Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) is a leading cause of hospital- and community-associated infections. The formation of adherent clusters of cells known as biofilms is an important virulence factor in MRSA pathogenesis. Previous studies showed that subminimal inhibitory (sub-MIC) concentrations of methicillin induce biofilm formation in the community-associated MRSA strain LAC. In this study we measured the ability sub-MIC concentrations of eight other β-lactam antibiotics and six non-β-lactam antibiotics to induce LAC biofilm. All eight β-lactam antibiotics, but none of the non-β-lactam antibiotics, induced LAC biofilm. The dose-response effects of the eight β-lactam antibiotics on LAC biofilm varied from biphasic and bimodal to near-linear. We also found that sub-MIC methicillin induced biofilm in 33 out of 39 additional MRSA clinical isolates, which also exhibited biphasic, bimodal and linear dose-response curves. The amount of biofilm formation induced by sub-MIC methicillin was inversely proportional to the susceptibility of each strain to methicillin. Our results demonstrate that induction of biofilm by sub-MIC antibiotics is a common phenotype among MRSA clinical strains and is specific for β-lactam antibiotics. These findings may have relevance to the use of β-lactam antibiotics in clinical and agricultural settings.
Keywords: antibiotic, bimodal, biofilm, biphasic, MRSA, Staphylococcus aureus, subminimal inhibitory
INTRODUCTION
Staphylococcus aureus is a major human pathogen that represents a growing public health burden in both hospital and community environments. The emergence of antibiotic-resistant clones such as methicillin-resistant S. aureus (MRSA) has contributed to the spread of this bacterium (Otto, 2012). In addition, S. aureus often forms matrix-encased biofilms on tissues and medical devices which confer additional antibiotic resistance and further complicate treatment (Kiedrowski and Horswill, 2011).
Previous studies showed that subminimal inhibitory (sub-MIC) concentrations of methicillin and other β-lactam antibiotics can induce biofilm formation in some MRSA strains (Kaplan et al., 2012). Antibiotic-induced biofilm formation was evidently dependent on the release of extracellular DNA (eDNA) since (i) the amount of eDNA in the biofilm matrix increased upon exposure to low-level methicillin, (ii) a strain carrying a mutation in the atl gene, which encodes the major S. aureus autolysin responsible for eDNA release, did not exhibit the biofilm induction phenotype, and (iii) the addition of exogenous DNase inhibited the biofilm induction phenotype (Kaplan et al., 2012). Antibiotic-induced biofilm formation may have clinical relevance because bacteria are exposed to low concentrations of antibiotics during the course of routine antibiotic chemotherapy (Craig, 1998; Odenholt, 2001). In addition, bacterial cells buried deep within a biofilm may be exposed to low levels of antibiotics due to diffusion gradients (Singh et al., 2010). The routine use of low-level antibiotics as growth promoters in agriculture may also expose bacteria to low levels of the drugs (Smith et al., 2002; Marshall and Levy, 2011).
The aim of the present study was to determine the specificity, prevalence and dose-response effects of low-level antibiotic-induced MRSA biofilm formation. In this report we present evidence that induction of biofilm by sub-MIC antibiotics is a common phenotype among MRSA clinical strains and is specific for β-lactam antibiotics. Our findings may shed light on the recalcitrance of some bacterial infections to antibiotic treatment in clinical settings and the evolution of antibiotic-resistant bacteria in agricultural settings.
MATERIALS AND METHODS
Antibiotics
Daptomycin powder was obtained from Cubist Pharmaceuticals, Inc. (Lexington, MA). All other antibiotics were purchased from Sigma-Aldrich (St. Louis, MO). Antibiotic stock solutions were prepared at a concentration of 10 mg/ml in sterile distilled water and then diluted in fresh broth as described below. MIC testing was carried out using the broth dilution method.
