Abstract
Concentration-dependent effects of aqueous extract from R. rosea root on long-term survival and stress resistance of budding yeast Saccharomyces cerevisiae were studied. At low concentrations, R. rosea aqueous extract extended yeast chronological lifespan, enhanced oxidative stress resistance of stationary-phase cells and resistance to number stressors in exponentially growing cultures. At high concentrations, R. rosea extract sensitized yeast cells to stresses and shortened yeast lifespan. These biphasic concentration-responses describe a common hormetic phenomenon characterized by a low-dose stimulation and a high-dose inhibition. Yeast pretreatment with low doses of R. rosea extract enhanced yeast survival and prevented protein oxidation under H2O2-induced oxidative stress. Positive effect of R. rosea extract on yeast survival under heat shock exposure was not accompanied with changes in antioxidant enzyme activities and levels of oxidized proteins. The deficiency in transcriptional regulators, Msn2/Msn4 and Yap1, abolished the positive effect of low doses of R. rosea extract on yeast viability under stress challenges. Potential involvement of Msn2/Msn4 and Yap1 regulatory proteins in realization of R. rosea beneficial effects is discussed.
Keywords: Saccharomyces cerevisiae, Rhodiola rosea, lifespan, stress resistance, Yap1p, Msn2/Msn4p
INTRODUCTION
The yeast Saccharomyces cerevisiae is a widely used model organism to study cell biology, metabolism, cell cycle and signal transduction. The availability of different yeast mutants makes possible evaluation of the role for any gene in cell metabolism and adaptation to environmental changes (Longo 1999; Fabrizio and Longo 2003; Semchyshyn 2009; Lushchak 2006; 2010; 2011). Some studies have reported that bakery’s yeast, similarly to nematode Caenorhabditis elegans and fruit fly Drosophila melanogaster, is a good model system for development of anti-aging approaches. Life-extending effect of some extracts or purified compounds from plants, such as resveratrol from grape skin (Howitz et al. 2003), quercetin from oak bark (Belinha et al. 2007) and Rhodiola rosea extract (Bayliak and Lushchak 2011) was demonstrated for S. cerevisiae cells.
Rhodiola rosea is a well-known plant with adaptogenic properties widely used in folk medicine in Eastern Europe and Asia, particularly in Western Ukraine. The extract from R. rosea rhizome was reported to have numerous health benefits in humans and animals, including adaptogenic, antioxidant, cardioprotective, anticancer and anti-aging activities, stimulation of central nervous system and enhancement of work performance (Jafari et al. 2007; Wiegant et al. 2008; Panossian et al. 2010; Gospodaryov et al. 2013). Despite documentation of a variety of beneficial effects, molecular mechanisms of R. rosea action are not clear yet. Some authors consider effects of R. rosea on organisms are partly attributed to antioxidant properties of its phenolic compounds (Lee et al. 2004; Kim et al. 2006b; Schriner et al. 2009b). However these compounds are not only antioxidants, but may be suggested to also stimulate antioxidant system of organisms. These both properties are considered to be one of the mechanisms for lifespan extension along with enhancement of stress resistance (Boon-Niermeijer et al. 2000; Calcabrini et al. 2010; Mao et al. 2010). However, stress-protective effects of R. rosea on cell viability without activation of antioxidant defense were also demonstrated (Schriner et al. 2009a).
The beneficial effects of R. rosea extract depended on its concentration and physiological state of organisms. Being beneficial at low concentrations, R. rosea may become toxic at higher levels (Wiegant et al. 2009; Schriner et al. 2009b; Bayliak and Lushchak 2011; Gospodaryov et al. 2013). This phenomenon has been widely observed in toxicology and medicine, and was termed “hormesis” (Wiegant et al. 2008; Calabrese and Mattson 2011). In this respect, phytochemicals are considered as hormesis-providing compounds, that induce an adaptive stress response making organisms resistant not only to high (and normally harmful) doses of the same agent, but also against other stressors including oxidants, nutrients and heat (Son et al. 2008). In previous study we found that R. rosea rhizome extract at concentrations extending S. cerevisiae lifespan did not demonstrate protective effect against oxidative stress induced by H2O2 and significantly increased sensitivity of “young” exponentially growing cells to this oxidant (Bayliak and Lushchak 2011).
In animal studies, some mechanisms of protective activity of R. rosea have been identified. There were a regulation of chaperone biosynthesis (Panossian et al. 2009) and influence on insulin/IGF-1-like/daf-2 pathway by activating DAF-16, a FOXO-family transcription factor, that controls the synthesis of proteins involved in stress resistance and thereby declines the rate of aging in C. elegans (Wiegant et al. 2009). In studies performed on cell culture, R. rosea increased expression of heme-oxygenase-1 (HO-1), which can be activated via the antioxidant-responsive element (ARE) in response to oxidative challenge (Wiegant et al. 2008). In S. cerevisiae Ras2/Cyr1/cAMP/PKA and the Sch9 pathways are similar to that identified in worms (insulin /IGF-1-like/daf-2 pathway) and regulate stress resistance and longevity by modulating activity of transcription factors Msn2/Msn4, which induce the expression of similar proteins (heat shock proteins, catalase, Mn-SOD and other housekeeping proteins) (Fabrizio and Longo 2003; Lushchak 2010). Msn2/Msn4p proteins are considered to be components of general stress response activated under different stress conditions (Costa and Moradas-Ferreira 2001; Erkina et al. 2009). Another transcriptional factor, Yap1p, is a key player in S. cerevisiae resistance to oxidative stress and many cytotoxic agents (Lushchak 2010; 2011), and some plant polyphenols seems realize their action in yeast via Yap1p (Maeta et al. 2007; Escoté et al. 2012).
In the present study, we estimated concentration-dependent effects of aqueous extracts from R. rosea rhizome on nonspecific stress resistance of “young” exponentially growing S. cerevisiae cells, which are somewhat relevant to proliferating cells of metazoas, and lifespan of “old” stationary yeast cultures, which are considered as a model of ageing of postmitotic somatic cells of higher eukaryotes. The involvement of antioxidant enzymes and transcriptional factors Msn2/Msn4p and Yap1p in stress-protective action of R. rosea aqueous extract was also examined.
Materials and Methods
Strains and growth conditions
The S. cerevisiae strains used in this study were as it follows: YPH250 (MATa trp1-Δ1 his3-Δ200 lys2-801 leu2-Δ1 ade2-101 ura3-52) and YPH250 (Δyap1) (MATa trp1-Δ1 his3-Δ200 lys2-801 leu2-Δ1 ade2-101 ura3-52 yap1::HIS3) kindly provided by Dr. Y. Inoue (Kyoto University, Japan); W303-1A (MATa leu2-3,112 trp1-1 can1-100 ura3-1 ade2-1 his3-11,15) and Wmsn2msn4 (MATa leu2-3,112 trp1-1 can1-100 ura3-1 ade2-1 his3-11,15 msn2::HIS3 msn4::TRP1) kindly provided by Dr K. Küchler (Vienna University, Austria). Cells were grown at 28°C with shaking at 175 rpm in liquid medium containing 1% yeast extract, 2% peptone, 2% glucose (YPD). The starting cell concentration in medium was about 0.3×106 cells ml–1. Exponential-phase and stationary-phase cells were harvested after cultivation for 15 h and 72 h, respectively.
