Abstract
Rationale: Type 2 diabetes mellitus is a major risk factor for the development of active tuberculosis, although the biological basis underlying this susceptibility remains poorly characterized.
Objectives and Methods: To identify the influence of coincident diabetes mellitus on cytokine levels in pulmonary tuberculosis, we examined circulating levels of a panel of cytokines and chemokines in the plasma of individuals with tuberculosis with diabetes and compared them with those of individuals without diabetes.
Measurements and Main Results: Tuberculosis with diabetes is characterized by elevated circulating levels of type 1 (IFN-γ, tumor necrosis factor-α, and IL-2), type 2 (IL-5), and type 17 (IL-17A) cytokines but decreased circulating levels of IL-22. This was associated with increased systemic levels of other proinflammatory cytokines (IL-1β, IL-6, and IL-18) and an antiinflammatory cytokine (IL-10) but not type 1 IFNs. Moreover, tuberculosis antigen–stimulated whole blood also showed increased levels of proinflammatory cytokines. Finally, type 1 and type 17 cytokines in plasma exhibit a significant positive correlation with hemoglobin A1C levels, indicating that impaired control of diabetes is associated with this proinflammatory milieu. Multivariate analysis revealed that the association of proinflammatory cytokines with diabetes mellitus was not influenced by age, sex, or other metabolic parameters.
Conclusions: Our data reveal that tuberculosis with diabetes is characterized by heightened cytokine responsiveness, indicating that chronic inflammation underlying type 2 diabetes potentially contributes to increased immune pathology and poor control in tuberculosis infection.
Keywords: bacterial, cytokines, chemokines, tuberculosis, diabetes mellitus
Among the many risk factors for the development of tuberculosis, type 2 diabetes mellitus is one that has long been recognized through clinical and epidemiological studies (1). There is growing evidence that type 2 diabetes mellitus is an important risk factor for developing active pulmonary tuberculosis (2). In fact, a meta-analysis of 13 observational studies on the risk for tuberculosis disease in diabetics determined that diabetic patients were 3.1 times more likely to have tuberculosis than nondiabetic individuals (2). In addition to increasing the risk for reactivating latent tuberculosis infection, there is also evidence that type 2 diabetes mellitus is also associated with greater severity of tuberculosis disease affecting both disease presentation and response to treatment. Despite the clinical and public health significance posed by the dual burden of tuberculosis and diabetes mellitus, little is known about the immunological and biochemical mechanisms of susceptibility.
Enhanced susceptibility to tuberculosis in patients with diabetes mellitus has been ascribed to several factors including those related directly to hyperglycemia and insulin resistance as well as to indirect effects on macrophage and lymphocyte function (1). Several early studies reported reduced proinflammatory cytokines in patients with diabetes after infection with Mycobacterium tuberculosis (3, 4). Two studies, however, reported the elevation of helper T-cell type 1 (Th1) cytokines in tuberculosis-infected diabetic hosts; one was conducted in streptozocin-treated mice (5) and another in diabetic and nondiabetic patients with tuberculosis (6). In addition, we have previously shown that type 2 diabetes mellitus in tuberculosis disease is associated with elevated frequencies of Th1 and Th17 cells and cytokines (7). Cytokines of the innate and adaptive immune systems orchestrate the immune response to tuberculosis infection, with type 1, type 17, and the IL-1 family of cytokines having been implicated in protection against tuberculosis disease (8) in murine systems whereas type 2 and antiinflammatory cytokines along with type 1 IFNs have been associated with either increased susceptibility to disease and/or enhanced pathology (8).
To study the influence of type 2 diabetes mellitus on active pulmonary tuberculosis, we examined levels of a large panel of type 1, type 2, type 17, regulatory, and other proinflammatory cytokines and chemokines in individuals with active tuberculosis and coincident diabetes and compared them with those in individuals with active tuberculosis but without diabetes. We also examined tuberculosis antigen–stimulated levels of certain cytokines in the whole blood of individuals with tuberculosis and diabetes and in individuals with tuberculosis without diabetes. We show that those with tuberculosis and diabetes have increased systemic levels of most of the proinflammatory cytokines as well as IL-5 and IL-10 but have decreased levels of IL-22 and type 1 IFN. In addition, tuberculosis antigen–stimulated levels of most of these cytokines were also increased in individuals with tuberculosis and diabetes. Thus, our data suggest that active tuberculosis with coincident diabetes mellitus is associated with heightened cytokine levels, levels possibly contributing to immune-mediated pathology in tuberculosis.
