Abstract
Inter-animal signaling from irradiated to non-irradiated organisms has been demonstrated for whole body irradiated mice and also for fish. The aim of the current study was to look at radiotherapy style limited exposure to part of the body using doses relevant in preclinical therapy. High dose homogenous field irradiation and the use of irradiation in the microbeam radiation therapy mode at the European Synchrotron Radiation Facility (ESRF) at Grenoble was tested by giving high doses to the right brain hemisphere of the rat. The right and left cerebral hemispheres and the urinary bladder were later removed to determine whether abscopal effects could be produced in the animals and also whether effects occurred in cage mates housed with them. The results show strong bystander signal production in the contra-lateral brain hemisphere and weaker effects in the distant bladder of the irradiated rats. Signal strength was similar or greater in each tissue in the cage mates housed for 48hrs with the irradiated rats. Our results support the hypothesis that proximity to an irradiated animal induces signalling changes in an unirradiated partner. If similar signaling occurs between humans, the results could have implications for caregivers and hospital staff treating radiotherapy patients.
INTRODUCTION
For years the biological effects of radiotherapy were attributed only to the DNA damage caused by the energy deposition of ionizing radiation. However, this hypothesis was challenged by the confirmation that healthy cells show radiation-like responses when they are exposed to a medium from irradiated cells (Mothersill and Seymour 1997, Mothersill and Seymour 1998) or when they are located in the vicinity of irradiated cells (Azzam et al, 1998, Azzam et al, 2001). This phenomenon is called the radiation-induced bystander effect (RIBE).
Most research in the bystander field is focused on determining the mechanisms operating at low radiation doses where not every cell may be hit (Seymour and Mothersill, 2000). Radiotherapy doses and regimes have been examined in some laboratories but not in detail (Rzeszowska-Wolny et al, 2009, Burdak-Rothkamm and Prise, 2009, Sjostedt and Bezak, 2010, Shen et al, 2012). However in the old literature there are several reports in radiotherapy patients of abscopal effects i.e. effects in remote organs (Kaminski et al, 2005, Lakshmanagowda et al, 2009, Kroemer and Zitvogel 2012) or clastogenic effects i.e. the production of factors in patient blood which can cause chromosome damage in cultured cells exposed to the serum (Boyes and Koval, 1983, Faguet et al, 1984, Youssefi et al, 1994). Since these effects are detected in tissue or cell cultureswhich were not directly irradiated, both can be classified as types of bystander effect.
The aim of this work was to examine the effect of very high doses of synchrotron microbeam radiation (MRT) and homogenous field irradiation (HR) using a broad beam), two modalities being examined for therapeutic effect in animals bearing models of transplantable tumors, in particular of glioblastoma multiforme (GBM). GBM is a type of malignant human brain tumour which is highly resistant to treatment. MRT delivers radiation as an array of parallel microbeams instead of a single broad beam; Thus, this irradiation approach creates parallel tissue slices exposed to high X-ray doses (peak doses) alternating with slices not directly in the path of the microbeams but to scattered X-rays (valley dose). There is a large dose differential between the tissue slices directly in the path of the beam and the tissue slices between the irradiation tracks, called the peak-to-valley dose ratio (PVDR). This means that a slice of low dose irradiated tissue is present between all microbeams. Radiation-induced bystander effects in the so-called valleys between the tracks of irradiated cells become very relevant at this point because even though there is clearly a scatter dose to the tissue in the valley there is a large dose differential. The study of tissue slices from right brain (irradiated), left brain (unirradiated) and bladder (unirradiated and distant) was undertaken to try to determine the role of scatter dose in the valleys of the irradiated right brain in producing bystander signals, compared with signal production in unirradiated brain and distant bladder. To address the above problems we completed collaborative work over the course of 2 years, between autumn 2009 and spring 2011. The experiments were conducted in a small animal model (adult Wistar rats). After some of the animals were exposed to skin entry doses of either 35 Gy or 350 Gy in one single fraction for the purpose of radiosurgery, they shared a cage with naïve, non-irradiated animals. Two different beam modalities were used to compare the induction of bystander responses, MRT and HR. A comparison between both techniques was made because HR tries to emulate the broad-beam radioteraphy currently used in human brain cancer treatments. The ESRF staff has successfully demonstrated that MRT has great advantages in the treatment of brain cancer compared to HR after using same skin entry doses in rodents. Now, due to the interest in using MRT technique as a new alternative for the treatment of brain cancer in humans, we wanted to see whether MRT and HR also differ in inducing abscopal and bystander effects.
The confirmation of the presence of RIBE was made using a clonogenic HPV-G reporter assay, an intracellular calcium concentration assay and proteomics analysis (Fernandez-Palomo et al 2013, Smith et al, 2012,). The data did reveal that strong bystander signals were produced in the contra-lateral cerebral hemisphere and also in the urinary bladder of the irradiated rats but the issue of scatter and neuro-endocrine involvement in the production of these signals could not be excluded.
During the last decade, evidence has been accumulating that bystander signals can be transmitted from irradiated animals to non-irradiated animals (Surinov et al, 2004, Mothersill et al, 2006, Mothersill et al, 2007, Isaeva and Surinov, 2007, Isaeva and Surinov, 2011, Audette-Stuart, (2011) Woenckhaus (1930). It occurred to us that this model might be useful to exclude the possibility that the effects we saw were due to systemic factors because as the rat was never exposed to radiation and merely shared a cage with the irradiated rat, any effects had to be due to transmitted signals and not intra-animal signalling.
METHODS
Normal male adult Wistar rats in the weight range 260–280g (Charles River, France) were used as the animal model in our experiments. Animals were housed and cared for prior to the experiments by the ESRF Animal Facility in accordance with French and Canadian guidelines (Table 1).
TABLE 1.
Irradiation group schedule for Cage Mate Experiments
| Group | Irradiated Rats | Cage Mates (non-irradiated) | Modality | Dose | Dissection |
|---|---|---|---|---|---|
| A | 4 | 4 | MRT | 350 Gy | 48 hrs |
| B | 4 | 4 | MRT | 35 Gy | 48 hrs |
| C | 2 | 2 | HR | 350 Gy | 48 hrs |
| D | 2 | 2 | HR | 35 Gy | 48 hrs |
| Controls | 5 | 5 | Rats never left the cage |
In preparation for the irradiations, rats were deeply anesthetized using 3% isofluorane in 2L/min compressed air and maintained with a intraperitoneal injection of a Ketamine-Xylazine cocktail (Ketamine : Xylazine = 1: 0.625; Ket 1000 and Paxman from Virback France).
