker | Never smoker | Self-report | 9 | RR, 1.8 (1.2–2.8) | 1.6 (1.0–2.6) | 1,3,4,5,6,8 | 7 | 3 |
| Rudolph, 2009 (37) | Current + Former smoker: (a) 1–30 pack-years; (b) > 30 pack-years | Never smoker | Self-report | NR | 1–30 pack-years | — | 6 | 3 | |
| RR, 1.1 (0.76–1.6); > 30 pack-years | |||||||||
| RR, 0.8 (0.5–1.3) | |||||||||
| Yoshimura, 2004 (40) | “History of smoking” | Self-report | 44 | 2.78 (0.94–8.24) | 2.2 (0.6–8.8) | 1,3,6 | 7 | 3 | |
| Lucidarme, 2010 (34) | Current smoker, quit ≤ 6 months | Quit > 6 mo + never smoker | Self- or surrogate report | 31 | 1.84 (0.84–3.90) | Did not perform | 6 | 2 | |
| Bohner, 2003 (30) | “History of smoking” | NR | 43 | 1.98 (1.03–3.83) | — | 5 | 0 | ||
| Koster, 2008 (33) | “Cigarette use” | NR | 15 | 0.76 (0.20–2.87) | — | 6 | 0 | ||
| Mardani, 2012 (35) | “History of smoking” | NR | NR | 8.36 (1.9–37.9) | — | 5 | 0 | ||
| Zakriya, 2002 (42) | “Smoking” | NR | 16 | 1.09 (0.44–2.70) | — | 6 | 0 |
Definitions of abbreviations: NOS = Newcastle Ottawa Scale (see Table E2); NR = not reported; OR = odds ratio; RR=relative risk.
Studies that restricted the smoking group to current smokers only are placed above other studies in the table. Covariates included in multivariate model: (1) smoking; (2) alcohol; (3) age; (4) sex; (5) cognitive impairment or dementia; (6) comorbidity; (7) severity of illness; (8) intraoperative/postoperative factors; (9) benzodiazepines; (10) opiods; (11) hospital/intensive care unit factors. Quality of smoking assessment score (6 points maximum): assessment for active smoking: 2 = current smoker only; 0 = current and former smoker, or not reported; source of assessment: 4 = biomarker; 3 = self; 2 = self, surrogate, or chart; 1 = chart only; 0 = not reported.
Cigarette smoking not included in multivariate model.
No association between active smoking and delirium (OR not reported), but OR for delirium 1.05 (95% CI = 1.02–1.08) per pack-year smoked.
OR not reported but study stated active smoking was associated with increased risk for delirium.
Assessment of Active Cigarette Smoking
The prevalence of active smoking ranged from 9 to 44%, and was not reported in 5 of the 14 studies (Table 2). Six studies restricted the smoking group to active smokers only (29, 31, 32, 36, 39, 41), three studies included both active and former smokers (29, 34, 37, 38), and five did not define the smoking group (30, 33, 35, 40, 42). The source of smoking history also varied widely between studies, ranging from self-report by patients before surgery to chart review only. Four of the studies did not describe how smoking history was obtained (30, 33, 35, 42). None of the studies used cigarette smoke biomarkers to determine smoking status. Overall, five studies had moderate quality assessments for active smoking (score 4–5 out of 6), five studies had low quality assessments (score 2–3), and four studies had poor quality assessments (score 0–1).
Assessment of Delirium
The measured incidence of delirium ranged from 9 to 52%. Delirium was diagnosed by Diagnostic and Statistical Manual of Mental Disorders III or IV criteria, or by validated delirium screening tools. The frequency of delirium assessments ranged from once daily in most studies to several times a day. Three studies did not report the frequency of delirium assessments (31, 35, 41). The experience and training of the assessor performing delirium screening varied widely, from experienced study staff and study physicians to bedside nurses (34).