Methods
Study Population
We studied a group of 88 individuals with active pulmonary tuberculosis attending the Tuberculosis Clinic at Stanley Medical Hospital in Chennai, India—the first consecutive 44 with diabetes and the first 44 without. Active pulmonary tuberculosis was diagnosed on the basis of sputum smear and culture positivity. Type 2 diabetes mellitus was diagnosed on the basis of glycated hemoglobin (HbA1c) levels and random blood glucose, according to the American Diabetes Association criteria (all type 2 diabetes individuals had HbA1c levels > 6.5% and random blood glucose > 200 mg/dl). All the individuals were HIV negative. The two groups did not differ significantly in terms of radiological extent of disease or the site of disease as assessed by chest X-ray readings from three independent experts. The two groups did differ significantly in smear grades, with the diabetes group having worse smear grades as assessed by the Mann–Whitney test, corrected for ties (P = 0.005), indicating higher bacillary burdens. All individuals were anti–tuberculous treatment naive. Anthropometric measurements, including height, weight, and waist circumference, and biochemical parameters, including plasma glucose, lipid profile, and HbA1c, were obtained by standardized techniques as detailed elsewhere (9). Hematology was performed on all individuals, using the Ac·T 5diff hematology analyzer (Beckman Coulter, Brea, CA). This study comprised a separate set of individuals compared with our previous study on tuberculosis and diabetes (7). All individuals were examined as part of a clinical protocol approved by the Institutional Review Board of the National Institute of Research in Tuberculosis (NCT01154959), and informed written consent was obtained from all participants.
ELISA
Plasma cytokines and chemokines were measured with a Bio-Plex multiplex cytokine assay system (Bio-Rad, Hercules, CA). The parameters analyzed were IFN-γ, tumor necrosis factor (TNF)-α, IL-2, IL-17A, IL-4, IL-5, IL-13, IL-10, IL-6, IL-12, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-8, chemokine ligand-11 (CCL11), granulocyte colony-stimulating factor (G-CSF), chemokine ligand-2 and -4 (CCL2 and CCL4, respectively), and platelet-derived growth factor (PDGF). Plasma levels of transforming growth factor (TGF)-β, IL-1α, IL-1β, IL-18, IFN-γ–inducible protein-10 (IP-10) (all R&D Systems); IL-17F (BioLegend, San Diego, CA); IL-22 (eBioscience, San Diego, CA); type 1 IFNs—IFN-α (multiple subtypes) and IFN-β (PBL Interferon Source, Piscataway, NJ)—were measured by ELISA.
QuantiFERON Supernatant ELISA
Whole blood from a subset of individuals with tuberculosis and diabetes and individuals with tuberculosis without diabetes (n = 22 each) was incubated with either no antigen or tuberculosis antigen (ESAT-6, CFP-10, TB 7.7) or mitogen (phorbol ester/ionomycin) according to the manufacturer’s instructions, using a QuantiFERON In-Tube Gold kit (Qiagen, Valencia, CA). Unstimulated or tuberculosis antigen–stimulated whole blood supernatants were then used to analyze levels of IFN-γ, TNF-α, IL-17, IL-10, IL-1β, and IL-18, using DuoSet ELISA kits from R&D Systems (Minneapolis, MN).
Statistical Analysis
Geometric means were used for measurements of central tendency. Statistically significant differences between two groups were analyzed by nonparametric Mann–Whitney U test. Correlations were calculated by Spearman rank correlation. For discriminating diabetes from nondiabetes, using cytokines, we used a Holm’s adjusted stepwise logistic modeling procedure with 24 of the cytokines and chemokines. Briefly, the Holm’s adjusted stepwise logistic modeling procedure is a forward stepwise procedure in the spirit of Holm’s multiple comparison correction: for k = 24 variables, include the first variable if its P < 0.05/k, include second if its P < 0.05/(k − 1), and so on, stopping when no new variable may be included. This procedure ensures that the overall probability of selecting any significant variable by chance is bounded at the 0.05 level. Because highly correlated variables with selected variables may not be selected by the procedure, we also used logistic regression for each variable alone. We estimated the percentage of correct prediction by leave-one-out cross validation, and give the Holm’s adjusted P values, which correct for the multiple comparisons. Analyses were performed with GraphPad PRISM version 5.01 (GraphPad Software, San Diego, CA) (Mann–Whitney and correlations) or R version 2.15.2.
Results
Study Population Characteristics
The baseline characteristics including demographics and clinical and biochemical features of the study population are shown in Table 1. As can be seen, compared with subjects without diabetes, those with diabetes and tuberculosis had higher fasting blood glucose, glycated hemoglobin, serum cholesterol, low-density lipoprotein and triglyceride levels but lower high-density lipoprotein cholesterol levels. The groups did not significantly differ in their baseline hematological parameters (data not shown).
Table 1.
Demographics of individuals with tuberculosis and diabetes and of individuals with tuberculosis without diabetes
| TB–DM (n = 44) | TB–NDM (n = 44) | P Value | |
|---|---|---|---|
| Age, yr | 45 (33–70) | 43.5 (20–70) | 0.0016 |
| Sex, M/F | 31/13 | 38/6 | NS |
| BMI, kg/m2 | 23.90 (19.56–33.38) | 22.16 (14.01–31.22) | NS |
| Duration of diabetes, yr | 4.5 (1–28) | — | |
| Systolic blood pressure, mm Hg | 115 (82–152) | 117 (90–169) | NS |
| Diastolic blood pressure, mm Hg | 70 (59–90) | 79.5 (40–112) | NS |
| Random glucose, mg/dl | 287 (200–653) | 101.5 (76–177) | <0.0001 |
| Glycated hemoglobin, % | 11.3 (8.01−16.16) | 5.6 (4.46–6.30) | <0.0001 |
| Total cholesterol, mg/dl | 215 (124–259) | 182.5 (134–296) | 0.0079 |
| Serum triglycerides, mg/dl | 182 (57–679) | 84.5 (57–433) | <0.0001 |
| High-density lipoprotein cholesterol, mg/dl | 38 (22–82) | 47 (34–86) | 0.0002 |
| Low-density lipoprotein cholesterol, mg/dl | 124 (47–185) | 112 (65–205) | 0.1196 |
Definition of abbreviations: BMI = body mass index; F = female; M = male; NS = not significant; TB–DM = tuberculosis with diabetes mellitus; TB–NDM = tuberculosis without diabetes mellitus.
