Abstract
Background: Pneumonia is a major cause of death during induction chemotherapy for acute leukemia. The purpose of this study was to quantify the incidence, risk factors, and outcomes of pneumonia in patients with acute leukemia.
Methods: We conducted a retrospective cohort study of 801 patients with acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), or acute lymphocytic leukemia (ALL) who underwent induction chemotherapy.
Measurements and Main Results: Pneumonia was present at induction start in 85 patients (11%). Of the 716 remaining patients, 148 (21%) developed pneumonia. The incidence rate of pneumonia was higher in MDS and AML than in ALL (0.013 vs. 0.008 vs. 0.003 pneumonias per day, respectively; P < 0.001). In multivariate analysis, age greater than or equal to 60 years, AML, low platelet count, low albumin level, neutropenia, and neutrophil count greater than 7,300 were risk factors. The case fatality rate of pneumonia was 17% (40 of 233). Competing risk analysis demonstrated that in the absence of pneumonia, death was rare: 28-day mortality was 6.2% for all patients but only 1.26% in those without pneumonia. Compared with patients without pneumonia, patients with pneumonia had more intensive care unit days, longer hospital stays, and 49% higher costs (P < 0.001).
Conclusions: Pneumonia after induction chemotherapy for acute leukemia continues to be common, and it is the most important determinant of early mortality after induction chemotherapy. Given the high incidence, morbidity, mortality, and cost of pneumonia, interventions aimed at prevention are warranted in patients with acute leukemia.
Keywords: pneumonia, opportunistic infections, leukemia, fungal pneumonia, epidemiology
Early mortality after chemotherapy for the induction of remission in patients with acute leukemia continues to be significant despite advances in supportive care (1–5). Thirty-day mortality rates during induction chemotherapy for acute myeloid leukemia (AML) range from 5 to 60%, depending on patient age and performance status (1–4). For patients with high-risk myelodysplastic syndrome (MDS), early mortality rates range from 5 to 15% (5, 6), whereas for older patients with acute lymphoblastic leukemia (ALL), rates range from 6 to 18% (7, 8). In part, early mortality occurs because of infectious complications that arise during the induction period. Of the infectious complications, pneumonia is among the most common, developing in 13 to 31% of patients undergoing induction chemotherapy for leukemia (9–13). Pneumonia is also one of the most serious infectious complications in this context, with case fatality rates of 25 to 45% (9–15).
Recent advances in our understanding of innate immunity may make pneumonia prevention feasible in selected high-risk populations (16–20). Translation of these basic science advances into clinically effective interventions requires careful measurement of the incidence rate of pneumonia in the leukemia population and identification of its determinants. However, the available published data have significant limitations. Chemotherapy regimens and supportive care measures have evolved significantly over that time period, so it is not clear whether older studies are generalizable to today’s practice (12, 15, 21, 22). In addition, previous studies did not control for competing risks, such as death due to other causes. Both changing incidence rates and competing risks are important when it comes to designing clinical trials, because they affect sample size calculations. In addition, previous studies reported incidence proportions of pneumonia rather than incidence rates, without controlling for time-varying covariates such as neutropenia. As a result, the impact of neutropenia on the incidence of pneumonia has not been adequately analyzed. Finally, data are limited on how pneumonia in the leukemia population affects health-care resource use. In an era of increasingly limited resources, a complete picture of the consequences of a disease is important, because future interventions must be able to demonstrate not only efficacy but also value.
The purpose of this study was to quantify the incidence rate, hazard function, and risk factors for pneumonia in patients with acute leukemia. Secondary objectives were to assess the impact of pneumonia on early mortality and on health-care resource use.
Methods
Study Design
We conducted a retrospective cohort study of all patients with AML, ALL, or high-risk MDS, 18 years of age or older, who underwent their first induction chemotherapy as inpatients at The University of Texas MD Anderson Cancer Center. For patients with ALL, the time period was from January 1, 2005 until September 30, 2009 when the database was migrated to a new platform. For patients with AML the time period was from January 1, 2005 until May 31, 2009. For patients with MDS it was January 1, 2005 until December 31, 2009. The study was approved by the Institutional Review Board 4, and a waiver of informed consent was given.
Data Capture and Abstraction
Clinical and oncology data were prospectively collected on all patients as part of an ongoing database of patients with acute leukemia. Data on pneumonia diagnosis and its related features were obtained retrospectively by chart review using a standardized form for data abstraction. All definitions were developed a priori. Charts were abstracted by a pulmonary fellow, and three pulmonary attending physicians adjudicated each case based on the extracted information and direct review of all chest imaging studies. All patients had data abstracted to day 33 after induction or their day of death, whichever occurred first. Data on hospital length of stay, intensive care unit (ICU) use, hospital charges, and hospital costs were obtained from the institutional electronic data warehouse. Daily absolute neutrophil count (ANC) was imported from the Laboratory Information Systems Clinical Applications Database.
Definitions
We based our definition of pneumonia on the American Thoracic Society guidelines for health care–associated pneumonia (23). We defined a clinical pneumonia syndrome as being present when all of the following criteria were met (1): Chest imaging (either chest X-ray or computed tomography scan) consistent with pneumonia. (2) No evidence of pulmonary edema based on echocardiographic and pulmonary artery catheter data, fluid balance, and radiographic response to diuresis. Response to diuresis was defined as significant clearing of chest infiltrates within 48 hours of diuresis. No other special tests or algorithms were used other than a detailed chart review and radiographic review. (3) Temperature greater than 38.3°C, or dyspnea, or cough with purulent sputum. A microbiologically confirmed pneumonia syndrome was defined as the presence of the clinical pneumonia syndrome in conjunction with the isolation of a probable pulmonary pathogen from the blood, sputum, bronchoalveolar lavage fluid, or lung tissue. Coagulase-negative Staphylococcus and Candida species were excluded unless there was biopsy-proven invasion or concomitant isolation of the organism in respiratory secretions and blood in the absence of a more likely pulmonary pathogen. A virus was considered a pathogen if it was detected by antibody or culture or polymerase chain reaction from respiratory secretions in the absence of a more likely etiologic agent (24).
The primary outcome was time to development of a clinical pneumonia syndrome. Secondary outcomes were time to death, ICU days, length of hospital stay, hospital costs, and hospital charges. Hospital costs were defined as the actual cost of providing care for patients, including both technical and professional components. Hospital charges included both technical and professional components. Details of the methodology for computing costs are given in the online supplement. Acute respiratory distress syndrome (ARDS) was defined as being present when there were bilateral opacities occurring within 1 week of a known clinical insult, respiratory failure not fully explained by cardiac failure or fluid overload, and a PaO2/FiO2 less than or equal to 300 (25).
