Abstract
Rationale: Several studies suggest that nasal nitric oxide (nNO) measurement could be a test for primary ciliary dyskinesia (PCD), but the procedure and interpretation have not been standardized.
Objectives: To use a standard protocol for measuring nNO to establish a disease-specific cutoff value at one site, and then validate at six other sites.
Methods: At the lead site, nNO was prospectively measured in individuals later confirmed to have PCD by ciliary ultrastructural defects (n = 143) or DNAH11 mutations (n = 6); and in 78 healthy and 146 disease control subjects, including individuals with asthma (n = 37), cystic fibrosis (n = 77), and chronic obstructive pulmonary disease (n = 32). A disease-specific cutoff value was determined, using generalized estimating equations (GEEs). Six other sites prospectively measured nNO in 155 consecutive individuals enrolled for evaluation for possible PCD.
Measurements and Main Results: At the lead site, nNO values in PCD (mean ± standard deviation, 20.7 ± 24.1 nl/min; range, 1.5–207.3 nl/min) only rarely overlapped with the nNO values of healthy control subjects (304.6 ± 118.8; 125.5–867.0 nl/min), asthma (267.8 ± 103.2; 125.0–589.7 nl/min), or chronic obstructive pulmonary disease (223.7 ± 87.1; 109.7–449.1 nl/min); however, there was overlap with cystic fibrosis (134.0 ± 73.5; 15.6–386.1 nl/min). The disease-specific nNO cutoff value was defined at 77 nl/minute (sensitivity, 0.98; specificity, >0.999). At six other sites, this cutoff identified 70 of the 71 (98.6%) participants with confirmed PCD.
Conclusions: Using a standardized protocol in multicenter studies, nNO measurement accurately identifies individuals with PCD, and supports its usefulness as a test to support the clinical diagnosis of PCD.
Keywords: primary ciliary dyskinesia, Kartagener syndrome, ciliopathy, axoneme
Primary ciliary dyskinesia (PCD) is a rare, genetically heterogeneous, autosomal recessive disorder leading to impaired ciliary clearance from the lower airways, middle ear, and paranasal sinuses, and is associated with mucus retention and chronic infections of the respiratory tract (1, 2). Accurate diagnosis is important to initiate early treatment to delay onset and progression of irreparable damage in all sites, including bronchiectasis in the lung, which is the major factor impacting on quality of life and prognosis (3, 4).
The diagnosis of PCD has traditionally been made by demonstration of ciliary ultrastructural or functional abnormalities, using transmission electron microscopy (EM) or video microscopy of airway epithelial biopsies (4, 5), although these approaches have many limitations and are dependent on adequate sample processing and expertise to interpret axonemal defects or abnormal ciliary activity (6). Genetic testing of the 21 known genes associated with PCD is emerging, but only a minority of individuals can currently be identified using commercially available tests (2). An alternative, reproducible, and cost-effective test would help direct appropriate individuals to further testing and potentially lead to earlier therapy. Studies have suggested that nasal nitric oxide (nNO) measurement could be a useful test for PCD (7–12).
Most nitric oxide (NO) in exhaled air originates from the upper airways, predominantly from the sinuses (13, 14). In PCD, nNO values are only 10–20% of the average values in healthy control subjects (6). The American Thoracic Society (ATS), in cooperation with the European Respiratory Society (ERS), has published guidelines for nNO measurements, which recommend obtaining levels while the patient performs trained velum closure or exhales against resistance or other techniques to decrease dilution from the lower airways (15). Although several studies have demonstrated that nNO levels are significantly lower in PCD than those measured in healthy control subjects, these investigations had several limitations, including small numbers of PCD-affected subjects and few disease control subjects, and each of these single-site studies has used a different NO analyzer with different sampling flow rate and methodology, which restricts side-by-side comparisons to assess reproducibility at multiple sites with different operators (7–13). Further standardization is needed for its use as a test to support the clinical diagnosis of PCD.
For our study, nNO levels were measured in subjects at least 5 years of age by a standard operating procedure (SOP) at one site (University of North Carolina [UNC], Chapel Hill, NC), including children and adults with PCD, with documented axonemal defects in ciliary ultrastructure; healthy control subjects; and lung disease control subjects with asthma, cystic fibrosis, and COPD (including longitudinal measurements in some subjects) to generate a disease-specific cutoff value with 98% sensitivity. For further validation, nNO measurements were analyzed in individuals (≥5 yr of age) undergoing evaluation for PCD at six other sites in North America, using the same standardized approach with instrument-specific SOP.
Methods
Study Sites and Subjects
Investigators in the Genetic Disorders of Mucociliary Clearance Consortium (GDMCC; seven geographically dispersed sites in North America) systematically and prospectively evaluated adults and children for possible PCD, if they had respiratory features suggestive of PCD, including situs abnormalities, usually after cystic fibrosis (CF) and immunodeficiencies had been excluded. The evaluation included a standardized format for clinical information and SOPs for nNO measurements, ciliary ultrastructural analyses, and genetic testing for disease-causing mutations in known PCD-associated genes. Normal control subjects were recruited from the UNC undergraduate and medical campus. Disease control subjects with asthma, CF, and COPD were recruited from pediatric and adult pulmonary clinics at UNC.
nNO Measurement
Studies were performed during clinical stability with palate closure, according to ATS/ERS guidelines (6, 15, 16) and after prospective training in use of our SOP. Briefly, subjects achieved palate closure by exhaling through the mouth (20–40 s) into a disposable resistor (cardboard cylinder with 1-mm opening at distal end [DirectMed Inc., Glen Cove, NY] or party favor blowout toy with comparable resistance), while nasal gas was aspirated through a line with a disposable foam olive inserted into one nostril. All studies were performed on subjects during clinically stable periods, free of acute upper or lower respiratory illnesses or nasal instrumentation for at least 2 weeks before undergoing measurements. NO was measured with a chemiluminescence analyzer (Sievers 280i NOA [Sievers, Boulder, CO], CLD 88SP [ECO PHYSICS AG, Duernten, Switzerland], or NIOX Flex [Aerocrine AB, Solna, Sweden]) using SOPs with two sections—“Operation of Instrument” with details about calibration and settings specific for the instrument used and “Measurement Procedure,” which was the same for all three instruments. Criteria for an acceptable maneuver included a greater than 20-second exhalation with steady NO signal plateau for 3–10 seconds on the online display. Then, two or three reproducible maneuvers (with less than 10% difference) were used to calculate the mean value for each nostril. The value for each participant was the mean of the right and left nostril values. nNO production (nl/min) was calculated by multiplying nNO concentration (parts per billion) by the sampling flow rate (0.5, 0.33, and 0.3 L/min for the Sievers, CLD, and NIOX, respectively). Measurements on different analyzers at the same visit in a subset of participants had comparable values after adjusting for different sampling flow rates (see Figure E1 in the online supplement).