Values represent geometric means and range (except for age, for which median and range are shown). P values were calculated by Mann–Whitney test (except for sex, which was tested by Fisher exact test).
Tuberculosis with Diabetes Is Associated with Increased Circulating Levels of Type 1 and Type 17 Cytokines
To determine the influence of diabetes mellitus on type 1 and type 17 cytokines in active tuberculosis, we measured the circulating levels of IFN-γ, TNF-α, and IL-2 as well as IL-17A, IL-17F, and IL-22 in individuals with tuberculosis with or without diabetes (Figure 1). As shown in Figure 1A, the systemic levels of all three type 1 cytokines—IFN-γ (geometric mean of 780.5 pg/ml in tuberculosis with diabetes vs. 146.3 pg/ml in tuberculosis with no diabetes), TNF-α (geometric mean of 796.7 vs. 496.3 pg/ml), and IL-2 (geometric mean of 15.02 vs. 10.73 pg/ml)—were significantly higher in diabetic compared with nondiabetic individuals. Similarly, the systemic level of the prototypical type 17 cytokine IL-17A was also significantly higher in diabetic compared with nondiabetic individuals with tuberculosis (geometric mean of 218.3 vs. 110.2 pg/ml). In contrast, two other cytokines associated with the Th17 response—IL-17F (geometric mean of 84.4 pg/ml vs. 125.1 pg/ml) and IL-22 (geometric mean of 10.1 pg/ml vs. 31.3 pg/ml)—were found be present at significantly lower levels in diabetic compared with nondiabetic individuals with tuberculosis. Thus, tuberculosis with diabetes is associated with heightened levels of type 1 cytokines and IL-17A at the time of presentation of active pulmonary tuberculosis.
Figure 1.
Elevated systemic levels of type 1 and type 17 cytokines as well as IL-5 and IL-10 in tuberculosis with diabetes. Plasma levels of (A) type 1 (IFN-γ, tumor necrosis factor [TNF]-α, IL-2) and type 17 (IL-17A, IL-17F, IL-22) cytokines and (B) type 2 (IL-4, IL-5, IL-13) and regulatory (IL-10, transforming growth factor [TGF]-β) cytokines were measured by ELISA in individuals with tuberculosis and diabetes (n = 44) and in individuals with tuberculosis with no diabetes (n = 44). Data are represented as scatter plots, with each circle representing a single individual (light gray, diabetes mellitus [DM]; dark gray, non–diabetes mellitus [NDM]). P values were calculated by Mann–Whitney test.
Tuberculosis with Diabetes Is Associated with Increased Circulating Levels of IL-5 and IL-10
To determine the influence of diabetes on type 2 and regulatory cytokines in active tuberculosis, we measured the circulating levels of IL-4, IL-5, and IL-13 as well as IL-10 and TGF-β in diabetic and nondiabetic individuals with tuberculosis (Figure 1). As shown in Figure 1B, the systemic levels of IL-5 (geometric mean of 78 vs. 38 pg/ml) but not IL-4 and IL-13 were significantly higher in diabetic compared with nondiabetic individuals. Similarly, the systemic levels of the regulatory cytokine IL-10 (geometric mean of 161.8 vs. 102.8 pg/ml) were also significantly higher in diabetic compared with nondiabetic individuals. In contrast, the circulating levels of the other potent regulatory cytokine, TGF-β, was not significantly different between the two groups. Thus, tuberculosis with diabetes is not associated with a concomitant decrease in type 2 or regulatory cytokine levels at the time of presentation with active pulmonary tuberculosis.
Tuberculosis with Diabetes Is Associated with Increased Circulating Levels of IL-1 Family and Other Proinflammatory Cytokines
To determine the influence of diabetes on the IL-1 family and other proinflammatory cytokines as well as type 1 IFNs in active tuberculosis, we measured circulating levels of these in diabetic and nondiabetic individuals with tuberculosis (Figure 2). As shown in Figure 2, systemic levels of the IL-1 family cytokines IL-1β (geometric mean of 125.2 vs. 101.7 pg/ml) and IL-18 (geometric mean of 23.3 vs. 10.2 pg/ml), but not IL-1α, were significantly higher in diabetic compared with nondiabetic individuals. Similarly, the systemic levels of IL-6 (geometric mean of 731.8 vs. 565.4 pg/ml) but not IL-12 or GM-CSF were also significantly higher in diabetic compared with nondiabetic individuals. In contrast, the levels of IFN-β (geometric mean of 6.1 vs. 10.8 pg/ml) but not IFN-α were found be present at significantly lower levels in diabetic compared with nondiabetic individuals. Thus, tuberculosis with diabetes is associated with heightened levels of proinflammatory cytokines but not type 1 IFNs at the time of presentation.
