Abstract
Carbapenem-resistant Enterobacteriaceae are increasingly reported worldwide and cause therapeutic problem in health care facilities. In this study 28 imipenem-resistant K. pneumoniae were examined for expression of carbapenemases by phenotypic and genotypic methods. Modified Hodge Test (MHT), CarbaNP test were used for phenotypic detection, and PCR using specific primers for the detection of blaOXA-48-, blaKPC-, blaNDM- and blaVIM-type carbapenemases with specific primers were performed. MHT and CarbaNP tests were positive for all of imipenem-resistant K. pneumoniae. The blaOXA-48 gene was detected in 27/28 isolates. One isolate was positive for the presence of the blaVIM-4 gene. According to our results NP test and MHT have high sensitivity and specificity for detection of those carbapenemases. This study reports the first cases of OXA-48-producing K. pneumoniae in Iran.
Keywords: carbapenem, Klebsiella pneumoniae, CarbaNP test, MHT, OXA-48
Zusammenfassung
Carbapenem-resistente Enterobacteriaceae sind weltweit ein zunehmendes therapeutisches Problem in Einrichtungen des Gesundheitswesens. In dieser epidemiologischen Untersuchung wurden 28 Imipenem-resistente K. pneumoniae-Stämme aus klinischem Material phenotypisch und genotypisch gegenüber ihrer Expression von Carbapenemasen untersucht. Carbapenemase-Bildung wurde phenotypisch mittels eines modifizierten Hodge-Tests (MHT) und eines CarbaNP-Tests und genotypisch mittels PCR unter Verwendung spezifischer Primer zur Detektion von Carbapenemase-Genen der Typen blaOXA-48, blaKPC, blaNDM and blaVIM analysiert. MHT- und CarbaNP-Tests waren bei allen untersuchten Imipenem-resistenten K. pneumoniae-Stämmen positiv. Das blaOXA-48-Gen wurde in 27/28 Isolaten nachgewiesen. Bei einem Isolat wurde das blaVIM-4-Gen nachgewiesen. Basierend auf dieser Untersuchung zeigten der NP-Test und der MHT die höchste Sensitivität und Spezifität zum Nachweis des Carbapenemase-Status. Diese Studie ist der erste Bericht über das Vorkommen von OXA-48-produzierenden K. pneumoniae-Stämmen im Iran.
Introduction
Carbapenems are often considered as a last therapeutic choice for the treatment of infections due to multidrug-resistant Gram-negative rods [1], [2], [3], [4], [5]. Emergence of carbapenem-resistant Enterobacteriaceae is increasingly reported worldwide and is becoming an important issue in health care systems [4], [6]. Resistance to carbapenems in K. pneumoniae is related to two main mechanisms: i) production of extended-spectrum β-lactamase (cephalosporinase or ESBL) associated with porin loss, and ii) production of carbapenem-hydrolyzing β-lactamase such as Ambler’s class A carbapenemases (KPC-type), class B metallo-β-lactamase (VIM-, NDM- or IMP-type) or class D carbapenemase OXA-48 [1], [4], [6], [7]. A number of phenotypical methods were used for detection of carbapenemases producer strains such as Modified Hodge Test (MHT) according to CLSI recommendations [8]. One new phenotypic method was described for detection of carbapenemases producer bacteria [1]. This test is currently utilized to identify the carbapenemase production in Gram-negative bacteria, with a low cost and high sensitivity and specificity, and was performed for rapid detection (≤2 h) of carbapenemases [1], [2]. CarbaNP test is a biochemical test, applicable to isolated bacterial colonies, and is based on in vitro hydrolysis of a carbapenem compound (imipenem). The OXA-48 carbapenemase was first reported from a Turkish isolates in 2004 [7] but recently the OXA-48-producing Enterobacteriaceae is repeatedly reported from different parts of the world however mostly from Mediterranean countries [9]. The blaOXA-48 has been identified within the Tn1999 composite transposon bracketed by two copies of IS1999 responsible for its mobility and expression [10], [11]. This carbapenemase gene is harbored by an IncL/M-type plasmid in which the Tn1999 was inserted [12]. This highly conjugative plasmid has been found responsible for the widespread of the blaOXA-48 genes [12].
