Abstract
Objective
Endothelial dysfunction is associated with atherosclerosis in mice, but it is difficult to reduce cholesterol levels enough to study regression of atherosclerosis in genetically modified mice. The goal of this study was to examine vascular structure and function before and after reducing elevated plasma lipid levels with a “genetic switch” in Reversa mice, and identify novel mechanisms contributing to structural and functional improvements in the vasculature following reduction of blood lipids.
Methods and Results
Following 6 months of hypercholesterolemia (HCHOL), endothelial function (maximum relaxation to acetylcholine) in aorta was impaired and responses to nitric oxide were unaffected. Further impairment in endothelial function was observed after 12 months of HCHOL, and was associated with reductions in sensitivity to nitric oxide. Expression of dihydrofolate reductase (DHFR) was reduced at 6 and 12 months, and addition of the tetrahydrobiopterin precursor sepiapterin significantly improved endothelial function. Reducing cholesterol levels at 6 months normalized DHFR expression and prevented further impairment in endothelial function. Similar functional changes were observed after 12 months of hypercholesterolemia followed by 2 months of lipid lowering.
Conclusions
Our data suggest that endothelial dysfunction following prolonged hypercholesterolemia is the result of both impairment of sensitivity to NO and reduced NOS cofactor bioavailability. Both of these changes can be prevented by normalizing blood lipids during moderately severe or advanced atherosclerosis.
Introduction
Endothelial dysfunction in humans with atherosclerosis is due to an increase in oxidative stress, reduction in nitric oxide bioavailability, and depletion of essential nitric oxide synthase (NOS) cofactors1-5. Endothelial dysfunction is also evident in early stages of atherosclerosis in hypercholesterolemic mice. Pharmacological and genetic interventions indicate that both oxidative stress and nitric oxide bioavailability are important determinants of plaque initiation, progression, and stability6-11. It has been difficult, however, to study structural and functional consequences of regression of atherosclerosis in low density lipoprotein receptor-null (Ldlr-/-) and apolipoprotein E-null (ApoE-/-) mice. Dietary restriction, for example, does not adequately lower cholesterol levels to study plaque regression, and lipid-lowering drugs are relatively ineffective or have significant off-target effects12, 13.
In “Reversa” mice, when cholesterol levels are reduced through conditional deletion of the microsomal triglyceride transfer protein (mttp) at an early age, initiation of atherosclerotic plaque formation is prevented14. When moderately advanced plaques are present, reduction of blood lipids results in rapid macrophage emigration, reduced inflammation, decreased lipid content, and increased collagen content in atherosclerotic plaques15. Functional consequences of these changes, however, are not known.
In the present study, we used “Reversa” mice to examine effects of prolonged progression and regression of atherosclerosis on vascular function and gene expression. Our primary goal was to test the hypothesis that lipid lowering with a genetic switch would normalize endothelial function following prolonged progression of atherosclerosis. Our second goal was to identify mechanisms underlying changes in endothelial function following reduction of blood lipids, and to determine whether there is a strong relationship between improvements in endothelial function and reductions in expression of NAD(P)H oxidase.
Methods
Animals
We studied female LDL receptor–deficient mice that were homozygous for apolipoprotein B100–only allele, a conditional knockout allele of Mttp, and an Mx1-Cre transgene (Ldlr−/−/Apob100/100/Mttpfl/fl/Mx1Cre+/+, a gift from Dr. Stephen G. Young). In brief, cholesterol levels in these mice can be dramatically reduced by parenteral administration of polyinosinic-polycytidylic acid, which drives expression of the Cre recombinase gene, and thereby excises a portion of the Mttp gene (rendering it inactive). This model has been described in detail previously14, and we have previously examined the pathophysiology of calcific aortic valve stenosis in these mice16, 17.
At 6–8 weeks of age, mice were assigned to either “control”, “progression,” or “regression” groups. Control mice were given 4 injections of polyinosinic-polycytidylic acid (pI-pC, 225 μg, i.p.) at two-day intervals and maintained on a chow diet for 6 or 12 months. Progression mice were placed on a Western diet (Harlan Teklad #TD88137, 42% of calories from fat, 0.25% cholesterol) for 6 or 12 months. Regression mice were placed on a Western diet for 6 months, and then given 4 injections of pI-pC (225 μg, i.p.), switched to a chow diet, and followed for an additional 6 months.
Histological and immunohistochemical changes in aorta
Serial sections (10 μm thickness) were taken from tissue blocks frozen in OCT compound. Lipid deposition was measured using Oil Red O (Sigma, France)16, 17. Calcification was measured using Alizarin Red staining (Sigma, France)16, 17. Macrophages were identified using F4/80 staining (AbD Serotec)16. Images were obtained using light microscopy at 4x and 10x magnification (Olympus BX 51 Digital Light Microscope, Olympus, Japan). For analysis, we used Adobe Photoshop CS2 (version 7, Adobe Systems Inc. San Jose, CA) to select only pixels that express red staining as described previously16, 17. Data are expressed as the percentage of vessel area that displays positive staining.
Gene expression
Tissue from the aortic arch and proximal descending thoracic aorta was used to measure gene expression. Quantitative real-time RT-PCR was used to measure expression of genes related to calcification (Msx2, core binding factor alpha1 (CBFA1), osterix, and osteopontin(OPN)), lipid deposition and reverse cholesterol transport (ATP-binding cassette subfamily A1 and B1, (ABCA1 and ABCG1)), antioxidant defense mechanisms (Manganese superoxide dismutase (MnSOD), copper-zinc SOD (CuZnSOD), and extracellular SOD (ecSOD)), pro-oxidant mechanisms (NADPH oxidase catalytic subunits 1, 2, and 4 (Nox1, Nox2, and Nox4, respectively)), and enzymes related to synthesis of nitric oxide (nitric oxide synthase isoforms 1, 2, and 3 (NOS1, NOS2, NOS3), GTP cyclohydrolase-1 (GTPCH), and dihydrofolate reductase (DHFR)) using previously described methods18.