Association between Active Smoking and Delirium
Quantitative summary measures were not calculated, due to study heterogeneity and missing data. Of the six studies that restricted the smoking group to active smokers only (29, 31, 32, 36, 39, 41), a history of active smoking before hospital admission was independently associated with incident delirium in one study on elderly patients who had undergone coronary artery bypass graft surgery (odds ratio [OR], 4.19; 95% confidence interval [CI], 1.35–13.05; P = 0.019) (39), and trended toward an association in a study on medical and surgical ICU patients (OR, 2.2; 95% CI, 0.94–4.94) (32). In a third study on patients who had undergone aortic aneurysm repair, the total number of pack-years smoked was independently associated with delirium (OR, 1.05; 95% CI, 1.02–1.08; P = 0.001), although active smoking was not (29). Two of the six studies reported a statistically significant increased risk of incident delirium (P < 0.05) in active smokers, but that association was lost after controlling for confounders (36, 41). The study with the poorest quality assessment of active smoking did not find an association between active smoking and delirium (31). Although all six studies assessed for advanced age and alcohol as potential risk factors in univariate analyses, the selection of covariates in multivariate analyses varied between studies. Specifically, factors that have been previously demonstrated to have robust associations with a greater risk of delirium, such as age, alcohol use, pre-existing cognitive impairment or dementia, severity of illness, comorbidity burden, and sedation with benzodiazepines, were variably adjusted for (Table 3). Although five out of the six studies adjusted for age, only three adjusted for benzodiazepine use (29, 36, 41), and two adjusted for cognitive impairment or dementia (29, 41). An additional potential confounder for the association between smoking and delirium is prehospitalization depression (43). None of the included studies accounted for depression in their multivariate analyses.
Only 1 of the 14 studies was designed to assess for smoking as the primary risk factor for delirium (34). However, the study was powered for a combined outcome of ICU delirium and agitation, rather than delirium only, and included both active and former smokers in the smoking group. It did not find an association between active/former smoking and delirium in univariate analyses (OR, 1.84; 95% CI, 0.84–3.90), although active/former smoking was independently associated with increased agitation (OR, 3.13; 95% CI, 1.45–6.74). Of note, standardization of delirium assessments was variable in this study.
Of the eight low- to poor-quality assessment studies that either included both active and former smokers in the smoking group (37, 38, 40), or did not specify how cigarette smoke exposure was defined (30, 33–35, 42), smoking was associated with delirium in three studies (30, 35, 38), and trended toward an association in one (OR, 2.78; 95% CI, 0.94–8.24; P < 0.1) (40). Only two of these studies included smoking in their multivariate analyses. One reported a trend toward an association between smoking and delirium (OR, 1.6; 95% CI, 1.0–2.6) (38), and one found no association (OR, 2.2; 95% CI, 0.6–8.8) (40).
Discussion
Although case reports and reviews have suggested that cigarette smoking may increase the risk of delirium in hospitalized and ICU patients, the literature on this association has yielded conflicting results. In the first reported systematic review of the literature to date, we found that there is currently insufficient evidence to determine whether cigarette smoking is a risk factor for delirium in hospitalized and ICU patients.
Delirium is a common and serious form of acute brain injury in hospitalized and ICU patients that carries an enormous societal and financial burden (44–46). In view of the high prevalence of cigarette smoking in these populations, determining whether smoking is a risk factor for delirium is of extreme importance, as nicotine withdrawal in active smokers may be a potentially modifiable target for both delirium prevention and treatment via nicotine replacement therapy. This question is particularly timely, given the recent debate on the benefits and safety of nicotine replacement therapy in critically ill patients. In fact, a recent prospective cohort study of critically ill smokers suggested that nicotine replacement therapy was associated with increased delirium (47). Currently, no studies support the use of nicotine replacement therapy for delirium prevention or treatment, and, given the potential risks, it should not be routinely used for delirium prevention or treatment until further study.
