Abstract
The androgen receptor (AR) is expressed ubiquitously and plays a variety of roles in a vast number of physiological and pathophysiological processes. Recent studies of AR knockout (ARKO) mouse models, particularly the cell type- or tissue-specific ARKO models, have uncovered many AR cell type- or tissue-specific pathophysiological roles in mice, which otherwise would not be delineated from conventional castration and androgen insensitivity syndrome studies. Thus, the AR in various specific cell types plays pivotal roles in production and maturation of immune cells, bone mineralization, and muscle growth. In metabolism, the ARs in brain, particularly in the hypothalamus, and the liver appear to participate in regulation of insulin sensitivity and glucose homeostasis. The AR also plays key roles in cutaneous wound healing and cardiovascular diseases, including atherosclerosis and abdominal aortic aneurysm. This article will discuss the results obtained from the total, cell type-, or tissue-specific ARKO models. The understanding of AR cell type- or tissue-specific physiological and pathophysiological roles using these in vivo mouse models will provide useful information in uncovering AR roles in humans and eventually help us to develop better therapies via targeting the AR or its downstream signaling molecules to combat androgen/AR-related diseases.
Introduction
Since the testicular male sex hormone (later named testosterone) was discovered in the late 1920s, its structure has been revealed, purified, crystalized and synthesized successfully [Freeman et al., 2001]. Testosterone can be converted to the more potent metabolite, 5α-dihydrotestosterone (DHT) [Shimazaki et al., 1965a; Shimazaki et al., 1965b; Bruchovsky and Wilson, 1968a; Bruchovsky and Wilson, 1968b], and further studies led to the conclusion that testosterone/DHT might exert their functions via binding to the androgen receptor (AR) [Anderson and Liao, 1968; Bruchovsky and Wilson, 1968a; Bruchovsky and Wilson, 1968b]. The cloning of the AR in 1988 [Chang et al., 1988a; Chang et al., 1988b; Lubahn et al., 1988; Tilley et al., 1989; Trapman et al., 1988] revealed it belongs to the nuclear receptor superfamily. Binding of testosterone/DHT to the AR leads to a conformational change of the receptor and translocation of the androgen/AR complex from the cytosol to the nucleus to elicit transcriptional regulation of target genes, which can be further modulated by various AR coregulators [Heemers and Tindall, 2007; Heinlein and Chang, 2002a; Yeh and Chang, 1996]. Importantly, androgen/AR signaling can also function through interaction with other proteins to exert its non-genomic functions [Gonzalez-Montelongo et al., 2010; Heinlein and Chang, 2002b].
Studies of prostate cancer (PCa) patients with anti-androgen therapy and androgen ablation therapy with surgical/chemical castration in past decades have revealed that androgen/AR signaling mediates many physiological and pathophysiological processes in various tissues/organs. The discovery of X-linked testicular feminization (Tfm) mutation in mice [Lyon and Hawkes, 1970], which exibit insensitivity to androgens [Ohno et al., 1971] has provided an excellent model for studying human complete androgen insensitivity syndrome (CAIS). Both the Tfm mice and CAIS showed inactivation of AR and displayed female genital phenotypes with cryptic testis, and lack of male external genitalia and accessory sex organs. Despite differences in circulating levels of testosterone and estradiol (E2), many physiological and pathophysiological roles of AR in various specific tissues or cell types could not be addressed from the studies with Tfm mice. The successful generation of the first cell type- or tissue-specific AR knockout (ARKO) mouse model [Yeh et al., 2002] was thought to be the solution. Studies of these specific ARKO models would not only lead us to confirm the early in vitro studies, but also allow us to study in vivo pathophysiological roles of AR in specific cell types or tissues, which in turn, may help us to develop better therapies via targeting unique AR roles in selective cell types or tissues in the future.
This review summarizes physiological and pathophysiological roles of AR obtained from the various ARKO and a few AR knock-in (ARKI) mouse models (Supplementary File 1). The possible impacts of these in vivo studies on various androgen/AR-related diseases will also be discussed.
Generation of ARKO mouse models
The success of conditional gene knockout using Cre-loxP recombination strategy depends heavily on the choice of the promoter to drive Cre recombinase expression and proper design of the floxed target gene with appropriate insertion of loxP sites in the introns of the target gene. Several strategies to generate total ARKO mice are discussed below.
The first ARKO mouse was developed by applying human β-actin-Cre (ACTB-Cre) to knock out the AR in all tissues using an exon 2-floxed AR [Yeh et al., 2002]. The floxed AR transgene was formulated by inserting loxP sites near the end of intron 1 and the beginning of intron 2, so that the wild type (Wt) AR protein will be expressed in floxed AR mice without altering mRNA splicing. The floxed AR mice have not displayed any overt phenotype or abnormal hormonal profiles compared to Wt littermates in many studies. After Cre-mediated deletion of exon 2, exon 1 will be linked to exon 3 and create an mRNA sequence coding for several unrelated amino acids before reaching two premature stop codons at new amino acid positions 533 and 534 in exon 3. Such a mRNA structure was unstable and subject to nonsense-mediated RNA decay [Isken and Maquat, 2007]. Similar AR mRNA instability in Tfm mice has been reported [Charest et al., 1991]. Due to ubiquitous expression of actin in all cell types [Lewandoski et al., 1997], the ARKO mice generated with ACTB-Cre would lead to a total AR knockout in the whole body and the phenotype manifestations observed in these mice would be due to complete loss of the AR in all tissues. This mouse model will be referred to as ARKO(ACTB-Cre).
The second ARKO mouse model developed by Sato et al used the Cytomegalovirus promoter-Cre (CMV-Cre) and an exon 1-floxed AR [Sato et al., 2004]. The design of the exon 1-floxed AR also did not alter the AR mRNA splicing and allowed for expression of Wt AR protein. The exon l-floxed AR mice showed no overt phenotype compared to Wt mice. Similarly, Murashima et al employed a CMV enhancer/chicken β-actin promoter-driven Cre (CAG-Cre) mouse [Sakai and Miyazaki, 1997] to cross with the AR exon 1-floxed mice [Murashima et al., 2011]. These CMV and CAG enhancer-driven Cre-generated ARKO mice are referred to as ARKO(CMV-Cre) mice and ARKO(CAG-Cre) mice, respectively. Complete loss of AR functions was observed in all tissues with CMV-Cre or CAG-Cre expression in these mouse models.
De Gendt et al used phosphoglycerate kinase-Cre (PGK-Cre) mouse, in which the Cre activity is driven by an early acting PGK-1 promoter to mediate conditional gene knockout in all organs [Lallemand et al., 1998], and crossed with exon 2-floxed AR mouse to generate total ARKO mouse [De Gendt et al., 2004]. This mouse model is referred to as ARKO(PGK-Cre).
Notini et al employed a CMV-Cre mouse line [Notini et al., 2005], in which the Cre recombinase is driven by a minimal CMV enhancer-promoter [Schwenk et al., 1995], to cross with an exon 3-floxed AR mouse designed to excise AR exon 3 encoding the 2nd zinc finger of AR-DBD domain, but deletion of exon 3 will result in an in-frame translation of the remaining AR protein. Such knockout mice still express a mutant AR with almost the entire AR protein except missing the 2nd zinc finger in all tissues. As discussed later, this truncated AR-zf2 protein may interact with various AR coregulators and/or other nuclear receptors or transcription factors [Heemers and Tindall, 2007; Heinlein and Chang, 2002]. Therefore, the phenotypes displayed by this mouse model might not be similar to those found in other ARKO mice [Notini et al., 2005] . This mouse model is referred to as AR-EX3(CMV-Cre).
Holdcraft and Braun developed other ARKO mouse models using synaptonemal complex protein 1 promoter driven Cre (Sycp1-Cre) or adenovirus EIIa promoter driven Cre (EIIa-Cre) (both are capable of global recombination) [Holdcraft and Braun, 2004]. They used an AR exon 1-floxed mouse in which the transgene was constructed by inserting the loxP sites around AR exon 1 in a way that would result in inverted exon 1 transcription upon recombination, leading to the expression of a stably inverted AR exon 1-neomycin fusion mRNA with reading frame shift at the AR-neomycin junction for translation of only the AR N-terminal fragment [Holdcraft and Braun, 2004]. These ARKO(Sycp1-Cre) and ARKO(EIIa-Cre) mice exhibited Tfm phenotypes, but characteristics other than this phenotype difference have not been further studied.
Several important aspects pertaining to the strategies of generating various ARKO mice need to be addressed. First, specificity and strength of the Cre transgene need to be determined for proper interpretation of the different results obtained from various global ARKO or cell type-specific models. For example, it has been shown that the CMV enhancer-promoter could at best drive gene expression only in 24 out of 28 tissues examined, failing in liver, lung, muscle, and pancreas [Schmidt et al., 1990], a property contended by Su et al for their CMV-Cre mice. CMV enhancer-promoter [Su et al., 2002] also had various efficiencies, being relatively weaker in the uterus, ovary, and breast than in the testis, heart, and brain [Schmidt et al., 1990]. The CMV-Cre mice [Notini et al., 2005] were claimed to have effective ARKO in all organs [Schwenk et al., 1995] and another CMV-Cre line capable of mediating low levels of Cre expression in the liver, lung, and muscle has also been reported [Metzger and Chambon, 2001], suggesting that different CMV-Cre constructs might have different Cre expression. On the other hand, ACTB-Cre has been shown to mediate ubiquitous embryonic recombination of Rosa26 reporter transgene [Lewandoski et al., 1997], and PGK-Cre, Sycp1-Cre, and EIIa-Cre were capable of global gene knockout. These contrasting results suggest that each Cre transgene may have different excision strengths in different tissues/organs or cell types. As many studies reviewed here failed to determine the extent of AR knockout in the particular tissues/organs or cell types, it will not be easy to precisely describe the different phenotype manifestations among various global ARKO mouse models in the context of knockout efficiency. It should be noted that an ideal complete specific gene knockout may not be achieved in all specific ARKO models. However, the observation of a specific, but partial ARKO with phenotypes significantly different from the Wt, should allow the conclusion that the AR plays a significant role in the specific cell type to affect a phenotype manifestation.
Second, it should be noted that males in all of the above-mentioned ARKO mouse models exhibited female-like external genitalia, small undescended testes, and lack of male external genitalia and auxiliary sex organs as seen in Tfm mice and human males with CAIS due to AR knockout. It was noted that male general ARKO mice [Notini et al., 2005; Sato et al., 2004; Simanainen et al., 2012] and Tfm mice exhibited very low circulating testosterone levels [Murphy and O'Shaughnessy, 1991] as opposed to human CAIS patients known to display normal male testosterone and elevated estrogen levels. Together, the common feature of abnormal sex development seen in these mice and CAIS patients appears to be due to the loss of AR activity despite their different hormonal profiles. Moreover, since AR-EX3(CMV-Cre) [Notini et al., 2005] and AR-EX3(Sox2-Cre) [Simanainen et al., 2012] mice had the AR genomic function ablated, it appears that AR genomic function is essential for normal development of male sex organs as concluded earlier [Notini et al., 2005]. It should be pointed out that increasing numbers of human patients with Tfm phenotype, without involvement of any mutation in the AR gene, have been reported [Gottlieb et al., 2012]. It is not clear at present whether the Tfm phenotype in these patients is caused by mutations in other genes, such as AR coregulators, which result in complete inactivation of the AR. Therefore, presentation of the Tfm phenotype does not necessarily indicate the presence of AR-inactivating mutations. Similarly, incomplete AR knockout in tissues and organs may not prevent manifestation of Tfm phenotype. Therefore, presentation of Tfm phenotype alone may not be simply regarded as an indication of complete ARKO in the model.
Third, the exon 1-floxed AR transgene of Holdcraft and Braun could be a hypomorphic AR allele [Holdcraft and Braun, 2004], due to the presence of the PGK-neo cassette [Meyers et al., 1998]. Their floxed AR male mouse already exhibited small testes and seminal vesicles, abnormally high levels of testosterone, global reduction in AR levels, and substantial decrease in epididymal sperm counts compared to Wt mice [Holdcraft and Braun, 2004] before crossing with a Cre mouse to knock out the AR in all types of cells. With an undesired background AR ablation, this exon 1-floxed AR mouse may not be an ideal precursor or control for generation of specific ARKO models for studying AR functions.
In addition, the male exon 3-floxed AR mice of Notini et al exhibited unexplained increases in the mass of kidney, seminal vesicle, lavator ani muscle, and heart compared to Wt littermates [Notini et al., 2005]. The abnormality of the exon 3-floxed AR mice may also affect the phenotype manifestations of various ARKO mice subsequently generated using these mice.
Fourth, the mode of AR gene deletion and inactivation is also an important variable affecting the interpretation of the phenotype displayed in a particular ARKO mouse model. The exon 3-floxed AR transgene of Notini et al was designed to produce AR-zf2 protein in the knockout mice [Notini et al., 2005] to mimic a human AR-zf2 mutant found in specific CAIS cases [Quigley et al., 1992]. However, this AR-zf2 protein contains all the AR amino acid residues except for the 2nd zinc finger, and may still be able to interact with other proteins to exert its unusual or non-genomic functions. For example, it has been shown that interaction of AR with estrogen receptor α (ERα) resulted in inhibition of AR and ERα transactivation [Panet-Raymond et al., 2000], and the C619Y AR mutant, which lacks DNA binding, but retains androgen binding activity, was capable of sequestering the SRC1 coactivator [Nazareth et al., 1999]. Importantly, AR could also repress gene activation by AP1/RelA through sequestration of CREB binding protein [Aarnisalo et al., 1998; Fronsdal et al., 1998]. The AR-zf2 protein of the AR-EX3(CMV-Cre) mice [Lim et al., 2009] and some human AR mutants that are incapable of binding to androgen-responsive elements on target genes [Quigley et al., 1992] still can translocate to cell nuclei in a ligand-dependent fashion and modulate gene expression [Lobaccaro et al., 1996] and cell proliferation [Gonit et al., 2011]. Therefore, it is possible that the AR-zf2 protein may retain the capability of many AR non-genomic functions, even though these AR non-genomic actions may not occur physiologically in all cell types. Thus, the difference in phenotypes between Wt and AR-EX3(CMV-Cre) mice may not be attributed solely to the loss of AR genomic function, and can be, in part, due to a gain of non-genomic function through the expression of AR-zf2 protein. In contrast, the phenotype changes observed in other ARKO mouse models [De Gendt et al., 2004; Yeh et al., 2002] can be attributed to the complete loss of the AR protein and functions, although the relative contribution of AR genomic versus non-genomic function can not be immediately deduced from these ARKO mouse models.
Finally, the purpose for generating ARKO mice, particularly the cell type- or tissue-specific ARKO models, is to study the physiological and pathophysiological roles of the AR in specific cells, tissues, or organs rather than to compare the abnormal sex development and associated clinical treatments with human CAIS patients. Many AR physiological and pathophysiological roles studied with various ARKO mouse models discussed in this paper can not be studied or observed in CAIS patients. The CAIS in human patients involves a large number of AR-inactivating mutations including various degrees of deletion, nonsense mutations, and point mutations resulting in the expression of different forms of AR mutant proteins including the N-terminal only (equivalent to ARKO), partial loss of DBD or LBD segment, or the entire AR protein with inactivating amino acid substitutions with some of them retaining AR non-genomic functions. In addition, CAIS patients are generally subjected to hormone treatments with gonadectomy and estrogen supplementation, which have not been applied to ARKO mice. Due to the above reasons, phenotypes observed in ARKO mouse models can not always be comparable with CAIS patients. On the other hand, species differences between human and mouse need to be considered. For instance, global ARKO mouse models and Tfm mice displayed very low circulating testosterone and normal male estrogen levels, whereas CAIS patients have normal male testosterone levels yet high estrogen levels, suggesting that the two species may have some differences in the regulatory mechanism for secretion of sex hormones.
AR roles in the immune system
Both humans and experimental animals display sexual dimorphism in immune responses. Compared to males, females tend to have stronger cell-mediated immune response with higher antibody production upon antigen challenge [Lahita, 1997] and higher propensity to develop autoimmune diseases [Whitacre et al., 1999]. In contrast, the male is at a higher risk than the female of developing sepsis, as well as acute respiratory and multi-organ failure following soft tissue trauma hemorrhagic shock and thermal injury [Deitch, 1992; Moore et al., 1996]. They proposed that the development and maintenance of sexual dimorphism in immune response is the result of neuroendocrine-immune system interaction, in which sex hormones play pivotal roles [Moore et al., 1996]. For example, an early study by Graff et al indicated that female and castrated male mice developed earlier rejection of skin allografts than intact male mice [Graff et al., 1969], suggesting that female and male sex hormones have different immunomodulatory effects and androgens might play an immunosuppressive role. Also, androgens were proposed to modulate cell-mediated immune response through Th1 and Th2 T helper cell responses to influence the development of autoimmune response [Whitacre et al., 1999].
Although early studies with castration of the male experimental models have provided some insights into how androgen/AR signaling might modulate the immune response, the availability of ARKO mice has provided better experimental models for studying the effect of complete loss of androgen/AR signaling in the immune system. For example, recent studies with ARKO mice have indicated that androgen/AR signaling regulates the development of several lineages of immune cells. The immune response differences in T-ARKO or Tfm mice may not be comparable to the CAIS patients since most CAIS patients have orchidectomy after puberty and have supplemented E2 to maintain a female phenotype, and thus have a female-like immune response, but the T-ARKO or Tfm mice have low testosterone and normal low male E2 levels. Indeed, men with CAIS having near normal testosterone levels and high E2 levels also showed different immune responses. The AR role in immune system is summarized in Figure 1.
Figure 1. The summary of androgen-AR roles in the development of 4 immune cell types including neutrophils, macrophages, T lymphocytes, and B lymphocytes.
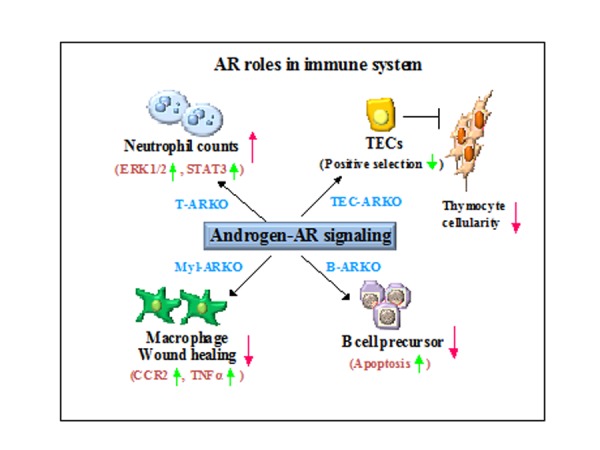
This cartoon describes the roles of androgen/AR signaling during the development of these immune cell types as deduced from phenotypic observations of the ARKO mice indicated in the figure. The potential AR downstream regulatory molecules or mechanisms in these 4 immune cell types are also illustrated.
AR roles in neutrophil development and acute infection
Mantalaris et al reported that in human the AR is broadly expressed in neutrophil-lineage cells from the myeloblast stage to the mature neutrophil stage with no difference between male and female [Mantalaris et al., 2001]. It was found that androgen stimulated proliferation of committed erythrocytic and granulocytic precursors in vitro [Byron, 1970; Udupa and Reissmann, 1974], and accelerated recovery of leukocytes after radiation therapy or chemotherapy for cancer in rats [Horn, 1971; Horn and Price, 1972]. Clinical observations with androgen treatments of aplastic anemia [Sanchez-Medal, 1971; Sanchez-Medal et al., 1964], Fanconi’s anemia [Alter, 1992], and during therapy with myelosuppressive agents [Kennedy, 1965; Rawbone and Bagshawe, 1972], suggest that androgen might increase neutrophil production. The polycystic ovary syndrome (PCOS) patients with high serum androgen levels exhibited higher neutrophil counts [Ibanez et al., 2005]. These observations suggest that androgen/AR signaling might be involved in granulopoiesis. However, it is still unclear how androgen/AR regulates neutrophil homeostasis.