During the period of this study, patients aged 50 years or older with AML or high-risk MDS and 60 years or older with ALL received induction chemotherapy in a laminar air flow room using precautions as previously described (26, 27). Chemotherapy was defined as intensive when cytarabine was used in a dose of 500 mg/m2 or greater daily as part of the regimen. The standard induction for adult patients with ALL at our institution is the HCVAD regimen: hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine. That includes cytarabine at a dose of 3 g/m2 every 12 hours × 2 days for younger patients and 1 g/m2 for older patients. For patients with AML, we do not use a 3+7 regimen but rather intermediate-dose cytarabine at a dose of 1.5 gm/m2/d for 4 days for younger patients and 3 days for patients aged 60 years or older. Similar regimens are used for younger patients with high-risk MDS. For laboratory values such as albumin and creatinine, we used either the lower or upper limit of normal to categorize results.
Statistics
We used an extended Cox model to analyze the factors associated with pneumonia syndrome incidence within the first 33 days after induction chemotherapy. We chose 33 days because calculation of hazard rates required a kernel smoothing technique, which requires that there be events both to the right and to the left of the time point of interest. By following patients to 33 days, we hoped to have sufficient events to calculate the hazard rate at day 28. For this analysis, ANC was represented as a time-dependent covariate, and death was treated as a censoring event. We used a last-observation-carry-forward method to impute the ANC for days when no tests were done. The population at risk for a pneumonia syndrome consisted of all patients undergoing induction chemotherapy without evidence of pneumonia in the 2 weeks up to and including the day of induction. Patients with evidence of a persistent pneumonia syndrome present at the start of induction were not included in that analysis, because they already had a pneumonia syndrome. For the time-to-death analysis, ANC and the presence of a pneumonia syndrome were treated as time-dependent covariates. Patients with a pneumonia syndrome present at the start of treatment were included in this analysis. There was no loss to follow-up. We also used a competing risk model to derive the cumulative incidence function for a pneumonia syndrome while considering death as a competing risk. Because charges and costs were not normally distributed, we used linear regression with the log of charges and the log of costs as the dependent variables to identify determinants. We decided a priori that variables significantly associated with outcomes at the 0.2 level in univariate analysis would be considered candidate variables for multivariate analysis. Backward selection was used to retain only candidate variables that had a level of significance less than 0.05. The Kaplan-Meier product-limit method was used to estimate the percentages of patients at risk with pneumonia stratified by leukemia type. P values less than 0.05 were considered statistically significant, and all tests were two-sided. No adjustment was made for multiple comparisons. Statistical analyses were performed using Stata/IC 12.1 (StataCorp LP, College Station, TX), SAS 9.3 (SAS Institute Inc., Cary, NC), S-Plus 8.2 (TIBCO Software Inc., Palo Alto, CA), and the contributed package cmprsk in R 2.15.2 (28).
Results
Baseline Characteristics and Clinical Features of Pneumonia
A total of 801 patients received their first induction chemotherapy as inpatients at our institution during this time period (Table 1). All patients received antibacterial, antifungal, and antiviral prophylaxis. This included fluconazole, voriconazole, or posaconazole; ciprofloxacin or levofloxacin; and acyclovir or valacyclovir. Signs and symptoms at the time of pneumonia syndrome diagnosis are summarized in Table 2. One or more pulmonary pathogens was identified in 79 (34%) of the 233 patients with the clinical pneumonia syndrome. Among these 79, gram-positive bacteria were present in 67% of patients, gram-negatives in 30%, fungi in 23%, and viruses in 10% (see Table E1 in the online supplement).
Table 1.
Study population characteristics
| Variable | AML (n = 645) | ALL (n = 121) | MDS (n = 35) | Total (n = 801) |
|---|---|---|---|---|
| Year of Treatment | ||||
| 2005 | 172 (27) | 49 (40) | 6 (17) | 227 (28) |
