Abstract
The nuclear receptor Liver Receptor Homolog-1, LRH-1, plays an important role in controlling lipid and cholesterol homeostasis and is a potential target for treatment of diabetes and hepatic diseases. LRH-1 is known to bind phospholipids (PLs) but the role of PLs in controlling LRH-1 activation remains highly debated. Here we describe the structure of both apo LRH-1 and the protein in complex with the antidiabetic dilauroylphosphatidylcholine (DLPC). Our studies show that DLPC binding is a novel dynamic process that alters coregulator selectivity and that the lipid-free receptor interacts with widely expressed corepressors. These observations greatly enhance our understating of LRH-1 regulation and highlight its importance as a novel therapeutic target for controlling diabetes.
Keywords: nuclear receptor, stem cells, phospholipids, NR5A, Diabetes, phosphatidylcholine
The regulation of lipid and glucose homeostasis is of central importance to human physiology. Governing this process are both extra- and intracellular receptors that sense hormones and nutrients, namely fatty acids and glucose, to control behavior and nutrient homeostasis. Hepatic lipid metabolism and glucose regulation are intimately related and lipid accumulation can lead to metabolic diseases such as steatosis and diabetes. Recently, the dietary PL, DLPC (PC 12:0/12:0), was shown to lower serum lipid levels and reduce blood glucose levels in diabetic mice1. This effect is completely dependent on the LRH-1, a PL binding orphan nuclear receptor (NR)1. This medium chain PC selectively activates LRH-1 mediated transcription in luciferase assays, increases the ability of LRH-1 to interact with coactivators, and increases the production of LRH-1 target genes. We have shown using mass spectrometry that DLPC is able to competitively displace larger PLs from the ligand binding domain of hLRH-1 in vitro while longer chain PLs such as DPPC (C16:0/C16:0) can not1. Thus, while a range of PLs bind to LRH-12-4, acyl chain length and head group composition dictate transcriptional activation1. The mechanism governing this selective activation, however, is unclear since the binding mode of DLPC is unknown.
LRH-1 is a member of the NR5A class of NRs regulating the expression of genes central to embryonic development, reproduction, lipid homeostasis and energy metabolism5,6. LRH-1 is required to maintain Oct4 expression in undifferentiated embryonic stem cells to maintain pluripotency7,8. In breast cancer, LRH-1 regulates both estrogen synthesis and estrogen receptor (ER) expression9-14. This, along with direct transcriptional regulation of LRH-1 expression by ER, makes LRH-1 a key element in the feed-forward loop driving sustained estrogen biosynthesis and signalling in ER+ breast cancer9-14. In hepatic tissues, LRH-1 regulates genes central to lipid and bile acid homeostasis6.
LRH-1, like most NRs, interacts with coactivators via a LXXLL motif (where X is any amino acid) at the interaction surface formed by an “active” AF-H packed against helices 3 and 415. However, LRH-1 also uses this same “active” surface to interact with the atypical NRs SHP and Dax-1, which generally act to repress LRH-1 in hepatic tissues16,17. This, combined with the fact that all NR5A receptors have crystallized with the AF-H in the active orientation, has led to the belief that LRH-1 is not optimized to interact with typical corepressors such as SMRT and NCoR and the position of the AF-H is not altered by ligand binding18,19. Indeed, recombinant LRH-1 loaded with co-purified E. coli lipids is incapable of binding to SMRT, despite being specifically repressed by SMRT in a dose dependent manner in vivo20.
