Abstract
Rationale: Clinical practice guidelines recommend spirometry to diagnose chronic obstructive pulmonary disease (COPD) and facilitate management. National trends in spirometry use in older adults with newly diagnosed COPD are not known.
Objectives: To examine the rate and beneficiary characteristics associated with spirometry use in subjects with newly diagnosed COPD between 1999 and 2008.
Methods: We examined newly diagnosed beneficiaries with COPD using a 5% Medicare population from 1999 to 2008. A new COPD diagnosis required two outpatient visits or one hospitalization with primary International Classification of Diseases, 9th edition code 491.xx, 492.xx, or 496 occurring at least 30 days apart with none in the prior 12 months. The primary measurement was spirometry performed within 365 days (±) of the first claim with a COPD diagnosis.
Measurements and Main Results: Between 1999 and 2008, 64,985 subjects were newly diagnosed with COPD. Of these, 35,739 (55%) had spirometry performed within 1 year before or after the initial diagnosis of COPD. Spirometry use increased from 51.3% in 1999 to 58.3% in 2008 (P < 0.001). Subjects with younger age, men, whites, those with higher socioeconomic status, and those with a greater number of comorbidities were more likely to have spirometry. In a multivariable analysis, compared with 1999, subjects diagnosed in 2008 had 10% higher odds (odds ratio, 1.10; 95% confidence interval, 1.06–1.13) of having spirometry performed.
Conclusions: Despite an increase in the use of spirometry over time in newly diagnosed older adults with COPD, spirometry use remains low. Clinical practice guidelines and educational efforts should focus on increasing the use of spirometry to diagnose and manage COPD.
Keywords: spirometry, chronic obstructive pulmonary disease, Medicare, older adults, pulmonary function test
Chronic obstructive pulmonary disease (COPD) affects 14.8 million people in the United States. Currently the third leading cause of death, COPD is the only one of the top five causes of morbidity and mortality that continues to increase in prevalence and mortality (1).
By definition, irreversible airflow obstruction must be present to make a diagnosis of COPD; determining this requires objective spirometry assessment. Diagnosis based on physician’s subjective assessment yields less than 50% diagnostic accuracy for COPD and occurs after significant loss of lung function (2–5). Yet, 76% of U.S. primary care physicians (PCPs) surveyed use subjective clinical data alone to diagnose or manage COPD (6). Although the prevalence of symptoms is high among smokers with airway obstruction, they are not sensitive, specific, or predictive (5). Thus, COPD is often both overdiagnosed and underdiagnosed. COPD is also misclassified, especially in the elderly, because many common comorbidities may cause similar symptoms to those caused by COPD (1, 7). Spirometry can be easily performed in older adults and remains a useful tool to diagnose and manage COPD, even in this population.
To improve COPD care, international efforts through the Global Initiative for Chronic Obstructive Lung Disease (GOLD) and such groups as the COPD Alliance have developed clinical practice guidelines over the last decade (8, 9). These clinical practice guidelines, which initially recommended spirometry, now require spirometry to diagnose COPD in persons more than 40 years of age with symptoms (chronic cough, breathlessness, sputum production, frequent exacerbations of bronchitis) and a history of exposure to risk factors, especially tobacco smoke, occupational dusts, home cooking, and biomass fuels (8–12). Spirometry data further classify severity of obstruction into stages to discern prognosis and to guide therapy.
Using a 5% national sample of fee-for-service Medicare beneficiaries, we examined national trends in the use of spirometry in older adults with newly diagnosed COPD between 1999 and 2008. We hypothesize that, since the initial publication of GOLD guidelines in 2001, the use of spirometry will increase over the study period. We also looked for variations in rates of spirometry use by age, sex, race, or number of comorbidities.
Methods
Data Source
This is a retrospective study of spirometry use in newly diagnosed subjects with COPD using a 5% Medicare beneficiary population between 1999 and 2008. More than 98% of adults in the United States aged 65 years and older are enrolled in Medicare, which serves more than 45 million beneficiaries. The Centers for Medicare and Medicaid Services (CMS) select a random sample of 5% Medicare beneficiaries based on the eighth and ninth digits (05, 20, 45, 70, 95) of their health insurance claim number, and this sample has been shown to be representative of the whole cohort (13).
Data from multiple files were used for this study: (1) Denominator File (Medicare enrollment information and demographic data), (2) Medicare Provider Analysis and Review file (claims for hospital inpatient and skilled nursing facility stays), (3) Outpatient Standard Analytic File (hospital outpatient services), and (4) 100% Physician/Supplier File (physician and other medical services) (13).
Study Cohort
A separate denominator file of beneficiaries with COPD was created for each calendar year (1999–2008). Each file was composed of participants in the year of interest who: (1) had a diagnosis of COPD in 1999 to 2008, (2) was aged 67 years or older at the time of diagnosis, (3) had no COPD diagnosis during the 365 days before the index date, (4) survived more than 180 days after initial COPD diagnosis, (5) had complete enrollment (Part A, Part B) in the 2 years before and the year after the index date or until death or December 2009, (6) were not enrolled in a health maintenance organization [HMO]), and (7) were not a resident of a nursing facility in the 2 years before the initial diagnosis.