| 2006 | 168 (26) | 48 (40) | 8 (23) | 224 (28) |
| 2007 | 151 (23) | 24 (20) | 10 (29) | 185 (23) |
| 2008 | 109 (17) | 8 (23) | 117 (15) | |
| 2009 | 45 (7) | 3 (9) | 48 (6) | |
| Age | ||||
| Mean ± SD, yr | 58 ± 15 | 45 ± 19 | 64 ± 14 | 56 ± 16 |
| Age < 60 yr | 335 (52) | 90 (74) | 14 (40) | 439 (55) |
| Age ≥ 60 yr | 310 (48) | 31 (26) | 21 (60) | 362 (45) |
| Sex | ||||
| Male | 337 (52) | 66 (55) | 21 (60) | 424 (53) |
| Female | 308 (48) | 55 (45) | 14 (40) | 377 (47) |
| Ethnicity | ||||
| White | 474 (73) | 70 (58) | 29 (83) | 573 (72) |
| Hispanic | 74 (11) | 36 (30) | 4 (11) | 114 (14) |
| Black | 57 (9) | 7 (6) | 1 (3) | 65 (8) |
| Asian | 27 (4) | 5 (4) | 0 (0) | 32 (4) |
| Other | 13 (2) | 3 (2) | 1 (3) | 17 (2) |
| Baseline laboratory values, mean ± SD | ||||
| Hemoglobin, g/dl | 8.6 ± 1.6 | 9.1 ± 3.2 | 9.2 ± 1.5 | 8.7 ± 1.9 |
| Platelets, ×103/μL | 68 ± 69 | 85 ± 97 | 70 ± 70 | 70 ± 74 |
| β2-microglobulin, mg/L* | 3.2 ± 2.1 | 3.1 ± 1.5 | 3.1 ± 1.2 | 3.2 ± 2.0 |
| Albumin low, g/dl | 3.5 ± 0.7 | 3.5 ± 0.6 | 3.8 ± 0.6 | 3.5 ± 0.7 |
| Bilirubin elevated, mg/dl | 0.7 ± 0.8 | 0.7 ± 0.6 | 0.5 ± 0.3 | 0.7 ± 0.8 |
| Creatinine elevated, mg/dl | 1.0 ± 0.4 | 1.0 ± 0.4 | 1.0 ± 0.3 | 1.0 ± 0.4 |
| ECOG performance status | ||||
| 0–1 | 529 (82) | 96 (79) | 34 (97) | 659 (82) |
| 2–4 | 116 (18) | 25 (21) | 1 (3) | 142 (18) |
| Laminar flow room | ||||
| Yes | 414 (64) | 31 (26) | 18 (52) | 463 (58) |
| No | 231 (36) | 90 (74) | 17 (49) | 338 (42) |
| Intensive chemotherapy | ||||
| Yes | 393 (61) | 0 (0) | 7 (20) | 521 (65) |
| No | 252 (39) | 121 (100) | 28 (80) | 280 (35) |
| Length of stay, d† | ||||
| Mean | 23.4 | 13.4 | 21.7 | 21.9 |
| Median (IQR) | 24 (13–30) | 9 (6–17) | 24 (8–31) | 23 (10–29) |
| ICU days, mean† | ||||
| Mean | 1.0 | 0.5 | 0.6 | 0.9 |
| Median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Charges, mean† | ||||
| Mean | $185,604 | $123,200 | $149,273 | $174,753 |
| Median (IQR) | $154,043 (97,408–220,497) | $90,244 (64,363–149,276) | $109,521 (64,527–190,183) | $143,694 (87,070–206,768) |
| Pneumonia syndrome‡ | ||||
| Present at start of induction | 76 (12) | 8 (7) | 1 (3) | 85 (11) |
| Occurring after induction | 125 (19) | 11 (9) | 12 (34) | 148 (18) |
| None | 444 (69) | 102 (84) | 22 (63) | 569 (71) |
| Response to treatment§ | ||||
| Complete response | 406 (63) | 108 (89) | 14 (40) | 528 (66) |
| CRP | 27 (4) | 1 (1) | 4 (11) | 32 (4) |
| Partial response | 13 (2) | 1 (1) | 1 (3) | 15 (2) |
| Resistant | 149 (23) | 3 (3) | 11 (31) | 163 (20) |
| Failed | 50 (8) | 8 (7) | 5 (14) | 63 (8) |
| Survival status at 33 d | ||||
| Dead | 41 (6) | 8 (7) | 1 (3) | 50 (6) |
| Alive | 604 (94) | 113 (93) | 34 (97) | 751 (94) |
Definition of abbreviations: ANC = absolute neutrophil count; CRP = complete response with insufficient platelet recovery; IQR = interquartile range.
Data are presented as n (%) unless otherwise noted.
Data on β2-microglobulin were only available for 474 patients and therefore were not included in the multivariate model. For all other covariates, n = 801.
This variable is not normally distributed. Mean value is given for budget purposes only and is not as valid as medians as a measure of central tendency.
Pneumonia occurring either within 2 weeks before induction or during the 33 days subsequent to the first day of induction chemotherapy.
Complete response defined as < 5% blasts with ANC >1,000 and platelet count > 100,000; CRP defined as the first two of these three criteria with < 100,000 platelets. Resistant defined as failure to achieve remission with induction regimen, treatment subsequently changed. Failed defined as failure to achieve remission, with death occurring before any documented response to induction regimen.
Table 2.
Clinical characteristics of pneumonia syndrome presentations
| Variable | No. of Patients (%) (n = 233) |
|---|---|
| Cough | |
| Absent | 22 (9) |
| Dry cough | 98 (42) |
| Productive cough, green or yellow sputum | 18 (8) |
| Productive cough, white sputum | 25 (11) |
| Not recorded | 70 (30) |
| Fever (temperature ≥ 38.3°C) | |
| Yes | 209 (90) |
| No | 24 (10) |
| Dyspnea | |
| Yes | 167 (72) |
| No | 66 (28) |
| Hypotension | |
| Yes | 9 (4) |
| No | 224 (96) |
| Pleuritic pain or chest pain | |
| Yes | 38 (16) |
| No | 195 (84) |
| Blood cultures | |
| Positive | 30 (13) |
| Negative | 198 (85) |
| Not done | 5 (2) |
| Sputum culture | |
| Positive | 43 (18) |