This systematic review has identified several deficiencies in the existing literature that likely account for the inconclusive nature of the findings. First and foremost, the existing literature was limited by suboptimal assessment of active smoking status. Because risk estimates can be significantly biased with only minor degrees of misclassification (48), an accurate quantitative assessment of active smoking is imperative when investigating the relationship between smoking and delirium. In this systematic review, none of the studies used biomarkers to assess for smoking status, which have been clearly demonstrated to identify more active smokers than self-report in both inpatient and ICU studies (17–19). An alternative measure of cigarette smoking is to determine the degree of nicotine dependence by a validated scale. However, these scales require patient participation, and have not been validated for surrogate use. Therefore, they could not be feasibly administered to delirious patients nor to critically ill patients who are comatose or in respiratory failure, and would not be useful in guiding management. Of note, only one of the reviewed studies assessed nicotine dependence using a validated scale (34). However, it did not investigate the association between nicotine dependence and delirium. In sum, to determine the true impact of acute cessation of active smoking on the development of delirium, future studies should consider using more quantitative and objective measures of active smoking than self-, surrogate-, and medical record report.
Analyzed studies were also limited by incomplete adjustment for potential confounders of delirium. Factors such as pre-existing cognitive impairment, opioid and benzodiazepine use, and severity of illness have been consistently identified as predisposing and precipitating factors for delirium in both hospitalized and ICU patients (49). These factors were variably measured in the analyzed studies. Depression, an important potential confounder for the relationship between smoking and delirium, was not adjusted for in any of the studies. Because all analyzed studies were observational by design, this limitation could have biased the results.
In studies of critically ill patients, an additional limitation was the lower incidence of delirium (19–32%) compared with previously reported studies (46–80%) (2, 50). This discordance occurred despite the use of validated methods to assess delirium, and may be due to variable delirium definitions (51), variable delirium assessment tools (36), variability in sedation practices, and lower severity of illness of the cohorts (32). Furthermore variability in training for delirium screening (34) can lead to decreased identification of delirious patients, as demonstrated by recent work that showed that detection of delirium by bedside ICU nurses was lower compared with trained researchers (52).
There are some additional limitations to our conclusions. First, none of the studies was powered to investigate the association between active smoking and delirium. Second, nonsignificant ORs were not reported. This not only precludes the generation of a quantitative summary measure, but also biases interpretation of the results. Third, although evidence based, the quality scoring system used for this review has not been validated. However, it has face validity, because biomarker assessment for active smoking has been demonstrated to be a superior measure for cigarette smoke exposure than smoking history in both hospitalized and ICU patients. Fourth, although the intended scope of the review was inpatient and critically ill populations, no inpatient studies met search criteria. Future studies should investigate the association between active smoking and delirium in this important patient population. Fifth, because of individual variation in nicotine withdrawal symptoms, symptom severity and presence of delirium may not be directly associated with intensity of smoking. This question would best be answered with a prospective study. Finally, gray literature was not included in the systematic review, and thus our findings may be limited by publication lag.
These limitations notwithstanding, the strengths of this study are: (1) the comprehensive search of the evidence; (2) the relatively stringent inclusion criteria; and (3) the evidence-based assessment scale to assess the quality of smoking history.
Conclusions and Implications
In this first systematic review of the literature on active cigarette smoking as a risk factor for delirium in surgical and ICU patients, we found insufficient evidence to determine whether an association exists. Considering the high prevalence of cigarette smokers and delirium in hospitalized and critically ill patients, and the potential preventative and therapeutic implications, a study is needed to carefully and decisively determine whether this association exists. Future studies should be designed to specifically investigate this association, and should use biochemical measures of cigarette smoking to objectively quantify smoking behavior.
Acknowledgments
Acknowledgments
The authors acknowledge Dr. Hillel Cohen and Louise Falzon for their contributions to this project.
