Abstract
Although severe stress can elicit toxicity, mild stress often elicits adaptations. Here we review the literature on stress-induced adaptations versus stress sensitization in models of neurodegenerative diseases. We also describe our recent findings that chronic proteotoxic stress can elicit adaptations if the dose is low but that high-dose proteotoxic stress sensitizes cells to subsequent challenges. In these experiments, long-term, low-dose proteasome inhibition elicited protection in a superoxide dismutase-dependent manner. In contrast, acute, high-dose proteotoxic stress sensitized cells to subsequent proteotoxic challenges by eliciting catastrophic loss of glutathione. However, even in the latter model of synergistic toxicity, several defensive proteins were upregulated by severe proteotoxicity. This led us to wonder whether high-dose proteotoxic stress can elicit protection against subsequent challenges in astrocytes, a cell type well known for their resilience. In support of this new hypothesis, we found that the astrocytes that survived severe proteotoxicity became harder to kill. The adaptive mechanism was glutathione dependent. If these findings can be generalized to the human brain, similar endogenous adaptations may help explain why neurodegenerative diseases are so delayed in appearance and so slow to progress. In contrast, sensitization to severe stress may explain why defenses eventually collapse in vulnerable neurons.
Keywords: dual hit, two hit, Parkinson’s disease, Alzheimer’s disease, preconditioning, hormesis, U-shaped
INTRODUCTION
It has long been observed that organisms can adapt to mild stress but are weakened by exposure to severe stress. Many decades ago, the father of toxicology, Phillipus von Hohenheim (also known as Paracelsus), observed that the dose makes the poison (Ottoboni 1997). Despite the dearth of scientific data in the 1500s, Paracelsus suggested that anything can be toxic in high doses and, conversely, that many poisons are not toxic in low doses. Hans Selye, who described biological responses to stress in the first half of the 20th century, also categorized stress into two forms: eustress and distress (Selye 1975). Eustress was defined as mild stressors that improve behavioral function and could be contrasted with severe stressors that exert a negative impact on adaptive behavior. Selye’s seminal studies demonstrated that the response to stress is largely duration-dependent. Stress initially elicits a phase of resistance in animals, whereas chronic stress that is unyielding results in distress. Both of these pioneers, Paracelsus and Selye, therefore described two features that strongly influence the direction of the response to stress: dose and duration. A plethora of more recent studies have largely confirmed their initial suspicions and also identified underlying mechanisms.
Our recent mechanistic studies on adaptations as well as sensitizations to stress are the subject of the present review. It will become evident from our studies that even long duration stress can elicit adaptations in some cellular models provided the stressor dose is low, and that even high dose stress can elicit adaptations in some cell types. However, in other cellular models, severe stress elicits synergistic toxicity when combined with a second challenge. Thus, the response to stress is not unidirectional and is probably highly dependent on stressor dose, stressor duration, cell type, brain region, prosurvival protein profile, organismal age, and many other factors. Our models of stress all revolve around proteotoxicity, stress caused by protein misfolding and aggregations. Proteotoxic stress is a hallmark pathology of neurodegenerative diseases (Walker and LeVine 2000; Walker et al. 2006; Morimoto 2008; Dickson 2009; Jellinger 2009; Uversky 2009; Angot et al. 2010; Gundersen 2010; Morimoto 2011). Many neurodegenerative conditions, including the more common Alzheimer’s and Parkinson’s diseases, are therefore known as proteinopathies. Each of these diseases is characterized by signature protein aggregations or inclusions in specific brain regions. Not surprisingly, there are also reductions in proteasome activity in both Parkinson’s and Alzheimer’s disease (Keller et al. 2000; McNaught et al. 2003; McNaught 2004). The barrel-shaped ubiquitin-proteasome system degrades misfolded proteins that are tagged with a polyubiquitin tail. Although proteasome inhibition cannot mimic the full extent of the pathologies in neurodegenerative diseases, inhibition of proteasome activity with pharmacological tools elicits some of the salient features of these disorders, such as the formation of protein aggregations and cell death (Rideout et al. 2001; Rideout and Stefanis 2002; Sawada et al. 2004; Rideout et al. 2005; Sun et al. 2006; Xie et al. 2010). Another important caveat of current models of neurodegeneration is that they fail to mimic the decades-long pathophysiology of chronic neurodegenerative diseases. This is a difficult obstacle to overcome for the entire field and can be attributed to our lack of knowledge of the primary cause of these disorders, the short rodent lifespan, and the acute nature of insults applied to in vitro models of neurodegeneration. On the other hand, studies on genetic or familial forms of Alzheimer’s and Parkinson’s disease strongly support the hypothesis that abnormally shaped proteins are causally linked to neurodegeneration. Despite this significant advance, our lack of knowledge of the original stimulus that first precipitates protein misfolding in sporadic forms of Alzheimer’s and Parkinson’s disease has stalled the identification of curative therapies and hindered the development of animal and cellular models. Until we identify the reason for protein aggregation in sporadic forms of these diseases, eliciting proteotoxicity with proteasome inhibition in cellular models or by direct infusion into the brain appears to be a reasonable and practical model of protein misfolding stress (Fornai et al. 2006; Pan et al. 2008; Vernon et al. 2010; Zhang et al. 2012). Another form of proteotoxicity in neurodegenerative disorders is the presence of autophagic stress (Nixon and Yang 2012; Son et al. 2012; Salminen et al. 2013). Autophagy by the lysosome is an alternative means to clear cellular debris such as misfolded proteins, and can be mobilized in self-defense when the proteasome is inhibited (Iwata et al. 2005; Ding et al. 2007; Rubinsztein et al. 2007; Janen et al. 2010; Wong and Cuervo 2010). MG132, the toxin that we have used to inhibit the proteasome, also inhibits lysosomal cathepsins (Lee and Goldberg 1998). Thus, treatment with MG132 mimics both the proteasomal and the autophagic stress of proteinopathies. In this respect it is similar to lactacystin, a proteasome inhibitor that also inhibits cathepsin A (Lee and Goldberg 1998; Aikawa et al. 2006).
In this review, we use the term “mild” to describe stressors that are short enough in duration or low enough in dose to be sublethal. We use the term “severe” to describe stressors that are long enough in duration or high enough in dose to be lethal to some fraction of the cellular population. We envision that the response to stress in the human brain may switch from enhanced resistance to increased vulnerability with a shift in either stressor dose or duration. Our earlier work was based on the hypothesis that adaptations to mild cellular stress may partly explain the delayed onset and protracted nature of neurodegenerative conditions (Leak et al. 2006; Leak and Zigmond 2007; Leak et al. 2008). We speculated that endogenous defenses against stress may keep full-blown neurodegenerative illnesses at bay in young individuals and may also brake disease progression in those who do finally develop Parkinson’s or Alzheimer’s disease in old age. Such favorable reactions elicited by low dose stressors or eustress are defined as hormetic responses and are well established in the toxicology literature (Calabrese 2008c; Giordano et al. 2008; Mattson 2008; Calabrese 2010). Hormesis can be viewed as the prototypical homeostatic reaction to environmental fluctuations, in accordance with the original definition of homeostasis by Claude Bernard and Walter Cannon. The ability to respond dynamically to challenges that threaten internal homeostasis is also described as plasticity and is more evident in some cell types than others. With higher levels of stress, however, adaptive responses may fail and the response to subsequent challenges can be compromised. In the latter situation, pre-stressed cells become sensitized to a second hit instead of protected. This toxic type of response may predominate in vulnerable brain regions or in aged animals (Boger et al. 2010). The biphasic nature of the response to stress is reflected in the U-shaped, hormetic dose-response curves that are often reported in toxicology. With some exceptions (Calabrese et al. 2007; Calabrese 2008b), discussions of U-shaped dose response curves and hormesis do not always include reference to a second hit. However, adaptation or sensitization to stress can be quantitatively measured by the response of pre-stressed cells to a second challenge, as will be discussed further below. If previously stressed cells are protected against a second challenge relative to naïve cells, they are said to be preconditioned or in a state of tolerance. On the other hand, if pre-stressed cells respond to two hits with synergistic toxicity they are said to be sensitized to the second challenge. In contrast, if two toxic hits are additive and not synergistic, neither adaptation nor sensitization is at work and the first hit is, in a sense, neutral, because it leaves behind cells that are as vulnerable to the second hit as naïve cells. In short, a two hit protocol is extremely useful to gauge both the direction and magnitude of the stress response.
Our long term goal is to characterize in detail the protein profile of stressed, but adapted neuronal and glial cells and to contrast this profile with cells that are sensitized to subsequent challenges. A better understanding of this “adaptive proteome” in the brain and how it responds to homeostatic challenges might hasten the development of CNS pharmacotherapies or identify dietary/lifestyle changes that mimic these stress-responses without causing any harm. Estimates place 15% of highly conserved proteins in the stress responsive category (Kultz 2005); their abundance and phylogenetic conservation can be viewed as a testament to their importance in homeostasis. Stress responsive proteins include (1) sensors to recognize perturbations, (2) transducers to amplify and integrate signals and (3) effectors to counteract stress (Kultz 2005; Babar et al. 2008). The effector proteins that battle challenges to homeostasis include the antioxidant enzyme systems and folding chaperone machinery as well as the prosurvival signaling cascades. Although these prosurvival effectors have often been observed to be lower in postmortem tissue from Alzheimer’s and Parkinson’s victims, the occasions on which they are higher in disease states are instructive. Well-established examples of antioxidant defenses that are lowered early in the course of Parkinson’s and Alzheimer’s disease include loss of the essential tripeptide glutathione (Sofic et al. 1992; Sian et al. 1994; Calabrese et al. 2006; Baldeiras et al. 2008; Zeevalk et al. 2008; Lloret et al. 2009; Martin and Teismann 2009; Johnson et al. 2012). Based on our two hit model of proteasome inhibition in N2a cells (discussed below), catastrophic drops in glutathione may reflect synergistic proteotoxicity within vulnerable cell types. One should note that these prodeath cellular changes are not necessarily maladaptive to the organism as a whole. It may benefit the organism to clear dysfunctional cells that are damaged beyond repair. Such removal may decrease mutagenesis risk and preserve energy for other cellular systems that can be salvaged. In other words, even toxic responses to stress may play an evolutionarily adaptive role under some circumstances.
In contrast to the loss in glutathione, some prosurvival molecules are raised in the brains of victims of neurodegenerative diseases. Some of the more enlightening examples of these changes will be discussed here. One might speculate that increases in prosurvival molecules reflect those remaining cells that have either successfully battled proteotoxic stress or are able to prolong their lives by delaying the harmful sequelae of proteotoxicity. It is also important to characterize regional variations in the adaptive proteome in this context, as differences in endogenous defense strategies across brain regions may underlie the topographic nature of neurodegenerative disorders, where all neurons are not equally vulnerable to inclusion formation and cell death (Mattson et al. 1989; Mattson and Kater 1989; Braak et al. 2000; Braak et al. 2003; Posimo et al. 2013). An example of regional differences in glutathione content has been published by Mythri and colleagues (Mythri et al. 2011). Although glutathione levels in the nigra are reduced in Parkinson’s disease, glutathione levels are raised in the less vulnerable frontal cortex, caudate, and putamen (Mythri et al. 2011). This increase in glutathione was accompanied by a decrease in the activity of gamma glutamyl transpeptidase, the enzyme that breaks down glutathione. Glutathione peroxidase activity levels were also raised in the caudate and putamen, supporting the hypothesis that more resilient brain regions are protected from oxidative damage in Parkinson’s disease (Mythri et al. 2011). Another instructive study of glutathione peroxidase 4 expression within neuromelanin-containing cells in the nigra reveals an important caveat of these types of experiments (Bellinger et al. 2011). In the Bellinger study, total glutathione peroxidase 4 immunoreactivity was decreased in the substantia nigra of Parkinson’s victims. However, when glutathione peroxidase 4 immunoreactivity was expressed relative to cell density, there was an upregulation of glutathione peroxidase 4 levels within the remaining nigral neurons in Parkinson’s brains. Thus, some previous studies that have reported losses in prosurvival systems in neurodegenerative disorders may actually reflect an overall loss in neuron number. Some studies have dealt with this caveat by quantifying immunostaining intensities within remaining neurons. One example of this type of study shows that the ferroxidase ceruloplasmin is higher in the remaining CA1 hippocampal neurons in Alzheimer’s brains (Loeffler et al. 2001). Ceruloplasmin is a serum copper chaperone, but recent studies show that it also plays a protective role in the central nervous system (Kaneko et al. 2008; Hineno et al. 2011; Texel et al. 2011). Ceruloplasmin concentrations in the brain are increased in Alzheimer’s and Parkinson’s disease (Loeffler et al. 1996). Given its ability to rise with stress in other human conditions, the rise in ceruloplasmin in neurodegenerative diseases is not surprising and may reflect the endogenous defense capacities of the human brain (Mezzetti et al. 1996; Mezzetti et al. 1998; Louro et al. 2000; Taysi et al. 2002; Memisogullari and Bakan 2004; Chacko and Cheluvappa 2010).
Other defensive proteins that are upregulated in neurodegenerative diseases include the well-studied heat shock family of proteins. The heat shock response to stress is a primordial defense against denatured proteins (Verbeke et al. 2001). Heat shock proteins battle apoptosis, refold misfolded proteins, and escort damaged proteins to the proteasome or lysosome for degradation (Kalia et al. 2010; Lanneau et al. 2010; Aridon et al. 2011). One might therefore speculate that an endogenous rise in heat shock proteins is a self-defense mechanism that slows down proteotoxicity in proteinopathies. Heat shock protein 90 is raised in Parkinson’s disease and is colocalized with α-synuclein in Lewy bodies (Uryu et al. 2006). Furthermore, heme oxygenase 1 (also known as heat shock protein 32) is raised in hippocampal and cortical tissue in Alzheimer’s disease (Schipper 2000; Schipper et al. 2006) and is also increased in astrocytes in Parkinson’s disease (Schipper et al. 1998). In mild cognitive impairment, a possible precursor to dementia, heat shock proteins 70 and 27 are both increased in the inferior parietal lobule (Di Domenico et al. 2010). Furthermore, heat shock protein 27 is raised in the nigrostriatal pathway in Parkinson’s disease (Zhang et al. 2005). The major risk factor for neurodegenerative diseases is aging. Although the induction of heat shock proteins is impaired with aging, chaperones in general are increased with aging (Fargnoli et al. 1990; Maiello et al. 1998; Soti and Csermely 2000; Schultz et al. 2001; Walters et al. 2001; Gupte et al. 2010). For example, heat shock proteins 40, 27, 60, 70, and constitutive heat shock protein 70 are known to increase in the aged central nervous system (Lee et al. 2000; Lu et al. 2004). Clearly, different aspects of endogenous defenses can be impaired or increased in individuals at risk for neurodegenerative diseases or with full-blown disorders. Thus, both types of responses can coexist in the same human brain, perhaps not the least because of striking regional variations in vulnerability (Mattson et al. 1989; Mattson and Kater 1989; Braak et al. 2000; Braak et al. 2003; Posimo et al. 2013).
