Abstract
Rationale: Prior studies have found that cognitive dysfunction is common in intensive care unit (ICU) survivors. Yet, relatively little is known about potentially modifiable risk factors for longer-term post-ICU cognitive impairment.
Objectives: To determine if in-hospital acute stress symptoms were associated with impaired 12-month cognitive functioning among ICU survivors.
Methods: We prospectively enrolled 150 nontrauma patients without cognitive impairment or a dementia diagnosis who were admitted to an ICU for more than 24 hours. Patients were interviewed before hospital discharge and again via telephone at 12 months post-ICU.
Measurements and Main Results: Demographics and clinical information were obtained through medical record reviews and in-person interviews. In-hospital acute stress symptoms were assessed with the Posttraumatic Stress Disorder Checklist-Civilian Version. Twelve-month post-ICU cognition was assessed with the modified Telephone Interview for Cognitive Status. Follow-up interviews were completed with 120 (80%) patients. Patients’ mean age at hospitalization was 48.2 years (SD, 13.7). In unadjusted analyses, a greater number of in-hospital acute stress symptoms was associated with significantly greater impairment in 12-month cognitive functioning (β, −0.1; 95% confidence interval, −0.2 to −0.004; P = 0.04). After adjusting for patient and clinical factors, in-hospital acute stress symptoms were independently associated with greater impairment in 12-month cognitive functioning (β, −0.1; 95% CI, −0.2 to −0.01; P = 0.03).
Conclusions: In-hospital acute stress symptoms may be a potentially modifiable risk factor for greater impairment in cognitive functioning post-ICU. Early interventions for at-risk ICU survivors may improve longer-term outcomes.
Keywords: critical care, acute stress symptoms, cognitive impairment
Quality of survivorship is becoming increasingly important for the millions of Americans surviving hospitalizations in intensive care units (ICUs) for the treatment of critical illnesses annually (1). Critical illnesses are life-threatening experiences that expose patients to enormous physiological and psychological stressors, including hypoxia, extreme pain, release of inflammatory cytokines, and delirium (2). More than one-fourth of ICU survivors have clinically meaningful posttraumatic stress disorder (PTSD) and depressive symptoms (2–5), and considerable physical impairments over the course of recovery are common (5–7).
A growing body of literature has identified that ICU survivors are at increased risk for incident cognitive impairment and overall cognitive decline (8, 9). Yet, there is limited understanding of potential risk factors for this adverse outcome. Prior studies have identified that preexisting depression as well as ICU-related exposures, such as hypoxia, delirium, and conservative fluid management, were associated with risk of post-ICU cognitive impairment (10–13). In addition, anxiety and depressive symptoms have been found to be correlated with post-ICU cognitive impairment (13, 14).
Recently, PTSD has been found to be associated with greater risk of dementia in later life (15, 16). This literature is of particular interest in the context of critical illness survival, because ICU survivors have high rates of both long-term cognitive dysfunction and PTSD (2, 8, 9). An important risk factor for the development of longer-term PTSD after a traumatic stressor is the presence of acute stress symptoms (17), which are PTSD symptoms that develop less than 1 month after the occurrence of the traumatic stressor. However, no studies have examined if post-ICU acute stress symptoms are prospectively associated with longer-term cognitive impairment. Determining if such an association is present is crucial, because cognitive impairment is an important contributor to preventable hospitalizations, rising healthcare costs, caregiver burden, and mortality (8, 18). Importantly, in-hospital acute stress symptoms in critical illness survivors are potentially modifiable if identified.
The present study is a prospective cohort investigation of potentially modifiable in-hospital risk factors for impaired cognition 12 months after medical-surgical ICU admission. We hypothesized that a greater number of in-hospital acute stress symptoms would be associated with impaired cognitive functioning post-ICU even after adjusting for baseline patient characteristics and other in-hospital clinical factors.
Methods
Study Setting, Participants, and Procedures
The present investigation took place at Harborview Medical Center (HMC) in Seattle, Washington. HMC is an urban, public medical center operated by the University of Washington (UW). The cohort has been previously described (19). In summary, between September 2010 and August 2011, we prospectively recruited 150 patients admitted to any of the ICUs at HMC. Key exclusion criteria for the study were: (1) initial admission diagnosis of traumatic injury, (2) preexisting cognitive impairment or dementia diagnosis noted in the medical record, (3) non-English speaking, (4) ICU length of stay less than or equal to 24 hours, (5) preexisting medical illness with life expectancy of less than 12 months noted in the medical record (e.g., terminal cancer, terminal HIV disease), and (6) admission for a suicide attempt. The study protocol was approved by the UW Institutional Review Board, and all participants provided informed consent before enrollment.
Study research assistants identified eligible subjects through review of HMC’s electronic admission records. Eligible subjects were approached for study consent before transfer from the ICU to the general medical-surgical ward. Because the UW Institutional Review Board did not allow surrogate consent for study participation, if the patient was unable to provide consent, typically due to ongoing delirium and/or cognitive impairment, consent was not obtained, and they were approached again once their delirium and/or cognitive impairment had resolved. Cognitive impairment was assessed using a cutoff score of three or more errors on the Six-Item Cognitive Screen (20). The area under the receiver operating characteristic curve for the Six-Item Cognitive Screen was 0.86 for a community-based sample compared with the Community Screening Instrument for Dementia and 0.91 for a clinical sample based on a battery including the Mini-Mental State Examination and the Consortium for Establishment of Registry for Alzheimer Disease battery (20).
