Abstract
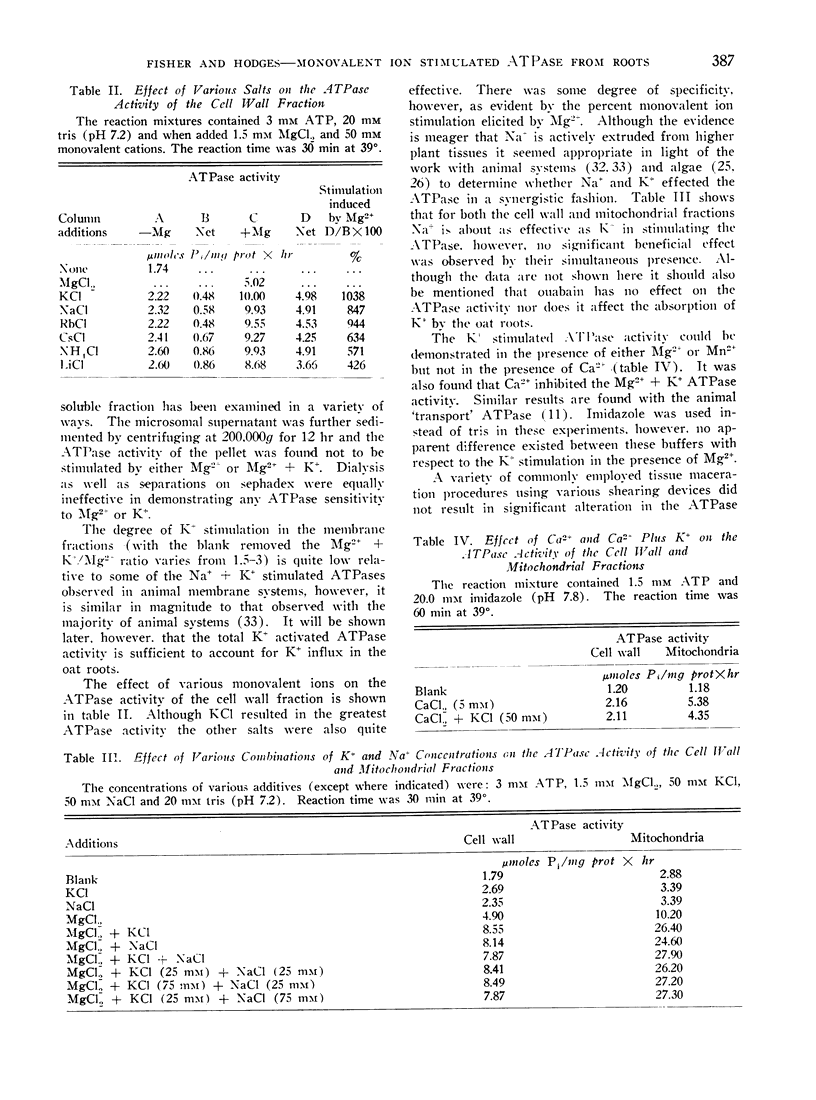
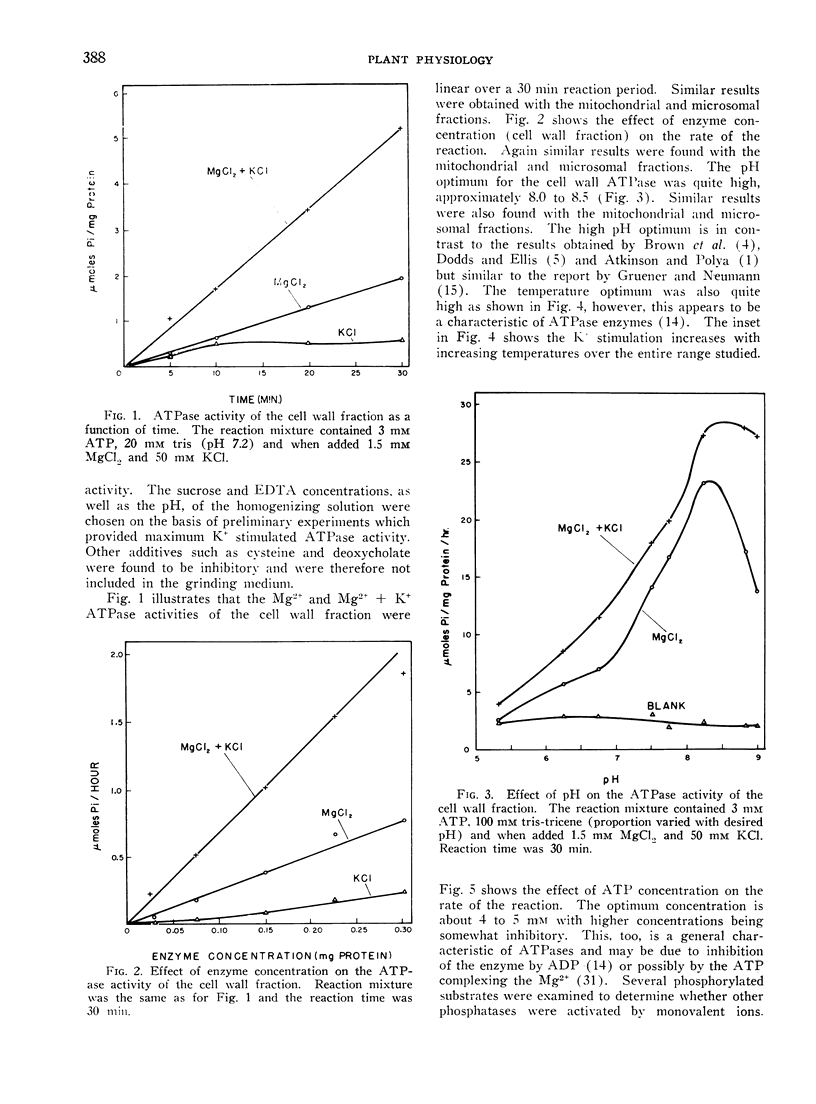
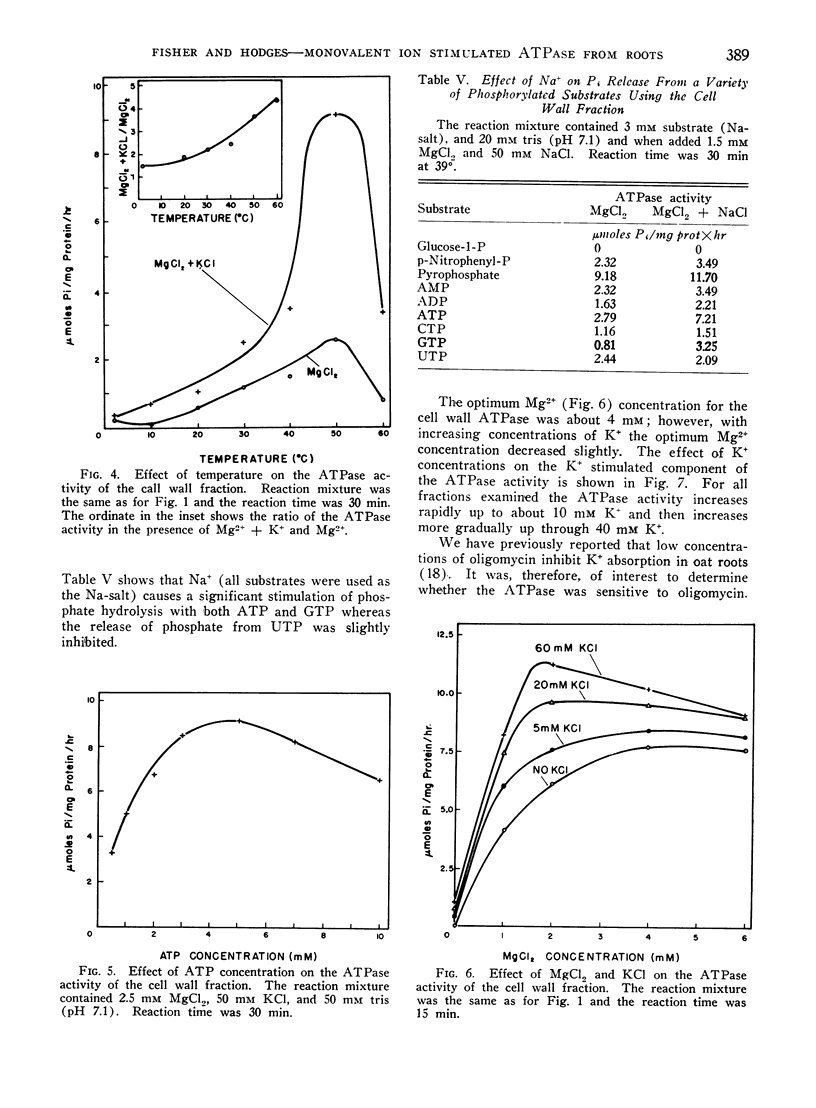
Monovalent ion stimulated ATPase activity from oat (Avena sativa) roots has been found to be associated with various membrane fractions (cell wall, mitochondrial and microsomal) of oat roots. The ATPase requires Mg2+ (or Mn+2) but is further stimulated by K+ and other monovalent ions. The monovalent ions are ineffective in the absence of the divalent activating cation. The ATPase has been described with respect to monovalent ion specificity, temperature, pH, substrate specificity, and Mg2+ and K+ concentrations. It was further shown that oligomycin inhibits a part of the total ATPase activity and on the basis of the oligomycin sensitivity it appears that at least 2 membrane associated ATPases are being measured. The mitochondrial