Preparation of R. rosea aqueous extracts and determination of salidroside concentration
Dried roots of R. rosea, collected at high altitudes in Ukrainian Carpathians, were used in this work. The rhizomes were comminuted to obtain particle fraction of 1–2 mm in size. The aqueous extraction was performed on the boiled water-bath in the ratio of 1:20 (comminuted dried rhizome material:distilled water) for 30 min. All liquid extracts were filtered, sterilized by boiling for 20 min, and kept at 4°C for 2 days. The content of salidroside in aqueous extracts and in dried rhizome material was estimated spectrophotometrically at 486 nm according to method approved by the Soviet Pharmacopoeia (National Pharmacopoeia of the USSR 1990). The content of salidroside in dried rhizomes of R. rosea was 17.7±0.5 mg/g, and in prepared aqueous extract from R. rosea rhizomes the concentration of salidroside was 0.080±0.009 mg/ml.
Stationary-phase survival
After 72 h of growth, the yeast cells were washed twice and then resuspended in sterile distilled water. The appropriate amounts of aqueous extracts from R. rosea root were added to cell suspensions, followed by incubation at regimen described above. The cells were washed twice every 2 days to remove all nutrients released by dead yeast, and resuspended in sterile water with addition of R. rosea preparations. To determine the number of viable yeast cells, starting at day 2 of incubation, every 2 days the aliquots were taken from each flask, serially diluted and plated. The viability was defined as the ability of an individual yeast cell to reproduce and form a colony within 72 h (colony forming unit, or CFU) on YPD agar plates (Fabrizio and Longo 2003). The amount of dead cells in yeast culture was measured by methylene blue staining (Smart et al. 1999).
Stress treatment
Exponential-phase cells were harvested and resuspended in fresh medium, and incubated with different concentrations of R. rosea aqueous extract during 2 h. Then the cells were harvested, washed and resuspended in equal volume of 50 mM potassium phosphate buffer (pH 7.0). Aliquots of the experimental cultures were exposed to different stressors during 1 h at 28°C. To test stress resistance in stationary-phase cells, aliquots were removed from experimental cultures incubated in water for 2 days, and challenged with hydrogen peroxide for 1 h at 28°C. Cell survival after stress exposure was monitored measuring a number of colony-forming units’ as described above.
Preparation of cell-free extracts, assay of enzyme activities, protein carbonyls, and protein concentration
The preparation of cells extracts, measurement of activities of superoxide dismutase (SOD) and catalase, and determination of the content of carbonyl groups in proteins were conducted as described earlier (Lushchak et al. 2005). Protein concentration was determined by the Coomassie brilliant blue G-250 dye-binding method (Bradford 1976) with bovine serum albumin as a standard.
Statistical analysis
Experimental data are expressed as the mean value of 4–6 independent experiments ± the standard error of the mean (SEM), and statistical analysis used Student’s t-test.
RESULTS AND DISCUSSION
Stationary-phase survival
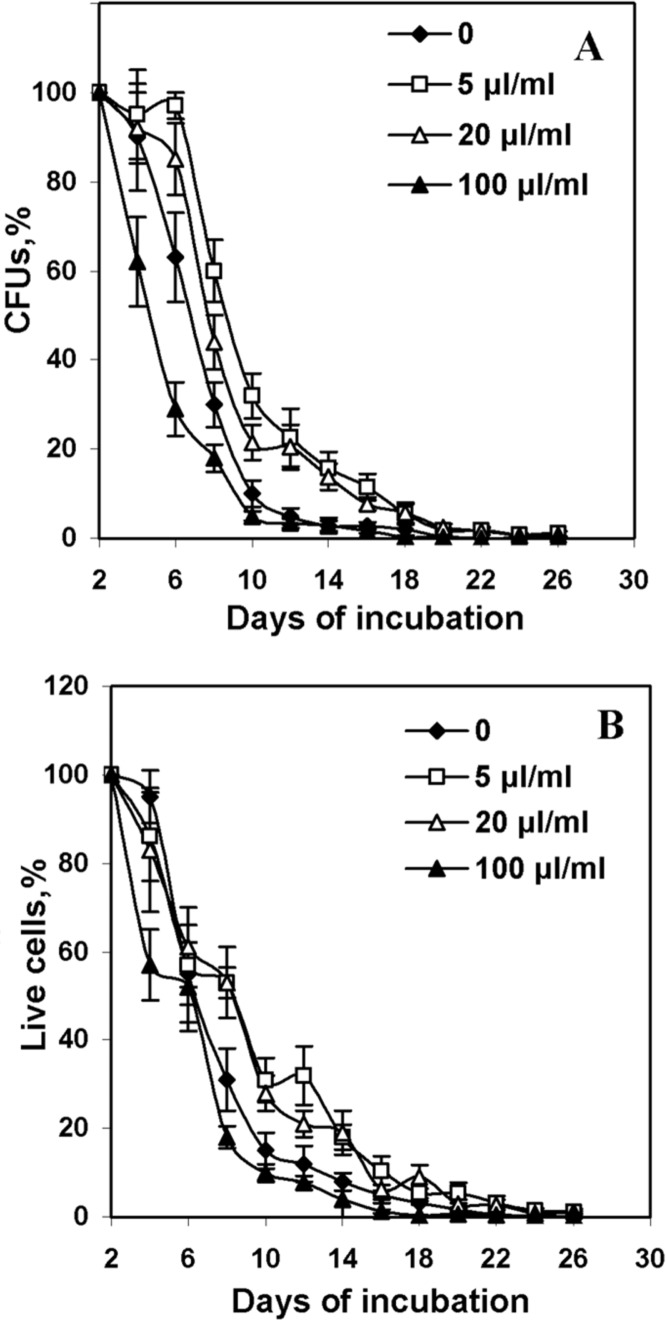
Recently we demonstrated that supplementation of YPD medium with aqueous R. rosea extract at the concentration of 20 μl/ml, which corresponded to the final concentration of salidroside of 16 μg/ml, increased viability and reproduction ability of yeast cells at stationary phase (Bayliak and Lushchak 2011). In YPD medium yeast cells are actively budding and produced large amount of young cells. So, the population contains every time a mixture of young and old cells. It makes difficult accurate estimation of maximal yeast lifespan. Growth would create a mixed population containing both young and old organisms, which would invalidate the survival studies (Fabrizio and Longo 2003). The incubation of yeast cells in water is another approach used to investigate chronological yeast lifespan. Yeasts incubated in water have low respiration, enter a non-dividing hypometabolic stationary phase in which organisms are highly resistant to multiple stresses and can survive for up to 3 months (Longo 1999; Fabrizio and Longo 2003). We used this approach, i.e. yeast incubation in distilled water, to determine the effect of R. rosea aqueous extract at different concentrations on lifespan of S. cerevisiae strain YPH250. During incubation, the cells were washed every 2 days to remove all nutrients released by dead yeasts and re-suspended them in sterile water. Incubation in water and the removal of nutrients released by dead organisms minimizes the chance of growth during long-term survival in stationary phase. Figure 1A demonstrates the effect of R. rosea extract on the yeast viability evaluated by monitoring cell ability to form colonies (colony forming units – CFUs) on complete medium. CFUs were monitored until 99.5% of the population died. The number of viable cells which could reproduce and form colonies decreased in cultures with and without R. rosea extracts over time. A concentration-dependent modulation of yeast reproductive capability by the extracts was observed. Low concentrations of roseroot extract (5 and 20 μl/ml) increased CFUs by 20–25% at days 6–12 and by 5–10% (P < 0.05) at days 14–18. Significant difference in yeast viability between experimental and control cultures was not observed after day 12. The highest concentration (100 μl/ml) of R. rosea preparation significantly decreased viability at first 8 days of incubation. Particularly, the amount of CFUs in cultures with 100 μl/ml of R. rosea extract was lower by 40% than in control cultures at day 6. At further cultivation, the percentage of cells which kept reproductive capability was similar in cultures with roseroot preparations and in control.