Enrolled patients were administered an in-person interview before hospital discharge and were reinterviewed via telephone at 12 months post-ICU.
Primary Independent Variable
The primary independent variable of interest in our analyses was the presence of in-hospital acute stress symptoms as ascertained by the PTSD Checklist-civilian version (PCL-C) administered in-person before hospital discharge. The 17-item PCL-C includes questions regarding five symptoms in the intrusive symptom cluster (e.g., intrusive thoughts, nightmares), seven symptoms in the avoidant symptom cluster (e.g., avoidance of thoughts or activities that remind the patient of the stressor, emotional numbing), and five symptoms in the arousal symptom cluster (e.g., impaired sleep, hypervigilance), and symptom severity is rated on a 5-point Likert scale (21). Scores range from 0 to a maximum of 85, with higher scores indicating greater severity of symptoms. Substantial acute stress symptoms, defined as PTSD symptoms present within 1 month of a traumatic stressor that are suggestive of a diagnosis of acute stress disorder, can be ascertained with the PCL-C in one of two ways: (1) following an algorithm that considers a score of 3 or more on at least one intrusive symptom, three avoidant symptoms, and two arousal symptoms as consistent with Diagnostic and Statistical Manual of Mental Disorders-IV diagnostic criteria; or (2) a total PCL-C score greater than or equal to 45 (21).
Covariates of Interest
Covariates of interest included baseline patient characteristics and ICU clinical factors, which were obtained through medical record review and in-person interview with the patient. Medical record–obtained covariates included demographics (e.g., age, sex, race); ICU admission diagnosis; baseline medical comorbidity information to compute a Charlson Comorbidity Score (22); illness severity measures at ICU admission to compute a Simplified Acute Physiology Score II (SAPS II) (23); ICU length of stay; requirements for mechanical ventilation, major surgery, or blood product transfusion; days of exposure (both in the ICU and on the medical-surgical ward) to benzodiazepine, opioid, antipsychotic, and antidepressant medications; presence of delirium in the ICU per nursing-documented assessment using the Confusion Assessment Method-ICU (CAM-ICU) (24); and presence of confusion/disorientation/difficulty following commands in nursing documentation. We defined probable delirium as a documented positive CAM-ICU assessment in the ICU- or nursing-documented presence of confusion/disorientation/difficulty following commands at any point during the hospitalization.
Additional covariates of interest obtained from the baseline interviews included demographics not obtained from medical records (e.g., marital/partnered status, education); assessment of prior trauma exposure with the National Comorbidity Survey-Replication Trauma History Screen (25); lifetime history of major depression with the Mini International Neuropsychiatric Interview major depression module (26); alcohol use in the previous year with the Alcohol Use Disorders Identification Test, with baseline problem drinking defined as a score greater than or equal to 8 (27); and drug use in the previous year with the Drug Abuse Screening Test-10, with baseline problem drug use defined as a score greater than or equal to 3 (28).
As part of the 12-month telephone follow-up interview, post-ICU PTSD symptoms were assessed with the PCL-C, and post-ICU depressive symptoms were assessed with the Patient Health Questionnaire-9 (29).
Outcome of Interest
Our outcome of interest was post-ICU cognitive functioning as assessed with the modified Telephone Interview for Cognitive Status (TICSm) at the 12-month telephone follow-up interview. The TICSm is a 13-item validated measure of cognition that is composed of four domains: (1) orientation; (2) registration, free recall, and delayed recall; (3) attention/calculation; and (4) semantic memory, comprehension, and repetition (30). The maximum score possible is 39, with higher scores indicating better cognitive functioning (30).
Statistical Analysis
We present descriptive data as means and SDs, medians and interquartile ranges (IQRs), or proportions.
We used multiple linear regression analyses to examine potential risk factors for worse cognitive functioning at 12 months post-ICU. The dependent variable for these analyses was the continuous TICSm score. Initially, we tested the association of in-hospital acute stress symptoms, defined by the continuous PCL-C score, with post-ICU TICSm score without adjustment. We then added two groups of potential confounding variables to the regression models all chosen a priori because they have been found to be important in critical illness-related research (2–4, 10, 12, 13): (1) demographics and baseline clinical characteristics (age, sex, race, education, marital/partnered status, lifetime history of major depression, number of prior traumatic event exposures, presence of unhealthy alcohol use during the year pre-ICU, problem drug use during the year pre-ICU, and Charlson score); and (2) clinical characteristics of the ICU admission (SAPS II score, presence of probable delirium during the hospitalization, and requirements for mechanical ventilation, major surgery, blood product transfusion, as well as receipt of benzodiazepines, opioids, antipsychotics, and antidepressants). Finally, in sequential models we adjusted for 12-month Patient Health Questionnaire-9 scores, 12-month PCL-C scores, and both, to determine if any association between in-hospital acute stress symptoms might be mediated by post-ICU depressive and/or PTSD symptoms.
We used Student t tests to examine associations between in-hospital acute stress symptoms and individual 12-month TICSm domains.
To examine the extent to which our results might be biased by nonresponse, we conducted a series of sensitivity analyses in which we repeated the regressions with assignment of the lowest observed TICSm score, the mean observed TICSm score, and the highest observed TICSm score, to patients who were missing 12-month follow-up.
We used two-sided significance tests for all analyses with statistical significance set at a P value of 0.05. Analyses were performed with appropriate components of the IBM SPSS Statistics 18 (SPSS Inc., Chicago, IL) and STATA 11.2 (Stata Corporation, College Station, TX) statistical software programs.