Bacterial strains
The 40 MRSA strains used in this study are listed in Table 1. A total of 27 strains were obtained from the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA). These strains (designated ‘NRS’) were isolated from patients in California, Connecticut, Georgia, Minnesota, New York, Oregon and Tennessee, and are representative of 11 different S. aureus PFGE (pulse field gel electrophoresis) types. An additional 12 strains (designated ‘GW’) were isolated from blood, wound and respiratory infections of patients at the George Washington University Hospital in Washington, DC. To characterize these strains, colonies that exhibited beta hemolysis on 5% sheep blood agar (BBL) were tested using a BactiStaph® latex agglutination assay (Remel # R21143) to distinguish S. aureus from coagulase negative staphylococci. Coagulase positive isolates were then tested for antibiotic susceptibility using the Vitek 2 system (# AST-GP67) that included cefoxitin which acts as a surrogate test for oxacillin. Strains that exhibited a MIC for cefoxitin that was ≥8 μg/ml were considered MRSA. No further genotyping was carried out on these 12 strains. S. aureus strain LAC, a well-characterized community-associated MRSA strain isolated from the Los Angeles County Jail (Miller et al., 2005), was obtained from Kenneth Bayles of the University of Nebraska Medical Center, Omaha, NE.
TABLE 1.
MRSA strains analyzed in this study.
| Strain | PFGE typea | Maximum biofilm induction in sub-MIC methicillin (A620 nm)b | Sensitivity to 0.7 μg/ml methicillin(ΔA450 nm)c | Methicillin concentration at maximum biofilm induction (μg/ml) |
|---|---|---|---|---|
| GW06 | n.d. | 21.0* | 0.09 | 3.9 |
| NRS751 | USA100 | 20.8* | 0.15 | 2.5 |
| GW05 | n.d. | 19.4* | 0.09 | 3.9 |
| NRS702 | USA300 | 18.8* | 0.11 | 3.2 |
| NRS716 | USA300 | 18.3* | 0.11 | 3.2 |
| NRS715 | USA600 | 18.1* | 0.04 | 6.0 |
| NRS745 | USA1000 | 18.1* | 0.09 | 3.5 |
| NRS739 | USA300 | 17.8* | 0.11 | 4.2 |
| NRS678 | USA500 | 17.3* | 0.07 | 4.3 |
| NRS685 | USA500 | 16.8* | 0.12 | 3.0 |
| NRS670 | USA100 | 16.6* | 0.12 | 2.5 |
| NRS645 | IBERIAN | 16.5* | 0.09 | 3.7 |
| LAC | USA300 | 16.2* | 0.09 | 2.5 |
| GW11 | n.d. | 16.2* | 0.04 | 2.8 |
| NRS123 | USA400 | 15.6* | 0.07 | 4.2 |
| NRS648 | USA600 | 15.5* | 0.20 | 1.0 |
| NRS686 | IBERIAN | 15.4* | 0.07 | 3.9 |
| GW07 | n.d. | 15.3* | 0.15 | 1.4 |
| NRS643 | USA300 | 15.1* | 0.10 | 3.5 |
| NRS734 | USA800 | 14.9* | 0.15 | 2.1 |
| NRS689 | USA700 | 14.7* | 0.09 | 6.0 |
| NRS732 | USA300 | 14.4* | 0.07 | 2.8 |
| GW10 | n.d. | 14.3* | 0.12 | 3.5 |
| NRS714 | USA800 | 14.3* | 0.07 | 3.7 |
| GW02 | n.d. | 12.8* | 0.10 | 6.0 |
| NRS662 | USA300 | 12.0* | 0.11 | 4.0 |
| GW03 | n.d. | 11.6* | 0.04 | 6.0 |
| NRS722 | USA200 | 11.1* | 0.11 | 3.3 |
| GW12 | n.d. | 11.0* | 0.11 | 5.6 |
| NRS484 | USA1100 | 8.2* | 0.02 | 6.3 |
| GW01 | n.d. | 6.4* | 0.06 | 5.3 |
| NRS717 | USA100 | 5.3* | 0.06 | 5.0 |
| NRS483 | USA1000 | 5.1* | 0.07 | 4.6 |
| NRS723 | USA100 | 4.4* | 0.09 | 5.7 |
| NRS651 | USA200 | 2.7 | 0.04 | 4.9 |
| GW09 | n.d. | 2.5 | 0.02 | 6.0 |
| GW08 | n.d. | 2.5 | 0.03 | 6.0 |
| GW04 | n.d. | 1.7 | 0.01 | 6.0 |
| NRS701 | USA200 | 1.6 | 0.07 | 5.0 |
| NRS100 | USA300 | 1.1 | 0.01 | 4.9 |
n.d., not determined.