FIGURE 1.
Viability of S. cerevisiae YPH250 incubated in water at the presence of R. rosea aqueous extracts. Yeast viability was determined by counting the number of colony-forming units (CFUs) (A) and by staining of dead cells with methylene blue (B). CFU was calculated as the ratio between the numbers of colonies formed to the total number of cells plated. As 100% accepted number of viable cells at day 2. (n=4–6).
The yeast viability was also evaluated by counting dead cells after methylene blue staining. The age-dependent decrease in the amount of live cells in cultures was observed (Fig. 1B). The results were similar to those obtained by CFU method. In cultures supplemented with 5 and 20 μl/ml of R. rosea aqueous extract percentage of live cells was higher by 15–20% on days 8–14, than control ones. The fastest death of yeast cells was observed for the cultures incubated with 100 μl/ml R. rosea extract. Any of R. rosea extract concentrations did not influence maximal lifespan of yeast cultures. A strong positive correlation was found between CFUs and a number of alive cells (R2 = 0.952). It is consistent with previous studies reported that the loss of the ability to reproduction was closely related with yeast cell death, but not with loss of ability to produce new buds (Fabrizio and Longo 2003; Bayliak and Lushchak 2011). These results confirm that R. rosea aqueous extract affects long-term viability of yeast cells in concentration-dependent manner. Low concentrations of R. rosea extract prevented cell death and increased the reproductive potential in population of aged yeast cells, while high concentrations of R. rosea extract decreased cell viability. This is in the line with studies demonstrated the similar dose-dependent action of R. rosea extract on lifespan of fruit flies (Jafari et al. 2007; Schriner et al. 2009a; Gospodaryov et al. 2013) and nematodes (Wiegant et al. 2009). It was proposed that at low concentrations R. rosea may act as a mild stressor, inducing adaptive response, which leads to an increase in cellular resistance, and thereby increasing maintenance and extending yeast lifespan. Higher R.rosea extract concentrations may be toxic inducing yeast cell death.
Susceptibility to H2O2 of stationary-phase cells
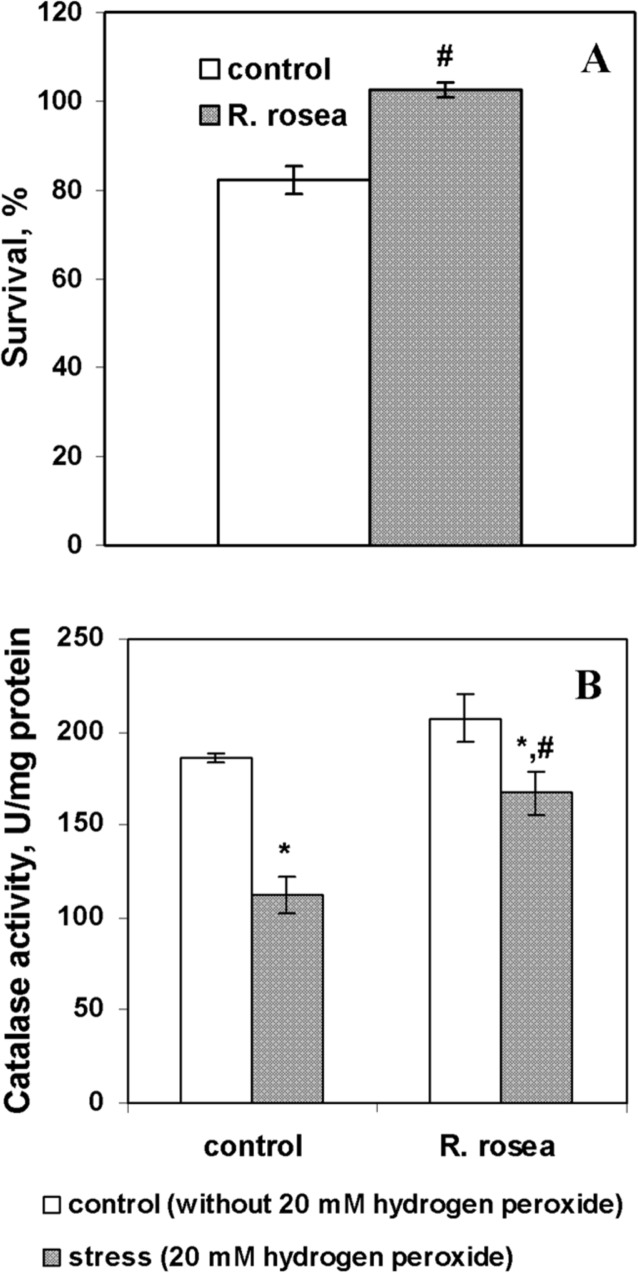
It is known, that yeast cells in stationary phase acquire resistance to different stresses, such as oxidative, heat and osmotic stresses, where the boost in antioxidant defense plays an important role (Lushchak 2010; Semchyshyn et al. 2011). It is supported by multiple reports demonstrating that starving yeast cells were capable to withstand additional environmental oxidative stress. For example, the expression of genes encoding antioxidant enzymes, superoxide dismutases and glutathione reductase, was increased when yeast cells at stationary stage were exposed to menadione, a compound producing superoxide anion at autoxidation (Cyrne et al. 2003). Catalase activity was also increased in starving cells upon treatment with low concentrations of hydrogen peroxide (Bayliak et al. 2005). The ability of R. rosea to increase resistance against different stresses in a variety of model systems was shown (Boon-Niermeijer et al. 2000; De Sanctis 2004; Wiegant et al. 2009; Calcabrini et al. 2010; Mao et al. 2010). Therefore, we addressed the question whether incubation with R. rosea preparations could influence an acquirement of stationary yeast cells to additional resistance to oxidative challenge with H2O2. The concentration of R. rosea extract (5 μl/ml) that showed a strong positive effect on yeast viability was chosen to check the ability to provide resistance to hydrogen peroxide. Cell survival upon treatment with 20 mM H2O2 decreased in control cultures by 20%, but was not changed in cells, preincubated with 5 μl/ml of R. rosea aqueous extract (Fig. 2A). The activity of catalase, a major H2O2-detoxifying enzyme in yeast, was similar in cells from control and roseroot-supplemented cultures (Fig. 2B). Exposure to 20 mM H2O2 decreased catalase activity in control cells by 40% and did not affect the enzyme activity in cells, incubated with R. rosea extract (Fig. 2B). The reduced catalase activity in control cells incubated with 20 mM H2O2 could result from oxidative inactivation of this enzyme as it was observed earlier in yeasts (Bayliak et al. 2006; Semchyshyn and Lozinska 2012) and bacteria (Semchyshyn et al. 2001). Hence, incubation with R. rosea extract at the concentration 5 μl/ml enhanced viability of stationary-phase yeast cells by possible prevention of oxidative inactivation of catalase under H2O2-induced stress. The strong positive relationship (R2 = 0.75) between catalase activity and yeast surviving under H2O2-induced stress was described by us earlier (Bayliak et al. 2006).
