Abstract
Rationale: Adults with chronic critical illness (tracheostomy after ≥ 10 d of mechanical ventilation) have a high burden of palliative needs, but little is known about the actual use and potential need of palliative care services for the larger population of older intensive care unit (ICU) survivors discharged to post–acute care facilities.
Objectives: To determine whether older ICU survivors discharged to post–acute care facilities have potentially unmet palliative care needs.
Methods: We examined electronic records from a 1-year cohort of 228 consecutive adults ≥ 65 years of age who had their first medical-ICU admission in 2009 at a single tertiary-care medical center and survived to discharge to a post–acute care facility (excluding hospice). Use of palliative care services was defined as having received a palliative care consultation. Potential palliative care needs were defined as patient characteristics suggestive of physical or psychological symptom distress or anticipated poor prognosis. We examined the prevalence of potential palliative needs and 6-month mortality.
Measurements and Main Results: The median age was 78 years (interquartile range, 71–84 yr), and 54% received mechanical ventilation for a median of 7 days (interquartile range, 3–16 d). Six subjects (2.6%) received a palliative care consultation during the hospitalization. However, 88% had at least one potential palliative care need; 22% had chronic wounds, 37% were discharged on supplemental oxygen, 17% received chaplaincy services, 23% preferred to not be resuscitated, and 8% were designated “comfort care.” The 6-month mortality was 40%.
Conclusions: Older ICU survivors from a single center who required postacute facility care had a high burden of palliative care needs and a high 6-month mortality. The in-hospital postcritical acute care period should be targeted for palliative care assessment and intervention.
Keywords: aged, critically ill, palliative medicine, nursing homes
Older adults (age ≥ 65 yr) comprise almost half of all intensive care unit (ICU) admissions in the United States (1), receive more intensive treatment than in the past (2), and survive what were previously fatal critical illnesses (2, 3). Despite these epidemiologic changes, outcomes can still be poor. One third of older adult ICU survivors (∼ 500,000 patients annually in the United States) are discharged to post–acute care facilities, nearly half are rehospitalized, and 25 to 65% die within 6 months (4, 5). The Institute of Medicine has prioritized research aimed at improving coordination of end-of-life care with skilled care for the elderly population (6), and one of the key research priorities of the 2012 Multisociety Taskforce for Critical Care Research in America was to identify improvement strategies for palliative and end-of-life care through the acute and chronic stages of critical illness and into recovery (7).
Palliative care is focused on providing patients with serious illness relief from pain and stress, and it should be available to patients at all stages of illness (8). Palliative care is often provided simultaneously with curative or life-sustaining treatments and is not hospice or end-of-life care (9). However, palliative care seeks to align treatment plans with patients’ goals and therefore may facilitate transitions to hospice in older ICU survivors who often have high end-of-life healthcare resource utilization (4, 5). Indeed, previous studies have demonstrated that palliative care can have a meaningful effect on patients’ quality of life and end-of-life care (10, 11), reduce end-of-life utilization of acute care resources (12), and prolong survival (13).
The need for palliative care services among older ICU survivors who have been transferred from the ICU to the general hospital ward and are awaiting discharge to a post–acute care facility is not well described. Studies have shown that patients with chronic critical illness (CCI), defined as placement of a tracheostomy after ≥ 10 days of mechanical ventilation (14), have a high burden of physical and psychological symptoms that persist after the ICU while in ventilator weaning facilities (15–18). CCI most often occurs in older adults and is characterized by prolonged mechanical ventilation via tracheostomy, which is its hallmark, and functional dependence due to some combination of profound weakness, endocrinopathy, poor nutrition, skin breakdown, and brain dysfunction manifesting as coma or delirium (14). We have observed that older ICU survivors at our institution who are discharged to post–acute care facilities have physical symptom distress, delirium, weakness, and wasting without necessarily meeting all of the criteria (e.g., prolonged mechanical ventilation) for CCI. Therefore, we hypothesized that among all older ICU survivors who are discharged to post–acute care facilities, physical symptoms, psychological distress, and anticipated poor prognosis that warrant palliative care are common and that few patients for whom palliative care is appropriate receive those services before hospital discharge.
The objectives of this study were (1) to explore the potential palliative care needs of older ICU survivors being discharged to post–acute care facilities from our institution and (2) to describe the potential need for a palliative care intervention after the ICU among debilitated survivors of critical illness who are awaiting hospital discharge.
Methods
Subjects, Setting, and Data Sources
This was a single-center retrospective cohort study. The cohort included consecutive adults aged ≥ 65 years at hospital admission who had their first medical-ICU (MICU) admission between January 1, 2009 and December 31, 2009 at Columbia University Medical Center, were not admitted to any Columbia ICU in 2008, and were discharged to a post–acute care facility (excluding those discharged to hospice). Post–acute care facilities included skilled-nursing facilities, long-term care facilities, long-term acute care facilities, inpatient rehabilitation centers, or subacute rehabilitation centers.
We excluded those with an ICU stay of ≤ 24 hours because these patients were most likely admitted for observation only. We focused on the patients admitted to the MICU rather than the surgical or cardiac ICU because MICU patients are less likely to have an elective intensive care admission and tend to have higher long-term mortality.