Maximum A620 nm value in the crystal violet binding assay. *, significantly different from no antibiotic control (P < 0.05).
(A450 nm [no antibiotic]) – (A450 nm [0.7 μg/ml methicillin]).
Bacterial growth and biofilm assays
Bacterial inocula were prepared in fresh broth from 18-h-old agar colonies as previously described (Izano et al., 2008). Aliquots of inocula (180 μl each, ca. 105 to 106 CFU/ml) were transferred to the wells of a 96-well microtiter plate (catalog no. 353936; Falcon) containing 20 μl of antibiotic solution at a concentration equal to ten times the desired final concentration. The final concentrations tested in the biofilm assay ranged from 0 × to 1 × MIC. Plates were incubated at 37°C for 18 to 24 h. To quantitate bacterial growth, the absorbance of the broth was measured in a microplate spectrophotometer set to 450 nm. The sensitivity of each strain to sub-MIC methicillin (Table 1) was quantitated by measuring the difference in the absorbance values (A450 nm) of broth cultures supplemented with 0 or 0.7 μg/ml methicillin (< 0.1 × MIC). For quantitation of biofilm formation, wells were rinsed with water to remove loosely adherent cells and then stained for 1 min with 200 μl of Gram’s crystal violet. The wells were then rinsed with water and dried. The amount of biofilm biomass was quantitated by destaining the wells with 200 μl of 33% acetic acid and then measuring the absorbance of the crystal violet solution in a microplate spectrophotometer set at 620 nm. The maximum biofilm induction value for each strain (Table 1) was equal to the highest observed absorbance value (A620 nm) divided by the absorbance value at 0 μg/ml methicillin.
Statistics and reproducibility of results
All biofilm assays were carried out in duplicate or triplicate wells, which exhibited an average variation in absorbance values of <10%. All assays were repeated 2 to 5 times, and in all cases, the observed patterns of growth inhibition and biofilm induction were reproducible. The significance of differences between absorbance values was calculated using a Student’s t-test. Correlation was measured using a Pearson correlation coefficient.
RESULTS
The MRSA biofilm induction phenotype is specific for β-lactam antibiotics
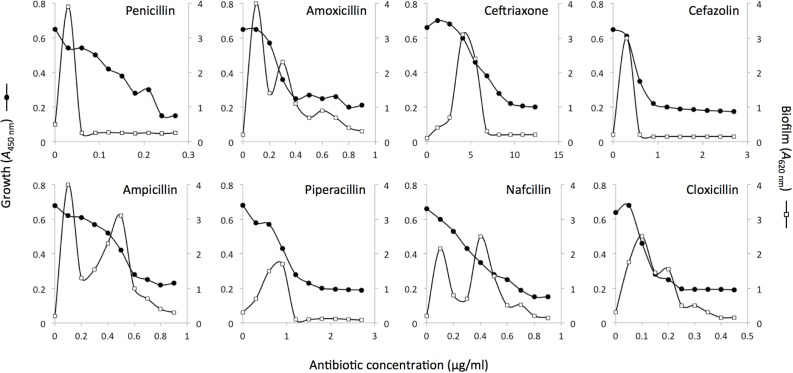
Previous studies showed that biofilm formation by MRSA strain LAC is strongly induced by sub-MIC methicillin (Kaplan et al., 2012). We tested whether sub-MIC concentrations of eight other β-lactam antibiotics also induced LAC biofilm. The β-lactam antibiotics we tested included six of the penicillin class (penicillin, amoxicillin, ampicillin, pipericillin, nafcillin and cloxicillin) and two of the cephalosporin class (ceftriaxone and cefazolin). Low-levels of all eight antibiotics significantly induced LAC biofilm (Figure 1). The amount of biofilm induction ranged from a 9- to 21-fold increase over background biofilm in the absence of antibiotic. The shapes of the dose-response curves for all eight antibiotics were biphasic, with four antibiotics (amoxicillin, ampicillin, nafcillin and cloxicillin) exhibiting a bimodal dose-response effect. Biofilm induction occurred over a wide range of sub-MIC concentrations ranging from <0.2 × MIC for penicillin and ampicillin to >0.5 × MIC for nafcillin and cloxicillin (Figure 1).