Although it seems reasonable to speculate that rises in prosurvival molecules in human neurodegenerative disorders slow down disease progression, this is not known for certain, as the human studies are correlational and do not establish causation. Furthermore, the analysis of postmortem brain tissue is a cross-sectional snapshot of one moment in time. As a result, we do not know whether changes in prosurvival proteins within individual neurons are long term or transient in nature. Finally, it is also not known how long stress-induced protection or sensitization can linger in the human brain. Most of the answers to these questions come from experimental model systems. The best way to experimentally address whether a response to stress is adaptive or toxic is to challenge cells with a second hit and to quantify the degree of protection or sensitization. As mentioned earlier, sublethal stress-induced protection against subsequent challenges is known as preconditioning. The mechanisms underlying preconditioning have been well defined in studies of ischemia but are often neglected in the study of neurodegenerative diseases. Only a small number of studies have considered the possibility of preconditioning in models of neurodegenerative diseases and some of these will be described here. First, Mark Mattson has proposed that dietary and behavioral manipulations such as exercise and food restriction may protect against models of neurodegeneration by activating stress-responsive pathways (Duan and Mattson 1999; Guo et al. 2000; Mattson et al. 2004; Son et al. 2008). For example, dietary restriction increases levels of brain derived neurotrophic factor, neurogenesis, and heat shock proteins (Mattson et al. 2004). Mattson has further proposed that dietary phytochemicals ingested from plants can precondition against multiple diseases, including Parkinson’s and Alzheimer’s disease (Son et al. 2008). From an evolutionary point of view, phytochemicals may activate stress-responsive pathways because they are designed to repel insects, molds, and even mammals. Two examples are nicotine and caffeine, both of which have been associated with reduced risk for neurodegeneration in epidemiological studies (Tan et al. 2003; Powers et al. 2008). Calabrese and colleagues have argued that hormetic phytochemicals work through vitagenes, genes which encode for heat shock proteins, sirtuin, and thioredoxin (Calabrese et al. 2012). If phytochemicals continue to have these effects for the long term, chronic adaptation may be possible in humans. Second, dietary habits such as moderate alcohol consumption are also associated with lower risks of Alzheimer’s disease and may also be effective over the long term (Peters et al. 2008; Anstey et al. 2009). In support of this notion, ethanol can precondition against models of Alzheimer’s disease (Mitchell et al. 2009; Collins et al. 2010). Other natural dietary compounds, such as the green tea polyphenol epigallocate-chin-3-gallate and the red wine ingredient resveratrol have also been proposed as preconditioning agents in Alzheimer’s and Parkinson’s disease models (Raval et al. 2008; Tai and Truong 2010; Tang et al. 2011; Wu et al. 2012). Resveratrol is thought to protect cells in a sirtuin-dependent manner (Farghali et al. 2012; Wu et al. 2012). Third, anesthetics induce tolerance against subsequent challenges in Alzheimer’s disease models and raise levels of phosphorylated tau (Wei and Xie 2009; Tang et al. 2011). Even β-amyloid itself can be used as a preconditioning tool against subsequent challenges, such as glutamate excitotoxicity, by promoting endocytosis of the NMDA receptor (Goto et al. 2006).
Another example of long term preconditioning in humans may be the lifelong benefits of exercise. Exercise can be viewed as a natural, mild stress. Exercise is known to raise free radical content and can precondition against ischemia (Frasier et al. 2011; Powers et al. 2011; Zhang et al. 2011). Many studies have supported the long-term benefits of exercise in humans, even in Alzheimer’s and Parkinson’s patients (Chen et al. 2005; Xu et al. 2010; Erickson et al. 2012; Mayeux and Stern 2012; Fisher et al. 2013; Intlekofer and Cotman 2013; Winchester et al. 2013). Animal studies have also shown convincingly that exercise is protective in experimental models of neurodegeneration (for some examples, see Adlard et al. 2005; Nichol et al. 2009; Pothakos et al. 2009; Zigmond et al. 2009; Gerecke et al. 2010; Vuckovic et al. 2010; Intlekofer and Cotman 2013; Souza et al. 2013). The studies on the benefits of long term exercise, dietary phytochemicals such as nicotine and caffeine, and moderate alcohol consumption all suggest that chronic adaptation to stress may be achievable in humans. Furthermore, exercise is known to generate an adaptive proteome. For example, we have observed that treadmill exercise raises ceruloplasmin in primates and that levels of physical activity are positively correlated with ceruloplasmin levels (Leak et al. 2012). Despite its stress-responsive nature, ceruloplasmin has not been extensively explored in connection with severe proteotoxic stress and brain neuroprotection. Further studies on this protein are therefore warranted.
In a cellular Parkinson’s disease model, we have shown that sublethal oxidative stress from 6-hydroxydopamine can precondition dopaminergic cells against subsequent lethal exposures to higher concentrations of the same toxin (Leak et al. 2006). The protection in this model was kinase dependent, as inhibitors of ERK1/2, Akt, and JNK activation all attenuated the preconditioning-induced protection. Besides sublethal 6-hydroxy-dopamine, another means of eliciting protection against lethal doses of 6-hydroxydopamine is by thrombin pretreatment in vivo (Cannon et al. 2005). Dopaminergic terminal loss in the striatum and ventricular enlargement were both attenuated by thrombin preconditioning. Third, hyperoxia preconditioning can protect animals against the behavioral symptoms of 6-hydroxydopamine toxicity, such as apomorphine-induced rotations and motor performance on the rotarod (Hamidi et al. 2012). Fourth, preconditioning can also be elicited by the bacterial endotoxin lipopolysaccharide. Lipopolysaccharide is well known to elicit inflammatory responses but, in low concentrations, can precondition organotypic midbrain cultures against subsequent lipopolysaccharide challenges (Ding and Li 2008). Lipopolysaccharide preconditioning protected against dopamine neuron loss as well as lactate dehydrogenase release in this organotypic slice model. Lipopolysaccharide preconditioning also prevented the microglial activation and tumor necrosis factor-α release in response to the second, higher concentration of lipopolysaccaride. In addition to these inflammation-suppressing functions of preconditioning, homeostatic crosstalk between endoplasmic reticulum stress and autophagy may also mediate the benefits of preconditioning in Drosophila and mouse Parkinson’s disease models (Fouillet et al. 2012; Matus et al. 2012). In these studies, inhibition of autophagy was found to impair endoplasmic reticulum stress-induced protection. Matus and colleagues have recently reviewed these hormetic responses to protein-misfolding stress (Matus et al. 2012). Fifth, activation of the antioxidant response element by endoplasmic reticulum stress inducers can also precondition against 6-hydroxydopamine toxicity (Hara et al. 2011). Sixth, low dose methamphetamine challenges can protect dopaminergic cells against 6-hydroxydopamine toxicity (El Ayadi and Zigmond 2011). Finally, in vitro studies also provide evidence that heat shock can precondition against 1-methyl-4-phenylpyridinium (MPP+), the active metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), another well-established model of Parkinson’s disease (Quigney et al. 2003; Fan et al. 2005). These studies, while not numerous, reveal that preconditioning can indeed occur in models of neurodegenerative diseases.
In contrast to the small numbers of studies on preconditioning in neurodegeneration, several seminal papers on short duration ischemic episodes initiated a flood of investigations on preconditioning in stroke models (Murry et al. 1986; Kitagawa et al. 1990; Kirino et al. 1991; Liu et al. 1992; Kirino 2002; Dirnagl et al. 2003). These studies consistently showed that short, sublethal ischemic episodes elicit tolerance of subsequent, longer duration ischemic attacks that would otherwise be lethal. As argued by Valina Dawson, ischemic preconditioning offers a way to “mine for survival genes” (Dawson and Dawson 2006) and has been a productive field of research for many decades. Ischemic preconditioning also has translational potential; remote ischemic preconditioning of an arm or leg with a tightened blood pressure cuff may protect distant organs from ischemic events such as stroke and cardiac bypass surgery (Fairbanks and Brambrink 2010; Candilio et al. 2011). The state of our knowledge on ischemic preconditioning has been discussed in many recent reviews (Dirnagl and Meisel 2008; Della-Morte et al. 2012; Kitagawa 2012; Prabhakar and Semenza 2012; Thompson et al. 2012) and will not be described further here.
As mentioned above, if the stressor is severe, it can exacerbate the toxic response to future insults and result in greater than additive cell loss. For example, previous exposures to thromboembolic events can combine with a subsequent ischemic insult to produce larger areas of ischemic injury (Dietrich et al. 1999; Danton et al. 2002). In the field of neurodegeneration, the synergistic toxicity of multiple challenges is the subject of the “two hit” or “dual hit” hypothesis (Zhu et al. 2004; Carvey et al. 2006; Manning-Bog and Langston 2007; Sulzer 2007; Zhu et al. 2007; Weidong et al. 2009; Boger et al. 2010; Gao and Hong 2011; Unnithan et al. 2012). A few reports of dual-hit insults in Parkinson’s and Alzheimer’s disease will be described below. First, Carvey and Di Monte have discussed the dual hit concept with reference to Parkinson’s disease in that sufficient cell loss to elicit symptoms may require multiple stress exposures (Di Monte et al. 2002; Ling et al. 2004b). For example, toxic environmental agents might interact with endogenous factors such as α-synuclein and aging. Indeed, gene-environment interactions are particularly relevant to the two hit hypothesis (Gao and Hong 2011) and can be studied by fusing animal models (Manning-Bog and Langston 2007). Genetic disruptions that result in fewer dopamine neurons at birth may also result in Parkinsonian symptoms when combined with age-related neuronal attrition (Weidong et al. 2009). Other models of the two hit hypothesis have examined loss in trophic factors such as glial cell derived neurotrophic factor (GDNF) and its impact on the response to aging and to methamphetamine challenges (Boger et al. 2010). As expected, genetic reductions in GDNF exacerbate age-related changes in dopaminergic systems and increase vulnerability to methamphetamine. Several studies have examined the two hit hypothesis in the context of inflammatory changes to the brain. The authors of these studies consistently report that the pesticide rotenone or the neurotoxin MPTP can both combine with the inflammogen lipopolysaccharide to elicit synergistic neurotoxicity in dopamine neurons (Gao et al. 2003a; Gao et al. 2003b; Ling et al. 2004a). Dopamine oxidation and mitochondrial dysfunction have also been suggested to combine with loss of function gene mutations or autophagic self-degradation to underlie cell death in Parkinson’s disease (Sulzer 2007). Smith and colleagues have put forth a two hit hypothesis for Alzheimer’s disease in that oxidative stress and mitogenic dysregulation may combine to increase risk for Alzheimer’s pathology (Zhu et al. 2001; Zhu et al. 2004; Zhu et al. 2007). In their model, oxidative stress and abnormalities in mitotic signaling can both initiate pathology, but both must be present to propagate the full extent of the pathology. A multiple hit model of changes in tau function has also been suggested to promote tau assembly (DeTure et al. 2006). Furthermore, traumatic brain injury has been hypothesized to predispose individuals to both Parkinson’s and Alzheimer’s disease (Kiraly and Kiraly 2007). Finally, a two hit study from the stroke literature is particularly edifying (Qiao et al. 2009). In this study by Tuor and colleagues, a 40 min stroke resulting in focal necrosis was combined with a 60 min stroke three days later. Proximal to the ischemic core, where loss of blood flow was the most severe, the damage exceeded that of the first insult, whereas distally, there was tolerance to the insult. These findings reveal that adaptation and sensitization can occur within the same brain and show elegantly that the direction of the response depends on the magnitude of the insult.
The two hit terminology has not generally been applied to studies of preconditioning although preconditioning protocols also apply two sequential stressors. The word ‘hit’ is not typically used in reference to sublethal stress even though it elicits transient damage (for an example, see Dembinski et al. 2006). Sublethal preconditioning stimuli also increase reactive oxygen species and activate the caspase cascade (McLaughlin et al. 2003; Thompson et al. 2012). Without sublethal injury, there would be no stress response because the sensors would not recognize any perturbations. Before setting semantic issues aside, we argue here that the two hit hypothesis should, by definition, encompass any protocol that involves two hits, be they sublethal or lethal, and that the hypothesis must account for the biphasic nature of stress responses. Therefore, we propose that the response to two hits can involve the following: 1) preconditioning-style adaptations following sublethal stressor hits, 2) additive toxic responses to two severe stressor hits in which the first hit does not change the response to the second hit, 3) synergistic toxic responses to two severe stressor hits in which the first hit magnifies the response to the second hit, and 4) adaptive responses to severe stress so that the impact of a second hit is blunted in the cells that manage to survive the first hit. To explore some of these possibilities, below we describe our recent work investigating responses to proteotoxicity in various two hit cellular models. We begin with a description of adaptations to chronic low dose proteotoxic challenges. A high-throughput two hit model of synergistic neurodegeneration is also presented. Finally, we summarize our recent findings that astrocytes, a glial cell type well known for stress-induced plasticity, can adapt to proteotoxic stress delivered at a high enough concentration to kill half the population. This was the first demonstration that the glial survivors of severe proteotoxic stress are more resistant than naïve cells. The mechanisms underlying the adaptations and sensitization are also presented.
EVEN LONG-TERM STRESS CAN ELICIT ADAPTATIONS
As mentioned above, neurodegenerative conditions are characterized by inhibition of the normal role of the proteasome. Indeed, the chronic nature of neurodegenerative conditions raises the possibility that diseased brains are exposed to proteotoxic stress for an extended time-frame. Of course, Selye had argued that chronic stress weakens defenses. Nonetheless, we wondered if cells are able to adapt to chronic stress if the dose is low enough. If this was true, mild stress-induced plasticity might explain why neurodegenerative conditions are so slow to progress despite evidence of long-term proteotoxicity.