Columbia University Medical Center consists of the Presbyterian Hospital that has 724 adult beds including two MICUs, each with 12 beds, and the Allen Pavilion that has 225 adult beds, including one MICU with 12 beds. When subjects were admitted to the MICUs, the primary responsibility for the patient was transferred to the intensivist. Each ICU team consisted of an attending physician who was board certified in critical care medicine (none was board certified in palliative care medicine), a pulmonary and critical care fellow, internal medicine residents, acute care nurse practitioners or physician assistants, critical care nurses, respiratory therapists, and a social worker. After leaving the ICU, subjects were transferred to an intermediate care unit or to a general medical ward or were discharged directly to a post–acute care facility or home. There was no dedicated ventilator weaning unit or long-term acute care facility at the institution. The palliative care team consisted of physicians who were board certified in pain and palliative care medicine, a nurse practitioner, and a social worker, and they did not make rounds regularly with the ICU team. The palliative care team performed 353, 360, and 364 inpatient consultations during the 2008, 2009, and 2010 calendar years, respectively. Palliative care consultations could be requested by physicians, nurse practitioners, or physician assistants.
We used institutional administrative and claims data prepared for submission to Medicare and the University Health Consortium (19) supplemented with data and notes abstracted from the electronic medical record. We did not review the paper component of the medical records, although patients’ pain reports were included there with the vital signs. The dataset contained claims data in the form of International Classification of Diseases, Ninth Revision, clinical modification codes demographic data, including admission source and discharge location, hospital length of stay, electronic notes and orders from treating physicians and consultants, and medications prescribed at hospital discharge.
Measurements
We ascertained certain demographic variables (age, sex, race, and admission from or discharge to a post–acute care facility) and clinical variables (principal diagnosis for hospitalization, Charlson comorbidities, use of mechanical ventilation, and hospital and ICU length of stay) using claims data. CCI was defined as placement of a tracheostomy after ≥ 10 days of mechanical ventilation because this is the suggested definition of CCI for research purposes (14). Further details of the methods of classification and grouping of these variables have been published previously (20).
The primary outcomes were subject characteristics suggestive of potential palliative care needs, actual use of palliative care services defined as having received a palliative care consultation during the hospitalization, and 6-month mortality from the date of hospital discharge. Date of death came from the National Death Index. The retrospective nature of the study did not allow us to assess palliative and/or hospice needs directly from patients and their families. Therefore, we identified characteristics suggestive of physical or psychological symptom distress and/or anticipated poor prognosis that may be compatible with the need for palliative care services on the basis of multidisciplinary input, clinical practice standards, and previous studies. Characteristics suggestive of a high physical symptom burden were chronic wounds (21), supplemental oxygen use (22), use of mechanical ventilation at the time of hospital discharge (5, 14, 16, 22), or prescription of opioids at hospital discharge (23). Characteristics suggestive of psychological symptom distress were consultation with a hospital chaplain during the hospitalization (24); brain dysfunction (delirium or dementia) at discharge (17); or prescription of anxiolytics, tricyclic antidepressants, γ-aminobutyric acid analogs, or antipsychotics at discharge (23). Characteristics suggestive of an anticipated poor prognosis were a diagnosis of active malignancy (22), a “do-not-resuscitate” order at the time of hospital discharge (25), or a designation of “comfort care” as the goal of care at the time of discharge to the post–acute care facility. We assessed these proxies for potential palliative care needs by reviewing discharge summaries (discharge diagnoses, assessment, and plan sections), institutional claims data, and electronic medical record orders and prescriptions. Physician Orders for Life-Sustaining Treatment forms, which state treatment preferences of patients toward the end of their lives, were not used in this study because these forms are not permitted for use at our institution.
The secondary outcomes were hospital and ICU readmissions to Columbia University Medical Center within 6 months after discharge after the index hospitalization. We ascertained readmissions from the electronic medical record. We could not determine whether patients were admitted to other hospitals during this time.
Statistical Analyses
Summary analyses were performed on demographic and clinical variables and are expressed as mean ± standard deviation or median and interquartile range (IQR). We determined the prevalence of characteristics suggestive of palliative care needs at hospital discharge for the entire cohort and for those who received or did not receive mechanical ventilation. There were three (1.3%) subjects with missing data who were excluded from the analyses (Figure 1). Analyses were performed with Stata 12.0 (Stata Corp., College Station, TX). The study was approved by the Institutional Review Board of Columbia University Medical Center.