The blaOXA-48 gene is spreading worldwide but not yet reported from Iran. In this study, we report the very first cases of OXA-48-producing K. pneumoniae identified in Iran.
Methods
Bacterial strains and susceptibility testings
In this study, a collection of 28 non-duplicated carbapenem-resistant K. pneumoniae isolated from 18 patients has been analyzed. All of these invasive strains were obtained from clinical specimens taken from patients hospitalized in a burn unit from Motahari Hospital, Teheran, Iran between February to August 2011. More than one K. pneumonia strain was isolated from 10 patients, but different antibiograms were observed in these isolates. On the other hand different specimens were collected from patients in different days after their hospitalization.
All of these 28 strains were isolated from infected burn wounds. Fifteen patients received meropenem as monotherapy whereas the three remaining patients received cefepime as monotherapy.
These strains were isolated from wounds of burn patients hospitalized in Motahari Hospital (referral center for burn patients in Tehran, Iran) from February to August 2011. These isolates were identified by using the API 20E system (bioMérieux, Marcy l’Etoile, France).
Antibiotic susceptibility testing
The antibiotic susceptibilities of the isolates were determined by the agar disc diffusion method on Mueller-Hinton agar with antibiotic discs MAST Company, UK and interpreted according to Clinical and Laboratory Standards Institute CLSI guidelines [8]. Tested antibiotics included; cefotaxime (30 µg), cefepime (30 µg), imipenem (10 µg), meropenem (10 µg), amoxicillin-clavulanic acid (20/10 µg), aztreonam (30 µg), tobramycin (10 µg), gentamicin (10 µg), amikacin (30 µg), trimethoprim-sulfamethoxazole (1.25/23.75 μg), chloramphenicol (30 µg) and tetracycline (30 µg). MIC of imipenem, meropenem and cefepime were determined by agar dilution method.
Phenotypic methods for detection of carbapenemase
Modified Hodge Test (MHT): This test was conducted according to the CLSI 2011 guidelines for search of carbapenemase production [8]. K. pneumoniae ATCC BAA-1705 were used as positive and K. pneumoniae ATCC BAA-1706 as negative controls. Strains with cloverleaf images of inhibition zone were considered as a carbapenemase-producing K. pneumoniae.
Use of boronic acid as a KPC inhibitor: Use of boronic acid (BA) as an inhibitor of KPC in combined-disk test with 400 µg/disc BA was carried out.
The stock solution of BA (benzene boronic acid; Sigma-Alderich, Germany) in dimethyl sulfoxcid and distilled water were mixed at a concentration of 20 mg/ml. From this solution 20 µl (containing 400 µg/disk) was added on to commercially meropenem disks.
The test was considered positive when the inhibition zone diameter around the disk containing meropenem and boronic acid was ≥5 mm compared with meropenem alone.
CarbaNP test (Carbapenemase Nordmann-Poirel test): One loop of strains was resuspended to Tris-HCL mmol/L as a lysis buffer from antibiogram plates, vortex for one minute and then incubated at room temperature for 30 minutes. Bacterial suspension was centrifuged at 10,000 xg at room temperature for 5 minutes. 30 µl of supernatant was mixed in 96 tray with 100 µl of imipenem monohydrate solusion (3 mg per ml) pH 7.8, phenolred solution and 0.1 mmol/L ZnSO4.
PCR amplification and sequencing
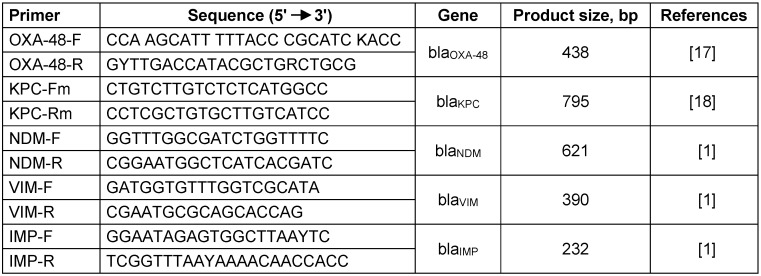
Rapid DNA extractions were prepared by boiling extraction. PCR experiments were performed using standard conditions and specific primers to search for carbapenemase genes that have been identified previously in K. pneumoniae i.e. the blaVIM, blaIMP, blaNDM, blaKPC and blaOXA-48 (Table 1 (Tab. 1)). Direct sequencing of PCR amplified products was carried out using ABI 3730X capillary sequencer (Genfanavaran, Macrogen, Seoul, Korea).