Irradiations: Animals were transported from the Animal Facility to the biomedical beam line ID17, which takes less than 5 minutes. Each rat was then individually placed on the goniometer and the corresponding radiation dose for its treatment group was applied exclusively to the right cerebral hemisphere by setting one edge of the irradiation field 2mm towards the right from the midline. The left non-irradiated cerebral hemispheres and the urinary bladder served as fields for study of bystander effects. Details of the irradiation modalities were as follows:
MRT mode
Animals were exposed in a single treatment session of 35 or 350 Gy skin-entry doses. Although multi-directional treatment is more successful in increasing survival, the geometry of the unidirectional beam works better for understanding RIBE. Unidirectional irradiation creates a less complicated 3D geometrical pattern of dose peaks and dose valleys within the brain tissue than bidirectional irradiation and therefore makes it easier to understand whether the normal unirradiated tissue slices tissue present between the microbeams increase the induction of bystander effects. Therefore, a 10mm wide array of 14mm high monochromatic anteroposterior beam was separated by a multislit collimator (Bräuer-Krisch E., Requardt H, Brochard T, Berruyer G, Renier M, Laissue JA, and Bravin A: New technology enables high precision multislit collimators for microbeam radiation therapy. Review Scientific Instruments (2009) 80: 074301; published online July 31, 2009). The array was composed of 50 quasi-parallel rectangular planar microbeams, which were 25μm thick with 200μm centre-to-centre distance. Additionally, the synchrotron was set to deliver a multi-chromatic synchrotron beam with a dose rate of 16,000 Gy/sec.
HR mode
To determine whether the bystander response produced by the spatially fractionated microbeams differs from the response to a single broad beam, a uniform radiation dose was delivered to another group of rats, with an equivalent dose to the right brain hemisphere delivered with the corresponding MRT protocols. HR was administered in one single treatment session at the same skin entry doses. The geometry, direction to the target, and energy of the homogenous beam was the same as for the MRT array.
Scatter Radiation
In order determine the extent of bystander responses of unirradiated tissue scatter radiation due to scatter two rats were selected as scatter controls. A PTW semiflex ion chamber was used to measure the dose received at the urinary bladder after irradiation with 350Gy delivered in the HR and the 350Gy MRT modes. The dose at the site of the urinary bladder was calculated as 30.6mGy for the HR configuration and 5.8 mGy for MRT. An X-ray generator was adapted with different additional filters to obtain an adequate dose rate, in order to deliver the whole body dose of 5.8 mGy to both rats. HD-610 and MD-55 Gafchromic Films (ISP Advanced Materials, http://online1.ispcorp.com/) were used to verify all irradiation doses and modalities applied.
After irradiation, rats were put in individual cages with a marked, unirradiated rat.
Untreated and Sham Controls
Untreated controls stayed in the ESRF animal facility and never left the cage. They received anaesthesia before euthanasia. The control rats were paired with cage mates and were held two to a cage as were the experimental groups. We previously demonstrated that a sham irradiation didn’t induce abscopal effects or affect the protein expression of brain compared to un-irradiated controls (Smith et al 2013). To exclude the possibility that sham irradiation and anaesthesia could be having an effect on the inter-animal transmission of signals, an additional group of animals were sham irradiated then paired with cage mates.
All irradiated rats were transported back to the ESRF animal facility after irradiation; after 48hrs they were deeply anesthetised, beheaded and dissected.
Dissections and Sampling for Explant Culture and Proteomics
The rats’ brains were extracted from the skull. Dissection of the brain was performed in a biosafety cabinet. Two pieces of brain tissue (5mm × 5mm × 3mm) were taken from both the right and the left cerebral hemispheres using sterile instruments. The tissue sample from the right (irradiated) hemisphere was taken from the center of the irradiation array and the sample from the left (unirradiated) hemisphere was taken from the anatomically corresponding (mirror) location. Samples were placed in a 5ml sterile tube containing 1mL of Roswell Park Memorial Institute (RPMI 1640, Gibco) growth medium, supplemented with 10% FBS, 5ml of Penicillin-Streptomycin (Gibco), 5ml of L-glutamine (Gibco), 0.5 mg/ml of Hydrocortisone (Sigma-Aldrich), and 12.5 ml of 1M HEPES buffer solution (Gibco). Samples were immediately transported on ice to the tissue culture laboratory. The remaining brain tissue was snap-frozen in liquid nitrogen and stored at −80ºC for proteomic studies. The entire extracted urinary bladder was also placed in a sterile 5ml tube containing 1ml of complete growth medium and used to set up tissue explants.
Explant Tissue Culture and culture medium harvest
Explant tissue culture was performed in the biosafety level 2 laboratory of the ESRF animal Facility. Brain and bladder tissues were cut in 3 equal-size pieces of approximately 2mm3 in a biosafety cabinet. Pieces were plated as single explants in the centre of a 25cm2 growth area in a 50 ml volume flask (Falcon), containing 2ml of complete growth medium. Flasks were then left undisturbed in a tissue culture incubator set at 37ºC, with an atmosphere of 5% CO2 in air and 95% humidity. Growth medium from each of the three explant pieces (total approximately 5ml) was harvested 48 hours later by pouring it off into a sterile plastic container. This was then filtered through a sterile 0.22μm filter (Acrodisc Syringe Filter with HT Tuffryn Membrane, Pall Life Sciences) to ensure that cells or other debris were not present in the harvested medium, and placed in a 7mL tube. Conditioned growth medium was kept in 4ºC until all medium was collected and then transported to McMaster University for clonogenic reporter bioassays.
Clonogenic Reporter Cell Line
HPV-G cells have been used as reporters for explanted tissue assays by our laboratory for over 10 years (Mothersill et al, 2001). The cell line consists of epithelial cells derived originally from human foreskin primary culture and immortalized through transfections of complete Human Papillomavirus (HPV) 16 genes (Woodworth et al, 1988, Pirisi et al, 1988). The HPV16 genes that directly participate in the immortalization of the epithelial cells are E6 and E7 (Münger et al., 1989).
HPV-G cells were given by Professor J. DiPaolo, NIH, Bethedsa, MD, and have been used in a wide range of experiments due to their reliable and stable response to bystander signals. They show a reduction of around 40% in colony survival in response to addition of autologous irradiated cell conditioned medium (ICCM) over a wide range of exposure conditions (Seymour and Mothersill 1997). The HPV-G cells were cultured in T75 flasks (Falcon) with RPMI 1640 supplemented as above. Once the cells reached about 90–95% confluence they were detached using 1:1 (v:v) solution of 0.02 % Trypsin/EDTA (1mM) (Gibco) and Dulbecco’s Phosphate-Buffered Solution (1x) (Gibco). The concentration of cells was determined using a Coulter Counter (Beckman Coulter model Zn).
Clonogenic HPV-G Reporter Bioassay: Upon arrival at McMaster University, the conditioned medium harvested in France was transferred into 25cm2 flasks containing the HPV-G reporter cells. Reporter flasks were seeded with 500 HPV-G cells and set up 6 hours prior to the medium transfer from T75 flasks which were 90–95% confluent. Plating efficiency and medium transfer controls were also set up. The flasks were then placed in an incubator for 10–12 days to allow for colony formation using the Puck and Marcus technique (Puck and Marcus, 1956). Once colonies reached a suitable size they were stained using 2mL of a 1:4 solution of Carbol Fuchsin in water.