Results
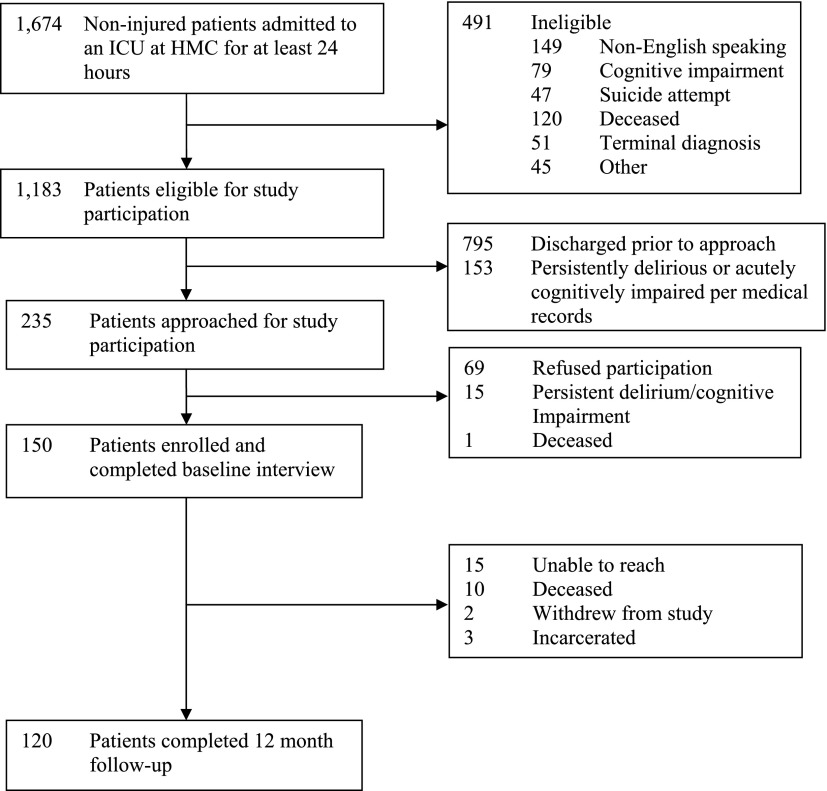
Nearly 1,200 patients were eligible for study participation (Figure 1). Of the 150 patients originally enrolled in the study, 10 were deceased and 3 were incarcerated at 12 months post-ICU, leaving 137 patients eligible to complete follow-up. One hundred twenty patients (88% of those eligible to complete follow-up, 80% of the original cohort) completed a 12-month telephone follow-up interview. Among the entire cohort, patients’ mean age at hospitalization was 48.2 years (SD, 13.7), their median Charlson Comorbidity score was 1.0 (IQR, 0.0–2.0), and 129 (86%) had graduated from high school. Their median ICU length of stay was 5.0 days (IQR, 3.0–9.0), and their median SAPS II score on admission was 23.0 (IQR, 13.0–37.0). Nearly one-half required mechanical ventilation during their ICU admission, with a median duration of 2.0 days (IQR, 1.0–4.0). The prevalence of probable delirium during the course of hospitalization was 51%.
Figure 1.
Study flow diagram. HMC = Harborview Medical Center; ICU = intensive care unit.
Baseline interviews were completed at a mean of 11.1 days after hospital admission (SD, 9.5). At baseline, patients’ mean PCL-C score was 30.8 (SD, 12.3), 15% had substantial acute stress symptoms based on the PCL-C algorithm, and 29% met criteria for lifetime history of major depression based on the Mini International Neuropsychiatric Interview.
Table 1 presents the baseline and hospitalization-related clinical characteristics of patients who completed 12-month follow-up.
Table 1.
Characteristics of participants who completed 12-month follow-up
| Variables | N = 120 |
|---|---|
| Patient baseline characteristics | |
| Age | 49.0 ± 14.6 |
| Female | 51 (42.5) |
| Nonwhite | 30 (25.2) |
| < High school graduate | 15 (12.6) |
| Married/partnered | 63 (52.5) |
| Lifetime major depression | 32 (26.9) |
| Lifetime traumatic event exposures | 4.1 ± 2.8 |
| Problem drinking (AUDIT ≥ 8) | 14 (11.7) |
| Problem drug use (DAST-10 ≥ 3) | 14 (11.7) |
| Charlson Comorbidity score | 1.0 (0–2.0) |
| Admission clinical characteristics | |
| ICU LOS, d | 5.0 (3.0–9.0) |
| SAPS II | 22.0 (13.0–36.8) |
| Admission diagnosis | |
| Cardiovascular | 11 (9.2) |
| Pulmonary | 18 (15.0) |
| Infectious disease | 31 (25.8) |
| Neurologic | 33 (27.5) |
| Vascular surgery | 19 (15.8) |
| Gastrointestinal | 14 (11.7) |
| Endocrine/renal | 5 (4.2) |
| Orthopedic | 10 (8.3) |
| Oncologic | 3 (2.5) |
| Other | 3 (2.5) |
| Mechanically ventilated | 56 (46.7) |
| Had major surgery | 56 (46.7) |
| Received blood product transfusion | 29 (24.2) |
| Probable delirium | 63 (52.5) |
| Days of benzodiazepines | 2.0 (0.3–4.8) |
| Days of opioids | 10.0 (5.0–19.0) |
| Days of antipsychotics | 0 (0–0) |
| Days of antidepressants | 0 (0–0) |
| PCL-C score at baseline interview | 29.8 ± 11.7 |
Definition of abbreviations: AUDIT = Alcohol Use Disorders Identification Test; DAST-10 = Drug Abuse Screening Test-10; ICU = intensive care unit; LOS = length of stay; MV = mechanical ventilation; PCL-C = Posttraumatic Stress Disorder Checklist-civilian version; SAPS II = Simplified Acute Physiology Score II.