A patient met the diagnosis of COPD who had any of the following: (1) at least two outpatient or consultation visits (Evaluation and Management codes 99201–99205, 99211–99215, or 99241–99245) with an encounter diagnosis of COPD (International Classification of Diseases, 9th revision [ICD-9] codes: 491.x [chronic bronchitis], 492.x [emphysema], or 496 [chronic airway obstruction]) at least 30 days apart within 365 days; or (2) one acute care hospitalization with primary discharge diagnosis of COPD. The index date was defined as the earliest COPD encounter during the study period.
Variables
Medicare enrollment files were used to categorize subjects by age (67–74, 75–84, ≥85 yr), sex (male, female), and race (white, Black, other). Medicaid eligibility (state buy-in) in the enrollment file was used as a proxy for low socioeconomic status. A comorbidity score (0, 1, 2, ≥3) was generated using the 27-item Elixhauser comorbidity score (excluding COPD) from inpatient and outpatient billing data (14, 15). Geographic region was divided into nine CMS regions.
Assessment of Model of Care
Outpatient physician visits to PCPs during the 730 days before the index date and to a pulmonary specialist during the 365 days before the index date were calculated. PCPs included physicians in any of the following specialties: family medicine, general practice, internal medicine, and geriatrics. Beneficiaries who had a PCP visit within 2 years and a pulmonary physician visit within 1 year of the index date were considered as being comanaged by a PCP and a pulmonary specialist; those who had a visit to a non-PCP or a nonpulmonary physician were considered as having “other” physician.
Outcome Measure
Our outcome of interest was spirometry performed ±365 days of the index date. Spirometry was identified through current procedural terminology codes (94010, 94014, 94015, 94016, 94060, 94070, and 94620). Sensitivity analyses were performed for participants diagnosed with COPD in 1999, first by extending the look-back period for spirometry use for 760 days and also by determining that the participant had no prior diagnosis of COPD during the same look-back period.
Statistical Analysis
The proportion of subjects who received spirometry by year of diagnosis were calculated and plotted by age, sex, race, and number of comorbidities. Patient characteristics were summarized using counts and percentages of categorical variables. The Chi-square test was used to compare patient characteristics of those who did and did not receive spirometry. A generalized estimate equation with modified Poisson model analysis was used to assess the trend in spirometry use adjusted for patient characteristics and health care measures (type of provider) (16). We tested the a priori interaction of age, sex, race, and comorbidities with the year of diagnosis and its effect on spirometry use. Changes in rates of spirometry use over time by age, sex, race, and number of comorbidities were evaluated using the Cochran Armitage trend test. All analyses were performed using SAS version 9.2 (SAS Inc., Cary, NC). All reported P values were two sided with P less than 0.05 considered statistically significant.
Results
Between 1999 and 2008, 64,985 beneficiaries were newly diagnosed with COPD. Of these, 35,739 (55%) had spirometry performed within 1 year of the date of initial diagnosis of COPD (±365 d). Table 1 shows the baseline characteristics of the study cohort. A majority of those in the cohort were younger than 75 years, white, had higher socioeconomic status, and were managed only by a PCP. Spirometry use increased from 51.3% in 1999 to 58.3% in 2008. Receipt of spirometry was more likely in younger subjects, whites, men, and those with higher socioeconomic status (Table 2).
Table 1.
Comparison of baseline characteristics of Medicare beneficiaries with newly diagnosed chronic obstructive pulmonary disease who had spirometry ±365 days, 1999–2008
| Characteristics | Spirometry* |
|||
|---|---|---|---|---|
| N | Yes | No | P Value† | |
| All subjects | 64,985 | 35,739 (55.0) | 29,246 (45.0) | |
| Age at diagnosis, yr | ||||
| 67–74 | 32,945 (50.7) | 19,234 (53.8) | 13,711 (46.9) | <0.0001 |
| 75-84 | 27,037 (41.6) | 14,534 (40.7) | 12,503 (42.8) | |