| Negative | 55 (24) |
| Not done | 135 (58) |
| Bronchoalveolar lavage | |
| Positive | 27 (11) |
| Negative | 55 (24) |
| Not done | 151 (65) |
| Chest radiograph results | |
| Bilateral patchy airspace disease | 128 (55) |
| Unilateral unilobar airspace disease | 69 (30) |
| Bilateral diffuse airspace disease | 22 (9) |
| Unilateral multilobar airspace disease | 10 (4) |
| Bilateral interstitial pattern | 4 (2) |
| Source of pathogens identified: | |
| Respiratory sources only | 49 (21) |
| Respiratory sources and blood cultures | 11 (5) |
| Blood cultures only | 19 (8) |
| No pathogens identified | 154 (66) |
Incidence and Risk Factors for Pneumonia Syndrome
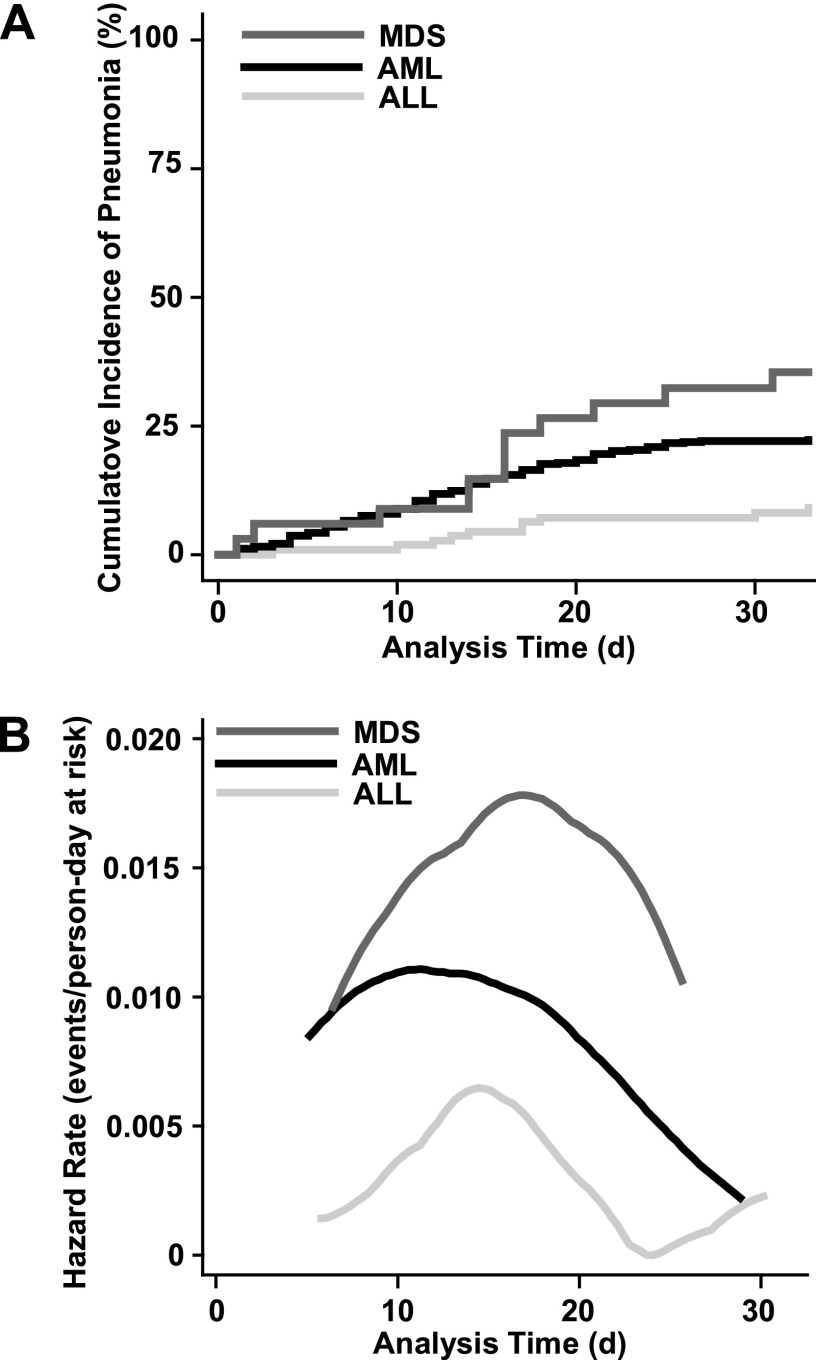
A pneumonia syndrome had been diagnosed within the 2 weeks before induction and was still present in 85 patients (11%). Fifteen of these 85 patients died (case fatality rate, 17.6%). Of the 716 patients without a pneumonia syndrome at the start of induction, 148 (21%) went on to develop a pneumonia syndrome during the next 33 days. Twenty-five of these 147 patients died (case fatality rate, 16.3%). The incidence rate of pneumonia syndromes was higher in patients with MDS and AML than in patients with ALL (Table 3).
Table 3.
Pneumonia incidence rate and 28-day cumulative incidence
| Incidence Rate (Pneumonias/day-at-risk) | 28-d Cumulative Incidence (%) | 95% CI | P Value | |
|---|---|---|---|---|
| Type of Leukemia | ||||
| AML | 0.0078 | 21.6 | 18.2–25 | <0.001 |
| ALL | 0.0028 | 7.1 | 2.3–11.8 | |
| MDS | 0.0133 | 29.4 | 13.8–45 | |
| Total cohort | 0.0072 | 19.84 | 16.9–22.8 |
Definition of abbreviations: ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia; CI = confidence interval; MDS = acute myelodysplastic syndrome.
Multiple variables were associated with pneumonia syndromes on univariate analysis (Table 4), but on multivariate analysis only age greater than or equal to 60 years, AML-type leukemia, low platelet count, low albumin level, neutropenia, and ANC greater than 7,300 were significant risk factors. Analysis of Kaplan-Meier curves and hazard functions for time to pneumonia syndrome development by type of leukemia (Figure 1) demonstrated that the period of maximum risk was on Days 10 to 20.
Table 4.
Univariate and multivariate analysis of time to pneumonia syndrome
| Variable | Univariate Analysis |
Multivariate Analysis |
||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Year of treatment | 1.32 (0.93-1.86) | 0.12 | ||
| Age ≥ 60 yr | 1.75 (1.23–2.43) | 0.001 | 1.60 (1.15–2.23) | 0.005 |
| Male sex | 1.14 (0.82–1.57) | 0.44 | ||
| Ethnicity (white vs. all others) | 1.18 (0.81–1.72) | 0.38 | ||
| Type of leukemia | ||||
| ALL (vs. AML reference) | 0.37 (0.19–0.70) | 0.002 | 0.42 (0.22–0.82) | 0.01 |
| MDS (vs. AML reference) | 1.66 (0.92–2.99) | 0.10 | 1.75 (0.96–3.18) | 0.07 |
| Baseline laboratory studies | ||||
| Hemoglobin, per g/dl | 0.95 (0.87–1.04) | 0.27 | ||
| Platelets, per ×103/μL | 0.995 (0.992–0.999) | 0.004 | 0.996 (0.993–0.999) | 0.02 |
| β2-microglobulin, per mg/L* | 1.07 (0.97–1.18) | 0.20 | ||
| Albumin low, <3.5 g/dl | 1.58 (1.14–2.18) | 0.006 | 1.52 (1.09–2.11) | 0.01 |
| Bilirubin elevated, >1.0 mg/dl | 0.92 (0.54–1.57) | 0.76 | ||
| Creatinine elevated, >1.5 mg/dl | 1.42 (0.72–2.79) | 0.31 | ||
| ECOG ≥ 2 | 1.72 (1.16–2.56) | 0.007 | ||
| Laminar flow room | 1.35 (0.96–1.89) | 0.08 | ||
| Intensive chemotherapy | 0.96 (0.70–1.33) | 0.81 | ||
| ANC† | ||||
| ANC < 500 ×103/μL (vs. ANC > 500 and ≤ 7,300) | 2.16 (1.32–3.55) | 0.002 | 1.86 (1.13–3.07) | 0.01 |
| ANC > 7,300 ×103/μL (vs. ANC > 500 and ≤ 7,300) | 2.04 (0.92–4.51) | 0.08 | 2.52 (1.14–5.57) | 0.02 |
Definition of abbreviations: ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia; ANC = absolute neutrophil count; CI = confidence interval; MDS = acute myelodysplastic syndrome.
Data on β2-microglobulin were only available for 474 patients and therefore were not included in the multivariate model. For all other covariates, n = 801.
Time-varying covariate.
Figure 1.
(A) Pneumonia syndrome incidence during induction chemotherapy in patients with acute leukemia. (B) Hazard function by type of leukemia. There is left and right truncation of the curves because the hazard functions are calculated over the range of observed failure times for each group using a weighted kernel-density estimate. ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia; MDS = acute myelodysplastic syndrome.