fraction is most sensitive to oligomycin and the microsomal fraction is least sensitive to oligomycin. The oligomycin insensitive ATPase appears to be stimulated more by K+ than the oligomycin sensitive ATPase.
It was further shown that per gram fresh weight of roots, approximately 0.7 to 0.8 μmoles of K+ were absorbed per μmole of K+ stimulated ATP hydrolysis. This result was obtained for a variety of K+ concentrations and was taken to mean that sufficient membrane associated ATPase exists to account for K+ transport in the oat roots.
Full text
PDF

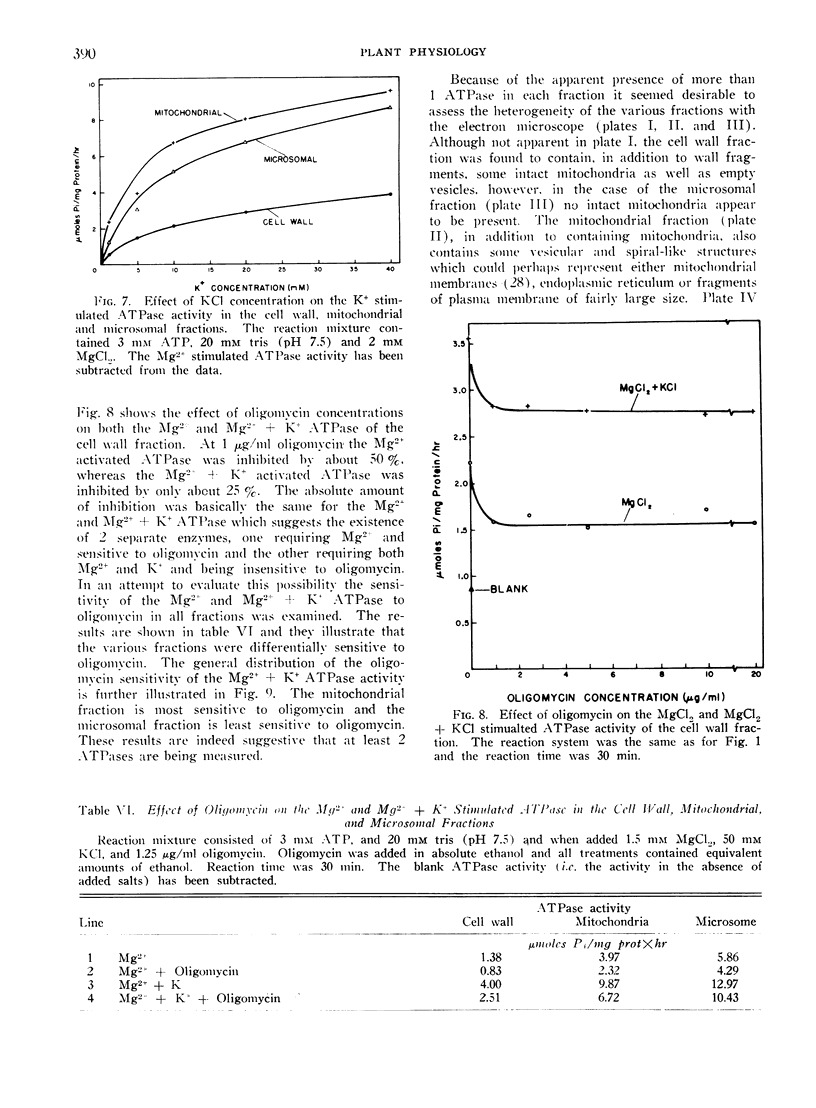
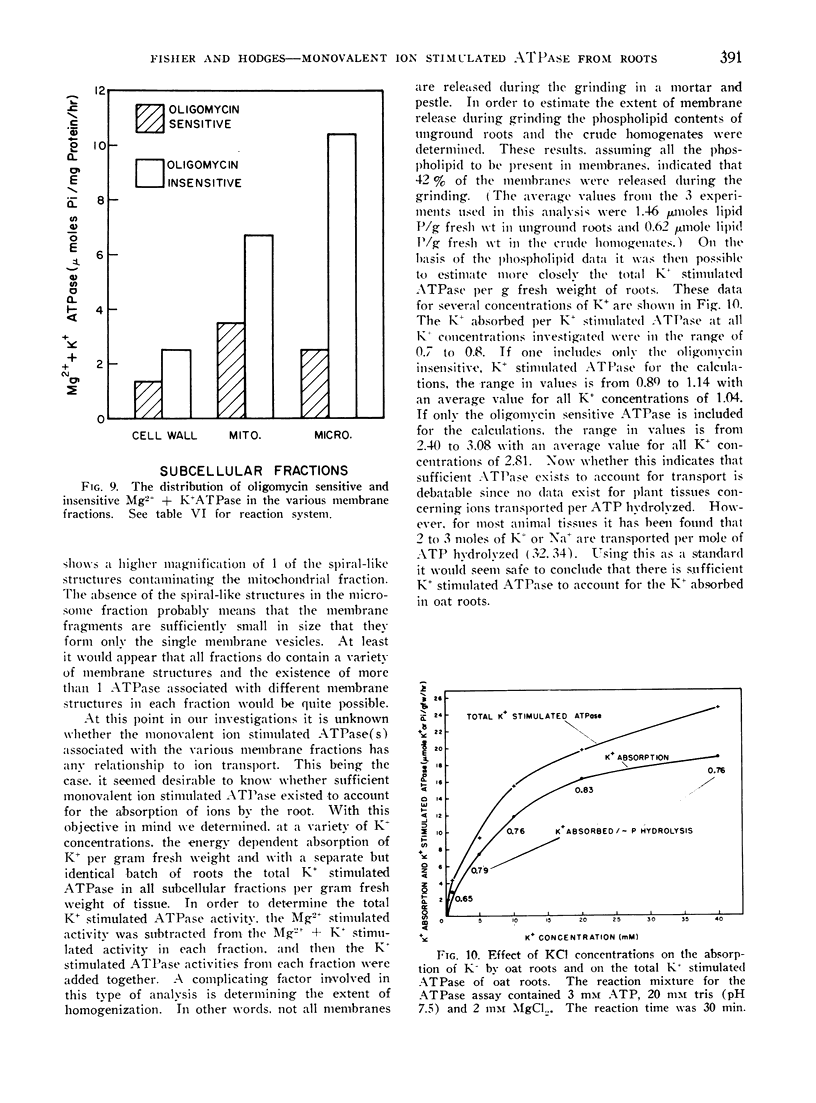

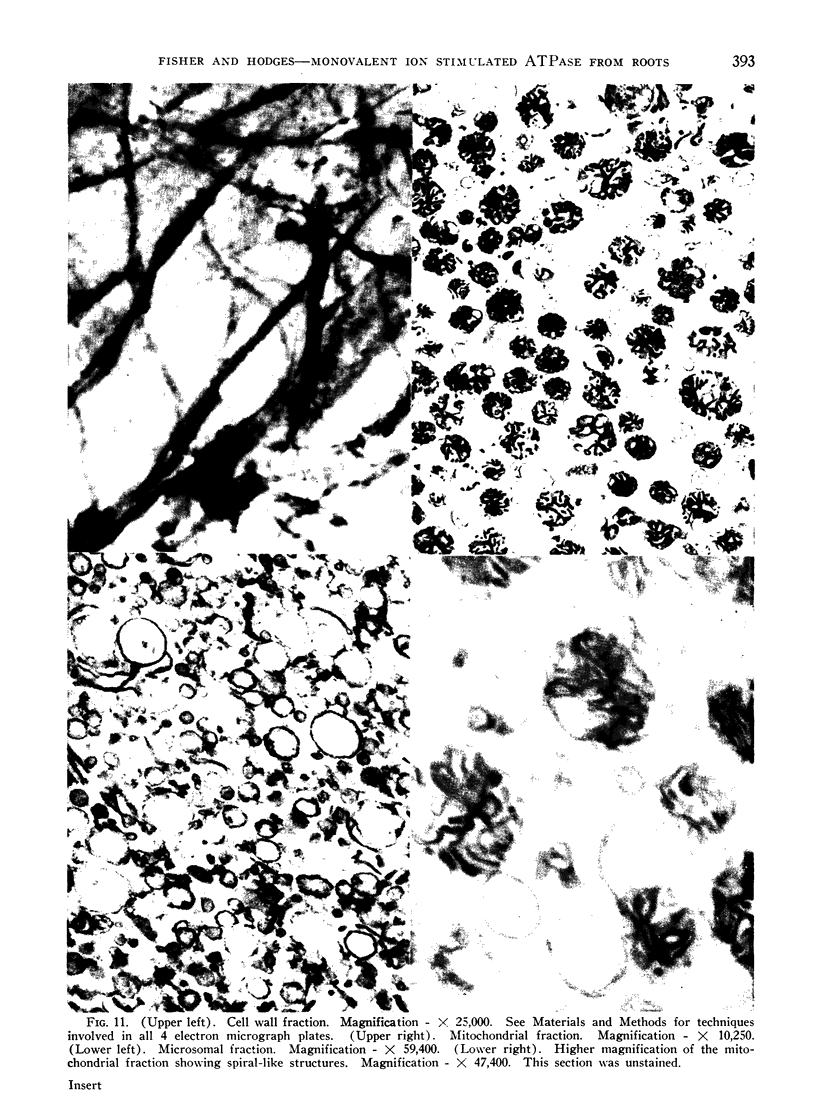

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BONTING S. L., CARAVAGGIO L. L. Studies on sodium-potassium-activated adenosinetriphosphatase. V. Correlation of enzyme activity with cation flux in six tissues. Arch Biochem Biophys. 