FIGURE 2.
Effect of exposure to 20 mM H2O2 for 1 h on cell survival (A) and catalase activity (B) in S. cerevisiae cells YPH250 incubated in water with 5 μl/ml of R. rosea aqueous extract during 2 days (n=4–6). * Significantly different from respective values of untreated cells (without H2O2) with P<0.05. #Significantly different from respective values in control cultures with P<0.05. (n=4–6).
Stress resistance of exponential-phase cells
In the experiments described above we observed that yeast incubation with 5 μl/ml of R. rosea aqueous extract enhanced the resistance of yeast cells in stationary phase to H2O2. Recently we found that yeast growth in the presence of R. rosea aqueous extract at concentration of 20 μl/ml resulted in sensitizing of exponential-phase yeast cells to H2O2 (Bayliak and Lushchak 2011). The difference in the results can reflect a concentration-dependent action of R. rosea observed above in yeast lifespan experiments. In addition, the difference in yeast physiological state at stationary and exponential culture phases could be important. To elucidate this situation, we examined the effect of pre-incubation with different concentrations of R. rosea aqueous extract on resistance of the exponentially growing S. cerevisiae cells to a number of stressors, which are known to have adverse effects on yeast viability. Table 1 presents the results of pre-incubation with R. rosea extract during 2 h on yeast survival under followed exposure to different stress factors: 10 mM H2O2, 2 mM Cu2+, 2 mM Cu2+/4 mM H2O2, 20% C2H5OH, 200 mM CH3COOH, and heat shock (40°C). It was shown that survival of S. cerevisiae YPH250 cells in control cultures without R. rosea extract was substantially reduced by treatment with these stressors. Pre-incubation with R. rosea extract at all used concentrations had no effect on yeast resistance to 20% of ethanol. In the case of hydrogen peroxide, effect of R. rosea extract was concentration-depended. Pre-treatment with 1 μl/ml of R. rosea extract resulted enhanced cell survival upon next exposure to 10 mM H2O2 by about 25% compared to viability of H2O2-treated control culture. In contrast, higher concentrations of R. rosea aqueous extract, 5 and 20 μl/ml, enhanced the sensitivity of cells to hydrogen peroxide and reduced survival to 9 and 17% from control viability, respectively. The preparation at 1 μl/ml concentration also enhanced survival of yeast cells under 2 mM Cu2+, 2 mM Cu2+/4 mM H2O2 and heat shock (40°C) treatments by 22, 13, and 16%, respectively. At higher concentrations of R. rosea extract, yeast cells showed virtually the same sensitivity to these stressors as the control cells. However, R. rosea extract at the highest concentration used in these experiments, 20 μl/ml, increased by 26% survival of yeast cell culture treated with 200 mM acetic acid while lower concentrations did not affect the resistance of yeast cells to the weak acid stress. Thus, these results demonstrate that R. rosea aqueous extract improves adaptive capability in S. cerevisisae, enhancing resistance to multiple stressors in dose-dependent manner. Similar results were shown in animal (Schriner et al. 2009a) and cell culture (Schriner et al. 2009b) studies reporting that R. rosea extract being beneficial at low doses, at high doses facilitated the toxic action of stressors.
TABLE 1.
Effect of different stressors on survival (%) of exponentially growing S. cerevisiae YPH250 (wt) and YPH250 (Δyap 1) cells after pre-incubation with R. rosea aqueous extracts.
| Concentration of R. rosea aqueous extract, μl/ml medium | ||||
|---|---|---|---|---|
|
| ||||
| Stressor | 0 | 1 | 5 | 20 |
| YPH250 | ||||
| 10 mM H2O2 | 61±7 | 81±7* | 39±7* | 39±6* |
| 2 mM Cu2+ | 27±6 | 49±9* | 28±7 | 22±6 |
| 2 mM Cu2+/4 mM H2O2 | 10±4 | 23±5* | 17±2 | 16±3 |
| 20% C2H5OH | 14±4 | 11±4 | 7±2 | 10±2 |
| 200 mM CH3COOH | 65±7 | 80±10 | 78±12 | 91±5* |
| Heat shock (40°C) | 54±5 | 70±7* | 66±6 | 57±9 |
| YPH250 (Δyap1) | ||||
| 10 mM H2O2 | 39±6 | 32±7 | 30±6 | NS |
| Heat shock (40°C) | 86±11 | 94±3 | 85±5 | NS |
Cells growing exponentially in YPD medium were pre-incubated with R. rosea aqueous extracts during 2 h, and then they were collected and resuspended in 50 mM K-phosphate buffer (pH 7.0) and exposed to different stressors for 1 h at 28°C, n=5–6.
Significantly different from respective control values with P<0.01 using Student’s t-test. Results are shown as means ± SEM (n = 4–6). NS – not studied.