Other Tests
Nasal ciliated cells obtained by nasal curettage were placed in fixative and shipped to UNC for transmission EM and examined for PCD-specific ultrastructural abnormalities as described, including (1) absent or truncated outer dynein arms (ODA), (2) absent ODA plus absent inner dynein arms (IDA), and (3) absent IDAs coupled with microtubular disorganization (7, 17, 18). An isolated IDA defect was not diagnostic, as this can be nonspecific (19). PCD was confirmed by identification of biallelic DNAH11 mutations in patients with normal ultrastructure (20). Data were entered in a web-based Data Management Coordinating Center (Director, J. Krischer), including laterality defects and clinical features. A respiratory symptom score (0 to 4) was calculated by assigning one point for clinical features seen in patients with PCD more than 5 years of age: (1) history of unexplained neonatal respiratory distress, (2) year-round nasal congestion, (3) year-round wet cough, and (4) more than five episodes of otitis media by age 2 years. For nNO validation studies at six (non-UNC) sites, PCD was confirmed by PCD-specific ciliary EM defects and, by the presence of biallelic mutations in PCD genes (20–31). Informed consent was obtained at the University of North Carolina at Chapel Hill and collaborating institutions under the auspices of Committees on the Protection of the Rights of Human Subjects.
Statistical Methods
Generalized estimating equation (GEE) methodology (32) was applied to fit prediction equations for nNO measurements (loge nNO) as a function of age, as detailed in the online supplement. The cutoff value was computed as the 98th percentile of the normal distribution with mean and standard error estimated under the GEE model. Model-based parameter estimates were used to compute positive and negative predictive values and specificity, assuming three different population prevalences of PCD in pulmonary specialty clinics (1 in 150, 1 in 50, and 1 in 20). Statistical analyses were performed with SAS (Cary, NC) and the R language.
Results
Subjects
The demographic characteristics for the study populations are shown in Table 1. For the lead site (UNC), the number of nNO measurements and summary statistics for age, sex, and nNO level are provided for 143 patients with PCD, as confirmed by the presence of PCD-specific ultrastructural defects detected by transmission EM, and 78 healthy control subjects. These data form the basis of the model fitting used to determine the cutoff diagnostic value and to compute specificity, positive predictive values (PPVs), and negative predictive values (NPVs) for the proposed nNO test. Table 1 also summarizes the characteristics of disease-control subjects at UNC, and the individuals evaluated at the other sites, which were used to evaluate the proposed nNO test.
Table 1.
Characteristics of study subjects
| Subjects: | Measurements* | Age in Years† |
nNO (nl/min) |
|||
|---|---|---|---|---|---|---|
| Total No. (No. Females) | No. | Mean (SD) | (min, max) | Mean (SD) | (min, max) | |
| UNC | ||||||
| PCD (with PCD-specific EM defect) | ||||||
| Subjects with cross-sectional data only | 103 (61) | 103 | 29.3 (17.6) | (5.2, 73.0) | 20.0 (19.8) | (1.5, 125.3) |
| Subjects with longitudinal data | 40 (21) | 132 | 9.0 (3.2) | (5.1, 16.7) | 21.0 (27.5) | (1.8, 207.3) |
| PCD (with biallelic DNAH11 mutations) | ||||||
| Subjects with cross-sectional data only | 4 (2) | 4 | 26.2 (9.2) | (12.4, 31.9) | 21.0 (7.4) | (9.8, 24.5) |
| Subjects with longitudinal data | 2 (2) | 6 | 14.2 (0.3) | (13.9, 14.4) | 25.9 (19.4) | (12.3, 65.0) |
| All PCD measurements at UNC | 149 (86) | 245 | 19.1 (14.8) | (5.1, 73.0) | 20.7 (24.1) | (1.5, 207.3) |
| Healthy control subjects | 78 (45) | 78 | 20.9 (15.7) | (5, 73.6) | 304.6 (118.8) | (125.5, 867.0) |
| Disease control subjects | ||||||
| Subjects with asthma | 37 (16) | 37 | 14.8 (11.5) | (5.4, 53.5) | 267.8 (103.2) | (125.0, 589.7) |
| Subjects with cystic fibrosis | 77 (41) | 77 | 16.0 (9.4) | (5.5, 56.0) | 134.0 (73.5) | (15.6, 386.1) |
| Subjects with COPD | 32 (10) | 32 | 61.1 (8.9) | (43.2, 77.8) | 223.7 (87.1) | (109.7, 449.1) |
| Validation subgroups (non-UNC sites) | ||||||
| Confirmed PCD‡ | 71(45) | 71 | 23.3 (18.0) | (5.1, 69.0) | 19.6 (16.6) | (0.66, 31.3) |
| Indeterminate§ | 84 (46) | 84 | 31.8 (22.3) | (5.5, 79.6) | 216.4 (174.5) | (1.8, 991.7) |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; EM = electron microscopy; nNO = nasal nitric oxide; PCD = primary ciliary dyskinesia; UNC = University of North Carolina.
Unless otherwise noted, subjects contribute cross-sectional measurements only.
For longitudinal data, age is summarized on the basis of age at first measurement.
PCD is confirmed by EM defects (n = 65) or biallelic mutations in PCD genes but indeterminate EM (n = 6).
These subjects were referred because of symptoms, but EM did not show hallmark ultrastructural defect in cilia.