Although Parkinson’s disease involves degeneration of multiple brain regions, such as noradrenergic (Forno 1996; Gesi et al. 2000), serotonergic (Halliday et al. 1990; Gai et al. 1992; Politis et al. 2012), and hypocretin/orexin systems (Fronczek et al. 2007; Thannickal et al. 2007), the motor deficits are largely attributed to massive dopaminergic cell loss in the nigrostriatal tract. Parkinson’s disease is also characterized by its late age of onset and progressive nature, suggesting that endogenous adaptations may be hard at work. In order to test the hypothesis that cells can adapt to chronic stress in a dopaminergic model, PC12 cells were exposed to long durations (14 days - 6 months) of the proteasome inhibitor MG132 (Leak et al. 2008). In our model, MG132 (0.1 μM) effectively reduced chymotrypsin proteasome activity by 47%. Furthermore, there was a statistical trend towards higher levels of ubiquitin-conjugated proteins with MG132 treatment. These findings suggest that MG132 was proteotoxic in this model. However, no impact of chronic MG132 on overt morphology or tyrosine hydroxylase expression was observed. Tyrosine hydroxylase is the rate-limiting enzyme for dopamine biosynthesis. We also measured viability at 4–5 day intervals for two weeks after initiation of MG132 treatment and found no change. MG132 (0.1 μM) was left in the media at all times for up to 6 months. Viability was measured within two days after plating cells throughout this entire procedure. We discovered that chronic pretreatment with 0.1 μM MG132 did indeed protect against subsequent challenges. Fourteen days or longer exposure to sublethal MG132 protected against either 6-hydroxydopamine or higher-dose MG132 (40 μM). Protection in this system was verified by two independent assays for cell viability: the Cell Titer Glo assay for ATP and counts of Hoechst-stained nuclei. We typically perform at least two viability assays in order to reduce the likelihood of false positives and to measure protection of both cell numbers and metabolic activity. This helps us ascertain the impact of treatments on cellular structures as well as their function. However, a potential confound of our interpretation that chronic MG132 was protective would be if MG132 decreased activity of the dopamine transporter that shuttles 6-hydroxydopamine into the cytoplasm from the extracellular medium. In other words, we were concerned that the rise in viability in chronically stressed cells might be an artifact of reduced influx of the 6-hydroxydopamine toxin. In contrast to this expectation, we observed a 36% rise in dopamine transporter activity after chronic treatment with MG132, not a fall. This suggested that the pre-stressed cells were protected despite slightly greater exposure to 6-hydroxydopamine. Notably, when MG132 was removed from the media for more than two weeks, the protection disappeared, suggesting that the stress had to be continuous to elicit an adaptive response. Conversely, this low concentration of MG132 was not sufficient to elicit protection if administered for less than two weeks. Taken together, all of these observations are consistent with the hypothesis that dopaminergic cells have the capacity to adapt to chronic sublethal stress but that the protection disappears when the stressful stimulus is removed.
Next we proceeded to examine the mechanism underlying long term adaptive defenses. First, we scrutizined the role of the ubiquitous thiol glutathione in this model. Thiol defenses are so important to cells that glutathione is present in millimolar concentrations in most tissues (Cooper and Kristal 1997; Wilson 1997; Dringen 2000; Pompella et al. 2003; Pocernich and Butterfield 2011). Proteotoxicity and oxidative stress are inextricably intertwined in neurodegenerative conditions because oxidized proteins can become misfolded and must be degraded by clearance systems such as the proteasome. We initially speculated that glutathione would stave off the negative impact of chronic proteotoxic stress. Thus, we hypothesized that inhibiting glutathione synthesis would abolish or attenuate the stress-induced protection against 6-hydroxy-dopamine. In contrast to this expectation, inhibition of glutathione synthesis with buthionine sulfoximine exacerbated 6-hydroxydopamine toxicity to the same degree in naïve cells and cells treated with chronic MG132 and did not attenuate the MG132-induced protection at all. Chronic MG132 also did not raise glutathione levels. This suggested that glutathione defenses were not responsible for the adaptation to long term MG132.
As a result of these negative findings, we proceeded to examine levels of other antioxidant molecules and folding chaperones. We found an increase in CuZn superoxide dismutase enzymatic activity and protein levels with chronic MG132. In contrast, a small rise in Mn superoxide dismutase protein levels was not accompanied by a parallel rise in enzyme activity. Superoxide dismutases catalyze the dismutation of superoxide into hydrogen peroxide and oxygen. Catalase and heat shock protein 70 levels were also raised by chronic MG132. Catalase aids the breakdown of hydrogen peroxide and thus may act in conjunction with the superoxide dismutase enzymes. Heat shock protein 70 actively battles apoptosis in addition to its chaperone functions and is thought to be protective against neurodegeneration (Koren et al. 2009; Nagai et al. 2010; Witt 2010; Aridon et al. 2011; Silver and Noble 2012). We decided to focus on the change in CuZn superoxide dismutase because previous investigations had shown that CuZn superoxide dismutase overexpression is protective in models of Parkinson’s disease whereas deficiencies in this protein exacerbate dopaminergic neurodegeneration (Przedborski et al. 1992; Asanuma et al. 1998; Barkats et al. 2002; Sturtz and Culotta 2002; Van Remmen et al. 2004; Barkats et al. 2006; Wang et al. 2006). Notably, CuZnSOD levels are lowered in Parkinson’s and Alzheimer’s patients (Boll et al. 2008; Torsdottir et al. 2010), and CuZnSOD is found in Lewy bodies (Nishiyama et al. 1995). We therefore hypothesized that knockdown of CuZn superoxide dismutase with RNA interference would attenuate the MG132-induced protection against 6-hydroxydopamine. As expected, CuZn superoxide dismutase knockdown with either of two short interfering RNA (siRNA) sequences attenuated MG132-induced protection. Using two independent siRNA sequences in place of one sequence alone reduced the likelihood of false positive results from non-specific effects of RNA interference. Taken together, these findings revealed multiple adaptive changes in stressed cells and showed that CuZn superoxide dismutase was responsible for long term stress-induced protection against oxidative toxicity.
In summary, these studies describe the protective nature of chronic but mild proteotoxic stress. The protection lasted for at least 6 months (the latest timepoint we tested), but removal of the stimulus caused loss of defenses. Adaptive proteins such as anti-apoptotic chaperones and antioxidant enzymes were raised by chronic stress and CuZn superoxide dismutase mediated protection of dopaminergic cells against oxidative toxicity. It must be acknowledged here that our definition of chronic falls short of the decade-long march of neurodegeneration in Parkinson’s and Alzheimer’s disease. The only conceivable model systems in which decades-long insults might be applied are the nonhuman primates. Whether mild, low dose proteotoxic stress can protect the primate brain for the truly long term remains to be seen. Second, although they express tyrosine hydroxylase, PC12 cells are not always predictive of dopaminergic neurons because PC12 cells originate from the adrenal gland and not the brain. Although PC12 cells can be differentiated to a neuronal phenotype, differentiated PC12 cells are unsuitable for the long term studies conducted here because they begin to die. For our next high-throughput cellular model, we switched to a neuroblastoma cell line, N2a, which originates from the mouse spinal cord. Although N2a cells are not dopaminergic, Parkinson’s disease is now well known to extend beyond the ventral midbrain, as mentioned earlier. Many extranigral brain regions, including the spinal cord, are affected with synuclein inclusions (Braak et al. 2002; Braak et al. 2003; Del Tredici and Braak 2012). In the studies discussed below, we examined the N2a response to acute, severe proteotoxic stress in a cellular model of synergistic neurodegeneration.
CELLS CAN BE SENSITIZED TO INJURY BY SEVERE STRESS
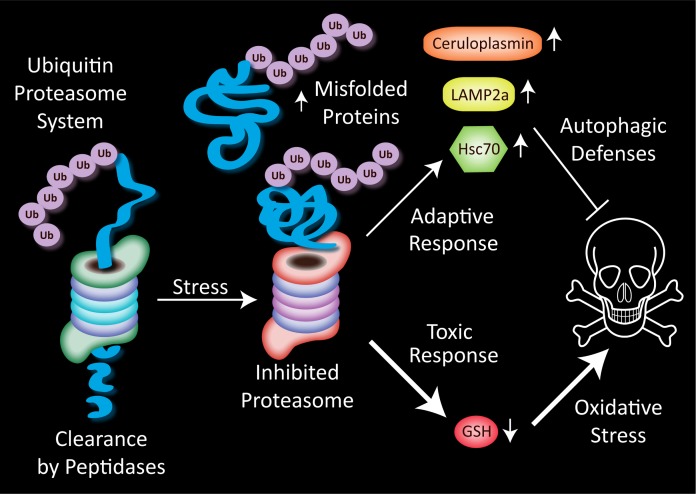
Although stressors that potentiate the response to subsequent challenges may occur decades prior to the second hit, two hits may also occur in rapid succession, such as exposure to toxicants in careers in agriculture or industry. Many agricultural workers, for example, are exposed to pesticides and herbicides on a daily basis. Some of these pulsatile challenges to humans may elicit oxidative damage and protein misfolding. In support of this notion, pesticide and herbicide exposures increase the risk for Parkinson’s disease and cause protein aggregations and cell death in animal models (Liou et al. 1997; Betarbet et al. 2000; Alam and Schmidt 2002; Manning-Bog et al. 2002; McCormack et al. 2002; Franco et al. 2010; Tanner et al. 2011). We hypothesized that synergistic responses to dual proteotoxic challenges should be dose dependent and only elicited by severe stress, such as high concentrations of MG132. In support of this hypothesis, lethal, but not sublethal concentrations of MG132 were found to synergize in their negative impact on N2a viability when administered 24 hours apart (Unnithan et al. 2012). Three independent and unbiased viability assays, conducted on the third day, illustrated this effect. For assaying viability in a high-throughput manner, we stained the nucleus and cytoplasm with a combination of two infrared stains (DRAQ5 and Sapphire) and measured levels of the cytoskeletal protein α-tubulin with immunocytochemistry. The third viability assay, Cell Titer Glo, measured ATP levels. Interestingly, the Cell Titer Glo assay demonstrated that sub-lethal concentrations of MG132 raised ATP without a parallel change in cell numbers. This favorable metabolic reaction to low level proteotoxic stress may allow slightly stressed cells to battle sublethal injury more effectively and is an example of hormesis. As hypothesized, higher concentrations of MG132 elicited cell loss and increased the toxic response to a second MG132 hit by all three viability assays. Low or subtoxic concentrations of MG132 did not elicit this synergistic response. Toxic, but not subtoxic concentrations of MG132 greatly raised ubiquitin-conjugated proteins in this model, suggesting that higher concentrations of MG132 effectively hindered the clearance of misfolded proteins (Fig. 1).
FIGURE 1.
Misfolded proteins are tagged with an ubiquitin tail, linearized, and fed through one end of the barrel-shaped proteasome. Peptides exit the other end of the proteasome and are further degraded by cytoplasmic peptidases into amino acids for recycling into fresh proteins. Stress on the proteasome, such as that induced by proteasome inhibitors, causes the buildup of misfolded proteins that can no longer be degraded. In N2a cells treated with high concentrations of the proteasome inhibitor MG132, two types of responses are elicited in response to the reduced clearance of damaged proteins. A rise in the antioxidant ceruloplasmin and the chaperone-mediated autophagy proteins lysosome-associated membrane protein type-2a (LAMP2a) and heat shock cognate 70 (Hsc70) may serve to defend the cell. Inhibiting autophagic defenses increases the toxicity of both single and dual MG132 hits in this model, suggesting that cells use autophagy as an alternative clearance mechanism when the proteasome is inhibited. Cells also respond to dual hits of severe proteotoxicity with a synergistic loss in glutathione (GSH) defenses. This loss of GSH may increase oxidative toxicity and enhance cell death.
Adaptive responses to low level stress in our dopaminergic model of the previous section included rises in several antioxidant proteins and the anti-apoptotic folding chaperone heat shock protein 70. Conversely, we expected toxic responses to severe stress to involve loss of antioxidant and chaperone defenses. Contrary to these hypotheses, we found no loss in heat shock protein 70 with toxic MG132 concentrations. In addition, toxic, but not subtoxic MG132 elicited a rise in ceruloplasmin, not a loss (Fig. 1). Our data showing an MG132-induced rise in ceruloplasmin in neuronal cells is consistent with previous studies that it increases with stress (Mezzetti et al. 1996; Mezzetti et al. 1998; Louro et al. 2000; Taysi et al. 2002; Memisogullari and Bakan 2004; Chacko and Cheluvappa 2010) and demonstrate that this response can be elicited by proteotoxicity even when the stress is severe.
Next we examined whether toxic MG132 would elicit loss of autophagic markers, because concomitant failure of autophagic and proteasome defenses might underlie the synergistic toxicity of two MG132 hits. However, toxic concentrations of MG132 raised proteins involved in chaperone-mediated autophagy such as heat shock cognate 70 (Hsc70) and the lysosome-associated membrane protein type-2a (LAMP2a). These responses again reflect adaptive responses to severe proteotoxic stress, not toxic responses to stress (Fig. 1). The notion that the chaperone-mediate autophagy markers reflect cellular engagement in self-defense was supported by the finding that ammonium chloride, an inhibitor of autophagic protease activity (Kaushik and Cuervo 2009), increased the toxicity of both single and dual hits of MG132 in our model. Thus, cells exposed to toxic concentrations of proteasome inhibitors may rely on autophagy as an alternative mechanism to clear cellular debris. Of course, adaptive responses such as rises in ceruloplasmin or autophagic markers failed to explain why two toxic MG132 hits were synergistic in nature. We therefore proceeded to test the hypothesis that loss of thiol defenses underlay the toxic impact of two hits.
In contrast to the rises in autophagic proteins and ceruloplasmin, we found that two hits elicited a synergistic loss of glutathione. The response of glutathione to two hits therefore paralleled the synergistic loss of viability and supported the hypothesis that loss of thiol defenses might underlie the toxicity of two hits (Fig. 1). As a result of these findings, we examined whether raising glutathione levels would protect the N2a cells against two toxic hits of MG132. The glutathione precursor N-acetyl cysteine prevented glutathione loss and almost completely abolished the toxic response to two MG132 hits by all three viability assays. N-acetyl cysteine is an over-the-counter supplement well known to be protective in animal models (for some examples, see Perry et al. 1985; Martinez Banaclocha 2000; Pocernich et al. 2000; Farr et al. 2003; Fu et al. 2006; Tucker et al. 2006; Sharma et al. 2007; Clark et al. 2010). It has even been shown to benefit cognitive status in Alzheimer’s patients (Adair et al. 2001). N-acetyl cysteine is therefore currently being tested in clinical trials of Parkinson’s disease (Clinicaltrials.gov ID: NCT01470027). Our findings are consistent with a protective effect of N-acetyl cysteine against proteinopathies, even when the proteotoxic stress is high in concentration and unremitting in nature.
In summary, the response of neuronal cells to two MG132 hits reveals an exquisite dose-sensitivity of synergistic effects. Low concentrations of MG132 did not elicit synergistic toxicity; only severely toxic concentrations of MG132 potentiated the response to the second hit. Despite the toxic effects of high concentrations of MG132, highly stressed cells nonetheless appeared to raise adaptive defenses in the form of autophagic markers and ceruloplasmin (Fig. 1). One might speculate that stressed cells would be even worse off without such defenses. This speculation is supported by our observation that the toxicity of MG132 was increased with an autophagy inhibitor. These studies showed for the first time that the two hit neurodegenerative phenomenon can be extended to protein-misfolding stress from proteasome inhibition. Furthermore, the data on glutathione support the classic notion that oxidative and proteotoxic stressors propel and propagate each other. Oxidative stress has been associated with neurodegenerative proteinopathies for many decades ever since Denham Harman drew attention to free radicals in aging in the 1950s (Harman 1956; Floyd and Hensley 2002; Harman 2006, 2009).