RESULTS
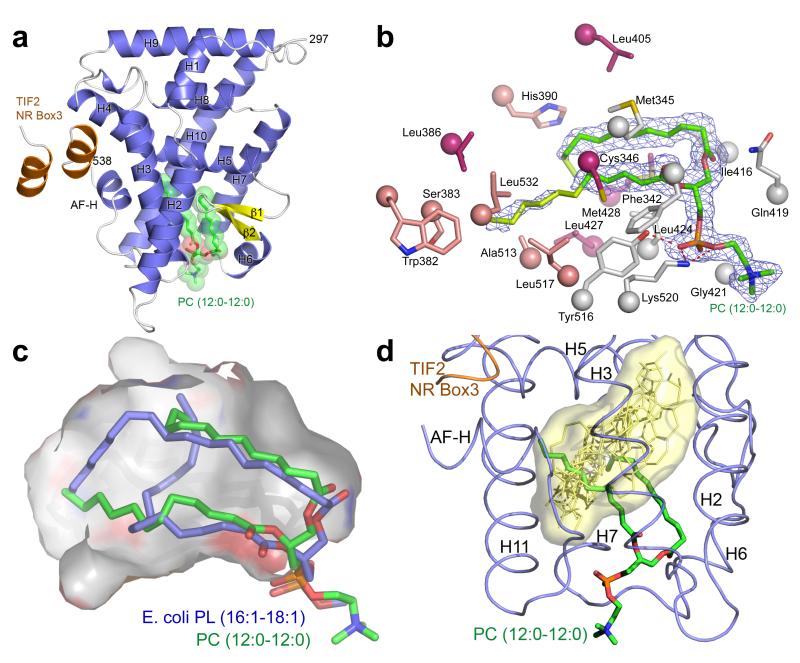
To visualize the molecular mechanism driving DLPC biology we determined the structure of the hLRH-1 LBD in complex with DLPC and a fragment of the human coactivator Transcriptional Intermediary Factor-2 (TIF2) to a resolution of 1.8 Å (Fig. 1A and Supplementary Table 1). Electron density within the ligand binding pocket (LBP) showed clear evidence for bound DLPC (Fig. 1B). Electron density was considerably weaker for the distal portion of the lipid tails, indicating that the terminal 2 atoms on the sn-1 acyl tail and 6 atoms on the sn-2 acyl tail are mobile. This is in stark contrast to the larger co-purified E. coli PLs reported previously (C16:1/C18:1) which show fully ordered lipid tails that intertwine to fully occupy the LBP (Fig. 1C)21-23. We hypothesized, that DLPC, with 10 fewer acyl chain carbons, would insert itself deeper in the LBP. Instead, the phosphoglycerol backbone of DLPC binds to LRH-1’s ~1300 Å3 LBP in a similar position to the phosphoglycerol backbone of E. coli lipids in previously reported structures 2,3,24. The ~240 Å3 difference in molecular volume between the LBP occupying atoms of DLPC and E. coli PL translates directly to additional unoccupied space in the deepest regions of the LBP, increasing the unoccupied pocket volume to ~870 Å3. Previous LRH-1 structures have done little to identify which portions of the LBP are important for coordinating receptor activation. The binding mode of DLPC is radically different from the endogenous ligands of other classes of NRs as the space used to coordinate ligands in nearly all other family members is left almost completely unfilled (Fig. 1D). Instead, the most ordered regions of the bound DLPC are near the “mouth” of the LBP (defined by loops between helices 2-3, 6-7 and 10-AF-H). Comparing differential lipid – LRH-1 residue contacts between the atoms of DLPC and bacterial PL in PDB 1YUC, reveals that DLPC maintains contacts at the mouth of the pocket and loses contacts with residues on H5, H7 and the AF-H (Supplementary Fig. 1 and Fig. 1B). Taken together, these results suggest that a unique set of protein-ligand interactions outside of the canonical LBP and a lack of interactions in the deepest regions of the pocket governs LRH-1 transcriptional activation by DLPC.
FIGURE 1. DLPC binds directly to LRH-1 and promotes activation through unique interactions.
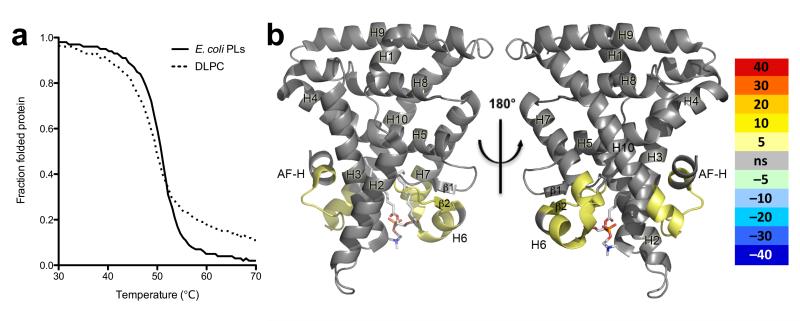
(A) Ribbon diagram of DLPC bound hLRH-LBD (α-helices, blue; β-strands, yellow) with the human TIF (hTIF) NR box 3 peptide (orange). The bound phospholipid is depicted as sticks (C, green; O, red; P, magenta; N, blue) surrounded by transparent spheres. (B) Fo – Fc omit electron density (contoured at 1 σ) for the bound ligand DLPC (C12:0/C12:0) well ordered lipid atoms are colored in green while poorly ordered atoms are colored in lime, along with side chains lining the LBP of hLRH-1 that contact DLPC atoms with strong electron density (grey), DLPC atoms with weak electron density (salmon), and side chains that contact bacterial PL (pdb 1YUC) but not DLPC (pink). (C) Superposition of the bacterial C16:1/C18:1 phospholipid from 1YUC (blue) with DLPC (green) with the surface of the LBP outlined in grey. (D) Superposition of DLPC (green) bound hLRH-1 (blue) with the endogenous ligands of representative NR family members (RXR, VDR, ERα, PPARα, FXR, and TR) shown as yellow sticks with their combined overall surface highlighted in light yellow. (E) Thermal melting monitored by circular diochroism showing native LRH-1 LBD (solid line) Tm= 50.90 +/− 0.021 C° and DLPC bound LRH-1 (dashed line) Tm= 49.12 +/− 0.0384. Native and DLPC bound LRH-1 LBD are at identical concentrations. (F) Differential HDX between native LRH-1 and DLPC bound LRH-1 LBD mapped onto PDB ID code 1YUC. Percent deuterium incorporation is indicated by color scale bar.