Development of nNO Testing at the Lead (UNC) Site
Development of the nNO test was based on 235 nNO measurements from 143 (82 female) patients with confirmed PCD at UNC. Ages ranged from 5.1 to 73.0 years. Forty younger patients with PCD (age, 5.1–16.7 yr) contributed repeated measurements to the analysis and demonstrated excellent reproducibility (see Figure E2). The mean nNO level was 20.0 ± 19.8 nl/minute (mean ± SD) for patients contributing single measurements and 21.0 ± 27.5 nl/minute for patients with multiple measurements. The average nNO value in 78 healthy control subjects was 304.6 ± 118.8 nl/minute.
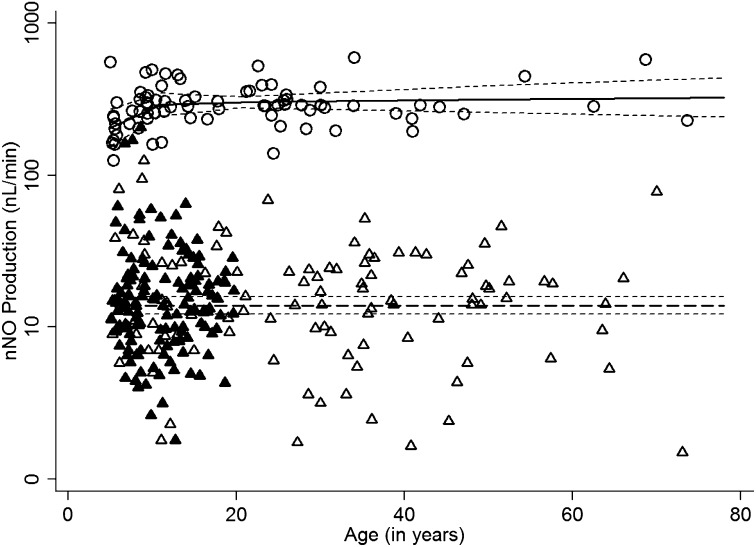
nNO production (nl/min) remained relatively flat for the 143 patients with PCD across the full age range studied (Figure 1). Thus, the cutoff value to detect disease for the proposed test was based on an intercept-only model fit to patients with PCD that did not include trends with respect to age. For the 78 healthy control subjects, nNO production increases with age in the younger age range. The model providing the best fit for healthy control subjects includes separate linear slopes with respect for loge age for patients aged 5 to 11 years versus patients aged 11 to 70 years (Figure 1).
Figure 1.
Scatter plot of nasal nitric oxide (nNO) values (natural log scale; nl/min) versus age for individuals with primary ciliary dyskinesia (PCD) and healthy control subjects, using model-predicted values and a 95% confidence band. For nNO measurements in subjects with PCD and ciliary ultrastructure defects evaluated at the University of North Carolina site (open triangles, single measurements; solid triangles, repeated measurements), no trends with respect to age were observed; consequently, an intercept-only model adequately captured the variation in nNO levels. For healthy control subjects (open circles), nNO increased with age in a nonlinear fashion; consequently, the model that appeared to provide the best fit included separate linear slopes with respect to loge age for subjects between 5 and 11 years and subjects older than 11 years of age.
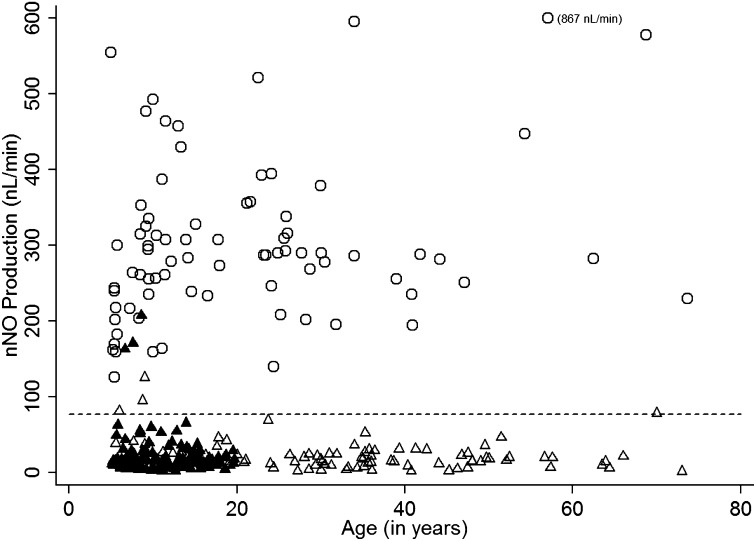
The cutoff value for nNO was determined to be 76.9 nl/minute (equivalent to 4.34 loge nNO). This cutoff value provides good separation between patients with PCD and healthy control subjects (Figure 2) with only a few nNO levels from younger subjects with PCD above the cutoff value. None of the healthy control subjects approached the cutoff value.
Figure 2.
Scatter plot of nasal nitric oxide (nNO) values (linear scale; nl/min) versus age for individuals with primary ciliary dyskinesia (PCD) and healthy control subjects with nNO cutoff (same data as in Figure 1, shown on a linear scale). All nNO values from healthy control subjects (open circles) were well above the cutoff (77 nl/min) and most of the nNO measurements in subjects with PCD and ciliary ultrastructure defects (open triangles, single measurements; solid triangles, repeated measurements) were below the cutoff. The three solid triangles above the cutoff are repeated measurements in the same individual with PCD.
Table 2 provides specificity and PPVs stratified by age at the time of the proposed nNO test based on nNO values in subjects with PCD and healthy control subjects. By design, the sensitivity of the test is 98% across all ages. Specificity increases with age, as would be expected from Figure 2, but is never below 99.9% across the ages studied. PPV increases with increasing population prevalence. For example, at age 5 years, PPV is 88.4% assuming a prevalence of 1 in 150, 95.9% assuming 1 in 50, and 98.4% assuming 1 in 20. By age 9, PPV is greater than 99.0% regardless of the underlying prevalence and remains so across the remaining age range. The NPV is 99.9% for the three prevalence values considered.
Table 2.