Figure 2.
Elevated systemic levels of IL-1 family and other proinflammatory cytokines in tuberculosis with diabetes. Plasma levels of IL-1 family members (IL-1α, IL-1β, IL-18), type 1 IFNs (IFN-α and IFN-β), and other proinflammatory (IL-6, IL-12, granulocyte-macrophage colony-stimulating factor [GM-CSF]) cytokines were measured by ELISA in individuals with tuberculosis and diabetes (n = 44) and in individuals with tuberculosis with no diabetes (n = 44). Data are represented as scatter plots, with each circle representing a single individual (light gray, diabetes mellitus [DM]; dark gray, non–diabetes mellitus [NDM]). P values were calculated by Mann–Whitney test.
Tuberculosis with Diabetes Is Associated with Increased Tuberculosis Antigen–Stimulated Levels of Type 1 and Other Proinflammatory Cytokines
To determine the influence of diabetes on tuberculosis antigen–stimulated levels of type 1 and other proinflammatory cytokines in active tuberculosis, we measured circulating levels of these cytokines after stimulation with no antigen or a cocktail of tuberculosis antigens (ESAT-6, CFP-10, TB 7.7) in a subset (n = 22 each) of diabetic and nondiabetic individuals with tuberculosis (Figure 3). As shown in Figure 3A, the spontaneously produced levels of IFN-γ (geometric mean of 93.7 vs. 46.7 pg/ml), TNF-α (geometric mean of 30.7 vs. 20.1 pg/ml), IL-17A (geometric mean of 8.8 vs. 6.2 pg/ml), and IL-1β (geometric mean of 743.9 vs. 401.3 pg/ml) were significantly higher in diabetic compared with nondiabetic individuals. Similarly, as shown in Figure 3B, the tuberculosis antigen–stimulated (net cytokine) levels of IFN-γ (geometric mean of 948 vs. 389.3 pg/ml), TNF-α (geometric mean of 36.9 vs. 23.4 pg/ml), IL-17A (geometric mean of 15.3 vs. 6.9 pg/ml), and IL-10 (geometric mean of 84.7 vs. 61.6 pg/ml) were also significantly higher in diabetic compared with nondiabetic individuals. Mitogen stimulation showed elevated levels of all cytokines, but these were not significantly different between the two groups (data not shown). Thus, tuberculosis with diabetes is associated with heightened levels of tuberculosis antigen–stimulated proinflammatory cytokines at the time of presentation.
Figure 3.
Elevated tuberculosis (TB) antigen–stimulated and unstimulated levels of type 1, type 17, and other proinflammatory cytokines in tuberculosis with diabetes. (A) Unstimulated or (B) TB antigen–stimulated levels of type 1, type 17, and other proinflammatory cytokines were measured by ELISA in whole blood of a subset of individuals with tuberculosis and diabetes (n = 22) and in individuals with tuberculosis with no diabetes (n = 22). TB antigen–stimulated cytokines are shown as net cytokines with the baseline subtracted out. Data are represented as scatter plots, with each circle representing a single individual (light gray, diabetes mellitus [DM]; dark gray, non–diabetes mellitus [NDM]). P values were calculated by Mann–Whitney test.
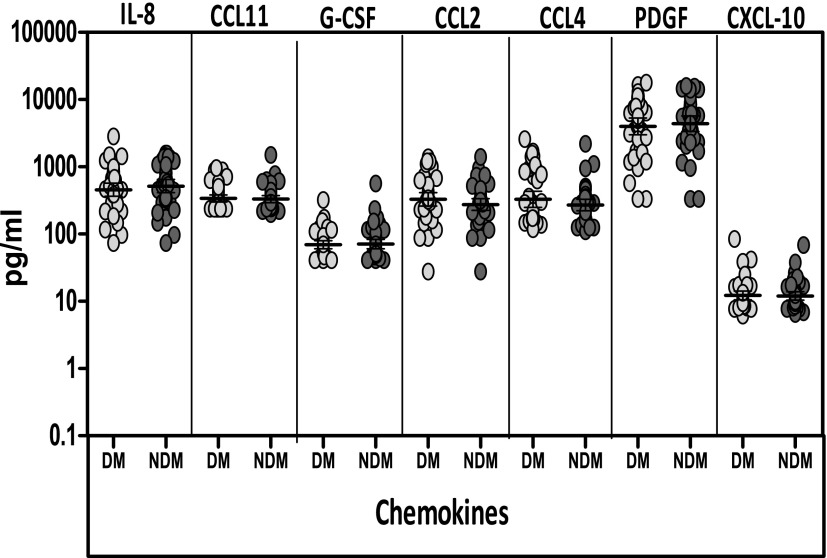
Tuberculosis with Diabetes Is Not Associated with Altered Circulating Levels of Chemokines
To determine the influence of diabetes on chemokines in active tuberculosis, we measured the circulating levels of IL-8, G-CSF, CCL2, CCL4, CCL11, PDGF, and CXCL-10 in diabetic and nondiabetic individuals with tuberculosis. As shown in Figure 4, the systemic levels of all the chemokines examined were not significantly different between the two groups. Thus, tuberculosis with diabetes is not associated with altered levels of circulating chemokines.