A few caveats of our neuroblastoma studies are worth mentioning here. Our N2a studies of two proteotoxic hits were extremely short in duration compared to the decades-long exposure to stress in human neurodegenerative conditions. Our protocol is better suited to model the toxic impact of rapid, successive hits of proteotoxic stress. Second, the protein profile and concentration of reactive oxygen species in tumor cells is often different from that of normal cells, especially when contrasted to differentiated cells that have exited the cell cycle some time ago. Studies on immortalized lines cannot fully recapitulate the highly heterogenous and differentiated pool of neurons in the brain. Primary cultures are therefore probably more predictive of in vivo brain function and were used in the next series of experiments. Because we had observed a number of defensive responses to severe proteotoxic stress in N2a cells, we wondered whether primary astrocytes might adapt to high dose proteotoxic stress. A previous study by Friedman and colleagues provides precedence to examine the protective impact of severe stress. In that study, exposure to a moderately toxic hit of glutamate (eliciting 30% cell loss) protected hippocampal neurons against exposure to a lethal glutamate challenge 7 days later (Friedman and Segal 2010).
ASTROCYTES CAN ADAPT TO SEVERE STRESS
Thus far we had shown that dopaminergic cells can adapt to low level proteotoxic stress in a CuZn superoxide dismutase-dependent and glutathione-independent manner but that neuronal cells cannot survive multiple bouts of high level proteotoxic stress because of catastrophic glutathione loss. Notably, highly stressed neuronal cells still responded to toxic MG132 with some adaptations, such as a rise in autophagic markers and ceruloplasmin. All of these findings led us to wonder whether severe proteotoxicity would elicit adaptations in cells known for their stress resistance, astrocytes (Shao and McCarthy 1994). Astrocytes are well known to interact with neighboring neurons, providing them with trophic support and metabolic precursors such as lactate (Westergaard et al. 1995; Rathbone et al. 1999; Benarroch 2005; Barres 2008). Furthermore, astrocytes probably serve as sentinels, as they express many types of neurotransmitter receptors (Fuller et al. 2010; Verkhratsky et al. 2012). Astrocytes are also critical for the production of glutathione in the brain (Dringen et al. 2000). In Parkinson’s disease, astrocytes in the amygdala, septum, cortex, thalamus, and striatum become immunoreactive for the neuronal protein α-synuclein (Wakabayashi et al. 2000; Braak et al. 2007). These findings support the hypothesis that astrocytes engulf α-synuclein from the extracellular space through endocytosis to protect neighboring neurons (Lee et al. 2010). Furthermore, astrocytes are also known to engulf extracellular β-amyloid (Wyss-Coray et al. 2003). Because of their plasticity and critical roles in maintaining neuronal viability, astrocytes have been proposed to define homeostasis in the central nervous system (Parpura et al. 2012).
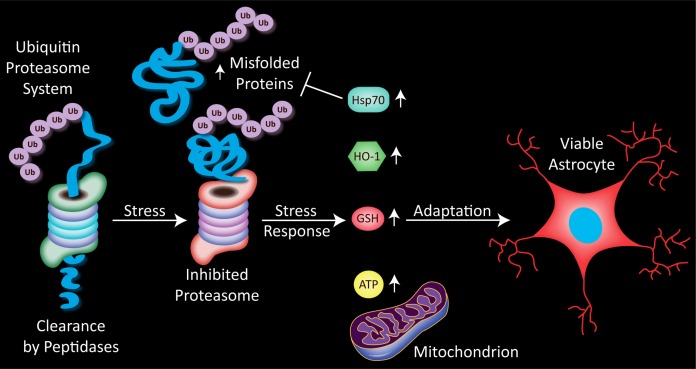
Given the plasticity of astrocytes, we wondered whether adaptive astrocytic responses could be elicited even with LC50 concentrations of MG132 that were lethal to half the cellular population. To our knowledge this question had not been answered in astrocytes before, although previous studies had shown that astrocytes can adapt to sublethal stressors (Rajapakse et al. 2003; Calabrese 2008a; Chu et al. 2010; Du et al. 2010; Du et al. 2011; Johnsen and Murphy 2011). In our model, we delivered two hits of toxic concentrations of MG132 one day apart to primary cortical astrocytes (Titler et al. 2013). Cells were assayed for viability on the third day by counting the remaining Hoechst-stained nuclei and measuring ATP. Toxic MG132 concentrations that killed approximately half the population of astrocytes did render the remaining cells resistant to a second MG132 hit. In other words, the toxicity of two hits was neither additive nor synergistic and the response to the second hit was blocked. Both cell count and ATP level viability assays verified that pre-stressed astrocytes were protected against a second hit. Although the cell count data confirmed the lethal nature of the first hit, the ATP assay actually revealed a slightly different pattern than cell counts. That is, the first hit did not elicit any ATP loss as it had loss of cell numbers; ATP output per cell had risen instead. Second, the first hit completely prevented the usual ATP loss in response to the second, higher dose challenge. The ATP dose-response curve therefore looked very similar to a traditional preconditioning curve with sublethal stress protecting against a second, otherwise lethal challenge. Because the pre-stressed cells were protected against ATP loss in response to a second MG132 challenge, we concluded that astrocytes exhibit active metabolic adaptations in response to severe proteotoxic stress. The rise in ATP output with the first hit may help fuel anti-apoptotic signaling cascades and preserve homeostasis.
As a potential confound to our interpretations on active astrocytic adaptations, we initially wondered whether the first MG132 hit was simply leaving behind astrocytes that were refractory to the toxin and therefore also unresponsive to a second hit. If this was the case, we could not claim that astrocytes mounted any adaptations to MG132. Alternatively, a second MG132 hit could continue to have an impact on the proteasome in pre-stressed cells even though it did not lead to additional cell death. We tried to distinguish between these two possibilities by assaying for ubiquitin-conjugated proteins. We found that two hits of MG132 caused a synergistic rise in ubiquitin-conjugated proteins (Fig. 2). This finding was incompatible with the hypothesis that the remaining cells were simply refractory to MG132. Even though the downstream impact of proteotoxic stress on cell viability itself was abrogated, these data demonstrate that the stress on the proteasome itself was not prevented. The potentiation of this proteasomal response verifies the continued impact of MG132 in the survivors of the first hit and also reflects the severity of the proteotoxicity.
FIGURE 2.
Stress on the proteasome, in the form on inhibition of its normal proteolytic function, increases the cellular burden of damaged proteins. Astrocytes respond to such proteasome inhibition with a rise in the anti-apoptotic heat shock protein 70 (Hsp70). Numerous studies reveal that Hsp70 refolds misfolded proteins or enhances their degradation by the proteasome. A parallel rise in heme oxygenase 1 (HO-1), a generally protective phase 2 enzyme, is also apparent. Astrocytes respond to severe proteotoxicity with glutathione (GSH) loss, unless they have been pre-stressed with MG132, in which case GSH levels are restored. This thiol adaptation serves to increase the number of viable astrocytes and is accompanied by a rise in ATP. Pre-stressed astrocytes are thus both structurally and functionally protected against further proteotoxicity.
In order to probe for adaptive rises in pro-survival molecules in stressed astrocytes, we measured heat shock protein 70 and heme oxygenase 1. Heme oxygenase 1 degrades heme into biliverdin and carbon monoxide (Grochot-Przeczek et al. 2012). Astrocytes express high levels of this protein, perhaps reflecting their inherent resilience (Dwyer et al. 1995). Both heat shock protein 70 and heme oxygenase 1 were raised by MG132, as expected from cells that are attempting to battle toxicity (Fig. 2). We also probed for glutathione levels in this model, and discovered that pre-stressed astrocytes failed to respond to the second hit with the usual glutathione loss, unlike naïve astrocytes challenged with high dose MG132. In other words, glutathione levels following the MG132 challenge were higher in pre-stressed astrocytes than in naïve controls. This finding suggested that severely stressed astrocytes might use thiol defenses to protect themselves against future insults. Consistent with this hypothesis, depletion of glutathione stores with buthionine sulfoximine unmasked the cumulative impact of two hits; pretreated astrocytes now became vulnerable to the second MG132 hit and responded with additional cell loss. The unmasking of the vulnerability to two hits following glutathione depletion was also inconsistent with the notion that the first MG132 hit simply left behind cells that were unresponsive to the poison. As in the N2a two hit model, the findings reveal that antioxidant defenses help defend cells against proteotoxic stress. However, astrocytes and the neuronal N2a cells responded to severe proteotoxicity in opposite fashion. The response to the second proteotoxic hit was blocked in astrocytes but potentiated in N2a cells. We do not claim here that neuronal cells only respond to dual challenges with synergistic toxicity or that only astrocytes can adapt to severe stress. Instead, we have preliminary data in primary cortical cultures that neurons are able to adapt to severe oxidative stress from hydrogen peroxide pretreatment. Thus, whether severe stress elicits adaptations or exacerbates further insults may depend on the nature of the stress as well as the specific cell type, in addition to dose and duration.
In summary, astrocytes are a highly plastic cell type that can adapt to stress even when it is toxic enough to kill half the population. The astrocytes that survive the initial hit are less, not more, vulnerable to further proteotoxicity and have higher levels of glutathione upon the second hit (Fig. 2). We speculate that astrocytes that are exposed to similarly severe protein-misfolding stress in the human brain may fulfill their roles as neurosupportive cells better than if they had no such defenses. As mentioned above, astrocytes and neurons are well known to interact in the brain, probably in conjunction with oligodendrocytes (Amaral et al. 2013). It remains to be determined whether stressed astrocytes provide increased trophic, metabolic, or antioxidant support for neighboring neurons or whether stressed astrocytes engulf more misfolded proteins in proteotoxic conditions. Many have argued instead that activated astrocytes neglect their neurosupportive roles, particularly in the presence of chronic inflammation (Fuller et al. 2010). Thus, examinations of the protective or toxic impact of severe proteotoxic stress on neuronal-astrocytic interactions are highly warranted.
CONCLUSIONS
The mammalian brain enjoys manifold robust defenses. Even the simple observation that a large fraction of dopamine must be lost before movement deficits emerge reflects the impressive compensatory adaptations of the human brain (Hornykiewicz 1975; Zigmond et al. 1990; Hornykiewicz 1998). Another form of adaptation is the ability to raise anti-apoptotic proteins in response to stress. The studies detailed in this review, as well as many others not discussed here, have slowly begun to define this adaptive proteome. Our studies add to this body of work by specifically revealing that the ubiquitous tripeptide glutathione is responsible for adaptation against severe proteotoxicity. Conversely, when it is reduced in levels, a lack in glutathione is responsible for synergistic proteotoxicity.
As mentioned above, the long term goal of our studies is to characterize the adaptive proteome so that it can be mimicked with pharmacological tools. Clinical preconditioning with pharmacotherapies is not unprecedented or futuristic. Many FDA approved agents such as aspirin, isoflurane, and statins are already thought to precondition against ischemia (Gidday 2010). Defining the molecular targets of preconditioning and the effectors to counteract stress may also guide studies on lifestyle and dietary factors that elicit a naturally therapeutic protein profile. However, all the findings presented must be examined in further detail in whole animals and over longer timeframes. Even partial inhibition of the proteasome for many months does not recapitulate the full extent of the pathophysiology of neurodegenerative disorders. More chronic models than presented here are therefore required to rigorously test the hypothesis that the cells that remain behind after neurodegeneration has commenced are either resistant or sensitized to further toxicity. Studies of this nature would build upon previous in vivo reports showing that repeated stressors can provide persistent protection against ischemia/reperfusion injury (Hoshida et al. 2002). Bearing these important gaps in our knowledge and the experimental caveats in mind, we predict that both neurons and glia in vivo will react to severe stress in a way that deeply affects their response to subsequent challenges, but that the response will depend on dose, duration, and perhaps brain region (Mattson et al. 1989; Mattson and Kater 1989; Braak et al. 2000; Braak et al. 2003; Posimo et al. 2013). More specifically, we propose that distinct adaptations and vulnerabilities to proteotoxic stress across different brain regions may underlie the signature topographies of protein inclusions in neurodegenerative diseases.
Finally, the two hit model is probably a simplification of the injuries that occur in neurodegenerative conditions because the diseased human brain may be exposed to a rolling landscape of hits, and not just two sequential stressors. Furthermore, some stressors may not even appear as a hit because they are not transient, but chronic in nature. Nevertheless, the two hit treatment protocol with MG132 is a useful tool to probe whether proteotoxic stress (the first hit) elicits adaptations or toxic responses. If more examples of adaptations can be collected in vivo in chronic proteotoxicity models, they would be consistent with the delayed onset and slowly progressive nature of neurodegenerative conditions. Conversely, toxic neuronal responses to severe proteotoxic stress may overwhelm defenses when the stress is unyielding, as originally postulated by Selye, and are consistent with the eventual collapse of vulnerable brain regions in Parkinson’s and Alzheimer’s disease.