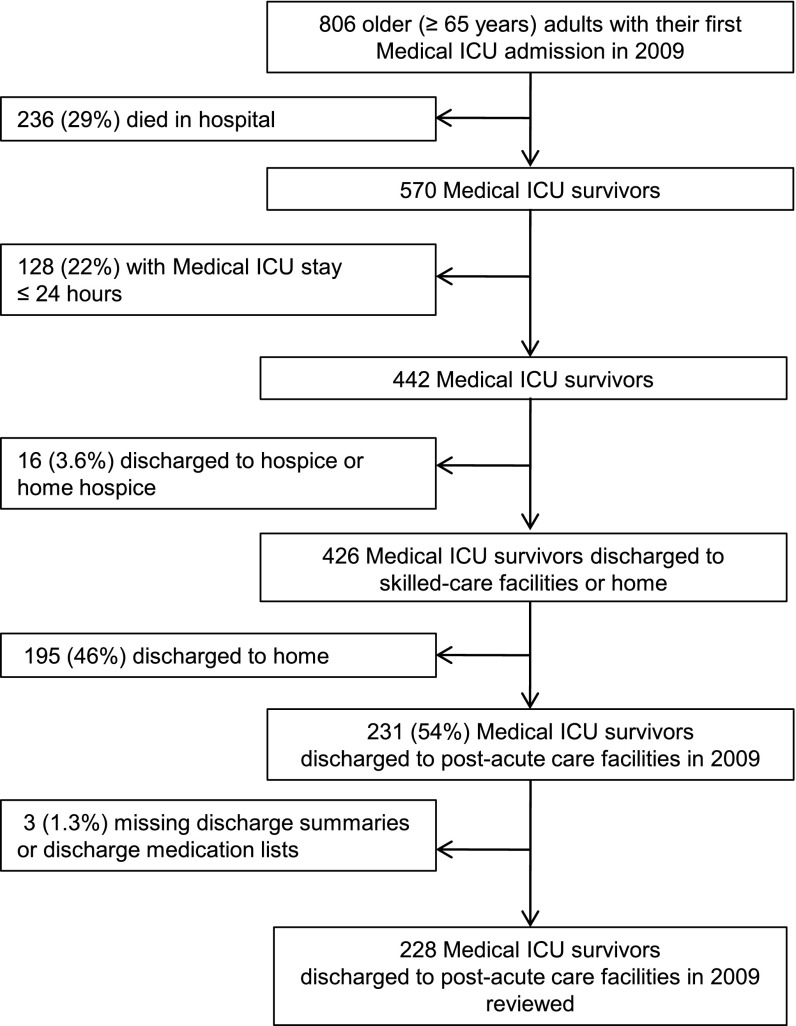
Figure 1.
Flow chart of medical intensive care unit (ICU) admissions.
Results
Subject Characteristics
Among 442 consecutive older MICU subjects who had an ICU length of stay > 24 hours and survived to hospital discharge, 16 (3.6%) were discharged to hospice or home hospice, 195 (44%) were discharged home, and 231 (52%) were discharged to post–acute care facilities. Among the 231 participants who were discharged to post–acute care facilities, 228 (99%) had complete data available for analysis (Figure 1). Demographic and hospitalization characteristics of the 228 subjects with complete data are shown in Table 1. The median (age of subjects was 78 years (IQR, 71–84 yr), and 44% were male. Subjects were of diverse races/ethnicities, and 48 (21%) were admitted from post–acute care facilities. A total of 122 subjects (54%) received mechanical ventilation for a median duration of 7 days (IQR, 3–16 d). Thirty-eight mechanically ventilated subjects (31%) required ≥ 10 days of mechanical ventilation with tracheostomy and therefore met criteria for CCI. The prevalence of CCI in the entire cohort was 17% (38/228). The median ICU and hospital lengths of stay were 5 days (IQR, 3–11 d) and 17 days (IQR, 11–26), respectively. Three percent of subjects (7/228) were discharged directly from the ICU to post–acute care facilities, whereas 97% of subjects (221/228) were first transferred from the ICU to the intermediate care unit or general ward and spent a median of 8 days (IQR, 5–14 d) on these wards before being discharged to a post–acute care facility.
Table 1.
Demographics, hospitalization characteristics, and 6-month mortality of older medical intensive care unit survivors discharged to post–acute care facilities (n = 228)
| Characteristic | n (%)* |
|---|---|
| Demographics | |
| Age, median (IQR) | 79 (73–86) |
| Male | 100 (44) |
| Race/ethnicity | |
| White | 72 (32) |
| Black | 45 (20) |
| Hispanic | 42 (18) |
| Other | 58 (25) |
| Unknown | 11 (4.8) |
| Prehospitalization residence | |
| Home | 166 (73) |
| Other hospital | 14 (6.1) |
| Skilled-care facility | 48 (21) |
| Use of MV | 122 (54) |
| MV days, median (IQR) | 7 (3–16) |
| Chronic critical illness† | 38 (17) |
| Length of stay | |
| MICU days, median (IQR) | 5 (3–11) |
| Hospital days, median (IQR) | 17 (11–26) |
| 6-mo mortality‡ | 92 (40) |
Definition of abbreviations: IQR = interquartile range; MICU = medical intensive care unit; MV = mechanical ventilation.
Values are n (%) unless otherwise noted.
Chronic critical illness is defined by MV ≥ 10 d with tracheostomy.
Death within 6-mo after date of hospital discharge.
The clinical characteristics of older MICU survivors discharged to post–acute care facilities are shown in Table 2. Nearly half of the subjects (48%) were admitted for treatment of infections or pulmonary disease, 7 (3%) were admitted for exacerbations of congestive heart failure, and 10 (4.4%) were admitted for treatment of or complications from malignancies. Most suffered from multiple comorbid conditions, with a majority having a Charlson comorbidity index score between 2 and 5.
Table 2.