Table 1. Primers sequencing.
Results
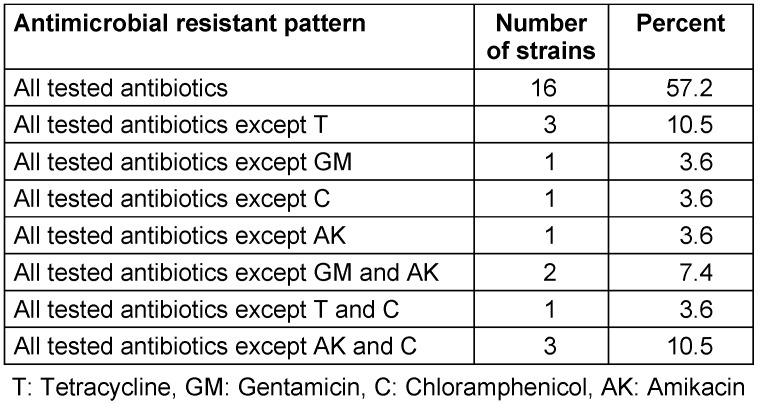
Among these 28 isolates, all were resistant to broad-spectrum cephalosporins, carbapenems (imipenem, ertapenem, meropenem), trimethoprim-sulfamethoxazole and quinolones (Table 2 (Tab. 2)). Some of them remained susceptible to amikacin and/or gentamicin, respectively (resistance rates for gentamicin at 90% and resistance rates to chloramphenicol, tetracycline and amikacin at 82%, 86% and 79%, respectively). Antimicrobial susceptibility testing results showed that all of the strains were resistant to imipenem, meropenem, and cefepime. Nine and eleven out of 28 isolates had MIC more than 64 µg/ml against imipenem and meropenem, respectively. Nineteen of 28 strains had MIC more than 128 µg/ml against cefepime.
Table 2. Antibiotic susceptibility patterns.
Eight different antimicrobial resistant patterns were observed among isolates likely indicating several clone are disseminating within the burn unit (Table 2 (Tab. 2)). The results show that MHT and CarbaNP tests were positive for all carbapenem-resistant K. pneumoniae but none of them showed at least 5 mm increase in diameter of inhibition zone around meropenem plus BA comparison with meropenem alone.
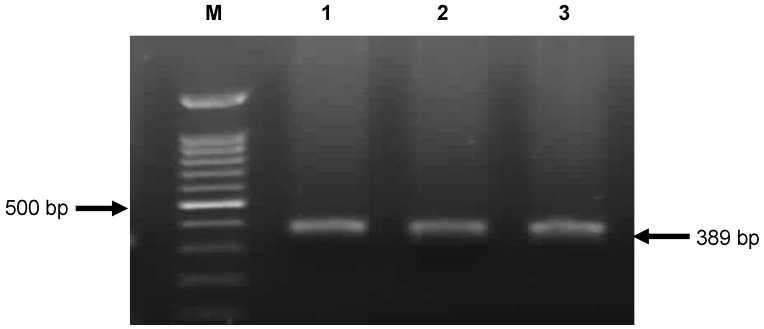
PCR analysis following by sequencing showed the presence of the blaOXA-48 gene in 27 isolates (Figure 1 (Fig. 1)).
Figure 1. PCR amplification fragments for detection of OXA-48 gene among Klebsiella pneumoniae isolates .
M: 1kb DNA size marker; lane 1: Positive control; lane 2 and 3: Positive strains.
Whereas a single isolate was positive for blaVIM-4 gene. No blaKPC, blaNDM or blaIMP genes were detected in this collection.
Discussion
Different K. pneumionieae with different antibiograms were isolated from one patient. Despite the K. pneumonia were isolated with same antibiogram among different patients. Isolates with same antibiotic-resistant pattern may derive from identical clone.