Figure 4.
No alterations in systemic levels of common chemokines in tuberculosis with diabetes. Plasma levels of some of the common chemokines—IL-8, chemokine ligand-11 (CCL11), granulocyte colony-stimulating factor (G-CSF), chemokine ligand-2 and -4 (CCL2 and CCL4, respectively), platelet-derived growth factor (PDGF), and chemokine (C-X-C motif) ligand-10 (CXCL-10)—were measured by ELISA in individuals with tuberculosis and diabetes (n = 44) and in individuals with tuberculosis with no diabetes (n = 44). Data are represented as scatter plots, with each circle representing a single individual (light gray, diabetes mellitus [DM]; dark gray, non–diabetes mellitus [NDM]). P values were calculated by Mann–Whitney test.
Relationship between Systemic Cytokines and HbA1c Levels
HbA1c is an accurate indicator of the level of diabetic control and increased values reflect poor control. Thus, to examine the relationship between the systemic levels of type 1, type 17, and regulatory cytokines with the degree of diabetic control, we assessed the association of IFN-γ, TNF-α, IL-17A, and IL-10 with HbA1c levels (in %) in all the individuals in the study. As shown in Figure 5, the systemic levels of IFN-γ, TNF-α, IL-17A, and IL-10 each exhibited a significant positive association with the HbA1c levels in the tuberculosis-infected individuals.
Figure 5.
Positive relationship between systemic levels of cytokines and glycated hemoglobin (HbA1c) levels in tuberculosis-infected individuals. The relationship between the plasma levels of IFN-γ, tumor necrosis factor (TNF)-α, IL-17A, and IL-10 and HbA1c levels was examined in tuberculosis-infected (n = 88) individuals. Data are represented as scatter plots, with each circle representing a single individual. P values were calculated by Spearman rank correlation.
Discriminating Diabetes from Nondiabetes in Active Pulmonary Tuberculosis
Because the systemic levels of a variety of pro- and antiinflammatory cytokines were significantly altered in tuberculosis with diabetes, we wanted to estimate the contribution of any of these cytokines toward our ability to discriminate diabetes from nondiabetes in individuals with tuberculosis. Using a Holm’s adjusted forward stepwise modeling procedure and a P value less than 0.05, we concluded that IFN-γ and IL-22 significantly discriminate diabetes from nondiabetes. This is plotted in Figure 6A, with the lines (contours from the model) representing the probability that an individual is a diabetic. Interestingly, even after correcting for age, sex, and smear grade, IFN-γ levels continue to be a significant predictor in this model. To demonstrate the value of the other parameters, we represent the discriminatory power from each of the logistic regressions for each cytokine alone, using a heat map for the cytokines whose adjusted P value is less than 0.05 (Figure 6B). Thus, our data suggest that multiple cytokines can be useful discriminators of diabetes in tuberculosis-infected individuals, including IL-17A (adjusted P = 0.0001478); IFN-β (adjusted P = 0.0005502); TNF-α (adjusted P = 0.0099083); IL-10 (adjusted P = 0.0014021), and IL-18 (adjusted P = 0.0073989) with IFN-γ (adjusted P = 0.0000821) and IL-22 (adjusted P = 0.0000078) being the most powerful and therefore potentially associated diabetes-mediated effects in the pathogenesis of active pulmonary tuberculosis.
Figure 6.
Discriminatory value of cytokines in tuberculosis-infected individuals. (A) Contribution of IFN-γ and IL-22 to the discrimination of diabetes from no diabetes in tuberculosis-infected individuals (n = 88) at a P value less than 0.05 is plotted, using a logistic model. The red dots are those with diabetes and active pulmonary tuberculosis. The lines are contours from the model, representing the probability that an individual is diabetic. (B) Heat map of predicted values from logistic regressions with a single cytokine predicting diabetes. The first column is the diabetes mellitus (DM) status (dark blue, DM; white, no DM). The rows are subjects sorted first by diabetes status, and then by predicted diabetes status by IL-22, the best predictor. We have shown only cytokines with unadjusted P values less than 0.05. GM-CSF = granulocyte-macrophage colony-stimulating factor; TNF-α = tumor necrosis factor-α.
Discussion
Components of the immune system are altered in type 2 diabetes mellitus, including altered levels of specific cytokines and chemokines, changes in the number and activation state of various immune cell subsets, and increased apoptosis and tissue fibrosis (10). Together, these changes suggest that inflammation participates in the pathogenesis of type 2 diabetes, but how these changes affect the immune response to bystander antigens or newly acquired infections remains unclear. The immunological basis for susceptibility to tuberculosis among those with diabetes mellitus is not well understood. One possible mechanism is that an impaired immune response in diabetic patients facilitates either primary infection with tuberculosis or reactivation of latent tuberculosis (11). Studies examining the innate and adaptive immune response to microbial antigens in diabetic patients suggest that these responses are compromised, particularly in patients with chronic hyperglycemia (12–14). Whether this applies to tuberculosis infection remains unclear.