Colonies were counted using a 50 cell threshold and the percentage survival fraction was calculated using the plating efficiency of the reporter cells as shown below.
Fura-2 measurements to determine intracellular free calcium in HPV-G cells: HPV-G cells were seeded in glass bottomed dish (MatTek) at a density of approximately 500 000 cells and incubated at 37°C and 5% CO2 for 18–24 hours prior to measurement to achieve 50% confluence. Cells were washed 3 times with buffer (130mM NaCl, 5 mM KCl, 1 mM Na2HPO4, 1 mM CaCl2, 1 mM MgCl2 and 25 mM Hepes (pH 7.4)) followed by incubation with 1ml of 4.17uM Fura-2 AM (Sigma) at 37°C for 30min. Cells were washed 3 times with buffer to remove residual Fura-2 and 300uL of fresh buffer added to the dish for imaging. An Olympus 1X81 microscope was used with a 40X oil objective and Fura filter cube with 510nm emission. Fura-2 was excited at 380 and 340nm and the ratio images were recorded every 4s for 5 minutes with addition of 100ul of ICCM or control media after a stable baseline was reached approaching 30s. All measurements were conducted in the dark at room temperature.
STATISTICAL ANALYSIS
Data are presented as standard deviation of the mean for the specific n value of each experiment. Significance was determined using the unpaired or paired Student t-test as appropriate. In all cases p values ≤ 0.05 were selected as significant.
RESULTS
Scatter and sham controls
There was no effect of scatter irradiation (plus anaesthesia) and no effect of sham irradiation on either directly irradiated rats or their cage mates.
Comparison of clonogenic reporter survivals between non-irradiated rats who shared the cage with irradiated rats
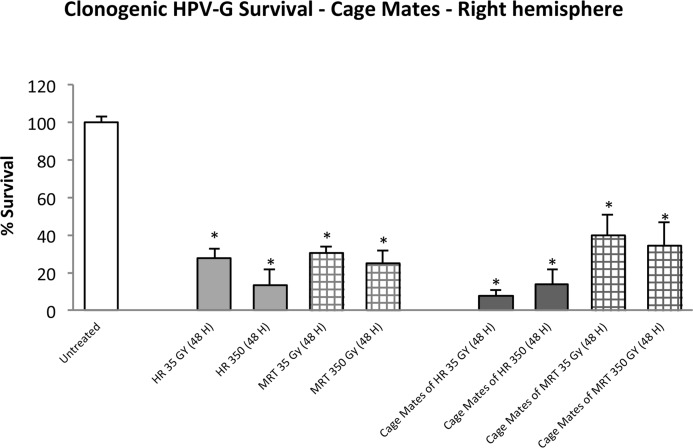
Figure 1 shows the clonogenic survival of HPV-G cells when they were exposed to explant conditioned medium from the right cerebral hemisphere of directly irradiated and non-irradiated cage-mate rats. A significant decrease in survival (p≤0.001) was observed in all directly irradiated rat groups no matter the dose or radiation modality. In the cage-mate rats, a significant decrease in survival (p≤0.001) was also observed in all groups.
FIGURE 1.
Clonogenic survival of HPV-G cells grown in explant-conditioned medium taken from the right cerebral hemisphere of irradiated rats and their non-irradiated cage mates. Irradiated rats were exposed to either MRT or HR in the right cerebral hemisphere. Non-irradiated rats were placed in the cage containing the irradiated rats during a 48 hours period and then all rats were killed and dissected. (Error bars indicate SEM for: untreated n=5; MRT and their cage mates n=4; HR and their cage mates n=2).
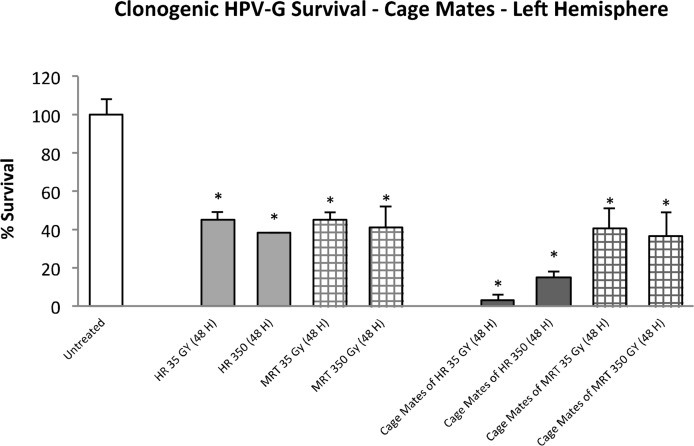
Clonogenic survival showed a significant reduction (p≤0.01) when HPV-G cells were grown in conditioned-explant medium transferred from the non-irradiated (left) cerebral hemisphere of both directly irradiated and cage-mate rats (Fig. 2). The directly irradiated groups (MRT and HR) showed an average of 40% of survival; while the cage-mate groups showed a survival of 40% when the medium was taken from the MRT cage-mate explants and 20% (or lower) when the medium was obtained from the HR cage-mates.
FIGURE 2.
Clonogenic survival of HPV-G cells grown in explant-conditioned medium taken from the left cerebral hemisphere of irradiated rats and their non-irradiated cage-mates. Irradiated rats were exposed to either MRT or HR to the right cerebral hemisphere. Non-irradiated rats were placed in the cage containing the irradiated rats during a 48 hours period and then all rats were killed and dissected. (Error bars indicate SEM for: untreated n=5; MRT and their cage-mates n=4; HR and their cage mates n=2).
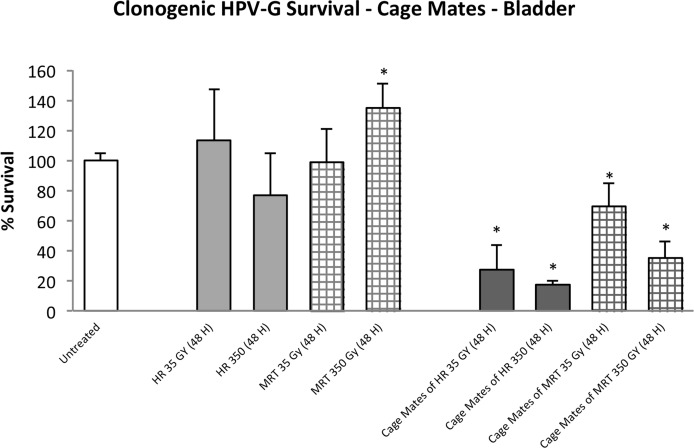
The data were more variable when medium was harvested from bladder explants. Clonogenic survival of reporters receiving medium from the directly irradiated and the cage-mate groups are shown in Figure 3. Within the direct-irradiated groups, survival ranged from 75% to 130% with the 350Gy HR group showing the lowest survival and the 350Gy MRT group showing the highest, which is also the only one significantly different from the control group (p≤0.05). The 4 unirradiated cage-mate groups show a significant decrease in survival compared to the control (p≤0.05). In details it can be observed an average of 20% of survival in both the 35Gy and 350Gy HR mates, and 35% and 60% of survival in the 350Gy and 35Gy MRT cage-mate groups.