1963 Apr;101:37–46. doi: 10.1016/0003-9861(63)90531-9. [DOI] [PubMed] [Google Scholar]
- Brown H. D., Altschul A. M. Glycoside-sensitive ATPase from Arachis hypogaea. Biochem Biophys Res Commun. 1964 Apr 22;15(5):479–483. doi: 10.1016/0006-291x(64)90490-5. [DOI] [PubMed] [Google Scholar]
- Brown H. D., Neucere N. J., Altschul A. M., Evans W. J. Activity patterns of purified ATPase from Arachis hypogaea. Life Sci. 1965 Jul;4(14):1439–1447. doi: 10.1016/0024-3205(65)90023-8. [DOI] [PubMed] [Google Scholar]
- Elzam O. E., Hodges T. K. Calcium inhibition of potassium absorption in corn roots. Plant Physiol. 1967 Nov;42(11):1483–1488. doi: 10.1104/pp.42.11.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzam O. E., Hodges T. K. Characterization of energy-dependent ca transport in maize mitochondria. Plant Physiol. 1968 Jul;43(7):1108–1114. doi: 10.1104/pp.43.7.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein F. H., Whittam R. The mode of inhibition by calcium of cell-membrane adenosine-triphosphatase activity. Biochem J. 1966 Apr;99(1):232–238. doi: 10.1042/bj0990232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBS R., RODDY P. M., TITUS E. PREPARATION, ASSAY, AND PROPERTIES OF AN NA+- AND K+-REQUIRING ADENOSINE TRIPHOSPHATASE FROM BEEF BRAIN. J Biol Chem. 1965 May;240:2181–2187. [PubMed] [Google Scholar]
- Hall J. R., Hodges T. K. Phosphorus metabolism of germinating oat seeds. Plant Physiol. 1966 Nov;41(9):1459–1464. doi: 10.1104/pp.41.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Elzam O. E. Effect of azide and oligomycin on the transport of calcium ions in corn mitochondria. Nature. 1967 Aug 26;215(5104):970–972. doi: 10.1038/215970a0. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Hanson J. B. Calcium Accumulation by Maize Mitochondria. Plant Physiol. 1965 Jan;40(1):101–109. doi: 10.1104/pp.40.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACROBBIE E. A. THE NATURE OF THE COUPLING BETWEEN LIGHT ENERGY AND ACTIVE ION TRANSPORT IN NITELLA TRANSLUCENS. Biochim Biophys Acta. 1965 Jan 25;94:64–73. doi: 10.1016/0926-6585(65)90008-7. [DOI] [PubMed] [Google Scholar]
- McClurkin I. T., McClurkin D. C. Cytochemical demonstration of a sodium-activated and a potassium-activated adenosine triphosphatase in loblolly pine seedling root tips. Plant Physiol. 1967 Aug;42(8):1103–1110. doi: 10.1104/pp.42.8.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezbindowski K. S., Ruzicka F. J., Sun F. F., Crane F. L. A double layer of protein in mitochondrial cristae. Biochem Biophys Res Commun. 1968 Apr 19;31(2):164–169. doi: 10.1016/0006-291x(68)90724-9. [DOI] [PubMed] [Google Scholar]
- SEN A. K., POST R. L. STOICHIOMETRY AND LOCALIZATION OF ADENOSINE TRIPHOSPHATE-DEPENDENT SODIUM AND POTASSIUM TRANSPORT IN THE ERYTHROCYTE. J Biol Chem. 1964 Jan;239:345–352. [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Schoner W., von Ilberg C., Kramer R., Seubert W. On the mechanism of Na+- and K+-stimulated hydrolysis of adenosine triphosphate. 1. Purification and properties of a Na+-and K+-activated ATPase from ox brain. Eur J Biochem. 1967 May;1(3):334–343. doi: 10.1007/978-3-662-25813-2_45. [DOI] [PubMed] [Google Scholar]
- Torii K., Laties G. G. Dual mechanisms of ion uptake in relation to vacuolation in corn roots. Plant Physiol. 1966 May;41(5):863–870. doi: 10.1104/pp.41.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]