REFERENCES
- Adair JC, Knoefel JE, Morgan N. Controlled trial of N-acetylcysteine for patients with probable Alzheimer’s disease. Neurology. 2001;57:1515–1517. doi: 10.1212/wnl.57.8.1515. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa S, Matsuzawa F, Satoh Y, Kadota Y, Doi H, Itoh K. Prediction of the mechanism of action of omuralide (clasto-lactacystin beta-lactone) on human cathepsin A based on a structural model of the yeast proteasome beta5/PRE2-subunit/omuralide complex. Biochimica et biophysica acta. 2006;1764:1372–1380. doi: 10.1016/j.bbapap.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Alam M, Schmidt WJ. Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res. 2002;136:317–324. doi: 10.1016/s0166-4328(02)00180-8. [DOI] [PubMed] [Google Scholar]
- Amaral AI, Meisingset TW, Kotter MR, Sonnewald U. Metabolic aspects of neuron-oligodendrocyte-astrocyte interactions. Front Endocrinol (Lausanne) 2013;4:54. doi: 10.3389/fendo.2013.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angot E, Steiner JA, Hansen C, Li JY, Brundin P. Are synucleinopathies prion-like disorders? Lancet neurology. 2010;9:1128–1138. doi: 10.1016/S1474-4422(10)70213-1. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Mack HA, Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am J Geriatr Psychiatry. 2009;17:542–555. doi: 10.1097/JGP.0b013e3181a2fd07. [DOI] [PubMed] [Google Scholar]
- Aridon P, Geraci F, Turturici G, D’Amelio M, Savettieri G, Sconzo G. Protective role of heat shock proteins in Parkinson’s disease. Neurodegener Dis. 2011;8:155–168. doi: 10.1159/000321548. [DOI] [PubMed] [Google Scholar]
- Asanuma M, Hirata H, Cadet JL. Attenuation of 6-hydroxydopamine-induced dopaminergic nigrostriatal lesions in superoxide dismutase transgenic mice. Neuroscience. 1998;85:907–917. doi: 10.1016/s0306-4522(97)00665-9. [DOI] [PubMed] [Google Scholar]
- Babar IA, Slack FJ, Weidhaas JB. miRNA modulation of the cellular stress response. Future oncology (London, England) 2008;4:289–298. doi: 10.2217/14796694.4.2.289. [DOI] [PubMed] [Google Scholar]
- Baldeiras I, Santana I, Proenca MT, Garrucho MH, Pascoal R, Rodrigues A, Duro D, Oliveira CR. Peripheral oxidative damage in mild cognitive impairment and mild Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2008;15:117–128. doi: 10.3233/jad-2008-15110. [DOI] [PubMed] [Google Scholar]
- Barkats M, Horellou P, Colin P, Millecamps S, Faucon-Biguet N, Mallet J. 1-methyl-4-phenylpyridinium neurotoxicity is attenuated by adenoviral gene transfer of human Cu/Zn superoxide dismutase. J Neurosci Res. 2006;83:233–242. doi: 10.1002/jnr.20696. [DOI] [PubMed] [Google Scholar]
- Barkats M, Millecamps S, Bilang-Bleuel A, Mallet J. Neuronal transfer of the human Cu/Zn superoxide dismutase gene increases the resistance of dopaminergic neurons to 6-hydroxy-dopamine. J Neurochem. 2002;82:101–109. doi: 10.1046/j.1471-4159.2002.00952.x. [DOI] [PubMed] [Google Scholar]
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Bellinger FP, Bellinger MT, Seale LA, Takemoto AS, Raman AV, Miki T, Manning-Bog AB, Berry MJ, White LR, Ross GW. Glutathione Peroxidase 4 is associated with Neuromelanin in Substantia Nigra and Dystrophic Axons in Putamen of Parkinson’s brain. Mol Neurodegener. 2011;6:8. doi: 10.1186/1750-1326-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Neuron-astrocyte interactions: partnership for normal function and disease in the central nervous system. Mayo Clin Proc. 2005;80:1326–1338. doi: 10.4065/80.10.1326. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nature neuroscience. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Boger HA, Granholm AC, McGinty JF, Middaugh LD. A dual-hit animal model for age-related parkinsonism. Progress in neurobiology. 2010;90:217–229. doi: 10.1016/j.pneurobio.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll MC, Alcaraz-Zubeldia M, Montes S, Rios C. Free copper, ferroxidase and SOD1 activities, lipid peroxidation and NO(x) content in the CSF. A different marker profile in four neurodegenerative diseases. Neurochem Res. 2008;33:1717–1723. doi: 10.1007/s11064-008-9610-3. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rub U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages) J Neurol. 2002;249(Suppl 3):III/1–5. doi: 10.1007/s00415-002-1301-4. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Schultz C, Braak E. Vulnerability of select neuronal types to Alzheimer’s disease. Ann N Y Acad Sci. 2000;924:53–61. doi: 10.1111/j.1749-6632.2000.tb05560.x. [DOI] [PubMed] [Google Scholar]
- Braak H, Sastre M, Del Tredici K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta neuropathologica. 2007;114:231–241. doi: 10.1007/s00401-007-0244-3. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Astrocytes: adaptive responses to low doses of neurotoxins. Critical reviews in toxicology. 2008a;38:463–471. doi: 10.1080/10408440802004023. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Converging concepts: adaptive response, preconditioning, and the Yerkes-Dodson Law are manifestations of hormesis. Ageing research reviews. 2008b;7:8–20. doi: 10.1016/j.arr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Pharmacological enhancement of neuronal survival. Critical reviews in toxicology. 2008c;38:349–389. doi: 10.1080/10408440801981973. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Hormesis is central to toxicology, pharmacology and risk assessment. Hum Exp Toxicol. 2010;29:249–261. doi: 10.1177/0960327109363973. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L, Cedergreen N, Cherian MG, Chiueh CC, Clarkson TW, Cook RR, Diamond DM, Doolittle DJ, Dorato MA, Duke SO, Feinendegen L, Gardner DE, Hart RW, Hastings KL, Hayes AW, Hoffmann GR, Ives JA, Jaworowski Z, Johnson TE, Jonas WB, Kaminski NE, Keller JG, Klaunig JE, Knudsen TB, Kozumbo WJ, Lettieri T, Liu SZ, Maisseu A, Maynard KI, Masoro EJ, McClellan RO, Mehendale HM, Mothersill C, Newlin DB, Nigg HN, Oehme FW, Phalen RF, Philbert MA, Rattan SI, Riviere JE, Rodricks J, Sapolsky RM, Scott BR, Seymour C, Sinclair DA, Smith-Sonneborn J, Snow ET, Spear L, Stevenson DE, Thomas Y, Tubiana M, Williams GM, Mattson MP. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicology and applied pharmacology. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Dinkova-Kostova AT, Iavicoli I, Di Paola R, Koverech A, Cuzzocrea S, Rizzarelli E, Calabrese EJ. Cellular stress responses, hormetic phytochemicals and vitagenes in aging and longevity. Biochimica et biophysica acta. 2012;1822:753–783. doi: 10.1016/j.bbadis.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Sultana R, Scapagnini G, Guagliano E, Sapienza M, Bella R, Kanski J, Pennisi G, Mancuso C, Stella AM, Butterfield DA. Nitrosative stress, cellular stress response, and thiol homeostasis in patients with Alzheimer’s disease. Antioxidants & redox signaling. 2006;8:1975–1986. doi: 10.1089/ars.2006.8.1975. [DOI] [PubMed] [Google Scholar]
- Candilio L, Hausenloy DJ, Yellon DM. Remote ischemic conditioning: a clinical trial’s update. J Cardiovasc Pharmacol Ther. 2011;16:304–312. doi: 10.1177/1074248411411711. [DOI] [PubMed] [Google Scholar]
- Cannon JR, Keep RF, Hua Y, Richardson RJ, Schallert T, Xi G. Thrombin preconditioning provides protection in a 6-hydroxydopamine Parkinson’s disease model. Neurosci Lett. 2005;373:189–194. doi: 10.1016/j.neulet.2004.10.089. [DOI] [PubMed] [Google Scholar]
- Carvey PM, Punati A, Newman MB. Progressive dopamine neuron loss in Parkinson’s disease: the multiple hit hypothesis. Cell Transplant. 2006;15:239–250. doi: 10.3727/000000006783981990. [DOI] [PubMed] [Google Scholar]
- Chacko SK, Cheluvappa R. Increased ceruloplasmin and fibrinogen in type 2 diabetes corresponds to decreased anti-oxidant activity in a preliminary tertiary South Indian hospital study. Exp Clin Endocrinol Diabetes. 2010;118:64–67. doi: 10.1055/s-0029-1225647. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64:664–669. doi: 10.1212/01.WNL.0000151960.28687.93. [DOI] [PubMed] [Google Scholar]
- Chu PW, Beart PM, Jones NM. Preconditioning protects against oxidative injury involving hypoxia-inducible factor-1 and vascular endothelial growth factor in cultured astrocytes. European journal of pharmacology. 2010;633:24–32. doi: 10.1016/j.ejphar.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Clark J, Clore EL, Zheng K, Adame A, Masliah E, Simon DK. Oral N-acetyl-cysteine attenuates loss of dopaminergic terminals in alpha-synuclein overexpressing mice. PLoS One. 2010;5:e12333. doi: 10.1371/journal.pone.0012333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MA, Neafsey EJ, Wang K, Achille NJ, Mitchell RM, Sivaswamy S. Moderate ethanol preconditioning of rat brain cultures engenders neuroprotection against dementia-inducing neuroinflammatory proteins: possible signaling mechanisms. Molecular neurobiology. 2010;41:420–425. doi: 10.1007/s12035-010-8138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AJ, Kristal BS. Multiple roles of glutathione in the central nervous system. Biol Chem. 1997;378:793–802. [PubMed] [Google Scholar]
- Danton GH, Prado R, Watson BD, Dietrich WD. Temporal profile of enhanced vulnerability of the postthrombotic brain to secondary embolic events. Stroke; a journal of cerebral circulation. 2002;33:1113–1119. doi: 10.1161/hs0402.105554. [DOI] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM. Mining for survival genes. Biochem Soc Trans. 2006;34:1307–1309. doi: 10.1042/BST0341307. [DOI] [PubMed] [Google Scholar]
- Del Tredici K, Braak H. Spinal cord lesions in sporadic Parkinson’s disease. Acta neuropathologica. 2012;124:643–664. doi: 10.1007/s00401-012-1028-y. [DOI] [PubMed] [Google Scholar]
- Della-Morte D, Guadagni F, Palmirotta R, Ferroni P, Testa G, Cacciatore F, Abete P, Rengo F, Perez-Pinzon MA, Sacco RL, Rundek T. Genetics and genomics of ischemic tolerance: focus on cardiac and cerebral ischemic preconditioning. Pharmacogenomics. 2012;13:1741–1757. doi: 10.2217/pgs.12.157. [DOI] [PubMed] [Google Scholar]
- Dembinski A, Warzecha Z, Ceranowicz P, Dembinski M, Cieszkowski J, Pawlik WW, Tomaszewska R, Konturek SJ, Konturek PC. Effect of ischemic preconditioning on pancreatic regeneration and pancreatic expression of vascular endothelial growth factor and platelet-derived growth factor-A in ischemia/reperfusion-induced pancreatitis. J Physiol Pharmacol. 2006;57:39–58. [PubMed] [Google Scholar]
- DeTure M, Granger B, Grover A, Hutton M, Yen SH. Evidence for independent mechanisms and a multiple-hit model of tau assembly. Biochemical and biophysical research communications. 2006;339:858–864. doi: 10.1016/j.bbrc.2005.11.087. [DOI] [PubMed] [Google Scholar]
- Di Domenico F, Sultana R, Tiu GF, Scheff NN, Perluigi M, Cini C, Butterfield DA. Protein levels of heat shock proteins 27, 32, 60, 70, 90 and thioredoxin-1 in amnestic mild cognitive impairment: an investigation on the role of cellular stress response in the progression of Alzheimer disease. Brain research. 2010;1333:72–81. doi: 10.1016/j.brainres.2010.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Monte DA, Lavasani M, Manning-Bog AB. Environmental factors in Parkinson’s disease. Neurotoxicology. 2002;23:487–502. doi: 10.1016/s0161-813x(02)00099-2. [DOI] [PubMed] [Google Scholar]
- Dickson DW. Neuropathology of non-Alzheimer degenerative disorders. Int J Clin Exp Pathol. 2009;3:1–23. [PMC free article] [PubMed] [Google Scholar]
- Dietrich WD, Danton G, Hopkins AC, Prado R. Thromboembolic events predispose the brain to widespread cerebral infarction after delayed transient global ischemia in rats. Stroke; a journal of cerebral circulation. 1999;30:855–861. doi: 10.1161/01.str.30.4.855. discussion 862. [DOI] [PubMed] [Google Scholar]
- Ding WX, Ni HM, Gao W, Yoshimori T, Stolz DB, Ron D, Yin XM. Linking of autophagy to ubiquitin-proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. The American journal of pathology. 2007;171:513–524. doi: 10.2353/ajpath.2007.070188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Li L. Lipopolysaccharide preconditioning induces protection against lipopolysac-charide-induced neurotoxicity in organotypic midbrain slice culture. Neurosci Bull. 2008;24:209–218. doi: 10.1007/s12264-008-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Meisel A. Endogenous neuroprotection: mitochondria as gateways to cerebral preconditioning? Neuropharmacology. 2008;55:334–344. doi: 10.1016/j.neuropharm.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Dringen R. Metabolism and functions of glutathione in brain. Progress in neurobiology. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. European journal of biochemistry / FEBS. 2000;267:4912–4916. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- Du F, Qian ZM, Zhu L, Wu XM, Yung WH, Ke Y. A synergistic role of hyperthermic and pharmacological preconditioning to protect astrocytes against ischemia/reperfusion injury. Neurochemical research. 2011;36:312–318. doi: 10.1007/s11064-010-0327-8. [DOI] [PubMed] [Google Scholar]
- Du F, Zhu L, Qian ZM, Wu XM, Yung WH, Ke Y. Hyperthermic preconditioning protects astrocytes from ischemia/reperfusion injury by up-regulation of HIF-1 alpha expression and binding activity. Biochimica et biophysica acta. 2010;1802:1048–1053. doi: 10.1016/j.bbadis.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J Neurosci Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Dwyer BE, Nishimura RN, Lu SY. Differential expression of heme oxygenase-1 in cultured cortical neurons and astrocytes determined by the aid of a new heme oxygenase antibody. Response to oxidative stress. Brain research. Molecular brain research. 1995;30:37–47. doi: 10.1016/0169-328x(94)00273-h. [DOI] [PubMed] [Google Scholar]
- El Ayadi A, Zigmond MJ. Low concentrations of methamphetamine can protect dopaminergic cells against a larger oxidative stress injury: mechanistic study. PLoS One. 2011;6:e24722. doi: 10.1371/journal.pone.0024722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Weinstein AM, Lopez OL. Physical activity, brain plasticity, and Alzheimer’s disease. Arch Med Res. 2012;43:615–621. doi: 10.1016/j.arcmed.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks SL, Brambrink AM. Preconditioning and postconditioning for neuroprotection: the most recent evidence. Best Pract Res Clin Anaesthesiol. 2010;24:521–534. doi: 10.1016/j.bpa.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Fan GH, Qi C, Chen SD. Heat shock proteins reduce toxicity of 1-methyl-4-phenylpyri-dinium ion in SK-N-SH cells. J Neurosci Res. 2005;82:551–562. doi: 10.1002/jnr.20656. [DOI] [PubMed] [Google Scholar]