Cytokines are known to play a major role in determining the outcome of infection in host defense against mycobacterial infections (8). Of major importance are IFN-γ and TNF-α, the functions of which have been well documented in both mouse models and human infections (15–17). The prototypical type 17 cytokine, IL-17A, has been shown to play a central role in mediating immunity to both extra- and intracellular bacteria, including Mycobacterium tuberculosis (18, 19). However, the role of IL-17F, another member of the type 17 cytokine family, has not been examined. On the other hand, IL-22 has been shown not to play an important role in resistance to mycobacterial infection (20). Our data on individuals with tuberculosis and concomitant diabetes reveal an interesting pattern of cytokine expression at baseline. First, the cytokines known to be involved in protection against infection and/or disease, that is, IFN-γ, TNF-α, and IL-17A, are all present at much higher levels in diabetic individuals. Second, cytokines not known to play a role in resistance, that is, IL-17F and IL-22, are both present at much lower levels. Whereas IL-17A is the prototypical cytokine marking CD4+ T cells as Th17 cells, IL-22 is the main cytokine produced by Th22 cells. The regulation of these subsets as well as CD4+ T cells producing IL-17F is not well understood (21). Our data would therefore suggest that in tuberculosis with diabetes, IL-17A is differentially regulated in comparison with IL-17F and IL-22. T cell–mediated immune responses are known to be altered in individuals with insulin resistance, which could indicate that T-cell factors are linked to disturbed insulin sensitivity. Indeed, it has been previously demonstrated that CD4+ T cells producing IL-17A and IFN-γ are increased in frequency and magnitude and promote inflammation and that several cytokines produced by Th1 or Th17 cells have been linked to insulin resistance (22–24). In addition, patients with type 2 diabetes also have been shown to exhibit decreased frequencies of natural regulatory T cells, indicating the presence of an unbalanced proinflammatory milieu in type 2 diabetes (7, 24). Thus, underlying poorly controlled diabetes by itself could contribute to the increase in type 1 and 17 cytokines observed in individuals with tuberculosis and diabetes.
Interestingly, the enhanced production of type 1 and 17 cytokines is associated with significantly higher systemic levels of IL-1 family and other proinflammatory cytokines. Cytokines of the IL-1 family, especially IL-1α and IL-1β, are important for resistance to infection, with mice deficient in IL-1α, IL-1β, and IL-1 receptor all showing greater susceptibility to tuberculosis (25, 26). In addition, IL-18 has also been shown to play a role in protective immunity to tuberculosis (27). Similarly, IL-12 is also known to be crucial in immunity to tuberculosis both in mouse models and human infections (28, 29). More recently, IL-6 has been shown to mediate inhibition of disease progression (30). Therefore, we examined a whole panel of proinflammatory cytokines expecting to observe impaired induction of one or more of these host protective cytokines. However, our data suggest that contrary to our initial hypothesis, host-protective cytokines do not exhibit impaired production at homeostasis but in fact are present at significantly heightened levels in individuals with tuberculosis and diabetes. Therefore, tuberculosis with diabetes is not associated with a down-modulation of host-protective proinflammatory cytokines. Whether this is the cause of increased susceptibility to tuberculosis or the result of increased bacterial burden or severity of disease in diabetic individuals remains to be ascertained. Our data did reveal minor differences in smear grades of tuberculous disease between the two groups (data not shown); however, more detailed analysis of the bacterial burden or severity of disease was not performed in this study. Finally, chemokines including CCL2, CCL4, IL-8, and CXCL-10 are all known to play a role in recruitment of immune cells to the mycobacteria-infected lung (31). In addition, CCL2 is also known to play a role in resistance to infection (32). However, our data reveal that alteration in the systemic levels of these chemokines is not a major feature of tuberculosis with diabetes.
One potential mechanism for the increase in baseline levels of proinflammatory cytokines in individuals with tuberculosis and diabetes could be a concomitant decrease in the levels of systemic type 2 or regulatory cytokines. However, as revealed by the increased systemic levels of IL-5 and IL-10 and unaltered levels of IL-4, IL-13, and TGF-β, it is not only cytokines with inflammatory potential that are elevated in diabetic individuals but also cytokines with immune-regulatory potential. Moreover, both type 2 cytokines and regulatory cytokines are known to play a role in enhancing susceptibility to infection (33). For example, IL-10 is elevated in samples obtained from patients with tuberculosis (34), and an increased ability to innately produce IL-10 is associated with increased incidence of active tuberculosis (35). Our data demonstrating increases in both pro- and antiinflammatory cytokines suggest that the alteration of the balance between these groups of cytokines is not clearly associated with diabetes in tuberculous disease. Other cytokines known to enhance susceptibility to infection and/or disease are the type 1 IFNs. Indeed, type 1 IFN receptor–deficient mice show lower bacterial burdens compared with wild-type controls (36), whereas mycobacteria-infected mice treated with substances known to induce type 1 IFN exhibit exacerbated lung pathology and increased bacterial burden (37). Therefore, we examined the baseline expression of IFN-α and IFN-β in tuberculosis with diabetes and showed that type 1 IFNs are not elevated in these individuals, indicating a minimal (if any) role for type 1 IFN in diabetes-mediated immune pathology in active tuberculosis.