FIGURE 3.
Clonogenic survival of HPV-G cells grown in explant-conditioned medium taken from the bladder of irradiated rats and their non-irradiated cage-mates. Irradiated rats were exposed to either MRT or HR to the right cerebral hemisphere. Non-irradiated rats were placed in the cage containing the irradiated rats during a 48 hour period and then all rats were killed and dissected. (Error bars indicate SEM for: untreated n=5; MRT and their cage mates n=4; HR and their cage mates n=2).
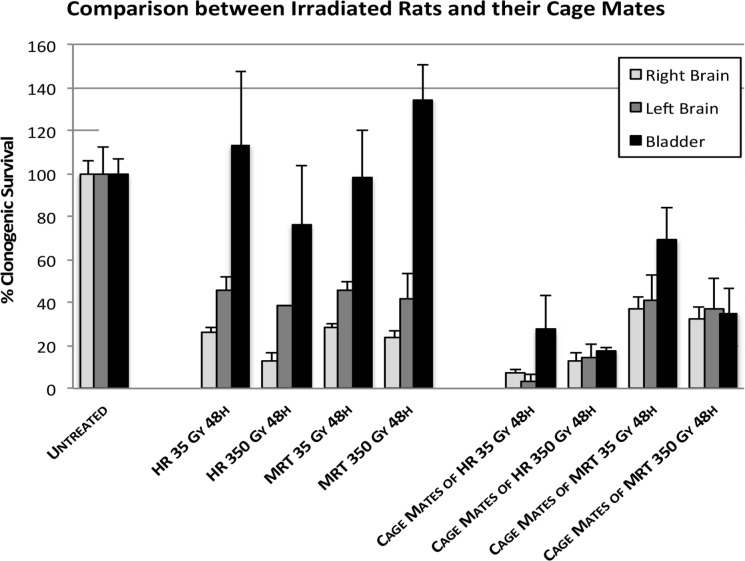
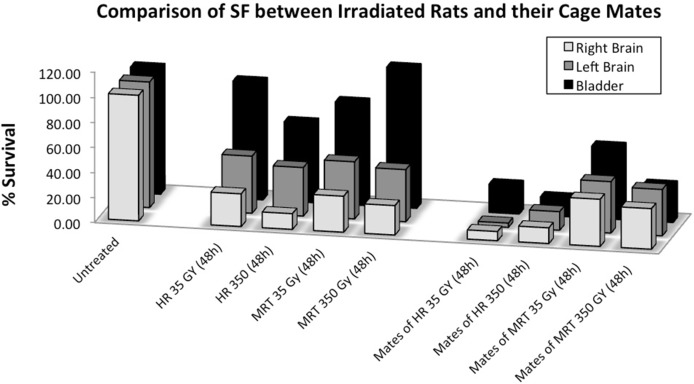
All the data are presented in one graph (Fig. 4) to allow easy comparison of the results and the statistical analyses are presented on Table 2. These analyses show that for all the cage-mate groups housed with irradiated partners, the data are significantly different from the controls (p≤0.05). In the directly irradiated groups, all but the urinary bladder produced signals, which led to a significant reduction in the reporter cells.
FIGURE 4.
Comparison of reporter clonogenic survival between all the explant organs. Rats from the untreated group received anaesthesia but did not received radiation. Irradiated rats were exposed to either MRT or HR in the right cerebral hemisphere. Non-irradiated rats were placed in the cage containing the irradiated rats during a 48 hours period and then all rats were killed and dissected. (Error bars indicate SEM for: untreated n=5; MRT and their cage-mates n=4; HR and their cage mates n=2).
TABLE 2.
– Statistical Analysis of direct irradiated treatment groups and their cage mates: Clonogenic survival of HPV-G cells.
| Treatment | Right Cerebral Hemisphere
|
Left Cerebral Hemisphere
|
Bladder
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean SF | Standv | t-test | Significant (p<0.05) | Mean SF | Standv | t-test | Significant (p<0.05) | Mean SF | Standv | t-test | Significant (p<0.05) | |
| Untreated | 45.2 | ±3.3 | - | - | 44.2 | ±7.8 | - | - | 87.4 | ±8.7 | - | - |
| HR 35 Gy (48 H) | 12.5 | ±3.5 | 0.000043 | Yes | 20.0 | ±4.2 | 0.005058 | Yes | 99.0 | ±42.4 | 0.264423 | No |
| MRT 35 Gy (48 H) | 13.8 | ±3.0 | 0.000001 | Yes | 20.0 | ±3.7 | 0.000377 | Yes | 86.3 | ±38.3 | 0.474583 | No |
| HR 350 Gy (48 H) | 6.0 | ±5.7 | 0.000036 | Yes | 17.0 | ±0.0 | 0.002706 | Yes | 67.0 | ±33.9 | 0.106025 | No |
| MRT 350 Gy (48 H) | 11.3 | ±6.7 | 0.000010 | Yes | 18.3 | ±10.4 | 0.001792 | Yes | 117.8 | ±28.4 | 0.027725 | Yes |
| Cage Mates of | ||||||||||||
| MRT 35 Gy (48 H) | 18.0 | ±9.9 | 0.000322 | Yes | 18.0 | ±10.5 | 0.001713 | Yes | 60.8 | ±25.7 | 0.031714 | Yes |
| MRT 350 Gy (48 H) | 15.5 | ±11.0 | 0.000336 | Yes | 16.3 | ±12.7 | 0.002275 | Yes | 30.8 | ±19.4 | 0.000297 | Yes |
| HR 35 Gy (48 H) | 3.5 | ±2.1 | 0.000009 | Yes | 1.5 | ±2.1 | 0.000381 | Yes | 24.0 | ±19.8 | 0.000673 | Yes |
| HR 350 Gy (48 H) | 6.0 | ±5.7 | 0.000036 | Yes | 6.5 | ±3.5 | 0.000725 | Yes | 15.5 | ±2.1 | 0.000054 | Yes |
Statistical analysis of experiment 3 showing how significantly different the treatments are compared to the untreated group. The study was performed using an unpaired t-test analysis.