The mechanisms of action of stressors used in this study are different, but they have one common feature. All of them are accompanied by development of oxidative stress, the situation, when the rate of reactive oxygen species (ROS) production exceeds the rate of their elimination by cellular defense mechanisms, leading to intensification of oxidation of biomolecules (Lushchak 2010; 2011). The rhizome of R. rosea contains a range of biologically active substances including several polyphenols, which were proposed to function as antioxidants (Panossian et al. 2010). In particular, it was shown, that R. rosea extract protected snail larvae against menadione (Boon-Niermeijer et al. 2000) and human erythrocytes against hypochlorous acid through antioxidant mechanism (De Sanctis et al. 2004). In order to determine whether in our experiments the positive effect of R. rosea extract at low concentrations on the stress resistance of S. cerevisiae was associated with the modulation of oxidative processes in yeast cells, we measured the activity of major antioxidant enzymes and the level of oxidized proteins. The content of carbonyl groups in proteins was taken as a marker of ROS-induced protein oxidation. Free radical oxidation of several amino acids, namely cysteine, histidine and some others, results in the formation of additional carbonyl groups in proteins. Due to many reasons, carbonyl groups are used commonly to evaluate the intensity of ROS attack to proteins (Stadtman and Levine 2000; Lushchak 2006; 2007). Figure 3 demonstrates the effects of pre-incubation with 1 μl/ml of R. rosea extract on the activities of SOD and catalase and level of carbonyl proteins in YPH250 cells treated with 10 mM H2O2 and heat shock (40°C). The pre-incubation itself did not influence neither activities of catalase and SOD, nor level of carbonyl proteins in yeast cells (Fig. 3A–C). At the same time, these parameters were different in the cells upon exposure to oxidative and heat stresses. The data presented in Fig. 3A–B show that exposure of YPH250 yeast cells to H2O2 decreased SOD and catalase activities by 50 % and 70% in control cells, respectively, but roseroot extract efficiently prevented these changes. Figure 3C demonstrates the results of carbonyl proteins measurement in cells treated with different stressors. Upon exposure to 10 mM H2O2, the content of carbonyl groups in proteins increased in cells from both, control and experimental, cultures. However, in cells pre-treated with R. rosea extract level of CP consisted of 58% of ones not preincubated with the extract.
FIGURE 3.
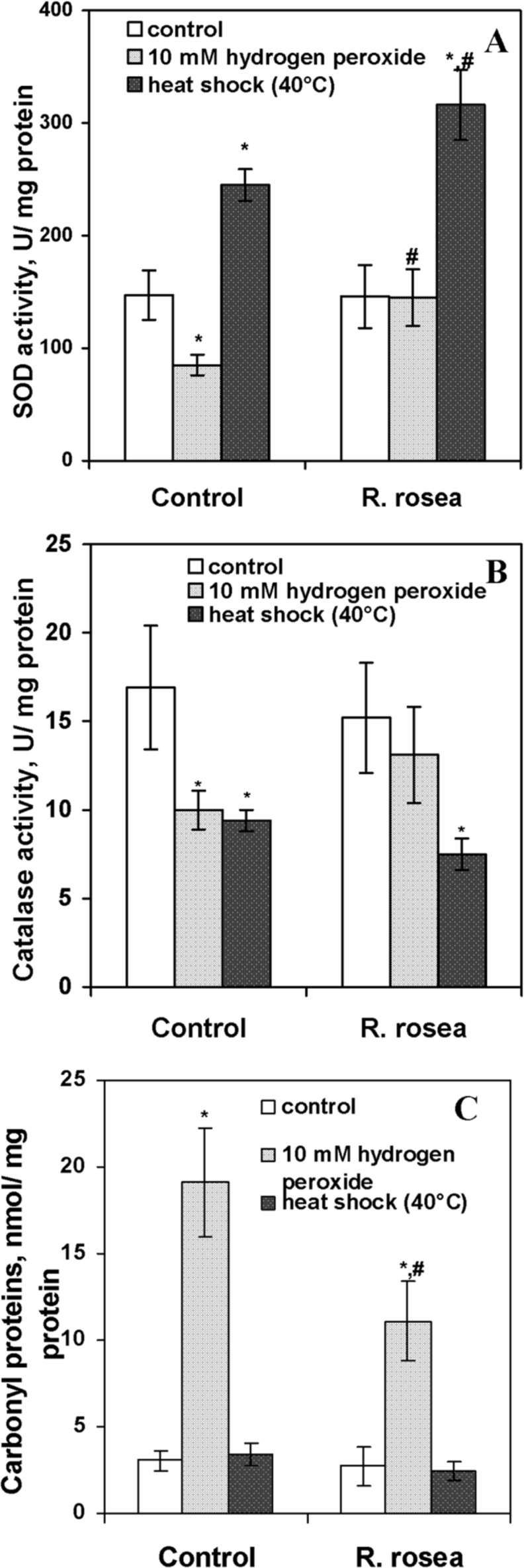
Effect of exposure by 10 mM H2O2 and heat shock for 1 h on SOD activity (A), catalase activity (B), and level of carbonyl proteins (C) in exponentially growing S. cerevisiae YPH250 cells after pre-incubation with R. rosea aqueous extracts during 2 h. *Significantly different from respective values of untreated cells (without H2O2) with P<0.05. #Significantly different from respective values in control cultures with P<0.05. (n=4–6).
In our case, hydrogen peroxide treatment was probably too rigorous for cells, leading to possible oxidative inactivation of sensitive enzymes, including antioxidant ones. Increase in ROS-induced protein oxidation is also confirmed by the elevated level of protein carbonyls. The possibility of inactivation of antioxidant enzymes due to oxidative modification was already shown in our previous works and in studies of other authors (Costa at al. 2002; Bayliak et al. 2006; Lushchak 2006, Semchyshyn and Lozinska 2012). In addition, lower activities of antioxidant enzymes may be due down-regulation of defense mechanisms under lethal levels of stressors. In the cells pre-incubated with R. rosea aqueous extract hydrogen peroxide did not affect activities of SOD and catalase and resulted in significantly lower CP level compared with control. In line, yeast viability was higher than that in control. These results suggested that R. rosea aqueous extract in low concentrations could enhance the resistance of exponentially growing yeast cells to hydrogen peroxide preventing inactivation of antioxidant enzymes and oxidation of proteins. The same situation might take place during the stationary phase. As it was noted above, the R. rosea aqueous extract at low concentrations enhanced survival of stationary-phase yeast cells and prevented inactivation of catalase upon hydrogen peroxide exposure.