All values are mean ± SD, median (IQR) or N (%) unless otherwise indicated.
At 12 months post-ICU (mean, 356.4 d; SD, 29.2), patients had a mean total TICSm score of 23.9 (SD, 4.7; range, 12–39). Patients with in-hospital substantial acute stress symptoms had a mean total TICSm score of 22.2 (SD, 4.8; range, 12–29) compared with 24.2 (SD, 4.6; range, 12–39) among those without in-hospital substantial acute stress symptoms.
In unadjusted analyses, an additional point on the in-hospital PCL-C was associated with a significantly lower 12-month TICSm score (β, −0.1; 95% confidence interval (CI), −0.2 to 0.004; P = 0.04). After adjusting for patient baseline characteristics, in-hospital PCL-C remained associated with a significantly lower 12-month TICSm score (β,−0.1; 95% CI, −0.2 to −0.01; P = 0.04) (Table 2). Patient characteristics associated with a lower 12-month TICSm score included less than high school education (β, −4.0; 95% CI, −6.4 to −1.5; P = 0.002) and greater medical comorbidity (β, −0.5; 95% CI, −0.9 to −0.04; P = 0.03). When we adjusted for both patient and in-hospital clinical characteristics of the ICU admission, in-hospital PCL-C score was independently associated with a lower 12-month TICSm score (β, −0.1; 95% CI, −0.2 to −0.01; P = 0.03). No other hospitalization-related factors were independently associated with 12-month TICSm scores. Less than high school education (β, −4.2; 95% CI, −6.8 to −1.6; P = 0.002) and greater medical comorbidity (β, −0.6; 95% CI, −1.1 to −0.1; P = 0.03) were also independently associated with a lower 12-month TICSm score.
Table 2.
Adjusted associations of in-hospital acute stress symptoms and cognitive performance at 12 months after medical-surgical intensive care unit admission
| Variables | Adjusted for Patient Characteristics | Adjusted for Hospitalization Characteristics | Adjusted for 12-Month Post-ICU Depressive Symptoms | Adjusted for 12-Month Post-ICU PTSD Symptoms | Adjusted for 12-Month Post-ICU Depressive and PTSD Symptoms |
|---|---|---|---|---|---|
| In-hospital acute stress symptoms (PCL-C) | −0.1 (−0.2 to −0.01)* | −0.1 (−0.2 to −0.01)* | −0.1 (−0.2 to −0.01)* | −0.1 (−0.2 to 0.01) | −0.1 (−0.2 to 0.03) |
| Age | −0.01 (−0.1 to 0.1) | −0.03 (−0.1 to 0.1) | −0.02 (−0.1 to 0.1) | −0.03 (−0.1 to 0.1) | −0.02 (−0.1 to 0.1) |
| Male | 0.1 (−1.6 to 1.8) | −0.02 (−1.8 to 1.8) | −0.01 (−1.8 to 1.8) | −0.03 (−1.8 to 1.8) | −0.03 (−1.8 to 1.8) |
| Nonwhite | 0.5 (−1.4 to 2.5) | 0.6 (−1.6 to 2.6) | 0.5 (−1.6 to 2.6) | 0.5 (−1.6 to 2.6) | 0.5 (−1.6 to 2.6) |
| Education less than high school | −4.0 (−6.4 to −1.5)† | −4.2 (−6.8 to −1.6)† | −4.2 (−6.8 to −1.5)† | −4.2 (−6.8 to −1.6)† | −4.2 (−6.8 to −1.5)† |
| Married | 0.3 (−1.4 to 2.0) | 0.1 (−1.6 to 1.9) | 0.1 (−1.8 to 1.9) | 0.2 (−1.6 to 2.0) | 0.1 (−1.8 to 1.9) |
| Lifetime major depression | 0.6 (−1.3 to 2.5) | 0.4 (−1.7 to 2.5) | 0.3 (−1.8 to 2.4) | 0.4 (−1.7 to 2.5) | 0.4 (−1.8 to 2.5) |
| Problem drinking | 0.6 (−2.1 to 3.2) | 0.2 (−2.8 to 3.1) | 0.2 (−2.8 to 3.1) | 0.1 (−2.8 to 3.1) | 0.2 (−2.8 to 3.1) |
| Problem drug use | −1.1 (−3.8 to 1.7) | −1.2 (−4.2 to 1.7) | −1.2 (−4.2 to 1.7) | −1.2 (−4.2 to 1.7) | −1.2 (−4.2 to 1.8) |
| Number of prior traumatic event exposures | 0.1 (−0.2 to 0.4) | 0.1 (−0.2 to 0.5) | 0.1 (−0.2 to 0.5) | 0.1 (−0.2 to 0.5) | 0.1 (−0.2 to 0.5) |
| Charlson Comorbidity Score | −0.5 (−0.9 to −0.04)* | −0.6 (−1.1 to −0.1)* | −0.6 (−1.1 to −0.1)* | −0.6 (−1.1 to−-0.1)* | −0.6 (−1.1 to −0.1)* |
| SAPS II | 0.01 (−0.03 to 0.1) | 0.1 (−0.04 to 0.1) | 0.1 (−0.04 to 0.1) | 0.1 (−0.04 to 0.1) | |
| Mechanical ventilated | −0.3 (−2.9 to 2.3) | −0.3 (−2.9 to 2.4) | −0.3 (−2.9 to 2.3) | −0.3 (−2.9 to 2.4) | |
| Required major surgery | −0.3 (−2.2 to 1.6) | −0.3 (−2.2 to 1.6) | −0.3 (−2.2 to 1.6) | −0.3 (−2.2 to 1.6) | |
| Required blood product transfusion | 0.3 (−1.9 to 2.5) | 0.4 (−1.9 to 2.6) | 0.5 (−1.9 to 2.5) | 0.4 (−1.9 to 2.6) | |
| Probable delirium | −0.3 (−2.6 to 2.0) | −0.3 (−2.6 to 2.0) | −0.3 (−2.6 to 2.1) | −0.2 (−2.6 to 2.2) | |
| Received benzodiazepines | −0.2 (−2.4 to 2.0) | −0.2 (−2.4 to 2.0) | −0.2 (−2.4 to 2.0) | −0.2 (−2.4 to 2.0) | |
| Received opioids | −5.2 (−14.5 to 4.2) | −5.2 (−14.7 to 4.2) | −5.0 (−14.5 to 4.4) | −5.1 (−14.6 to 4.5) | |