| ≥85 | 5,003 (7.70) | 1,971 (5.51) | 3,032 (10.4) | |
| Sex | ||||
| Male | 31,547 (48.5) | 17,794 (49.8) | 13,753 (47.0) | <0.0001 |
| Female | 33,438 (51.5) | 17,945 (50.2) | 15,493 (53.0) | |
| Race | ||||
| White | 58,402 (89.9) | 32,417 (90.7) | 25,985 (88.9) | <0.0001 |
| Black | 3,709 (5.70) | 1,936 (5.42) | 1,773 (6.10) | |
| Other | 2,874 (4.42) | 1,386 (3.90) | 1,488 (5.10) | |
| Medicaid eligible‡ | ||||
| No | 54,997 (84.6) | 31,265 (87.5) | 23,732 (81.1) | <0.0001 |
| Yes | 9,988 (15.4) | 4,474 (12.5) | 5,514 (18.9) | |
| Region | ||||
| New England | 3,526 (5.43) | 1,972 (5.52) | 1,554 (5.31) | <0.0001 |
| Middle Atlantic | 9,411 (14.5) | 5,426 (15.2) | 3,985 (13.6) | |
| East North Central | 1,0892 (16.9) | 6,186 (17.3) | 4,706 (16.1) | |
| West North Central | 4,542 (7.00) | 2,511 (7.03) | 2,031 (6.94) | |
| South Atlantic | 14,686 (22.6) | 8,329 (23.3) | 6,357 (21.7) | |
| East South Central | 4,666 (7.18) | 2,314 (6.47) | 2,352 (8.04) | |
| West South Central | 6,985 (10.7) | 3,678 (10.3) | 3,307 (11.3) | |
| Mountain | 3,686 (5.67) | 1,921 (5.38) | 1,765 (6.04) | |
| Pacific | 6,591 (10.1) | 3,402 (9.52) | 3,189 (10.9) | |
| Elixhauser comorbidity,§ excluding COPD | ||||
| 0 | 17,997 (27.7) | 8,970 (25.1) | 9,027 (30.9) | <0.0001 |
| 1 | 16,303 (25.1) | 8,867 (24.8) | 7,436 (25.4) | |
| 2 | 12,532 (19.3) | 7,043 (19.7) | 5,489 (18.8) | |
| ≥3 | 18,153 (27.9) | 10,859 (30.4) | 7,294 (24.9) | |
| Diagnosis year | ||||
| 1999 | 6,170 (9.50) | 3,164 (8.85) | 3,006 (10.3) | <0.0001 |
| 2000 | 6,149 (9.50) | 3,139 (8.78) | 3,010 (10.3) | |
| 2001 | 6,890 (10.6) | 3,525 (9.86) | 3,365 (11.5) | |
| 2002 | 7,074 (10.9) | 3,727 (10.4) | 3,347 (11.4) | |
| 2003 | 6,686 (10.3) | 3,665 (10.3) | 3,021 (10.3) | |
| 2004 | 6,803 (10.5) | 3,841 (10.8) | 2,962 (10.1) | |
| 2005 | 6,767 (10.4) | 3,866 (10.8) | 2,901 (9.92) | |
| 2006 | 6,179 (9.50) | 3,558 (10.0) | 2,621 (8.96) | |
| 2007 | 6,116 (9.40) | 3,668 (10.3) | 2,448 (8.37) | |
| 2008 | 6,151 (9.50) | 3,586 (10.0) | 2,565 (8.77) | |
| Provider | ||||
| PCP only | 44,870 (69.0) | 23,184 (64.9) | 21,686 (74.2) | <0.0001 |
| Other physician | 9,551 (14.7) | 4,321 (12.1) | 5,230 (17.9) | |
| Pulmonary only | 1,782 (2.74) | 1,208 (3.38) | 574 (1.96) | |
| PCP + pulmonary | 8,782 (13.5) | 7,026 (19.7) | 1,756 (6.00) | |
| PCP clinic visit | ||||
| 0 | 11,333 (17.4) | 5,529 (15.5) | 5,804 (19.9) | <0.0001 |
| 1–3 | 11,519 (17.7) | 6,061 (17.0) | 5,458 (18.7) | |
| 4–6 | 10,576 (16.3) | 5,853 (16.4) | 4,723 (16.1) | |
| >6 | 31,557 (48.6) | 18,296 (51.2) | 13,261 (45.3) | |
| Pulmonary clinic visit | ||||
| 0 | 54,421 (83.7) | 27,505 (77.0) | 26,916 (92.0) | <0.0001 |
| 1 | 4,464 (6.90) | 3,590 (10.1) | 874 (2.99) | |
| 2 | 2,506 (3.90) | 1,958 (5.48) | 548 (1.87) | |
| ≥3 | 3,594 (5.50) | 2,686 (7.52) | 908 (3.10) | |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; PCP = primary care physician.
A COPD diagnosis is defined as having International Classification of Diseases, ninth revision codes 491.x (chronic bronchitis), 492.x (emphysema), or 496 (chronic airway obstruction).
Spirometry was identified through current procedural terminology (CPT) codes (94010, 94014, 94015, 94016, 94060, 94070, and 94s620) from −365 days to 365 days of the first claim date with COPD diagnosis.
P values represent Chi-square tests to compare the distribution of each covariate for those with and without receipt of spirometry.
Socioeconomic status was based on whether the patient was eligible for state buy-in coverage provided by the Medicaid program for at least 1 month during the index year.
Components of Elixhauser comorbidity (excludes chronic pulmonary disease): congestive heart failure, valvular disease, pulmonary circulation disorders, peripheral vascular disorders, hypertension, paralysis, other neurological disorders, diabetes-uncomplicated, diabetes-complicated, hypothyroidism, renal failure, liver disease, peptic ulcer disease excluding bleeding, AIDS, lymphoma, metastatic cancer, solid tumor without metastasis, rheumatoid arthritis/collagen vascular diseases, coagulopathy, obesity, weight loss, fluid and electrolyte disorders, blood loss anemia, deficiency anemia, alcohol abuse, drug abuse, psychoses, and depression (14).
Table 2.