Mortality during Induction Chemotherapy
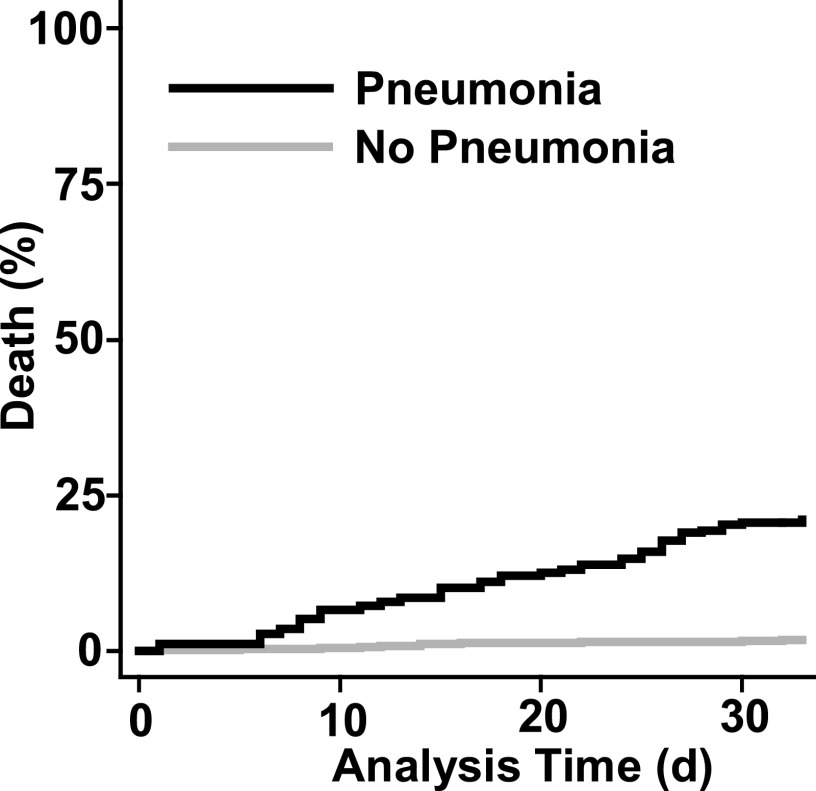
Of the 801 patients undergoing induction chemotherapy, 50 (6.2%) died during the 33 days after induction. Multiple variables were associated with death (Table 5), but on multivariate analysis only a pneumonia syndrome (Figure 2), age greater than or equal to 60 years, elevated baseline creatinine, low performance status (ECOG > 2), laminar flow room, and year of treatment remained significant. Among patients with a pneumonia syndrome, the case fatality rate was 14% for patients who were culture negative, 24% for those who had negative blood cultures with a pathogen identified from a respiratory source, and 24% for those who had a positive blood culture.
Table 5.
Univariate and multivariate analysis of time to death within 33 days of induction
| Variable | Univariate Analysis |
Multivariate Analysis |
||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Year of treatment | 0.72 (0.56–0.93) | 0.01 | 0.74 (0.57–0.96) | 0.02 |
| Age ≥ 60 yr | 2.66 (1.47–4.83) | 0.001 | 2.85 (1.49–5.44) | 0.001 |
| Type of leukemia | ||||
| ALL (vs. AML reference) | 1.03 (0.48–2.19) | 0.95 | ||
| MDS (vs. AML reference) | 0.44 (0.06–3.21) | 0.42 | ||
| Baseline laboratory studies | ||||
| Hemoglobin, per g/dl | 0.95 (0.81–1.11) | 0.56 | ||
| Platelets, per ×103/μL | 0.99 (0.98–0.997) | 0.009 | ||
| β2-microglobulin, per mg/L* | 1.23 (1.12–1.36) | <0.001 | ||
| Albumin low, < 3.5 g/dl | 3.77 (1.97–7.21) | <0.001 | ||
| Bilirubin elevated, >1.0 mg/dl | 2.55 (1.36–4.81) | 0.004 | ||
| Creatinine elevated, > 1.5 mg/dl | 3.74 (1.75–7.96) | 0.001 | 2.31 (1.07–4.99) | 0.03 |
| ECOG ≥ 2 | 5.04 (2.90–8.78) | <0.001 | 2.23 (1.23–4.03) | 0.008 |
| Laminar flow room | 0.48 (0.27–0.84) | 0.01 | 0.32 (0.18–0.59) | <0.001 |
| Intensive chemotherapy | 0.67 (0.38–1.16) | 0.15 | ||
| Neutropenia (ANC < 500 ×103/μL)† | 0.75 (0.40–1.43) | 0.39 | ||
| Pneumonia syndrome* | 14.99 (7.46–30.15) | <0.001 | 12.23 (6.05–24.75) | <0.001 |
For definition of abbreviations, see Table 4.
Data on β2-microglobulin were only available for 474 patients and were therefore not included in the multivariate model. For all other covariates, n = 801.
Time-varying covariate.
Figure 2.
Extended Cox model plot of time to death during induction chemotherapy for acute leukemia. Patients with pneumonia syndrome had an increased risk of death (log rank P < 0.001).
Competing Risk Model: Development of Pneumonia Syndrome Versus Death
Among the 716 patients who started induction chemotherapy without a prior pneumonia syndrome, the cumulative incidences of a pneumonia syndrome at Days 14 and 28 were 10.75% (95% confidence interval [CI], 8.5–13%) and 19.84% (95% CI, 16.9–22.8%), respectively. In the absence of a pneumonia syndrome, death was rare: a total of 10 patients died within the 33-day evaluation period without having had a pneumonia syndrome (Figure E1). The overall cumulative incidences of death without a prior pneumonia syndrome at Days 14 and 28 were 0.70% (95% CI, 0.09–1.3%) and 1.26% (95% CI, 0.44–2.07%).
Health-Care Resource Use
Among the 233 patients with a pneumonia syndrome, 62 (27%) required positive pressure ventilation, 20 (9%) met criteria for ARDS, 43 (18%) had septic shock, and 74 (32%) required ICU care. Patients with a pneumonia syndrome spent more days in the ICU and had an increased length of hospital stay as compared with patients without a pneumonia syndrome (P < 0.001, Table 6). In both univariate analysis and multivariate analysis, year of treatment, increasing age, AML, higher ECOG score, and pneumonia syndrome were associated with increased hospital charges and costs (Tables E2–E5). In multivariate analysis, development of a pneumonia syndrome was associated with a 62% increase in hospital charges and a 49% increase in hospital costs.
Table 6.