Calcium assay
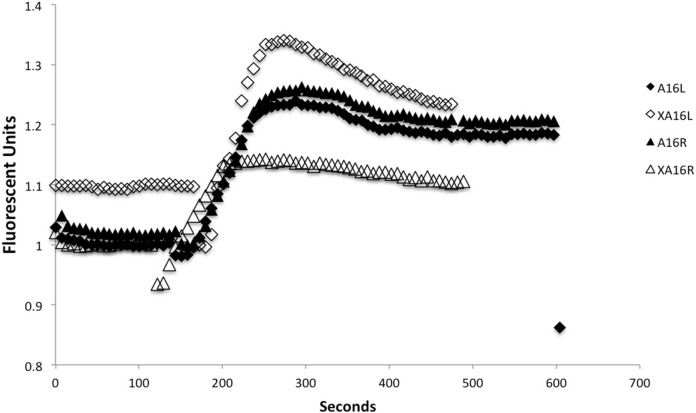
The data showing that calcium signalling occurs in tissues harvested from directly irradiated rats or their cage mates are presented in Figure 5 and Table 3 (a and b). In Table 3a, the initial slope and maximum value for intracellular calcium fluorescence are tabulated. These parameters define the rate and extent of calcium influx into the reporter cells. In Figure 5 a sample graph is presented showing the calcium pulse obtained when media from explanted brain tissues were added to reporter cells. The transient pulse is one of the earliest indicators that a bystander signal is present and shows that intracellular calcium levels have suddenly increased when the test medium is applied. The detection of a signal varies for different animals so the figure shows a sample pair of traces for a directly irradiated animal and its cage mate. Clearly the data on the tables and figure show that where a rat right brain hemisphere was directly irradiated, reporter medium harvested from its right and left brain hemisphere and from its cage mate show similar calcium responses. The controls never showed a calcium response. Because the bladder clonogenic data were so variable, the individual data were correlated with the magnitude of the calcium pulse. These results are presented in Table 3b and show that where the clonogenic survival was most affected, the calcium pulse was strong. Data obtained from bladders taken from rats receiving direct irradiation yielded the following linear regression statistics: Clonogenic survival (CS) vs slope R2 = 0.8473, a = 0.008 (0.0012), b = −6.613 × 10−5 (1.146 × 10−5), p = 0.0012. CS vs plateau R2 = 0.5120, a = 1.0144 (0.1640), b = −0.0038 (0.0015), p = 0.046. CS vs rise R2 = 0.7779, a = 1.0106 (0.199), b = −0.0085 (0.0019), p = 0.0038. For the cage mates similar highly significant correlations were found as follows: CS vs slope R2 = 0.9201, a = 0.0115 (6.638 × 10−5), b = −1.060 × 10−4 (1.275 × 10−5), p = 0.0007. CS vs plateau R2 = 0.8740, a = 1.8306 (0.1134), b = −0.01406 (0.0027), p = 0.0007. Clonogenics vs rise R2 = 0.8760, a = 1.3736 (0.1064), b = −0.0133 (0.0021), p = 0.006.
FIGURE 5.
The slope for all is 0.003 and the “rise” or change in the value of Y from the lowest to highest point is 0.25 approximately for all. Legend for symbols A16L = Left hemisphere from rat 16 directly irradiated to the right hemisphere, A16R = Right hemisphere from rat 16 directly irradiated to the right hemisphere. XA16L = left hemisphere of unirradiated cage mate of rat 16. XA16R = right hemishphere of unirradiated cage mate of rat 16.
TABLE 3A.
Slope, plateau and rise parameters for calcium fluxes measured using a reporter bioassay on samples harvested from rats directly irradiated to the right cerebral hemisphere, the left cerebral hemisphere from these rats and the right and left cerebral hemispheres from their cage mates.
| Group/sample number | Direct MRT Right Hemisphere |
Cage mate MRT Right Hemisphere |
Direct MRT (unirradiated Left Hemisphere) |
Cage mate MRT (unirradiated Left Hemisphere) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| slope | plateau | rise | slope | plateau | rise | slope | plateau | rise | slope | plateau | rise | |
| 1 | 0.003 | 1.4 | 0.25 | 0.0027 | 1.2 | 0.23 | 0.0027 | 0.9 | 0.23 | 0.003 | 0.8 | 0.25 |
| 2 | 0.006 | 1.2 | 0.46 | 0.0063 | 1.1 | 0.5 | 0.0076 | 1.3 | 0.72 | 0.0063 | 1.4 | 0.51 |
| 3 | 0.005 | 0.9 | 0.39 | 0.0048 | 1.4 | 0.4 | 0.006 | 1.1 | 0.47 | 0.0053 | 1.3 | 0.40 |
| 4 | 0.008 | 1.7 | 0.77 | 0.0085 | 1.9 | 0.47 | 0.007 | 1.7 | 0.69 | 0.008 | 1.7 | 0.73 |
| Group/sample number | Direct HR Right Hemisphere |
Cage mate HR Right Hemisphere |
Direct HR (unirradiated Left Hemisphere) |
Cage mate HR (unirradiated Left hemisphere) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| slope | plateau | rise | slope | plateau | rise | slope | plateau | rise | slope | plateau | rise | |
| 1 | 0.005 | 1.4 | 0.4 | 0.0052 | 1.2 | 0.4 | 0.005 | 1.6 | 0.45 | 0.005 | 1.5 | 0.48 |
| 2 | 0.003 | 1.0 | 0.25 | 0.0029 | 0.8 | 0.26 | 0.0028 | 0.9 | 0.26 | 0.003 | 0.9 | 0.26 |
TABLE 3B.
Individual data for the calcium reporter assay and for the clonogenic assay for bladder tissue from the rats described in Table 3a.
| Group/sample | MRT direct (calcium assay) |
MRT Cage mate (calcium assay) |
MRT direct (clonogenic survival % of PE control) |
MRT Cage mate (clonogenic survival % of PE control) |
||||
|---|---|---|---|---|---|---|---|---|
| slope | plateau | rise | slope | plateau | rise | |||
| Handling control (mean data) | 0 | 0.5±0.03 | 0 | 0 | 0.47±0.03 | 0 | 87.6±8.7 | 96±8.9 |
| 1 350Gy | 0 | 0.7 | 0 | 0.008 | 1.4 | 0.98 | 144 | 35 |
| 2 350Gy | 0 | 0.4 | 0 | 0.007 | 1.3 | 0.87 | 130 | 29 |
| 3 350Gy | 0 | 0.46 | 0 | 0.006 | 1.0 | 0.67 | 119 | 53 |
| 4 350Gy | 0.003 | 0.67 | 0.15 | 0.012 | 1.8 | 1.4 | 78 | 6 |
| 1 35Gy | 0.007 | 1.0 | 0.98 | 0.008 | 1.4 | 0.84 | 29 | 27 |
| 2 35Gy | 0 | 0.7 | 0 | 0.003 | 0.6 | 0.26 | 110 | 74 |
| 3 35Gy | 0 | 0.49 | 0 | 0.003 | 0.7 | 0.26 | 101 | 86 |
| 4 35Gy | 0 | 0.56 | 0 | 0.006 | 1.3 | 0.84 | 105 | 56 |
| Group/sample
|
HR direct (calcium assay) |
HR Cage mate (calcium assay) |
HR direct (clonogenic survival) |
HR Cage mate (clonogenic survival) |
||||
|---|---|---|---|---|---|---|---|---|
| slope | plateau | rise | slope | plateau | rise | |||
| 1 350Gy | 0.002 | 0.46 | 0.01 | 0.009 | 0.98 | 0.7 | 91 | 17 |
| 2 350Gy | 0.006 | 0.86 | 0.57 | 0.013 | 1.5 | 1.1 | 43 | 14 |
| 1 35Gy | 0 | 0.5 | 0 | 0.009 | 1.1 | 0.8 | 129 | 38 |
| 2 35Gy | 0.004 | 0.8 | 0.68 | 0.14 | 1.4 | 1.1 | 69 | 10 |
DISCUSSION
The results indicate that a single exposure of radiosurgical doses of MRT and HR to one cerebral hemisphere caused the release of signals from both hemispheres and from the distant bladder that altered the biological response in non-irradiated HPV-G cells. Moreover, these data confirm the communication of bystander factors from the irradiated rats which induced signal production in the brain and bladder of completely un-irradiated rats.