Multivariate analysis of odds of spirometry use ±365 days in patients with newly diagnosed chronic obstructive pulmonary disease
| Odds Ratio (95% CI) | |
|---|---|
| Age, yr | |
| 66–74 | Reference |
| 75–84 | 0.89 (0.88–0.91) |
| ≥85 | 0.66 (0.64–0.68) |
| Sex | |
| Male | Reference |
| Female | 0.96 (0.95–0.97) |
| Race | |
| White | Reference |
| Black | 0.98 (0.95–1.01) |
| Other | 0.99 (0.95–1.03) |
| Medicaid eligible | |
| No | Reference |
| Yes | 0.82 (0.80–0.84) |
| Elixhauser comorbidity, excluding COPD* | |
| 0 | Reference |
| 1 | 1.06 (1.04–1.08) |
| 2 | 1.08 (1.06–1.10) |
| ≥3 | 1.10 (1.08–1.12) |
| Year | |
| 1999 | Reference |
| 2000 | 0.99 (0.96–1.03) |
| 2001 | 1.00 (0.97–1.03) |
| 2002 | 1.03 (1.00–1.06) |
| 2003 | 1.05 (1.02–1.08) |
| 2004 | 1.08 (1.04–1.11) |
| 2005 | 1.08 (1.04–1.11) |
| 2006 | 1.09 (1.06–1.12) |
| 2007 | 1.13 (1.10–1.17) |
| 2008 | 1.10 (1.06–1.13) |
| Region | |
| New England | Reference |
| Middle Atlantic | 1.00 (0.96–1.03) |
| East North Central | 0.99 (0.96–1.02) |
| West North Central | 0.98 (0.94–1.01) |
| South Atlantic | 0.97 (0.94–1.00) |
| East South Central | 0.87 (0.83–0.90) |
| West South Central | 0.92 (0.89–0.96) |
| Mountain | 0.92 (0.88–0.95) |
| Pacific | 0.94 (0.90–0.97) |
| Provider | |
| Other physician | 0.90 (0.87–0.92) |
| PCP only | Reference |
| Pulmonary only | 1.31 (1.26–1.35) |
| PCP + pulmonary | 1.50 (1.48–1.52) |
Definition of abbreviations: CI = confidence interval; COPD = chronic obstructive pulmonary disease; PCP = primary care physician.
Components of Elixhauser comorbidity (excludes chronic pulmonary disease): congestive heart failure, valvular disease, pulmonary circulation disorders, peripheral vascular disorders, hypertension, paralysis, other neurological disorders, diabetes-uncomplicated, diabetes-complicated, hypothyroidism, renal failure, liver disease, peptic ulcer disease excluding bleeding, AIDS, lymphoma, metastatic cancer, solid tumor without metastasis, rheumatoid arthritis/collagen vascular diseases, coagulopathy, obesity, weight loss, fluid and electrolyte disorders, blood loss anemia, deficiency anemia, alcohol abuse, drug abuse, psychoses, and depression (14).
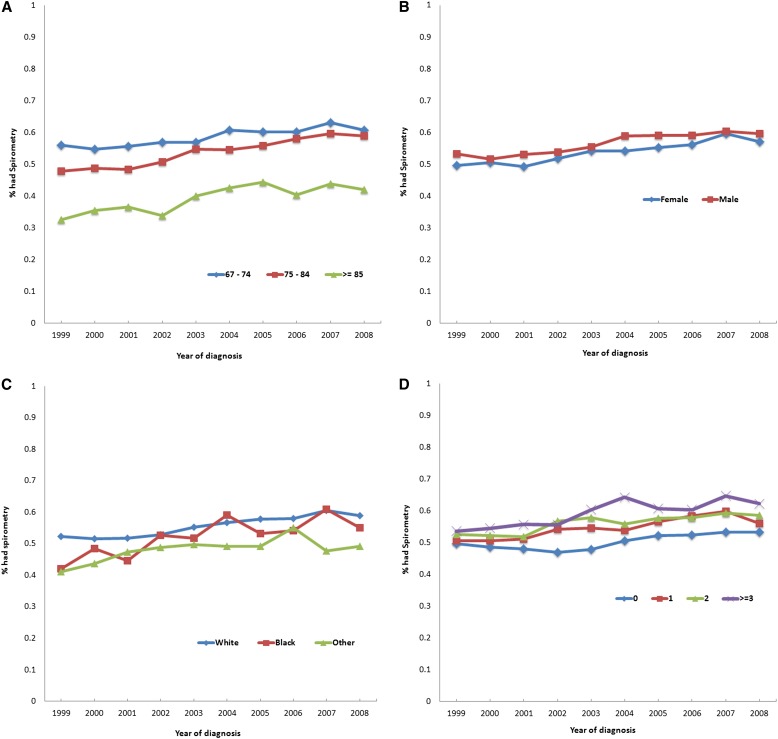
Figure 1 show spirometry rates by year categorized for age, sex, race, and Elixhauser comorbidity score (P value of trend < 0.001). During the study period, spirometry use increased 4.7% among subjects age 67 to 74 years compared with 11.1% in those 75 to 84 years and 9.6% in those 85 years and older. Spirometry use increased 7.5% in women compared with 6.5% in men during the study period. The largest increases in spirometry use from 1999 to 2008 were seen in Blacks (13.0%) compared with whites (6.6%) and others (8.1%). However, overall use of spirometry remained low, albeit much lower in nonwhites. Increase in spirometry was greater in subjects with more comorbidities. For example, subjects with zero comorbidities had a 3.6% increase, compared with 5.4, 6.0, and 8.7% for those with one, two, and three or more comorbidities, respectively, between 1999 and 2008.
Figure 1.
Percent of patients with spirometry within one year (±365 d) of diagnosis of chronic obstructive pulmonary disease (COPD) by (A) age, (B) sex, (C) race, and (D) number of comorbidities and year of diagnosis of COPD. All P values of trend test were < 0.0001.