The impact of these differential contacts on the conformational dynamics of LRH-1 is difficult to predict via the crystal structure alone; therefore, we used thermal unfolding and hydrogen deuterium exchange coupled to mass spectrometry (HDX) to assess DLPC’s effect on LRH-1’s LBD in solution. Consistent with the observation that DLPC occupied less space and contacted fewer amino acids than E. coli PLs, DLPC binding decreased LRH-1’s ability to resist thermal denaturation (Fig. 1E). To identify which regions of the protein were being specifically destabilized by DLPC, we used high resolution HDX to compare the E. coli PL bound receptor and the LRH-1 DLPC complex. This solution based structure probing revealed that the β-sheet – helix 6 region and surprisingly helix 10 and the AF-H were more dynamic in the DLPC complex suggesting that differences PL acyl tail length may affect the ability of LRH-1 to interact with co-regulators (Fig. 1F and Supplementary Fig. 2).
To date LRH-1 has only been characterized in complex with contaminating E. coli PLs2,3. We therefore used organic solvents to denature and strip recombinantly expressed LRH-1 LBD of bound E. coli PLs followed by refolding to generate apo receptor. Lipid phosphorous assays confirmed that the receptor contains only trace amounts of PL (Fig. 3B, and Supplementary Fig. 4)2 while circular dichroism detected similar secondary structure composition albeit with less overall secondary structure than native LRH-1 (Supplementary Fig. 3).
FIGURE 3. DLPC binding affects LRH-1 dynamics and coregulator preference.
(A) Activity of the wild-type, G398A, and G421A LRH-1 mutants in HeLa cells using a SHP luc reporter. Data are represented as mean +/− SEM; * denotes p < 0.05. (B) Total phospholipid quantification of LRH-1 variants following chloroform: methanol extraction. (C) Kds from Table S2 plotted in bar graph form as the inverse log of the Kd +/− SEM. Apo LRH-1 is chloroform:methanol extracted and refolded as detailed in Material and Methods, PG/PE (14-20) are the range of PLs bound to LRH-1 when purified from E. coli and DLPC is LRH-1 that has been loaded with DLPC and re-purified as detailed in Material and Methods. The wedges at top represent LRH-1’s preference for coactivators (PGC-1α, SRC1, TIF2) vs. corepressors (SHP, SMRT) in varying liganded states.
To identify novel mobile regions that are sensitive to ligand status, we performed a low-resolution proteolysis protection assay coupled to quantitative mass spectrometry. By mapping the chymotrypsin proteolysis patterns in DLPC bound vs. apo receptor we were able to identify regions of the protein that were highly mobile and protease sensitive that are stabilized upon DLPC binding (Supplementary Fig. 5 and Supplementary Table 2). Contrary to our expectations we found that the most mobile portion of apo LRH-1 is helix 10/11 and the AF-H. This observation directly refutes the idea that the AF-H of LRH-1 is rigid and insensitive to ligand status2-4,18. To quantify the relative conformational mobility of the receptor in the both the apo and ligand bound states we again employed high resolution HDX, revealing that the β-sheet – helix 6 region is even more dynamic than the AF-H (Fig. 2A-B and Supplementary Fig. 6). We were also surprised to find regions of the receptor, such as helix 9 and 10, specifically stabilized upon lipid binding. These helices have been recently reported to be the interaction site for β-catenin, which serves as a coactivator for LRH-125. These data suggest that β-catenin’s coactivation of LRH-1 may be ligand regulated.
FIGURE 2. Structure of apo LRH-1 identifies a novel mobile activation function region.