Specificity and positive predicted values of the nasal nitric oxide screening test*
| Age in Years | Specificity | PPV (Prevalence 1/150)† | PPV (Prevalence 1/50)‡ | PPV (Prevalence 1/20)§ |
|---|---|---|---|---|
| 5 | 0.99914 | 0.884 | 0.959 | 0.983 |
| 11 | 0.99997 | 0.995 | 0.998 | 0.999 |
| 21 | 0.99998 | 0.996 | 0.999 | 0.999 |
| 50 | 0.99998 | 0.998 | 0.999 | 0.999 |
| 70 | 0.99999 | 0.998 | 0.999 | 0.999 |
Definition of abbreviations: NPV = negative predicted value; PPV = positive predicted value.
The nasal nitric oxide cutoff value is 76.9, based on an assumed sensitivity of 0.98.
NPV = 0.99987 over all ages.
NPV = 0.99959 over all ages.
NPV = 0.99895 over all ages.
Performance of Proposed nNO Test in Other Lung Disease Groups Evaluated at the Lead Site
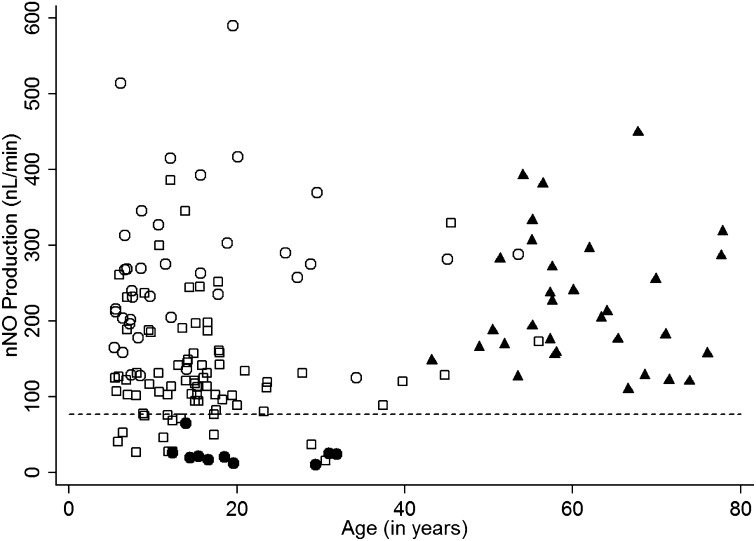
The nNO screening test was applied to other lung disease cohorts (Table 1 and Figure 3). All of the subjects with asthma and COPD exceeded the cutoff line, but 12% of the patients with CF fell below the cutoff value. All 10 measurements from six patients with PCD with normal ciliary ultrastructure, but a PCD diagnosis confirmed by biallelic, disease-causing DNAH11 mutations, had nNO levels below the cutoff value.
Figure 3.
Scatter plot of nasal nitric oxide (nNO) values (linear scale; nl/min) versus age for University of North Carolina disease control subjects and a subset of subjects with primary ciliary dyskinesia (PCD) with normal electron micrographs but biallelic mutations in DNAH11. Individual nNO measurements are shown for disease control subjects: asthma (open circles), cystic fibrosis (open squares), and chronic obstructive pulmonary disease (solid triangles). In addition, 10 measurements are shown for a special subset of PCD (solid circles) with PCD confirmed by the presence of biallelic mutations in DNAH11 despite normal ultrastructure.
Performance of Proposed nNO Testing at Six Other Sites
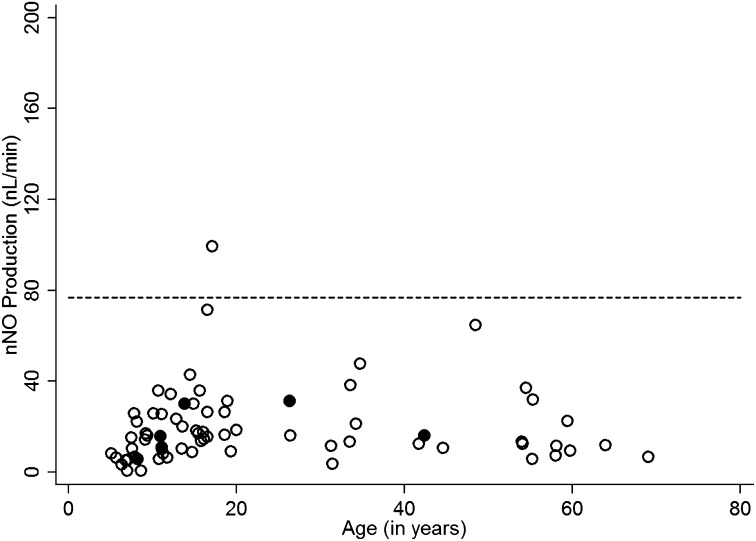
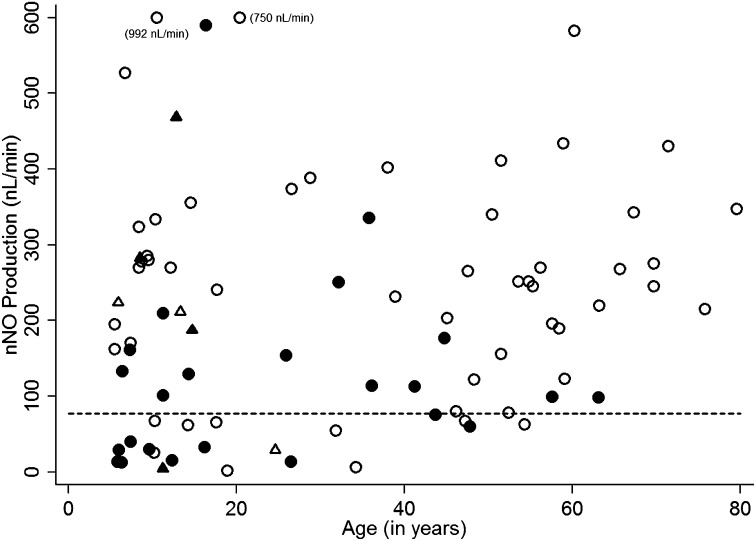
Of the 155 consecutive individuals evaluated at the six other sites, 71 (45.8%) had confirmed PCD based on clinical features and the presence of typical ultrastructural anomalies, and/or biallelic mutations in PCD genes, including 6 with genetic diagnosis but indeterminate ultrastructure. Of the participants with confirmed PCD, 98.6% had nNO below the cutoff value (Figure 4). The one participant with an nNO level (99 nl/min) above the cutoff value had clinical features consistent with PCD, including situs inversus totalis, and a clinical score of 4. For this confirmed PCD group, the prevalence of a laterality defect (35 of 71, or 49.3%) and the symptom scores (2.87 ± 0.86; mean ± SD) were similar to those in 99 patients with PCD from the lead site (49.5% and 3.04 ± 0.80, respectively) (Table 3). There were 84 individuals at other sites in whom we were unable to demonstrate EM defects or genetic mutations (Figure 5); thus, we could not determine whether they had PCD, or not. Of those 84 “indeterminate” or “undefined” individuals, 21 of 84 (25%) had nNO levels below the cutoff (77 nl/min), and 63 of 84 (75%) had nNO levels above the cutoff (Figure 5). Those with nNO values below the cutoff of 77 nl/minute (n = 21) had respiratory symptom scores similar to those of patients with confirmed PCD at other sites, but higher than those with nNO values above the cutoff (n = 63) (2.48 ± 0.93 vs. 1.94 ± 0.93, respectively; P < 0.027). The prevalence of laterality defects was low in these individuals with nNO above the cutoff of 77 nl/minute (7.9%), as well as in those with nNO in the PCD range (9.5%), which might not be expected; however, the low prevalence of laterality defects in this latter group may reflect an enrichment of PCD mutations that are not associated with dynein arm defects or laterality abnormalities (see Discussion).