FIGURE 1.
Bacterial growth and biofilm formation by S. aureus MRSA strain LAC in the presence of sub-MIC concentrations of eight different β-lactam antibiotics. Bacterial growth (A490 nm) is indicated along the left-hand y axes, biofilm formation (A620 nm) is indicated along the right-hand y axes, and antibiotic concentration is indicated along the x axes. Values show mean absorbance values for duplicate wells. Error bars were omitted for clarity.
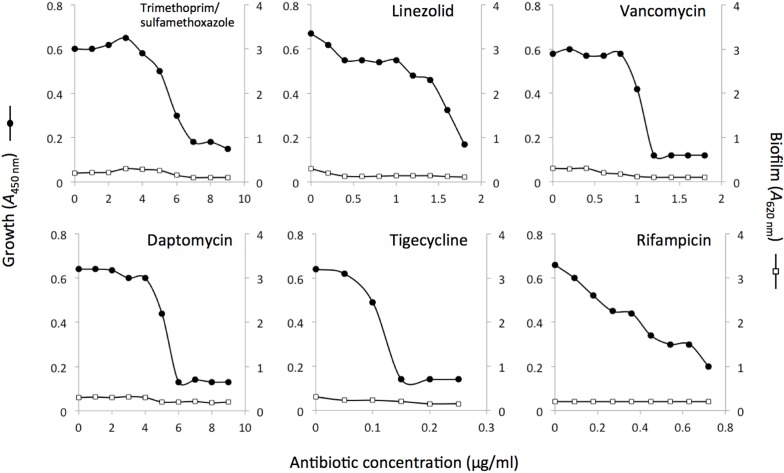
We also measured the ability of six non-β-lactam antibiotics to induce biofilm formation in strain LAC. The antibiotics we tested included trimethoprim/sulfamethoxazole, linezolid, vancomycin, daptomycin, tigecycline, and rifampicin. Strain LAC exhibited little or no biofilm induction when cultured in sub-MIC concentrations of these six antibiotics (Figure 2).
FIGURE 2.
Bacterial growth and biofilm formation by S. aureus MRSA strain LAC in the presence of sub-MIC concentrations of six different non-β-lactam antibiotics. Bacterial growth (A490 nm) is indicated along the left-hand y axes, biofilm formation (A620 nm) is indicated along the right-hand y axes, and antibiotic concentration is indicated along the x axes. Values show mean absorbance values for duplicate wells. Error bars were omitted for clarity.