The major limitation in analyzing the immune parameters in circulation is the nonspecific nature of the analysis. We have therefore sought to corroborate the plasma cytokine results by measuring the levels of a subset of pro- and antiinflammatory cytokines in tuberculosis antigen–stimulated whole blood supernatants. Our data on both pro- and antiinflammatory cytokine responses after tuberculosis antigen stimulation clearly show that diabetes has a profound effect on altering antigen-specific innate and adaptive immune responses in tuberculosis. Thus, elevation in circulating levels of type 1 and type 17 cytokines as well as IL-10 is reflected by similar elevations of in vitro–measured cytokines after stimulation with mycobacterial antigens in tuberculosis with diabetes.
Our study provides important insights into the influence of poorly controlled type 2 diabetes mellitus on the pathogenesis of pulmonary tuberculosis. Our data supporting the effects of an excessive inflammatory response in diabetes provide a rational basis for testing combined antimicrobial and antiinflammatory therapies in patients with diabetes and tuberculosis. Our study also provides an impetus to perform longitudinal studies examining the role of immunological biomarkers in the development of tuberculosis in diabetic patients, especially the roles of IFN-γ and IL-22 as predictors of diabetes influence on active tuberculosis. Finally, by dissecting the innate and adaptive cytokine response at homeostasis in diabetic individuals with tuberculosis, our study also suggests that immunomodulatory strategies already in clinical trials for the treatment of type 2 diabetes mellitus, such as IL-1 antagonists (10), could potentially aid in combating the increased susceptibility to exogenous infections and their subsequent pathology.
Acknowledgments
Acknowledgment
The authors thank the staff of Department of Clinical Research and the Department of Social Work, NIRT, especially Ms. Kalaiselvi and Government Stanley Hospital, Chennai, for valuable assistance in recruiting the patients for this study; and R. Anuradha, V. Gopinath, and Jovvian George of the NIH-ICER for technical assistance.
Footnotes
Supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Author Contributions: Conceived and designed the study and wrote the manuscript: T.B.N., M.P.F., S.B.; performed the experiments and analyzed the data: N.P.K., M.P.F., S.B.; contributed materials and samples: R.S., V.V.B., M.S.J.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9:737–746. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Attiyah RJ, Mustafa AS. Mycobacterial antigen-induced T helper type 1 (Th1) and Th2 reactivity of peripheral blood mononuclear cells from diabetic and non-diabetic tuberculosis patients and Mycobacterium bovis bacilli Calmette-Guérin (BCG)–vaccinated healthy subjects. Clin Exp Immunol. 2009;158:64–73. doi: 10.1111/j.1365-2249.2009.04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stalenhoef JE, Alisjahbana B, Nelwan EJ, van der Ven-Jongekrijg J, Ottenhoff TH, van der Meer JW, Nelwan RH, Netea MG, van Crevel R. The role of interferon-γ in the increased tuberculosis risk in type 2 diabetes mellitus. Eur J Clin Microbiol Infect Dis. 2008;27:97–103. doi: 10.1007/s10096-007-0395-0. [DOI] [PubMed] [Google Scholar]
- 5.Vallerskog T, Martens GW, Kornfeld H. Diabetic mice display a delayed adaptive immune response to Mycobacterium tuberculosis. J Immunol. 2010;184:6275–6282. doi: 10.4049/jimmunol.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Restrepo BI, Fisher-Hoch SP, Pino PA, Salinas A, Rahbar MH, Mora F, Cortes-Penfield N, McCormick JB. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin Infect Dis. 2008;47:634–641. doi: 10.1086/590565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Nutman TB, Babu S.Expansion of pathogen-specific Th1 and Th17 cells in pulmonary tuberculosis with coincident type 2 diabetes mellitus J Infect Dis 2013;208:739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper AM, Mayer-Barber KD, Sher A. Role of innate cytokines in mycobacterial infection. Mucosal Immunol. 2011;4:252–260. doi: 10.1038/mi.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deepa M, Pradeepa R, Rema M, Mohan A, Deepa R, Shanthirani S, Mohan V. The Chennai Urban Rural Epidemiology Study (CURES)—study design and methodology (urban component) (CURES-I) J Assoc Physicians India. 2003;51:863–870. [PubMed] [Google Scholar]
- 10.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 11.Ponce-De-Leon A, Garcia-Garcia Md MdeL, Garcia-Sancho MC, Gomez-Perez FJ, Valdespino-Gomez JL, Olaiz-Fernandez G, Rojas R, Ferreyra-Reyes L, Cano-Arellano B, Bobadilla M, et al. Tuberculosis and diabetes in southern Mexico. Diabetes Care. 2004;27:1584–1590. doi: 10.2337/diacare.27.7.1584. [DOI] [PubMed] [Google Scholar]