Figure 4.
Validation data from six other sites: primary ciliary dyskinesia (PCD) confirmed with ultrastructural defect or biallelic mutations in gene-associated electron microscopy (EM) defect. Single nasal nitric oxide (nNO) measurements are shown for the six other Genetic Disorders of Mucociliary Clearance Consortium (GDMCC) sites: National Institute of Allergy and Infectious Diseases (Bethesda, MD; 43 participants); Washington University (Saint Louis, MO; 39 participants); Hospital for Sick Children (Toronto, ON, Canada; 26 participants); University of Colorado (Denver, CO; 23 participants); University of Washington (Seattle, WA; 18 participants); and Stanford University (Palo Alto, CA; 6 participants). The open circles represent individuals with PCD confirmed by identification of ultrastructural defect. The solid circles represent individuals with PCD confirmed by genetic testing alone, demonstrating biallelic mutations in a PCD gene.
Table 3.
Laterality defect and respiratory symptoms
| Prevalence of Laterality Defect | Prevalence of Symptom Score ≥ 3 | Symptom Score Mean ± SD | |
|---|---|---|---|
| Confirmed at lead site (n = 99) | 49.5% | 73.7% | 3.04 ± 0.80 |
| Validation groups | 49.3% | 62.0% | |
| Confirmed PCD (n = 71) | 2.87 ± 0.86 | ||
| Indeterminate (n = 84) | 9.5% | 52.4% | 2.48 ± 0.93* |
| Below cutoff (n = 21) | |||
| Above cutoff (n = 63) | 7.9% | 27.0% | 1.94 ± 0.93* |
Definition of abbreviation: PCD = primary ciliary dyskinesia.
P = 0.027 by two-tailed t test.
Figure 5.
Validation data from six other sites: Participants undergoing primary ciliary dyskinesia (PCD) evaluation but no confirmatory ultrastructural defect or genetic mutations. The triangles represent individuals with a laterality defect (situs inversus totalis or heterotaxy) and the circles represent individuals with PCD with no laterality defect. Participants represented by the open symbols have low symptom scores (0–2) and participants represented by the solid symbols have high symptom scores (3 or 4).
Discussion
Measurement of nNO levels holds promise as a test to identify patients who have PCD, but there has been no single standardized procedure for measurement, even though ATS/ERS guidelines have outlined the general methods for nNO measurements (15). Studies that followed ATS/ERS guidelines have used different methods to isolate gases from the nasopharynx, some using a prolonged breath hold that varied from 10 to 60 seconds, and others using a variety of palate closure maneuvers (7–13). Moreover, most of these studies reported results as measured nNO concentrations (parts per billion) without adjustment for sampling flow rate. These studies have used different chemiluminescent analyzers that aspirate nasal gas at different flow rates (varying from 200 to 500 ml/min), which can greatly influence the measured concentration of nNO levels, because NO concentrations in samples are inversely related to flow rate. Consequently, it is difficult to directly compare data from these different studies. In most cases, these studies have also been limited to a single site with relatively small numbers of individuals with PCD, healthy control subjects, and disease control subjects and variations in diagnostic criteria to define PCD. Because these studies demonstrate consistently that measured nNO levels are markedly lower in PCD than in healthy control subjects, several institutions across Europe are using nNO measurements as part of their evaluation for PCD (4). Further standardization of technical aspects of the measurement, identification of specific cutoff value, and validation of testing across multiple sites is needed before implementing nNO levels as an adjunctive test in selected centers to identify individuals with PCD.
In this study, we systematically measured nNO levels during palate closure in a sizable number of healthy control subjects and individuals with PCD across a wide age range (5–73 yr old) and in several control populations with airway diseases (CF, asthma, and COPD). The nNO values are reported as nNO production (product of nNO concentration and transnasal sampling flow rate) to correct for the flow rate for different analyzers. For subjects with PCD, nNO production remains low and relatively flat across ages. For healthy control subjects, however, nNO increases slightly with age for subjects 5–10 years of age, but with little change thereafter. Because the majority of nNO is thought to arise from the paranasal sinuses (13, 14), the age-related increase in nNO levels in the younger cohort likely reflects development and growth of paranasal sinuses. Studies have demonstrated that nNO can be measured in younger children during tidal breathing (33); however, nNO values during tidal breathing are lower than during palate closure maneuvers. Other preliminary studies suggest that nNO measured during tidal breathing with a hand-held device has promise as a diagnostic test for PCD (34). For these other approaches, studies are needed to standardize the protocol, establish appropriate cutoff values, and validate across multiple sites.