Prevalence of biofilm induction among MRSA clinical isolates
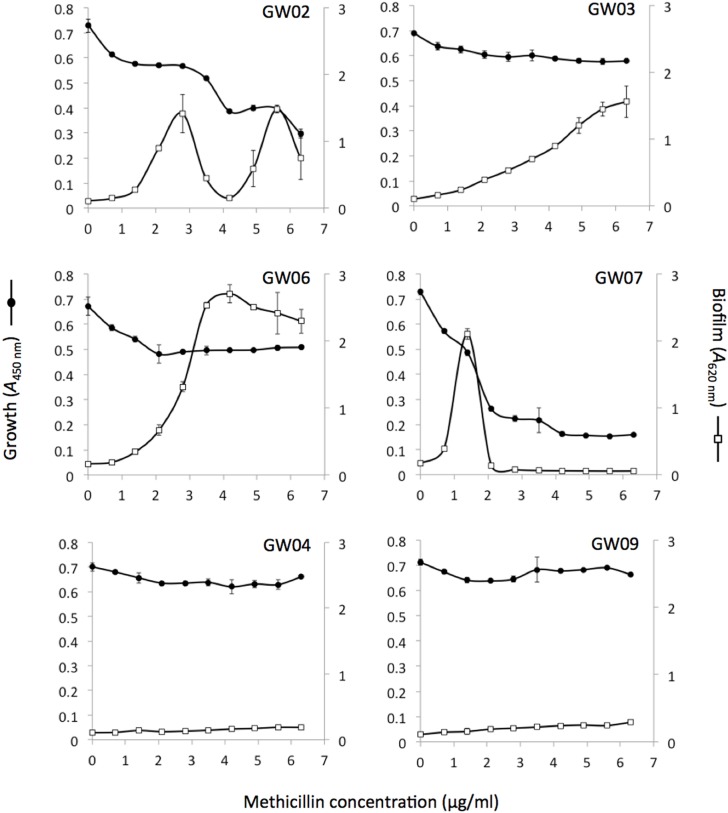
To determine the prevalence of the biofilm induction phenotype among MRSA clinical strains, we measured the ability of sub-MIC methicillin (0–6.3 μg/ml) to induce biofilm formation among 39 additional MRSA clinical isolates (Table 1). The MIC of methicillin against most of these strains was >8 μg/ml. A total of 33/39 strains (85%) exhibited significant biofilm induction at one or more sub-MIC concentrations of methicillin. Figure 3 shows examples of dose-response curves for four inducible strains (GW02, GW03, GW06, GW07) and two non-inducible strains (GW04, GW09). Inducible strains exhibited various patterns of dose-dependent biofilm formation including biphasic (strain GW07), bimodal (strain GW02) and near-linear (strain GW03). A total of four strains (GW02, GW12, NRS734 and NRS751) exhibited a bimodal dose-response effect.
FIGURE 3.
Bacterial growth and biofilm formation six clinical MRSA strains in the presence of sub-MIC concentrations of sub-MIC methicillin. Bacterial growth (A490 nm) is indicated along the left-hand y axes, biofilm formation (A620 nm) is indicated along the right-hand y axes, and antibiotic concentration is indicated along the x axes. Values show mean absorbance values for duplicate wells and error bars indicate range.
MRSA biofilm induction correlates with methicillin susceptibility
MRSA strains that were more sensitive to methicillin (e.g., strain GW07; Figure 3) appeared to exhibit biofilm induction at lower methicillin concentrations than strains that were more resistant to methicillin (e.g., strain GW03; Figure 3). To confirm this relationship, we graphed the sensitivity of each strain to methicillin against the methicillin concentration at which maximum biofilm induction occurred (Figure 4A). Methicillin sensitivity was quantitated by measuring the change in the absorbance of the broth caused by 0.7 μg/ml methicillin (< 0.1 × MIC). A significant negative correlation was observed (R2 = 0.517; P < 0.01). Similarly, a significant positive correlation was observed when methicillin sensitivity was graphed against the maximum amount of biofilm induction (R2 = 0.347; P < 0.05; Figure 4B).
FIGURE 4.
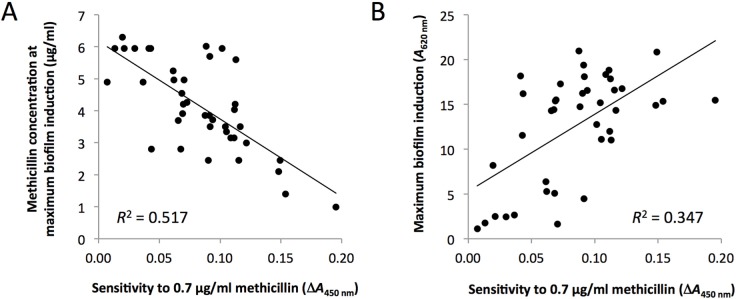
Relationship between methicillin sensitivity and biofilm induction among 39 MRSA clinical strains. (A) Relationship between methicillin sensitivity (x axis) and the methicillin concentration that induced maximum biofilm induction (y axis). (B) Relationship between methicillin sensitivity (x axis) and the maximum amount of biofilm induced by sub-MIC methicillin (y axis).