A recent study by Chuang et al using male ARKO(ACTB-Cre) and Wt mice [Chuang et al., 2009] have provided an understanding of the AR roles in neutrophil production and function. ARKO(ACTB-Cre) mice were found to have lower neutrophil counts in the blood and bone marrow, and were more susceptible to acute bacterial infection. Cell sorting analyses of bone marrow cells indicated that the levels of granulocyte/macrophage myeloid progenitors (GMP) and common myeloid progenitors (CMP) were similar in ARKO(ACTB-Cre) mice compared to Wt mice, suggesting similar neutrophil lineage commitments between the two genotypes. However, a decrease in myelocytes/metamyelocytes and a marked decrease in mature neutrophils were found in bone marrow cells of ARKO(ACTB-Cre) mice compared to those of Wt mice. These results suggest that the defect in granulopoiesis of ARKO(ACTB-Cre) mice occurs during the transition between proliferation of precursors (myeloblasts, promyelocytes, and myelocytes) and maturation of neutrophils (metamyelocytes, band forms, and neutrophils), leading to a difference in terminal differentiation of neutrophils. The GMPs isolated from ARKO(ACTB-Cre) mice produced few neutrophils after granulocyte colony-stimulating factor (G-CSF) stimulation, but acquired the capability after retroviral transfection with a functional AR gene, whereas the GMPs from Wt mice significantly reduced neutrophil production after knockdown of the AR by transfection with AR-siRNA [Chuang et al., 2009]. These in vitro results suggest a pivotal role of AR in neutrophil differentiation.
Further analyses have indicated that bone marrow granulocytes from ARKO(ACTB-Cre) mice had lower mitotic cell division rate and were more susceptible to apoptosis compared to those from Wt mice. Furthermore, neutrophil production in response to G-CSF injection was found decreased in ARKO(ACTB-Cre) mice compared to Wt mice. The mRNA levels of several G-CSF downstream genes were also found decreased in ARKO(ACTB-Cre) mice compared to those of Wt mice without affecting the G-CSF receptor level. Bone marrow granulocytes of Wt mice treated with AR-siRNA had decreased ERK1/2 activation and cell proliferation in response to G-CSF, whereas retroviral expression of the AR in ARKO(ACTB-Cre) granulocytes promoted ERK1/2 activation and cell proliferation [Chuang et al., 2009]. Further in vitro studies indicated that ablation of the AR in granulocytes resulted in impairment of G-CSF signaling to influence neutrophil precursor proliferation and differentiation.
The loss of the AR in neutrophils also influenced their functional development [Del Rio et al., 2001]. Neutrophils from ARKO(ACTB-Cre) mice remained capable of phagocytosis and oxidative burst, but showed reduced production of chemokines and cytokines. Moreover, impaired chemokine receptor CXCR2-mediated neutrophil migration, but not neutrophil degranulation, was observed in ARKO(ACTB-Cre) neutrophils compared to Wt mice [Chuang et al., 2009]. However, there is no evidence that CAIS patients have impaired neutrophil function.
Interestingly, the AR in neutrophils seemed to play an important role in the innate immune system to defend against microbial infection based on the observation that ARKO(ACTB-Cre) mice showed increased death rates compared to Wt mice upon pathogenic bacterial and septic challenges [Chuang et al., 2009]. The ARKO(ACTB-Cre) mouse model could be a useful mouse model for future studies of neutrophil development in humans.
AR physiological roles in monocyte/macrophage production and inflammation
The early myeloid progenitors of the bone marrow give rise to monocyte/macrophage-restricted progeny under the influence of GM-CSF and macrophage colony-stimulating factor (M-CSF), and eventually develop into monocytes, which are then transported to the circulation. Upon stimulation, blood monocytes migrate to peripheral tissues, particularly the inflammatory sites, and differentiate into tissue macrophages [Takahashi, 2001].
Analysis of the peripheral blood mononuclear cells (PBMCs) found the monocyte population (CD11b+F4/80+), especially in the inflammatory monocyte population (CD11b+F4/80+Gr1+), was reduced in male ARKO(ACTB-Cre) mice compared to Wt mice. In contrast, the resident monocyte subset (CD11b+F4/80+Gr1-) in PBMC was similar between Wt and ARKO(ACTB-Cre) mice, suggesting that the AR might specifically increase the inflammatory monocyte pool in the peripheral blood. The mRNA expression of CCR2, a key chemokine receptor, was decreased in the bone marrow-derived monocytes/macrophages of ARKO(ACTB-Cre) mice compared to Wt mice, and the AR, in the presence of DHT, could induce CCR2 expression at the transcriptional level. In addition, the expression levels of TNFα in bone marrow-derived macrophages from ARKO(ACTB-Cre) and myeloid-specific ARKO (Myl-ARKO) mice were lower than those from Wt mice and the AR was able to induce transcription of TNFα [Lai et al., 2009]. These observations indicate that AR is involved in modulation of inflammatory monocyte/macrophage production, migration, and function. The mechanism through which the AR modulates these processes remains to be elucidated.
The reduction in inflammatory monocyte production in ARKO(ACTB-Cre) and Myl-ARKO mice had a profound influence on cutaneous wound healing due to the reduced macrophage infiltration at the wound site. The reduced macrophage infiltration in Myl-ARKO mice also resulted in lower incidence of inflammation-associated atherosclerosis (to be discussed in the subsection titled “AR roles in cardiovascular function”).
AR physiological roles in B lymphopoiesis and autoimmune diseases
Early studies have indicated that androgen/AR signaling might modulate the production of B lymphocytes and influence autoimmunity. For instance, Viselli et al observed that in castrated male mice there were enlarged spleens with expansion of B cells in the spleen and bone marrow compared to intact mice [Viselli et al., 1997]. Androgen replacement resulted in reversal of B cell expansion. The AR-inactivated Tfm mice also exhibited B cell expansion in the bone marrow and spleen [Smithson et al., 1998]. Further analysis of bone marrow B lineage cells have indicated that castration and inactivation of the AR resulted in increased pro-B, pre-B, and immature B cells in the bone marrow, suggesting that androgen/AR signaling modulates B lymphopoiesis [Ellis et al., 2001]. Cultured splenocytes from the castrated mice also produced higher levels of autoantibodies in response to pokeweed mitogen stimulation [Viselli et al., 1995b], suggesting that androgen/AR signaling might also influence autoimmunity.
To determine the role of the AR in B cell homeostasis, Altuwaijri et al compared the B cell population in the bone marrow and spleens of male ARKO(ACTB-Cre), B cell-specific ARKO (Bsp-ARKO), Tfm, castrated, and intact Wt mice and observed that all four models of AR functional ablation displayed increased population of B cells in the bone marrow and the spleen [Altuwaijri et al., 2009]. Flow cytometric analysis indicated that bone marrow cells from ARKO(ACTB-Cre) and Bsp-ARKO mice had a statistically higher proportion of pre-B cells and a lower proportion of pro-B cells than Wt bone marrow cells. The changes of B precursor cells appeared to be associated with decreased apoptosis of B cells and increased proliferation of B precursor cells in ARKO(ACTB-Cre) and Bsp-ARKO mice than Wt mice. The resistance of AR-deficient B cells to apoptosis was accompanied by altered levels of several modulators of apoptosis. Since apoptosis plays a crucial role during B cell development and tolerance [Hayakawa et al., 1999; Montes et al., 2006], the increase in apoptosis in AR-deficient B cells would be likely to affect B cells’ specificity and autoimmunity. Both ARKO(ACTB-Cre) and Bsp-ARKO mice were found to contain higher levels of serum IgG2a, IgG3 and basal anti-double-stranded DNA IgG antibodies, and were more susceptible than Wt mice [Altuwaijri et al., 2009] to collagen-induced arthritis [Hayakawa et al., 1999], a disease regulated by IL-10-producing B cells [Klinker and Lundy, 2012]. Thus, the AR in bone marrow B lineage cells appears to modulate B cell development and tolerance.
Since the greater extent of alteration in B cell development was observed in ARKO(ACTB-Cre) than in Bsp-ARKO mice, we may speculate that the AR in bone marrow stromal cells may also contribute to B cell development. This notion is consistent with the conclusion of Olsen et al, derived from their reciprocal bone marrow transplantation studies using bone marrow cells from Tfm and Wt mice [Olsen et al., 2001a], that the stromal AR plays an important role in B cell development. However, the detailed mechanism of this regulation by AR remains to be elucidated.
AR physiological roles in thymocyte development and bone marrow transplantation
T lineage-committed hematopoietic progenitor cells formed in bone marrow migrate to the thymus where they differentiate into mature thymocytes and emigrate from the organ and home to various lymphoid tissues so that mature T lymphocytes can trigger adaptive immune response. It has been known for decades that orchidectomy in adult male animals resulted in thymic enlargement or rejuvenation, which is reversed by androgen replacement [Hince et al., 2008], suggesting that androgen/AR signaling is involved in regulating thymic function. Similar thymic enlargements were observed in Tfm [Olsen et al., 2001b] or ARKO(ACTB-Cre) [Fan et al., 2005] male mice. Early studies have indicated that AR is expressed in thymic epithelial reticular cells, thymocytes, and other stromal cells [Kovacs and Olsen, 1987; Olsen and Kovacs, 2001; Viselli et al., 1995a]. Using bone marrow cells from Wt and Tfm mice in a reciprocal bone marrow transplantation study on thymocyte development, Olsen et al concluded that the AR in thymic epithelial cells (TECs) is an important modulator of thymocyte development [Olsen et al., 2001b]. However, in which thymic stromal cells the AR is involved in modulating thymocyte development and its mechanism of function are not clear at present, but the studies of various ARKO mouse models by Lai et al have provided further insight into AR roles in thymocyte development [Lai et al., 2013].
Reciprocal bone marrow transplantation studies indicated thymic epithelial AR modulates T cell population
The total thymocyte numbers in ARKO(ACTB-Cre) mice were found increased compared to Wt mice [Lai et al., 2013]. Cell population analyses indicated that the relative CD4-CD8- double negative (DNs), CD4+CD8+ double positive (DPs), and CD4+ or CD8+ single positive (SPs) thymocyte populations in Wt and ARKO(ACTB-Cre) mice were comparable. These results suggested that knockout of the AR in the whole body resulted in thymus enlargement and increased thymocyte cellularity.
To dissect AR roles in different cellular compartments in thymus, reciprocal transplantation of bone marrow cells from Wt and ARKO(ACTB-Cre) mice into γ-irradiated ARKO(ACTB-Cre) and Wt recipient mice were conducted. The results revealed that transplantation of bone marrow cells from either Wt or ARKO(ACTB-Cre) mice into irradiated ARKO(ACTB-Cre) recipients resulted in enlarged thymus as compared to the thymus of Wt recipients of the same bone marrow cells. Conversely, Wt or ARKO(ACTB-Cre) mice bone marrow cells transplanted into Wt recipient mice did not lead to thymic enlargement [Lai et al., 2013]. These results were in agreement with the observation of Olson et al that the AR in thymic residential stromal cells, but not in the hematopoietic-derived cells, determines T cell repopulation in the thymus [Olsen et al., 2001b].
To further define AR expression in which thymus residential stromal cells have more prominent roles in T cell repopulation, several cell type-specific ARKO mouse models were generated by crossing the exon 2-floxed AR mice with various cell type-specific Cre mice, including lck-Cre [Hennet et al., 1995] for T cell-specific ARKO (Tsp-ARKO) mice, bovine CK5-Cre [Berton et al., 2003] for TEC-specific ARKO (TEC-ARKO) mice, and fibroblast-specific protein 1 promoter-driven Cre (fsp1-Cre) [Bhowmick et al., 2004] for Fsp-ARKO mice. Among these three cell-specific ARKO mice, only TEC-ARKO mice had increased total thymocyte numbers over Wt mice, indicating that loss of thymic epithelial AR was critical to mediate thymus enlargement by increasing total thymocytes.
Mechanistically, only ARKO(ACTB-Cre) and TEC-ARKO mice exhibited less apoptotic thymocytes as compared to their Wt littermates [Lai et al., 2013]. Further studies of TEC-ARKO mice in the context of thymocyte positive and negative selections indicated that cortical TEC AR regulated positive selection to enhance thymocyte survival without disturbing negative selection after breeding with AND-TCR tg and HY-TCR tg mice [Lai et al., 2013].
Together, these results indicated that the AR loss in TECs led to thymic enlargement and to increase of thymocytes through decrease in cell apoptosis without alteration in thymocyte differentiation.
Ablation of the AR in TECs improves grafting efficiency of bone marrow transplantation
Since ablation of the AR increases T cell cellularity, it was postulated that bone marrow cells transplanted in TEC-ARKO mice would improve the grafting efficiency, especially in T cell reconstitution. To mimic human autologous bone marrow transplantation, bone marrow transplantation between two congenic mice, FVB/C57BL/6 (CD45.1+CD45.2+) as recipient and FVB/C57BL/6.SJL (CD45.1+CD45.2-) as donor, was performed in lethally γ-irradiated recipients of either Wt or TEC-ARKO mice. By 3 and 6 weeks post-transplantation, an increase of donor derived (CD45.1+CD45.2-) bone marrow cells, thymocytes, and splenocytes were detected in the TEC-ARKO compared to Wt recipients. Importantly, the reconstituted splenic CD8+ T cells from TEC-ARKO recipient showed the similar capability to produce cytokine IFN-γ compared to Wt recipient mice [Lai et al., 2013].
Male Wt B6 mice were treated with the newly-developed AR degradation enhancer, ASC-J9® [Emerling et al., 2008; Miyamoto et al., 2007a; Yang et al., 2007] or vehicle for two weeks, γ-irradiated, and then subjected to congenic bone marrow transplantation. Simlar patterns of increased cellularity of donor-derived bone marrow cells, thymocytes, and splenocytes were observed in ASC-J9®-treated mice over control mice [Lai et al., 2013]. These observations clearly suggest that inactivation of TEC AR in recipients prior to bone marrow transplantation can improve grafting efficiency. Treatment of recipients with an agent such as ASC-J9® before autologous bone marrow transplantation may be tested in the future to improve grafting efficiency in humans.
AR pathophysiological roles in metabolic syndrome and cardiovascular diseases
Male hypogonadism is associated with sexual dysfunction, decreased lean body mass and muscle strength, increased fat mass, decreased quality-of-life, and osteoporosis [Basaria and Dobs, 2001]. PCa patients undergoing androgen deprivation therapy (ADT) also displayed these complications and have higher risks of skeletal fracture, metabolic syndrome, and diabetes, as well as cardiovascular diseases [Basaria, 2008; Taylor et al., 2009]. Androgen supplementation can improve these complications, indicating that reducing androgen/AR signaling in males plays an important role in development of these complications. Similarly, Kennedy’s Disease patients, who have poly Q-expanded AR with lower transcriptional activity, frequently displayed glucose intolerance, hepatic dysfunction, and hyperlipidemia [Li et al., 1995; Sobue et al., 1989]. In contrast, PCOS women with hyperandrogenemia also have higher risk of developing visceral obesity, metabolic syndrome, type 2 diabetes, and cardiovascular diseases, in addition to hirsutism and infertility [Pasquali and Gambineri, 2006]. Recent studies have led to the suggestion that hyperandrogenemia in these patients arises from insulin resistance and hyperinsulinemia that caused increased androgen production and reduced levels of steroid hormone binding globulin (SHBG). Improving the insulin resistance state with low-calorie diet or insulin-sensitizing drugs also led to reduced androgen levels and visceral obesity in these patients. Treatment with insulin-sensitizing metformin in combination with the antiandrogen, hydroxyflutamide (HF), results in an additive effect on clinical improvement (reviewed in [Pasquali, 2006]). Livingstone and Collison proposed that sex hormones, within the window of their physiological concentrations, are important for maintaining insulin sensitivity in various target tissues to regulate their metabolism and functions [Livingstone and Collison, 2002]. Thus, the interplay of estrogen/ER signaling and androgen/AR signaling in females may play a different role from males. The wide expression of AR in various insulin target tissues has suggested that androgen/AR signaling might play significant roles in metabolism, body mass homeostasis, and cardiovascular functions. Recent generation of various ARKO male mice have provided useful models for studying the AR roles in these complex physiological processes (Figure 2).
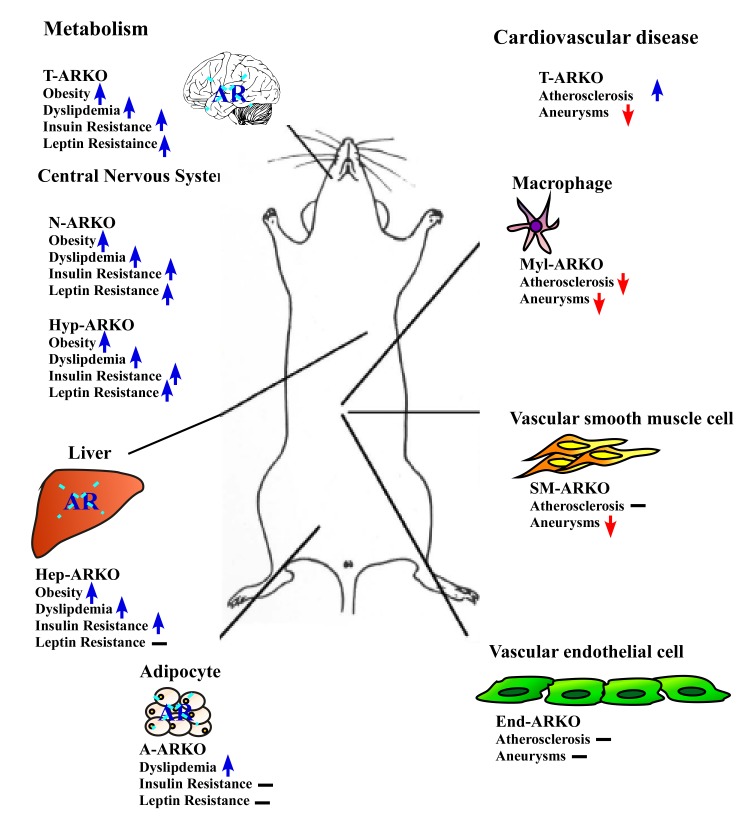
Figure 2. AR roles in metabolic syndrome and cardiovascular diseases (CADs).
Total ARKO (T-ARKO) mice displayed a greater extent of metabolic abnormalities including obesity, dyslipidemia, insulin resistance, and leptin resistance compared to Wt mice. The AR roles in various cell types for development of these metabolic abnormalities are deduced from the phenotype observations in neuron-specific ARKO (N-ARKO), hypothalamus-specific ARKO (Hyp-ARKO), hepatocyte-specific ARKO (Hep-ARKO), and adipocyte-specific ARKO (A-ARKO) mice. In CADs, T-ARKO mice have increased atherosclerosis plaque formation, but completely abolished aneurysm development. The AR roles in different cell types for CADs are deduced from observations in myeloid cell-specific ARKO (Myl-ARKO), smooth muscle cell-specific ARKO (SM-ARKO), and endothelial cell-specific ARKO (End-ARKO) mice. An up arrow indicates increase, whereas a down arrow denotes decrease in a metabolic abnormality or CAD symptom compared to Wt mice. The minus sign indicates no significant difference was observed between the specific ARKO and Wt mice.