In the case of heat shock resistance, the situation was different from H2O2 treatment. Heat stress increased SOD activity by 1.7-fold and 2.1-fold in control cells and cells pre-treated with R. rosea aqueous extract, respectively (Fig. 3A). Catalase activity decreased in similar manner in cells from both cultures under heat shock exposure (Fig. 3B). At the same time, no effect on carbonyl protein level in yeast cells was observed (Fig. 3C). Earlier it was found in S. cerevisiae increase in temperature elevated ROS production and antioxidant defense (Pereira et al. 2001; Kim et al. 2006a). Taking it into account, the increase in SOD activity in cells exposed to 40°C may be an adaptive response to increased ROS level, probably superoxide anion one. Superoxide anion at high concentrations may inactivate some enzymes, particularly catalase (Kono and Fridovich 1982). It may explain the lower activity of catalase in cells exposed to heat shock. It cannot be excluded that decrease in catalase activity could result also from down regulation. As in the case with hydrogen peroxide, R. rosea extract showed protective effect on yeast resistance against heat stress without activation of antioxidant defense. Similar results were shown in studies with animals (Schringer et al. 2009a) and cell cultures (Schringer et al. 2009b). Benefit to yeast cells can be conferred by antioxidant properties of R. rosea active compounds (Calcabrini et al. 2010; Panossian et al. 2010). However, yeast cells were pre-incubated with very low concentrations of aqueous R. rosea extract, and unlikely, that the cells accumulated the phytochemicals in amounts sufficient to scavenge H2O2 or products of its metabolisms at high concentrations. At the same time, there is emerging evidence suggesting that hormetic mechanisms of action may account for many of the health benefits of different phytochemicals (Mattson 2008; Calabrese and Mattson 2011). This explanation be applicable to our experiments, as it was mentioned above with lifespan experiments. In this case R. rosea extract at low concentrations acts as a mild stressor, which activates defense mechanisms, providing resistance to followed lethal stress. It can be supposed, that components of R. rosea preparation may stabilize DNA or interact with mediators of signaling pathways that up-regulate resistance/defense mechanisms.
Yap1 and Msn2/Msn4 proteins may modulate adaptogenic effect of R. rosea extract
In S. cerevisae, several signalling pathways were identified to play a role in regulation of stress resistance and yeast longevity. The transcriptional factors Msn2/4p and Yap1p are involved in these pathways. The synthesis of glutathione, heat shock and other stress proteins are under their control (Erkina et al. 2009; Lushchak 2010; 2011). To establish the involvement of these transcription regulators in stress-protective effects of R. rosea, we used yeast strains, defective for genes MSN2/4 and YAP1. The strain Δyap1 is isogenic derivative strain of YPH250, for which we have observed the positive effects of R. rosea described above. The sensitivity of Δyap1 mutant to hydrogen peroxide was higher, than parental strain YPH250 (Table 1). In addition, Δyap1 cells were more sensitive to hydrogen peroxide than to heat shock under selected conditions, which could be expected because protein Yap1 regulates yeast resistance to hydrogen peroxide (Lushchak 2010). Pre-incubation with R. rosea extract at low concentrations did not affect yeast resistance to oxidative and heat stresses.
The strain Δmsn2Δmsn4 was the derivative of wild-type S. cerevisae strain W303-1A. Since yeast strains with different genetic background may differ in stress resistance (Bayliak et al. 2006; Semchyshyn 2009), we also examined survival under stress exposure in parental W303-1A strain. The survival of W303-1A yeast cells, pre-treated with 1 and 5 μl/ml of R. rosea, did not differ from control upon heat shock (Table 2). In the case of exposure to hydrogen peroxide, W303-1A behaved similarly to YPH250 strain. At concentration of 1 μl/ml, R. rosea extract enhanced the resistance of yeast cells to 10 mM H2O2, but at higher concentration (5 μl/ml), R. rosea extract enhanced sensitivity of W303-1A cells to hydrogen peroxide. The Δmsn2Δmsn4 mutant, similarly to Δyap1 mutant, was more susceptible to hydrogen peroxide, than to heat shock (Table 2). Pre-treatment with R. rosea extract did not influence the sensitivity of Δmsn2Δmsn4 strain to 10 mM H2O2, but enhanced the resistance to heat shock.
TABLE 2.
Effect of hydrogen peroxide and heat shock on survival (%) of exponentially growing S. cerevisiae W303-1A (wt) and Wmsn2msn4 (Δmsn2Δ msn4) cells after pre-incubation with R. rosea aqueous extracts.
| Concentration of R. rosea aqueous extract, μl/ml medium
|
||||
|---|---|---|---|---|
| Stressor | Strain | 0 | 1 | 5 |
| 10 mM H2O2 | W303-1A (wt) | 83±1 | 95±5* | 49±10 |
| Wmsn2msn4 (Δmsn2Δ msn4) | 5±2 | 3±1 | 3±1 | |
| Heat shock (40°C) | W303-1A (wt) | 87±6 | 88±8 | 72±4 |
| Wmsn2msn4 (Δmsn2Δ msn4) | 57±12 | 85±8* | 76±12 | |
Cells growing exponentially in YPD medium were pre-incubated with R. rosea aqueous extracts during 2 h, and then they were collected by centrifugation and resuspended in 50 mM K-phosphate buffer (pH 7.0) and exposed to stresses for 1 h, n=5–6.
Significantly different from respective control values (without R. rosea extract) with P<0.01 using Student’s t-test. Results are shown as means ± SEM (n=5–6).
In general, the obtained results suggest that regulatory proteins, Yap 1 and Msn2/Msn4, are required for realization of complete protective action of R. rosea on yeast resistance at least to selected stressors. It may be supposed, that in our experiments R. rosea extract might act due to connection with Msn2/Msn4 and Yap1 transcription factors inducing synthesis of protective proteins, including primary antioxidant enzymes. Most likely, it is not direct interaction of components of R. rosea extract with Yap1, or with Msn2/4. This assumption is supported by reports which showed indirect activation of Yap1p by low concentrations of some plant compounds, such as resveratrol and green tea extract (Maeta et al. 2007; Escoté et al. 2012). In some studies, it was shown that R. rosea may exert its positive effects preventing depletion of glutathione (Calcabrini et al. 2010; Kim et al. 2006b). This situation may take place in our experiments, because both yeast transcriptional regulators activate expression of genes of glutathione biosynthesis. R. rosea components may also affect other pathways, in particular, biosynthesis of heat shock proteins, as it was observed in C. elegans (Panossian et al. 2009). This mechanism may explain the enhanced resistance of Δmsn2Δmsn4 cells, pre-incubated with R. rosea extract, to heat shock. Particularly, induction of heat shock proteins in S. cerevisiae is controlled predominantly by Hsf1p (heat shock factor)and can be additionally regulated by Yap1 and Msn2/4 (Costa and Moradas-Ferreira 2001). One may suggest also that the effects observed in this study might be associated somewhat with antioxidant properties of R. rosea compounds. Earlier it was shown, that the direct and indirect antioxidant action of salidroside, a marker compound of R. rosea rhizome, can partly protected fibroblasts from H2O2-induced premature senescence (Mao et al. 2010) and attenuated H2O2-induced cell damage by down-regulation of intracellular levels of Ca2+ and ROS via a cAMP-dependent pathway (Guan et al. 2011).