- 12.Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26:259–265. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 13.Nikolajczyk BS, Jagannathan-Bogdan M, Shin H, Gyurko R. State of the union between metabolism and the immune system in type 2 diabetes. Genes Immun. 2011;12:239–250. doi: 10.1038/gene.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–1248. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 15.Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev. 2008;226:191–204. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris J, Keane J. How tumour necrosis factor blockers interfere with tuberculosis immunity. Clin Exp Immunol. 2010;161:1–9. doi: 10.1111/j.1365-2249.2010.04146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang SY, Boisson-Dupuis S, Chapgier A, Yang K, Bustamante J, Puel A, Picard C, Abel L, Jouanguy E, Casanova JL. Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-α/β, IFN-γ, and IFN-λ in host defense. Immunol Rev. 2008;226:29–40. doi: 10.1111/j.1600-065X.2008.00698.x. [DOI] [PubMed] [Google Scholar]
- 18.Khader SA, Cooper AM. IL-23 and IL-17 in tuberculosis. Cytokine. 2008;41:79–83. doi: 10.1016/j.cyto.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torrado E, Cooper AM. IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 2010;21:455–462. doi: 10.1016/j.cytogfr.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson MS, Feng CG, Barber DL, Yarovinsky F, Cheever AW, Sher A, Grigg M, Collins M, Fouser L, Wynn TA. Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth infections. J Immunol. 2010;184:4378–4390. doi: 10.4049/jimmunol.0903416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252:116–132. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- 22.Matarese G, Procaccini C, De Rosa V. At the crossroad of T cells, adipose tissue, and diabetes. Immunol Rev. 2012;249:116–134. doi: 10.1111/j.1600-065X.2012.01154.x. [DOI] [PubMed] [Google Scholar]
- 23.Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8:709–716. doi: 10.1038/nrendo.2012.114. [DOI] [PubMed] [Google Scholar]
- 24.Jagannathan-Bogdan M, McDonnell ME, Shin H, Rehman Q, Hasturk H, Apovian CM, Nikolajczyk BS. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol. 2011;186:1162–1172. doi: 10.4049/jimmunol.1002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayer-Barber KD, Andrade BB, Barber DL, Hieny S, Feng CG, Caspar P, Oland S, Gordon S, Sher A. Innate and adaptive interferons suppress IL-1α and IL-1β production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity. 2011;35:1023–1034. doi: 10.1016/j.immuni.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugawara I, Yamada H, Hua S, Mizuno S. Role of interleukin (IL)-1 type 1 receptor in mycobacterial infection. Microbiol Immunol. 2001;45:743–750. doi: 10.1111/j.1348-0421.2001.tb01310.x. [DOI] [PubMed] [Google Scholar]
- 27.Schneider BE, Korbel D, Hagens K, Koch M, Raupach B, Enders J, Kaufmann SH, Mittrücker HW, Schaible UE. A role for IL-18 in protective immunity against Mycobacterium tuberculosis. Eur J Immunol. 2010;40:396–405. doi: 10.1002/eji.200939583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Döffinger R, Bernaudin F, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 29.Hölscher C, Atkinson RA, Arendse B, Brown N, Myburgh E, Alber G, Brombacher F. A protective and agonistic function of IL-12p40 in mycobacterial infection. J Immunol. 2001;167:6957–6966. doi: 10.4049/jimmunol.167.12.6957. [DOI] [PubMed] [Google Scholar]
- 30.Martinez AN, Mehra S, Kaushal D. Role of interleukin 6 in innate immunity to Mycobacterium tuberculosis infection. J Infect Dis. 2013;207:1253–1261. doi: 10.1093/infdis/jit037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slight SR, Khader SA. Chemokines shape the immune responses to tuberculosis. Cytokine Growth Factor Rev. 2013;24:105–113. doi: 10.1016/j.cytogfr.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kipnis A, Basaraba RJ, Orme IM, Cooper AM. Role of chemokine ligand 2 in the protective response to early murine pulmonary tuberculosis. Immunology. 2003;109:547–551. doi: 10.1046/j.1365-2567.2003.01680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rook GA. Th2 cytokines in susceptibility to tuberculosis. Curr Mol Med. 2007;7:327–337. doi: 10.2174/156652407780598557. [DOI] [PubMed] [Google Scholar]
- 34.Barnes PF, Lu S, Abrams JS, Wang E, Yamamura M, Modlin RL. Cytokine production at the site of disease in human tuberculosis. Infect Immun. 1993;61:3482–3489. doi: 10.1128/iai.61.8.3482-3489.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Awomoyi AA, Marchant A, Howson JM, McAdam KP, Blackwell JM, Newport MJ. Interleukin-10, polymorphism in SLC11A1 (formerly NRAMP1), and susceptibility to tuberculosis. J Infect Dis. 2002;186:1808–1814. doi: 10.1086/345920. [DOI] [PubMed] [Google Scholar]
- 36.Manca C, Tsenova L, Bergtold A, Freeman S, Tovey M, Musser JM, Barry CE, III, Freedman VH, Kaplan G. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-α/β. Proc Natl Acad Sci USA. 2001;98:5752–5757. doi: 10.1073/pnas.091096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antonelli LR, Gigliotti Rothfuchs A, Gonçalves R, Roffê E, Cheever AW, Bafica A, Salazar AM, Feng CG, Sher A. Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J Clin Invest. 2010;120:1674–1682. doi: 10.1172/JCI40817. [DOI] [PMC free article] [PubMed] [Google Scholar]