Health-care resource use
| No Pneumonia Syndrome (n = 569) | Pneumonia Syndrome (n = 232) | P Value | |
|---|---|---|---|
| Length of stay, d* | |||
| Mean | 20.1 | 26.2 | |
| Median (IQR) | 22 (9–27) | 26 (15–34) | <0.001† |
| ICU d, mean* | |||
| Mean | 0.2 | 2.6 | |
| Median (IQR) | 0 (0–0) | 0 (0–1.7) | <0.001† |
| Charges*‡ | |||
| Mean ± SD | $141,656 ± $93,969 | $256,267 ± $182,666 | |
| Median (IQR) | $120,767 ($78,991–$178,362) | $206,157 ($142,018–$331,973) | <0.001† |
The variable is not normally distributed. Mean value is given for budget purposes only and is not as valid as median as a measure of central tendency.
Mann-Whitney rank sum test.
Charge data were missing on 8 patients (3 with and 5 without pneumonia syndrome), so n = 793.
Discussion
This retrospective cohort study of pneumonia in patients with acute leukemia after induction chemotherapy demonstrates that pneumonia continues to be a major problem associated with significant morbidity, mortality, and health-care resource use. A pneumonia syndrome was either present at the start of induction or developed during the subsequent 33 days in 29% of the 801 patients studied. The incidence rate of a pneumonia syndrome was highest in patients with high-risk MDS, followed by AML and ALL. Other risk factors included advanced age, poor performance status, low albumin level, low baseline platelet count, and neutropenia. The case fatality rate of pneumonia syndromes was 17%, and it was by far the largest risk factor associated with death within the first month after induction chemotherapy. In terms of health-care resource use, pneumonia syndromes were associated with an increase in length of hospital stay and ICU use, and as a result it was the single most important factor in terms of impact on hospital charges and costs.
Our study is consistent with and adds to the existing body of knowledge regarding pneumonia in patients with acute leukemia. The cumulative incidence rates of a pneumonia syndrome after induction in this study—21.6% in AML and 7.1% in ALL—are consistent with those of prior studies (9–13). However, previous studies used logistic regression with incidence proportions to analyze outcomes. Logistic regression has a significant limitation in this setting in that it assumes that the exposure status of patients does not change during the period of observation, and it assumes equal time at risk for all patients. As such, it cannot control for clinically important variables that vary over time, such as neutropenia. For example, patients with neutropenia initially who develop pneumonia after the neutropenia resolves will be classified the same as patients with neutropenia initially who develop pneumonia while still neutropenic. Similarly, a patient who is not neutropenic initially who becomes neutropenic later and develops pneumonia while neutropenic will be misclassified.
Indeed, the only two prior studies that evaluated the impact of ANC on pneumonia risk in patients with acute leukemia used logistic regression and evaluated ANC only at the onset of leukemia rather than allowing ANC to vary over time (10, 11). Both studies failed to demonstrate an association between initial ANC and pneumonia risk. Similarly, if we had used logistic regression with our data, using ANC at the time of induction as the covariate, we would have had similar results (neutropenia odds ratio, 0.78; P = 0.21). In contrast, the use of extended Cox models with time-varying covariates allows us to properly control for both varying exposure status (i.e., going from nonneutropenic to neutropenic and back again) and varying time at risk. Our results show that there is a relationship between neutropenia status on any given day and the incidence of pneumonia on that day. Clinically it is much more logical to adjust for neutropenia on a day-by-day basis than to predict pneumonia based solely on the Day 0 ANC, because neutropenia status changes over time. The extended Cox models with time-varying covariates allowed us to quantify the impact of neutropenia on pneumonia syndrome risk and to obtain more precise estimates of the incidence rate and hazard function for pneumonia syndromes in this population. To our knowledge, this is the first study to do so.
Of course, the finding that neutropenia is indeed a risk factor for pneumonia in patients with acute leukemia is hardly unexpected. However, being able to adjust for neutropenia and other time-varying covariates has significant value, because it allows more accurate quantification of the risk associated with other covariates as well. For instance, although prior studies had suggested a higher incidence of pneumonia in AML than in ALL, whether AML was a risk factor for pneumonia could not truly be determined, because the treatments for AML were associated with more prolonged neutropenia. Our findings suggest that AML and/or its treatments are indeed associated with a higher risk of pneumonia syndrome, even after adjusting for other factors, including neutropenia.
The use of extended Cox models with time-varying covariates also allows a more systematic evaluation of the determinants of early mortality in patients with acute leukemia. Simple multivariate logistic regression using 28-day survival cannot accurately quantify the risk of death due to pneumonia, because there is no way to represent the time-varying status of patients (i.e., a patient either always has pneumonia from Day 0 onward or never has pneumonia). Our findings are consistent with previous studies that suggested that age and performance status are key baseline risk factors for early mortality (1, 2, 29). The main finding of interest, however, is the magnitude of the effect of pneumonia syndromes on mortality risk. Although we identified a variety of other risk factors associated with early mortality on univariate analysis, none were even close in terms of effect size. This finding is also supported by the competing risk analysis. Among patients with leukemia who never developed a pneumonia syndrome, early mortality was rare, with a cumulative incidence of 1.26% at 28 days.
The information from this competing risk analysis should also prove useful for designing future clinical trials (16–18). Efficient study design requires knowledge of competing risks that may result in censoring in addition to the cumulative incidence of disease. Although many studies have reported on pneumonia incidence proportions, to our knowledge, no studies have used a competing risk model. The competing risk model adds valuable additional insights in this area, because it provides a conservative estimate of the minimum amount of censoring that will occur due to death from other causes. Our study suggests that with current practice patterns, early mortality due to other causes in the absence of a pneumonia syndrome will be rare (<2.1% at 28 days) and that the cumulative incidence of a pneumonia syndrome will be 21% at 28 days.
Finally, this study provides useful information on the impact of pneumonia syndromes on health-care resource use. Quantifying this is important, because interventions to decrease pneumonia incidence may be costly. However, our analysis suggests that prevention in this setting may be very cost effective. In particular, because the pneumonia syndrome has a high cumulative incidence (21%) and a high case fatality rate (17%), and because it increases costs by 49%, a strategy of pneumonia prevention in this population may save lives while simultaneously reducing resource use.