For the confirmed PCD group with defined ultrastructural defects in the ciliary axonemes, the mean nNO level (20.0 nl/min) is less than 10% of the mean nNO measurement for healthy control subjects (304.6 nl/min) with minimal overlap. Previous reports in smaller subsets show a similar magnitude of difference between PCD and healthy control subjects (7–12). The cutoff value defined in this study (77 nl/min) is slightly lower than what was identified in our earlier study that examined a smaller patient population (7).
nNO values for subjects with asthma and COPD do not overlap with values in PCD, and are comparable to values in normal control subjects. The nNO values in some of the patients with CF, however, did overlap with PCD values, which has been described in other studies (7–9, 11). Therefore, CF must be excluded by a quantitative pilocarpine iontophoresis sweat test or CFTR genotype testing in all individuals with nNO measurements below the cutoff values. As newborn screening for CF extends to more universal application, the potential for CF confounders should decrease, because early diagnosis of CF will limit their referral at older ages to specialty clinics for diagnostic evaluation.
Using a standardized approach to evaluate PCD, including a uniform nNO protocol and cutoff value at six other GDMCC clinical research sites, nNO measurements identified virtually every participant with confirmed PCD (defined by the presence of a “hallmark” ultrastructural defect of the ciliary axonemes, or biallelic mutations in genes known to cause PCD). All but one of the 71 individuals with confirmed PCD had nNO values below the cutoff.
We propose that a substantial portion of the 21 participants at other (non-UNC) sites without identified PCD-specific ultrastructural defects or biallelic mutations, but nNO levels below the cutoff value (77 nl/min), may ultimately prove to have PCD, based on the strong clinical phenotype and high respiratory scores (Figure 5 and Table 3). The lower prevalence of laterality defects (9.5%) in these 21 individuals most likely reflects a low prevalence of dynein arm defects that are (more) easily defined on ciliary ultrastructural analysis, and that are associated with laterality defects in 50% of patients. These 21 individuals likely have a high prevalence of central apparatus defects, which are less apparent on EM analysis, and not associated with laterality defects. Therefore, the confirmed PCD group is highly enriched with dynein arm defects that are associated with laterality defects, whereas the undefined group has normal dynein arms and is more likely enriched with central apparatus defects that are not associated with laterality defects. The genetic basis for PCD is rapidly emerging, and a growing number of mutations in multiple genes that encode proteins involved in ciliary structure, function, and assembly have been implicated in disease. We speculate that many patients with nondiagnostic ciliary ultrastructure, but a strong clinical phenotype and low nNO levels, at the other six sites will have mutations in PCD-associated genes that affect the central apparatus. Similarly, with further advances in our understanding of PCD genes and genotype–phenotype correlations, novel genetic variants may emerge that are not associated with low nNO.
As with any biological test, nNO testing has limitations and confounders that must be considered. Acute respiratory infections can damage the respiratory epithelium, resulting in lower nNO values. Therefore, nNO levels should be measured during periods of clinical stability and at least 2 weeks after an acute respiratory illness. Blood in the nasal cavity can rapidly bind NO, so the nasal mucosa must be examined before nNO measurements; plus, nNO measurements should be delayed if there has been a recent nosebleed. Because of these potential confounders, measurements should be repeated at least once on a separate day to ensure that reduced nNO levels are reproducible. Standardized procedures must be followed to ensure that measurements are accurate, including routine analyzer calibration, ambient NO level measurements, examination of tubing for secretions in tubing that can alter flow rate, and monitoring flow rates.
The usefulness of the nNO test, especially the PPV, depends on the prevalence of PCD within the target populations of patients that are tested. As demonstrated in Table 2, the PPV decreases as the prevalence of PCD within the tested population decreases from 1 in 20 to 1 in 150, particularly in younger children. For target populations such as those with chronic oto-sino-pulmonary disease starting in early childhood or those with situs inversus totalis (in which the prevalence of PCD is likely greater than 1 in 50), the PPV is strong (0.96 or better); but, for other populations such as those with recurrent otitis media or chronic cough, in which the prevalence of PCD is much less than 1 in 150, the PPV will be less robust. At this point, nNO testing is not ready for broad-scale clinical use, but we expect it to be employed in specialized centers that are evaluating complex patients and therefore selecting appropriate target populations for testing, using SOPs. This study provides data that will be important for applications to the U.S. Food and Drug Administration (and other regulatory agencies) for approval of nNO devices for future clinical use. Unfortunately, infants and young children cannot cooperate with velum closure maneuvers described in our SOP. Preliminary studies have shown that nNO can be measured during tidal breathing (33); however, further standardization and definition of disease-specific cutoff values are needed for application of this tidal breathing approach in very young children to aid in early identification of PCD.
In summary, we have defined a standardized protocol for measuring nNO levels during palate closure in adults and children at least 5 years of age that can be used as a noninvasive test for PCD. In healthy children, nNO values increase progressively with age between 5 and 10 years of age, likely reflecting sinus development. In PCD, nNO values remain extremely low across all ages. The defined nNO cutoff value has robust ability to discriminate between “classic” forms of PCD (confirmed only in patients with specific ultrastructural defects and/or biallelic mutations in selected PCD genes) from other pulmonary conditions, with the exception of CF. Preliminary validation of this approach was confirmed at six other sites, which accurately identified subjects with PCD using the same protocol for nNO measurement and the same cutoff value. These results provide the basis for a noninvasive test to support the clinical diagnosis of PCD.
Acknowledgments
Acknowledgment
The authors are grateful to the patients with PCD and their families for their participation. The authors thank Michele Manion and the U.S. PCD Foundation, all additional investigators, and the coordinators of the Genetic Disorders of Mucociliary Clearance Consortium (GDMCC), which is part of the Rare Disease Clinical Research Network, including Tanya Glaser, Meghan O’Connell, Heather Root, Andrea Henkel, Caroline Smith (NIAID/NIH, Bethesda, MD), Jane Quante (Washington University, St. Louis, MO), Shelley Mann and Carol Kopecky (Children’s Hospital Colorado, Aurora, CO), Sharon McNamara, Liz Cochrane, Molly Elliott, Jennifer Soper, and Robert Johnson (Children’s Hospital, Seattle, WA), Jacquelyn Zirbes (Stanford University Medical Center, Stanford, CA), Donna Wilkes and Melody Miki (Hospital for Sick Children, Toronto, ON, Canada), and Susan Minnix and Caroline LaFave (UNC-Chapel Hill). The authors thank Kimberly Burns, Whitney Wolf, Rhonda Pace, and Michael Patrone for technical assistance; Elizabeth Godwin for administrative support; and Syanne Olson for editorial assistance.