(A) % deuterium incorporation over time for apo LRH-1 LBD and (B) native LRH-1 LBD mapped to PDB ID code 1YUC. (C) Ribbon diagram of apo hLRH-LBD (α-helices, blue) with the human SHP (hSHP) NR box 1 peptide (red). Residues 398-420 lack traceable main chain density and have been omitted from the structure (dashed line). (D) Molecular surface of apo LRH-1 (grey) with residues 398-420 (pink) and DLPC (green) superposed from DLPC bound hLRH-1.
To directly visualize these structural differences we determined a structure of apo LRH-1, stabilized by a fragment of the atypical corepressor SHP, to a resolution of 1.9 Å representing an inactivated form of the receptor to juxtapose with the active LRH-1 – DLPC complex (Fig. 2C and Supplementary Table 1). Unexpectedly, we found that in the absence of PL, residues 397-421 are completely disordered including both β-strands and helix 6 which form one wall of the LBP and one half of the mouth of the receptor (Fig. 2C-D and Supplementary Fig. 7). Since this region forms extensive contacts with DLPC, and was identified as having an altered HDX profile when complexed with different length PLs, we hypothesized that this mobile element may be important for sensing and transmitting ligand status. To test whether conformational flexibility in this region is required for efficient PL binding and transcriptional activation we individually mutated two conserved flanking glycines at positions 398 and 421 to alanine (Fig. 2C). In support of our hypothesis we found that the both mutations significantly reduced LRH-1’s ability to activate transcription (Fig. 3A). Only the G421A mutation significantly reduced the ability of the protein to bind PLs when purified from E. coli (Fig. 3B); however, G421 participates in a backbone amide H-bond with the PL phosphate. While a corresponding alanine mutation should preserve this interaction, based on the allowed backbone torsion angles, the greater conformational mobility of G241 clearly plays a significant role in recognizing PLs. Thus, we have identified the β-sheet – helix 6 region as a novel activation function of LRH-1, critical for sensing ligand status and for driving receptor activation.
DISCUSSION
The traditional model explaining NR transcriptional activation describes a system where high affinity ligand binding drives a conformational switch from an inactive to an active state involving a repositioning of the AF-H. Appropriate NR activation is the result of a fine balance between receptor stability and ligand affinity. In the absence of ligand, NRs populate a partially unfolded or “molten globule” state where ligand binding catalyzes proper receptor folding and activation26,27. The binding energy gained from contacts with the ligand is not enough to explain the high affinity; rather, ligand binding allows for additional intramolecular contacts at the mouth of the LBP between helices 2-3, 6-7, and 10-AF-H which ultimately supports receptor activation26. In contrast, it is clear from the LRH-1 – DLPC structure that activating PLs do not facilitate equivalent direct interactions. Rather, LRH-1 relies on PLs to bridge these critical intramolecular interactions with intermolecular interactions to achieve receptor activation. In this way, LRH-1 has tinkered with the canonical molecular switch – adapting it to respond to diverse PL ligands by using the phosphoglycerol backbone to transmit a signal from helix 6 to the AF-H while relying on deep pocket interaction with the lipid tails to fine tune receptor dynamics and thus co-regulator specificity.
Since our data show that the AF-H is mobile in the absence of ligand, we investigated whether apo LRH-1 is capable of binding to a traditional corepressor such as SMRT, which requires the AF-H to leave the active orientation. Contrary to studies using receptor co-purified with contaminating E. coli PLs20, we found that a peptide derived from SMRT containing the corepressor motif (LxxxIxxxI/L) binds to apo LRH-1 with a 49.6 nM affinity (Fig. 3C and Supplementary Table 3). This is by far the highest affinity interaction between LRH-1 and a coregulator derived peptide that we have tested, supporting our observation that in the absence of ligand, the AF-H is free to rotate away from helix 3 and helix 4 to allow traditional corepressor binding. This data is in line with previous data showing that SMRT is capable of repressing LRH-1 in vivo in a dose dependent manner and provides the first direct evidence that LRH-1 may be sensitive to repression by direct interactions with traditional corepressors18,20. Since, SMRT binding requires displacement of the AF-H, it is likely that at least a portion of LRH-1 remains in the apo form in cells. In addition, SHP is able to bind both the apo receptor and the receptor loaded with bacterial PLs; however, SHP binding is completely lost upon DLPC addition. Since LRH-1’s repression in the liver dictates much of LRH-1’s effects on gene expression 6,28, the effects of DLPC in the liver may be due in part to relieved SHP and SMRT repression rather than enhanced coactivator interaction.