Clinical characteristics of older medical intensive care unit survivors discharged to post–acute care facilities (n = 228)
| Principal Diagnoses for Hospitalization | n (%) |
|---|---|
| Infections | 69 (30) |
| Sepsis | 63 (28) |
| Meningitis | 3 (1.3) |
| Infection related to HIV | 3 (1.3) |
| Pulmonary | 42 (18) |
| Respiratory failure | 28 (12) |
| Pneumonia | 5 (2.2) |
| Aspiration pneumonitis | 5 (2.2) |
| COPD/asthma exacerbation | 4 (1.8) |
| Cardiac | 25 (11) |
| Acute myocardial infarction | 10 (4.3) |
| Congestive heart failure exacerbation | 7 (3.0) |
| Pulmonary heart disease | 4 (1.8) |
| Arrhythmia | 4 (1.3) |
| Gastrointestinal | 20 (8.8) |
| GI hemorrhage | 8 (3.5) |
| Diverticulosis and diverticulitis | 5 (2.2) |
| Intestinal obstruction without hernia | 3 (1.3) |
| Liver or biliary disease | 4 (1.8) |
| Treatment of or complication from malignancy | 10 (4.4) |
| Hematologic malignancy | 0 (0) |
| Solid malignancy | 10 (4.4) |
| Complications from organ transplant or other care | 9 (3.9) |
| Complication of organ transplant | 7 (3.1) |
| Complication of surgery or medical care | 2 (0.9) |
| Renal | 7 (3.1) |
| Acute renal failure | 7 (3.1) |
| Neurological | 3 (1.3) |
| Acute stroke | 3 (1.3) |
| Endocrine | 3 (1.3) |
| Diabetes with complications | 3 (1.3) |
| Injuries | 10 (4.4) |
| Hip fracture | 4 (1.8) |
| Back and other injuries | 6 (2.6) |
| Diagnoses occurring in < 1% of the subjects | 30 (13) |
| Charlson index score | |
| Overall, median (IQR) [range] | 2 (1–4) [0–10] |
| 0–1 | 85 (37) |
| 2–5 | 127 (56) |
| 6–7 | 13 (5.7) |
| ≥ 8 | 3 (1.3) |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; GI = gastrointestinal; HIV = human immunodeficiency virus; IQR = interquartile range.
Actual Use and Potential Need of Palliative Care Services
Six subjects (2.6%) received a palliative care consultation during the hospitalization involving intensive care. The most common characteristics at hospital discharge that suggested the potential need for palliative care services were chronic wounds (22%), supplemental oxygen use at discharge (37%), chaplain consultation (17%), delirium or dementia (39%), and a preference to not be resuscitated (23%). Less common characteristics suggestive of palliative care needs were use of noninvasive mechanical ventilation at discharge (6.6%); prescription of opioids (14%), tricyclic antidepressants or GABA analogs (6.6%), and antipsychotics (12%); and other characteristics suggesting an anticipated poor prognosis, including active malignancy (9.6%) or a designation of “comfort care” at discharge (8.3%) (Table 3). Subjects who received or did not receive mechanical ventilation demonstrated similar proportions of chronic wounds, delirium or dementia, characteristics suggesting an anticipated poor prognosis, and 6-month mortality (Table 3).
Table 3.
Frequencies of characteristics at discharge that are suggestive of palliative care needs among older medical intensive care unit survivors discharged to post–acute care facilities and for those who received or did not receive mechanical ventilation
| Patient Characteristics | All (n = 228) | Mechanical Ventilation* (n = 122) | No Mechanical Ventilation (n = 106) |
|---|---|---|---|
| Characteristics suggesting physical symptom distress | |||
| Chronic wounds | 51 (22)† | 29 (24) | 22 (21) |
| Supplemental oxygen use at discharge | 84 (37) | 65 (53) | 19 (18) |
| Noninvasive mechanical ventilation at discharge | 15 (6.6) | 11 (9.0) | 4 (3.8) |
| Opioids prescribed at discharge | 33 (14) | 14 (11) | 19 (18) |
| Characteristics suggesting psychological symptom distress | |||
| Chaplain consultation | 38 (17) | 29 (24) | 9 (8.5) |
| Dementia or delirium | 88 (39) | 42 (34) | 46 (44) |
| Tricyclic antidepressants or GABA analogs prescribed at discharge | 16 (6.6) | 9 (7.3) | 7 (6.6) |
| Anxiolytics (benzodiazepines) prescribed at discharge | 15 (6.5) | 13 (11) | 2 (1.9) |
| Antipsychotics prescribed at discharge‡ | 29 (12) | 19 (16) | 10 (9.4) |
| Characteristics suggesting an anticipated poor prognosis | |||
| Active malignancy or metastatic disease | 22 (9.6) | 12 (10) | 10 (9.4) |
| Designated “do not resuscitate” at discharge | 53 (23) | 26 (21) | 27 (25) |
| Designated “comfort care” at discharge | 19 (8.3) | 10 (8.2) | 9 (8.5) |
| 6-mo mortality§ | 92 (40) | 49 (40) | 43 (41) |
Definition of abbreviation: GABA = γ-aminobutyric acid.
Mechanical ventilation of any duration.
Values are number of subjects with percentage in parentheses.
Haloperidol, olanzapine, risperidone, or quetiapine.