DISCUSSION
Several previous studies demonstrated that sub-MIC levels of β-lactam antibiotics can induce S. aureus biofilm formation (Haddadin et al., 2010; Mirani and Jamil, 2011; Subrt et al., 2011; Kaplan et al., 2012). One study demonstrated that S. aureus MRSA strains exhibit higher levels of biofilm induction than methicillin-sensitive S. aureus (MSSA) strains, which usually exhibit high levels of biofilm formation in the absence of antibiotics (Kaplan et al., 2012). In the present study we found that sub-MIC concentrations of β-lactam antibiotics, but not of non-β-lactam antibiotics, induced biofilm formation in the MRSA strain LAC. Strain LAC is a member of the USA300 clonal lineage that is commonly associated with community-acquired skin and soft tissue infections (Otto, 2010). The specificity of the biofilm induction phenotype for β-lactam antibiotics suggests that β-lactam antibiotics have the potential to act as signal molecules independent of their antimicrobial activity. Consistent with this hypothesis, vancomycin, which also inhibits bacterial cell wall biosynthesis, did not induce LAC biofilm when present at sub-MIC concentrations (Figure 2). Interestingly, previous studies have shown that resistance to β-lactam antibiotics predates the modern antibiotic era (Davies, 2006; Bernier and Surette, 2013; Sengupta et al., 2013), which also suggests that antibiotics and antibiotic resistance determinants have other roles in nature.
The amount of biofilm induction exhibited by strain LAC in response to sub-MIC concentrations of eight different β-lactam antibiotics (Figure 1) was 9- to 21-fold, consistent with the amount of biofilm induction exhibited by strain LAC in response to sub-MIC methicillin, and by strain FPR3757, another USA300 strain, in response to sub-MIC methicillin and ampicillin (Kaplan et al., 2012). All of the β-lactam antibiotics tested in the present study exhibited a biphasic dose-response curve, with low concentrations inducing biofilm and higher concentrations inhibiting biofilm. Some β-lactam antibiotics (amoxicillin, ampicillin, nafcillin and cloxicillin) exhibited a bimodal dose-response effect on LAC biofilm (Figure 1), consistent with the bimodal dose-response effects of methicillin on other MRSA strains (Kaplan et al., 2012). Previous studies showed that the bimodal dose-response curve might be a superposition of two biphasic dose-effect curves because DNase I was able to inhibit the first peak of biofilm formation but not the second peak in several MRSA and MSSA strains (Kaplan et al., 2012). Since biofilm induction usually occurs over a narrow range of antibiotic concentrations, more doses will need to be tested in order to determine whether the bimodal biofilm induction response in common for all β-lactam antibiotics.
β-lactam antibiotics are among the most common antibiotics used for the treatment of severe infections (Talbot, 2013). As a general rule, most patients who receive empiric antibiotics for sepsis prior to identification of the offending organism receive a β-lactam antibiotic such as piperacillin/tazobactam to kill Gram-negative bacteria, and vancomycin to kill MRSA. It is possible that concomitant β-lactam antibiotics induce MRSA biofilm formation and reduce the effectiveness of vancomycin in some patients.
Transmission of MRSA from livestock to humans has recently been confirmed using whole genome sequencing combined with single nucleotide polymorphism analysis (Harrison et al., 2013). These findings highlight the role of farm animals as a reservoir for MRSA. It is tempting to speculate that administering low-level penicillin to farm animals may facilitate the emergence of multidrug-resistant bacteria by means of eDNA release and biofilm formation.
Acknowledgments
We thank Bradley Katz of Cubist Pharmaceuticals (Lexington, MA) for providing daptomycin, and the Network on Antimicrobial Resistance in Staphylococcus aureus (NIH) and Kenneth Bayles (University of Nebraska Medical Center, Omaha, NE) for providing S. aureus strains. This work was supported by funds from the American University College of Arts and Sciences.