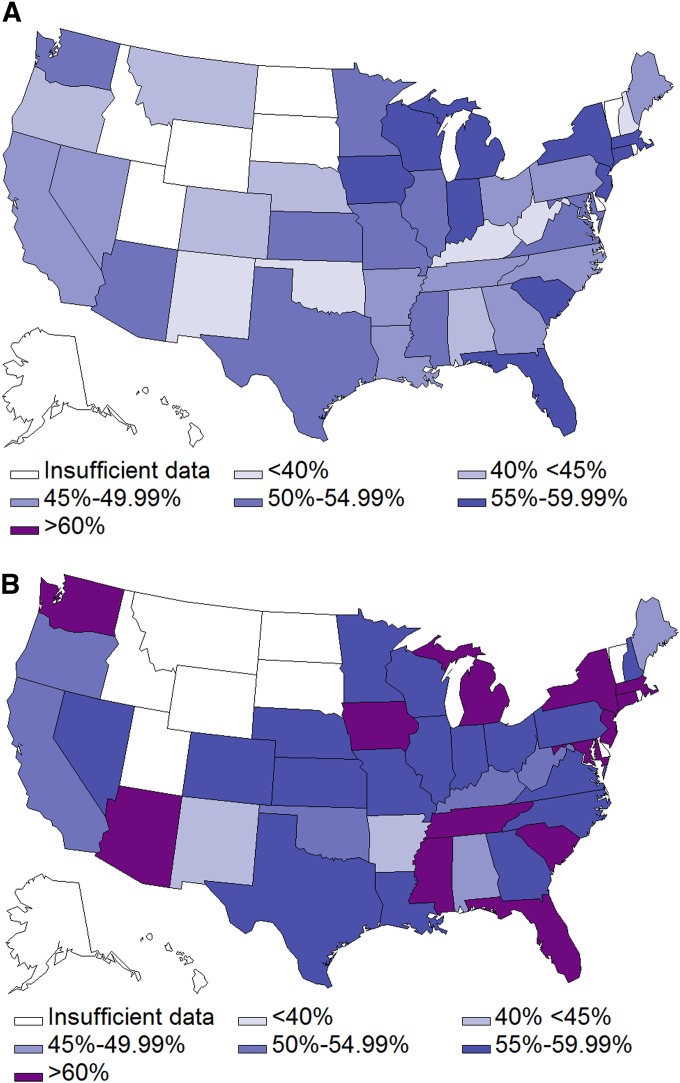
New COPD cases documented by ICD-9 codes varied fourfold across nine United States regions, ranging from 3,526 in New England to 14,686 in the South Atlantic. However, regional variation in spirometry use did not correlate with or account for the variance in new COPD diagnoses for the sample as a whole (Table 1). To examine the growth in spirometry use by state, we censored states with fewer than 100 participants during the study period. Overall, the rates of spirometry use in subjects with newly diagnosed COPD were higher in 2006 to 2008 across most states compared with those diagnosed in 1999 to 2001 (Figure 2). However, the absolute increases in spirometry use vary widely. The largest increase in spirometry use during the study period was seen in Delaware (45.7% in 1999–2001 to 68.6% in 2006–2008), and the smallest increase was seen in South Carolina, from 58.6 to 58.9%. In general, states with low spirometry use during the earlier years showed substantial increases compared with states with already high spirometry use at baseline.
Figure 2.
(A) Percent of patients newly diagnosed with chronic obstructive pulmonary disease (COPD) who had spirometry (±365 d) by U.S. states (1999–2001). (B) Percent of patients newly diagnosed with COPD who had spirometry (±365 d) by U.S. states (2006–2008). Insufficient data is defined as fewer than 100 patients in the region.
A majority of subjects with newly diagnosed COPD were seen by a PCP only (69%), whereas 13.5% were comanaged with a pulmonary physician; among subjects who were comanaged, 80% had a spirometry test compared with 52% managed by a PCP. From 1999 to 2008, comanagement increased from 10.2 to 16.4%, whereas PCP management alone decreased from 71.2 to 67.6%. Of those who had spirometry performed, 51.2% had six or more visits to a PCP.
Table 2 presents the multivariable analysis of factors associated with spirometry use in subjects with a new COPD diagnosis. Beneficiaries with more comorbidities were more likely to receive spirometry. Compared with 1999, subjects diagnosed in 2008 had 10% higher odds (odds ratio [OR], 1.10; 95% confidence interval [CI], 1.06–1.13) of having spirometry. The East South Central region had slightly lower odds (OR, 0.87; 95% CI, 0.83–0.9) of spirometry use than the New England region. After adjusting for other relevant factors, beneficiaries comanaged by both a PCP and a pulmonary physician had increased odds of spirometry use (OR, 1.50; 95% CI, 1.48–1.52) over those seen by a PCP only. However, only 13.5% of the cohort was comanaged. When we tested a priori interactions, only age and year of diagnosis were significant factors (P < 0.001).
For beneficiaries diagnosed with COPD in 1999, when we extended the look-back period for spirometry or no prior diagnosis of COPD to 760 days, spirometry use was 52.3% compared with 51.3%. Finally, when we expanded the time frame for receipt of spirometry from the index date to 2009 or death for subjects newly diagnosed with COPD between 1999 and 2008, 68% had ever received spirometry.
Discussion
Our study demonstrated that the use of spirometry increased over time based on ICD-9 codes. The largest increases in spirometry use over time were seen in the oldest subgroup of adults using a large, nationally representative sample of the United States. Despite this increase, roughly 4 out of 10 subjects were coded as a new diagnosis of COPD without spirometry testing as an objective measurement.