AR roles in insulin sensitivity and metabolism
General loss of the AR results in insulin and leptin resistance, dyslipidemia, and obesity
Early studies of male ARKO(CMV-Cre) mice indicated that they became obese with increased body white adipose tissue mass without increase in food uptake or dyslipidemia at age beyond 10 weeks old [Sato et al., 2003]. A more detailed study by Lin et al indicated that, along with increased body weight, male ARKO(ACTB-Cre) mice developed hyperinsulinemia, hyperleptinemia, hyperglycemia, hypo-adiponectinemia, increased serum levels of triglycerides and free fatty acids, and impaired glucose tolerance at advanced ages [Lin et al., 2005]. ARKO(ACTB-Cre) male mice exhibited AR deletion, as well as very low levels of circulating testosterone. Restoring androgen level with DHT in ARKO(ACTB-Cre) male mice did not reverse these changed parameters, suggesting the observed phenotypes were due to loss of AR function. ARKO(ACTB-Cre) mice also became insulin- and leptin-resistant, and had increased triglyceride contents in the skeletal muscle and the liver. PI3K activity, which mediates the metabolic effect of insulin, was found reduced in the skeletal muscle and the liver of ARKO(ACTB-Cre) mice compared to Wt mice. The increase in lipid deposition in white adipose tissue was associated with increased expression of 4 genes that stimulate late adipocyte differentiation and lipid accumulation, namely peroxisome proliferator-activated receptor gamma (PPAR-γ), CCAAT/enhancer-binding protein-α (C/EBPα), adipocyte fatty acid-binding protein 4/adipocyte protein 2 (aP2), and sterol regulatory element-binding protein 1c (SREBP1c) in white adipose tissue of male ARKO(ACTB-Cre) mice compared to Wt mice. In contrast, the expression levels of PPARα gene, which modulates lipid oxidation through activation of several peroxisomal and mitochondrial fatty acid oxidation genes [Guerre-Millo et al., 2000], were found reduced in skeletal muscle and the liver of ARKO(ACTB-Cre) compared to Wt mice. Collectively, these data suggest that loss of the AR results in insulin and leptin resistance, dysregulation of lipid metabolism and favors adipocyte differentiation and fat deposition leading to obesity. However, the roles of AR genomic and non-genomic functions in these phenotype manifestations and gene expression changes remain to be elucidated, as both functions are lost in the ARKO mice.
Yanase et al reported that their ARKO(CMV-Cre) mice, in comparison with Wt mice, displayed late onset obesity with a mild hyperinsulinemia without insulin resistance, hyperadiponectinemia, less dynamic energy consummation, lower expressions of thermogenic uncoupling protein 1 (UCP1), and hormone-sensitive lipase without alteration in expressions of several lipogenetic genes, including FASN, ACC, and SRBP1c [Yanase et al., 2008]. The lack of insulin resistance was attributed to the elevated adiponectin, known to increase insulin sensitivity, while the development of obesity was attributed to reduced lipolysis. However, a study of ARKO(CMV-Cre) indicated the absence of obesity in this mouse model up to 52 weeks old [Kawano et al., 2003].
Rana et al reported that AR-EX3(CMV-Cre) male mice exhibited increased adipocity without obesity, along with reduced voluntary activity, decreased food uptake, hyperleptinemia, and hyperadiponectinemia, without insulin resistance [Rana et al., 2011]. The lack of insulin resistance might be due to elevated adiponectin levels. In contrast, the decreased food intake and lack of late onset obesity observed only in AR-EX3(CMV-Cre) male mice could be due to the unknown non-genomic effects of the AR-zf2 protein.
It is interesting to note that both ARKO(CMV-Cre) and AR-EX3(CMV-Cre) mice exhibited hyperadiponectinemia that might contribute to the lack of insulin resistance in these mice. It would be very interesting to examine other male ARKO models such as the ARKO(PGK-Cre) [De Gendt et al., 2004] and AR-EX3(Sox2-Cre) mice [Simanainen et al., 2012] to determine if hyperadiponectinemia is unique to CMV-Cre-generated ARKO models. In contrast, the different genetic backgrounds of ARKO(ACTB-Cre), ARKO(CMV-Cre), and AR-EX3(CMV-Cre) do not appear to play a significant role in different phenotype expression of insulin sensitivity, since hep-ARKO mice [Lin et al., 2008] and neuron-specific ARKO (N-ARKO) mice [Yu et al., 2013], which were generated in the same genetic backgroud as AR-EX3(CMV-Cre) mice, displayed various degrees of insulin resistance (to be discussed below).
It should be noted that the observation of late onset obesity and insulin resistance in ARKO(ACTB-Cre) mice appears to be in good agreement with these phenotypes observed in humans with low androgen/AR signaling activity, including hypogonadal men, PCa patients treated with ADT, and Kennedy’s Disease patients, as mentioned earlier. Moreover, it has been reported that CAIS patients, particularly those without orchidectomy, have higher propensity to develop obesity and abnormal insulin sensitivity than normal male and female subjects [Dati et al., 2009]. If this observation made in a small cohort of CAIS patients can be confirmed with a larger number of CAIS patients, it would lend additional support to AR’s role in modulating insulin sensitivity in males in both human and mouse. In contrast, the lack of development of obesity and insulin resistance in female ARKO mice appears to suggest that metabolic responses to androgen/AR signaling are different between males and females.
There are complex inter-organ communications among various sites of insulin action [Accili, 2004; Okamoto et al., 2004] and the AR is expressed in many key organs involved in insulin sensitivity. Therefore, it would be necessary to study tissue- or organ-specific ARKO models to dissect the AR role(s) in regulation of insulin sensitivity and glucose homeostasis.
Loss of the AR in the liver leads to hepatic insulin resistance
The liver plays an important role in controlling glucose metabolism and maintaining glucose homeostasis. A major contributor to the hyperglycemia of diabetes mellitus is the chronic elevation of hepatic glucose production [Bouche et al., 2004; Muoio and Newgard, 2006; Wu et al., 2005]. Regulating lipid metabolism is also an important role of the liver. Dysregulation of hepatic fatty acid oxidation and synthesis led to hepatic steatosis (fatty liver) [Postic et al., 2004], which contributes to the development of hepatic insulin resistance with hepatic glucose over-production [Samuel et al., 2004; Wang et al., 2001]. The roles of the AR in hepatic insulin sensitivity, glucose, and fatty acid metabolism are not clear at present.
The hep-ARKO mouse model was generated by crossing the exon 2-floxed AR mice [Yeh et al., 2002] with albumin-Cre mice to study the AR roles in hepatic metabolism and insulin sensitivity [Lin et al., 2008]. The floxed AR mice used in this study had been back crossed several generations to have C57BL/6J background. When fed with a normal mouse chow diet, hep-ARKO mice exhibited a normal growth curve as compared to Wt littermates, but male, and not female, hep-ARKO mice developed liver steatosis at an old age (40 weeks). Upon feeding with a high fat diet, male hep-ARKO mice gained more weight and become more obese than Wt mice. Obese male hep-ARKO mice developed more severe liver steatosis than male Wt mice and hyperlipidemia, indicating dysregulation of lipid metabolism as confirmed by the observations of reduced fatty acid oxidation and increased de novo fatty acid synthesis, suggesting that it may be due to reduced PPAR-γ and increased SREBP1c expression levels, and alteration of their target genes involved in these metabolic activities. Consequently, obese male hep-ARKO mice developed hepatic insulin resistance through decreased PI3K activity, increased levels of phosphotyrosyl phosphatase 1B (PTP1B) and phosphoenopyruvate carboxykinase (PEPCK) expression in the liver, leading to increased hepatic glucose production. These observations suggested that the hepatic AR plays a pivotal role in regulation of hepatic glucose, lipid metabolism, and insulin sensitivity. However, it remains unclear whether the hepatic AR functions through genomic or non-genomic pathways to mediate these metabolic regulations.
Loss of the AR in adipocytes leads to hyperleptinemia without obesity and leptin resistance
An adipose tissue-specific ARKO (A-ARKO) mouse model was generated [Yu et al., 2008] by crossing aP2-Cre (fatty acid binding protein 4-Cre) mice [He et al., 2003] and the exon 2-floxed AR mice [Lin et al., 2008]. Male A-ARKO mice exhibited normal body weight with hyperleptinemia, hypotriglyceridemia, hypocholesterolemia, and normal central leptin sensitivity. The hyperleptinemia was attributed to an increased production of leptin from white adipose tissue of A-ARKO mice, as a result of increased levels of estrogen/ER signaling in this tissue. The expression of transcription factors involved in lipid oxidation, including PPAR-γ coactivator-1α (PGC-1α) and CPT-1, were elevated in white adipose tissues of A-ARKO compared to those of Wt mice. In addition, the expression of UCP-2 was increased, whereas that of aP2 was decreased, indicating an increased energy expenditure and decreased fatty acid transportation in white adipose tissues of A-ARKO mice. Since leptin is known to enhance lipid oxidation in the liver and skeletal muscle [Gallardo et al., 2007; Minokoshi et al., 2002], the decreased fatty acid transportation into adipose tissues could lead to increased lipid oxidation in these organs that might account for the observed hypotriglyceridemia and hypocholesterolemia [Yu et al., 2008]. These observations suggested that hyperleptinemia resulting from adipocyte-specific ARKO might be beneficial to body weight control. However, the effect of ARKO on energy expenditure in brown adipose tissue has not been investigated. Moreover, there might be a significant difference in body weight gain between A-ARKO and Wt mice when fed with high fat diet and this might point to a beneficial effect of adipose-specific ablation of AR signaling.
It should be noted that aP2-Cre, although predominantly expressed in adipose tissues postnatally [Bluher et al., 2002], is also expressed in other cells prenatally, especially in cell types such as chondrocytes, myocytes, neurons, and osteocytes that are derived from a common mesenchymal stem cell progenitor population [Urs et al., 2006]. Therefore, despite the claim of Yu et al that ARKO in A-ARKO mice was specific for adipose tissues [Yu et al., 2008], it remains possible that partial ARKO in other tissues might have occurred beyond their detection limit. Further investigation may be needed using the recently-developed and more specific adiponectin-Cre transgene [Wang et al., 2010].
Loss of AR in central nervous system leads to hypothalamic insulin and leptin resistance
The brain is an insulin target organ that plays a key role in regulation of energy balance and glucose homeostasis [Obici and Rossetti, 2003; Schwartz and Porte, 2005; Woods et al., 1979]. Various studies have shown that there is greater insulin sensitivity in the male than female central nervous system [Clegg et al., 2006; Clegg et al., 2003]. Higher levels of the AR are found in the hypothalamus of male than female mice and the AR was found to colocalize with insulin receptor, suggesting that the brain AR might be involved in regulation of central insulin sensitivity in males [Yu et al., 2013].
To study the role of neuronal AR in metabolic regulation, Yu et al have generated neuron-specific ARKO (N-ARKO) mice by crossing Synapsin I-Cre mice [Ferguson et al., 2007] with exon 2-floxed AR mice, both in C57BL/6J background [Yu et al., 2013]. Administration of insulin in the fasting state reduced food intake in Wt, but not in N-ARKO male mice, suggesting that the latter had central insulin resistance. N-ARKO male mice also exhibited increasing systemic insulin resistance and glucose intolerance with increasing age. Aged N-ARKO mice had greater body weight with increased visceral fat, liver lipid deposition, and hepatic glucose production than Wt mice. The hepatic lipid deposition was associated with elevated levels of SCD1 and SREBP-1c, indicating increased lipogenesis. The increased hepatic glucose production was associated with increased expression of gluconeogenesis genes, PEPCK, and glucose-6-phosphatase, in N-ARKO mice. In addition, insulin-induced activation of hepatic STAT3, which mediates central insulin regulation of hepatic glucose production [Inoue et al., 2006] was decreased in N-ARKO mice. Hypothalamic insulin signaling is known to suppress hepatic glucose production [Obici et al., 2002; Pocai et al., 2005] through suppression of agouti related peptide (AgRP) release from hypothalamic arcuate nucleus [Konner et al., 2007]. The expression levels of hypothalamic AgRP in N-ARKO mice were found to be upregulated. Moreover, hypothalamic Akt and insulin receptor phosphorylation in response to insulin administration were significantly reduced in N-ARKO mice compared to Wt mice, apparently due to reduced expression of hypothalamic PTP1B. Incidentally, the AR was found to suppress NFκB-induced PTP1B expression in neuronal cells [Yu et al., 2013]. These results indicate that ablation of neuronal AR resulted in hypothalamic insulin resistance that leads to systemic insulin resistance, dysregulation of glucose homeostasis and lipid metabolism, and visceral obesity.
Using rat insulin promoter 2-Cre (RIP2-Cre) mice to achieve hypothalamus-specific gene knockout [Choudhury et al., 2005; Kubota et al., 2004; Lin et al., 2004; Mori et al., 2009], Yu also generated male mice with hypothalamus-specific ARKO, the Hyp-ARKO mice [Yu, 2010]. Insulin signaling activity in the arcuate nucleus of Hyp-ARKO mice was found to be reduced compared to the control RIP2-Cre mice. Hyp-ARKO mice also displayed lower insulin sensitivity to regulate circulating glucose level than the control mice. In addition, leptin signaling activity in arcuate nucleus neurons was also reduced compared to the controls. Consequently, leptin had less suppressive effect on body weight and food intake in Hyp-ARKO than control mice. These observations suggest that the AR plays a pivotal role in modulating hypothalamic insulin and leptin signal cascades. However, it should be noted that RIP2-Cre also mediated AR knockout in pancreatic islet β cells. It is not clear if this affects the expression and secretion levels of the two β cell hormones, insulin and amylin (also a regulator of food intake and body weight) [Trevaskis et al., 2008], which might obscure the interpretation of observations made in Hyp-ARKO mice.
In an attempt to explain the obesity with lack of insulin resistance in ARKO(CMV-Cre) mice, Miyamoto et al [Miyamoto et al., 2007b] reported that male ARKO(CMV-Cre) mice displayed hypertrophic adrenal glands and overproduction of glucocorticoids. In the pituitary glands of ARKO(CMV-Cre) mice there were increased expression of proopiomelanocortin (POMC) and decreased expression of the glucocorticoid receptor (GR). Since the AR regulates expression of the GR in pituitary cells, they concluded that ablation of the AR in the pituitary resulted in dysregulation of feedback control of glucocorticoid production that led to obesity in this mouse model. However, in view of the results obtained from N-ARKO and Hyp-ARKO mice, the lack of insulin resistance in ARKO(CMV-Cre) mice might be due to their high levels of adiponectin. Incidentally, Tfm male mice have been shown to have elevated corticosterone levels and increased stress indices suggesting hyperactivation of the hypothalamic-pituitary-adrenal axis [Zuloaga et al., 2008a]. Therefore, the possibility that this AR-regulated glucocorticoid feedback circuit might also play a significant role in the development of obesity in ARKO(ACTB-Cre) mice remains to be investigated. The roles of the AR in metabolism, as determined from various tissue/cell type-specific ARKO mice so far investigated, are depicted in Figure 2, left side.
AR roles in cardiovascular function
The AR and cardiac growth
Ikeda et al examined the effect of AR knockout on heart function and structure in their male ARKO(CMV-Cre) mice in comparison with male Wt littermates at 25 weeks old [Ikeda et al., 2005]. There were no differences in systolic blood pressures and heart rates between the two genotypes, but the size of heart or heart weight/body weight was smaller in ARKO(CMV-Cre) than in Wt mice, a difference observed only after 6 weeks of age suggesting the involvement of post-puberty event(s). Cross-sectional analyses of the hearts indicated also that the volume and wall thickness of the left ventricles were smaller in ARKO(CMV-Cre) than in Wt mice. In addition, the extent of cardiac hypertrophy induced by angiotensin II (Ang II) was less in ARKO(CMV-Cre) mice than Wt mice. Furthermore, increased fibrosis and attenuated systolic function in left ventricle upon Ang II stimulation in ARKO(CMV-Cre) mice compared to Wt mice at old age was observed. Based on these observations, it was suggested that androgen/AR signaling participates in regulation of normal cardiac growth and modulates cardiac adaptive hypertrophy and fibrosis during cardiac remodeling upon hypertrophic stress [Ikeda et al., 2005], but their molecular mechanisms remain to be elucidated.
MacLean et al reported that male and female AR-EX3(CMV-Cre) mice exhibited decreased heart mass compared to their corresponding Wt mice [MacLean et al., 2010]. After correction for body weights, there were no differences between Wt and AR-EX3 males. Since exon 3-floxed AR male mice had higher heart mass than Wt controls [MacLean et al., 2008a], it remains possible to speculate that AR may play a role in cardiac growth in male mice. Since the heart masses of homozygous floxed AR females were not compared, the effect of ablating genomic AR function on heart growth in females might be even greater. Therefore, the AR role in cardiac growth can not be validly concluded from this study.
AR and vascular remodeling and atherosclerosis
Jones et al reported that the diameters of various arterial vessels were similar between Tfm and Wt littermates [Jones et al., 2003]. In contrast, in vitro vasoconstriction in response to KCl (mediated through voltage-gated calcium channels) and vasodilation in response to acetylcholine (which is endothelium-dependent) were reduced in femoral arteries of Tfm mice compared to Wt mice, suggesting that androgen/AR signaling might modulate arterial functions. Nettleship et al reported that a high cholesterol diet for 4 weeks in Tfm and castrated male Wt mice resulted in significantly higher fat deposition in the aortic root than in intact Wt mice [Nettleship et al., 2007], suggesting that androgen/AR signaling might protect against atherosclerosis.
The effect of ARKO on vascular remodeling was also investigated in ARKO(CMV-Cre) mice [Ikeda et al., 2009]. Comparing male ARKO(CMV-Cre) mice and male Wt littermates at 25 weeks old, no morphological differences in the coronary artery and thoracic aorta were found between the two genotypes. Despite nitric oxide (NO) availability, aortic endothelial NO synthase expression and phosphorylation, as well as Akt phosphorylation, were significantly reduced in ARKO(CMV-Cre) mice. Upon Ang II stimulation, however, ARKO(CMV-Cre) mice exhibited marked increases over Wt littermates in medial thickness and perivascular fibrosis in the aorta that were accompanied with increased expressions of TGF-β1, collagen type I, collagen type III, and nicotinamide adenine dinucleotide phosphate oxidase component genes, as well as increased superoxide production and lipid peroxidation. In addition, phosphorylation of c-Jun N-terminal kinase (JNK) and Smad2/3 were enhanced in ARKO(CMV-Cre) over Wt mice after Ang II stimulation. The authors concluded that the androgen/AR system is required for the preservation of NO bioavailability through activation of the Akt-endothelial NO synthase system and has protective effects against Ang II-induced vascular remodeling by regulating oxidative stress, JNK signaling, and the TGF-β1/phospho-Smad signal cascade. A subsequent study demonstrated that doxorubicin-induced cardiotoxicity is increased in male ARKO(CMV-Cre) compared to Wt mice through exacerbation of mitochondrial damage and superoxide generation, leading to enhanced apoptosis of cardiomyocytes [Ikeda et al., 2010].
It should be noted that the above three studies were conducted in ARKO(CMV-Cre) mice generated through CMV-Cre-mediated gene knockout. As CMV-Cre has a strong expression in the heart [Schmidt et al., 1990], and the AR gene is ubiquitously deleted in all heart cells, it is not clear to what extent androgen/AR signaling in cardiac myocytes, and various vascular cell types contributes to the observed phenotypes. Thus, AR roles in various specific cardiac cell types remain to be investigated through cardiac cell type-specific ARKO mice. More importantly, male ARKO(CMV-Cre) mice have very low testosterone levels that might significantly affect tissue E2 levels and ER signaling. For example, in animal models of early stages of atherosclerosis, testosterone reduces plaque development in males through its conversion into E2 [Nathan et al., 2001]. In addition, testosterone supplementation in Tfm mice reduced aortic fatty streak formation which was partially reversed by an ERα blocker or aromatase inhibitor, also suggesting the involvement of ERα [Nathan et al., 2001]. There were no attempts to study the effect of testosterone supplementation in the above ARKO(CMV-Cre) studies. Thus, it is not clear to what extent the ARKO effects observed in these studies is attributable to reduced ER signaling. Moreover, since cardiac function is under the regulation of sympathetic and parasympathetic neural circuits, the AR roles in the CNS control of these neural circuits remain to be elucidated.