Death within 6-mo after date of hospital discharge.
For the entire cohort, the median number of characteristics suggestive of potential palliative care needs was 2 (IQR, 1–3), and 199 subjects (88%) had one or more, 138 (61%) had two or more, and 73 (32%) had three or more of these characteristics. The distribution of subjects based on number of characteristics suggestive of potential palliative care needs is described in Figure 2.
Figure 2.
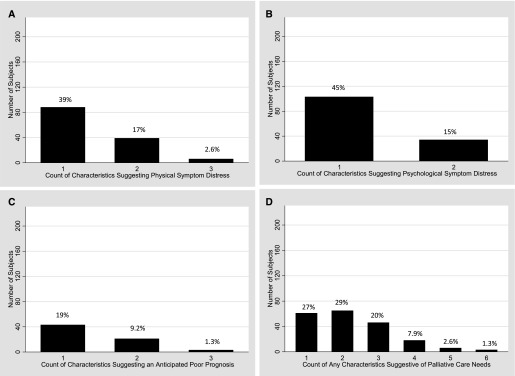
Distribution of older medical intensive care unit survivors by number of characteristics suggestive of potential palliative care needs at hospital discharge for physical symptom distress only (A), psychological symptom distress only (B), anticipated poor prognosis only (C), and any physical, psychological, or poor prognostic characteristic (D). Percentages of subjects are included above each bar.
Six-Month Mortality and Readmissions
Six-month mortality of the cohort was 40%. Counts and characteristics of readmissions to Columbia University Medical Center in the 6 months after the date of discharge for hospitalization involving intensive care are shown in Table 4. For the entire cohort, 84 (37%) were readmitted at least once, of whom 24 (11%) were readmitted to the ICU. Eighteen (8%) died during the hospital readmission. Readmissions to other hospitals were not captured in the study.
Table 4.
Readmissions to the same medical center in the following 6 months and outcomes from readmissions for older medical intensive care unit survivors discharged to post–acute care facilities
| Elderly ICU Survivors Discharged to Skilled-Care Facilities | All Patients (n = 228) |
|---|---|
| Readmitted at least once within 6 mo, n (%) | 84 (37) |
| Readmissions within 6 mo, mean (SD) [range] | 0.57 (0.93) [0–5] |
| Readmitted to ICU, n (%) | 24 (11) |
| Died during ICU readmission, n (%) | 9 (3.9) |
| Died during ICU or hospital readmission, n (%) | 18 (7.9) |
Definition of abbreviation: ICU = intensive care unit.
Discussion
We have shown that in a retrospective cohort of older medical-ICU survivors discharged to post–acute care facilities from our institution in 2009, few received specialist palliative care services during their hospitalization. Furthermore, characteristics indicative of physical and psychological symptom distress, an anticipated poor prognosis, and high 6-month mortality were common across this larger group of older ICU survivors awaiting discharge to post–acute care facilities. Our findings suggest that palliative care interventions for debilitated survivors of critical illness may be needed not only in the ICU but also during the postcritical acute care period at our institution and other institutions with similar models of clinical care.
There are several reasons why palliative care might have been underutilized among older ICU survivors who were discharged to post–acute care facilities from our institution. We have had an “integrative model” for ICU palliative care where intensivists embed palliative care principles into daily practice and rarely request palliative care consultations (26). However, the paucity of palliative care consultations remains concerning because almost every older ICU survivor was cared for on the general ward for days to weeks before discharge, and an integrative model of palliative care for general ward nononcologic care is only being developed now. Many older ICU survivors suffer from delirium and dementia and may be unable to voice their discomfort (17, 27). Healthcare providers may be reluctant to prescribe opioids, anxiolytics, and adjuvant analgesics to older patients with symptom distress out of concern for the deleterious effects on physical and cognitive function that may lead to setbacks in the recovery process (28–30). There is a lack of continuity of care from ICU to hospital ward and from hospital ward to post–acute care facility (31, 32). Therefore, healthcare providers may not observe trajectories of physical or psychological changes that may indicate a need for palliative services. We do not have nurse-initiated palliative care consultations at our institution, which could have increased the use of palliative care services by study subjects. Nurse-initiated palliative care is an important question for future studies of palliative care.
Older ICU survivors may not have received palliative care consultation at our institution in part because their healthcare providers underappreciated their high risk of 6-month mortality. A component of palliative care includes aligning treatments with patients’ goals toward the end of their lives, and some older ICU survivors with particularly poor prognoses might have preferred enrolling in hospice or home hospice after consulting with their healthcare provider and a palliative care specialist. Healthcare providers may not have realized the potential hospice needs in this cohort of older ICU survivors with a 40% 6-month mortality because only a minority of these patients had severe congestive heart failure or a progressive malignancy, the two most common reasons for enrollment in hospice (33). In addition, although some critical illness constitutes an acute event with minimal sequelae, many older ICU survivors who have multiple comorbidities, disability, and frailty suffer from a combination of repeated infections and respiratory failure that leads to progressive debilitation and death (14). Epidemiologic data on outcomes after hospital discharge and prediction models for these patients have been published only recently and may have yet to affect clinical practice (4, 5, 20, 34).