Vasomotor function
Vasomotor function of aorta was evaluated by measurement of isometric tension ex vivo, as we have described previously19. Briefly, mice were anesthetized and euthanized with an overdose of sevofluorane. The aorta was excised, loose connective and adipose tissue was removed, and the aorta was placed in oxygenated Krebs buffer. Vessels were suspended between two triangular hooks in an organ bath, and isometric tension was measured. Vessels were pre-constricted to 50-60% of maximum tension with prostaglandin-F2α, and responses to acetylcholine (endothelium-dependent), sodium nitroprusside (SNP, endothelium-independent), and papaverine (nitric oxide-independent) were examined.
We used the superoxide dismutase mimetic Tempo (1mM dissolved in saline; Sigma) and sepiapterin (100μM, dissolved in saline; Sigma). Adjacent regions of descending thoracic aorta were used as time controls for antioxidant/sepiapterin treatment conditions, and incubated in the organ bath with identical concentrations of vehicle. Because we did not detect a significant time effect across any of the conditions, data are presented as the mean of all time control vessels.
Statistical analyses
All data are expressed as mean ± SE. Differences in contraction and relaxation across groups were detected using an analysis of variance, with subsequent post-hoc testing using Bonferroni-corrected T-tests.
Results
Changes in blood lipids
In control mice, plasma cholesterol levels were 157 ± 20 at 6 months and 145 ± 27 mg/dl at 12 months. In hypercholesterolemic mice, plasma cholesterol levels were 997 ± 87 at 6 months and 828 ± 89 mg/dl at 12 months (p < 0.05 versus control mice at both time points). In the regression group,”switching off” the mttp gene reduced plasma cholesterol levels to 228 ± 30 mg/dl (p < 0.05 versus 6 month or 12 month hypercholesterolemic mice).
Histological and immunohistochemical changes in aorta
In control mice, there was negligible lipid deposition and intimal plaque formation at 6 and 12 months. In contrast, lipid deposition and intimal plaque formation were significantly increased in aorta from hypercholesterolemic mice at six months, and continued to increase at 12 months (Figure 1A). In the regression group, normalizing blood lipids after 6 months of hypercholesterolemia prevented increases in lipid content at 12 months (p < 0.05 versus 12-month hypercholesterolemic mice), and tended to reduce (p = n.s.) intimal plaque size at 12 months (Supplemental Figure I). Changes in macrophage infiltration paralleled changes in lipid content of intimal plaques (Supplemental Figure II).
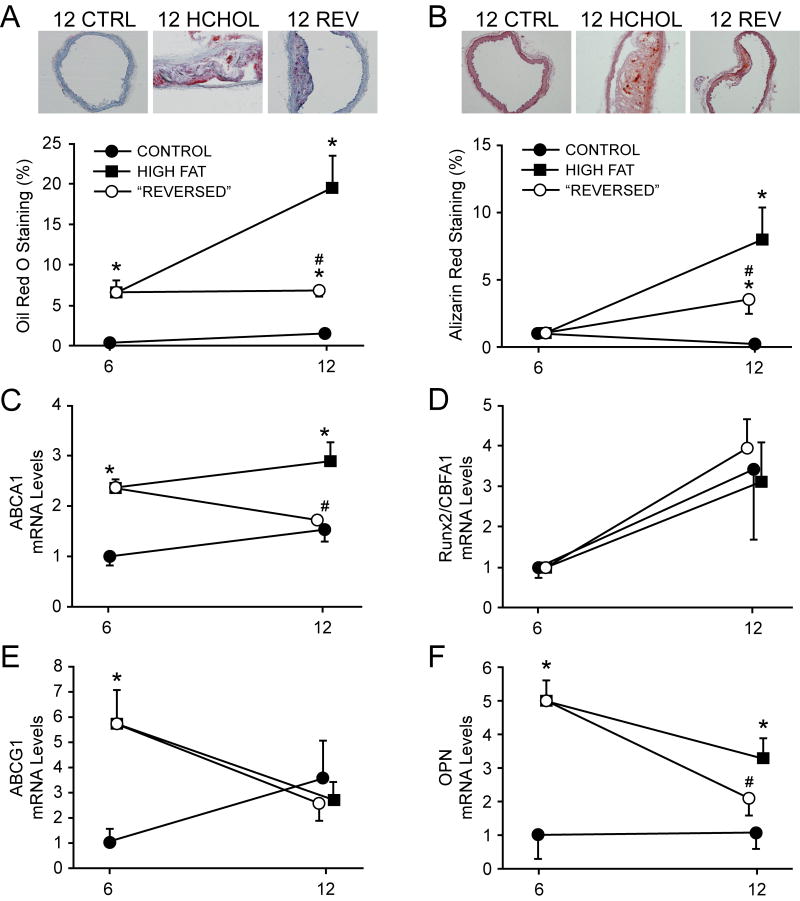
Figure 1.
Deposition of lipid (Oil Red O) and calcium (Alizarin red) in aorta, and expression of related genes. Lipid deposition and calcium deposition from 6 to 12 months are dramatically attenuated by reducing cholesterol levels. Increases in aortic lipid content were associated with alterations in ABCA1, but not ABCG1. Changes in calcium deposition were associated with osteopontin, but not Runx2 or other osteoblast-associated genes (see online supplementary data). CTRL = normocholesterolemic group, HCHOL = hypercholesterolemic group, REV = “reversed”/regression group. * = p < 0.05 versus iso-time CTRL group; # = p < 0.05 versus 6 month HCHOL group. For immunohistochemical/histological data, n = 5-11 per group at each time point; for gene expression data n = 5-13 per group at each time point). Mice were sacrificed at time points denoted on x-axis—offset is provided for clarity and minimization of error bar overlap.