Studies of atherosclerosis in male LDL receptor deficient (LDLR-/-) mice with Wt AR, ARKO(ACTB-Cre), SM-ARKO, Myl-ARKO, or endothelium-specific ARKO (End-ARKO) have revealed that the AR may play a positive role in promoting atherosclerosis in selective cell types [Huang, 2012]. ARKO(ACTB-Cre)/LDLR-/- mice were found to have higher plaque formation in the aorta than Wt LDLR-/- mice. In contrast, Myl-ARKO/LDLR-/- mice had lower atherosclerosis plaque forming rates than Wt LDLR-/- mice, whereas both SM-ARKO/LDLR-/- and End-ARKO/LDLR-/- mice had similar plaque forming rates as the control LDLR-/- mice. Although the aorta of both ARKO(ACTB-CRE)/LDLR-/- and Myl-ARKO/LDLR-/- mice had lower macrophage infiltration rates than Wt LDLR-/- mice, only ARKO(ACTB-Cre)/LDLR-/- mice had higher collagen disposition than Wt LDLR-/- mice. In addition, ARKO(ACTB-Cre)/LDLR-/-, but not Myl-ARKO/LDLR-/-, mice had higher circulating cholesterol, triglycerides, and LDL, but lower HDL levels than Wt LDLR-/- mice. Knocking down the AR in myeloid cell line, THP-1, resulted in suppression of cell migration and foam cell formation. Taken together, these observations indicated that the AR in non-myeloid cells such as smooth muscle and endothelial cells might not play any significant role in atherosclerosis, while AR in myeloid cells has a positive role in promoting atherosclerosis. Due to very low testosterone concentrations in ARKO(ACTB-Cre) mice, it is unclear whether ERα or the AR in extra-aortic cells might protect against atherosclerosis by modulating cholesterol and lipid metabolism. The underlying mechanisms of these AR roles remain to be elucidated.
Bourghardt et al reported that male ARKO(PGK-Cre)/ApoE-/- mice had substantially higher atherosclerosis areas in the aortic root than ApoE-/- mice with Wt AR when the animals were fed with a high fat diet [Bourghardt et al., 2010]. The higher atherosclerotic lesion areas in the ARKO(PGK-Cre)/ApoE-/- in comparison with Wt ApoE-/- mice were associated with elevated serum TNFα levels. Castration of Wt ApoE-/- mice, but not ARKO(PGK-Cre)/ApoE-/- mice, increased atherosclerotic lesions. Testosterone supplementation reduced atherosclerotic lesion areas in both castrated Wt ApoE-/- (by 50%) and ARKO(PGK-Cre)/ApoE-/- (by 24%) mice. It was concluded that testosterone atheroprotection involves both AR-dependent and AR-independent components. It is not clear if the AR-independent component is ERα-mediated, as observed in the Tfm mouse [Nettleship et al., 2007].
The AR and abdominal aorta aneurysm (AAA)
In ApoE-deficient (ApoE-/-) mice, treatment with angiotensin II induces AAA [Daugherty et al., 2000]. Total ablation of the AR in ARKO(ACTB-Cre)/ApoE-/- male mice resulted in complete suppression of AAAs compared to ApoE-/- mice with Wt AR. Castration of Wt ApoE-/- mice also resulted in suppression of AAAs, an effect reversed by supplementation with DHT. Specific knockout of AR in smooth muscle cells (SM-ARKO/ApoE-/- mice) and myeloid cells (Myl-ARKO/ApoE-/- mice), but not in vascular endothelial cells (End-ARKO/ApoE-/- mice), also significantly decreased AAAs [Huang, 2012]. These observations indicated that the AR in vascular smooth muscle cells and macrophages, but not in endothelial cells, might play exacerbating roles in initiation or progression of AAA (Figure 2, right side). Interestingly, treatment of the ApoE-/- mice with ASC-J9® also suppressed angiotensin II-induced AAAs. However, the AR roles in extra-aortic cells in AAA and the underlying mechanisms remain to be studied.
AR roles in bone and skeletal muscle
AR roles in development and maintenance of bone
Albright et al first pointed out that deficiencies in sex hormones had profound adverse effects on bone homeostasis in post-menopausal women and aging men [Albright, 1947]. Decades of extensive studies since then have indicated that androgens and estrogens are key regulators of growth and maintenance of both female and male skeleton [Callewaert et al., 2010b; Frenkel et al., 2010; Riggs et al., 2002; Vanderschueren et al., 2004; Vanderschueren et al., 2006]. A study of the effect of testosterone supplementation on bone metabolism in castrated Tfm male mice in comparison with castrated control male mice indicated that testosterone increased trabecular bone number, width, volume, and bone mineral density (BMD), while it reduced bone turnover in an AR-dependent manner [Vandenput et al., 2004]. The physiological role(s) of androgens/AR signaling in bone metabolism is not clear at present. The availability of various ARKO mouse models thus have provided some useful tools for studying the AR roles in bone metabolism.
Yeh et al observed that male ARKO(ACTB-Cre) mice had lower cancellous bone volumes than male and female Wt mice. Male ARKO(ACTB-Cre) mice also had higher osteoclast numbers, higher mineral deposition, and bone formation rates in the femoral metaphysis than Wt mice [Yeh et al., 2002]. The osteopenia in ARKO(ACTB-Cre) mice was attributed to a greater bone resorption rate than bone formation rate. Further microcomputed tomography scan analyses of skull bones from 8 to 10 week old male and female ARKO(ACTB-Cre) mice and Wt littermates indicated delayed mineralization of the skull vault, reduced bone volume and surface area, and thickness in the calvaria of both male and female ARKO(ACTB-Cre) mice compared to Wt littermates [Kang et al., 2008]. Histomorphometric analyses indicated that calvaria from 8 week old male and female ARKO(ACTB-Cre) mice were significantly narrower in width and had a smaller bone area, osteoid surface, lower osteoblast and osteocyte number, higher osteoclasts per bone area, and lower area of mineralization than Wt littermates.
DHT treatment of organ cultures of neonatal calvaria bones from Wt male mice increased the new bone area by increasing the number of bone-lining osteocytes, suture width, and area of calcification. These effects of DHT were impaired in calvaria bone organ culture of male ARKO(ACTB-Cre) mice, suggesting that the loss of the AR suppresses new bone formation and decreases osteoblast activity required for differentiation and mineralization. In primary calvarial osteoblasts, androgen-mediated calcium deposition was significantly decreased in ARKO(ACTB-Cre) cells compared to Wt cells. DHT stimulated the expression levels of AKP2 gene, which encodes tissue non-specific alkaline phosphatase (TNSALP), and the small integrin-binding ligand, N-linked glycoprotein (SIBLING) gene family were found decreased significantly in calvarial cells from ARKO(ACTB-Cre) mice compared to cells of Wt mice. These genes were found to be the target of the AR. Moreover, it was shown that the AR mediated the androgen-stimulated increase of TNSALP activity and intracellular inorganic phosphate (Pi) levels in the MC3T3-E1 model of osteoblast differentiation and mineralization and in primary calvarial osteoblasts. Ectopic expression of TNSLP or exogenous Pi partially reversed the decrease in bone formation in both MC3T3-E1 cells with AR knocked down and ARKO(ACTB-Cre) primary osteoblasts. Since the function of TNSLP is to increase Pi levels, it was concluded that the AR signaling plays an essential role in bone formation by coordinating the expression of genes associated with Pi regulation [Kang et al., 2008].
Tsai et al observed that male ARKO(ACTB-Cre) mice, in comparison with Wt mice, exhibited continuous loss of bone mass and deterioration of bone microarchitectures (decreased trabecular number and increased trabecular separation) in trabecular bones throughout the ages of 6 to 30 weeks old [Tsai et al., 2011]. Analysis of bone marrow stromal cells obtained from Wt and ARKO(ACTB-Cre) mice indicated that ARKO(ACTB-Cre) bone marrow stromal cells had higher numbers of colony formation unit-fibroblasts (CFU-F), a heterogeneous population of stem and progenitor cells that can be induced to differentiate in vitro [Frenkel et al., 2010], than Wt cells. Flow cytometric analyses of CFU-F indicated that ARKO(ACTB-Cre) cells contained higher proportions of CD44 (marker of mesenchymal stem cells, MSCs) and CD34 (marker of hematopoietic stem cells) positive cells than Wt cells. When MSCs were induced to differentiate into osteoblasts, the expression levels of several genes linked to osteogenesis were decreased in cells isolated from ARKO(ACTB-Cre) mice as compared to Wt mice. Thus, we may conclude that AR functions to regulate MSC proliferation and osteogenic differentiation.
Kawano et al observed that ARKO(CMV-Cre) mice, in which the AR was knocked out in the bone, also exhibited osteopenia with increased bone resorption rate [Kawano et al., 2003]. Testosterone supplementation restored BMD in orchidectomized Wt mice, but only partially in orchidectomized ARKO(CMV-Cre) mice. Supplementation with DHT partially restored BMD in orchidectomized Wt mice, but was ineffective in orchidectomized ARKO(CMV-Cre) mice. These observations were similar to those made in Tfm mice [Vandenput et al., 2004].
Venken et al also studied trabecular and cortical bone in intact and orchidectomized Wt and ARKO(PGK-Cre) pubertal male mice [Venken et al., 2006]. They observed that AR knockout or orchidectomy in male mice resulted in similar trabecular bone loss, which was prevented by testosterone or DHT supplementation in orchidectomized Wt, but not ARKO(PGK-Cre) mice. The effects of testosterone on trabecular bone in orchidectomized Wt mice was not affected by an aromatase inhibitor. ARKO and orchidectomy also produced decreasing cortical bone thickness and periosteal bone formation. Periosteal bone formation and cortical thickness were increased by testosterone or DHT supplementation in orchidectomized Wt mice, but not in ARKO(PGK-Cre) mice. The effect of testosterone on periosteal bone in orchidectomized Wt mice was partially reduced by an aromatase inhibitor that was associated with a decrease in serum IGF-1 level. These observations suggest that AR signaling, but not ER signaling, is required for normal development of trabecular bone, whereas both AR and ER signal cascades are required for optimal stimulation of periosteal bone growth.
MacLean et al observed that male AR-Ex3(CMV-Cre) mice exhibited decreased trabecular volume and number, bone mineralization surface, cortical bone thickness, periosteal, and medullary circumferences compared to Wt males [MacLean et al., 2010]. In contrast, female AR-Ex3(CMV-Cre) mice displayed only decreased trabecular number, periosteal, and medullary circumferences and an increase in trabecular thickness compared to Wt females. These observations indicated that the AR plays significant roles in bone metabolism in both male and female mice.
Tfm mice with inactivated AR retain long bone length comparable to Wt males, but combining Tfm with loss of the ERα resulted in reduced bone length. On the other hand, either inactivation of AR or loss of ERα or both, resulted in reduced BMD in femoral bone and cortical width of tibia compared to Wt male mice [Tozum et al., 2004]. These results indicated that both the AR and ERα activities are important for maintaining bone mineralization in males. Tfm mice, but not ERαKO males, also had decreased trabecular bone compared to Wt male mice, suggesting that AR signaling is important for maintaining trabecular bone mass [Tozum et al., 2004]. A recent study comparing bone and body composition among ARKO(PGK-Cre), ERαKO, ARKO(PGK-Cre)/ERαKO double knockout, and in Wt male mice has yielded the same conclusions [Callewaert et al., 2009].
The role of AR signaling in anabolic response of bone to mechanical loading was studied by comparing adaptive responses of in vivo ulna loading in ARKO(PGK-Cre), ERαKO, ARKO(PGK-Cre)/ERαKO double knockout, and Wt male mice [Tozum et al., 2004]. It was found that ulna loading induced greater periosteal bone formation in ARKO(PGK-Cre) and ARKO(PGK-Cre)/ERαKO double knockout than Wt male mice. Ulna loading decreased the expression of SOST/sclerostin, an inhibitor of bone formation [Callewaert et al., 2010a], to a greater extent in ARKO(PGK-Cre) than Wt mice [Del Rio et al., 2001]. Thus, the AR-mediated signaling appears to limit osteogenic response to mechanical loading in male mice. Interestingly, Ophoff et al reported that voluntary wheel running in ARKO(PGK-Cre) mice did not influence body weight, muscle mass, or periosteal bone expansion, but significantly reduced bone turnover and prevented cancellous bone loss due to a preservation of trabecular number [Ophoff et al., 2009a]. However, the mechanism by which physical activity compensates for the effect of ARKO on bone resorption is not clear at present.
Although the above studies with Tfm, ARKO(CMV-Cre), ARKO(ACTB-Cre), ARKO(PGK-Cre), and ERαKO models have demonstrated that the androgen/AR signaling plays important roles in regulation of pubertal bone growth and maintenance of bone homeostasis, it is unclear how the AR signaling might mediate these functions. As these mice have global inactivation or ablation of AR, generation and study of bone cell type- or tissue-specific ARKO models will be needed to delineate the roles of the AR in bone cells and bone metabolism.
Notini et al have generated an osteoblast-specific exon 3-truncated AR mouse model [Notini et al., 2007] by crossing Col2.3-Cre mice [Liu et al., 2004] with their exon 3-floxed AR mice [Notini et al., 2005]. The Cre-mediated recombination resulted in truncation of AR exon 3 in mature osteoblasts and the resultant transgenic mice exhibited reduced trabecular bone volume that was associated with a decrease in trabecular number and accompanied by a reduction in connectivity density and an increase in trabecular separation. They concluded that the AR genomic function in matured osteoblasts is involved in maintaining trabecular bone volume by regulating bone resorption in adult mice.
Chiang et al employed osteocalcin-Cre mice [Zhang et al., 2002] to achieve specific truncation of AR exon 3 in terminally differentiated, mineralizing osteoblasts (Ostbl-AR-EX3 mice) [Chiang et al., 2009]. Throughout all ages, the mutant mice displayed reduction in trabecular bone volume, decreased cortical thickness, and abnormal mineralization of bone matrix with increased unmineralized bone matrix and a decrease in the amount of mineralizing surface. The authors concluded that the AR in mineralizing osteoblasts functions to maintain bone by regulating bone resorption and the coordination of bone matrix synthesis and mineralization. However, as these two studies employ AR mutants with exon 3 truncation without total knockout of the AR protein expression, it is not certain that the observed phenotypes were the results of loss of AR genomic function and/or influence of the truncated AR-ZF2 protein that is not normally produced in cells. Moreover, the AR has been shown to mediate non-genomic activation of the PI3K/Akt cascade by androgen to influence proliferation of osteoblasts in vitro [Kang et al., 2004], and the truncated AR protein might retain such activity. Together with the recently developed osteoclast-specific Cre mice [Chiu et al., 2004], these mouse models should provide useful tools for generation and study of various bone tissue-specific ARKO mice. Further studies using these bone tissue-specific ARKO mice are expected to lead to full understanding of the AR roles in bone maintenance and eventually to preventive treatment for male hypogonadism-associated osteoporosis and bone fracture.
Sinnesael et al have generated an osteocyte-specific ARKO (Ocy-ARKO) by crossing dentin matrix protein 1 promoter-driven Cre (DMP1-Cre) mice [Lu et al., 2007] with exon 2-floxed AR mice [Sinnesael et al., 2012]. Expression levels of the AR in osteocytes increased with increasing age, but were reduced greater than 80% in Ocy-ARKO osteocytes compared to Wt osteocytes. Compared to Wt mice, Ocy-ARKO mice displayed lower trabecular number and volume in femora and tibia at 32 weeks old and decreased trabecular number in L5 vertebrae at 12 weeks old, whereas no difference was observed in trabecular bone formation or femoral cortical structure. The femora of Ocy-ARKO mice were stiffer and more sensitive to mechanical force-induced failure at 32 weeks old. These observations suggest that the AR in osteocytes plays an important role in maintaining the integrity of trabecular bone and are thus in agreement with a previous report of this AR role [Almeida et al., 2007].
AR roles in development and maintenance of skeletal muscle
Androgens are essential for development and maintenance of muscle mass and strength [Celotti and Negri Cesi, 1992; Chen et al., 2005]. Exogenous testosterone administration has been shown to increase the muscle mass and leg strength and decrease the fat mass in eugonadal young and aging men [Bhasin et al., 2001; Bhasin et al., 2005]. Herbst and Bhasin proposed that androgens promote the commitment of mesenchymal pluripotent cells into myogenic lineage and inhibit adipogenesis through an AR-mediated pathway [Herbst and Bhasin, 2004]. AR is expressed in various human and rodent skeletal muscle cell types, including fibroblasts, mesenchymal stem cells, myonuclei, satellite cells [Johansen et al., 2007; Monks et al., 2004; Sinha-Hikim et al., 2004; Yang and Arnold, 2000], and in motor neurons [Yang and Arnold, 2000]. Based on the fact that several actin-interacting proteins function as AR coregulators, Ting and Chang proposed androgen/AR signaling plays important roles during development and function of skeletal muscle [Ting and Chang, 2008]. However, it is not clear at present how the AR in these cells functions to modulate skeletal muscle mass and strength.
Altuwaijri et al observed that during in vitro differentiation of myoblasts into myotubes, expression of AR and myogenin increased, suggesting that AR signaling might be involved in muscle development [Altuwaijri et al., 2004]. However, when hind limb skeletal muscle from adult ARKO(ACTB-Cre) and Wt male mice were compared, there were no differences in muscle morphology, the protein levels of desmin (a muscle-specific protein), or β-dystroglycan (a muscle-specific membrane protein), between the two genotypes, suggesting a normal differentiation of muscle in ARKO(ACTB-Cre) mice compared to Wt mice. In contrast, the levels of myosin and troponin I specific for slow-twitch muscle fibers were decreased, whereas that of troponin T specific for fast-twitch muscle fibers was increased in quadricep muscles of ARKO(ACTB-Cre) mice compared to Wt mice. Thus, AR might play a role in skeletal muscle development by influencing the balance of muscle fiber type in favor of slow twitch fibers through upregulation of slow-twitch fiber-specific proteins and downregulation of fast-twitch fiber-specific proteins [Altuwaijri et al., 2004].
In the study of the effect of ARKO(PKG-Cre) on bone and body composition in male mice, Callewaiert et al observed that ablation of the AR resulted in decreased muscle mass [Callewaert et al., 2009], suggesting that AR signaling is important for growth and/or maintenance of muscle mass. In addition, MacLean et al examined AR-EX3(CMV-Cre) mice and observed that muscle mass of male, but not female, mutant mice was decreased compared to their Wt counterparts [MacLean et al., 2008b]. Male mutant mice failed to develop the levator ani (LA) muscle. Contractile analyses indicated that fast-twitch muscles from male mutants produced less force, whereas their slow-twitch muscles had increased fatigue resistance. Analysis of gene expression in the male mutant muscle indicated that slow-twitch muscle contractile protein was increased. Although some of the phenotypic traits observed in the male mutant mice might be due to loss of AR genomic function, it was uncertain whether perturbation of gene expression by the truncated AR mutant protein might have occurred.
Ophoff et al generated a myocyte-specific ARKO (M-ARKO) mouse [Ophoff et al., 2009b] using MCK-Cre [Bruning et al., 1998] and exon 2-floxed AR mice. AR protein expression in muscles from M-ARKO mice was reduced by 60-88% compared to muscles of the corresponding Wt mice. The residual AR expression was attributed to non-myocytic muscle cells. The weight of the LA muscle was significantly reduced by 46%, whereas the weights of other peripheral muscles were not significantly reduced. M-ARKO mice also exhibited lower intra-abdominal fat without alteration in cortical or trabecular bone mass. Compared to Wt mice, muscles of M-ARKO mice displayed increased proportion of slow-twitch fibers and reduced fast-twitch muscle fibers. On the other hand, there were no differences in muscle strength and fatigue between the two genotypes. Similar muscle phenotypes were observed when muscles of ARKO(PGK-Cre) mice were compared with the muscles of corresponding Wt mice of the same genetic background. It was concluded that myocytic AR functions to maintain muscle mass and regulate muscle fiber type.