Footnotes
Supported by (1) NIH 5 U54 HL09640958-09 and 5R01HL071798 funded by the Office of Rare Diseases Research (ORDR, NCATS) and administered by the NHLBI; (2) CTSA NIH/NCATS UNC ULTR000083; (3) CTSA NIH/NCATS Colorado UL1TR000154; (4) NIH T32ES007018; and (5) the Intramural Research Program of NIH/NIAID. The Genetic Disorders of Mucociliary Clearance Consortium (5 U54HL096458) is a part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN), supported through collaboration between the NIH Office of Rare Diseases Research (ORDR) at the National Center for Advancing Translational Science (NCATS) and the NIH National Heart, Lung, and Blood Institute (NHLBI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: M.W.L. and M.R.K., designed study; M.W.L., M.R.K., and L.M.L., prepared manuscript; M.J.H., developed SOPs for nNO measurements; K.K.C., B.R.B., A.J.S., and D.E.B., performed nNO measurements at UNC; L.M.L., B.J.H., and B.Q., performed biostatistical analysis with GEE methodology; J.L.C., reviewed EMs; M.W.L., M.R.K., S.D.Da., S.D.De., T.W.F., J.J.A., K.N.O., S.D.S., M.R., and C.M., recruited and evaluated patients at GDMCC sites; H.-S.L. and J.K., coordinated web-based data entry; M.A.Z., coordinated and validated genetic studies.
This article has an online supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Afzelius BA. A human syndrome caused by immotile cilia. Science. 1976;193:317–319. doi: 10.1126/science.1084576. [DOI] [PubMed] [Google Scholar]
- 2.Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW. Primary ciliary dyskinesia: recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med. 2013;188:913–922. doi: 10.1164/rccm.201301-0059CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown DE, Pittman JE, Leigh MW, Fordham L, Davis SD. Early lung disease in young children with primary ciliary dyskinesia. Pediatr Pulmonol. 2008;43:514–516. doi: 10.1002/ppul.20792. [DOI] [PubMed] [Google Scholar]
- 4.Barbato A, Frischer T, Kuehni CE, Snijders D, Azevedo I, Baktai G, Bartoloni L, Eber E, Escribano A, Haarman E, et al. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur Respir J. 2009;34:1264–1276. doi: 10.1183/09031936.00176608. [DOI] [PubMed] [Google Scholar]
- 5.Papon JF, Coste A, Roudot-Thoraval F, Boucherat M, Roger G, Tamalet A, Vojtek AM, Amselem S, Escudier E. A 20-year experience of electron microscopy in the diagnosis of primary ciliary dyskinesia. Eur Respir J. 2010;35:1057–1063. doi: 10.1183/09031936.00046209. [DOI] [PubMed] [Google Scholar]
- 6.Leigh MW, O’Callaghan C, Knowles MR. The challenges of diagnosing primary ciliary dyskinesia. Proc Am Thorac Soc. 2011;8:434–437. doi: 10.1513/pats.201103-028SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noone PG, Leigh MW, Sannuti A, Minnix SL, Carson JL, Hazucha M, Zariwala MA, Knowles MR. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med. 2004;169:459–467. doi: 10.1164/rccm.200303-365OC. [DOI] [PubMed] [Google Scholar]
- 8.Narang I, Ersu R, Wilson NM, Bush A. Nitric oxide in chronic airway inflammation in children: diagnostic use and pathophysiological significance. Thorax. 2002;57:586–589. doi: 10.1136/thorax.57.7.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wodehouse T, Kharitonov SA, Mackay IS, Barnes PJ, Wilson R, Cole PJ. Nasal nitric oxide measurements for the screening of primary ciliary dyskinesia. Eur Respir J. 2003;21:43–47. doi: 10.1183/09031936.03.00305503. [DOI] [PubMed] [Google Scholar]
- 10.Corbelli R, Bringolf-Isler B, Amacher A, Sasse B, Spycher M, Hammer J. Nasal nitric oxide measurements to screen children for primary ciliary dyskinesia. Chest. 2004;126:1054–1059. doi: 10.1378/chest.126.4.1054. [DOI] [PubMed] [Google Scholar]
- 11.Walker WT, Jackson CL, Lackie PM, Hogg C, Lucas JS. Nitric oxide in primary ciliary dyskinesia. Eur Respir J. 2012;40:1024–1032. doi: 10.1183/09031936.00176111. [DOI] [PubMed] [Google Scholar]
- 12.Santamaria F, De Stefano S, Montella S, Barbarano F, Iacotucci P, Ciccarelli R, Sofia M, Maniscalco M. Nasal nitric oxide assessment in primary ciliary dyskinesia using aspiration, exhalation, and humming. Med Sci Monit. 2008;14:CR80–CR85. [PubMed] [Google Scholar]
- 13.Lundberg JO, Rinder J, Weitzberg E, Lundberg JM, Alving K. Nasally exhaled nitric oxide in humans originates mainly in the paranasal sinuses. Acta Physiol Scand. 1994;152:431–432. doi: 10.1111/j.1748-1716.1994.tb09826.x. [DOI] [PubMed] [Google Scholar]
- 14.Lundberg JO. Nitric oxide and the paranasal sinuses. Anat Rec (Hoboken) 2008;291:1479–1484. doi: 10.1002/ar.20782. [DOI] [PubMed] [Google Scholar]
- 15.American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 16.Leigh MW, Zariwala MA, Knowles MR. Primary ciliary dyskinesia: improving the diagnostic approach. Curr Opin Pediatr. 2009;21:320–325. doi: 10.1097/MOP.0b013e328329cddb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carson JL, Collier AM, Fernald GW, Hu SC. Microtubular discontinuities as acquired ciliary defects in airway epithelium of patients with chronic respiratory diseases. Ultrastruct Pathol. 1994;18:327–332. doi: 10.3109/01913129409023201. [DOI] [PubMed] [Google Scholar]