- Farghali H, Kutinova Canova N, Lekic N. Resveratrol and related compounds as antioxidants with an allosteric mechanism of action in epigenetic drug targets. Physiol Res. 2012 doi: 10.33549/physiolres.932434. [DOI] [PubMed] [Google Scholar]
- Fargnoli J, Kunisada T, Fornace AJ, Jr, Schneider EL, Holbrook NJ. Decreased expression of heat shock protein 70 mRNA and protein after heat treatment in cells of aged rats. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:846–850. doi: 10.1073/pnas.87.2.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, Butterfield DA, Morley JE. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. Journal of neurochemistry. 2003;84:1173–1183. doi: 10.1046/j.1471-4159.2003.01580.x. [DOI] [PubMed] [Google Scholar]
- Fisher BE, Li Q, Nacca A, Salem GJ, Song J, Yip J, Hui JS, Jakowec MW, Petzinger GM. Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson’s disease. Neuroreport. 2013;24:509–514. doi: 10.1097/WNR.0b013e328361dc13. [DOI] [PubMed] [Google Scholar]
- Floyd RA, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002;23:795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Fornai F, Lazzeri G, Bandettini Di Poggio A, Soldani P, De Blasi A, Nicoletti F, Ruggieri S, Paparelli A. Convergent roles of alpha-synuclein, DA metabolism, and the ubiquitin-proteasome system in nigrostriatal toxicity. Annals of the New York Academy of Sciences. 2006;1074:84–89. doi: 10.1196/annals.1369.007. [DOI] [PubMed] [Google Scholar]
- Forno LS. Neuropathology of Parkinson’s disease. Journal of neuropathology and experimental neurology. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- Fouillet A, Levet C, Virgone A, Robin M, Dourlen P, Rieusset J, Belaidi E, Ovize M, Touret M, Nataf S, Mollereau B. ER stress inhibits neuronal death by promoting autophagy. Autophagy. 2012;8:915–926. doi: 10.4161/auto.19716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R, Li S, Rodriguez-Rocha H, Burns M, Panayiotidis MI. Molecular mechanisms of pesticide-induced neurotoxicity: Relevance to Parkinson’s disease. Chem Biol Interact. 2010;188:289–300. doi: 10.1016/j.cbi.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasier CR, Moore RL, Brown DA. Exercise-induced cardiac preconditioning: How exercise protects your achy-breaky heart. J Appl Physiol. 2011 doi: 10.1152/japplphysiol.00004.2011. [DOI] [PubMed] [Google Scholar]
- Friedman LK, Segal M. Early exposure of cultured hippocampal neurons to excitatory amino acids protects from later excitotoxicity. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2010;28:195–205. doi: 10.1016/j.ijdevneu.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Fronczek R, Overeem S, Lee SY, Hegeman IM, van Pelt J, van Duinen SG, Lammers GJ, Swaab DF. Hypocretin (orexin) loss in Parkinson’s disease. Brain : a journal of neurology. 2007;130:1577–1585. doi: 10.1093/brain/awm090. [DOI] [PubMed] [Google Scholar]
- Fu AL, Dong ZH, Sun MJ. Protective effect of N-acetyl-L-cysteine on amyloid beta-peptide-induced learning and memory deficits in mice. Brain research. 2006;1109:201–206. doi: 10.1016/j.brainres.2006.06.042. [DOI] [PubMed] [Google Scholar]
- Fuller S, Steele M, Munch G. Activated astroglia during chronic inflammation in Alzheimer’s disease—do they neglect their neurosupportive roles? Mutat Res. 2010;690:40–49. doi: 10.1016/j.mrfmmm.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Gai WP, Blumbergs PC, Geffen LB, Blessing WW. Age-related loss of dorsal vagal neurons in Parkinson’s disease. Neurology. 1992;42:2106–2111. doi: 10.1212/wnl.42.11.2106. [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS. Gene-environment interactions: key to unraveling the mystery of Parkinson’s disease. Progress in neurobiology. 2011;94:1–19. doi: 10.1016/j.pneurobio.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, Liu B. Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson’s disease. J Neurosci. 2003a;23:1228–1236. doi: 10.1523/JNEUROSCI.23-04-01228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, Hong JS. Synergistic dopaminergic neurotoxicity of MPTP and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson’s disease. Faseb J. 2003b;17:1957–1959. doi: 10.1096/fj.03-0203fje. [DOI] [PubMed] [Google Scholar]
- Gerecke KM, Jiao Y, Pani A, Pagala V, Smeyne RJ. Exercise protects against MPTP-induced neurotoxicity in mice. Brain research. 2010;1341:72–83. doi: 10.1016/j.brainres.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesi M, Soldani P, Giorgi FS, Santinami A, Bonaccorsi I, Fornai F. The role of the locus coeruleus in the development of Parkinson’s disease. Neurosci Biobehav Rev. 2000;24:655–668. doi: 10.1016/s0149-7634(00)00028-2. [DOI] [PubMed] [Google Scholar]
- Gidday JM. Pharmacologic Preconditioning: Translating the Promise. Transl Stroke Res. 2010;1:19–30. doi: 10.1007/s12975-010-0011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano J, Ives JA, Jonas WB. Hormetic responses in neural systems: consideration, contexts, and caveats. Critical reviews in toxicology. 2008;38:623–627. doi: 10.1080/10408440802026356. [DOI] [PubMed] [Google Scholar]
- Goto Y, Niidome T, Akaike A, Kihara T, Sugimoto H. Amyloid beta-peptide preconditioning reduces glutamate-induced neurotoxicity by promoting endocytosis of NMDA receptor. Biochemical and biophysical research communications. 2006;351:259–265. doi: 10.1016/j.bbrc.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Grochot-Przeczek A, Dulak J, Jozkowicz A. Haem oxygenase-1: non-canonical roles in physiology and pathology. Clin Sci (Lond) 2012;122:93–103. doi: 10.1042/CS20110147. [DOI] [PubMed] [Google Scholar]
- Gundersen V. Protein aggregation in Parkinson’s disease. Acta neurologica Scandinavica. 2010 doi: 10.1111/j.1600-0404.2010.01382.x. Supplementum 82–87. [DOI] [PubMed] [Google Scholar]
- Guo Z, Ersoz A, Butterfield DA, Mattson MP. Beneficial effects of dietary restriction on cerebral cortical synaptic terminals: preservation of glucose and glutamate transport and mitochondrial function after exposure to amyloid beta-peptide, iron, and 3-nitropropionic acid. Journal of neurochemistry. 2000;75:314–320. doi: 10.1046/j.1471-4159.2000.0750314.x. [DOI] [PubMed] [Google Scholar]
- Gupte AA, Morris JK, Zhang H, Bomhoff GL, Geiger PC, Stanford JA. Age-related changes in HSP25 expression in basal ganglia and cortex of F344/BN rats. Neuroscience letters. 2010;472:90–93. doi: 10.1016/j.neulet.2010.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM, Blumbergs PC, Cotton RG, Blessing WW, Geffen LB. Loss of brainstem serotonin- and substance P-containing neurons in Parkinson’s disease. Brain Res. 1990;510:104–107. doi: 10.1016/0006-8993(90)90733-r. [DOI] [PubMed] [Google Scholar]
- Hamidi GA, Faraji A, Zarmehri HA, Haghdoost-Yazdi H. Prolonged hyperoxia preconditioning attenuates behavioral symptoms of 6-hydroxydopamine-induced Parkinsonism. Neurol Res. 2012;34:636–642. doi: 10.1179/1743132812Y.0000000056. [DOI] [PubMed] [Google Scholar]
- Hara H, Kamiya T, Adachi T. Endoplasmic reticulum stress inducers provide protection against 6-hydroxydopamine-induced cytotoxicity. Neurochem Int. 2011;58:35–43. doi: 10.1016/j.neuint.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. Journal of gerontology. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harman D. Alzheimer’s disease pathogenesis: role of aging. Annals of the New York Academy of Sciences. 2006;1067:454–460. doi: 10.1196/annals.1354.065. [DOI] [PubMed] [Google Scholar]
- Harman D. About “Origin and evolution of the free radical theory of aging: a brief personal history, 1954–2009”. Biogerontology. 2009;10:783. doi: 10.1007/s10522-009-9253-z. [DOI] [PubMed] [Google Scholar]
- Hineno A, Kaneko K, Yoshida K, Ikeda S. Ceruloplasmin protects against rotenone-induced oxidative stress and neurotoxicity. Neurochemical research. 2011;36:2127–2135. doi: 10.1007/s11064-011-0537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornykiewicz O. Brain monoamines and parkinsonism. Natl Inst Drug Abuse Res Monogr Ser. 1975:13–21. doi: 10.1037/e472122004-001. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Biochemical aspects of Parkinson’s disease. Neurology. 1998;51:S2–9. doi: 10.1212/wnl.51.2_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- Hoshida S, Yamashita N, Otsu K, Hori M. Repeated physiologic stresses provide persistent cardioprotection against ischemia-reperfusion injury in rats. J Am Coll Cardiol. 2002;40:826–831. doi: 10.1016/s0735-1097(02)02001-6. [DOI] [PubMed] [Google Scholar]
- Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiology of disease. 2013;57:47–55. doi: 10.1016/j.nbd.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Iwata A, Riley BE, Johnston JA, Kopito RR. HDAC6 and microtubules are required for autophagic degradation of aggregated huntingtin. J Biol Chem. 2005;280:40282–40292. doi: 10.1074/jbc.M508786200. [DOI] [PubMed] [Google Scholar]
- Janen SB, Chaachouay H, Richter-Landsberg C. Autophagy is activated by proteasomal inhibition and involved in aggresome clearance in cultured astrocytes. Glia. 2010;58:1766–1774. doi: 10.1002/glia.21047. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Recent advances in our understanding of neurodegeneration. Journal of neural transmission. 2009;116:1111–1162. doi: 10.1007/s00702-009-0240-y. [DOI] [PubMed] [Google Scholar]
- Johnsen D, Murphy SJ. Isoflurane preconditioning protects astrocytes from oxygen and glucose deprivation independent of innate cell sex. J Neurosurg Anesthesiol. 2011;23:335–340. doi: 10.1097/ANA.0b013e3182161816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WM, Wilson-Delfosse AL, Mieyal JJ. Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients. 2012;4:1399–1440. doi: 10.3390/nu4101399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia SK, Kalia LV, McLean PJ. Molecular chaperones as rational drug targets for Parkinson’s disease therapeutics. CNS Neurol Disord Drug Targets. 2010;9:741–753. doi: 10.2174/187152710793237386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko K, Hineno A, Yoshida K, Ikeda S. Increased vulnerability to rotenone-induced neurotoxicity in ceruloplasmin-deficient mice. Neurosci Lett. 2008;446:56–58. doi: 10.1016/j.neulet.2008.08.089. [DOI] [PubMed] [Google Scholar]
- Kaushik S, Cuervo AM. Methods to monitor chaperone-mediated autophagy. Methods in enzymology. 2009;452:297–324. doi: 10.1016/S0076-6879(08)03619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN, Hanni KB, Markesbery WR. Impaired proteasome function in Alzheimer’s disease. J Neurochem. 2000;75:436–439. doi: 10.1046/j.1471-4159.2000.0750436.x. [DOI] [PubMed] [Google Scholar]
- Kiraly M, Kiraly SJ. Traumatic brain injury and delayed sequelae: a review—traumatic brain injury and mild traumatic brain injury (concussion) are precursors to later-onset brain disorders, including early-onset dementia. ScientificWorldJournal. 2007;7:1768–1776. doi: 10.1100/tsw.2007.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino T. Ischemic tolerance. J Cereb Blood Flow Metab. 2002;22:1283–1296. doi: 10.1097/01.WCB.0000040942.89393.88. [DOI] [PubMed] [Google Scholar]
- Kirino T, Tsujita Y, Tamura A. Induced tolerance to ischemia in gerbil hippocampal neurons. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1991;11:299–307. doi: 10.1038/jcbfm.1991.62. [DOI] [PubMed] [Google Scholar]
- Kitagawa K. Ischemic tolerance in the brain: endogenous adaptive machinery against ischemic stress. Journal of neuroscience research. 2012;90:1043–1054. doi: 10.1002/jnr.23005. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, Handa N, Fukunaga R, Kimura K, Mikoshiba K, et al. ‘Ischemic tolerance’ phenomenon found in the brain. Brain Res. 1990;528:21–24. doi: 10.1016/0006-8993(90)90189-i. [DOI] [PubMed] [Google Scholar]
- Koren J, 3rd, Jinwal UK, Lee DC, Jones JR, Shults CL, Johnson AG, Anderson LJ, Dickey CA. Chaperone signalling complexes in Alzheimer’s disease. J Cell Mol Med. 2009;13:619–630. doi: 10.1111/j.1582-4934.2008.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kultz D. Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol. 2005;67:225–257. doi: 10.1146/annurev.physiol.67.040403.103635. [DOI] [PubMed] [Google Scholar]
- Lanneau D, Wettstein G, Bonniaud P, Garrido C. Heat shock proteins: cell protection through protein triage. ScientificWorldJournal. 2010;10:1543–1552. doi: 10.1100/tsw.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak RK, Garbett KA, Dettmer AM, Zhang Z, Mirnics K, Cameron JL. Physical activity is linked to ceruloplasmin in the striatum of intact but not MPTP-treated primates. Cell and tissue research. 2012;350:401–407. doi: 10.1007/s00441-012-1488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak RK, Liou AK, Zigmond MJ. Effect of sublethal 6-hydroxydopamine on the response to subsequent oxidative stress in dopaminergic cells: evidence for preconditioning. J Neurochem. 2006;99:1151–1163. doi: 10.1111/j.1471-4159.2006.04149.x. [DOI] [PubMed] [Google Scholar]
- Leak RK, Zigmond MJ. “Parkinson’s Disease: Pathogenic and Therapeutic Insights from Toxin and Genetic Models”. Elsevier; 2007. Endogenous defenses that protect dopamine neurons: studies with 6-OHDA models of Parkinson’s disease. [Google Scholar]
- Leak RK, Zigmond MJ, Liou AK. Adaptation to chronic MG132 reduces oxidative toxicity by a CuZnSOD-dependent mechanism. J Neurochem. 2008;106:860–874. doi: 10.1111/j.1471-4159.2008.05459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nature genetics. 2000;25:294–297. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. The Journal of biological chemistry. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Z, Chang QA, Tong CW, Leurgans SE, Lipton JW, Carvey PM. Rotenone potentiates dopamine neuron loss in animals exposed to lipopolysaccharide prenatally. Experimental neurology. 2004a;190:373–383. doi: 10.1016/j.expneurol.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Ling ZD, Chang Q, Lipton JW, Tong CW, Landers TM, Carvey PM. Combined toxicity of prenatal bacterial endotoxin exposure and postnatal 6-hydroxydopamine in the adult rat midbrain. Neuroscience. 2004b;124:619–628. doi: 10.1016/j.neuroscience.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, Chen RC. Environmental risk factors and Parkinson’s disease: a case-control study in Taiwan. Neurology. 1997;48:1583–1588. doi: 10.1212/wnl.48.6.1583. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kato H, Nakata N, Kogure K. Protection of rat hippocampus against ischemic neuronal damage by pretreatment with sublethal ischemia. Brain research. 1992;586:121–124. doi: 10.1016/0006-8993(92)91380-w. [DOI] [PubMed] [Google Scholar]