Taken together, our results suggest, that action of R. rosea on S. cerevisiae lifespan and stress resistance could involve hormetic mechanisms (Wiegant et al. 2008). That is because R. rosea extract only at low concentrations (1 and 5 μl/ml) boosted the resistance of yeast cells against different stresses and provided life-prolonging effect, whereas at high concentrations (20 μl/ml) it had adverse effects on yeast stress resistance. It seems that the stress-protective action of R. rosea is connected with involvement of transcription factors Msn2/Msn4 and Yap1. These findings suggest that a shift towards stress resistance may be due to changes in gene expression. At high concentrations, R. rosea extract sensitizes S. cerevisiae cells to stressors and shorts yeast lifespan. Nevertheless, the “beneficial” doses for “young” exponential-phase cells and “old” stationary-phase cells are different, clearly demonstrating importance of physiological state in R. rosea extract effects. These results support the utilization of yeast as a useful model to study in vivo molecular mechanisms responsible for putative health beneficial effects of natural phytochemicals.
Acknowledgments
The research was partially supported by a grant from the Ministry of Education, Science, Youth and Sports of Ukraine (#0109U001412) to V. Lushchak. We are grateful to Drs. Y. Inoue and K. Küchler for the providing S. cerevisiae strains, and Dr. D. Gospodaryov for critical reading of the manuscript.
REFERENCES
- Bayliak MM, Abrat OB, Semchyshyn HM, Lushchak VI. Survival and antioxidant defense of the yeast Saccharomyces cerevisiae during starvation and oxidative stress. Ukr Biokhim Zh. 2005;77:93–98. [PubMed] [Google Scholar]
- Bayliak M, Semchyshyn H, Lushchak V. Effect of hydrogen peroxide on antioxidant enzyme activities in Saccharomyces cerevisiae is strain-specific. Biochemistry (Moscow) 2006;71:1013–1020. doi: 10.1134/s0006297906090100. [DOI] [PubMed] [Google Scholar]
- Bayliak MM, Lushchak VI. The golden root, Rhodiola rosea, prolongs lifespan but decreases oxidative stress resistance in yeast Saccharomyces cerevisiae. Phytomedicine. 2011;18:1262–1268. doi: 10.1016/j.phymed.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Belinha I, Amorim MA, Rodrigues P, De Freitas V, Moradas-Ferreira P, Mateus N, Costa V. Quercetin increases oxidative stress resistance and longevity in Saccharomyces cerevisiae. J Agric Food Chem. 2007;55:2446–2451. doi: 10.1021/jf063302e. [DOI] [PubMed] [Google Scholar]
- Boon-Niermeijer EK, van den Berg A, Wikman G, Wiegant FAC. Phyto-adaptogens protect against environmental stress-induced death of embryos from the freshwater snail Lymnaea stagnalis. Phytomedicine. 2000;7:389–399. doi: 10.1016/S0944-7113(00)80060-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Mattson MP. Hormesis provides a generalized quantitative estimate of biological plasticity. J Cell Commun Signal. 2011;5:25–38. doi: 10.1007/s12079-011-0119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcabrini C, De Bellis R, Mancini U, Cucchiarini L, Potenza L, De Sanctis R, Patrone V, Scesa C, Dachà M. Rhodiola rosea ability to enrich cellular antioxidant defenses of cultured human keratinocytes. Arch Dermatol Res. 2010;302:191–200. doi: 10.1007/s00403-009-0985-z. [DOI] [PubMed] [Google Scholar]
- Costa VM, Amorim MA, Quintanilha A, Moradas-Ferreira P. Hydrogen peroxide-induced carbonylation of key metabolic enzymes in Saccharomyces cerevisiae: the involvement of the oxidative stress response regulators Yap1 and Skn7. Free Rad Biol Med. 2002;33:1507–1515. doi: 10.1016/s0891-5849(02)01086-9. [DOI] [PubMed] [Google Scholar]
- Costa VM, Moradas-Ferreira P. Oxidative stress and signal transduction in Saccharomyces cerevisiae: insights into aging, apoptosis and diseases. Mol Asp Med. 2001;22:217–246. doi: 10.1016/s0098-2997(01)00012-7. [DOI] [PubMed] [Google Scholar]
- Cyrne L, Martins L, Fernandes L, Marinho HS. Regulation of antioxidant enzymes gene expression in the yeast Saccharomyces cerevisiae during stationary phase. Free Radic Biol Med. 2003;34:385–393. doi: 10.1016/s0891-5849(02)01300-x. [DOI] [PubMed] [Google Scholar]
- De Sanctis R, De Bellis R, Scesa C, Mancini U, Cucchiarini L, Dacha M. In vitro protective effect of Rhodiola rosea extract against hypochlorous acid-induced oxidative damage in human erythrocytes. BioFactors. 2004;20:147–159. doi: 10.1002/biof.5520200304. [DOI] [PubMed] [Google Scholar]
- Erkina TI, Lavrova MV, Erkin AM. Alternative ways of stress regulation in cells of Saccharomyces cerevisiae: transcriptional activators Msn2 and Msn4. Tsitologiia (in Russian) 2009;51:271–278. [PubMed] [Google Scholar]
- Escoté X, Miranda M, Menoyo S, Rodríguez-Porrata B, Carmona-Gutiérrez D, Jungwirth H, Madeo F, Cordero RR, Mas A, Tinahones F, Clotet J, Vendrell J. Resveratrol induces antioxidant defense via transcription factor Yap1p. Yeast. 2012;29:251–263. doi: 10.1002/yea.2903. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Longo VD. The chronological life span of Saccharomyces cerevisiae. Aging Cell. 2003;2:73–81. doi: 10.1046/j.1474-9728.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- Gospodaryov DV, Yurkevych IS, Jafari M, Lushchak VI, Lushchak OV. Lifespan extension and delay of age-related functional decline caused by Rhodiola rosea depends on dietary macronutrient balance. Longevity & Healthspan. 2013;2:5. doi: 10.1186/2046-2395-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan S, Wang W, Lu J, Qian W, Huang G, Deng X, Wang X. Salidroside attenuates hydrogen peroxide-induced cell damage through a cAMP-dependent pathway. Molecules. 2011;16:3371–3379. doi: 10.3390/molecules16043371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang L, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Jafari M, Jeffrey S, Felgner JS, Bussel II, Hutchili T, Khodayari B, Rose MR, Vince-Cruz C, Mueller LD. Rhodiola: a promising anti-aging Chinese herb. Rejuvenation Res. 2007;10:587–602. doi: 10.1089/rej.2007.0560. [DOI] [PubMed] [Google Scholar]