Vascular calcium deposition was negligible in control mice at 6 and 12 months (Figure 1B), but increased progressively from 6 to 12 months in hypercholesterolemic mice (expressed as absolute calcium levels [872 ± 173 and 1870 ± 324 pixels, respectively] and percentage of vessel positively stained [Figure 1B]). Normalizing lipid levels after 6 months of hypercholesterolemia markedly attenuated increases in calcium in aorta from 6 to 12 months (absolute calcium levels = 360 ± 160 pixels; percentage of vessel positively stained, Figure 1B).
Gene expression in aorta
Expression of ABCA1 was significantly elevated after 6 and 12 months of hypercholesterolemia compared to control mice (Figure 1C). Reducing cholesterol levels after 6 months of hypercholesterolemia significantly reduced ABCA1 expression at 12 months (Figure 1C). ABCG1 expression was significantly elevated after 6 months of hypercholesterolemia, but did not remain elevated following 12 months of hypercholesterolemia or after normalizing cholesterol levels at 6 months (figure 1E).
Expression of Runx2 was not significantly affected by any of the interventions used in this study (Figure 1D). Osteopontin, however, was significantly increased after 6 or 12 months of hypercholesterolemia relative to normocholesterolemic control mice (Figure 1F). Normalizing cholesterol levels after 6 months of hypercholesterolemia significantly reduced expression of osteopontin at 12 months (Figure 1F).
Changes in vasomotor function
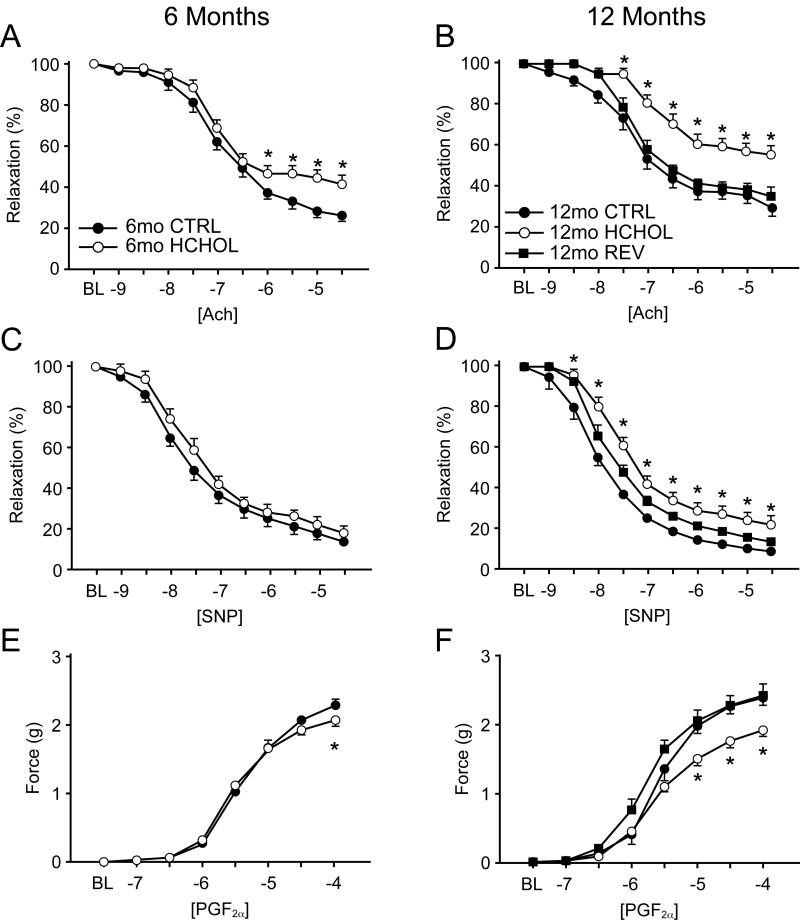
Endothelial function and responses to sodium nitroprusside (SNP) were normal in 6 and 12 month old control mice (maximum relaxation to acetylcholine and SNP were ∼75% and ˜90%, respectively; see Figure 2A-D). After 6 months of hypercholesterolemia, endothelial function was significantly impaired (Figure 2A) with no detectable impairment in vascular relaxation to sodium nitroprusside (see Figure 2C). Following 12 months of hypercholesterolemia, endothelial function was profoundly impaired (Figure 2B) and was also associated with impaired relaxation to sodium nitroprusside (Figure 2D). Normalizing cholesterol levels following 6 months of hypercholesterolemia prevented impairment in vascular responses to acetylcholine and sodium nitroprusside observed in hypercholesterolemic mice at 12 months. Responses to PGF2α were impaired only in 12 month hypercholesterolemic mice (Figure 2E-F). Similar changes were observed in other mice in which cholesterol was normalized for two months following 12 months of hypercholesterolemia (see online supplement).
Figure 2.
Changes in vasomotor function following progression and regression of atherosclerosis. Vasomotor responses to acetylcholine (Ach) in control, hypercholesterolemic, and “reversed” mice at 6 (left panels) and 12 (right panels) months. Vascular responses to sodium nitroprusside (SNP) and prostaglandin F2α (PGF2α) in control, hypercholesterolemic, and “reversed” mice at 6 and 12 months. * = p < 0.05 versus CTRL at each concentration; # = p < 0.05 versus HCHOL at each concentration. For all panels, n = 13-21 for each group at each time point.