REFERENCES
- Bernier SP, Surette MG. Concentration-dependent activity of antibiotics in natural environments. Front Microbiol. 2013;4:20. doi: 10.3389/fmicb.2013.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antimicrobial dosing of mice and men. Clin Infect Dis. 1998;26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- Davies J. Are antibiotics naturally antibiotics? J Ind Microbiol Biotechnol. 2006;33:496–499. doi: 10.1007/s10295-006-0112-5. [DOI] [PubMed] [Google Scholar]
- Haddadin RN, Saleh S, Al-Adham IS, Buultjens TE, Collier PJ. The effect of subminimal inhibitory concentrations of antibiotics on virulence factors expressed by Staphylococcus aureus biofilms. J Appl Microbiol. 2010;108:1281–1291. doi: 10.1111/j.1365-2672.2009.04529.x. [DOI] [PubMed] [Google Scholar]
- Harrison EM, Paterson GK, Holden MT, Larsen J, Stegger M, Larsen AR, Petersen A, Skov RL, Christensen JM, Bak Zeuthen A, Heltberg O, Harris SR, Zadoks RN, Parkhill J, Peacock SJ, Holmes MA. Whole genome sequencing identifies zoonotic transmission of MRSA isolates with the novel mecA homologue mecC. EMBO Mol Med. 2013;5:509–515. doi: 10.1002/emmm.201202413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izano EA, Amarante MA, Kher WB, Kaplan JB. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl Environ Microbiol. 2008;74:470–476. doi: 10.1128/AEM.02073-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JB, Izano EA, Gopal P, Karwacki MT, Kim S, Bose JL, Bayles KW, Horswill AR. Low levels of β-lactam antibiotics induce extracellular DNA release and biofilm formation in Staphylococcus aureus. mBio. 2012;3:e00198–12. doi: 10.1128/mBio.00198-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiedrowski MR, Horswill AR. New approaches for treating staphylococcal biofilm infections. Ann N Y Acad Sci. 2011;1241:104–121. doi: 10.1111/j.1749-6632.2011.06281.x. [DOI] [PubMed] [Google Scholar]
- Marshall BM, Levy SB. Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev. 2011;24:718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LG, Perdreau-Remington F, Rieg G, Mehdi S, Perlroth J, Bayer AS, Tang AW, Phung TO, Spellberg B. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–1453. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- Mirani ZA, Jamil N. Effect of sub-lethal doses of vancomycin and oxacillin on biofilm formation by vancomycin intermediate resistant Staphylococcus aureus. J Basic Microbiol. 2011;51:191–195. doi: 10.1002/jobm.201000221. [DOI] [PubMed] [Google Scholar]
- Odenholt I. Pharmacodynamic effects of subinhibitory antibiotic concentrations. Int J Antimicrob Agents. 2011;17:1–8. doi: 10.1016/s0924-8579(00)00243-0. [DOI] [PubMed] [Google Scholar]
- Otto M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu Rev Microbiol. 2010;64:143–162. doi: 10.1146/annurev.micro.112408.134309. [DOI] [PubMed] [Google Scholar]
- Otto M. MRSA virulence and spread. Cell Microbiol. 2012;14:1513–1521. doi: 10.1111/j.1462-5822.2012.01832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Chattopadhyay MK, Grossart H-P. The multifaceted roles of antibiotics and antibiotic resistance in nature. Front Microbiol. 2013;4:47. doi: 10.3389/fmicb.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Ray P, Das A, Sharma M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Antimicrob Chemother. 2010;65:1955–1958. doi: 10.1093/jac/dkq257. [DOI] [PubMed] [Google Scholar]
- Smith DL, Harris AD, Johnson JA, Silbergeld EK, Morris JG., Jr Animal antibiotic use has an early but important impact on the emergence of antibiotic resistance in human commensal bacteria. Proc Nat Acad Sci U S A. 2002;99:6434–6439. doi: 10.1073/pnas.082188899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subrt N, Mesak LR, Davies J. Modulation of virulence gene expression by cell wall active antibiotics in Staphylococcus aureus. J Antimicrob Chemother. 2011;66:979–984. doi: 10.1093/jac/dkr043. [DOI] [PubMed] [Google Scholar]
- Talbot GH. β-lactam antimicrobials: what have you done for me lately? Ann N Y Acad Sci. 2013;1277:76–83. doi: 10.1111/j.1749-6632.2012.06809.x. [DOI] [PubMed] [Google Scholar]