| Received antipsychotics | −0.3 (−2.9 to 2.3) | −0.3 (−3.0 to 2.3) | −0.3 (−2.9 to 2.4) | −0.3 (−3.0 to 2.3) | |
| Received antidepressants | −0.4 (−2.6 to 1.8) | −0.4 (−2.6 to 1.9) | −0.4 (−2.7 to 1.8) | −0.4 (−2.6 to 1.9) | |
| 12-mo PHQ-9 Scores | 0.02 (−0.2 to 0.2) | 0.1 (−0.2 to 0.3) | |||
| 12-mo PCL-C Scores | −0.01 (−0.1 to 0.1) | −0.03 (−0.1 to 0.1) |
Definition of abbreviations: PCL-C = Posttraumatic Stress Disorder Checklist-civilian version; PHQ-9 = Patient Health Questionniare-9; PTSD = posttraumatic stress disorder; SAPS II = Simplified Acute Physiology Score II.
Data are presented as β coefficient (95% confidence interval).
P < 0.05.
P < 0.01.
After we adjusted for 12-month depressive symptoms, in-hospital PCL-C score remained independently associated with a lower 12-month TICSm score (β, −0.1; 95% CI, −0.2 to −0.01; P = 0.03). Adjustment for 12-month PTSD symptoms appeared to attenuate the association of in-hospital acute stress symptoms with 12-month TICSm score slightly (β, −0.1; 95% CI, −0.2, 0.01; P = 0.11). In the final model, which adjusted for both 12-month depressive and PTSD symptoms, the magnitude of the association between in-hospital acute stress symptoms per PCL-C with lower 12-month TICSm score remained similar (β,−0.1; 95% CI, −0.2 to 0.03; P = 0.13).
Of the individual 12-month TICSm domains, higher in-hospital PCL-C scores were associated with worse performance in attention/calculation (t = −2.81, P = 0.006) and delayed recall (t = −2.1, P = 0.04), but not with any other TICSm domain.
In our sensitivity analysis in which we replaced missing 12-month TICSm scores with the lowest observed TICSm score, an additional point on the in-hospital PCL-C remained independently associated with a significantly lower 12-month TICSm score (β, −0.1; 95% CI, −0.2 to −0.04; P = 0.005). When we reran our fully adjusted linear regression model after replacing missing 12-month TICSm scores with the mean observed TICSm score, the point estimates suggested a similar magnitude of association but were less precise (β, −0.1; 95% CI, −0.1 to 0.001; P = 0.05). When we replaced missing 12-month TICSm scores with the highest observed TICSm score, there was no longer an independent association between the in-hospital PCL-C score and 12-month TICSm score (β, 0.03; 95% CI, −0.1 to 0.1; P = 0.62).
Discussion
In this prospective investigation of cognitive functioning in medical-surgical ICU survivors, we highlight a potentially modifiable risk factor for post-ICU cognitive dysfunction. We found that developing a greater number of acute stress symptoms before hospital discharge was independently associated with greater impairment in 12-month cognitive functioning, even after adjusting for patient characteristics and other ICU and hospital-related exposures. This finding builds on our prior work identifying in-hospital acute stress symptoms as independently associated with both longer-term post-ICU PTSD and depressive symptoms as well as increased alcohol use (19, 31). Furthermore, the association we found between in-hospital acute stress symptoms and greater impairment in 12-month cognitive functioning was independent of post-ICU depressive symptoms. Our results suggest that the association between in-hospital acute stress symptoms and greater risk of post-ICU cognitive impairment could be partially mediated by post-ICU PTSD; however, it is possible that we simply lacked sufficient statistical power, because the point estimates after controlling for 12-month PTSD symptoms were quite similar.
Our study adds to the growing body of literature identifying an association between PTSD symptoms and potential risk of cognitive impairment. Two large prospective cohort studies of combat veterans found significant associations between PTSD diagnosis and increased risk of later dementia diagnosis (15, 16). A third national multisite investigation of outcomes after traumatic injuries found that PTSD symptoms at 12 months postinjury were independently associated with worse 12-month cognitive functioning for patients with mild, moderate, or severe traumatic brain injury (32). Incorporating our findings into this larger context, PTSD symptoms appear to be an important risk factor for cognitive impairment across a diverse group of patient populations.