- 18.Olin JT, Burns K, Carson JL, Metjian H, Atkinson JJ, Davis SD, Dell SD, Ferkol TW, Milla CE, Olivier KN, et al. for the Genetic Disorders of Mucociliary Clearance Consortium. Diagnostic yield of nasal scrape biopsies in primary ciliary dyskinesia: a multicenter experience. Pediatr Pulmonol. 2011;46:483–488. doi: 10.1002/ppul.21402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Callaghan C, Rutman A, Williams GM, Hirst RA. Inner dynein arm defects causing primary ciliary dyskinesia: repeat testing required. Eur Respir J. 2011;38:603–607. doi: 10.1183/09031936.00108410. [DOI] [PubMed] [Google Scholar]
- 20.Knowles MR, Leigh MW, Carson JL, Davis SD, Dell SD, Ferkol TW, Olivier KN, Sagel SD, Rosenfeld M, Burns KA, et al. Genetic Disorders of Mucociliary Clearance Consortium. Mutations of DNAH11 in patients with primary ciliary dyskinesia with normal ciliary ultrastructure. Thorax. 2012;67:433–441. doi: 10.1136/thoraxjnl-2011-200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noone PG, Zariwala M, Sannuti A, Minnix S, Leigh MW, Carson J, Knowles MR. Mutations in DNAI1 (IC78) cause primary ciliary dyskinesia. Chest. 2002;121(3, Suppl):97S. [PubMed] [Google Scholar]
- 22.Fliegauf M, Olbrich H, Horvath J, Wildhaber JH, Zariwala MA, Kennedy M, Knowles MR, Omran H. Mislocalization of DNAH5 and DNAH9 in respiratory cells from patients with primary ciliary dyskinesia. Am J Respir Crit Care Med. 2005;171:1343–1349. doi: 10.1164/rccm.200411-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horváth J, Fliegauf M, Olbrich H, Kispert A, King SM, Mitchison H, Zariwala MA, Knowles MR, Sudbrak R, Fekete G, et al. Identification and analysis of axonemal dynein light chain 1 in primary ciliary dyskinesia patients. Am J Respir Cell Mol Biol. 2005;33:41–47. doi: 10.1165/rcmb.2004-0335OC. [DOI] [PubMed] [Google Scholar]
- 24.Hornef N, Olbrich H, Horvath J, Zariwala MA, Fliegauf M, Loges NT, Wildhaber J, Noone PG, Kennedy M, Antonarakis SE, et al. DNAH5 mutations are a common cause of primary ciliary dyskinesia with outer dynein arm defects. Am J Respir Crit Care Med. 2006;174:120–126. doi: 10.1164/rccm.200601-084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zariwala MA, Leigh MW, Ceppa F, Kennedy MP, Noone PG, Carson JL, Hazucha MJ, Lori A, Horvath J, Olbrich H, et al. Mutations of DNAI1 in primary ciliary dyskinesia: evidence of founder effect in a common mutation. Am J Respir Crit Care Med. 2006;174:858–866. doi: 10.1164/rccm.200603-370OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loges NT, Olbrich H, Becker-Heck A, Häffner K, Heer A, Reinhard C, Schmidts M, Kispert A, Zariwala MA, Leigh MW, et al. Deletions and point mutations of LRRC50 cause primary ciliary dyskinesia due to dynein arm defects. Am J Hum Genet. 2009;85:883–889. doi: 10.1016/j.ajhg.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lie H, Zariwala MA, Helms C, Bowcock AM, Carson JL, Brown DE, III, Hazucha MJ, Forsen J, Molter D, Knowles MR, et al. Primary ciliary dyskinesia in Amish communities. J Pediatr. 2010;156:1023–1025. doi: 10.1016/j.jpeds.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berg JS, Evans JP, Leigh MW, Omran H, Bizon C, Mane K, Knowles MR, Weck KE, Zariwala MA. Next generation massively parallel sequencing of targeted exomes to identify genetic mutations in primary ciliary dyskinesia: implications for application to clinical testing. Genet Med. 2011;13:218–229. doi: 10.1097/GIM.0b013e318203cff2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horani A, Druley TE, Zariwala MA, Patel AC, Levinson BT, Van Arendonk LG, Thornton KC, Giacalone JC, Albee AJ, Wilson KS, et al. Whole-exome capture and sequencing identifies HEATR2 mutation as a cause of primary ciliary dyskinesia. Am J Hum Genet. 2012;91:685–693. doi: 10.1016/j.ajhg.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antony D, Becker-Heck A, Zariwala MA, Schmidts M, Onoufriadis A, Forouhan M, Wilson R, Taylor-Cox T, Dewar A, Jackson C, et al. Mutations in CCDC39 and CCDC40 are the major cause of primary ciliary dyskinesia with axonemal disorganization and absent inner dynein arms. Hum Mutat. 2013;34:462–472. doi: 10.1002/humu.22261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knowles MR, Leigh MW, Ostrowski LE, Huang L, Carson JL, Hazucha MJ, Yin W, Berg JS, Davis SD, Dell SD, et al. Genetic Disorders of Mucociliary Clearance Consortium. Exome sequencing identifies mutations in CCDC114 as a cause of primary ciliary dyskinesia. Am J Hum Genet. 2013;92:99–106. doi: 10.1016/j.ajhg.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diggle PJ, Heagerty P, Liang KY, Zeger SL. Generalized linear models for longitudinal data: analysis of longitudinal data. 2nd ed. Oxford: Oxford University Press; 2002. [Google Scholar]
- 33.Mateos-Corral D, Coombs R, Grasemann H, Ratjen F, Dell SD. Diagnostic value of nasal nitric oxide measured with non–velum closure techniques for children with primary ciliary dyskinesia. J Pediatr. 2011;159:420–424. doi: 10.1016/j.jpeds.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Marthin JK, Nielsen KG. Hand-held tidal breathing nasal nitric oxide measurement—a promising targeted case-finding tool for the diagnosis of primary ciliary dyskinesia. PLoS One. 2013;8:e57262. doi: 10.1371/journal.pone.0057262. [DOI] [PMC free article] [PubMed] [Google Scholar]