Chambon et al also generated male M-ARKO and inducible M-ARKO (ind-M-ARKO) mice [Chambon et al., 2010] using human skeletal actin-Cre (HSA-Cre), [Miniou et al., 1999]) and HSA-Cre-ERT2 [Schuler et al., 2005] mice, respectively, to cross with exon 1-floxed AR mice. The mass of perineal skeletal muscles, including bulbocavernosus (BC) and LA together (BC/LA), in M-ARKO mice was found to be 50% that of the corresponding floxed AR male mice at age of 6 weeks. The mass of BC/LA increased with age, but the increase in M-ARKO mice was significantly less than the control Wt mice, suggesting a retardation in muscle growth. Ablation of the AR was induced with tamoxifen in ind-M-ARKO mice at 7 weeks old and found to reduce muscle AR expression effectively. After induction of ARKO, the mean mass of BC/LA in ind-M-ARKO mice was 20% lower at 8 weeks old and 25% lower at 25 weeks old than the corresponding tamoxifen-treated control mice, suggesting that AR signaling is required for maintaining adult skeletal muscle. These observations were accompanied with a decreased expression of the IGF-I splice variant, IGF-IEa, which might mediate the effect of AR signaling. In contrast, there were no significant differences between M-ARKO and control mice in limb muscle mass and gross morphology. However, the strength of fast-twitch and intermediate-twitch, but not slow-twitch, muscles were decreased without change in fatigue resistance. The decrease in muscle strength was also observed in ind-M-ARKO mice after tamoxifen induction. Ultrastructural analyses of the affected muscles indicated the disruption of myofibrils in about 10% of sarcomeres from M-ARKO mice with loss of myofilaments, rupture of Z lines, and enlarged sarcoplasm that might be the cause of reduced muscle strength.
AR roles in central nervous system function
Sex hormones play an important role in organization and differentiation of neural circuits that mediate many sexually dimorphic behaviors [Negri-Cesi et al., 2004; Phoenix et al., 1959] and neuroendocrine functions [Simerly, 1998]. The prenatal and neonatal testosterone surges in rodents masculinize neural circuits, leading to an enhancement of male sex behavioral patterns. Sex hormones also exhibit a wide array of neuroprotective and neurotherapeutic effects [Jones, 1993; Jones et al., 2001; Tronche et al., 1999]. The exhibition of feminine behavior by human subjects with CAIS and previous studies in Tfm rodent models have indicated that the AR plays significant roles in masculinization of several sexually dimorphic brain regions and various behaviors, including cognitive processing, sexual behavior, and stress response [Bodo, 2008; Zuloaga et al., 2008b]. Although the AR roles in these neural effects of androgens in males are not clear at present, they can be further delineated by studying various ARKO mouse models.
An early study with ARKO(CMV-Cre) mice suggested that perinatal brain masculinization requires AR function [Sato et al., 2004]. Raskin et al generated male N-ARKO by crossing Nestin-Cre male mice [Tronche et al., 1999] with female exon 2-floxed AR mice [Raskin et al., 2009]. In male N-ARKO mice the AR was selectively knocked out in the brain, including regions important in the regulation of reproduction-related neuroendocrine and behavioral functions, such as mating and aggression. Sexual motivation and performance, as well as aggressive behaviors, in N-ARKO mice were less vigorous with only a low percentage of these mice exhibiting complete sexual behavior and offensive attacks. The erectile activity during mating was also altered, whereas olfactory preference and neuronal activity were normal in N-ARKO mice compared to Wt controls. It was concluded from these observations that central AR signaling is involved in modulation of masculine behaviors and regulation of neuroendocrine functions of gonadotropic and somatotropic axes [Raskin et al., 2009].
In studies with the same male N-ARKO mouse model, Juntti et al observed that these mice exhibited decreased sexual and territorial behaviors compared to Wt mice [Juntti et al., 2010] in a similar fashion as reported earlier [Raskin et al., 2009]. However, Juntti et al did not observe elevated testosterone levels and growth retardation in their N-ARKO mice [Juntti et al., 2010], which were observations also made by others [Yu et al., 2013]. Since both labs [Juntti et al., 2010; Raskin et al., 2009] used the same nestin-Cre and AR exon 2-floxed mice to generate N-ARKO mice, the role of central AR in regulation of gonadotropic and somatotropic neuroendocrine functions remains to be clarified. Juntti et al also examined the AR expression in the brain of developing mice and found that during testosterone surges in prenatal and neonatal stages, very little AR was expressed in the medial amygdala, bed nucleus of stria terminalis (BNST), and the hypothalmic medial preoptic area [Juntti et al., 2010], which are important in the regulation of reproduction-related neuroendocrine and behavioral functions, such as mating and aggression. These investigators thus concluded that the AR is not involved in regulating organization and differentiation of neural circuits controlling sexual and masculine behavior in mice. Instead, the AR is involved only in modulating the execution of these behaviors.
Apart from modulating brain masculinization and the aforementioned regulation of central insulin and leptin sensitivity, the roles of the AR in other aspects of CNS functions remain to be studied with appropriate CNS region-specific ARKO mice.
AR roles in skin physiology and dermatology
The skin contains all the enzymes required to convert the adrenal androgens, dehydroepiandrosterone (DHEA) and dehydroepiandrosterone-sulfate (DHEA-S), into DHT and thus may be a local target of DHT-AR actions [Gilliver et al., 2003]. In human skin, the AR is expressed in epidermal keratinocytes, dermal papilla cells, hair follicles, sebaceous gland sebocytes, sweat glands, vascular endothelial and smooth muscle cells, and fibroblasts [Gilliver et al., 2003; Pochi and Strauss, 1974]. Therefore, androgen/AR signaling may well modulate the functions of these cell types and tissues, and affect their involvement in skin physiology and pathophysiology. A previous study has indicated that Tfm mice, in comparison with male Wt mice, displayed decreased collagen content in the skin, kidney, and lung [Markova et al., 2004], thereby suggesting androgen/AR signaling is involved in modulation of collagen synthesis in these tissues/organs.
Androgens are known to mediate sebum secretion and hair growth. Acne, androgenetic alopecia (male-pattern baldness), and hirsutism are the three main androgen-related diseases in dermatology [Hamilton, 1942; Kaufman, 1996; Rittmaster, 1997]. It has been known that prepubertal castration prevents androgenetic alopecia and sebum secretion, whereas adult castration stops progression of androgenic alopecia [Hamilton, 1942]. These effects of castration can be reversed by testosterone supplementation, suggesting that testosterone is involved in these dermatological diseases. Patients without a functional AR retain the sebum profile of preadrenarchal children and never development acne, facial hair, or androgenetic alopecia [Imperato-McGinley et al., 1993]. It has been found that having the AR with shorter CAG repeats in the exon 1 (hence more active AR) is more prevalent in patients with androgenetic alopecia [Gilliver et al., 2003]. In contrast, Kennedy’s Disease patients with longer CAG repeats in their AR gene have thicker hair than normal men [Sinclair et al., 2007]. Furthermore, the AR is expressed in fibroblasts, keratinocytes, and inflammatory cells during wound healing and thus might regulate the inflammation and repair processes involving these cells [Ashcroft and Mills, 2002]. These observations suggest that AR is involved in the development of androgen-mediated skin diseases, and development of various ARKO mice models has provided a golden opportunity to study the AR roles in these aspects as presented below.
AR roles in development of hair follicles
In humans after puberty, androgens stimulate the growth of beard, axillary, and pubic hairs, but cause vellus transformation in the frontal scalp hair in some genetic backgrounds, suggesting that the hair follicles have differential susceptibility to androgen/AR signaling. In contrast, hair follicles in the back of the head appeared to be insensitive to androgen/AR signaling [Inui et al., 2002].
In rat and mice, hair growth is stimulated by gonadectomy and inhibited by testosterone [Davis, 1963; Ebling and Johnson, 1964; Ebling and Hale, 1970] and thus they have androgen sensitivity similar to the frontal scalp of humans. ARKO(CMV-Cre) mice were found to grow longer and thicker hair than Wt mice. DHT inhibited hair regrowth in Wt, but not in ARKO(CMV-Cre) mice, suggesting that the effect of DHT is mediated by the AR [Naito et al., 2008]. Thus, hair growth in response to DHT in mouse skin appears to resemble hair growth in the frontal scalp in men. However, in which cell type the AR is involved and the molecular mechanisms of AR modulation remain to be studied.
AR roles in cutaneous wound healing
Several studies have indicated that androgen/AR signaling may play an important role in cutaneous wound healing. For instance, androgen deprivation by castration in mice resulted in accelerated wound healing associated with attenuated inflammation that can be reversed by administration of exogenous androgen [Ashcroft and Mills, 2002]. Also, the 5α-reductase inhibitor, which blocks conversion of testosterone to DHT, accelerated wound healing similar to castration, dampening the local inflammatory response of the wounds [Ashcroft and Mills, 2002]. Attenuation of wound healing and a decline in the permeability barrier homeostasis in elderly individuals are associated with local androgen excess [Ashcroft and Mills, 2002; Gilliver et al., 2008] and wound healing in elderly males is slower than elderly females [Ashcroft and Mills, 2002; Gilliver et al., 2008]. All these observations suggest that androgen/AR signaling plays a suppressive role in cutaneous wound healing.
A recent study in various ARKO mice has provided some understanding of how androgen/AR signaling might influence cutaneous wound healing [Lai et al., 2009]. In male ARKO(ACTB-Cre) mice, cutaneous wound healing was accelerated with faster re-epithelialization, attenuated inflammation, and increased collagen deposition. Intriguingly, male Myl-ARKO mice showed a similar acceleration of wound healing, suggesting that the AR in inflammatory cells, especially macrophages (as both ARKO models have reduced inflammatory monocytes, see the subsection titled “AR physiological roles in monocyte/macrophage production and inflammation”), plays an important role to suppress cutaneous wound repair, as previously suggested [Gilliver et al., 2008]. This hypothesis was further confirmed by the reduced wound healing rate in ARKO(ACTB-Cre) mice that received Wt bone marrow transplantation (so that only the immune cells at the wound site express the AR). TNFα, which is mainly expressed by infiltrating macrophages, was reduced in ARKO(ACTB-Cre) wounds compared to Wt wounds, while local restoration of TNFα could delay wound healing in ARKO(ACTB-Cre) mice, implicating TNFα as a critical mediator of AR function in wound healing suppression [Lai et al., 2009]. Further studies showed that the AR in macrophages could enhance TNFα expression independent of androgen binding to AR, and this effect was possibly mediated through augmenting ERK1/2 activation. Treatment with ASC-J9® to promote AR degradation led to suppressed TNFα expression in macrophages, and this effect could be reflected in the in vivo wound healing acceleration after topical ASC-J9® application on Wt mice wounds [Lai et al., 2009].
The roles of the AR in keratinocytes and dermal fibroblasts in cutaneous wound healing were also studied. In Ker-ARKO mice, the wound healing rate was similar to that in Wt littermates, however, the re-epithelialization was delayed, suggesting that AR in keratinocytes suppresses the proliferation and/or migration of epithelial cells in the cutaneous wounds, but is not critical to suppress the overall repair process. In contrast, there was no difference in wound healing rates between Fsp-ARKO and Wt mice although there was accelerated re-epithelialization in the mutant mice [Lai et al., 2009].
AR roles revealed in ARKI mouse models
The ARKO mouse models are useful for studying the effect of losing the AR activity on targeted physiological and pathophysiological processes. However, their use for studying pathophysiological roles of various AR mutants, particularly those with gain of function, are limited. In contrast, ARKI mouse models are particularly useful for studying pathophysiology of genetic diseases involving AR mutations. The number of reported AR mutations has increased steadily and rose from 605 in 2004 [Gottlieb et al., 2004] to 1,029 in September 2011 [Gottlieb et al., 2012]. Thus, global ARKI models will be particularly useful for studying pathogenesis of diseases associated with AR germ-line mutations; whereas tissue-specific ARKI models will be useful for the pathophysiological roles of AR somatic mutations. However, only a few ARKI studies have been reported to date and will be discussed below.
Polymorphism in the length of glutamine repeat sequence (poly Q) at the N-terminal domain encoded by the CAG trinucleotide repeat of the hAR gene has been implicated in several diseases, including male infertility, Kennedy’s Disease (spinal and bulbar muscular atrophy, SBMA), and prostate cancer [Casella et al., 2001]. The normal poly Q repeat ranged from 9 to 36 residues, while expansion to more than 40 residues results in Kennedy’s Disease. The AR transactivation is influenced by the length of the poly Q tract, increasing with shorter repeat length while decreasing with longer poly Q length [Chamberlain et al., 1994; Tut et al., 1997]. Albertelli et al generated 3 humanized AR mouse models in which hAR N-terminal domain containing 12, 21 or 48 glutamines were knocked in to replace mouse NTD of mAR gene in mouse embryonic stem cells to generate transgenic mice carrying the chimeric ARs, 12Q-h/mAR, 21Q-h/mAR, and 48Q-h/mAR, respectively [Albertelli et al., 2006]. All three types of mutant male mice displayed comparable behavior, growth, reproductive morphology, and fertility as Wt littermates. The weights of the SVs in 12Q-h/mAR mice tend to be higher than in mAR mice with increasing age and the differences became statistically significant at 24 months of age. The prostate of all h/mAR mice had normal appearace, although there was a tendency of decreasing AR mRNA and probasin expression, along with increasing poly Q length. Significantly higher expression levels of NKX3.1 (an AR-stimulated gene) and lower levels of clusterin (an AR-suppressed gene) were observed in 48Q-h/mAR, compared to mAR and the other mutant mice, suggesting reduced AR activity. These observations suggested that, similar to the in vitro observations [Chamberlain et al., 1994; Tut et al., 1997], the in vivo AR activity might be inversely related to the poly Q length, a notion subsequently confirmed in these h/mAR mutants [Simanainen et al., 2011]. Interestingly, after crossing with TRAMP mice, 12Q-h/mAR/TRAMP mice exhibited significantly higher tumor incidence than mAR/TRAMP and 21Q-h/mAR/TRAMP mice, which were comparable, whereas 48Q-h/mAR mice had significantly lower tumor incidence than Wt TRAMP mice [Albertelli et al., 2006]. Thus, this observation fits well with the observations made in AR(E231G) transgenic mice [Han et al., 2005] and hARKI(Osr1-Cre) mice [Zhu et al., 2011].
Yu et al generated 113Q-h/mAR mice [Yu et al., 2006b] using the same strategy as Albertelli et al [Albertelli et al., 2006]. Male 113Q-h/mAR mice displayed testis atrophy, reduced fertility, and systemic signs (high LH, low FSH) of partial androgen insensitivity that occur in Kennedy’s Disease patients. Testes of 113Q-h/mAR mice exhibited morphological abnormalities of germ cell maturation, decreased intracellular mutant AR solubility, increased apoptotic cells, and abnormal Sertoli cell cytoskeleton. Further study of 113Q-h/mAR mice indicated that they also developed neuromuscular pathology [Yu et al., 2006a]. Thus, male 113Q-h/mAR mice were smaller and had weaker muscle strength than Wt mice and their skeletal muscle displayed both neurogenic and myogenic pathology including age-dependent increase of AR immunoreactive nuclear inclusions in the skeletal muscle fibers. Eighty percent of male 113Q-h/mAR mice died between 2 to 4 months of age and this was caused by obstruction of the urinary tract. Castration of 113Q-h/mAR mice improved their muscle strength and prolonged their survival, suggesting that their neuromuscular pathology and motility were due to androgen-dependent AR poly Q-mediated defects.
Conclusions and future prospects
From the studies of the many ARKO mouse models summarized in this article, it can be concluded that the AR indeed plays many important physiological and pathophysiological roles in various organs and tissues. Although studies in Tfm and global ARKO mice have provided some useful information about the involvement of the AR in a particular physiological function, the studies in various cell type- or tissue-specific ARKO mice provided more conclusive results pertaining to the AR roles. However, it is important to ensure that the Cre mouse is really cell type- or tissue-specific and the floxed AR mice have no inherent abnormal phenotypes for generation of these specific ARKO mice. It should also be noted that the endogenous hormone level changes due to AR knockout in the mice may affect the phenotypes of the mice developed. So, careful interpretations should be made. However, tissue levels of testosterone or E2 have not been determined except for a few ARKO models.
Although various studies of ARKO mice, particularly the cell type- or tissue-specific ARKO mice, have established AR physiological roles in several organs/tissues, there are many aspects of AR physiological roles remaining to be elucidated. In addition, most of the mechanisms by which AR regulates physiological and pathophysiological phenotypes have not been studied in detail, so these mechanism studies using these valuable mouse models may be another important thing that needs to be done. The possible involvement of AR non-genomic function needs to be investigated, particularly when expression changes of certain non-AR target genes are associated with the observed phenotype of a specific ARKO mouse. In the cases in which interaction of AR signaling cascade with another nuclear receptor cascade is involved, proper double knockout mice need to be developed and studied to delineate the exact AR roles.
Most importantly, we need to be careful as to how we apply results from these mice studies to human cases.
Acknowledgements
Grant support: NIH Grants (CA127300 and CA156700), and Taiwan Department of Health Clinical Trial and Research Center of Excellence Grant DOH99-TD-B-111-004 (China Medical University, Taichung, Taiwan). Disclosure summary: ASC-J9® was patented by the University of Rochester, the University of North Carolina, and AndroScience, and then licensed to AndroScience. Both the University of Rochester and C.C. own royalties and equity in AndroScience.