Although these findings are useful, it is important to recognize the limitations of this study. First, a diagnosis of pneumonia is difficult to establish with certainty in this population (23). Many possible mimics of pneumonia, including leukemic infiltrates, drug toxicity, hydrostatic pulmonary edema, and pulmonary edema due to increased capillary permeability, might have contributed to misclassification. To mitigate this possibility, we used a definition of pneumonia based on established guidelines, used a standardized approach to rule out alternative causes of radiographic infiltrates, and used multiple reviewers. We did identify a probable pathogen in one-third of cases, which is consistent with reports in the literature (9–13). In addition, the strong relationship of neutropenia to pneumonia syndromes incidence suggests that many of these events were indeed infectious, because neutropenia would not be a risk factor for drug toxicity or hydrostatic pulmonary edema. Even so, persistent misclassification bias may exist and, if it does, then likely the bias is upward, resulting in falsely high estimates of pneumonia.
It is also important to recognize that some of these associations may not be causal. For example, the association of elevated ANC with pneumonia syndrome is probably not causal, because leukocytosis is a frequent response to infection rather than a cause. Indeed, all but three of the patients who developed pneumonia with an elevated ANC did so on Day 16 or later, after their neutropenia resolved. Other possible explanations for this association would include granulocyte colony-stimulating factor–associated ARDS or capillary leak, but the low number of events in this stratum and the retrospective nature of the study limit the inferences that can be drawn regarding these alternative possibilities.
Other findings, such as the association of low baseline platelet count with higher risk of pneumonia syndrome, should be viewed as preliminary, given the retrospective nature of the study. It is quite possible that more severe thrombocytopenia in this setting is just a marker for more profound marrow involvement by leukemia, resulting in higher rates of infection. However, recent work suggests that platelets have an important function in inflammation and the immune response (30). We could not adequately study these possibilities using a retrospective design, so future studies will be needed to clarify these issues. Similarly, the highest risk of infection occurred during Days 10 to 20, which corresponds to when transfusions are most frequent. Transfusions are a known source of lung injury and impact the immune response, so this may also be contributing to the incidence of pneumonia syndrome but will require additional study.
In addition, this was a single-center study, and we focused on inpatients only, so the results may not be generalizable to other settings. In particular, it may be that the incidence of pneumonia is significantly lower in patients with leukemia undergoing outpatient induction than in the population that we studied. The incidence of pneumonia syndrome in our study was similar to that of other centers (9–13), although in some previous reports it is not clear whether the population consisted of inpatients or outpatients.
In conclusion, we found that a pneumonia syndrome after induction chemotherapy for acute leukemia continues to be common, with an overall cumulative incidence of 21.6% in AML and 7.1% in ALL at 28 days. The case fatality rate was 17%. Pneumonia syndrome was the most important determinant of early mortality after induction chemotherapy. Overall, early mortality was 6.2%, but in the absence of a pneumonia syndrome early mortality was only 1.26% at 28 days. Pneumonia syndromes also had a high impact on health-care resource use, increasing costs by 49%. Given the high incidence, morbidity, mortality, and cost of pneumonia syndrome in the acute leukemic population, interventions aimed at pneumonia prevention are warranted.
Acknowledgments
Acknowledgment
The authors thank Bina Patel, Brian Yacovone, Michael Black, Joyce Roquemore, Robert Trujillo, and Paula Moynihan from Clinical Decision Support Services and Clinical Operations Informatics for help with data from the electronic information warehouse and Cathy Price and Xia Li from Pathology and Laboratory Medicine for providing data from the laboratory medicine information warehouse.
Footnotes
Supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
Author Contributions: All authors contributed to the writing and editing of this manuscript. D.E.O. was the Principal Investigator, developed the concept, hypotheses, and design and oversaw the project and data auditing and conducted the statistical analysis with X.L. J.B.G. was responsible for chart abstraction and data collection. D.E.O., S.E.E., J.B.G., and B.F.D. were responsible for adjudicating pneumonia cases. W.W. and J.E.C. were responsible for database management.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, Anderson JE, Petersdorf SH. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estey EH. Therapeutic options for acute myelogenous leukemia. Cancer. 2001;92:1059–1073. doi: 10.1002/1097-0142(20010901)92:5<1059::aid-cncr1421>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara F, Schiffer CA. Acute myeloid leukaemia in adults. Lancet. 2013;381:484–495. doi: 10.1016/S0140-6736(12)61727-9. [DOI] [PubMed] [Google Scholar]
- 4.Löwenberg B, Ossenkoppele GJ, van Putten W, Schouten HC, Graux C, Ferrant A, Sonneveld P, Maertens J, Jongen-Lavrencic M, von Lilienfeld-Toal M, et al. Dutch-Belgian Cooperative Trial Group for Hemato-Oncology (HOVON); German AML Study Group (AMLSG); Swiss Group for Clinical Cancer Research (SAKK) Collaborative Group. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361:1235–1248. doi: 10.1056/NEJMoa0901409. . [Published erratum appears in N Engl J Med 362:1155.] [DOI] [PubMed] [Google Scholar]
- 5.de Witte T, Suciu S, Verhoef G, Labar B, Archimbaud E, Aul C, Selleslag D, Ferrant A, Wijermans P, Mandelli F, et al. Intensive chemotherapy followed by allogeneic or autologous stem cell transplantation for patients with myelodysplastic syndromes (MDSs) and acute myeloid leukemia following MDS. Blood. 2001;98:2326–2331. doi: 10.1182/blood.v98.8.2326. [DOI] [PubMed] [Google Scholar]
- 6.Itzykson R, Thépot S, Quesnel B, Dreyfus F, Beyne-Rauzy O, Turlure P, Vey N, Recher C, Dartigeas C, Legros L, et al. Groupe Francophone des Myelodysplasies(GFM) Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117:403–411. doi: 10.1182/blood-2010-06-289280. [DOI] [PubMed] [Google Scholar]