Expression of nitric oxide synthase isoforms and genes related to redox balance
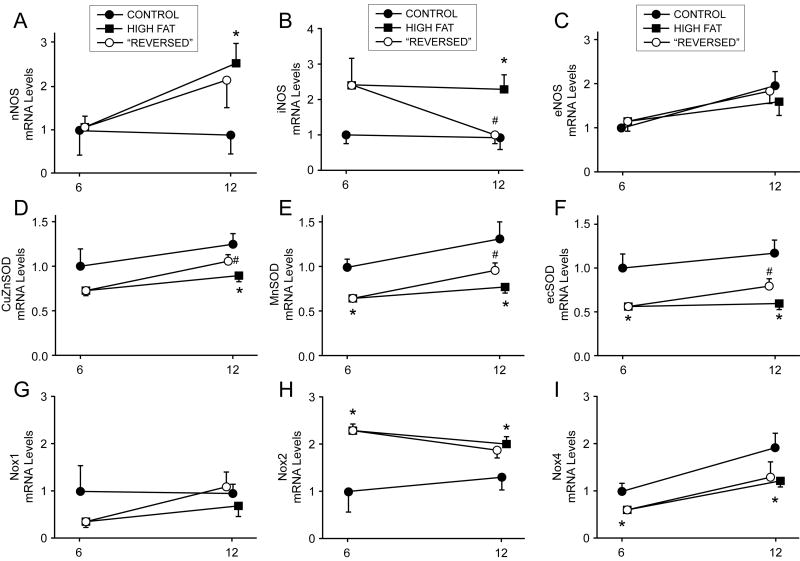
Expression of eNOS was not affected by any interventions in this study (Figure 3C). Expression of iNOS was significantly increased following 6 or 12 months of hypercholesterolemia. Normalizing cholesterol levels following 6 months of hypercholesterolemia significantly reduced iNOS expression at the 12 month time point (Figure 3B). Expression of nNOS was significantly increased following 12 months of hypercholesterolemia (Figure 3A), and but was not altered by normalizing cholesterol levels for 6 months.
Figure 3.
Changes in gene expression of nitric oxide synthase isoforms and pro- and anti-oxidant genes. Changes in expression of NOS isoforms (top row), superoxide dismutase isoforms (middle row), and NAD(P)H oxidase catalytic subunits were highly isoform specific in hypercholesterolemic and “reversed” mice at 6 and 12 months. Specifically, iNOS and CuZnSOD-containing SOD isoforms were most labile following reduction of blood lipids, whereas Nox isoforms were remarkably insensitive to lipid lowering. For each gene, n = 5-13 per group at each time point. Mice were sacrificed at time points denoted on x-axis—offset is provided for clarity and minimization of error bar overlap.
Expression of CuZnSOD, MnSOD, and ecSOD was significantly reduced in 6 and 12 month hypercholesterolemic mice compared to iso-time normocholesterolemic mice (Figure 3D-F). Normalizing cholesterol levels following 6 months of hypercholesterolemia significantly increased expression of CuZnSOD and ecSOD, but not MnSOD, at 12 months (Figure 3D-F).
Expression of Nox1 was not affected by any interventions in this study (Figure 3G). Expression of Nox2 was significantly increased in hypercholesterolemic mice at 6 and 12 months when compared to control mice (Figure 3H). In contrast, expression of Nox4 was significantly reduced following 6 or 12 months of hypercholesterolemia (Figure 3I). Reducing cholesterol levels for 6 months did not affect Nox2 and Nox4 expression compared to 12 month hypercholesterolemic mice (Figure 3H-I).
Effects of exogenous antioxidants on vascular function
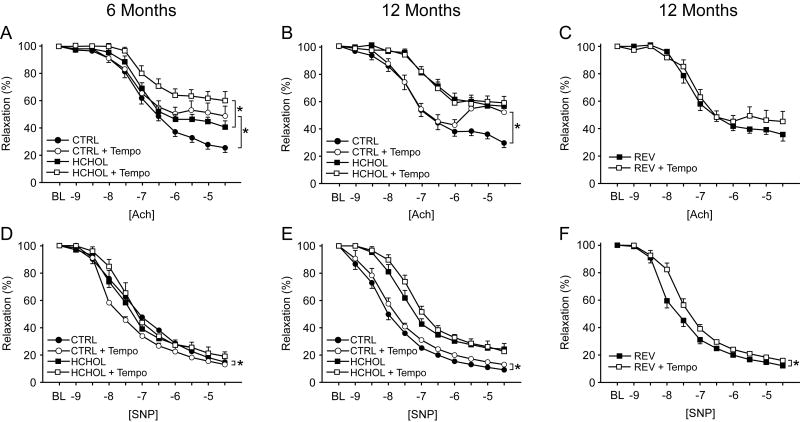
Addition of Tempo to the organ chamber baths did not improve vascular function in 6 or 12 month control animals (Figure 4A-B). Furthermore, Tempo tended to impair vascular function in some groups of mice at 6 and 12 months (Figure 4A-B), but these changes did not reach statistical significance. Responses to sodium nitroprusside were not significantly affected by Tempo.
Figure 4.
Changes in endothelial function and responses to sodium nitroprusside after incubation of vessel segments with a superoxide dismutase mimetic (Tempo) in control, hypercholesterolemic, and “reversed” mice at 6 and 12 months. Acute treatment with Tempo does not improve responses to acetylcholine in normocholesterolemic or hypercholesterolemic mice, and does not alter responses to nitroprusside in any of the groups. For all panels, n = 9-21 for each treatment group at each time point.
Expression of genes related to tetrahydrobiopterin production
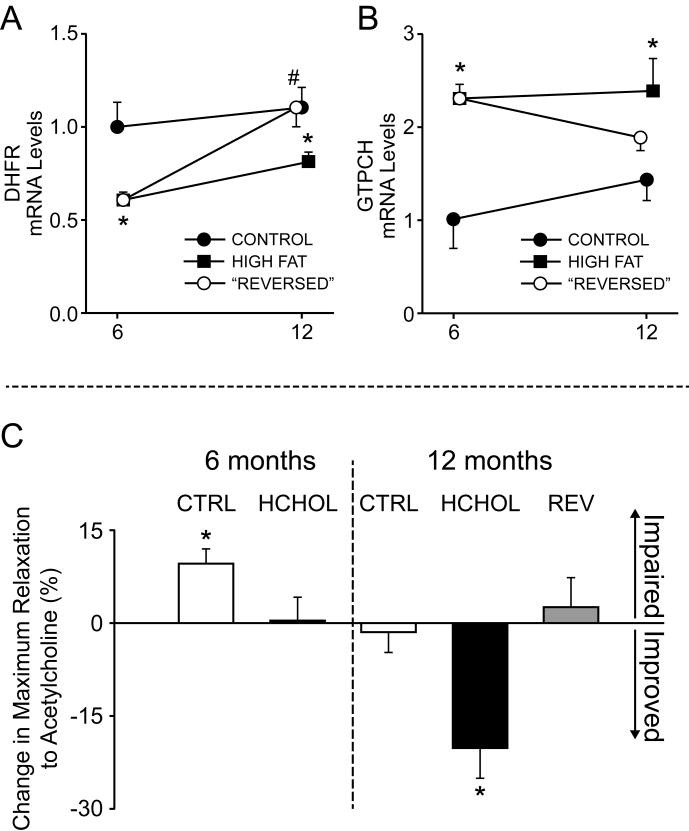
Expression of GTPCH was significantly increased following 6 and 12 months of hypercholesterolemia when compared to control animals (Figure 5A). Normalizing cholesterol levels following 6 months of hypercholesterolemia reduced expression of GTPCH compared to 12 month hypercholesterolemic mice (Figure 5A). In contrast, expression of DHFR was significantly reduced following 6 and 12 months of hypercholesterolemia compared to iso-time control animals (Figure 5B). Reducing cholesterol levels at 6 months normalized DHFR expression at 12 months (Figure 5B).