- Lloret A, Badia MC, Mora NJ, Pallardo FV, Alonso MD, Vina J. Vitamin E paradox in Alzheimer’s disease: it does not prevent loss of cognition and may even be detrimental. Journal of Alzheimer’s disease : JAD. 2009;17:143–149. doi: 10.3233/JAD-2009-1033. [DOI] [PubMed] [Google Scholar]
- Loeffler DA, LeWitt PA, Juneau PL, Sima AA, Nguyen HU, DeMaggio AJ, Brickman CM, Brewer GJ, Dick RD, Troyer MD, Kanaley L. Increased regional brain concentrations of ceruloplasmin in neurodegenerative disorders. Brain research. 1996;738:265–274. doi: 10.1016/s0006-8993(96)00782-2. [DOI] [PubMed] [Google Scholar]
- Loeffler DA, Sima AA, LeWitt PA. Ceruloplasmin immunoreactivity in neurodegenerative disorders. Free Radic Res. 2001;35:111–118. doi: 10.1080/10715760100300651. [DOI] [PubMed] [Google Scholar]
- Louro MO, Cocho JA, Mera A, Tutor JC. Immunochemical and enzymatic study of ceruloplasmin in rheumatoid arthritis. J Trace Elem Med Biol. 2000;14:174–178. doi: 10.1016/S0946-672X(00)80007-3. [DOI] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Maiello M, Boeri D, Sampietro L, Pronzato MA, Odetti P, Marinari UM. Basal synthesis of heat shock protein 70 increases with age in rat kidneys. Gerontology. 1998;44:15–20. doi: 10.1159/000021977. [DOI] [PubMed] [Google Scholar]
- Manning-Bog AB, Langston JW. Model fusion, the next phase in developing animal models for Parkinson’s disease. Neurotoxicity research. 2007;11:219–240. doi: 10.1007/BF03033569. [DOI] [PubMed] [Google Scholar]
- Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J Biol Chem. 2002;277:1641–1644. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- Martin HL, Teismann P. Glutathione—a review on its role and significance in Parkinson’s disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23:3263–3272. doi: 10.1096/fj.08-125443. [DOI] [PubMed] [Google Scholar]
- Martinez Banaclocha M. N-acetylcysteine elicited increase in complex I activity in synaptic mitochondria from aged mice: implications for treatment of Parkinson’s disease. Brain research. 2000;859:173–175. doi: 10.1016/s0006-8993(00)02005-9. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Hormesis defined. Ageing research reviews. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Wan R, Guo Z. Prophylactic activation of neuroprotective stress response pathways by dietary and behavioral manipulations. NeuroRx : the journal of the American Society for Experimental NeuroTherapeutics. 2004;1:111–116. doi: 10.1602/neurorx.1.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Guthrie PB, Kater SB. Intrinsic factors in the selective vulnerability of hippocampal pyramidal neurons. Progress in clinical and biological research. 1989;317:333–351. [PubMed] [Google Scholar]
- Mattson MP, Kater SB. Development and selective neurodegeneration in cell cultures from different hippocampal regions. Brain Res. 1989;490:110–125. doi: 10.1016/0006-8993(89)90436-8. [DOI] [PubMed] [Google Scholar]
- Matus S, Castillo K, Hetz C. Hormesis: protecting neurons against cellular stress in Parkinson disease. Autophagy. 2012;8:997–1001. doi: 10.4161/auto.20748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeux R, Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA. Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis. 2002;10:119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- McLaughlin B, Hartnett KA, Erhardt JA, Legos JJ, White RF, Barone FC, Aizenman E. Caspase 3 activation is essential for neuroprotection in preconditioning. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:715–720. doi: 10.1073/pnas.0232966100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaught KS. Proteolytic dysfunction in neurodegenerative disorders. International review of neurobiology. 2004;62:95–119. doi: 10.1016/S0074-7742(04)62003-4. [DOI] [PubMed] [Google Scholar]
- McNaught KS, Belizaire R, Isacson O, Jenner P, Olanow CW. Altered proteasomal function in sporadic Parkinson’s disease. Exp Neurol. 2003;179:38–46. doi: 10.1006/exnr.2002.8050. [DOI] [PubMed] [Google Scholar]
- Memisogullari R, Bakan E. Levels of ceruloplasmin, transferrin, and lipid peroxidation in the serum of patients with Type 2 diabetes mellitus. J Diabetes Complications. 2004;18:193–197. doi: 10.1016/S1056-8727(03)00032-1. [DOI] [PubMed] [Google Scholar]
- Mezzetti A, Lapenna D, Romano F, Costantini F, Pierdomenico SD, De Cesare D, Cuccurullo F, Riario-Sforza G, Zuliani G, Fellin R. Systemic oxidative stress and its relationship with age and illness. Associazione Medica “Sabin”. J Am Geriatr Soc. 1996;44:823–827. doi: 10.1111/j.1532-5415.1996.tb03741.x. [DOI] [PubMed] [Google Scholar]
- Mezzetti A, Pierdomenico SD, Costantini F, Romano F, De Cesare D, Cuccurullo F, Imbastaro T, Riario-Sforza G, Di Giacomo F, Zuliani G, Fellin R. Copper/zinc ratio and systemic oxidant load: effect of aging and aging-related degenerative diseases. Free radical biology & medicine. 1998;25:676–681. doi: 10.1016/s0891-5849(98)00109-9. [DOI] [PubMed] [Google Scholar]
- Mitchell RM, Neafsey EJ, Collins MA. Essential involvement of the NMDA receptor in ethanol preconditioning-dependent neuroprotection from amyloid-betain vitro. Journal of neurochemistry. 2009;111:580–588. doi: 10.1111/j.1471-4159.2009.06351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes & development. 2008;22:1427–1438. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI. The heat shock response: systems biology of proteotoxic stress in aging and disease. Cold Spring Harbor symposia on quantitative biology. 2011;76:91–99. doi: 10.1101/sqb.2012.76.010637. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Mythri RB, Venkateshappa C, Harish G, Mahadevan A, Muthane UB, Yasha TC, Srinivas Bharath MM, Shankar SK. Evaluation of markers of oxidative stress, antioxidant function and astrocytic proliferation in the striatum and frontal cortex of Parkinson’s disease brains. Neurochemical research. 2011;36:1452–1463. doi: 10.1007/s11064-011-0471-9. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Fujikake N, Popiel HA, Wada K. Induction of molecular chaperones as a therapeutic strategy for the polyglutamine diseases. Curr Pharm Biotechnol. 2010;11:188–197. doi: 10.2174/138920110790909650. [DOI] [PubMed] [Google Scholar]
- Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5:287–294. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama K, Murayama S, Shimizu J, Ohya Y, Kwak S, Asayama K, Kanazawa I. Cu/Zn superoxide dismutase-like immunoreactivity is present in Lewy bodies from Parkinson disease: a light and electron microscopic immunocytochemical study. Acta Neuropathol. 1995;89:471–474. doi: 10.1007/BF00571500. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Yang DS. Autophagy and neuronal cell death in neurological disorders. Cold Spring Harbor perspectives in biology. 2012;4 doi: 10.1101/cshperspect.a008839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottoboni MA. The Dose Makes the Poison. John Wiley & Sons, Inc; New York: 1997. [Google Scholar]
- Pan T, Kondo S, Zhu W, Xie W, Jankovic J, Le W. Neuroprotection of rapamycin in lactacystin-induced neurodegeneration via autophagy enhancement. Neurobiology of disease. 2008;32:16–25. doi: 10.1016/j.nbd.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF, Jr, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A. Glial cells in (patho)physiology. Journal of neurochemistry. 2012;121:4–27. doi: 10.1111/j.1471-4159.2012.07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry TL, Yong VW, Clavier RM, Jones K, Wright JM, Foulks JG, Wall RA. Partial protection from the dopaminergic neurotoxin N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine by four different antioxidants in the mouse. Neuroscience letters. 1985;60:109–114. doi: 10.1016/0304-3940(85)90229-0. [DOI] [PubMed] [Google Scholar]
- Peters R, Peters J, Warner J, Beckett N, Bulpitt C. Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age Ageing. 2008;37:505–512. doi: 10.1093/ageing/afn095. [DOI] [PubMed] [Google Scholar]
- Pocernich CB, Butterfield DA. Elevation of glutathione as a therapeutic strategy in Alzheimer disease. Biochimica et biophysica acta. 2011 doi: 10.1016/j.bbadis.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocernich CB, La Fontaine M, Butterfield DA. In-vivo glutathione elevation protects against hydroxyl free radical-induced protein oxidation in rat brain. Neurochemistry international. 2000;36:185–191. doi: 10.1016/s0197-0186(99)00126-6. [DOI] [PubMed] [Google Scholar]
- Politis M, Wu K, Loane C, Quinn NP, Brooks DJ, Oertel WH, Bjorklund A, Lindvall O, Piccini P. Serotonin neuron loss and nonmotor symptoms continue in Parkinson’s patients treated with dopamine grafts. Sci Transl Med. 2012;4:128ra141. doi: 10.1126/scitranslmed.3003391. [DOI] [PubMed] [Google Scholar]
- Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochemical pharmacology. 2003;66:1499–1503. doi: 10.1016/s0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- Posimo JM, Titler AM, Choi HJ, Unnithan AS, Leak RK. Neocortex and allocortex respond differentially to cellular stress in vitro and aging in vivo. PLoS One. 2013;8:e58596. doi: 10.1371/journal.pone.0058596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothakos K, Kurz MJ, Lau YS. Restorative effect of endurance exercise on behavioral deficits in the chronic mouse model of Parkinson’s disease with severe neurodegeneration. BMC Neurosci. 2009;10:6. doi: 10.1186/1471-2202-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers KM, Kay DM, Factor SA, Zabetian CP, Higgins DS, Samii A, Nutt JG, Griffith A, Leis B, Roberts JW, Martinez ED, Montimurro JS, Checkoway H, Payami H. Combined effects of smoking, coffee, and NSAIDs on Parkinson’s disease risk. Movement disorders : official journal of the Movement Disorder Society. 2008;23:88–95. doi: 10.1002/mds.21782. [DOI] [PubMed] [Google Scholar]
- Powers SK, Talbert EE, Adhihetty PJ. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol. 2011;589:2129–2138. doi: 10.1113/jphysiol.2010.201327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiological reviews. 2012;92:967–1003. doi: 10.1152/physrev.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S, Kostic V, Jackson-Lewis V, Naini AB, Simonetti S, Fahn S, Carlson E, Epstein CJ, Cadet JL. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J Neurosci. 1992;12:1658–1667. doi: 10.1523/JNEUROSCI.12-05-01658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao M, Zhao Z, Barber PA, Foniok T, Sun S, Tuor UI. Development of a model of recurrent stroke consisting of a mild transient stroke followed by a second moderate stroke in rats. Journal of neuroscience methods. 2009;184:244–250. doi: 10.1016/j.jneumeth.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Quigney DJ, Gorman AM, Samali A. Heat shock protects PC12 cells against MPP+ toxicity. Brain Res. 2003;993:133–139. doi: 10.1016/j.brainres.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Rajapakse N, Kis B, Horiguchi T, Snipes J, Busija D. Diazoxide pretreatment induces delayed preconditioning in astrocytes against oxygen glucose deprivation and hydrogen peroxide-induced toxicity. Journal of neuroscience research. 2003;73:206–214. doi: 10.1002/jnr.10657. [DOI] [PubMed] [Google Scholar]
- Rathbone MP, Middlemiss PJ, Gysbers JW, Andrew C, Herman MA, Reed JK, Ciccarelli R, Di Iorio P, Caciagli F. Trophic effects of purines in neurons and glial cells. Progress in neurobiology. 1999;59:663–690. doi: 10.1016/s0301-0082(99)00017-9. [DOI] [PubMed] [Google Scholar]
- Raval AP, Lin HW, Dave KR, Defazio RA, Della Morte D, Kim EJ, Perez-Pinzon MA. Resveratrol and ischemic preconditioning in the brain. Curr Med Chem. 2008;15:1545–1551. doi: 10.2174/092986708784638861. [DOI] [PubMed] [Google Scholar]
- Rideout HJ, Lang-Rollin IC, Savalle M, Stefanis L. Dopaminergic neurons in rat ventral midbrain cultures undergo selective apoptosis and form inclusions, but do not up-regulate iHSP70, following proteasomal inhibition. J Neurochem. 2005;93:1304–1313. doi: 10.1111/j.1471-4159.2005.03124.x. [DOI] [PubMed] [Google Scholar]
- Rideout HJ, Larsen KE, Sulzer D, Stefanis L. Proteasomal inhibition leads to formation of ubiquitin/alpha-synuclein-immunoreactive inclusions in PC12 cells. J Neurochem. 2001;78:899–908. doi: 10.1046/j.1471-4159.2001.00474.x. [DOI] [PubMed] [Google Scholar]
- Rideout HJ, Stefanis L. Proteasomal inhibition-induced inclusion formation and death in cortical neurons require transcription and ubiquitination. Mol Cell Neurosci. 2002;21:223–238. doi: 10.1006/mcne.2002.1173. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nature reviews. Drug discovery. 2007;6:304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, Hiltunen M. Impaired autophagy and APP processing in Alzheimer’s disease: The potential role of Beclin 1 interactome. Progress in neurobiology. 2013 doi: 10.1016/j.pneurobio.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Sawada H, Kohno R, Kihara T, Izumi Y, Sakka N, Ibi M, Nakanishi M, Nakamizo T, Yamakawa K, Shibasaki H, Yamamoto N, Akaike A, Inden M, Kitamura Y, Taniguchi T, Shimohama S. Proteasome mediates dopaminergic neuronal degeneration, and its inhibition causes alpha-synuclein inclusions. J Biol Chem. 2004;279:10710–10719. doi: 10.1074/jbc.M308434200. [DOI] [PubMed] [Google Scholar]