Previous cross-sectional studies using survey data and administrative claims showed that 30 to 40% of subjects with newly diagnosed COPD received spirometry (17–19). The lower rates seen in these studies are likely related to patient population and eligibility criteria. Veteran population studies are limited inherently by being composed of more than 98% men, having a high proportion of smokers, and having spirometry performed mainly in a laboratory setting, which likely limits access for most patients (18). Another study that included subjects enrolled in commercial, Medicare, and Medicaid healthcare coverage also included asthma diagnosis codes (17). Both of these studies were conducted retrospectively over 1 year and include a significantly larger number of younger subjects than our cohort. Our results showed that 55% of Medicare beneficiaries received spirometry, a much higher proportion than previously reported, and these rates increased over time. Higher rates of spirometry use in our study of Medicare beneficiaries are consistent with a recent Danish study of adults with universal health coverage.
Age-associated decline in the immunological and physiological function of the respiratory system predisposes older adults to an increased risk of developing COPD (7). Although older adults and women had larger increases in spirometry use during the study period, overall use of spirometry in these groups remains low (17, 19). COPD is often misclassified in the elderly (1, 7). Older adults are often perceived as unable to perform the test; however, a majority can complete spirometry according to international guidelines (20, 21). Cognitive and functional impairment, not age, are predictors of poor test performance (20, 21). Similarly, women are less likely than men to receive spirometry when presenting with comparable symptoms (2, 22, 23). Women who received spirometry reported more severe symptoms, had more advanced disease stages when diagnosed, and had steeper declines in FEV1 than men (23–25). Future studies should address sex disparities in the care of patients with COPD. Spirometry can be easily performed in older adults and remains a useful tool to diagnose and manage COPD even in this population.
In our study, two-thirds of beneficiaries with COPD were managed solely by a PCP. Spirometry use increased over the study period; our study was not designed to identify factors contributing to changes in spirometry use. One possibility is increased comanagement with a pulmonary physician. However, this increase comprises a small proportion of the cohort and does not account for the overall increase in spirometry use. Prior studies examining physician comanagement for COPD yielded mixed results in the quality of care received (17–19). Additionally, severity of disease and the availability of pulmonary physicians may influence whether a particular patient receives spirometry or referral to a pulmonary specialist or spirometry testing. This information is not captured in the administrative data files.
The reasons for underuse of spirometry by PCPs in patients with COPD are likely multifactorial. When surveyed with a clinical scenario for chronic bronchitis, only 38% of PCPs requested spirometry as a part of the diagnostic evaluation (26). Surveys among physicians with office spirometry found that nearly one-half were uncertain of the impact of results, were unfamiliar with the test, or lacked training to perform and interpret the test (27). A recent focus group of PCPs in an urban academic center characterized the perspectives of PCPs on the use of spirometry. In general, PCPs felt confident in their clinical judgment to diagnose COPD, and spirometry was neither necessary to confirm the diagnosis nor helpful in changing their management. Additionally, most participants believed there was lack of evidence that medication used in COPD leads to improved outcomes (28). The validity of office spirometry has been questioned, but with appropriate training it is accurate, reproducible, and easy to administer (29–32). Moreover, newer, more sophisticated spirometers now provide immediate results to PCPs, eliminating the time lag required for testing at pulmonary function laboratories and interpretation by a pulmonary physician. Provider nihilism (the view that COPD is a self-induced disease with ineffective treatment) may also contribute to low rates of spirometry use (33). These reasons highlight the need for future education efforts geared toward improving PCP acceptance of spirometry to diagnose COPD and PCP recognition of its role in providing evidence-based care to patients with COPD.
Spirometry in persons aged 40 years or older within 730 days before or 180 days after a diagnosis of COPD was included in 2006 as a measure in the National Committee for Quality Assurance’s Healthcare Effectiveness Data and Information Set (NCQA HEDIS) (34). We did not see a sharp increase in spirometry rates in 2007 or 2008 compared with previous years. In recent years, pay-for-performance initiatives have been used to standardize and improve health care quality while reducing costs. Adoption in 2010 by the NCQA HEDIS and CMS of spirometry as a quality performance measure serves as a financial incentive to practicing physicians and will undoubtedly continue to encourage the use of spirometry in the evaluation of COPD (18, 34, 35). The effectiveness of these initiatives remains unknown.
Finally, studies examining the impact of spirometry for COPD diagnosis on clinical outcomes are lacking. However, patients with spirometry are more likely to have an accurate diagnosis, receive combination medication therapy, and show better concordance with guideline-based therapy (18, 31, 36). These treatments have been shown to improve quality of life as well as decrease hospitalizations, acute exacerbations of COPD, and mortality. Such results provide indirect evidence of improved outcomes with use of spirometry to guide decision making.