- 7.Huguet F, Leguay T, Raffoux E, Thomas X, Beldjord K, Delabesse E, Chevallier P, Buzyn A, Delannoy A, Chalandon Y, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. 2009;27:911–918. doi: 10.1200/JCO.2008.18.6916. . [Published erratum appears in J Clin Oncol 27:2574.] [DOI] [PubMed] [Google Scholar]
- 8.Sive JI, Buck G, Fielding A, Lazarus HM, Litzow MR, Luger S, Marks DI, McMillan A, Moorman AV, Richards SM, et al. Outcomes in older adults with acute lymphoblastic leukaemia (ALL): results from the international MRC UKALL XII/ECOG2993 trial. Br J Haematol. 2012;157:463–471. doi: 10.1111/j.1365-2141.2012.09095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannas G, Pautas C, Raffoux E, Quesnel B, de Botton S, de Revel T, Reman O, Gardin C, Elhamri M, Boissel N, et al. Infectious complications in adult acute myeloid leukemia: analysis of the Acute Leukemia French Association-9802 prospective multicenter clinical trial. Leuk Lymphoma. 2012;53:1068–1076. doi: 10.3109/10428194.2011.636812. [DOI] [PubMed] [Google Scholar]
- 10.Rossini F, Verga M, Pioltelli P, Giltri G, Sancassani V, Pogliani EM, Corneo G. Incidence and outcome of pneumonia in patients with acute leukemia receiving first induction therapy with anthracycline-containing regimens. Haematologica. 2000;85:1255–1260. [PubMed] [Google Scholar]
- 11.Specchia G, Pastore D, Carluccio P, Mele G, Montagna MT, Liso A, Rizzi R, Ianora AS, Liso V. Pneumonia in acute leukemia patients during induction therapy: experience in a single institution. Leuk Lymphoma. 2003;44:97–101. doi: 10.1080/1042819021000040297. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida M, Akiyama N, Fujita H, Miura K, Miyatake J, Handa H, Kito K, Takahashi M, Shigeno K, Kanda Y, et al. Analysis of bacteremia/fungemia and pneumonia accompanying acute myelogenous leukemia from 1987 to 2001 in the Japan Adult Leukemia Study Group. Int J Hematol. 2011;93:66–73. doi: 10.1007/s12185-010-0746-y. [DOI] [PubMed] [Google Scholar]
- 13.Wilhelm M, Kantarjian HM, O’Brien S, Pierce S, Keating MJ, Freireich EJ, Estey EH. Pneumonia during remission induction chemotherapy in patients with AML or MDS. Leukemia. 1996;10:1870–1873. [PubMed] [Google Scholar]
- 14.Chaoui D, Legrand O, Roche N, Cornet M, Lefebvre A, Peffault de Latour R, Sanhes L, Huchon G, Marie JP, Rabbat A. Incidence and prognostic value of respiratory events in acute leukemia. Leukemia. 2004;18:670–675. doi: 10.1038/sj.leu.2403270. [DOI] [PubMed] [Google Scholar]
- 15.Offidani M, Corvatta L, Malerba L, Marconi M, Bichisecchi E, Cecchini S, Manso E, Principi T, Gasparini S, Leoni P. Risk assessment of patients with hematologic malignancies who develop fever accompanied by pulmonary infiltrates: a historical cohort study. Cancer. 2004;101:567–577. doi: 10.1002/cncr.20406. [DOI] [PubMed] [Google Scholar]
- 16.Clement CG, Evans SE, Evans CM, Hawke D, Kobayashi R, Reynolds PR, Moghaddam SJ, Scott BL, Melicoff E, Adachi R, et al. Stimulation of lung innate immunity protects against lethal pneumococcal pneumonia in mice. Am J Respir Crit Care Med. 2008;177:1322–1330. doi: 10.1164/rccm.200607-1038OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuvim MJ, Gilbert BE, Dickey BF, Evans SE. Synergistic TLR2/6 and TLR9 activation protects mice against lethal influenza pneumonia. PLoS ONE. 2012;7:e30596. doi: 10.1371/journal.pone.0030596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuvim MJ, Evans SE, Clement CG, Dickey BF, Gilbert BE. Augmented lung inflammation protects against influenza A pneumonia. PLoS ONE. 2009;4:e4176. doi: 10.1371/journal.pone.0004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans SE, Tuvim MJ, Fox CJ, Sachdev N, Gibiansky L, Dickey BF. Inhaled innate immune ligands to prevent pneumonia. Br J Pharmacol. 2011;163:195–206. doi: 10.1111/j.1476-5381.2011.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duggan JM, You D, Cleaver JO, Larson DT, Garza RJ, Guzmán Pruneda FA, Tuvim MJ, Zhang J, Dickey BF, Evans SE. Synergistic interactions of TLR2/6 and TLR9 induce a high level of resistance to lung infection in mice. J Immunol. 2011;186:5916–5926. doi: 10.4049/jimmunol.1002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodey GP, Powell RD, Jr, Hersh EM, Yeterian A, Freireich EJ. Pulmonary complications of acute leukemia. Cancer. 1966;19:781–793. doi: 10.1002/1097-0142(196606)19:6<781::aid-cncr2820190607>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 22.Ewig S, Glasmacher A, Ulrich B, Wilhelm K, Schäfer H, Nachtsheim KH. Pulmonary infiltrates in neutropenic patients with acute leukemia during chemotherapy: outcome and prognostic factors. Chest. 1998;114:444–451. doi: 10.1378/chest.114.2.444. [DOI] [PubMed] [Google Scholar]
- 23.American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 24.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 25.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 26.Estey E, Thall P, Andreeff M, Beran M, Kantarjian H, O’Brien S, Escudier S, Robertson LE, Koller C, Kornblau S, et al. Use of granulocyte colony-stimulating factor before, during, and after fludarabine plus cytarabine induction therapy of newly diagnosed acute myelogenous leukemia or myelodysplastic syndromes: comparison with fludarabine plus cytarabine without granulocyte colony-stimulating factor. J Clin Oncol. 1994;12:671–678. doi: 10.1200/JCO.1994.12.4.671. [DOI] [PubMed] [Google Scholar]
- 27.Estey E, Thall P, Beran M, Kantarjian H, Pierce S, Keating M. Effect of diagnosis (refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, or acute myeloid leukemia [AML]) on outcome of AML-type chemotherapy. Blood. 1997;90:2969–2977. [PubMed] [Google Scholar]
- 28.Gray B. Vienna, Austria: Vienna University of Economics and Business Administration; 2007. The cmprsk package. The Comprehensive R Archive Network. [Google Scholar]
- 29.Al Ameri A, Koller C, Kantarjian H, Ravandi F, Verstovsek S, Borthakur G, Pierce S, Mattiuzzi G. Acute pulmonary failure during remission induction chemotherapy in adults with acute myeloid leukemia or high-risk myelodysplastic syndrome. Cancer. 2010;116:93–97. doi: 10.1002/cncr.24711. [DOI] [PubMed] [Google Scholar]
- 30.Li C, Li J, Li Y, Lang S, Yougbare I, Zhu G, Chen P, Ni H. Crosstalk between platelets and the immune system: old systems with new discoveries. Adv Hematol. 2012;2012:384685. doi: 10.1155/2012/384685. [DOI] [PMC free article] [PubMed] [Google Scholar]