We found that in-hospital acute stress symptoms were associated with greater impairment in 12-month performance in the attention/calculation and delayed recall domains of the TICSm. These results are in line with prior studies, which have found associations between PTSD and impairments in sustaining attention, learning, and verbal memory (33–35). However, unlike these studies, we show that PTSD symptoms in the acute aftermath of a medical traumatic stressor are associated with poorer cognitive functioning 12 months later. If our findings are replicated in larger cohorts of critical illness survivors, then this distinction has important clinical implications, because these patients could be identified before hospital discharge to facilitate receipt of evidence-based treatments with the aim of preventing the development of PTSD (36, 37). Additional research is warranted to determine if implementing such interventions for medical-surgical ICU survivors could also prevent post-ICU cognitive impairment.
For clinicians working with critical illness survivors in the early aftermath of the ICU, our findings suggest that patients exhibiting symptoms such as insomnia with accompanying nightmares, anxiety with hyperarousal, and/or intrusive recollections of the ICU experience, whether of real events or of psychotic experiences (i.e., “delusional memories”), are at risk for the development of PTSD and possibly longer-term cognitive impairment. These patients warrant referral for careful evaluation for acute stress disorder by a specialist to receive evidence-based treatments if needed.
Although observational studies such as this one cannot prove causation, credible biological pathways exist that reduce the concern that the association presented here between in-hospital acute stress symptoms and impairment in 12-month post-ICU cognitive functioning is due to residual confounding or a chance finding. Exposure to profound stress has been associated with damage to the hippocampus, as evidenced by decreased levels of the hippocampal neuronal marker, N-acetyl aspartate (38). Furthermore, neuroimaging studies have found that adults exposed to trauma may have smaller hippocampal volumes (39, 40). In addition, critical illnesses and PTSD symptoms have been found to be associated with increased release of proinflammatory cytokines (41, 42), which has been hypothesized to lead to neurodegenerative changes (43).
In addition to in-hospital acute stress symptoms, we also found that less than a high school level of education and greater medical comorbidity were also associated with greater impairment in cognitive functioning at 1 year post-ICU. These findings are consistent with previous studies identifying lower education and chronic medical conditions as potential risk factors for cognitive decline and dementia (44–46). Importantly, improved management of chronic medical conditions could prevent ICU admissions and subsequent post–critical illness cognitive decline.
Our study has several potential limitations. Because we were unable to obtain surrogate consent from patients who were persistently delirious and therefore could not include them in our study, we could not effectively evaluate whether prolonged delirium was associated with post-ICU cognitive dysfunction. However, requiring patients to pass a validated cognitive screen before providing consent for study participation likely minimized the possibility that our assessment of in-hospital acute stress symptoms was confounded by residual hyperactive delirium, reduced the chance that patients with undiagnosed substantial cognitive impairment were recruited into the study, and decreased the likelihood that the acute stress symptoms seen were a response to acute cognitive deficits. Although we cannot definitively rule out the possibility that in-hospital acute stress symptoms in our cohort are a marker for pre-ICU cognitive impairment, this possibility is minimized by the procedures we used to enroll patients. Furthermore, a recent study found that in-hospital cognitive testing results did not predict longer-term cognitive dysfunction in critical illness survivors (47), suggesting that in-hospital acute stress symptoms could be a unique early predictor of later cognitive impairment in this patient population.
Although we cannot completely rule out that our findings are biased by nonresponse, we conducted multiple sensitivity analyses to quantify the potential effects of such bias and found that our results were only substantively affected by the extreme situation in which all participants missing 12-month follow-up had perfect cognitive functioning. Also, in our prior examinations of predictors of greater severity of post-ICU PTSD and depressive symptoms as well as increased alcohol use in this cohort, we found that patients with in-hospital substantial acute stress symptoms were more likely to be lost to follow-up (19, 31), suggesting the possibility that the magnitude of the association we describe here between in-hospital acute stress symptoms and impairment in post-ICU cognition may be underestimated. Information on prior traumatic event exposure, lifetime major depression, and pre-ICU substance use may be subject to recall bias, as these data were obtained from patients while they were still in the hospital and is therefore retrospective in nature. Also, although the TICSm has not been specifically validated as an assessment of cognitive functioning in medical-surgical ICU survivors or among patients under the age of 50, it has been used previously in similar populations (8, 48) and has been validated against neuropsychiatric interview (49). Moreover, our study was conducted in a single center serving a safety net population, and a majority of patients potentially eligible for our study were discharged before approach. Because we only have data from patients who consented to participate in our study, we cannot characterize potential differences between our cohort and all eligible ICU survivors from our institution. Therefore, these factors limit the generalizability of our results to the entire population of medical-surgical ICU survivors. Because our sample size was relatively small, our findings warrant replication in larger prospective cohort studies. Finally, the possibility of residual confounding remains, as in any observational study.
In conclusion, we identified that in-hospital acute stress symptoms are independently associated with greater impairment in cognitive functioning 12 months after a medical-surgical ICU admission. Efforts to incorporate screening of medical-surgical ICU survivors for acute stress symptoms into routine care after critical illnesses, as well as additional research into the mechanisms linking PTSD symptoms, critical illnesses, and cognitive impairment, are crucial in light of the enormous toll that critical illnesses, PTSD, and cognitive dysfunction take on patients, their families, and society.