The previous data from our group (Fernandez-Palomo et al 2013, Smith et al, 2013) show that bystander signals are produced in vivo in rats after delivery of controlled radiosurgical doses of MRT and HR to their right cerebral hemispheres. In this paper, the data are extended to include measurement of signals in animals which were not irradiated at all but merely shared a cage for 48hrs with directly irradiated animals. Bystander responses were measured using the clonogenic survival assay of Puck and Marcus (1956), and by determining the size of the calcium pulse using HPV-G cells as the read out (Mothersill et al 2005). The results confirm previous studies done by Mothersill et al (2005) in which soluble factors present in medium from explanted mouse bladder tissue had the capacity to cause death in reporter recipient cells in vitro. O’Dowd et al (2006), also showed that bystander signals were produced in vivo after irradiating rainbow trout. However the data produced using the cage mates takes the study to a new level by demonstrating the induction in a completely unirradiated animal of bystander–like signals. These data are discussed after discussion of the directly irradiated animal data.
The results reported here clearly show that explant-conditioned media from the right and left cerebral hemispheres of directly irradiated rats significantly reduced the survival of reporters and led to generation in reporter cells of a strong and transient calcium pulse as described by Lyng et al 2002. Less than 40% of the control reporter cell clonogenic survival was observed when explant-conditioned medium was harvested from the right cerebral hemisphere was tested and less than 50% survival when extracted from the left cerebral hemisphere. However as shown in Table 2, the medium extracted from bladder explants contained much weaker signals and produced significant results in only one treatment group. Although there are not significant differences between the reporter assay results for both radiation modalities, there seems to be a correlation between the dose and the clonogenic survival in the HR group. As is shown in Figure 4, 350 Gy of HR produced a stronger reduction of reporter survival than 35 Gy of HR in both cerebral hemispheres and bladder. MRT groups -, did not show that dose effect relationship.
Earlier work by our group tested the time over which fish produce bystander factors after irradiation (Mothersill et al 2007). Fish irradiated in early life-stages continue producing signals during their entire life span. The rat experiments show that adult rats conserved their capacity to produce signals for at least 48 hours post-irradiation. It may be important to determine whether rats have a prolonged capacity of signal-production, and whether this capacity extends to other mammals—including humans.
Non-irradiated rats placed in the same cage as irradiated rats over a 48-hour period showed a significant reduction in clonogenic reporter survival (Table 2). The data showing that the intracellular calcium concentration rises in the reporters receiving medium from directly irradiated rats and their cage mates clearly confirm the transmission of signals from irradiated to non-irradiated animals in vivo. Other preliminary data published as yet only as a meeting abstract (Mothersill et al 2012) show that some proteomic changes can also be seen in the cage mates and that in the case of 3 of the proteins either an identical protein or an isoform as significantly elevated or reduced in both the directly irradiated animal and its cage mate. The rationale for these experiments was to observe if the transmission of bystander signals occurs between irradiated mammals given very high radiosurgical doses and their cage mates. Previous studies done by our group showed that directly irradiated rainbow trout, medaka, fathead minnow and zebrafish all released signals into their water that affected non-irradiated fish (Mothersill et al, 2006, Mothersill et al, 2007, Mothersill et al, 2009, Mothersill et al, 2012). Similar effects were seen in amphibians where tadpoles from contaminated lakes induced adaptive responses in tadpoles from pristine lakes (Audette-Stuart 2011). Aqueous transmission of signals was suspected although no chemical species has yet been identified. Work published by Isaeva and Surinov (2007), provides the first data that such in vivo inter-animal signal transmission might be important also in mammals. They showed, using blood analysis that irradiated mice induced immunosuppression in non-irradiated mice of various genotypes. Our results show that all cage-mate groups showed a significant decrease in clonogenic reporter survival and a correspondingly strong calcium signal. The very strong correlation between clonogenic survival reduction and calcium signal strength occurs in all groups irrespective of treatment suggesting that these endpoints are linked mechanistically. This is particularly evident in Table 3b where the bladder data are presented for each rat. The correlation coeficients for each calcium parameter are very strongly correlated with the clonogenic survival for each animal assay. In the case of the bladder tissue, the response in the cage mates is greater that the response in the bladder of the rats receiving direct cerebral irradiation suggesting an interesting mechanistic difference in either the reception of signals by unirradiated cage mates, or a difference in the strength of the signals produced. Furthermore, the cage-mate response seems to be related to the radiation modality but independent of the radiation dose. In fact, as is shown in Figure 6 the cage-mates of the HR groups showed a more pronounced decrease in clonogenic survival compared to the cage-mates of the MRT group, suggesting that as the heavily irradiated tissue volume increases by a factor of 8, the depression of clonogenic survival of reporter cells in cage-mate animals also increases. This could suggest that non-irradiated rats are more likely to detect these signals and therefore more sensitive to their effects. Alternatively, once signals are detected, rats start their own bystander signal machinery that would enhance the final effect. A trend of survival can be easily observed in Figure 6 following the directly irradiated group, in which survival increases as we increase the distance from the irradiated right hemisphere. On the contrary, the cage-mate clonogenic survival response was equal in all organs but dependent on dose and type of irradiation. These findings suggest that the production of the signal(s) in the irradiated rat is directly related to the distance between the organ involved in the bystander signal production and the radiation-target organ. Thus, once the factor(s) is/are expelled from the animal the systemic response in the non-irradiated rat may be a more complicated process, which could be related to the amount of signal intake. According to Mothersill et al (2007), the signals seem to be both stable and soluble in water, which is confirmed in part by our results. Isaeva and Surinov (2007) proposed that the signals were transmitted through urine and involved volatile compounds which were detected by nasal receptors. Daev proved this in part by exposing mice to the straw only from irradiated mouse cage (Daev et al 2007). If we accept that urine is the means of transmission, the intake of the signals by the non-irradiated rats could result from two mechanisms. First, the signals may be ingested through the gastrointestinal system as a result of rats grooming to each other as part of their social behavior; and second, if Surinov’s hypothesis is correct, the signals may be volatile, and the intake of the factors would be through the olfactory/respiratory system.