Abbreviations
- AAA
abdominal aorta aneurysm
- ACTB-Cre
human β-actin-Cre
- ADT
androgen deprivation therapy
- aP2
adipocyte protein 2
- AR
androgen receptor
- ARKI
androgen receptor knock-in
- ARKO
androgen receptor knockout
- BC/LA
bulbocavernosus (BC) and levator ani (LA)
- BMD
bone mineral density
- C/EBP
CCAAT/enhancer-binding protein
- CAG-Cre
chicken β-actin promoter driven-Cre
- CAIS
complete androgen insensitivity syndrome
- CMP
common myeloid progenitors
- CMV-Cre
Cytomegalovirus promoter driven-Cre
- DBD
DNA binding domain
- DHEA-S
dehydroepiandrosterone-sulfate
- DHT
5α-dihydrotestosterone
- ella-Cre
EIIa promoter driven-Cre
- fsp1-Cre
fibroblast-specific protein 1 promoter-driven-Cre
- GMP
granulocyte/macrophage myeloid progenitors
- LBD
ligand binding domain
- NO
nitric oxide
- Osr1-Cre
odd-skipped related 1 promoter driven-Cre
- PBMC
peripheral blood mononuclear cell
- PCOS
polycystic ovary syndrome
- PGK-Cre
phosphoglycerate kinase-Cre
- PPAR
peroxisome proliferation activated receptor
- SREBP
sterol regulatory element-binding protein
- Sycp1-Cre
synaptonemal complex protein 1 promoter driven-Cre
- TECs
thymic epithelial cells
- Tfm
testicular feminization
- UCP1
uncoupling protein 1
- WT
wild type
Supplementary Material
References
- Aarnisalo P., Palvimo J. J., Janne O. A. CREB-binding protein in androgen receptor-mediated signaling. Proc Natl Acad Sci U S A . 1998;95:2122–7. doi: 10.1073/pnas.95.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accili D. Lilly lecture 2003: the struggle for mastery in insulin action: from triumvirate to republic. Diabetes. 2004;53:1633–42. doi: 10.2337/diabetes.53.7.1633. [DOI] [PubMed] [Google Scholar]
- Albertelli M. A., Scheller A., Brogley M., Robins D. M. Replacing the mouse androgen receptor with human alleles demonstrates glutamine tract length-dependent effects on physiology and tumorigenesis in mice. Mol Endocrinol. 2006;20:1248–60. doi: 10.1210/me.2006-0021. [DOI] [PubMed] [Google Scholar]
- Albright F. The effect of hormones on osteogenesis in man. Recent Prog Horm Res. 1947;1:293–353. doi: 10.1016/b978-1-4831-9840-8.50014-4. [DOI] [PubMed] [Google Scholar]
- Almeida M., Han L., Martin-Millan M., Plotkin L. I., Stewart S. A., Roberson P. K., Kousteni S., O'Brien C. A., Bellido T., Parfitt A. M., Weinstein R. S., Jilka R. L., Manolagas S. C. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282:27285–97. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter B. P. Fanconi's anemia. Current concepts. Am J Pediatr Hematol Oncol. 1992;14:170–6. [PubMed] [Google Scholar]
- Altuwaijri S., Lee D. K., Chuang K. H., Ting H. J., Yang Z., Xu Q., Tsai M. Y., Yeh S., Hanchett L. A., Chang H. C., Chang C. Androgen receptor regulates expression of skeletal muscle-specific proteins and muscle cell types. Endocrine. 2004;25:27–32. doi: 10.1385/endo:25:1:27. [DOI] [PubMed] [Google Scholar]
- Altuwaijri S., Chuang K. H., Lai K. P., Lai J. J., Lin H. Y., Young F. M., Bottaro A., Tsai M. Y., Zeng W. P., Chang H. C., Yeh S., Chang C. Susceptibility to autoimmunity and B cell resistance to apoptosis in mice lacking androgen receptor in B cells. Mol Endocrinol. 2009;23:444–53. doi: 10.1210/me.2008-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. M., Liao S. Selective retention of dihydrotestosterone by prostatic nuclei. Nature. 1968;219:277–9. doi: 10.1038/219277a0. [DOI] [PubMed] [Google Scholar]
- Ashcroft G. S., Mills S. J. Androgen receptor-mediated inhibition of cutaneous wound healing. J Clin Invest. 2002;110:615–24. doi: 10.1172/JCI15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaria S. Androgen deprivation therapy, insulin resistance, and cardiovascular mortality: an inconvenient truth. J Androl. 2008;29:534–9. doi: 10.2164/jandrol.108.005454. [DOI] [PubMed] [Google Scholar]
- Basaria S., Dobs A. S. Hypogonadism and androgen replacement therapy in elderly men. Am J Med. 2001;110:563–72. doi: 10.1016/s0002-9343(01)00663-5. [DOI] [PubMed] [Google Scholar]
- Berton T. R., Matsumoto T., Page A., Conti C. J., Deng C. X., Jorcano J. L., Johnson D. G. Tumor formation in mice with conditional inactivation of Brca1 in epithelial tissues. Oncogene. 2003;22:5415–26. doi: 10.1038/sj.onc.1206825. [DOI] [PubMed] [Google Scholar]
- Bhasin S., Woodhouse L., Casaburi R., Singh A. B., Mac R. P., Lee M., Yarasheski K. E., Sinha-Hikim I., Dzekov C., Dzekov J., Magliano L., Storer T. W. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678–88. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- Bhasin S., Woodhouse L., Casaburi R., Singh A. B., Bhasin D., Berman N., Chen X., Yarasheski K. E., Magliano L., Dzekov C., Dzekov J., Bross R., Phillips J., Sinha-Hikim I., Shen R., Storer T. W. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:E1172–81. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- Bhowmick N. A., Chytil A., Plieth D., Gorska A. E., Dumont N., Shappell S., Washington M. K., Neilson E. G., Moses H. L. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–51. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- Bluher M., Michael M. D., Peroni O. D., Ueki K., Carter N., Kahn B. B., Kahn C. R. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- Bodo C. A role for the androgen receptor in the sexual differentiation of the olfactory system in mice. Brain Res Rev. 2008;57:321–31. doi: 10.1016/j.brainresrev.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouche C., Serdy S., Kahn C. R., Goldfine A. B. The cellular fate of glucose and its relevance in type 2 diabetes. Endocr Rev. 2004;25:807–30. doi: 10.1210/er.2003-0026. [DOI] [PubMed] [Google Scholar]
- Bourghardt J., Wilhelmson A. S., Alexanderson C., De Gendt K., Verhoeven G., Krettek A., Ohlsson C., Tivesten A. Androgen receptor-dependent and independent atheroprotection by testosterone in male mice. Endocrinology. 2010;151:5428–37. doi: 10.1210/en.2010-0663. [DOI] [PubMed] [Google Scholar]
- Bruchovsky N., Wilson J. D. The conversion of testosterone to 5-alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J Biol Chem. 1968a;243:2012–21. [PubMed] [Google Scholar]
- Bruchovsky N., Wilson J. D. The intranuclear binding of testosterone and 5-alpha-androstan-17-beta-ol-3-one by rat prostate. J Biol Chem. 1968b;243:5953–60. [PubMed] [Google Scholar]
- Bruning J. C., Michael M. D., Winnay J. N., Hayashi T., Horsch D., Accili D., Goodyear L. J., Kahn C. R. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2:559–69. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- Byron J. W. Effect of steroids on the cycling of haemopoietic stem cells. Nature. 1970;228:1204–1204. doi: 10.1038/2281204a0. [DOI] [PubMed] [Google Scholar]
- Callewaert F., Bakker A., Schrooten J., Van Meerbeek B., Verhoeven G., Boonen S., Vanderschueren D. Androgen receptor disruption increases the osteogenic response to mechanical loading in male mice. J Bone Miner Res. 2010b;25:124–31. doi: 10.1359/jbmr.091001. [DOI] [PubMed] [Google Scholar]
- Callewaert F., Venken K., Ophoff J., De Gendt K., Torcasio A., van Lenthe G. H., Van Oosterwyck H., Boonen S., Bouillon R., Verhoeven G., Vanderschueren D. Differential regulation of bone and body composition in male mice with combined inactivation of androgen and estrogen receptor-alpha. Faseb J. 2009;23:232–40. doi: 10.1096/fj.08-113456. [DOI] [PubMed] [Google Scholar]
- Callewaert F., Boonen S., Vanderschueren D. Sex steroids and the male skeleton: a tale of two hormones. Trends Endocrinol Metab. 2010a;21:89–95. doi: 10.1016/j.tem.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Casella R., Maduro M. R., Lipshultz L. I., Lamb D. J. Significance of the polyglutamine tract polymorphism in the androgen receptor. Urology. 2001;58:651–6. doi: 10.1016/s0090-4295(01)01401-7. [DOI] [PubMed] [Google Scholar]
- Celotti F., Negri Cesi P. Anabolic steroids: a review of their effects on the muscles, of their possible mechanisms of action and of their use in athletics. J Steroid Biochem Mol Biol. 1992;43:469–77. doi: 10.1016/0960-0760(92)90085-w. [DOI] [PubMed] [Google Scholar]
- Chamberlain N. L., Driver E. D., Miesfeld R. L. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 1994;22:3181–6. doi: 10.1093/nar/22.15.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon C., Duteil D., Vignaud A., Ferry A., Messaddeq N., Malivindi R., Kato S., Chambon P., Metzger D. Myocytic androgen receptor controls the strength but not the mass of limb muscles. Proc Natl Acad Sci U S A. 2010;107:14327–32. doi: 10.1073/pnas.1009536107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. S., Kokontis J., Liao S. T. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988a;240:324–6. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- Chang C. S., Kokontis J., Liao S. T. Structural analysis of complementary DNA and amino acid sequences of human and rat androgen receptors. Proc Natl Acad Sci U S A. 1988b;85:7211–5. doi: 10.1073/pnas.85.19.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest N. J., Zhou Z. X., Lubahn D. B., Olsen K. L., Wilson E. M., French F. S. A frameshift mutation destabilizes androgen receptor messenger RNA in the Tfm mouse. Mol Endocrinol. 1991;5:573–81. doi: 10.1210/mend-5-4-573. [DOI] [PubMed] [Google Scholar]
- Chen Y., Zajac J. D., MacLean H. E. Androgen regulation of satellite cell function. J Endocrinol. 2005;186:21–31. doi: 10.1677/joe.1.05976. [DOI] [PubMed] [Google Scholar]
- Chiang C., Chiu M., Moore A. J., Anderson P. H., Ghasem-Zadeh A., McManus J. F., Ma C., Seeman E., Clemens T. L., Morris H. A., Zajac J. D., Davey R. A. Mineralization and bone resorption are regulated by the androgen receptor in male mice. J Bone Miner Res. 2009;24:621–31. doi: 10.1359/jbmr.081217. [DOI] [PubMed] [Google Scholar]
- Chiu W. S., McManus J. F., Notini A. J., Cassady A. I., Zajac J. D., Davey R. A. Transgenic mice that express Cre recombinase in osteoclasts. Genesis. 2004;39:178–85. doi: 10.1002/gene.20041. [DOI] [PubMed] [Google Scholar]
- Choudhury A. I., Heffron H., Smith M. A., Al-Qassab H., Xu A. W., Selman C., Simmgen M., Clements M., Claret M., Maccoll G., Bedford D. C., Hisadome K., Diakonov I., Moosajee V., Bell J. D., Speakman J. R., Batterham R. L., Barsh G. S., Ashford M. L., Withers D. J. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. J Clin Invest. 2005;115:940–50. doi: 10.1172/JCI24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang K. H., Altuwaijri S., Li G., Lai J. J., Chu C. Y., Lai K. P., Lin H. Y., Hsu J. W., Keng P., Wu M. C., Chang C. Neutropenia with impaired host defense against microbial infection in mice lacking androgen receptor. J Exp Med. 2009;206:1181–99. doi: 10.1084/jem.20082521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg D. J., Riedy C. A., Smith K. A., Benoit S. C., Woods S. C. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52:682–7. doi: 10.2337/diabetes.52.3.682. [DOI] [PubMed] [Google Scholar]
- Clegg D. J., Brown L. M., Woods S. C., Benoit S. C. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–87. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- Dati E., Baroncelli G. I., Mora S., Russo G., Baldinotti F., Parrini D., Erba P., Simi P., Bertelloni S. Body composition and metabolic profile in women with complete androgen insensitivity syndrome. Sex Dev. 2009;3:188–93. doi: 10.1159/000228719. [DOI] [PubMed] [Google Scholar]
- Daugherty A., Manning M. W., Cassis L. A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–12. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. K. Quantitative morphological studies upon the influence of the endocrine system on the growth of hair by white mice. Acta Endocrinol (Copenh) 1963;44:SUPPL85:81–102. doi: 10.1530/acta.0.044s009. [DOI] [PubMed] [Google Scholar]
- De Gendt K., Swinnen J. V., Saunders P. T., Schoonjans L., Dewerchin M., Devos A., Tan K., Atanassova N., Claessens F., Lecureuil C., Heyns W., Carmeliet P., Guillou F., Sharpe R. M., Verhoeven G. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A. 2004;101:1327–32. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitch E. A. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992;216:117–34. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio L., Bennouna S., Salinas J., Denkers E. Y. CXCR2 deficiency confers impaired neutrophil recruitment and increased susceptibility during Toxoplasma gondii infection. J Immunol. 2001;167:6503–9. doi: 10.4049/jimmunol.167.11.6503. [DOI] [PubMed] [Google Scholar]
- Ebling F. J., Hale P. A. The control of the mammalian moult. Mem Soc Endocrinol. 1970;18:215–37. [Google Scholar]
- Ebling F. J., Johnson E. The action of hormones on spontaneous hair growth cycles in the rat. J Endocrinol. 1964;29:193–201. doi: 10.1677/joe.0.0290193. [DOI] [PubMed] [Google Scholar]
- Ellis T. M., Moser M. T., Le P. T., Flanigan R. C., Kwon E. D. Alterations in peripheral B cells and B cell progenitors following androgen ablation in mice. Int Immunol. 2001;13:553–8. doi: 10.1093/intimm/13.4.553. [DOI] [PubMed] [Google Scholar]
- Emerling B. M., Weinberg F., Liu J. L., Mak T. W., Chandel N. S. PTEN regulates p300-dependent hypoxia-inducible factor 1 transcriptional activity through Forkhead transcription factor 3a (FOXO3a) Proc Natl Acad Sci U S A. 2008;105:2622–7. doi: 10.1073/pnas.0706790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W., Yanase T., Nomura M., Okabe T., Goto K., Sato T., Kawano H., Kato S., Nawata H. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes. 2005;54:1000–8. doi: 10.2337/diabetes.54.4.1000. [DOI] [PubMed] [Google Scholar]
- Ferguson C., Hardy S. L., Werner D. F., Hileman S. M., Delorey T. M., Homanics G. E. New insight into the role of the beta3 subunit of the GABAA-R in development, behavior, body weight regulation, and anesthesia revealed by conditional gene knockout. BMC Neurosci. 2007;8:8–8. doi: 10.1186/1471-2202-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman E. R., Bloom D. A., McGuire E. J. A brief history of testosterone. J Urol . 2001;165:371–3. doi: 10.1097/00005392-200102000-00004. [DOI] [PubMed] [Google Scholar]
- Frenkel B., Hong A., Baniwal S. K., Coetzee G. A., Ohlsson C., Khalid O., Gabet Y. Regulation of adult bone turnover by sex steroids. J Cell Physiol. 2010;224:305–10. doi: 10.1002/jcp.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronsdal K., Engedal N., Slagsvold T., Saatcioglu F. CREB binding protein is a coactivator for the androgen receptor and mediates cross-talk with AP-1. J Biol Chem. 1998;273:31853–9. doi: 10.1074/jbc.273.48.31853. [DOI] [PubMed] [Google Scholar]
- Gallardo N., Bonzon-Kulichenko E., Fernandez-Agullo T., Molto E., Gomez-Alonso S., Blanco P., Carrascosa J. M., Ros M., Andres A. Tissue-specific effects of central leptin on the expression of genes involved in lipid metabolism in liver and white adipose tissue. Endocrinology. 2007;148:5604–10. doi: 10.1210/en.2007-0933. [DOI] [PubMed] [Google Scholar]
- Gilliver S. C., Wu F., Ashcroft G. S. Regulatory roles of androgens in cutaneous wound healing. Thromb Haemost. 2003;90:978–85. doi: 10.1160/TH03-05-0302. [DOI] [PubMed] [Google Scholar]
- Gilliver S. C., Ruckshanthi J. P., Hardman M. J., Nakayama T., Ashcroft G. S. Sex dimorphism in wound healing: the roles of sex steroids and macrophage migration inhibitory factor. Endocrinology. 2008;149:5747–57. doi: 10.1210/en.2008-0355. [DOI] [PubMed] [Google Scholar]
- Gonit M., Zhang J., Salazar Md, Cui H., Shatnawi A., Trumbly R., Ratnam M. Hormone depletion-insensitivity of prostate cancer cells is supported by the AR without binding to classical response elements. Mol Endocrinol. 2011;25:621–34. doi: 10.1210/me.2010-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Montelongo M. C., Marin R., Gomez T., Diaz M. Androgens are powerful non-genomic inducers of calcium sensitization in visceral smooth muscle. Steroids. 2010;75:533–8. doi: 10.1016/j.steroids.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Gottlieb B., Beitel L. K., Wu J. H., Trifiro M. The androgen receptor gene mutations database (ARDB): 2004 update. Hum Mutat. 2004;23:527–33. doi: 10.1002/humu.20044. [DOI] [PubMed] [Google Scholar]
- Gottlieb B., Beitel L. K., Nadarajah A., Paliouras M., Trifiro M. The androgen receptor gene mutations database: 2012 update. Hum Mutat. 2012;33:887–94. doi: 10.1002/humu.22046. [DOI] [PubMed] [Google Scholar]
- Graff R. J., Lappe M. A., Snell G. D. The influence of the gonads and adrenal glands on the immune response to skin grafts. Transplantation. 1969;7:105–11. doi: 10.1097/00007890-196902000-00003. [DOI] [PubMed] [Google Scholar]
- Guerre-Millo M., Gervois P., Raspe E., Madsen L., Poulain P., Derudas B., Herbert J. M., Winegar D. A., Willson T. M., Fruchart J. C., Berge R. K., Staels B. Peroxisome proliferator-activated receptor alpha activators improve insulin sensitivity and reduce adiposity. J Biol Chem. 2000;275:16638–42. doi: 10.1074/jbc.275.22.16638. [DOI] [PubMed] [Google Scholar]
- Hamilton J. B. Male hormone stimulation is prerequisite and an incitant in common baldness. American Journal of Anatomy. 1942;71:451–480. [Google Scholar]
- Han G., Buchanan G., Ittmann M., Harris J. M., Yu X., Demayo F. J., Tilley W., Greenberg N. M. Mutation of the androgen receptor causes oncogenic transformation of the prostate. Proc Natl Acad Sci U S A. 2005;102:1151–6. doi: 10.1073/pnas.0408925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Asano M., Shinton S. A., Gui M., Allman D., Stewart C. L., Silver J., Hardy R. R. Positive selection of natural autoreactive B cells. Science. 1999;285:113–6. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- He W., Barak Y., Hevener A., Olson P., Liao D., Le J., Nelson M., Ong E., Olefsky J. M., Evans R. M. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100:15712–7. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemers H. V., Tindall D. J. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28:778–808. doi: 10.1210/er.2007-0019. [DOI] [PubMed] [Google Scholar]
- Heinlein C. A., Chang C. Androgen receptor (AR) coregulators: an overview. Endocr Rev. 2002a;23:175–200. doi: 10.1210/edrv.23.2.0460. [DOI] [PubMed] [Google Scholar]
- Heinlein C. A., Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002b;16:2181–7. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- Hennet T., Hagen F. K., Tabak L. A., Marth J. D. T-cell-specific deletion of a polypeptide N-acetylgalactosaminyl-transferase gene by site-directed recombination. Proc Natl Acad Sci U S A. 1995;92:12070–4. doi: 10.1073/pnas.92.26.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst K. L., Bhasin S. Testosterone action on skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7:271–7. doi: 10.1097/00075197-200405000-00006. [DOI] [PubMed] [Google Scholar]