Acknowledgments
Acknowledgment
The authors thank Collin McFadden, B.A., and Jeffrey Love, B.A., for assistance with patient recruitment and data collection, and Jin Wang, Ph.D., for assistance with data cleaning.
Footnotes
Supported by National Institutes of Health grants KL2 TR000421, R03 AA020146-02, NRSA-T32/MH20021-12, R01 AA01602, and K24 MH086814-03 and grant ADAI-1009-2 from the University of Washington Alcohol and Drug Abuse Institute.
Author Contributions: D.S.D. had full access to all of the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis. D.S.D. was responsible for the study concept and design, acquisition of data, analysis and interpretation of data, drafting and critical revision of the manuscript, statistical analysis, obtaining funding, and study supervision. D.Z. was responsible for the acquisition of data, interpretation of data, critical revision of the manuscript, obtaining funding, and administrative, technical, and material support. C.L.H. was responsible for interpretation of data and critical revision of the manuscript. W.J.K. was responsible for the study concept and design, interpretation of data, critical revision of the manuscript, and obtaining funding.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Iwashyna TJ. Survivorship will be the defining challenge of critical care in the 21st century. Ann Intern Med. 2010;153:204–205. doi: 10.7326/0003-4819-153-3-201008030-00013. [DOI] [PubMed] [Google Scholar]
- 2.Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008;30:421–434. doi: 10.1016/j.genhosppsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davydow DS, Gifford JM, Desai SV, Bienvenu OJ, Needham DM. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med. 2009;35:796–809. doi: 10.1007/s00134-009-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davydow DS, Desai SV, Needham DM, Bienvenu OJ. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med. 2008;70:512–519. doi: 10.1097/PSY.0b013e31816aa0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davydow DS, Hough CL, Langa KM, Iwashyna TJ. Symptoms of depression in survivors of severe sepsis: a prospective cohort study of older Americans. Am J Geriatr Psychiatry. doi: 10.1016/j.jagp.2013.01.017. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Needham DM, Feldman DR, Kho ME. The functional costs of ICU survivorship. Collaborating to improve post-ICU disability. Am J Respir Crit Care Med. 2011;183:962–964. doi: 10.1164/rccm.201012-2042ED. [DOI] [PubMed] [Google Scholar]
- 7.Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, Dinglas VD, Shanholtz C, Husain N, Dennison CR, Herridge MS, Pronovost PJ, Needham DM. Depressive symptoms and impaired physical function after acute lung injury: a 2-year longitudinal study. Am J Respir Crit Care Med. 2012;185:517–524. doi: 10.1164/rccm.201103-0503OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehlenbach WJ, Hough CL, Crane PK, Haneuse SJ, Carson SS, Curtis JR, Larson EB. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303:763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davydow DS, Hough CL, Langa KM, Iwashyna TJ. Presepsis depressive symptoms are associated with incident cognitive impairment in survivors of severe sepsis: a prospective cohort study of older Americans. J Am Geriatr Soc. 2012;60:2290–2296. doi: 10.1111/jgs.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson-LOHR V. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:50–56. doi: 10.1164/ajrccm.160.1.9708059. [DOI] [PubMed] [Google Scholar]
- 12.Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, Gordon SM, Canonico AE, Dittus RS, Bernard GR, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, Localio AR, Demissie E, Hopkins RO, Angus DC. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185:1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson JC, Hart RP, Gordon SM, Shintani A, Truman B, May L, Ely EW. Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med. 2003;31:1226–1234. doi: 10.1097/01.CCM.0000059996.30263.94. [DOI] [PubMed] [Google Scholar]
- 15.Qureshi SU, Kimbrell T, Pyne JM, Magruder KM, Hudson TJ, Petersen NJ, Yu HJ, Schulz PE, Kunik ME. Greater prevalence and incidence of dementia in older veterans with posttraumatic stress disorder. J Am Geriatr Soc. 2010;58:1627–1633. doi: 10.1111/j.1532-5415.2010.02977.x. [DOI] [PubMed] [Google Scholar]
- 16.Yaffe K, Vittinghoff E, Lindquist K, Barnes D, Covinsky KE, Neylan T, Kluse M, Marmar C. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67:608–613. doi: 10.1001/archgenpsychiatry.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zatzick DF, Kang SM, Müller HG, Russo JE, Rivara FP, Katon W, Jurkovich GJ, Roy-Byrne P. Predicting posttraumatic distress in hospitalized trauma survivors with acute injuries. Am J Psychiatry. 2002;159:941–946. doi: 10.1176/appi.ajp.159.6.941. [DOI] [PubMed] [Google Scholar]
- 18.Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of incident dementia with hospitalizations. JAMA. 2012;307:165–172. doi: 10.1001/jama.2011.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davydow DS, Zatzick D, Hough CL, Katon WJ. A longitudinal investigation of posttraumatic stress and depressive symptoms over the course of the year following medical-surgical intensive care unit admission. Gen Hosp Psychiatry. 2013;35:226–232. doi: 10.1016/j.genhosppsych.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Weathers FW, Huska JA, Keane TM.The PTSD Checklist – civilian version. Boston, MA: The National Center for PTSD, Boston VA Medical Center; 1991 [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 24.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 25.Ramstad SM, Russo J, Zatzick DF. Is it an accident? Recurrent traumatic life events in level I trauma center patients compared to the general population. J Trauma Stress. 2004;17:529–534. doi: 10.1007/s10960-004-5802-z. [DOI] [PubMed] [Google Scholar]