FIGURE 6.
Comparison of clonogenic reporter survival (SF) between irradiated rats and their cage-mates. Each treatment group includes the percentage of survival resulted from exposing HPV-G cells to the medium from right brain hemisphere, left brain hemisphere and bladder. (Error bars indicate mean standard deviation for: untreated n=5; MRT and their cage mates n=4; HR and their cage mates n=2).
The key novel aspect of this work is that it confirms the relevance of bystander effects in radiotherapy. It also confirms that scatter or systemic effects are not involved in production of the type of bystander effects reported here in cage mates, allowing a clear distinction between bystander and abscopal effects. Many authors deny the existence of bystander effects in vivo suggesting they are an in vitro artifact. This is particularly said of in vitro work involving medium transfer. In vivo manifestations mostly involve shielding part of the body while targeting another part (Pazzaglia et al, 2009, Koturbash et al, 2011,) or use of parabiotic animals, one partner being shielded by lead while the unshielded partner is exposed to X-irradiation (Woenckhaus 1930) and as such are really abscopal rather than true bystander effects. Other approaches involve irradiation in vivo followed by in vitro culture (Lorimore et al, 2011, Mukherjee et al, 2012, Rastogi et al, 2012). The data reported here are from high dose exposed rats. The doses used are extremely high and would be expected to induce immediate cell death in the target regions. This slightly complicates the interpretation of the results, at least within individual animals, where the mechanism of the abscopal effect may be the familiar ones associated with trauma. It is well established that local trauma can cause systemic inflammatory effects and cytokine storms and such a mechanism may be invoked here for distant effects within the same organism. Only the inter-animal signaling techniques allow the full demonstration development of bystander mechanisms and effects in vivo, in a whole animal which never received direct irradiation to any part; thus, transmission of radiation-induced substances and/or mechanisms can be examined in vivo in the absence of any direct damage to the test animal.
Apart from the value of this work in helping to identify specific bystander mechanisms and hence targets for “bystander therapy”, these results may have practical importance if such signals are produced by radiotherapy patients. While the bystander effects identified in the fish and amphibian models appear to suggest protective responses, it must be remembered that these experiments involved low or chronic direct doses. Surinov’s work with mice and Woenckhaus’ experiments with rats) showed adverse effects in the cage-mates when using ≥4Gy doses to the directly exposed animals. The adverse effects included immune suppression and chromosome damage (Daev et al, 2007) and leukopenia (Woenckhaus, 1930). The assays reported in this paper cannot be used to determine whether the bystander effects in the non-exposed rats are harmful but further experiments aimed at addressing this issue are clearly necessary.
Acknowledgments
We acknowledge financial support from the Canada Research Council Chairs programme and The Natural Science and Engineering Research Council (NSERC) Discovery Grant Programme. These experiments were performed on the ID17 beamline at the European Synchrotron Radiation Facility (ESRF), Grenoble, France. We are grateful for allocation of the beamtime and assistance by the ID17 staff.
REFERENCES
- Audette-Stuart M, Yankovich T. Bystander effects in Bullfrog tadpoles. Radioprotection. 2011;46:S497–S497. [Google Scholar]
- Azzam EI, de Toledo SM, Gooding T, Little JB. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. J Radiat Res. 1998;150:497–504. [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Little JB. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha -particle irradiated to nonirradiated cells. Proc Natl Acad Sci USA. 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes BG, Koval JJ. Clastogenic interactions of gamma radiation and caffeine in human peripheral blood cultures. Mutat Res. 1983;108:239–249. doi: 10.1016/0027-5107(83)90123-9. [DOI] [PubMed] [Google Scholar]
- Burdak-Rothkamm S, Prise KM. New molecular targets in radiotherapy: DNA damage signalling and repair in targeted and non-targeted cells. Eur J Pharmacol. 2009;625:151–155. doi: 10.1016/j.ejphar.2009.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daev EV, Surinov BP, Dukel’skaia AV, Marysheva TM. Chromosomal abnormalities and splenocyte production in laboratory mouse males after exposure to stress chemosignals. Tsitologiia. 2007;49:696–701. [PubMed] [Google Scholar]
- Faguet GB, Reichard SM, Welter DA. Radiation-induced clastogenic plasma factors. Cancer Genet Cytogenet. 1984;12:73–83. doi: 10.1016/0165-4608(84)90010-4. [DOI] [PubMed] [Google Scholar]
- Fernandez-Palomo C, Schültke E, Smith R, Bräuer-Krisch E, Laissue J, Schroll C, Fazzari J, Seymour C, Mothersill C. Radiation induced bystander effects in healthy and tumor-bearing rat brains following synchrotron microbeam radiation treatment. Int J Radiat Biol. 2013 doi: 10.3109/09553002.2013.766770. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Isaeva VG, Surinov BP. Postradiation volatile secretion and development of immunosu-pression effectes by laboratory mice with various genotype. Radiatsionnaia biologiia, radioecologiia. 2007;47:10–16. [PubMed] [Google Scholar]
- Isaeva VG, Surinov BP. Effect of natural and postradiation volatile secretions of mice on the immune reactivity and blood cellularity of irradiated animals. Radiatsionnaia biologiia, radioecologiia. 2011;51:444–450. [PubMed] [Google Scholar]
- Kaminski JM, Shinohara E, Summers JB, Niermann KJ, Morimoto A, Brousal J. The controversial abscopal effect. Cancer Treat Rev. 2005;31:159–172. doi: 10.1016/j.ctrv.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Koturbash I, Zemp F, Kolb B, Kovalchuk O. Sex-specific radiation-induced microRNAome responses in the hippocampus, cerebellum and frontal cortex in a mouse model. Mutat Res. 2011;722:114–118. doi: 10.1016/j.mrgentox.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Zitvogel L. Abscopal but desirable: The contribution of immune responses to the efficacy of radiotherapy. Oncoimmunology. 2012;1:407–408. doi: 10.4161/onci.20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanagowda PB, Viswanath L, Thimmaiah N, Dasappa L, Supe SS, Kallur P. Abscopal effect in a patient with chronic lymphocytic leukemia during radiation therapy: a case report. Cases Journal. 2009;2:204. doi: 10.1186/1757-1626-2-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimore SA, Mukherjee D, Robinson JI, Chrystal JA, Wright EG. Long-lived inflammatory signalling in irradiated bone marrow is genome dependent. J Cancer Res. 2011;71:6485–6491. doi: 10.1158/0008-5472.CAN-11-1926. [DOI] [PubMed] [Google Scholar]