Figure 5.
Effects of sepiapterin on vascular dysfunction with hypercholesterolemia. Changes in expression of dihydrofolate reductase (DHFR, a BH4 salvage pathway) and GTP cyclohydrolase (GTPCH, de novo synthesis of BH4) with prolonged hypercholesterolemia or lipid lowering. C) Changes in endothelial function after incubation of vessel segments with a precursor for BH4 synthesis (sepiapterin). Note that after 12 months, but not 6 months, HCHOL mice had significant improvement in endothelial function with sepiapterin. For gene expression data, n = 5-13 per group at each time point). For vascular function panels, n = 6-13 for each treatment group at each time point. For gene expression panels, mice were sacrificed at time points denoted on x-axis—offset is provided for clarity and minimization of error bar overlap.
Effects of exogenous tetrahydrobiopterin precursors on vascular function
Addition of sepiapterin to the organ chamber baths did not improve vascular function in 6 or 12 month control animals, 6 month hypercholesterolemic mice, or mice that underwent 6 months of regression/lipid lowering (Figure 5C and Supplemental Figure IV). After 12 months of hypercholesterolemia, however, maximum relaxation in response to acetylcholine was significantly improved by pre-incubation with sepiapterin (Figure 5C and Supplemental Figure IV).
Discussion
The goals of this study were to examine effects of reducing cholesterol levels on structure and function of the aorta following prolonged hypercholesterolemia in mice and examine mechanisms contributing to these changes. We observed in mice, as previously reported in other species, that reducing cholesterol levels prevents reduces atherosclerotic plaque lipid content, and prevents further endothelial dysfunction and losses in vascular NO sensitivity resulting from prolonged hyperlipidemia. The major novel findings of this study are: (1) dystrophic calcium accumulation in aorta is remarkably responsive to reductions in blood lipids in mice, 2) expression of antioxidant enzymes, but not pro-oxidant enzymes, is favorably affected by reducing blood lipids in mice, and (3) restoration of tetrahydrobiopterin synthesis and salvage pathways may be an important mechanism contributing to improvement in endothelial function following reduction in blood lipids.
Histological changes during progression and regression of atherosclerosis in mice
In the present study, hypercholesterolemia resulted in progressive increases in lipid in the aorta. ABCA1 and ABCG1, which are genes critical for reverse cholesterol transport and prevention of lipid accumulation in atherosclerosis, were significantly elevated following 6 months of atherosclerosis. Following 12 months of hypercholesterolemia, ABCA1 (but not ABCG1) was elevated over control levels. While global reduction of ABCA1 accelerates progression of atherosclerosis in hypercholesterolemic mice20, ABCA1 overexpression attenuates atherosclerotic lesion size only when overexpressed in endothelium21-23. Conversely, global reduction of ABCG1 slows progression of atherosclerosis24, but may exert atheroprotective effects if increased only in the vascular endothelium25, 26. Collectively, these data suggest that increases in endothelial ABCA1 and ABCG1 may be protective in early stages of atherogenesis, with progressive reductions in ABCG1 contributing to lipid accumulation in advanced stages of vascular disease.
We also observed calcium deposition in aorta with prolonged hypercholesterolemia, which was surprisingly responsive to lipid lowering. Although increases in calcium were not associated with increases in Runx2, Msx2, or osterix, osteopontin expression paralleled changes in vascular calcium levels. This pattern of gene expression is consistent with observations from dystrophically calcified tissue, where ectopic calcium accumulation progresses in the absence of increased expression of markers of osteoblast-like cells or evidence of bone matrix formation. These data suggest that even dystrophic calcification may be capable of regression following reductions in blood lipids in mice. These data differ from previous studies in humans and non-human primates, where calcification rarely undergoes resorption following reduction of blood lipids27-30. Understanding the differences between species and therapeutic interventions (e.g., dietary intervention versus statin treatment versus mttp inactivation) may be critical to application of these findings to therapeutic interventions in humans.
Vasomotor function and redox-related genes during progression and regression of atherosclerosis in mice
Endothelium-dependent relaxation
Relaxation to acetylcholine was significantly impaired following 6 and 12 months of hypercholesterolemia. Altered responses are likely to be the result of increases in oxidative stress resulting from increases in Nox2-derived radicals (see Figure 4) and reductions in expression of all three isoforms of superoxide dismutase. While increases in NAD(P)H oxidase are consistent with previous findings in hypercholesterolemic mice6, 15, reductions in SOD expression in our LDLr-deficient mice contrasts with reports from apolipoprotein E-null mice, where superoxide dismutase expression increases during progression of atherosclerosis7. Our mice, however, were exposed to prolonged hypercholesterolemia (6-12 months), compared to 3-4 months in other studies. Our findings may be explained by recent reports which suggest that older mice have an impaired ability to mount antioxidant responses to hyperlipidemic stressors8.