The OXA-48-type carbapenemases have been reported from France [4], Spain [6], Netherlands [13], Lebanon [4], Morocco [14] and Oman [15] and is becoming one of the main resistance mechanisms in K. pneumoniae [10]. The blaOXA-48 gene has been identified in 90.5% of K. pneumoniae isolates in six different Spanish hospitals [6]. To best of our knowledge, this study constitutes the very first report of OXA-48-producing K. pneumoniae from Iran. Detection of OXA-48-producing Enterobacteriaceae can be important, because such strains may often remain susceptible to third and fourth generation cephalosporins and monobactams and also this characteristic can make difficulty for detection of them [10]. After the recent identification of the blaKPC and the blaNDM-1 genes in a burn unit in Teheran, the identification of the blaOXA-48 carbapenemase gene is worrisome [5], [16].
Overall, this study identifies for the first time OXA-48-producing K. pneumoniae strains from Iran. Therefore, the spread of OXA-48 producers may be more widespread than expected and can be expected in any countries of the Middle East.
Notes
Competing interests
The authors declare that they have no competing interests.
Acknowledgment
This study was supported by a grant (M/T 91-04-134-20187) from Iran University of Medical Sciences, Tehran, Iran.
References
- 1.Nordmann P, Poirel L, Dortet L. Rapid detection of carbapenemase-producing Enterobacteriaceae. Emerging Infect Dis. 2012 Sep;18(9):1503–1507. doi: 10.3201/eid1809.120355. Available from: http://dx.doi.org/10.3201/eid1809.120355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordmann P, Poirel L. Strategies for identification of carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother. 2013 Mar;68(3):487–489. doi: 10.1093/jac/dks426. Available from: http://dx.doi.org/10.1093/jac/dks426. [DOI] [PubMed] [Google Scholar]
- 3.Navarro-San Francisco C, Mora-Rillo M, Romero-Gomez MP, Moreno-Ramos F, Rico-Nieto A, Ruiz-Carrascoso G, Gomez-Gil R, Arribas-Lopez JR, Mingorance J, Pano-Pardo J. Bacteraemia due to OXA-48-carbapenemase-producing Enterobacteriaceae: a major clinical challenge. Clin Microbiol Infect. 2013;19:E72–E79. doi: 10.1111/1469-069.12091. Available from: http://dx.doi.org/10.1111/1469-069.12091. [DOI] [PubMed] [Google Scholar]
- 4.Cuzon G, Ouanich J, Gondret R, Naas T, Nordmann P. Outbreak of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in France. Antimicrob Agents Chemother. 2011 May;55(5):2420–2423. doi: 10.1128/AAC.01452-10. Available from: http://dx.doi.org/10.1128/AAC.01452-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rastegar Lari A, Azimi L, Rahbar M, Fallah F, Alaghehbandan R. Phenotypic detection of Klebsiella pneumoniae carbapenemase among burns patients: first report from Iran. Burns. 2013 Feb;39(1):174–176. doi: 10.1016/j.burns.2012.02.025. Available from: http://dx.doi.org/10.1016/j.burns.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Oteo J, Hernández JM, Espasa M, Fleites A, Sáez D, Bautista V, Pérez-Vázquez M, Fernández-García MD, Delgado-Iribarren A, Sánchez-Romero I, García-Picazo L, Miguel MD, Solís S, Aznar E, Trujillo G, Mediavilla C, Fontanals D, Rojo S, Vindel A, Campos J. Emergence of OXA-48-producing Klebsiella pneumoniae and the novel carbapenemases OXA-244 and OXA-245 in Spain. J Antimicrob Chemother. 2013 Feb;68(2):317–321. doi: 10.1093/jac/dks383. Available from: http://dx.doi.org/10.1093/jac/dks383. [DOI] [PubMed] [Google Scholar]