- Hince M., Sakkal S., Vlahos K., Dudakov J., Boyd R., Chidgey A. The role of sex steroids and gonadectomy in the control of thymic involution. Cell Immunol. 2008;252:122–38. doi: 10.1016/j.cellimm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Holdcraft R. W., Braun R. E. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131:459–67. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- Horn Y. The effect of androgenic hormones on bone marrow of rats receiving chemotherapy. Oncology. 1971;25:512–9. doi: 10.1159/000224602. [DOI] [PubMed] [Google Scholar]
- Horn Y., Price D. C. The effect of androgens on erythroid and granulocytic marrow recovery in the irradiated rat. Acta Haematol. 1972;48:300–6. doi: 10.1159/000208474. [DOI] [PubMed] [Google Scholar]
- Huang C. -K. P. H., Lee S. O., Chang C. The male hormone signals in cardiovascular diseases. J Hypertension. 2012;30 (Suppl 1) Abstract #1069:e311–e311. [Google Scholar]
- Ibanez L., Jaramillo A. M., Ferrer A., de Zegher F. High neutrophil count in girls and women with hyperinsulinaemic hyperandrogenism: normalization with metformin and flutamide overcomes the aggravation by oral contraception. Hum Reprod. 2005;20:2457–62. doi: 10.1093/humrep/dei072. [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Aihara K., Akaike M., Sato T., Ishikawa K., Ise T., Yagi S., Iwase T., Ueda Y., Yoshida S., Azuma H., Walsh K., Tamaki T., Kato S., Matsumoto T. Androgen receptor counteracts Doxorubicin-induced cardiotoxicity in male mice. Mol Endocrinol. 2010;24:1338–48. doi: 10.1210/me.2009-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Aihara K., Sato T., Akaike M., Yoshizumi M., Suzaki Y., Izawa Y., Fujimura M., Hashizume S., Kato M., Yagi S., Tamaki T., Kawano H., Matsumoto T., Azuma H., Kato S., Matsumoto T. Androgen receptor gene knockout male mice exhibit impaired cardiac growth and exacerbation of angiotensin II-induced cardiac fibrosis. J Biol Chem. 2005;280:29661–6. doi: 10.1074/jbc.M411694200. [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Aihara K., Yoshida S., Sato T., Yagi S., Iwase T., Sumitomo Y., Ise T., Ishikawa K., Azuma H., Akaike M., Kato S., Matsumoto T. Androgen-androgen receptor system protects against angiotensin II-induced vascular remodeling. Endocrinology. 2009;150:2857–64. doi: 10.1210/en.2008-1254. [DOI] [PubMed] [Google Scholar]
- Imperato-McGinley J., Gautier T., Cai L. Q., Yee B., Epstein J., Pochi P. The androgen control of sebum production. Studies of subjects with dihydrotestosterone deficiency and complete androgen insensitivity. J Clin Endocrinol Metab. 1993;76:524–8. doi: 10.1210/jcem.76.2.8381804. [DOI] [PubMed] [Google Scholar]
- Inoue H., Ogawa W., Asakawa A., Okamoto Y., Nishizawa A., Matsumoto M., Teshigawara K., Matsuki Y., Watanabe E., Hiramatsu R., Notohara K., Katayose K., Okamura H., Kahn C. R., Noda T., Takeda K., Akira S., Inui A., Kasuga M. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab. 2006;3:267–75. doi: 10.1016/j.cmet.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Inui S., Fukuzato Y., Nakajima T., Yoshikawa K., Itami S. Androgen-inducible TGF-beta1 from balding dermal papilla cells inhibits epithelial cell growth: a clue to understand paradoxical effects of androgen on human hair growth. Faseb J. 2002;16:1967–9. doi: 10.1096/fj.02-0043fje. [DOI] [PubMed] [Google Scholar]
- Isken O., Maquat L. E. Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function. Genes Dev. 2007;21:1833–56. doi: 10.1101/gad.1566807. [DOI] [PubMed] [Google Scholar]
- Johansen J. A., Breedlove S. M., Jordan C. L. Androgen receptor expression in the levator ani muscle of male mice. J Neuroendocrinol. 2007;19:823–6. doi: 10.1111/j.1365-2826.2007.01592.x. [DOI] [PubMed] [Google Scholar]
- Jones R. D., Pugh P. J., Hall J., Channer K. S., Jones T. H. Altered circulating hormone levels, endothelial function and vascular reactivity in the testicular feminised mouse. Eur J Endocrinol. 2003;148:111–20. doi: 10.1530/eje.0.1480111. [DOI] [PubMed] [Google Scholar]
- Jones K. J. Gonadal steroids and neuronal regeneration. A therapeutic role. Adv Neurol. 1993;59:227–40. [PubMed] [Google Scholar]
- Jones K. J., Brown T. J., Damaser M. Neuroprotective effects of gonadal steroids on regenerating peripheral motoneurons. Brain Res Brain Res Rev. 2001;37:372–82. doi: 10.1016/s0165-0173(01)00107-2. [DOI] [PubMed] [Google Scholar]
- Juntti S. A., Tollkuhn J., Wu M. V., Fraser E. J., Soderborg T., Tan S., Honda S., Harada N., Shah N. M. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron. 2010;66:260–72. doi: 10.1016/j.neuron.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H. Y., Shyr C. R., Huang C. K., Tsai M. Y., Orimo H., Lin P. C., Chang C., Huang K. E. Altered TNSALP expression and phosphate regulation contribute to reduced mineralization in mice lacking androgen receptor. Mol Cell Biol. 2008;28:7354–67. doi: 10.1128/MCB.00582-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H. Y., Cho C. L., Huang K. L., Wang J. C., Hu Y. C., Lin H. K., Chang C., Huang K. E. Nongenomic androgen activation of phosphatidylinositol 3-kinase/Akt signaling pathway in MC3T3-E1 osteoblasts. J Bone Miner Res. 2004;19:1181–90. doi: 10.1359/JBMR.040306. [DOI] [PubMed] [Google Scholar]
- Kaufman K. D. Androgen metabolism as it affects hair growth in androgenetic alopecia. Dermatol Clin. 1996;14:697–711. doi: 10.1016/s0733-8635(05)70396-x. [DOI] [PubMed] [Google Scholar]
- Kawano H., Sato T., Yamada T., Matsumoto T., Sekine K., Watanabe T., Nakamura T., Fukuda T., Yoshimura K., Yoshizawa T., Aihara K., Yamamoto Y., Nakamichi Y., Metzger D., Chambon P., Nakamura K., Kawaguchi H., Kato S. Suppressive function of androgen receptor in bone resorption. Proc Natl Acad Sci U S A. 2003;100:9416–21. doi: 10.1073/pnas.1533500100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy B. J. Stimulation of hematopoiesis by androgenic hormones. Geriatrics. 1965;20:808–15. [PubMed] [Google Scholar]
- Klinker M. W., Lundy S. K. Multiple mechanisms of immune suppression by B lymphocytes. Mol Med. 2012;18:123–37. doi: 10.2119/molmed.2011.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konner A. C., Janoschek R., Plum L., Jordan S. D., Rother E., Ma X., Xu C., Enriori P., Hampel B., Barsh G. S., Kahn C. R., Cowley M. A., Ashcroft F. M., Bruning J. C. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–49. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Kovacs W. J., Olsen N. J. Androgen receptors in human thymocytes. J Immunol. 1987;139:490–3. [PubMed] [Google Scholar]
- Kubota N., Terauchi Y., Tobe K., Yano W., Suzuki R., Ueki K., Takamoto I., Satoh H., Maki T., Kubota T., Moroi M., Okada-Iwabu M., Ezaki O., Nagai R., Ueta Y., Kadowaki T., Noda T. Insulin receptor substrate 2 plays a crucial role in beta cells and the hypothalamus. J Clin Invest. 2004;114:917–27. doi: 10.1172/JCI21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahita R. G. Effects of gender on the immune system. Implications for neuropsychiatric systemic lupus erythematosus. Ann N Y Acad Sci. 1997;823:247–51. doi: 10.1111/j.1749-6632.1997.tb48396.x. [DOI] [PubMed] [Google Scholar]
- Lai J. J., Lai K. P., Chuang K. H., Chang P., Yu I. C., Lin W. J., Chang C. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression. J Clin Invest. 2009;119:3739–51. doi: 10.1172/JCI39335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K. P., Lai J. J., Chang P., Altuwaijri S., Hsu J. W., Chuang K. H., Shyr C. R., Yeh S., Chang C. Targeting thymic epithelia AR enhances T-cell reconstitution and bone marrow transplant grafting efficacy. Mol Endocrinol. 2013;27:25–37. doi: 10.1210/me.2012-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand Y., Luria V., Haffner-Krausz R., Lonai P. Maternally expressed PGK-Cre transgene as a tool for early and uniform activation of the Cre site-specific recombinase. Transgenic Res. 1998;7:105–12. doi: 10.1023/a:1008868325009. [DOI] [PubMed] [Google Scholar]
- Lewandoski M., Meyers E. N., Martin G. R. Analysis of Fgf8 gene function in vertebrate development. Cold Spring Harb Symp Quant Biol. 1997;62:159–68. [PubMed] [Google Scholar]
- Lim P., Robson M., Spaliviero J., McTavish K. J., Jimenez M., Zajac J. D., Handelsman D. J., Allan C. M. Sertoli cell androgen receptor DNA binding domain is essential for the completion of spermatogenesis. Endocrinology. 2009;150:4755–65. doi: 10.1210/en.2009-0416. [DOI] [PubMed] [Google Scholar]
- Lin X., Taguchi A., Park S., Kushner J. A., Li F., Li Y., White M. F. Dysregulation of insulin receptor substrate 2 in beta cells and brain causes obesity and diabetes. J Clin Invest. 2004;114:908–16. doi: 10.1172/JCI22217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. Y., Yu I. C., Wang R. S., Chen Y. T., Liu N. C., Altuwaijri S., Hsu C. L., Ma W. L., Jokinen J., Sparks J. D., Yeh S., Chang C. Increased hepatic steatosis and insulin resistance in mice lacking hepatic androgen receptor. Hepatology. 2008;47:1924–35. doi: 10.1002/hep.22252. [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Xu Q., Yeh S., Wang R. S., Sparks J. D., Chang C. Insulin and leptin resistance with hyperleptinemia in mice lacking androgen receptor. Diabetes. 2005;54:1717–25. doi: 10.2337/diabetes.54.6.1717. [DOI] [PubMed] [Google Scholar]
- Li M., Sobue G., Doyu M., Mukai E., Hashizume Y., Mitsuma T. Primary sensory neurons in X-linked recessive bulbospinal neuropathy: histopathology and androgen receptor gene expression. Muscle Nerve. 1995;18:301–8. doi: 10.1002/mus.880180306. [DOI] [PubMed] [Google Scholar]
- Liu F., Woitge H. W., Braut A., Kronenberg M. S., Lichtler A. C., Mina M., Kream B. E. Expression and activity of osteoblast-targeted Cre recombinase transgenes in murine skeletal tissues. Int J Dev Biol. 2004;48:645–53. doi: 10.1387/ijdb.041816fl. [DOI] [PubMed] [Google Scholar]
- Livingstone C., Collison M. Sex steroids and insulin resistance. Clin Sci (Lond) 2002;102:151–66. doi: 10.1042/cs1020151. [DOI] [PubMed] [Google Scholar]
- Lobaccaro J. M., Poujol N., Chiche L., Lumbroso S., Brown T. R., Sultan C. Molecular modeling and in vitro investigations of the human androgen receptor DNA-binding domain: application for the study of two mutations. Mol Cell Endocrinol. 1996;116:137–47. doi: 10.1016/0303-7207(95)03709-8. [DOI] [PubMed] [Google Scholar]
- Lubahn D. B., Joseph D. R., Sullivan P. M., Willard H. F., French F. S., Wilson E. M. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science. 1988;240:327–30. doi: 10.1126/science.3353727. [DOI] [PubMed] [Google Scholar]
- Lu Y., Xie Y., Zhang S., Dusevich V., Bonewald L. F., Feng J. Q. DMP1-targeted Cre expression in odontoblasts and osteocytes. J Dent Res. 2007;86:320–5. doi: 10.1177/154405910708600404. [DOI] [PubMed] [Google Scholar]
- Lyon M. F., Hawkes S. G. X-linked gene for testicular feminization in the mouse. Nature. 1970;227:1217–9. doi: 10.1038/2271217a0. [DOI] [PubMed] [Google Scholar]
- MacLean H. E., Chiu W. S., Ma C., McManus J. F., Davey R. A., Cameron R., Notini A. J., Zajac J. D. A floxed allele of the androgen receptor gene causes hyperandrogenization in male mice. Physiol Genomics. 2008a;33:133–7. doi: 10.1152/physiolgenomics.00260.2007. [DOI] [PubMed] [Google Scholar]
- MacLean H. E., Moore A. J., Sastra S. A., Morris H. A., Ghasem-Zadeh A., Rana K., Axell A. M., Notini A. J., Handelsman D. J., Seeman E., Zajac J. D., Davey R. A. DNA-binding-dependent androgen receptor signaling contributes to gender differences and has physiological actions in males and females. J Endocrinol. 2010;206:93–103. doi: 10.1677/JOE-10-0026. [DOI] [PubMed] [Google Scholar]
- MacLean H. E., Chiu W. S., Notini A. J., Axell A. M., Davey R. A., McManus J. F., Ma C., Plant D. R., Lynch G. S., Zajac J. D. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. Faseb J. 2008b;22:2676–89. doi: 10.1096/fj.08-105726. [DOI] [PubMed] [Google Scholar]
- Mantalaris A., Panoskaltsis N., Sakai Y., Bourne P., Chang C., Messing E. M., Wu J. H. Localization of androgen receptor expression in human bone marrow. J Pathol. 2001;193:361–6. doi: 10.1002/1096-9896(0000)9999:9999<::AID-PATH803>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Markova M. S., Zeskand J., McEntee B., Rothstein J., Jimenez S. A., Siracusa L. D. A role for the androgen receptor in collagen content of the skin. J Invest Dermatol. 2004;123:1052–6. doi: 10.1111/j.0022-202X.2004.23494.x. [DOI] [PubMed] [Google Scholar]
- Metzger D., Chambon P. Site- and time-specific gene targeting in the mouse. Methods. 2001;24:71–80. doi: 10.1006/meth.2001.1159. [DOI] [PubMed] [Google Scholar]
- Meyers E. N., Lewandoski M., Martin G. R. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat Genet. 1998;18:136–41. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- Miniou P., Tiziano D., Frugier T., Roblot N., Le Meur M., Melki J. Gene targeting restricted to mouse striated muscle lineage. Nucleic Acids Res. 1999;27:e27. doi: 10.1093/nar/27.19.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y., Kim Y. B., Peroni O. D., Fryer L. G., Muller C., Carling D., Kahn B. B. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–43. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Miyamoto H., Yang Z., Chen Y. T., Ishiguro H., Uemura H., Kubota Y., Nagashima Y., Chang Y. J., Hu Y. C., Tsai M. Y., Yeh S., Messing E. M., Chang C. Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst. 2007a;99:558–68. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- Miyamoto J., Matsumoto T., Shiina H., Inoue K., Takada I., Ito S., Itoh J., Minematsu T., Sato T., Yanase T., Nawata H., Osamura Y. R., Kato S. The pituitary function of androgen receptor constitutes a glucocorticoid production circuit. Mol Cell Biol. 2007b;27:4807–14. doi: 10.1128/MCB.02039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks D. A., O'Bryant E. L., Jordan C. L. Androgen receptor immunoreactivity in skeletal muscle: enrichment at the neuromuscular junction. J Comp Neurol. 2004;473:59–72. doi: 10.1002/cne.20088. [DOI] [PubMed] [Google Scholar]
- Montes C. L., Maletto B. A., Acosta Rodriguez E. V., Gruppi A., Pistoresi-Palencia M. C. B cells from aged mice exhibit reduced apoptosis upon B-cell antigen receptor stimulation and differential ability to up-regulate survival signals. Clin Exp Immunol. 2006;143:30–40. doi: 10.1111/j.1365-2249.2005.02969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore F. A., Sauaia A., Moore E. E., Haenel J. B., Burch J. M., Lezotte D. C. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma. 1996;40:501–10; discussion 510-2. doi: 10.1097/00005373-199604000-00001. [DOI] [PubMed] [Google Scholar]
- Mori H., Inoki K., Munzberg H., Opland D., Faouzi M., Villanueva E. C., Ikenoue T., Kwiatkowski D., MacDougald O. A., Myers M. G., Jr., Guan K. L. Critical role for hypothalamic mTOR activity in energy balance. Cell Metab. 2009;9:362–74. doi: 10.1016/j.cmet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoio D. M., Newgard C. B. Obesity-related derangements in metabolic regulation. Annu Rev Biochem. 2006;75:367–401. doi: 10.1146/annurev.biochem.75.103004.142512. [DOI] [PubMed] [Google Scholar]
- Murashima A., Miyagawa S., Ogino Y., Nishida-Fukuda H., Araki K., Matsumoto T., Kaneko T., Yoshinaga K., Yamamura K., Kurita T., Kato S., Moon A. M., Yamada G. Essential roles of androgen signaling in Wolffian duct stabilization and epididymal cell differentiation. Endocrinology. 2011;152:1640–51. doi: 10.1210/en.2010-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy L., O'Shaughnessy P. J. Testicular steroidogenesis in the testicular feminized (Tfm) mouse: loss of 17 alpha-hydroxylase activity. J Endocrinol. 1991;131:443–9. doi: 10.1677/joe.0.1310443. [DOI] [PubMed] [Google Scholar]
- Naito A., Sato T., Matsumoto T., Takeyama K., Yoshino T., Kato S., Ohdera M. Dihydrotestosterone inhibits murine hair growth via the androgen receptor. Br J Dermatol. 2008;159:300–5. doi: 10.1111/j.1365-2133.2008.08671.x. [DOI] [PubMed] [Google Scholar]
- Nathan L., Shi W., Dinh H., Mukherjee T. K., Wang X., Lusis A. J., Chaudhuri G. Testosterone inhibits early atherogenesis by conversion to estradiol: critical role of aromatase. Proc Natl Acad Sci U S A. 2001;98:3589–93. doi: 10.1073/pnas.051003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazareth L. V., Stenoien D. L., Bingman W. E., 3rd, James A. J., Wu C., Zhang Y., Edwards D. P., Mancini M., Marcelli M., Lamb D. J., Weigel N. L. A C619Y mutation in the human androgen receptor causes inactivation and mislocalization of the receptor with concomitant sequestration of SRC-1 (steroid receptor coactivator 1) Mol Endocrinol. 1999;13:2065–75. doi: 10.1210/mend.13.12.0382. [DOI] [PubMed] [Google Scholar]
- Negri-Cesi P., Colciago A., Celotti F., Motta M. Sexual differentiation of the brain: role of testosterone and its active metabolites. J Endocrinol Invest. 2004;27:120–7. [PubMed] [Google Scholar]
- Nettleship J. E., Jones T. H., Channer K. S., Jones R. D. Physiological testosterone replacement therapy attenuates fatty streak formation and improves high-density lipoprotein cholesterol in the Tfm mouse: an effect that is independent of the classic androgen receptor. Circulation. 2007;116:2427–34. doi: 10.1161/CIRCULATIONAHA.107.708768. [DOI] [PubMed] [Google Scholar]
- Notini A. J., Davey R. A., McManus J. F., Bate K. L., Zajac J. D. Genomic actions of the androgen receptor are required for normal male sexual differentiation in a mouse model. J Mol Endocrinol. 2005;35:547–55. doi: 10.1677/jme.1.01884. [DOI] [PubMed] [Google Scholar]
- Notini A. J., McManus J. F., Moore A., Bouxsein M., Jimenez M., Chiu W. S., Glatt V., Kream B. E., Handelsman D. J., Morris H. A., Zajac J. D., Davey R. A. Osteoblast deletion of exon 3 of the androgen receptor gene results in trabecular bone loss in adult male mice. J Bone Miner Res. 2007;22:347–56. doi: 10.1359/jbmr.061117. [DOI] [PubMed] [Google Scholar]
- Obici S., Zhang B. B., Karkanias G., Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–82. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- Obici S., Rossetti L. Minireview: nutrient sensing and the regulation of insulin action and energy balance. Endocrinology. 2003;144:5172–8. doi: 10.1210/en.2003-0999. [DOI] [PubMed] [Google Scholar]
- Ohno S., Dofuku R., Tettenborn U. More about X-linked testicular feminization of the mouse as a noninducible(is)mutation of a regulatory locus: 5-alpha-androstan-3-alpha-17-beta-diol as the true inducer of kidney alcohol dehydrogenase and beta-glucuronidase. Clin Genet. 1971;2:128–40. doi: 10.1111/j.1399-0004.1971.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Nakae J., Kitamura T., Park B. C., Dragatsis I., Accili D. Transgenic rescue of insulin receptor-deficient mice. J Clin Invest. 2004;114:214–23. doi: 10.1172/JCI21645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen N. J., Olson G., Viselli S. M., Gu X., Kovacs W. J. Androgen receptors in thymic epithelium modulate thymus size and thymocyte development. Endocrinology. 2001b;142:1278–83. doi: 10.1210/endo.142.3.8032. [DOI] [PubMed] [Google Scholar]