If oxidative stress contributed to impaired vasomotor relaxation in this study, it might seem surprising that incubation of aortic rings with Tempo (an antioxidant) did not improve endothelial function, and even impaired vascular function in some groups. We have previously reported that Tempo can increase levels of hydrogen peroxide, which can subsequently induce production of endothelium-derived contracting factors9. While we focused on superoxide dismutase expression in the current study, recent work also demonstrated adequate levels of catalase are critical for degradation of SOD-produced hydrogen peroxide and subsequent atheroprotection with SOD1 overexpression10. We do not have direct evidence for such a phenomenon in the current study, but it is an intriguing future direction to pursue in mice with hypercholesterolemia.
Reducing blood lipids following 6 months of hypercholesterolemia completely prevented progression of impairment in endothelial function from 6 to 12 months, and endothelial function in 12 month old “reversed” mice was nearly identical to 12 month old control mice. Furthermore, reducing blood lipids for only two months following 12 months of hypercholesterolemia resulted in similar changes (see online supplement). In contrast to our hypothesis, however, these changes were not associated with reduction in expression of Nox2 or Nox4, but were instead more closely associated with increases in expression of superoxide dismutase. Thus, lipid lowering preserves endothelial function even after prolonged hypercholesterolemia, and may do so in part by increasing antioxidant defense mechanisms.
Endothelium-independent relaxation
As reported previously in hypercholesterolemic animals31-33, impaired relaxation to acetylcholine following 12 months of hypercholesterolemia may be related in part to decreased nitric oxide bioavailability and to decreased responses to nitric oxide, as we observed reductions in relaxation to sodium nitroprusside. This observation is consistent with the concept that the nitric oxide binding site of soluble guanylate cyclase can be oxidized by NAD(P)H-derived radicals, thereby reducing its responsiveness to nitric oxide and/or nitric oxide donors11. If reductions in sensitivity to nitric oxide occur via this mechanism, however, our data suggest that acute treatment with an antioxidant (i.e., Tempo) is not sufficient to reverse oxidation of soluble guanylate cyclase and improve responses to nitric oxide donors.
Reducing blood lipids also prevented impairment in responses to nitroprusside. Similar results were observed when blood lipids were reduced for two months following 12 months of hypercholesterolemia (see online supplement). These data suggest that sensitivity of vascular smooth muscle cell to nitric oxide is remarkably labile even after a relatively short duration (2 months) of reduction in blood lipids.
Tetrahydrobiopterin deficiency contributes to endothelial dysfunction with hyperlipidemia
Following both 6 and 12 months of hypercholesterolemia, we found increased expression of GTP cyclohydrolase I, but reduced expression of dihydrofolate reductase, which are enzymes related to the de novo and salvage pathways of tetrahydrobiopterin synthesis, respectively. These data are consistent with previous observations from ApoE-deficient mice, in which GTPCH expression and activity were significantly increased following 5 months of severe hyperlipidemia and resulted in increased levels of vascular tetrahydrobiopterin12.
Incubation of vessels with sepiapterin (a dihydrofolate reductase-dependent BH4 precursor) at 6 months did not improve responses to acetylcholine. Treatment with sepiapterin, however, improved endothelium-dependent relaxation following 12 months of hyperlipidemia (Figure 5C). These data are consistent with studies which examined effects of administration of tetrahydrobiopterin to hypercholesterolemic mice (e.g., orally for > 2 weeks), where tetrahydrobiopterin reduces NOS uncoupling, attenuates inflammation, and improves endothelial function13. Increasing tetrahydrobiopterin also improved endothelial function in patients with atherosclerosis34-36. We speculate that, much like L-arginine (i.e., the “L-arginine paradox”17), tetrahydrobiopterin levels may need to exceed physiological levels by several fold in disease states to have a therapeutic benefit.
Limitations
In the current study, we used female mice for all experiments, as male ldlr--/apoB100/100 mice tend to develop severe skin lesions following 10-12 months of Western diet feeding. By using only female animals, we were able to avoid changes in systemic inflammation secondary to the presence of large skin lesions in male mice, which are likely to be a major confounding variable when examining progression/regression of atherosclerosis.
We did not conduct quantitative measurements of superoxide levels in this study (e.g., lucigenin-enhanced chemiluminescence), because most aortic tissue was used for studies of vascular function and gene expression, and because mice were treated for long periods of time (6-14 months).
We have not conducted extensive studies to examine cell type-specific changes in molecules during prolonged hypercholesterolemia. We recognize that the phenotypic consequence of altering expression of cholesterol transporters and numerous other enzymes may depend on the cell type in which the enzyme is expressed21-23, and are deserving of mechanistic investigation in future studies.
Conclusions
Endothelium-dependent relaxation and sensitivity of smooth muscle cells to nitric oxide can be markedly improved following reduction of blood lipids in mice. These functional improvements appear to be due, at least in part, to improvement in antioxidant defense mechanisms and de novo tetrahydrobiopterin production, but not reductions in NAD(P)H oxidase expression. Future studies examining regulation of these molecular changes may lead to novel therapeutic targets for treatment of endothelial dysfunction in atherosclerosis.
Supplementary Material
Acknowledgments
The authors would like to thank Kathy Walters at the University of Iowa Central Microscopy Research Facility for help with immunostaining.
Sources of Funding: This work was supported by NIH grants HL062984 (D.D.H.), HL092235 (J.D.M.), NS024621 (D.D.H.), and funds from a Carver Research Trust Program of Excellence at the University of Iowa.