The results of this study may have been influenced by several limitations. First, our cohort was limited to beneficiaries aged 67 years and older who had Medicare Parts A and B coverage, and our findings may not be relevant to younger patients, spirometry or healthcare visits to primary care or pulmonary specialists covered by non-Medicare insurance providers, or individuals enrolled in HMOs. However, prior similar studies that included Medicare, Medicaid, and HMOs demonstrated little difference in patients who received spirometry based on type of health insurance coverage (17). Additionally, we examined only the receipt of spirometry in beneficiaries with a COPD diagnosis as indicated by ICD-9 codes. It is not known how often testing is ordered by a physician but not completed. Also, administrative data do not contain spirometry values to confirm a COPD diagnosis and may include subjects incorrectly diagnosed. However, claims data using ICD-9 codes for COPD have been validated as reliable for extracting information (17). Furthermore, carrier files provide a reliable record of the care received but not necessarily the care needed. For instance, just as the accuracy of a diagnosis cannot be determined in subjects with a documented COPD diagnosis due to lack of specific spirometry values, the severity of disease―and, therefore, the appropriateness of management―also cannot be determined. Likewise, we cannot determine if subjects with more severe symptoms, late-stage disease, or with greater availability of pulmonary physicians were more likely to receive spirometry. Spirometry early in the course of COPD is likely most beneficial when specific interventions can be administered to minimize symptoms, optimize functional status, reduce exacerbations, and potentially slow the decline in lung function. Finally, several studies show an increase in spirometry use based on the number of respiratory medications a patient is prescribed for obstructive lung disease (17, 19, 22). We did not examine the effect of prescription medications on the receipt of spirometry. The increase in use of spirometry during the study period may be related, at least in part, to an increased availability of inhaler medications to treat COPD. We did not examine factors that may have contributed to greater use of spirometry during the study period.
In summary, spirometry use in older adults with newly diagnosed COPD has increased from 1999 to 2008. Factors contributing to greater use of spirometry over time remain unknown.
Acknowledgments
Acknowledgment
Sarah Toombs Smith, Ph.D., E.L.S., provided help in preparation of the manuscript.
Footnotes
Supported by the National Institutes of Health grants K08 AG31583, K05-CA134923, 5P30AG024832, and R01-AG033134 and by the Agency for Healthcare Research and Quality grant R01-HS020642.
Author Contributions: S.P.E.N.: substantial contributions to conception and design, interpretation of data, drafting the article or revising it critically for important intellectual content, and final approval of the version to be published. Y.W.: acquisition of data, analysis and interpretation of data, final approval of the version to be published. Y.-F.K.: substantial contributions to conception and design, analysis and interpretation of data, final approval of the version to be published. J.S.G.: substantial contributions to conception and design, interpretation of data, drafting the article or revising it critically for important intellectual content, and final approval of the version to be published. G.S.: substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, drafting the article or revising it critically for important intellectual content, and final approval of the version to be published.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.National Heart, Lung, and Blood Institute. Morbidity & mortality: 2012 chart book on cardiovascular, lung, and blood diseases. National Institutes of Health 2012 [accessed 2012 Dec]. Available from: http://www.nhlbi.nih.gov/resources/docs/2012_ChartBook.pdf
- 2.Arne M, Lisspers K, Ställberg B, Boman G, Hedenström H, Janson C, Emtner M. How often is diagnosis of COPD confirmed with spirometry? Respir Med. 2010;104:550–556. doi: 10.1016/j.rmed.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Joo MJ, Au DH, Fitzgibbon ML, McKell J, Lee TA. Determinants of spirometry use and accuracy of COPD diagnosis in primary care. J Gen Intern Med. 2011;26:1272–1277. doi: 10.1007/s11606-011-1770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chavez PC, Shokar NK. Diagnosis and management of chronic obstructive pulmonary disease (COPD) in a primary care clinic. COPD. 2009;6:446–451. doi: 10.3109/15412550903341455. [DOI] [PubMed] [Google Scholar]
- 5.Ohar JA, Sadeghnejad A, Meyers DA, Donohue JF, Bleecker ER. Do symptoms predict COPD in smokers? Chest. 2010;137:1345–1353. doi: 10.1378/chest.09-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore PL. Practice management and chronic obstructive pulmonary disease in primary care. Am J Med. 2007;120:S23–S27. doi: 10.1016/j.amjmed.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Sharma G, Hanania NA, Shim YM. The aging immune system and its relationship to the development of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:573–580. doi: 10.1513/pats.200904-022RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for diagnosis, management, and prevention of COPD. 2011 [accessed 2013 Jan 14]. Available from: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html
- 9.COPD Alliance. 2013 [accessed 2012 Jan 14]. Available from: http://www.copd.org/
- 10.American Thoracic Society. Standards for the diagnosis and management of patients with COPD 2004, updated 2005 September 8 [accessed 2013 Jan 14]. 1.2: Available from: http://www.thoracic.org/go/copd