- Olsen N. J., Gu X., Kovacs W. J. Bone marrow stromal cells mediate androgenic suppression of B lymphocyte development. J Clin Invest. 2001a;108:1697–704. doi: 10.1172/JCI13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen N. J., Kovacs W. J. Effects of androgens on T and B lymphocyte development. Immunol Res. 2001c;23:281–8. doi: 10.1385/IR:23:2-3:281. [DOI] [PubMed] [Google Scholar]
- Ophoff J., Van Proeyen K., Callewaert F., De Gendt K., De Bock K., Vanden Bosch A., Verhoeven G., Hespel P., Vanderschueren D. Androgen signaling in myocytes contributes to the maintenance of muscle mass and fiber type regulation but not to muscle strength or fatigue. Endocrinology. 2009b;150:3558–66. doi: 10.1210/en.2008-1509. [DOI] [PubMed] [Google Scholar]
- Ophoff J., Callewaert F., Venken K., De Gendt K., Ohlsson C., Gayan-Ramirez G., Decramer M., Boonen S., Bouillon R., Verhoeven G., Vanderschueren D. Physical activity in the androgen receptor knockout mouse: evidence for reversal of androgen deficiency on cancellous bone. Biochem Biophys Res Commun. 2009a;378:139–44. doi: 10.1016/j.bbrc.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Panet-Raymond V., Gottlieb B., Beitel L. K., Pinsky L., Trifiro M. A. Interactions between androgen and estrogen receptors and the effects on their transactivational properties. Mol Cell Endocrinol. 2000;167:139–50. doi: 10.1016/s0303-7207(00)00279-3. [DOI] [PubMed] [Google Scholar]
- Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril. 2006b;85:1319–40. doi: 10.1016/j.fertnstert.2005.10.054. [DOI] [PubMed] [Google Scholar]
- Pasquali R., Gambineri A. Polycystic ovary syndrome: a multifaceted disease from adolescence to adult age. Ann N Y Acad Sci. 2006a;1092:158–74. doi: 10.1196/annals.1365.014. [DOI] [PubMed] [Google Scholar]
- Phoenix C. H., Goy R. W., Gerall A. A., Young W. C. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–82. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Pocai A., Obici S., Schwartz G. J., Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab. 2005;1:53–61. doi: 10.1016/j.cmet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Pochi P. E., Strauss J. S. Endocrinologic control of the development and activity of the human sebaceous gland. J Invest Dermatol. 1974;62:191–201. doi: 10.1111/1523-1747.ep12676783. [DOI] [PubMed] [Google Scholar]
- Postic C., Dentin R., Girard J. Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes Metab. 2004;30:398–408. doi: 10.1016/s1262-3636(07)70133-7. [DOI] [PubMed] [Google Scholar]
- Quigley C. A., Evans B. A., Simental J. A., Marschke K. B., Sar M., Lubahn D. B., Davies P., Hughes I. A., Wilson E. M., French F. S. Complete androgen insensitivity due to deletion of exon C of the androgen receptor gene highlights the functional importance of the second zinc finger of the androgen receptor in vivo. Mol Endocrinol. 1992;6:1103–12. doi: 10.1210/mend.6.7.1508223. [DOI] [PubMed] [Google Scholar]
- Rana K., Fam B. C., Clarke M. V., Pang T. P., Zajac J. D., MacLean H. E. Increased adiposity in DNA binding-dependent androgen receptor knockout male mice associated with decreased voluntary activity and not insulin resistance. Am J Physiol Endocrinol Metab. 2011;301:E767–78. doi: 10.1152/ajpendo.00584.2010. [DOI] [PubMed] [Google Scholar]
- Raskin K., de Gendt K., Duittoz A., Liere P., Verhoeven G., Tronche F., Mhaouty-Kodja S. Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. J Neurosci. 2009;29:4461–70. doi: 10.1523/JNEUROSCI.0296-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawbone R. G., Bagshawe K. D. Anabolic steroids and bone marrow toxicity during therapy with methotrexate. Br J Cancer. 1972;26:395–401. doi: 10.1038/bjc.1972.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs B. L., Khosla S., Melton L. J., 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- Rittmaster R. S. Hirsutism. Lancet. 1997;349:191–5. doi: 10.1016/S0140-6736(96)07252-2. [DOI] [PubMed] [Google Scholar]
- Sakai K., Miyazaki Ji. A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochem Biophys Res Commun. 1997;237:318–24. doi: 10.1006/bbrc.1997.7111. [DOI] [PubMed] [Google Scholar]
- Samuel V. T., Liu Z. X., Qu X., Elder B. D., Bilz S., Befroy D., Romanelli A. J., Shulman G. I. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–53. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- Sanchez-Medal L., Pizzuto J., Torre-Lopez E., Derbez R. Effect of Oxymetholone in Refractory Anemia. Arch Intern Med. 1964;113:721–9. doi: 10.1001/archinte.1964.00280110101020. [DOI] [PubMed] [Google Scholar]
- Sanchez-Medal L. The hemopoietic action of androstanes. Prog Hematol. 1971;7:111–36. [PubMed] [Google Scholar]
- Sato T., Matsumoto T., Kawano H., Watanabe T., Uematsu Y., Sekine K., Fukuda T., Aihara K., Krust A., Yamada T., Nakamichi Y., Yamamoto Y., Nakamura T., Yoshimura K., Yoshizawa T., Metzger D., Chambon P., Kato S. Brain masculinization requires androgen receptor function. Proc Natl Acad Sci U S A. 2004;101:1673–8. doi: 10.1073/pnas.0305303101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Matsumoto T., Yamada T., Watanabe T., Kawano H., Kato S. Late onset of obesity in male androgen receptor-deficient (AR KO) mice. Biochem Biophys Res Commun. 2003;300:167–71. doi: 10.1016/s0006-291x(02)02774-2. [DOI] [PubMed] [Google Scholar]
- Schmidt E. V., Christoph G., Zeller R., Leder P. The cytomegalovirus enhancer: a pan-active control element in transgenic mice. Mol Cell Biol. 1990;10:4406–11. doi: 10.1128/mcb.10.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler M., Ali F., Metzger E., Chambon P., Metzger D. Temporally controlled targeted somatic mutagenesis in skeletal muscles of the mouse. Genesis. 2005;41:165–70. doi: 10.1002/gene.20107. [DOI] [PubMed] [Google Scholar]
- Schwartz M. W., Porte D., Jr. Diabetes, obesity, and the brain. Science. 2005;307:375–9. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- Schwenk F., Baron U., Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–1. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki J., Kurihara H., Ito Y., Shida K. Metabolism of testosterone in prostate. 2. Separation of prostatic 17-beta-ol-dehydrogenase and 5-alpha-reductase. Gunma J Med Sci. 1965a;14:326–33. [PubMed] [Google Scholar]
- Shimazaki J., Kurihara H., Ito Y., Shida K. Testosterone metabolism in prostate; formation of androstan-17-beta-ol-3-one and androst-4-ene-3, 17-dione, and inhibitory effect of natural and synthetic estrogens. Gunma J Med Sci. 1965b;14:313–25. [PubMed] [Google Scholar]
- Simanainen U., Gao Y. R., Walters K. A., Watson G., Desai R., Jimenez M., Handelsman D. J. Androgen resistance in female mice increases susceptibility to DMBA-induced mammary tumors. Horm Cancer. 2012;3:113–24. doi: 10.1007/s12672-012-0107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanainen U., Brogley M., Gao Y. R., Jimenez M., Harwood D. T., Handelsman D. J., Robins D. M. Length of the human androgen receptor glutamine tract determines androgen sensitivity in vivo. Mol Cell Endocrinol. 2011;342:81–6. doi: 10.1016/j.mce.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly R. B. Organization and regulation of sexually dimorphic neuroendocrine pathways. Behav Brain Res. 1998;92:195–203. doi: 10.1016/s0166-4328(97)00191-5. [DOI] [PubMed] [Google Scholar]
- Sinclair R., Greenland K. J., Egmond Sv, Hoedemaker C., Chapman A., Zajac J. D. Men with Kennedy disease have a reduced risk of androgenetic alopecia. Br J Dermatol. 2007;157:290–4. doi: 10.1111/j.1365-2133.2007.08026.x. [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I., Taylor W. E., Gonzalez-Cadavid N. F., Zheng W., Bhasin S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab. 2004;89:5245–55. doi: 10.1210/jc.2004-0084. [DOI] [PubMed] [Google Scholar]
- Sinnesael M., Claessens F., Laurent M., Dubois V., Boonen S., Deboel L., Vanderschueren D. Androgen receptor (AR) in osteocytes is important for the maintenance of male skeletal integrity: evidence from targeted AR disruption in mouse osteocytes. J Bone Miner Res. 2012;27:2535–43. doi: 10.1002/jbmr.1713. [DOI] [PubMed] [Google Scholar]
- Smithson G., Couse J. F., Lubahn D. B., Korach K. S., Kincade P. W. The role of estrogen receptors and androgen receptors in sex steroid regulation of B lymphopoiesis. J Immunol. 1998;161:27–34. [PubMed] [Google Scholar]
- Sobue G., Hashizume Y., Mukai E., Hirayama M., Mitsuma T., Takahashi A. X-linked recessive bulbospinal neuronopathy. A clinicopathological study. Brain. 1989;112 (Pt 1):209–32. doi: 10.1093/brain/112.1.209. [DOI] [PubMed] [Google Scholar]
- Su H., Mills A. A., Wang X., Bradley A. A targeted X-linked CMV-Cre line. Genesis. 2002;32:187–8. doi: 10.1002/gene.10043. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Development and differentiation of macrophages and related cells: historical review and current concept. J Clin Exp Hematopathol. 2001;41:1–33. [Google Scholar]
- Taylor L. G., Canfield S. E., Du X. L. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. 2009;115:2388–99. doi: 10.1002/cncr.24283. [DOI] [PubMed] [Google Scholar]
- Tilley W. D., Marcelli M., Wilson J. D., McPhaul M. J. Characterization and expression of a cDNA encoding the human androgen receptor. Proc Natl Acad Sci U S A. 1989;86:327–31. doi: 10.1073/pnas.86.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting H. J., Chang C. Actin associated proteins function as androgen receptor coregulators: an implication of androgen receptor's roles in skeletal muscle. J Steroid Biochem Mol Biol. 2008;111:157–63. doi: 10.1016/j.jsbmb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Tozum T. F., Oppenlander M. E., Koh-Paige A. J., Robins D. M., McCauley L. K. Effects of sex steroid receptor specificity in the regulation of skeletal metabolism. Calcif Tissue Int. 2004;75:60–70. doi: 10.1007/s00223-004-0119-8. [DOI] [PubMed] [Google Scholar]
- Trapman J., Klaassen P., Kuiper G. G., van der Korput J. A., Faber P. W., van Rooij H. C., Geurts van Kessel A., Voorhorst M. M., Mulder E., Brinkmann A. O. Cloning, structure and expression of a cDNA encoding the human androgen receptor. Biochem Biophys Res Commun. 1988;153:241–8. doi: 10.1016/s0006-291x(88)81214-2. [DOI] [PubMed] [Google Scholar]
- Trevaskis J. L., Coffey T., Cole R., Lei C., Wittmer C., Walsh B., Weyer C., Koda J., Baron A. D., Parkes D. G., Roth J. D. Amylin-mediated restoration of leptin responsiveness in diet-induced obesity: magnitude and mechanisms. Endocrinology. 2008;149:5679–87. doi: 10.1210/en.2008-0770. [DOI] [PubMed] [Google Scholar]
- Tronche F., Kellendonk C., Kretz O., Gass P., Anlag K., Orban P. C., Bock R., Klein R., Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Tsai M. Y., Shyr C. R., Kang H. Y., Chang Y. C., Weng P. L., Wang S. Y., Huang K. E., Chang C. The reduced trabecular bone mass of adult ARKO male mice results from the decreased osteogenic differentiation of bone marrow stroma cells. Biochem Biophys Res Commun. 2011;411:477–82. doi: 10.1016/j.bbrc.2011.06.113. [DOI] [PubMed] [Google Scholar]
- Tut T. G., Ghadessy F. J., Trifiro M. A., Pinsky L., Yong E. L. Long polyglutamine tracts in the androgen receptor are associated with reduced trans-activation, impaired sperm production, and male infertility. J Clin Endocrinol Metab. 1997;82:3777–82. doi: 10.1210/jcem.82.11.4385. [DOI] [PubMed] [Google Scholar]
- Udupa K. B., Reissmann K. R. Acceleration of granulopoietic recovery by androgenic steroids in mice made neutropenic by cytotoxic drugs. Cancer Res. 1974;34:2517–20. [PubMed] [Google Scholar]
- Urs S., Harrington A., Liaw L., Small D. Selective expression of an aP2/Fatty Acid Binding Protein 4-Cre transgene in non-adipogenic tissues during embryonic development. Transgenic Res. 2006;15:647–53. doi: 10.1007/s11248-006-9000-z. [DOI] [PubMed] [Google Scholar]
- Vandenput L., Swinnen J. V., Boonen S., Van Herck E., Erben R. G., Bouillon R., Vanderschueren D. Role of the androgen receptor in skeletal homeostasis: the androgen-resistant testicular feminized male mouse model. J Bone Miner Res. 2004;19:1462–70. doi: 10.1359/JBMR.040505. [DOI] [PubMed] [Google Scholar]
- Vanderschueren D., Vandenput L., Boonen S., Lindberg M. K., Bouillon R., Ohlsson C. Androgens and bone. Endocr Rev. 2004;25:389–425. doi: 10.1210/er.2003-0003. [DOI] [PubMed] [Google Scholar]
- Vanderschueren D., Venken K., Ophoff J., Bouillon R., Boonen S. Clinical Review: Sex steroids and the periosteum--reconsidering the roles of androgens and estrogens in periosteal expansion. J Clin Endocrinol Metab. 2006;91:378–82. doi: 10.1210/jc.2005-1766. [DOI] [PubMed] [Google Scholar]
- Venken K., De Gendt K., Boonen S., Ophoff J., Bouillon R., Swinnen J. V., Verhoeven G., Vanderschueren D. Relative impact of androgen and estrogen receptor activation in the effects of androgens on trabecular and cortical bone in growing male mice: a study in the androgen receptor knockout mouse model. J Bone Miner Res. 2006;21:576–85. doi: 10.1359/jbmr.060103. [DOI] [PubMed] [Google Scholar]
- Viselli S. M., Reese K. R., Fan J., Kovacs W. J., Olsen N. J. Androgens alter B cell development in normal male mice. Cell Immunol. 1997;182:99–104. doi: 10.1006/cimm.1997.1227. [DOI] [PubMed] [Google Scholar]
- Viselli S. M., Stanziale S., Shults K., Kovacs W. J., Olsen N. J. Castration alters peripheral immune function in normal male mice. Immunology. 1995a;84:337–42. [PMC free article] [PubMed] [Google Scholar]
- Viselli S. M., Olsen N. J., Shults K., Steizer G., Kovacs W. J. Immunochemical and flow cytometric analysis of androgen receptor expression in thymocytes. Mol Cell Endocrinol. 1995b;109:19–26. doi: 10.1016/0303-7207(95)03479-q. [DOI] [PubMed] [Google Scholar]
- Wang Z. V., Deng Y., Wang Q. A., Sun K., Scherer P. E. Identification and characterization of a promoter cassette conferring adipocyte-specific gene expression. Endocrinology. 2010;151:2933–9. doi: 10.1210/en.2010-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Obici S., Morgan K., Barzilai N., Feng Z., Rossetti L. Overfeeding rapidly induces leptin and insulin resistance. Diabetes. 2001;50:2786–91. doi: 10.2337/diabetes.50.12.2786. [DOI] [PubMed] [Google Scholar]
- Whitacre C. C., Reingold S. C., O'Looney P. A. A gender gap in autoimmunity. Science. 1999;283:1277–8. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- Woods S. C., Lotter E. C., McKay L. D., Porte D., Jr. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–5. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- Wu C., Okar D. A., Kang J., Lange A. J. Reduction of hepatic glucose production as a therapeutic target in the treatment of diabetes. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:51–9. doi: 10.2174/1568008053174769. [DOI] [PubMed] [Google Scholar]
- Yanase T., Fan W., Kyoya K., Min L., Takayanagi R., Kato S., Nawata H. Androgens and metabolic syndrome: lessons from androgen receptor knock out (ARKO) mice. J Steroid Biochem Mol Biol. 2008;109:254–7. doi: 10.1016/j.jsbmb.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Yang Z., Chang Y. J., Yu I. C., Yeh S., Wu C. C., Miyamoto H., Merry D. E., Sobue G., Chen L. M., Chang S. S., Chang C. ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation of androgen receptor. Nat Med. 2007;13:348–53. doi: 10.1038/nm1547. [DOI] [PubMed] [Google Scholar]
- Yang L. Y., Arnold A. P. Interaction of BDNF and testosterone in the regulation of adult perineal motoneurons. J Neurobiol. 2000;44:308–19. doi: 10.1002/1097-4695(20000905)44:3<308::aid-neu2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Yeh S., Chang C. Cloning and characterization of a specific coactivator, ARA70, for the androgen receptor in human prostate cells. Proc Natl Acad Sci U S A. 1996;93:5517–21. doi: 10.1073/pnas.93.11.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh S., Tsai M. Y., Xu Q., μ X. M., Lardy H., Huang K. E., Lin H., Yeh S. D., Altuwaijri S., Zhou X., Xing L., Boyce B. F., Hung M. C., Zhang S., Gan L., Chang C. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A. 2002;99:13498–503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Dadgar N., Albertelli M., Scheller A., Albin R. L., Robins D. M., Lieberman A. P. Abnormalities of germ cell maturation and sertoli cell cytoskeleton in androgen receptor 113 CAG knock-in mice reveal toxic effects of the mutant protein. Am J Pathol. 2006a;168:195–204. doi: 10.2353/ajpath.2006.050619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Dadgar N., Albertelli M., Gruis K., Jordan C., Robins D. M., Lieberman A. P. Androgen-dependent pathology demonstrates myopathic contribution to the Kennedy disease phenotype in a mouse knock-in model. J Clin Invest. 2006b;116:2663–72. doi: 10.1172/JCI28773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I. C., Lin H. Y., Liu N. C., Wang R. S., Sparks J. D., Yeh S., Chang C. Hyperleptinemia without obesity in male mice lacking androgen receptor in adipose tissue. Endocrinology. 2008;149:2361–8. doi: 10.1210/en.2007-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I. C. Multiple roles of the androgen receptor in insulin resistance and metabolic syndrome: tissue-specific perspectives. PhD thesis, University of Rochester School of Medicine and Dentistry, Department of Interdepartmental Graduate Program of Neuroscience. 2010 [Google Scholar]
- Yu I. C., Lin H. Y., Liu N. C., Sparks J. D., Yeh S., Fang L. Y., Chen L., Chang C. Neuronal androgen receptor regulates insulin sensitivity via suppression of hypothalamic NF-κB-mediated PTP1B expression. Diabetes. 2013;62:411–23. doi: 10.2337/db12-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Xuan S., Bouxsein M. L., von Stechow D., Akeno N., Faugere M. C., Malluche H., Zhao G., Rosen C. J., Efstratiadis A., Clemens T. L. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277:44005–12. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- Zhu C., Luong R., Zhuo M., Johnson D. T., McKenney J. K., Cunha G. R., Sun Z. Conditional expression of the androgen receptor induces oncogenic transformation of the mouse prostate. J Biol Chem. 2011;286:33478–88. doi: 10.1074/jbc.M111.269894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga D. G., Morris J. A., Jordan C. L., Breedlove S. M. Mice with the testicular feminization mutation demonstrate a role for androgen receptors in the regulation of anxiety-related behaviors and the hypothalamic-pituitary-adrenal axis. Horm Behav. 2008a;54:758–66. doi: 10.1016/j.yhbeh.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Zuloaga D. G., Puts D. A., Jordan C. L., Breedlove S. M. The role of androgen receptors in the masculinization of brain and behavior: what we've learned from the testicular feminization mutation. Horm Behav. 2008b;53:613–26. doi: 10.1016/j.yhbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.