- Lyng FM, Seymour CB, Mothersill C. Early events in the apoptotic cascade initiated in cells treated with medium from the progeny of irradiated cells. Radiat Prot Dosim. 2002;99:169–172. doi: 10.1093/oxfordjournals.rpd.a006753. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Bucking C, Smith RW, Agnihotri N, Oneill A, Kilemade M, Seymour CB. Communication of radiation-induced stress or bystander signals between fish in vivo. Environ Sci Technol. 2006;40:6859–6864. doi: 10.1021/es061099y. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Lyng F, Seymour C, Maguire P, Lorimore S, Wright E. Genetic factors influencing bystander signaling in murine bladder epithelium after low-dose irradiation in vivo. Radiat. Res. 2005;163:391–399. doi: 10.1667/rr3320. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Rea D, Wright EG, Lorimore SA, Murphy D, Seymour CB, O’Malley K. Individual variation in the production of a ‘bystander signal’ following irradiation of primary cultures of normal human urothelium. Carcinogenesis. 2001;22:1465–71. doi: 10.1093/carcin/22.9.1465. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour C. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. Int J Radiat Biol. 1997;71:421–427. doi: 10.1080/095530097144030. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. Cell-cell contact during gamma irradiation is not required to induce a bystander effect in normal human keratinocytes: evidence for release during irradiation of a signal controlling survival into the medium. Radiat Res. 1998;149:256–62. [PubMed] [Google Scholar]
- Mothersill C, Smith RW, Agnihotri N, Seymour CB. Characterization of a radiation-induced stress response communicated in vivo between zebrafish. Environ Sci Technol. 2007;41:3382–3387. doi: 10.1021/es062978n. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Smith RW, Hinton TG, Aizawa K, Seymour CB. Communication of radiation-induced signals in vivo between DNA repair deficient and proficient medaka (Oryzias latipes) Environ Sci Technol. 2009;43:3335–3342. doi: 10.1021/es8035219. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Smith R, Fernandez-Palomo C, Schültke E, Bräuer-Krisch E, Laissue J, Schroll C, Fazzari J, Seymour C. Transmission of signals from irradiated rats to cage mates: an inter-animal bystander effect. Gliwice Scientific Meetings, Poland (Abstract) 2012 doi: 10.2203/dose-response.13-011.Mothersill. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee D, Coates PJ, Lorimore SA, Wright EG. The in vivo expression of radiation-induced chromosomal instability has an inflammatory mechanism. Radiat Res. 2012;177:18–24. doi: 10.1667/rr2793.1. [DOI] [PubMed] [Google Scholar]
- Münger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989;8:4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dowd C, Mothersill CE, Cairns MT, Austin B, McClean B, Lyng FM, Murphy JE. The release of bystander factor(s) from tissue explant cultures of rainbow trout (Onchorhynchus mykiss) after exposure to gamma radiation. Radiat Res. 2006;166:611–617. doi: 10.1667/RR0606.1. [DOI] [PubMed] [Google Scholar]
- Pazzaglia S, Pasquali E, Tanori M, Mancuso M, Leonardi S, di Majo V, Rebessi S, Saran A. Physical, heritable and age-related factors as modifiers of radiation cancer risk in patched heterozygous mice. Int J Radiat Oncol, Biol, Phys. 2009;73:1203–10. doi: 10.1016/j.ijrobp.2008.10.068. [DOI] [PubMed] [Google Scholar]
- Pirisi L, Creek KE, Doniger J, DiPaolo JA. Continuous cell lines with altered growth and differentiation properties originate after transfection of human keratinocytes with human papillomavirus type 16 DNA. Carcinogenesis. 1988;9:1573–1579. doi: 10.1093/carcin/9.9.1573. [DOI] [PubMed] [Google Scholar]
- Puck TT, Marcus PI. Action of x-rays on mammalian cells. J Exp Med. 1956;103:653–666. doi: 10.1084/jem.103.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi S, Coates PJ, Lorimore SA, Wright EG. Bystander-type effects mediated by long-lived inflammatory signaling in irradiated bone marrow. Radiat Res. 2012;177:244–250. doi: 10.1667/rr2805.1. [DOI] [PubMed] [Google Scholar]
- Rzeszowska-Wolny J, Przybyszewski WM, Widel M. Ionizing radiation-induced bystander effects, potential targets for modulation of radiotherapy. Eur J Pharmacol. 2009;625:156–164. doi: 10.1016/j.ejphar.2009.07.028. [DOI] [PubMed] [Google Scholar]
- Seymour CB, Mothersill C. Delayed expression of lethal mutations and genomic instability in the progeny of human epithelial cells that survived in a bystander-killing environment. Radiat Oncol Invest. 1997;5:106–110. doi: 10.1002/(SICI)1520-6823(1997)5:3<106::AID-ROI4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Seymour CB, Mothersill C. Relative contribution of bystander and targeted cell killing to the low-dose region of the radiation dose-response curve. Radiat Res. 2000;153:508–511. doi: 10.1667/0033-7587(2000)153[0508:rcobat]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Shen H, Yu H, Liang PH, Cheng H, XuFeng R, Yuan Y, Zhang P, Smith CA, Cheng T. An acute negative bystander effect of γ-irradiated recipients on transplanted hematopoietic stem cells. Blood. 2012;119:3629–3637. doi: 10.1182/blood-2011-08-373621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostedt S, Bezak E. Non-targeted effects of ionising radiation and radiotherapy. Australas Phys Eng Sci Med. 2010;33:219–231. doi: 10.1007/s13246-010-0030-8. [DOI] [PubMed] [Google Scholar]
- Smith RW, Jiaxi Wang, Schültke E, Seymour CB, Bräuer-Krisch E, Laissue JA, Blattmann H, Mothersill C. Proteomic changes in the rat brain induced by homogenous irradiation and by the bystander effect resulting from high energy synchrotron X-ray microbeams. Int J Radiat Biol. 2013;89:118–127. doi: 10.3109/09553002.2013.732252. [DOI] [PubMed] [Google Scholar]
- Surinov BP, Isaeva VG, Dukhova NN. Postirradiation volatile secretions of mice: syngeneic and allogeneic immune and behavioral effects. Bull Exp Biol Med. 2004;138:384–386. doi: 10.1007/s10517-005-0048-1. [DOI] [PubMed] [Google Scholar]
- Woodworth CD, Bowden PE, Doniger J, Pirisi L, Barnes W, Lancaster WD, DiPaolo JA. Characterization of normal human exocervical epithelial cells immortalized in vitro by papillomavirus types 16 and 18 DNA. Cancer Research. 1988;48:4620–4628. [PubMed] [Google Scholar]
- Woenckhaus E. Beitrag zur Allgemeinwirkung der Röntgenstrahlen. Naunyn-Schmiedeberg’s Arch Pharmacol. 1930;150:182–197. (Contribution to the general effects of X rays) [Google Scholar]
- Youssefi AA, Arutyunyan R, Emerit I. Chromosome damage in PUVA-treated human lymphocytes is related to active oxygen species and clastogenic factors. Mutat Res. 1994;309:185–191. doi: 10.1016/0027-5107(94)90091-4. [DOI] [PubMed] [Google Scholar]