The retrospective nature of our study limits our ability to assess directly the presence and severity of symptom distress from subjects. In particular, the prevalence of many characteristics suggestive of symptom distress in our ICU cohort was lower than what was identified in a prospective cohort study of CCI subjects in a ventilator weaning unit, where 44% had physical pain and 60% had psychological distress at the highest levels (16, 17). Therefore, actual symptom distress in older ICU survivors being discharged to post–acute care facilities may be underestimated by using proxies to identify symptoms. Nevertheless, our methodology may provide a useful way to ascertain potential palliative care needs from administrative datasets and electronic health records in future studies.
Our study has several other limitations. We did not include data on subjects’ pain because it was recorded only in the written record during the study period and therefore was not readily analyzable. We cannot exclude the possibility that palliative care was provided by the treating physician or that consultation with a palliative care specialist was offered but declined by patients and their families. We also could not determine if patients received palliative or hospice care upon arrival to their post–acute care facilities. However, the use of palliative care and hospice services in nonhospice post–acute care facilities remains limited (35, 36). Long-term care facilities are a natural care setting for the integration of palliative and hospice care, and developing standards for incorporating and evaluating palliative care in these settings warrants further investigation. Our study subjects were treated in a single tertiary-care center in New York City, and the subjects and the treatment setting may not be broadly generalizable. We only examined subjects discharged to post–acute care facilities; future investigations should examine the palliative needs of older ICU survivors who are discharged home.
Previous trials of palliative care interventions within the ICU have not been shown to improve the quality of dying or decrease hospital length of stay (37–39), perhaps in part because surrogates have optimistic biases earlier in the course of critical illness (40). Our study shows that after discharge from the ICU, nearly all older ICU survivors who require postacute facility care spend a median of 8 days on our general wards before hospital discharge. Older ICU survivors and their surrogates may have significant palliative and/or hospice needs later in the patient’s course when recovery has stalled and anticipated outcome is poor. Therefore, the postcritical acute care period may be an important time to initiate or readdress a palliative care intervention.
Increasing recognition of the growing number of patients with CCI has led to a multicenter palliative intervention trial for patients with CCI that is assessing palliative needs and patient and surrogate satisfaction after the ICU (41, 42). Our study suggests that debilitated older ICU survivors at our institution may benefit from similar palliative care interventions regardless of their need for mechanical ventilation or its duration. However, given the limited number of palliative care specialists in the United States, there may be a shortage of such specialists to provide all palliative care services for all older ICU survivors awaiting discharge to a post–acute care facility. An alternative approach, therefore, may be to follow the recent recommendation for a sustainable model of palliative care where the primary physician first provides basic pain management and discussions of prognosis before consultation with a palliative care specialist. For older ICU survivors, basic palliative care may be provided by intensivists first in the ICU and then by general ward physicians during the postcritical acute care period; palliative care specialists may be consulted to help manage more complex and difficult cases either in the ICU or later in the hospital course on the general ward (43).
In conclusion, we show that older medical ICU survivors who were discharged to post–acute care facilities from a single institution had a high prevalence of characteristics associated with physical and psychological symptom distress and high short-term mortality, whereas few received specialist palliative care services. One possible implication of our findings is that healthcare providers at our institution and perhaps at others with similar clinical care models should consider anticipated discharge to a post–acute care facility for older medical ICU survivors to be a potential trigger for evaluating their palliative care needs. When pain, treatment of anxiety or depression, and concerns about goals of care are difficult to manage, a hospital-based palliative care team should be consulted. Prospective investigations are needed to determine the optimal timing, approach, and effect of palliative care interventions for the rapidly growing population of debilitated older ICU survivors. These data suggest that the time after stabilization of the critical illness and before discharge to a post–acute care facility may be an often missed opportunity.
Acknowledgments
Acknowledgment
The authors thank Alla Babina, M.S., and Jiang Yao, M.S., from the Columbia University Department of Biomedical Informatics for assistance with data abstraction.
Footnotes
This work was supported by National Institutes of Health grants UL1 RR024156 and 3P30AG022845–078 and by a Loan Repayment Grant from the National Institute on Aging (M.R.B.). The National Institute on Aging had no role in study design, analysis, or manuscript approval.