- 7.Carrër A, Poirel L, Eraksoy H, Cagatay AA, Badur S, Nordmann P. Spread of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in Istanbul, Turkey. Antimicrob Agents Chemother. 2008 Aug;52(8):2950–2954. doi: 10.1128/AAC.01672-07. Available from: http://dx.doi.org/10.1128/AAC.01672-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing. Twenty-first informational supplement. M100-S21. Wayne, PA: Clinical and Laboratory Standards Institute; 2011. [Google Scholar]
- 9.Poirel L, Potron A, Nordmann P. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother. 2012 Jul;67(7):1597–1606. doi: 10.1093/jac/dks121. Available from: http://dx.doi.org/10.1093/jac/dks121. [DOI] [PubMed] [Google Scholar]
- 10.Poirel L, Héritier C, Tolün V, Nordmann P. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumonia. Antimicrob Agents Chemother. 2004;8:15–22. doi: 10.1128/AAC.48.1.15-22.2004. Available from: http://dx.doi.org/10.1128/AAC.48.1.15-22.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aubert D, Naas T, Héritier C, Poirel L, Nordmann P. Functional characterization of IS1999, an IS4 family element involved in mobilization and expression of beta-lactam resistance genes. J Bacteriol. 2006 Sep;188(18):6506–6514. doi: 10.1128/JB.00375-06. Available from: http://dx.doi.org/10.1128/JB.00375-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poirel L, Bonnin RA, Nordmann P. Genetic features of the widespread plasmid coding for the carbapenemase OXA-48. Antimicrob Agents Chemother. 2012 Jan;56(1):559–562. doi: 10.1128/AAC.05289-11. Available from: http://dx.doi.org/10.1128/AAC.05289-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potron A, Kalpoe J, Poirel L, Nordmann P. European dissemination of a single OXA-48-producing Klebsiella pneumoniae clone. Clin Microbiol Infect. 2011 Dec;17(12):E24–E26. doi: 10.1111/j.1469-0691.2011.03669.x. Available from: http://dx.doi.org/10.1111/j.1469-0691.2011.03669.x. [DOI] [PubMed] [Google Scholar]
- 14.Hays C, Benouda A, Poirel L, Elouennass M, Nordmann O. Nosocomial occurrence of OXA-48-producing enterobacterial isolates in a Moroccan hospital. Int J Antimicrob Agents. 2012;39:545–547. doi: 10.1016/j.ijantimicag.2012.03.002. Available from: http://dx.doi.org/10.1016/j.ijantimicag.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Dortet L, Poirel L, Al Yaqoubi F, Nordmann P. NDM-1, OXA-48 and OXA-181 carbapenemase-producing Enterobacteriaceae in Sultanate of Oman. Clin Microbiol Infect. 2012 May;18(5):E144–E148. doi: 10.1111/j.1469-0691.2012.03796.x. Available from: http://dx.doi.org/10.1111/j.1469-0691.2012.03796.x. [DOI] [PubMed] [Google Scholar]
- 16.Shahcheraghi F, Nobari S, Rahmati Ghezelgeh F, Nasiri S, Owlia P, Nikbin VS, Imani Fooladi AA. First report of New Delhi metallo-beta-lactamase-1-producing Klebsiella pneumoniae in Iran. Microb Drug Resist. 2013 Feb;19(1):30–36. doi: 10.1089/mdr.2012.0078. Available from: http://dx.doi.org/10.1089/mdr.2012.0078. [DOI] [PubMed] [Google Scholar]
- 17.Voets GM, Fluit AC, Scharringa J, Cohen Stuart J, Leverstein-van Hall MA. A set of multiplex PCRs for genotypic detection of extended-spectrum β-lactamases, carbapenemases, plasmid-mediated AmpC β-lactamases and OXA β-lactamases. Int J Antimicrob Agents. 2011 Apr;37(4):356–359. doi: 10.1016/j.ijantimicag.2011.01.005. Available from: http://dx.doi.org/10.1016/j.ijantimicag.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Naas T, Cuzon G, Villegas MV, Lartigue MF, Quinn JP, Nordmann P. Genetic structures at the origin of acquisition of the beta-lactamase bla KPC gene. Antimicrob Agents Chemother. 2008 Apr;52(4):1257–1263. doi: 10.1128/AAC.01451-07. Available from: http://dx.doi.org/10.1128/AAC.01451-07. [DOI] [PMC free article] [PubMed] [Google Scholar]