Footnotes
Supported by National Institute on Aging grant RO3AG040673 (S.J.H.); National Center for Research Resources and the National Center for Advancing Translational Sciences (NCATS) (a component of the National Institutes of Health [NIH]) grant 8KL2TR0000088-05 (S.J.H.); National Heart, Blood, and Lung Institute grants R01 HL086667 and U01 HL108712 (M.N.G.); and, in part, by Clinical and Translational Science Awards grants UL1 RR025750, KL2 RR025749, and TL1 RR025748 from NCATS.
Author Contributions: study conception and design: S.J.H., M.N.G.; analysis and interpretation: S.J.H., M.S., A.N.L., F.H., M.N.G.; drafting the manuscript for important intellectual content: S.J.H., M.S., M.N.G.
The contents of this article are solely the responsibility of the authors, and do not necessarily represent the official view of the National Center for Research Resources or National Institutes of Health.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Inouye SK. Delirium in hospitalized older patients. Clin Geriatr Med. 1998;14:745–764. [PubMed] [Google Scholar]
- 2.Pisani MA, Araujo KL, Van Ness PH, Zhang Y, Ely EW, Inouye SK. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10:R121. doi: 10.1186/cc5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miranda M, Slachevsky A, Venegas PP. Delirium from nicotine withdrawal in a post-operative adult patient [in Spanish] Rev Med Chil. 2005;133:385–386. doi: 10.4067/s0034-98872005000300017. [DOI] [PubMed] [Google Scholar]
- 4.Givens JL, Sanft TB, Marcantonio ER. Functional recovery after hip fracture: the combined effects of depressive symptoms, cognitive impairment, and delirium. J Am Geriatr Soc. 2008;56:1075–1079. doi: 10.1111/j.1532-5415.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- 5.Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, Jones RN. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, Inouye SK, Bernard GR, Dittus RS. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 7.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180:1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skrobik Y. Broadening our perspectives on ICU delirium risk factors. Crit Care. 2009;13:160. doi: 10.1186/cc7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girard TD, Pandharipande PP, Ely EW. Delirium in the intensive care unit. Crit Care. 2008;12:S3. doi: 10.1186/cc6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noimark D. Predicting the onset of delirium in the post-operative patient. Age Ageing. 2009;38:368–373. doi: 10.1093/ageing/afp024. [DOI] [PubMed] [Google Scholar]
- 11.Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5:210–220. doi: 10.1038/nrneurol.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hshieh TT, Fong TG, Marcantonio ER, Inouye SK. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci. 2008;63:764–772. doi: 10.1093/gerona/63.7.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- 15.Papaioannou A, Fraidakis O, Michaloudis D, Balalis C, Askitopoulou H. The impact of the type of anaesthesia on cognitive status and delirium during the first postoperative days in elderly patients. Eur J Anaesthesiol. 2005;22:492–499. doi: 10.1017/s0265021505000840. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC) Current cigarette smoking prevalence among working adults—United States, 2004–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1305–1309. [PubMed] [Google Scholar]