Author’s Contributions: Study concept and design: M.R.B., H.W., W.R.N., N.W.S., C.D.B., M.C.R., M.S.M., N.G., D.J.L., and P.B. Data acquisition: M.R.B., W.R.N., and P.A.R. Data analysis: M.R.B., W.R.N., P.A.R., and P.B. Drafting the article: M.R.B. Critical revision of the article: H.W., P.A.R., C.D.B., N.W.S., M.C.R., M.S.M., N.G., D.J.L., and P.B. All authors have approved this version for publication.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Angus DC, Shorr AF, White A, Dremsizov TT, Schmitz RJ, Kelley MA. Critical care delivery in the united states: distribution of services and compliance with leapfrog recommendations. Crit Care Med. 2006;34:1016–1024. doi: 10.1097/01.CCM.0000206105.05626.15. [DOI] [PubMed] [Google Scholar]
- 2.Lerolle N, Trinquart L, Bornstain C, Tadie JM, Imbert A, Diehl JL, Fagon JY, Guerot E. Increased intensity of treatment and decreased mortality in elderly patients in an intensive care unit over a decade. Crit Care Med. 2010;38:59–64. doi: 10.1097/CCM.0b013e3181b088ec. [DOI] [PubMed] [Google Scholar]
- 3.Spragg RG, Bernard GR, Checkley W, Curtis JR, Gajic O, Guyatt G, Hall J, Israel E, Jain M, Needham DM, et al. Beyond mortality: future clinical research in acute lung injury. Am J Respir Crit Care Med. 2010;181:1121–1127. doi: 10.1164/rccm.201001-0024WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble WT. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010;303:849–856. doi: 10.1001/jama.2010.216. [DOI] [PubMed] [Google Scholar]
- 5.Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long-term acute care hospital utilization after critical illness. JAMA. 2010;303:2253–2259. doi: 10.1001/jama.2010.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sox HC, Greenfield S. Comparative effectiveness research: a report from the institute of medicine. Ann Intern Med. 2009;151:203–205. doi: 10.7326/0003-4819-151-3-200908040-00125. [DOI] [PubMed] [Google Scholar]
- 7.Deutschman CS, Ahrens T, Cairns CB, Sessler CN, Parsons PE. Multisociety task force for critical care research: key issues and recommendations. Am J Respir Crit Care Med. 2012;185:96–102. doi: 10.1164/rccm.201110-1848ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanken PN, Terry PB, Delisser HM, Fahy BF, Hansen-Flaschen J, Heffner JE, Levy M, Mularski RA, Osborne ML, Prendergast TJ, et al. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med. 2008;177:912–927. doi: 10.1164/rccm.200605-587ST. [DOI] [PubMed] [Google Scholar]
- 9.Morrison RS, Meier DE. Clinical practice: palliative care. N Engl J Med. 2004;350:2582–2590. doi: 10.1056/NEJMcp035232. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann C, Riechelmann R, Krzyzanowska M, Rodin G, Tannock I. Effectiveness of specialized palliative care: a systematic review. JAMA. 2008;299:1698–1709. doi: 10.1001/jama.299.14.1698. [DOI] [PubMed] [Google Scholar]
- 11.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 12.Casarett D, Karlawish J, Morales K, Crowley R, Mirsch T, Asch DA. Improving the use of hospice services in nursing homes: a randomized controlled trial. JAMA. 2005;294:211–217. doi: 10.1001/jama.294.2.211. [DOI] [PubMed] [Google Scholar]
- 13.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 14.Nelson JE, Cox CE, Hope AA, Carson SS. Chronic critical illness. Am J Respir Crit Care Med. 2010;182:446–454. doi: 10.1164/rccm.201002-0210CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox CE, Martinu T, Sathy SJ, Clay AS, Chia J, Gray AL, Olsen MK, Govert JA, Carson SS, Tulsky JA. Expectations and outcomes of prolonged mechanical ventilation. Crit Care Med. 2009;37:2888–2894. doi: 10.1097/CCM.0b013e3181ab86ed. , quiz 2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson JEMD, Litke A, Natale DA, Siegel RE, Morrison RS. The symptom burden of chronic critical illness. Crit Care Med. 2004;32:1527–1534. doi: 10.1097/01.ccm.0000129485.08835.5a. [DOI] [PubMed] [Google Scholar]
- 17.Nelson JE, Tandon N, Mercado AF, Camhi SL, Ely EW, Morrison RS. Brain dysfunction: another burden for the chronically critically ill. Arch Intern Med. 2006;166:1993–1999. doi: 10.1001/archinte.166.18.1993. [DOI] [PubMed] [Google Scholar]
- 18.Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008;30:421–434. doi: 10.1016/j.genhosppsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uhc.edu. Advance new knowledge [Internet]. Chicago: University Healthsystem Consortium [revised 2013 January 1; accessed 2011 July 20]. Available from: http://www.uhc.edu/UHCResearchInstitute.htm
- 20.Baldwin MR, Narain WR, Wunsch H, Schluger NW, Cooke JT, Maurer MS, Rowe JW, Lederer DJ, Bach PB. A prognostic model for 6-month mortality in elderly survivors of critical illness. Chest. 2013;143:910–919. doi: 10.1378/chest.12-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Letizia M, Uebelhor J, Paddack E. Providing palliative care to seriously ill patients with nonhealing wounds. J Wound Ostomy Continence Nurs. 2010;37:277–282. doi: 10.1097/WON.0b013e3181d8c9f7. [DOI] [PubMed] [Google Scholar]
- 22.Weissman DE, Meier DE. Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the center to advance palliative care. J Palliat Med. 2011;14:17–23. doi: 10.1089/jpm.2010.0347. [DOI] [PubMed] [Google Scholar]