- Schipper HM. Heme oxygenase-1: role in brain aging and neurodegeneration. Experimental gerontology. 2000;35:821–830. doi: 10.1016/s0531-5565(00)00148-0. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Bennett DA, Liberman A, Bienias JL, Schneider JA, Kelly J, Arvanitakis Z. Glial heme oxygenase-1 expression in Alzheimer disease and mild cognitive impairment. Neurobiology of aging. 2006;27:252–261. doi: 10.1016/j.neurobiolaging.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Liberman A, Stopa EG. Neural heme oxygenase-1 expression in idiopathic Parkinson’s disease. Experimental neurology. 1998;150:60–68. doi: 10.1006/exnr.1997.6752. [DOI] [PubMed] [Google Scholar]
- Schultz C, Dick EJ, Cox AB, Hubbard GB, Braak E, Braak H. Expression of stress proteins alpha B-crystallin, ubiquitin, and hsp27 in pallido-nigral spheroids of aged rhesus monkeys. Neurobiology of aging. 2001;22:677–682. doi: 10.1016/s0197-4580(01)00229-9. [DOI] [PubMed] [Google Scholar]
- Selye H. Stress without distress. Signet; Philadelphia: 1975. [Google Scholar]
- Shao Y, McCarthy KD. Plasticity of astrocytes. Glia. 1994;11:147–155. doi: 10.1002/glia.440110209. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kaur P, Kumar V, Gill KD. Attenuation of 1-methyl-4-phenyl-1, 2,3,6-tetrahy-dropyridine induced nigrostriatal toxicity in mice by N-acetyl cysteine. Cell Mol Biol (Noisy-le-grand) 2007;53:48–55. [PubMed] [Google Scholar]
- Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- Silver JT, Noble EG. Regulation of survival gene hsp70. Cell stress & chaperones. 2012;17:1–9. doi: 10.1007/s12192-011-0290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofic E, Lange KW, Jellinger K, Riederer P. Reduced and oxidized glutathione in the sub-stantia nigra of patients with Parkinson’s disease. Neurosci Lett. 1992;142:128–130. doi: 10.1016/0304-3940(92)90355-b. [DOI] [PubMed] [Google Scholar]
- Son JH, Shim JH, Kim KH, Ha JY, Han JY. Neuronal autophagy and neurodegenerative diseases. Experimental & molecular medicine. 2012;44:89–98. doi: 10.3858/emm.2012.44.2.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son TG, Camandola S, Mattson MP. Hormetic dietary phytochemicals. Neuromolecular Med. 2008;10:236–246. doi: 10.1007/s12017-008-8037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soti C, Csermely P. Molecular chaperones and the aging process. Biogerontology. 2000;1:225–233. doi: 10.1023/a:1010082129022. [DOI] [PubMed] [Google Scholar]
- Souza LC, Filho CB, Goes AT, Fabbro LD, de Gomes MG, Savegnago L, Oliveira MS, Jesse CR. Neuroprotective Effect of Physical Exercise in a Mouse Model of Alzheimer’s Disease Induced by beta-Amyloid1-40 Peptide. Neurotoxicity research. 2013;24:148–163. doi: 10.1007/s12640-012-9373-0. [DOI] [PubMed] [Google Scholar]
- Sturtz LA, Culotta VC. Superoxide dismutase null mutants of baker’s yeast, Saccharomyces cerevisiae. Methods in enzymology. 2002;349:167–172. doi: 10.1016/s0076-6879(02)49332-9. [DOI] [PubMed] [Google Scholar]
- Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends in neurosciences. 2007;30:244–250. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Sun F, Anantharam V, Zhang D, Latchoumycandane C, Kanthasamy A, Kanthasamy AG. Proteasome inhibitor MG-132 induces dopaminergic degeneration in cell culture and animal models. Neurotoxicology. 2006;27:807–815. doi: 10.1016/j.neuro.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Tai KK, Truong DD. (−)-Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, reduces dichlorodiphenyl-trichloroethane (DDT)-induced cell death in dopaminergic SHSY-5Y cells. Neuroscience letters. 2010;482:183–187. doi: 10.1016/j.neulet.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Tan EK, Tan C, Fook-Chong SM, Lum SY, Chai A, Chung H, Shen H, Zhao Y, Teoh ML, Yih Y, Pavanni R, Chandran VR, Wong MC. Dose-dependent protective effect of coffee, tea, and smoking in Parkinson’s disease: a study in ethnic Chinese. Journal of the neurological sciences. 2003;216:163–167. doi: 10.1016/j.jns.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Tang JX, Mardini F, Caltagarone BM, Garrity ST, Li RQ, Bianchi SL, Gomes O, Laferla FM, Eckenhoff RG, Eckenhoff MF. Anesthesia in presymptomatic Alzheimer’s disease: a study using the triple-transgenic mouse model. Alzheimers Dement. 2011;7:521–531. e521. doi: 10.1016/j.jalz.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, Comyns K, Richards MB, Meng C, Priestley B, Fernandez HH, Cambi F, Umbach DM, Blair A, Sandler DP, Langston JW. Rotenone, Paraquat and Parkinson’s Disease. Environ Health Perspect. 2011 doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taysi S, Polat F, Gul M, Sari RA, Bakan E. Lipid peroxidation, some extracellular antioxi-dants, and antioxidant enzymes in serum of patients with rheumatoid arthritis. Rheumatol Int. 2002;21:200–204. doi: 10.1007/s00296-001-0163-x. [DOI] [PubMed] [Google Scholar]
- Texel SJ, Zhang J, Camandola S, Unger EL, Taub DD, Koehler RC, Harris ZL, Mattson MP. Ceruloplasmin deficiency reduces levels of iron and BDNF in the cortex and striatum of young mice and increases their vulnerability to stroke. PLoS One. 2011;6:e25077. doi: 10.1371/journal.pone.0025077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal TC, Lai YY, Siegel JM. Hypocretin (orexin) cell loss in Parkinson’s disease. Brain. 2007;130:1586–1595. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JW, Narayanan SV, Perez-Pinzon MA. Redox signaling pathways involved in neuronal ischemic preconditioning. Curr Neuropharmacol. 2012;10:354–369. doi: 10.2174/157015912804143577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titler AM, Posimo JM, Leak RK. Astrocyte plasticity revealed by adaptations to severe proteotoxic stress. Cell and tissue research. 2013 doi: 10.1007/s00441-013-1571-4. [DOI] [PubMed] [Google Scholar]
- Torsdottir G, Kristinsson J, Snaedal J, Sveinbjornsdottir S, Gudmundsson G, Hreidarsson S, Johannesson T. Case-control studies on ceruloplasmin and superoxide dismutase (SOD1) in neurodegenerative diseases: a short review. J Neurol Sci. 2010;299:51–54. doi: 10.1016/j.jns.2010.08.047. [DOI] [PubMed] [Google Scholar]
- Tucker S, Ahl M, Cho HH, Bandyopadhyay S, Cuny GD, Bush AI, Goldstein LE, Westaway D, Huang X, Rogers JT. RNA therapeutics directed to the non coding regions of APP mRNA, in vivo anti-amyloid efficacy of paroxetine, erythromycin, and N-acetyl cysteine. Curr Alzheimer Res. 2006;3:221–227. doi: 10.2174/156720506777632835. [DOI] [PubMed] [Google Scholar]
- Unnithan AS, Choi HJ, Titler AM, Posimo JM, Leak RK. Rescue from a two hit, high-throughput model of neurodegeneration with N-acetyl cysteine. Neurochemistry international. 2012;61:356–368. doi: 10.1016/j.neuint.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Uryu K, Richter-Landsberg C, Welch W, Sun E, Goldbaum O, Norris EH, Pham CT, Yazawa I, Hilburger K, Micsenyi M, Giasson BI, Bonini NM, Lee VM, Trojanowski JQ. Convergence of heat shock protein 90 with ubiquitin in filamentous alpha-synuclein inclusions of alpha-synucleinopathies. The American journal of pathology. 2006;168:947–961. doi: 10.2353/ajpath.2006.050770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky VN. Intrinsic disorder in proteins associated with neurodegenerative diseases. Frontiers in bioscience : a journal and virtual library. 2009;14:5188–5238. doi: 10.2741/3594. [DOI] [PubMed] [Google Scholar]
- Van Remmen H, Qi W, Sabia M, Freeman G, Estlack L, Yang H, Mao Guo Z, Huang TT, Strong R, Lee S, Epstein CJ, Richardson A. Multiple deficiencies in antioxidant enzymes in mice result in a compound increase in sensitivity to oxidative stress. Free Radic Biol Med. 2004;36:1625–1634. doi: 10.1016/j.freeradbiomed.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Verbeke P, Fonager J, Clark BF, Rattan SI. Heat shock response and ageing: mechanisms and applications. Cell biology international. 2001;25:845–857. doi: 10.1006/cbir.2001.0789. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Rodriguez JJ, Parpura V. Neurotransmitters and integration in neuronal-astroglial networks. Neurochemical research. 2012;37:2326–2338. doi: 10.1007/s11064-012-0765-6. [DOI] [PubMed] [Google Scholar]
- Vernon AC, Johansson SM, Modo MM. Non-invasive evaluation of nigrostriatal neuropathology in a proteasome inhibitor rodent model of Parkinson’s disease. BMC Neurosci. 2010;11:1. doi: 10.1186/1471-2202-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuckovic MG, Li Q, Fisher B, Nacca A, Leahy RM, Walsh JP, Mukherjee J, Williams C, Jakowec MW, Petzinger GM. Exercise elevates dopamine D2 receptor in a mouse model of Parkinson’s disease: in vivo imaging with [(1)(8)F]fallypride. Movement disorders : official journal of the Movement Disorder Society. 2010;25:2777–2784. doi: 10.1002/mds.23407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Hayashi S, Yoshimoto M, Kudo H, Takahashi H. NACP/alpha-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson’s disease brains. Acta neuropathologica. 2000;99:14–20. doi: 10.1007/pl00007400. [DOI] [PubMed] [Google Scholar]
- Walker LC, LeVine H. The cerebral proteopathies: neurodegenerative disorders of protein conformation and assembly. Molecular neurobiology. 2000;21:83–95. doi: 10.1385/MN:21:1-2:083. [DOI] [PubMed] [Google Scholar]
- Walker LC, Levine H, 3rd, Mattson MP, Jucker M. Inducible proteopathies. Trends in neurosciences. 2006;29:438–443. doi: 10.1016/j.tins.2006.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters TJ, Ryan KL, Mason PA. Regional distribution of Hsp70 in the CNS of young and old food-restricted rats following hyperthermia. Brain research bulletin. 2001;55:367–374. doi: 10.1016/s0361-9230(01)00502-0. [DOI] [PubMed] [Google Scholar]
- Wang D, Qian L, Xiong H, Liu J, Neckameyer WS, Oldham S, Xia K, Wang J, Bodmer R, Zhang Z. Antioxidants protect PINK1-dependent dopaminergic neurons in Drosophila. Proc Natl Acad Sci U S A. 2006;103:13520–13525. doi: 10.1073/pnas.0604661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Xie Z. Anesthesia, calcium homeostasis and Alzheimer’s disease. Curr Alzheimer Res. 2009;6:30–35. doi: 10.2174/156720509787313934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidong L, Shen C, Jankovic J. Etiopathogenesis of Parkinson disease: a new beginning? Neuroscientist. 2009;15:28–35. doi: 10.1177/1073858408319974. [DOI] [PubMed] [Google Scholar]
- Westergaard N, Sonnewald U, Schousboe A. Metabolic trafficking between neurons and astrocytes: the glutamate/glutamine cycle revisited. Dev Neurosci. 1995;17:203–211. doi: 10.1159/000111288. [DOI] [PubMed] [Google Scholar]
- Wilson JX. Antioxidant defense of the brain: a role for astrocytes. Can J Physiol Pharmacol. 1997;75:1149–1163. [PubMed] [Google Scholar]
- Winchester J, Dick MB, Gillen D, Reed B, Miller B, Tinklenberg J, Mungas D, Chui H, Galasko D, Hewett L, Cotman CW. Walking stabilizes cognitive functioning in Alzheimer’s disease (AD) across one year. Arch Gerontol Geriatr. 2013;56:96–103. doi: 10.1016/j.archger.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt SN. Hsp70 molecular chaperones and Parkinson’s disease. Biopolymers. 2010;93:218–228. doi: 10.1002/bip.21302. [DOI] [PubMed] [Google Scholar]
- Wong E, Cuervo AM. Integration of clearance mechanisms: the proteasome and autophagy. Cold Spring Harbor perspectives in biology. 2010;2:a006734. doi: 10.1101/cshperspect.a006734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PF, Xie N, Zhang JJ, Guan XL, Zhou J, Long LH, Li YL, Xiong QJ, Zeng JH, Wang F, Chen JG. Resveratrol preconditioning increases methionine sulfoxide reductases A expression and enhances resistance of human neuroblastoma cells to neurotoxins. J Nutr Biochem. 2012 doi: 10.1016/j.jnutbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nature medicine. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- Xie W, Li X, Li C, Zhu W, Jankovic J, Le W. Proteasome inhibition modeling nigral neuron degeneration in Parkinson’s disease. Journal of neurochemistry. 2010;115:188–199. doi: 10.1111/j.1471-4159.2010.06914.x. [DOI] [PubMed] [Google Scholar]
- Xu Q, Park Y, Huang X, Hollenbeck A, Blair A, Schatzkin A, Chen H. Physical activities and future risk of Parkinson disease. Neurology. 2010;75:341–348. doi: 10.1212/WNL.0b013e3181ea1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevalk GD, Razmpour R, Bernard LP. Glutathione and Parkinson’s disease: is this the elephant in the room? Biomed Pharmacother. 2008;62:236–249. doi: 10.1016/j.biopha.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wu Y, Jia J. Exercise preconditioning and brain ischemic tolerance. Neuroscience. 2011;177:170–176. doi: 10.1016/j.neuroscience.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Zhang Y, James M, Middleton FA, Davis RL. Transcriptional analysis of multiple brain regions in Parkinson’s disease supports the involvement of specific protein processing, energy metabolism, and signaling pathways, and suggests novel disease mechanisms. Am J Med Genet B Neuropsychiatr Genet. 2005;137B:5–16. doi: 10.1002/ajmg.b.30195. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Li X, Xie WJ, Tuo H, Hintermann S, Jankovic J, Le W. Anti-parkinsonian effects of Nurr1 activator in ubiquitin-proteasome system impairment induced animal model of Parkinson’s disease. CNS Neurol Disord Drug Targets. 2012;11:768–773. doi: 10.2174/187152712803581155. [DOI] [PubMed] [Google Scholar]
- Zhu X, Castellani RJ, Takeda A, Nunomura A, Atwood CS, Perry G, Smith MA. Differential activation of neuronal ERK, JNK/SAPK and p38 in Alzheimer disease: the ‘two hit’ hypothesis. Mechanisms of ageing and development. 2001;123:39–46. doi: 10.1016/s0047-6374(01)00342-6. [DOI] [PubMed] [Google Scholar]
- Zhu X, Lee HG, Perry G, Smith MA. Alzheimer disease, the two-hit hypothesis: an update. Biochimica et biophysica acta. 2007;1772:494–502. doi: 10.1016/j.bbadis.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Zhu X, Raina AK, Perry G, Smith MA. Alzheimer’s disease: the two-hit hypothesis. Lancet neurology. 2004;3:219–226. doi: 10.1016/S1474-4422(04)00707-0. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Abercrombie ED, Berger TW, Grace AA, Stricker EM. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 1990;13:290–296. doi: 10.1016/0166-2236(90)90112-n. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Cameron JL, Leak RK, Mirnics K, Russell VA, Smeyne RJ, Smith AD. Triggering endogenous neuroprotective processes through exercise in models of dopamine deficiency. Parkinsonism & related disorders. 2009;15(Suppl 3):S42–45. doi: 10.1016/S1353-8020(09)70778-3. [DOI] [PubMed] [Google Scholar]