- 17.Benowitz NL, Schultz KE, Haller CA, Wu AH, Dains KM, Jacob P., III Prevalence of smoking assessed biochemically in an urban public hospital: a rationale for routine cotinine screening. Am J Epidemiol. 2009;170:885–891. doi: 10.1093/aje/kwp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calfee CS, Matthay MA, Eisner MD, Benowitz N, Call M, Pittet JF, Cohen MJ. Active and passive cigarette smoking and acute lung injury after severe blunt trauma. Am J Respir Crit Care Med. 2011;183:1660–1665. doi: 10.1164/rccm.201011-1802OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh SJ, Ware LB, Eisner MD, Yu L, Jacob P, III, Havel C, Goniewicz ML, Matthay MA, Benowitz NL, Calfee CS. Biomarkers increase detection of active smoking and secondhand smoke exposure in critically ill patients. Crit Care Med. 2011;39:40–45. doi: 10.1097/CCM.0b013e3181fa4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh SJ, Shum M, Lee A, Al-Othman F, Gong MN. Cigarette smoking as a risk factor for delirium in hospitalized patients: a systematic review [abstract] Am J Respir Crit Care Med. 2012:A1616. [Google Scholar]
- 21.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 22.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P.The Newcastle–Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses [Internet] Ottawa: Ottawa Hospital Research Institute; 2011. [accessed July 1, 2011]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 23.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. Atlanta: Centers for Disease Control and Prevention; 2004. The health consequences of smoking: a report of the Surgeon General. [Google Scholar]
- 24.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169:236–248. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 25.Navarro AM. Smoking status by proxy and self report: rate of agreement in different ethnic groups. Tob Control. 1999;8:182–185. doi: 10.1136/tc.8.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woo JG, Pinney SM. Retrospective smoking history data collection for deceased workers: completeness and accuracy of surrogate reports. J Occup Environ Med. 2002;44:915–923. doi: 10.1097/00043764-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Mant J, Murphy M, Rose P, Vessey M. The accuracy of general practitioner records of smoking and alcohol use: comparison with patient questionnaires. J Public Health Med. 2000;22:198–201. doi: 10.1093/pubmed/22.2.198. [DOI] [PubMed] [Google Scholar]
- 28.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benoit AG, Campbell BI, Tanner JR, Staley JD, Wallbridge HR, Biehl DR, Bradley BD, Louridas G, Guzman RP, Fromm RA. Risk factors and prevalence of perioperative cognitive dysfunction in abdominal aneurysm patients. J Vasc Surg. 2005;42:884–890. doi: 10.1016/j.jvs.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 30.Böhner H, Hummel TC, Habel U, Miller C, Reinbott S, Yang Q, Gabriel A, Friedrichs R, Müller EE, Ohmann C, et al. Predicting delirium after vascular surgery: a model based on pre- and intraoperative data. Ann Surg. 2003;238:149–156. doi: 10.1097/01.sla.0000077920.38307.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang YL, Tsai YF, Lin PJ, Chen MC, Liu CY. Prevalence and risk factors for postoperative delirium in a cardiovascular intensive care unit. Am J Crit Care. 2008;17:567–575. [PubMed] [Google Scholar]
- 32.Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27:1297–1304. doi: 10.1007/s001340101017. [DOI] [PubMed] [Google Scholar]
- 33.Koster S, Oosterveld FG, Hensens AG, Wijma A, van der Palen J. Delirium after cardiac surgery and predictive validity of a risk checklist. Ann Thorac Surg. 2008;86:1883–1887. doi: 10.1016/j.athoracsur.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 34.Lucidarme O, Seguin A, Daubin C, Ramakers M, Terzi N, Beck P, Charbonneau P, du Cheyron D. Nicotine withdrawal and agitation in ventilated critically ill patients. Crit Care. 2010;14:R58. doi: 10.1186/cc8954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mardani D, Bigdelian H. Predictors and clinical outcomes of postoperative delirium after administration of dexamethasone in patients undergoing coronary artery bypass surgery. Int J Prev Med. 2012;3:420–427. [PMC free article] [PubMed] [Google Scholar]
- 36.Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33:66–73. doi: 10.1007/s00134-006-0399-8. [DOI] [PubMed] [Google Scholar]