- Kim IS, Moon HY, Yun HS, Jin I. Heat shock causes oxidative stress and induces a variety of cell rescue proteins in Saccharomyces cerevisiae KNU5377. J Microbiol. 2006a;44:492–501. [PubMed] [Google Scholar]
- Kim SH, Hyun SH, Choung SY. Antioxidative effects of Cinnamomi cassiae and Rhodiola rosea extracts in liver of diabetic mice. Biofactors. 2006b;26:209–219. doi: 10.1002/biof.5520260306. [DOI] [PubMed] [Google Scholar]
- Kono Y, Fridovich I. Superoxide radical inhibits catalase. J Biol Chem. 1982;227:5751–5754. [PubMed] [Google Scholar]
- Lee EJ, Kwon YI, Shetty K, Jang HD. Antioxidant activity of Rhodiola rosea extract on human low-density lipoprotein oxidation and DNA strand scission. Food Sci Biotechnol. 2004;13:814–820. [Google Scholar]
- Longo VD. Mutations in signal transduction proteins increase stress resistance and longevity in yeast, nematodes, fruit flies, and mammalian neuronal cells. Neurobiology of Aging. 1999;20:476–486. doi: 10.1016/s0197-4580(99)00089-5. [DOI] [PubMed] [Google Scholar]
- Lushchak V, Semchyshyn H, Mandryk S, Lushchak O. Possible role of superoxide dismutases in the yeast Saccharomyces cerevisiae under respiratory conditions. Arch Biochem Biophys. 2005;441:35–40. doi: 10.1016/j.abb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Lushchak VI. Budding yeast Saccharomyces cerevisiae as a model to study oxidative modification of proteins in eukaryotes. Acta Biochim Pol. 2006;53:679–684. [PubMed] [Google Scholar]
- Lushchak VI. Free radical oxidation of proteins and its relationship with functional state of organisms. Biochemistry (Moscow) 2007;72:809–27. doi: 10.1134/s0006297907080020. [DOI] [PubMed] [Google Scholar]
- Lushchak VI. Oxidative stress in yeast. Biochemistry (Moscow) 2010;75:281–296. doi: 10.1134/s0006297910030041. [DOI] [PubMed] [Google Scholar]
- Lushchak VI. Adaptive response to oxidative stress: bacteria, fungi, plants and animals. Comp Biochem Physiol C. Toxicol Pharmacol. 2011;153:175–190. doi: 10.1016/j.cbpc.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Maeta K, Nomura W, Takatsume Y, Izawa S, Inoue Y. Green tea polyphenols function as prooxidants to activate oxidative-stress-responsive transcription factors in yeasts. Appl Environ Microbiol. 2007;73:572–580. doi: 10.1128/AEM.01963-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G, Wang Y, Qiu Q, Deng H, Yuan L, Li R, Song D, Li YY, Li D, Wang Z. Salidroside protects human fibroblast cells from premature senescence induced by H2O2 partly through modulating oxidative status. Mech Ageing Dev. 2010;131:723–731. doi: 10.1016/j.mad.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Pharmacopoeia of the USSR Pharmacopoeia paper 75, Rhizome and roots of Rhodiola rosea. The USSR Ministry of Health Moscow Meditsina. (11th Ed) 1990;(2):364–366. [Google Scholar]
- Panossian A, Wikman G, Kaur P, Asea A. Adaptogens exert a stress-protective effect by modulation of expression of molecular chaperones. Phytomedicine. 2009;16:617–622. doi: 10.1016/j.phymed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Panossian A, Wikman G, Sarris J. Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine. 2010;17:481–493. doi: 10.1016/j.phymed.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Pereira MD, Eleutherio ECA, Panek AD. Acquisition of tolerance against oxidative damage in Saccharomyces cerevisiae. BMC Microbiology. 2001;1:11. doi: 10.1186/1471-2180-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriner SE, Abrahamyan A, Avanessian A, Bussel I, Maler S, Gazarian M, Holmbeck MA, Jafari M. Decreased mitochondrial superoxide levels and enhanced protection against paraquat in Drosophila melanogaster supplemented with Rhodiola rosea. Free Radical Research. 2009a;43:836–843. doi: 10.1080/10715760903089724. [DOI] [PubMed] [Google Scholar]
- Schriner SE, Avanesian A, Liu Y, Luesch H, Jafari M. Protection of human cultured cells against oxidative stress by Rhodiola rosea without activation of antioxidant defences. Free Radic Biol Med. 2009b;47:577–584. doi: 10.1016/j.freeradbiomed.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Semchyshyn HM, Bayliak MM, Lushshak VI. Starvation in yeast: biochemical aspects. In: Merkin TC, editor. Biology of starvation in human and other organisms. Nova Science Publishers, Inc; 2011. pp. 103–150. Chapter 2. [Google Scholar]
- Semchyshyn H. Hydrogen peroxide-induced response in E. coli and S. cerevisiae: different stages of the flow of the genetic information. CEJB. 2009;4:142–153. [Google Scholar]
- Semchyshyn HM, Dyljovyj MV, Klymenko AO, Lushchak VI. Effect of Escherichia coli cell desintegration on some biochemical properties of catalase. Ukr Biokhim Zh J. 2001;73:24–28. [PubMed] [Google Scholar]
- Semchyshyn HM, Lozinska LM. Fructose protects baker’s yeast against peroxide stress: potential role of catalase and superoxide dismutase. FEMS Yeast Res. 2012;12:761–773. doi: 10.1111/j.1567-1364.2012.00826.x. [DOI] [PubMed] [Google Scholar]
- Smart K, Chambers K, Lambert I, Jenkins C. Use of methylene violet staining procedures to determine yeast viability and vitality. J Am Soc Brew Chem. 1999;57:18–23. [Google Scholar]
- Son TG, Camandola S, Mattson MP. Hormetic dietary phytochemicals. Neuromolecular Med. 2008;10:236–246. doi: 10.1007/s12017-008-8037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman E, Levine R. Protein oxidation. Ann NY Acad Sci USA. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- Wiegant FAC, Limandjaia G, de Poot SAH, Vorontsova ON, Zenina TA, Langelaar-Makkinje M, Post JA, Wikman G. Plant adaptogens activate cellular adaptive mechanisms by causing mild damage. In: Lukyanova L, Takeda N, Singal PK, editors. Adaptation Biology and Medicine: Health Potentials. Vol. 5. New Delhi: Narosa Publishers; 2008. pp. 319–332. [Google Scholar]
- Wiegant FAC, Surinova S, Ytsma E, Langelaar-Makkinje M, Wikman G, Post JA. Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogerontology. 2009;10:27–42. doi: 10.1007/s10522-008-9151-9. [DOI] [PubMed] [Google Scholar]