- 23.Delgado-Guay MO, Parsons HA, Li Z, Palmer LJ, Bruera E. Symptom distress, interventions, and outcomes of intensive care unit cancer patients referred to a palliative care consult team. Cancer. 2009;115:437–445. doi: 10.1002/cncr.24017. [DOI] [PubMed] [Google Scholar]
- 24.Sulmasy DP. Spirituality, religion, and clinical care. Chest. 2009;135:1634–1642. doi: 10.1378/chest.08-2241. [DOI] [PubMed] [Google Scholar]
- 25.Chen YY, Connors AF, Jr, Garland A. Effect of decisions to withhold life support on prolonged survival. Chest. 2008;133:1312–1318. doi: 10.1378/chest.07-1500. [DOI] [PubMed] [Google Scholar]
- 26.Nelson JE, Bassett R, Boss RD, Brasel KJ, Campbell ML, Cortez TB, Curtis JR, Lustbader DR, Mulkerin C, Puntillo KA, et al. Models for structuring a clinical initiative to enhance palliative care in the intensive care unit: a report from the IPAL-ICU project (improving palliative care in the ICU) Crit Care Med. 2010;38:1765–1772. doi: 10.1097/CCM.0b013e3181e8ad23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camhi SL, Mercado AF, Morrison RS, Du Q, Platt DM, August GI, Nelson JE. Deciding in the dark: advance directives and continuation of treatment in chronic critical illness. Crit Care Med. 2009;37:919–925. doi: 10.1097/CCM.0b013e31819613ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spitz A, Moore AA, Papaleontiou M, Granieri E, Turner BJ, Reid MC. Primary care providers' perspective on prescribing opioids to older adults with chronic non-cancer pain: a qualitative study. BMC Geriatr. 2011;11:35. doi: 10.1186/1471-2318-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner AK, Zhang F, Soumerai SB, Walker AM, Gurwitz JH, Glynn RJ. Ross-Degnan D. Benzodiazepine use and hip fractures in the elderly: who is at greatest risk? Arch Intern Med. 2004;164:1567–1572. doi: 10.1001/archinte.164.14.1567. [DOI] [PubMed] [Google Scholar]
- 30.Solomon DH, Rassen JA, Glynn RJ, Garneau K, Levin R, Lee J, Schneeweiss S. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med. 2010;170:1979–1986. doi: 10.1001/archinternmed.2010.450. [DOI] [PubMed] [Google Scholar]
- 31.Pham HH, O'Malley AS, Bach PB, Saiontz-Martinez C, Schrag D. Primary care physicians' links to other physicians through medicare patients: the scope of care coordination. Ann Intern Med. 2009;150:236–242. doi: 10.7326/0003-4819-150-4-200902170-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma G, Freeman J, Zhang D, Goodwin JS. Continuity of care and intensive care unit use at the end of life. Arch Intern Med. 2009;169:81–86. doi: 10.1001/archinternmed.2008.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caffrey C, Sengupta M, Moss A, Harris-Kojetin L, Valverde R. Home health care and discharged hospice care patients: United States, 2000 and 2007. Natl Health Stat Report. 2011;38:1–27. [PubMed] [Google Scholar]
- 34.Carson SS, Kahn JM, Hough CL, Seeley EJ, White DB, Douglas IS, Cox CE, Caldwell E, Bangdiwala SI, Garrett JM, et al. A multicenter mortality prediction model for patients receiving prolonged mechanical ventilation. Crit Care Med. 2012;40:1171–1176. doi: 10.1097/CCM.0b013e3182387d43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlson MD, Lim B, Meier DE. Strategies and innovative models for delivering palliative care in nursing homes. J Am Med Dir Assoc. 2011;12:91–98. doi: 10.1016/j.jamda.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meier DE, Lim B, Carlson MD. Raising the standard: palliative care in nursing homes. Health Aff (Millwood) 2010;29:136–140. doi: 10.1377/hlthaff.2009.0912. [DOI] [PubMed] [Google Scholar]
- 37.A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). The Support principal investigators. JAMA. 1995;274:1591–1598. [PubMed] [Google Scholar]
- 38.Curtis JR, Nielsen EL, Treece PD, Downey L, Dotolo D, Shannon SE, Back AL, Rubenfeld GD, Engelberg RA. Effect of a quality-improvement intervention on end-of-life care in the intensive care unit: a randomized trial. Am J Respir Crit Care Med. 2011;183:348–355. doi: 10.1164/rccm.201006-1004OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cassel JB, Kerr K, Pantilat S, Smith TJ. Palliative care consultation and hospital length of stay. J Palliat Med. 2010;13:761–767. doi: 10.1089/jpm.2009.0379. [DOI] [PubMed] [Google Scholar]
- 40.Zier LS, Sottile PD, Hong SY, Weissfield LA, White DB. Surrogate decision makers' interpretation of prognostic information: a mixed-methods study. Ann Intern Med. 2012;156:360–366. doi: 10.1059/0003-4819-156-5-201203060-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox CE, Carson SS, Holmes GM, Howard A, Carey TS. Increase in tracheostomy for prolonged mechanical ventilation in North Carolina, 1993–2002. Crit Care Med. 2004;32:2219–2226. doi: 10.1097/01.ccm.0000145232.46143.40. [DOI] [PubMed] [Google Scholar]
- 42.Informing decisions in chronic critical illness: a randomized controlled trial. Trial identifier: NCT 01230099 [revised 2013 January 1; accessed 2012 December 6]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01230099
- 43.Quill TE, Abernethy AP. Generalist plus specialist palliative care: creating a more sustainable model. N Engl J Med. 2013;368:1173–1175. doi: 10.1056/NEJMp1215620. [DOI] [PubMed] [Google Scholar]