- 37.Rudolph JL, Jones RN, Levkoff SE, Rockett C, Inouye SK, Sellke FW, Khuri SF, Lipsitz LA, Ramlawi B, Levitsky S, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119:229–236. doi: 10.1161/CIRCULATIONAHA.108.795260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudolph JL, Jones RN, Rasmussen LS, Silverstein JH, Inouye SK, Marcantonio ER. Independent vascular and cognitive risk factors for postoperative delirium. Am J Med. 2007;120:807–813. doi: 10.1016/j.amjmed.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 39.Santos FS, Velasco IT, Fráguas R., Jr Risk factors for delirium in the elderly after coronary artery bypass graft surgery. Int Psychogeriatr. 2004;16:175–193. [PubMed] [Google Scholar]
- 40.Yoshimura Y, Kubo S, Shirata K, Hirohashi K, Tanaka H, Shuto T, Takemura S, Kinoshita H. Risk factors for postoperative delirium after liver resection for hepatocellular carcinoma. World J Surg. 2004;28:982–986. doi: 10.1007/s00268-004-7344-1. [DOI] [PubMed] [Google Scholar]
- 41.Van Rompaey B, Elseviers MM, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Bossaert L. Risk factors for delirium in intensive care patients: a prospective cohort study. Crit Care. 2009;13:R77. doi: 10.1186/cc7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zakriya KJ, Christmas C, Wenz JF, Sr, Franckowiak S, Anderson R, Sieber FE.Preoperative factors associated with postoperative change in confusion assessment method score in hip fracture patients Anesth Analg 2002941628–1632. [Table of Contents]. [DOI] [PubMed] [Google Scholar]
- 43.McAvay GJ, Van Ness PH, Bogardus ST, Jr, Zhang Y, Leslie DL, Leo-Summers LS, Inouye SK. Depressive symptoms and the risk of incident delirium in older hospitalized adults. J Am Geriatr Soc. 2007;55:684–691. doi: 10.1111/j.1532-5415.2007.01150.x. [DOI] [PubMed] [Google Scholar]
- 44.Milbrandt EB, Deppen S, Harrison PL, Shintani AK, Speroff T, Stiles RA, Truman B, Bernard GR, Dittus RS, Ely EW. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 45.Breitbart W, Gibson C, Tremblay A. The delirium experience: delirium recall and delirium-related distress in hospitalized patients with cancer, their spouses/caregivers, and their nurses. Psychosomatics. 2002;43:183–194. doi: 10.1176/appi.psy.43.3.183. [DOI] [PubMed] [Google Scholar]
- 46.Leslie DL, Inouye SK. The importance of delirium: economic and societal costs. J Am Geriatr Soc. 2011;59:S241–S243. doi: 10.1111/j.1532-5415.2011.03671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cartin-Ceba R, Warner DO, Hays JT, Afessa B. Nicotine replacement therapy in critically ill patients: a prospective observational cohort study. Crit Care Med. 2011;39:1635–1640. doi: 10.1097/CCM.0b013e31821867b8. [DOI] [PubMed] [Google Scholar]
- 48.Copeland KT, Checkoway H, McMichael AJ, Holbrook RH. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol. 1977;105:488–495. doi: 10.1093/oxfordjournals.aje.a112408. [DOI] [PubMed] [Google Scholar]
- 49.Vasilevskis EE, Han JH, Hughes CG, Ely EW. Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26:277–287. doi: 10.1016/j.bpa.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guenther U, Popp J, Koecher L, Muders T, Wrigge H, Ely EW, Putensen C. Validity and reliability of the CAM-ICU Flowsheet to diagnose delirium in surgical ICU patients. J Crit Care. 2010;25:144–151. doi: 10.1016/j.jcrc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 51.van Eijk MM, van Marum RJ, Klijn IA, de Wit N, Kesecioglu J, Slooter AJ. Comparison of delirium assessment tools in a mixed intensive care unit. Crit Care Med. 2009;37:1881–1885. doi: 10.1097/CCM.0b013e3181a00118. [DOI] [PubMed] [Google Scholar]
- 52.van Eijk MM, van den Boogaard M, van Marum RJ, Benner P, Eikelenboom P, Honing ML, van der Hoven B, Horn J, Izaks GJ, Kalf A, et al. Routine use of the confusion assessment method for the intensive care unit: a multicenter study. Am J Respir Crit Care Med. 2011;184:340–344. doi: 10.1164/rccm.201101-0065OC. [DOI] [PubMed] [Google Scholar]