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gimbrone MA, Jr, Garcia-Cardena G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc Pathol. 2012 doi: 10.1016/j.carpath.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siasos G, Tousoulis D, Oikonomou E, Zaromitidou M, Stefanadis C, Papavassiliou AG. Inflammatory markers in hyperlipidemia: From experimental models to clinical practice. Curr Pharm Des. 2011;17:4132–4146. doi: 10.2174/138161211798764780. [DOI] [PubMed] [Google Scholar]
- 3.Leonarduzzi G, Gamba P, Gargiulo S, Biasi F, Poli G. Inflammation-related gene expression by lipid oxidation-derived products in the progression of atherosclerosis. Free radical biology & medicine. 2012;52:19–34. doi: 10.1016/j.freeradbiomed.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 4.Seals DR, Walker AE, Pierce GL, Lesniewski LA. Habitual exercise and vascular ageing. J Physiol. 2009;587:5541–5549. doi: 10.1113/jphysiol.2009.178822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antoniades C, Shirodaria C, Crabtree M, Rinze R, Alp N, Cunnington C, Diesch J, Tousoulis D, Stefanadis C, Leeson P, Ratnatunga C, Pillai R, Channon KM. Altered plasma versus vascular biopterins in human atherosclerosis reveal relationships between endothelial nitric oxide synthase coupling, endothelial function, and inflammation. Circulation. 2007;116:2851–2859. doi: 10.1161/CIRCULATIONAHA.107.704155. [DOI] [PubMed] [Google Scholar]
- 6.Kauser K, da Cunha V, Fitch R, Mallari C, Rubanyi GM. Role of endogenous nitric oxide in progression of atherosclerosis in apolipoprotein e-deficient mice. Am J Physiol Heart Circ Physiol. 2000;278:H1679–1685. doi: 10.1152/ajpheart.2000.278.5.H1679. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Kuhlencordt PJ, Astern J, Gyurko R, Huang PL. Hypertension does not account for the accelerated atherosclerosis and development of aneurysms in male apolipoprotein e/endothelial nitric oxide synthase double knockout mice. Circulation. 2001;104:2391–2394. doi: 10.1161/hc4501.099729. [DOI] [PubMed] [Google Scholar]
- 8.Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH, Huang PL. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein e/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104:448–454. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- 9.Shimada K, Murayama T, Yokode M, Kita T, Uzui H, Ueda T, Lee JD, Kishimoto C. N-acetylcysteine reduces the severity of atherosclerosis in apolipoprotein e-deficient mice by reducing superoxide production. Circ J. 2009;73:1337–1341. doi: 10.1253/circj.cj-08-1148. [DOI] [PubMed] [Google Scholar]
- 10.Ivanovski O, Szumilak D, Nguyen-Khoa T, Ruellan N, Phan O, Lacour B, Descamps-Latscha B, Drueke TB, Massy ZA. The antioxidant n-acetylcysteine prevents accelerated atherosclerosis in uremic apolipoprotein e knockout mice. Kidney Int. 2005;67:2288–2294. doi: 10.1111/j.1523-1755.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 11.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS. P47phox is required for atherosclerotic lesion progression in apoe(-/-) mice. The Journal of clinical investigation. 2001;108:1513–1522. doi: 10.1172/JCI11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sparrow CP, Burton CA, Hernandez M, Mundt S, Hassing H, Patel S, Rosa R, Hermanowski-Vosatka A, Wang PR, Zhang D, Peterson L, Detmers PA, Chao YS, Wright SD. Simvastatin has anti-inflammatory and antiatherosclerotic activities independent of plasma cholesterol lowering. Arteriosclerosis, thrombosis, and vascular biology. 2001;21:115–121. doi: 10.1161/01.atv.21.1.115. [DOI] [PubMed] [Google Scholar]
- 13.Scalia R, Gooszen ME, Jones SP, Hoffmeyer M, Rimmer DM, 3rd, Trocha SD, Huang PL, Smith MB, Lefer AM, Lefer DJ. Simvastatin exerts both anti-inflammatory and cardioprotective effects in apolipoprotein e-deficient mice. Circulation. 2001;103:2598–2603. doi: 10.1161/01.cir.103.21.2598. [DOI] [PubMed] [Google Scholar]
- 14.Lieu HD, Withycombe SK, Walker Q, Rong JX, Walzem RL, Wong JS, Hamilton RL, Fisher EA, Young SG. Eliminating atherogenesis in mice by switching off hepatic lipoprotein secretion. Circulation. 2003;107:1315–1321. doi: 10.1161/01.cir.0000054781.50889.0c. [DOI] [PubMed] [Google Scholar]
- 15.Feig JE, Parathath S, Rong JX, Mick SL, Vengrenyuk Y, Grauer L, Young SG, Fisher EA. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation. 2011;123:989–998. doi: 10.1161/CIRCULATIONAHA.110.984146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller JD, Weiss RM, Serrano KM, Brooks RM, 2nd, Berry CJ, Zimmerman K, Young SG, Heistad DD. Lowering plasma cholesterol levels halts progression of aortic valve disease in mice. Circulation. 2009;119:2693–2701. doi: 10.1161/CIRCULATIONAHA.108.834614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller JD, Weiss RM, Serrano KM, Castaneda LE, Brooks RM, Zimmerman K, Heistad DD. Evidence for active regulation of pro-osteogenic signaling in advanced aortic valve disease. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:2482–2486. doi: 10.1161/ATVBAHA.110.211029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu Y, Heistad DD, Knudtson KL, Lamping KG, Faraci FM. Quantification of mrna for endothelial no synthase in mouse blood vessels by real-time polymerase chain reaction. Arterioscler Thromb Vasc Biol. 2002;22:611–616. doi: 10.1161/01.atv.0000012663.85364.fa. [DOI] [PubMed] [Google Scholar]