- 11.U.S. Preventive Services Task Force. Screening for Chronic Obstructive Pulmonary Disease using spirometry: recommendation statement. 2008 [accessed 2013 Jan 14]; Available from: http://www.uspreventiveservicestaskforce.org/uspstf/uspscopd.htm [DOI] [PubMed]
- 12.Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, Marciniuk DD, Denberg T, Schünemann H, Wedzicha W, et al. American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 13.Medicare data file descriptions. [accessed 2013 Jan 24]. Available from: http://www.resdac.org/cms-data/search?f%5B0%5D=im_field_privacy_level%3A42
- 14.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Healthcare Cost and Utilization Project. Comorbidity software, Version 3.7. 2013 [accessed 2013 Jan]. Available from: http://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp
- 16.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 17.Han MK, Kim MG, Mardon R, Renner P, Sullivan S, Diette GB, Martinez FJ. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132:403–409. doi: 10.1378/chest.06-2846. [DOI] [PubMed] [Google Scholar]
- 18.Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134:38–45. doi: 10.1378/chest.08-0013. [DOI] [PubMed] [Google Scholar]
- 19.Lee TA, Bartle B, Weiss KB. Spirometry use in clinical practice following diagnosis of COPD. Chest. 2006;129:1509–1515. doi: 10.1378/chest.129.6.1509. [DOI] [PubMed] [Google Scholar]
- 20.Pezzoli L, Giardini G, Consonni S, Dallera I, Bilotta C, Ferrario G, Cristina Sandrini M, Annoni G, Vergani C. Quality of spirometric performance in older people. Age Ageing. 2003;32:43–46. doi: 10.1093/ageing/32.1.43. [DOI] [PubMed] [Google Scholar]
- 21.Bellia V, Pistelli R, Catalano F, Antonelli-Incalzi R, Grassi V, Melillo G, Olivieri D, Rengo F. Quality control of spirometry in the elderly. The SA.R.A. study. SAlute Respiration nell’Anziano = Respiratory Health in the Elderly. Am J Respir Crit Care Med. 2000;161:1094–1100. doi: 10.1164/ajrccm.161.4.9810093. [DOI] [PubMed] [Google Scholar]
- 22.Koefoed MM, dePont Christensen R, Søndergaard J, Jarbøl DE. Lack of spirometry use in Danish patients initiating medication targeting obstructive lung disease. Respir Med. 2012;106:1743–1748. doi: 10.1016/j.rmed.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Watson L, Vestbo J, Postma DS, Decramer M, Rennard S, Kiri VA, Vermeire PA, Soriano JB. Gender differences in the management and experience of Chronic Obstructive Pulmonary Disease. Respir Med. 2004;98:1207–1213. doi: 10.1016/j.rmed.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Xu X, Li B, Wang L. Gender difference in smoking effects on adult pulmonary function. Eur Respir J. 1994;7:477–483. doi: 10.1183/09031936.94.07030477. [DOI] [PubMed] [Google Scholar]
- 25.Mapel DW, Dalal AA, Blanchette CM, Petersen H, Ferguson GT. Severity of COPD at initial spirometry-confirmed diagnosis: data from medical charts and administrative claims. Int J Chron Obstruct Pulmon Dis. 2011;6:573–581. doi: 10.2147/COPD.S16975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kesten S, Chapman KR. Physician perceptions and management of COPD. Chest. 1993;104:254–258. doi: 10.1378/chest.104.1.254. [DOI] [PubMed] [Google Scholar]
- 27.Kaminsky DA, Marcy TW, Bachand M, Irvin CG. Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respir Care. 2005;50:1639–1648. [PubMed] [Google Scholar]
- 28.Joo MJ, Sharp LK, Au DH, Lee TA, Fitzgibbon ML. Use of spirometry in the diagnosis of COPD: a qualitative study in primary care. COPD. 2013;10:444–449. doi: 10.3109/15412555.2013.766683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolton CE, Ionescu AA, Edwards PH, Faulkner TA, Edwards SM, Shale DJ. Attaining a correct diagnosis of COPD in general practice. Respir Med. 2005;99:493–500. doi: 10.1016/j.rmed.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Schermer TR, Jacobs JE, Chavannes NH, Hartman J, Folgering HT, Bottema BJ, van Weel C. Validity of spirometric testing in a general practice population of patients with chronic obstructive pulmonary disease (COPD) Thorax. 2003;58:861–866. doi: 10.1136/thorax.58.10.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yawn BP, Enright PL, Lemanske RF, Jr, Israel E, Pace W, Wollan P, Boushey H. Spirometry can be done in family physicians’ offices and alters clinical decisions in management of asthma and COPD. Chest. 2007;132:1162–1168. doi: 10.1378/chest.06-2722. [DOI] [PubMed] [Google Scholar]
- 32.Mitra AD, Ogston S, Crighton A, Mukhopadhyay S. Lung function and asthma symptoms in children: relationships and response to treatment. Acta Paediatr. 2002;91:789–792. doi: 10.1080/08035250213230. [DOI] [PubMed] [Google Scholar]
- 33.Cooke CE, Sidel M, Belletti DA, Fuhlbrigge AL. Review: clinical inertia in the management of chronic obstructive pulmonary disease. COPD. 2012;9:73–80. doi: 10.3109/15412555.2011.631957. [DOI] [PubMed] [Google Scholar]
- 34.National Committee for Qualtiy Assurance. HEDIS & performance measurement. [Accessed 2013 Jan]. Available from: http://www.ncqa.org/HEDISQualityMeasurement.aspx
- 35.Claims-based quality measures. [Accessed 2013 Jan 14]. Available from: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeedbackProgram/downloads/claims_based_measures_with_descriptions_num_denom_excl.pdf
- 36.Asche CV, Leader S, Plauschinat C, Raparla S, Yan M, Ye X, Young D. Adherence to current guidelines for chronic obstructive pulmonary disease (COPD) among patients treated with combination of long-acting bronchodilators or inhaled corticosteroids. Int J Chron Obstruct Pulmon Dis. 2012;7:201–209. doi: 10.2147/COPD.S25805. [DOI] [PMC free article] [PubMed] [Google Scholar]