- 26.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. quiz 34–57. [PubMed] [Google Scholar]
- 27.Babor TF, de la Fuente JR, Saunders J, Grant M.The Alcohol Use Disorders Identification Test. Guidelines for use in primary health care. Geneva, Switzerland: World Health Organization; 1992 [Google Scholar]
- 28.Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- 29.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Jager CA, Budge MM, Clarke R. Utility of TICS-M for the assessment of cognitive function in older adults. Int J Geriatr Psychiatry. 2003;18:318–324. doi: 10.1002/gps.830. [DOI] [PubMed] [Google Scholar]
- 31.Davydow DS, Zatzick D, Hough CL, Katon WJ. A longitudinal investigation of alcohol use over the course of the year following medical-surgical intensive care unit admission. Psychosomatics. 2013;54:307–316. doi: 10.1016/j.psym.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zatzick DF, Rivara FP, Jurkovich GJ, Hoge CW, Wang J, Fan MY, Russo J, Trusz SG, Nathens A, Mackenzie EJ. Multisite investigation of traumatic brain injuries, posttraumatic stress disorder, and self-reported health and cognitive impairments. Arch Gen Psychiatry. 2010;67:1291–1300. doi: 10.1001/archgenpsychiatry.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vasterling JJ, Brailey K, Constans JI, Sutker PB. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12:125–133. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- 34.Yehuda R, Golier JA, Halligan SL, Harvey PD. Learning and memory in Holocaust survivors with posttraumatic stress disorder. Biol Psychiatry. 2004;55:291–295. doi: 10.1016/s0006-3223(03)00641-3. [DOI] [PubMed] [Google Scholar]
- 35.Johnsen GE, Asbjørnsen AE. Consistent impaired verbal memory in PTSD: a meta-analysis. J Affect Disord. 2008;111:74–82. doi: 10.1016/j.jad.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Shalev AY, Ankri Y, Israeli-Shalev Y, Peleg T, Adessky R, Freedman S. Prevention of posttraumatic stress disorder by early treatment: results from the Jerusalem Trauma Outreach And Prevention study. Arch Gen Psychiatry. 2012;69:166–176. doi: 10.1001/archgenpsychiatry.2011.127. [DOI] [PubMed] [Google Scholar]
- 37.Zatzick D, Jurkovich G, Rivara F, Russo J, Wagner A, Wang J, Dunn C, Lord SP, Petrie M, O’connor SS, et al. A randomized stepped care intervention trial targeting posttraumatic stress disorder for surgically hospitalized injury survivors. Ann Surg. 2013;257:390–399. doi: 10.1097/SLA.0b013e31826bc313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuff N, Neylan TC, Lenoci MA, Du AT, Weiss DS, Marmar CR, Weiner MW. Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol Psychiatry. 2001;50:952–959. doi: 10.1016/s0006-3223(01)01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hedges DW, Woon FL. Premorbid brain volume estimates and reduced total brain volume in adults exposed to trauma with or without posttraumatic stress disorder: a meta-analysis. Cogn Behav Neurol. 2010;23:124–129. doi: 10.1097/WNN.0b013e3181e1cbe1. [DOI] [PubMed] [Google Scholar]
- 40.Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1181–1188. doi: 10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 41.Jacob A, Brorson JR, Alexander JJ. Septic encephalopathy: inflammation in man and mouse. Neurochem Int. 2011;58:472–476. doi: 10.1016/j.neuint.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Plantinga L, Bremner JD, Miller AH, Jones DP, Veledar E, Goldberg J, Vaccarino V. Association between posttraumatic stress disorder and inflammation: a twin study. Brain Behav Immun. 2013;30:125–132. doi: 10.1016/j.bbi.2013.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katon WJ, Lyles CR, Parker MM, Karter AJ, Huang ES, Whitmer RA. Association of depression with increased risk of dementia in patients with type 2 diabetes: the Diabetes and Aging Study. Arch Gen Psychiatry. 2012;69:410–417. doi: 10.1001/archgenpsychiatry.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmand B, Smit J, Lindeboom J, Smits C, Hooijer C, Jonker C, Deelman B. Low education is a genuine risk factor for accelerated memory decline and dementia. J Clin Epidemiol. 1997;50:1025–1033. doi: 10.1016/s0895-4356(97)00121-2. [DOI] [PubMed] [Google Scholar]
- 45.Kivipelto M, Helkala EL, Hänninen T, Laakso MP, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and late-life mild cognitive impairment: a population-based study. Neurology. 2001;56:1683–1689. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- 46.Yip AG, Brayne C, Matthews FE MRC Cognitive Function and Ageing Study. Risk factors for incident dementia in England and Wales: the Medical Research Council Cognitive Function and Ageing Study. A population-based nested case-control study. Age Ageing. 2006;35:154–160. doi: 10.1093/ageing/afj030. [DOI] [PubMed] [Google Scholar]
- 47.Woon FL, Dunn CB, Hopkins RO. Predicting cognitive sequelae in survivors of critical illness with cognitive screening tests. Am J Respir Crit Care Med. 2012;186:333–340. doi: 10.1164/rccm.201112-2261OC. [DOI] [PubMed] [Google Scholar]
- 48.Davydow DS, Hough CL, Levine DA, Langa KM, Iwashyna TJ.Functional disability, cognitive impairment, and depression after hospitalization for pneumonia Am J Med 2013126615–624.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]