- 19.Miller JD, Peotta VA, Chu Y, Weiss RM, Zimmerman K, Brooks RM, Heistad DD. Mnsod protects against cox1-mediated endothelial dysfunction in chronic heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H1600–1607. doi: 10.1152/ajpheart.01108.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lammers B, Zhao Y, Hoekstra M, Hildebrand RB, Ye D, Meurs I, Van Berkel TJ, Van Eck M. Augmented atherogenesis in ldl receptor deficient mice lacking both macrophage abca1 and apoe. PLoS One. 2011;6:e26095. doi: 10.1371/journal.pone.0026095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaisman BL, Demosky SJ, Stonik JA, Ghias M, Knapper CL, Sampson ML, Dai C, Levine SJ, Remaley AT. Endothelial expression of human abca1 in mice increases plasma hdl cholesterol and reduces diet-induced atherosclerosis. J Lipid Res. 2011 doi: 10.1194/jlr.M018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joyce CW, Wagner EM, Basso F, Amar MJ, Freeman LA, Shamburek RD, Knapper CL, Syed J, Wu J, Vaisman BL, Fruchart-Najib J, Billings EM, Paigen B, Remaley AT, Santamarina-Fojo S, Brewer HB., Jr Abca1 overexpression in the liver of ldlr-ko mice leads to accumulation of pro-atherogenic lipoproteins and enhanced atherosclerosis. The Journal of biological chemistry. 2006;281:33053–33065. doi: 10.1074/jbc.M604526200. [DOI] [PubMed] [Google Scholar]
- 23.Joyce CW, Amar MJ, Lambert G, Vaisman BL, Paigen B, Najib-Fruchart J, Hoyt RF, Jr, Neufeld ED, Remaley AT, Fredrickson DS, Brewer HB, Jr, Santamarina-Fojo S. The atp binding cassette transporter a1 (abca1) modulates the development of aortic atherosclerosis in c57bl/6 and apoe-knockout mice. Proc Natl Acad Sci U S A. 2002;99:407–412. doi: 10.1073/pnas.012587699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarling EJ, Bojanic DD, Tangirala RK, Wang X, Lovgren-Sandblom A, Lusis AJ, Bjorkhem I, Edwards PA. Impaired development of atherosclerosis in abcg1-/- apoe-/- mice: Identification of specific oxysterols that both accumulate in abcg1-/- apoe-/- tissues and induce apoptosis. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:1174–1180. doi: 10.1161/ATVBAHA.110.205617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westerterp M, Koetsveld J, Yu S, Han S, Li R, Goldberg IJ, Welch CL, Tall AR. Increased atherosclerosis in mice with vascular atp-binding cassette transporter g1 deficiency--brief report. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:2103–2105. doi: 10.1161/ATVBAHA.110.212985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terasaka N, Westerterp M, Koetsveld J, Fernandez-Hernando C, Yvan-Charvet L, Wang N, Sessa WC, Tall AR. Atp-binding cassette transporter g1 and high-density lipoprotein promote endothelial no synthesis through a decrease in the interaction of caveolin-1 and endothelial no synthase. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:2219–2225. doi: 10.1161/ATVBAHA.110.213215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stary HC. The development of calcium deposits in atherosclerotic lesions and their persistence after lipid regression. The American journal of cardiology. 2001;88:16E–19E. doi: 10.1016/s0002-9149(01)01713-1. [DOI] [PubMed] [Google Scholar]
- 28.Stary HC. Natural history of calcium deposits in atherosclerosis progression and regression. Z Kardiol. 2000;89(Suppl 2):28–35. doi: 10.1007/s003920070097. [DOI] [PubMed] [Google Scholar]
- 29.Kovarnik T, Mintz GS, Skalicka H, Kral A, Horak J, Skulec R, Uhrova J, Martasek P, Downe RW, Wahle A, Sonka M, Mrazek V, Aschermann M, Linhart A. Virtual histology evaluation of atherosclerosis regression during atorvastatin and ezetimibe administration: Heaven study. Circ J. 2012;76:176–183. doi: 10.1253/circj.cj-11-0730. [DOI] [PubMed] [Google Scholar]
- 30.Terry JG, Carr JJ, Kouba EO, Davis DH, Menon L, Bender K, Chandler ET, Morgan T, Crouse JR., 3rd Effect of simvastatin (80 mg) on coronary and abdominal aortic arterial calcium (from the coronary artery calcification treatment with zocor [catz] study) The American journal of cardiology. 2007;99:1714–1717. doi: 10.1016/j.amjcard.2007.01.060. [DOI] [PubMed] [Google Scholar]
- 31.Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, H SA, Meurer S, Deile M, Taye A, Knorr A, Lapp H, Muller H, Turgay Y, Rothkegel C, Tersteegen A, Kemp-Harper B, Muller-Esterl W, Schmidt HH. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. The Journal of clinical investigation. 2006;116:2552–2561. doi: 10.1172/JCI28371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaghoubi M, Oliver-Krasinski J, Cayatte AJ, Cohen RA. Decreased sensitivity to nitric oxide in the aorta of severely hypercholesterolemic apolipoprotein e-deficient mice. J Cardiovasc Pharmacol. 2000;36:751–757. doi: 10.1097/00005344-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Weisbrod RM, Griswold MC, Du Y, Bolotina VM, Cohen RA. Reduced responsiveness of hypercholesterolemic rabbit aortic smooth muscle cells to nitric oxide. Arteriosclerosis, thrombosis, and vascular biology. 1997;17:394–402. doi: 10.1161/01.atv.17.2.394. [DOI] [PubMed] [Google Scholar]
- 34.Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Hara K, Jitsuiki D, Goto C, Oshima T, Chayama K, Yoshizumi M. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis. 2006;186:390–395. doi: 10.1016/j.atherosclerosis.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 35.Antoniades C, Cunnington C, Antonopoulos A, Neville M, Margaritis M, Demosthenous M, Bendall J, Hale A, Cerrato R, Tousoulis D, Bakogiannis C, Marinou K, Toutouza M, Vlachopoulos C, Leeson P, Stefanadis C, Karpe F, Channon KM. Induction of vascular gtp-cyclohydrolase i and endogenous tetrahydrobiopterin synthesis protect against inflammation-induced endothelial dysfunction in human atherosclerosis. Circulation. 2011;124:1860–1870. doi: 10.1161/CIRCULATIONAHA.111.029272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antoniades C, Shirodaria C, Warrick N, Cai S, de Bono J, Lee J, Leeson P, Neubauer S, Ratnatunga C, Pillai R, Refsum H, Channon KM. 5-methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: Effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation. 2006;114:1193–1201. doi: 10.1161/CIRCULATIONAHA.106.612325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.