Like a true agonist, DLPC simultaneously enhanced coactivator peptide recruitment while disfavoring both typical and atypical corepressor peptide interaction (Fig. 3C and Supplementary Table 2). However, the coactivators SRC-1 and TIF2 retain their ability to interact with LRH-1 in the absence of ligand explaining its low basal activity. These results clearly show that LRH-1 undergoes profound structural changes upon ligand binding and definitively confirms LRH-1’s viability as a therapeutic target for both agonist and antagonist design. Our results also suggest that dietary PLs may have signaling effects outside hepatic tissues. For example, if DLPC is trafficked out of the liver it may exacerbate LRH-1’s malicious role in breast cancer by selectively recruiting PGC-1α to Aromatase and Estrogen Receptor promoters, driving increased estrogen synthesis to fuel local tumor growth. Finally, these findings may facilitate the development of tissue selective LRH-1 modulators by revealing discrete regions of the LBP required for the recruitment of distinct coregulators.
METHODS
Reagents
Chemicals were purchased from Sigma, Fisher or Avanti Phospholipids. pMALCH10T and the vector for His tagged TEV was a gift from John Tesmer (UT Austin). pLIC_MBP and pLIC_HIS were gifts from John Sondek (UNC, Chapel Hill). Peptides were synthesized by RS Synthesis (Louisville, KY).
Protein Expression and Purification
The human LRH-1 LBD (residues 291-541) was cloned and purified as described previously 2. The drosophila Ftz-F1 LBD, residues 791-1025, was cloned into the pLIC_MBP vector C-terminal to a cassette containing a 6xHis tag, maltose binding protein (MBP) and a TEV protease cleavage site. A second construct of hLRH-1 (residues 299-541) was cloned into the pLIC_HIS vector C-terminal to a cassette containing a 6xHis tag and a TEV protease cleavage site. The fusion proteins were expressed in BL21(DE3) pLysS cells using standard methods and purified using affinity chromatography (His Select, Probond) with TEV cleavage of the fusion partners. For the DLPC LRH-1 complex, purified LRH-1 LBD (residues 299-541) was incubated with DLPC vesicles, prepared by sonication to optical clarity, at a 1:20 (protein:lipid) molar ratio for 24 hours 22 °C. Receptor was purified away from unbound lipids by size exclusion chromatography, dialyzed against 60mM NaCl, 100mM ammonium acetate, pH7.4, 1mM DTT, 1mM EDTA, and 2mM CHAPS and concentrated to 5-7 mg ml−1. For apo LRH-1 crystallization, purified LRH-1 LBD (residues 291-541) was incubated with 1,2-ditetracosanoyl-sn-glycero-3-phosphocholine (PC 24:0-24:0) (Avanti Polar Lipids) and (RJW101) at a final PC24:ligand:protein ratio of 20:3:1 29. The receptor was purified away from unbound PC 24:0-24:0 and the weakly bound agonist by size exclusion chromatography, dialyzed against 60mM NaCl, 100mM ammonium acetate, pH7.4, 1mM DTT, 1mM EDTA, and 2mM CHAPS and concentrated to 5-7 mg ml−1 Structure Determination- Crystals of the LRH-1 LBD – DLPC – hTIF2 complex were grown by hanging drop vapor diffusion at 20 °C from solutions containing 3 μL of protein at 6.5 mg/mL protein complexed with a peptide derived from human TIF2 NR Box 3 (+H3N- KENALLRYLLDKDD-CO2−) at a 1:4 molar ratio and 1 μL of the following crystallant: 18%-24% PEG 400, 5% glycerol, 0.1M lithium sulfate, and 0.1M sodium acetate pH 5.2. Crystals were cryoprotected in crystallant containing 12% glycerol and 12% ethylene glycol and flash-frozen in liquid N2. Crystals of apo LRH-1 LBD hSHP complex were grown by hanging drop vapor diffusion at 20 °C from solutions containing 1 μL of protein at 4 mg/mL protein complexed with a peptide derived from human SHP NR box 1 (+H3N-QGAASRPAILYALLSSSLK-CO2−) at a 1:4 molar ratio and 1 μL of the following crystallant: 9.5%-15% PEG 3350, 5% glycerol, and 50 mM Bis-Tris, pH 6.4. Crystals were cryoprotected in crystallant containing 20% glycerol and flash-frozen in liquid N2. Data to 1.8 Å (DLPC) and 1.9 Å (apo) resolution were collected at 100 K at the South East Regional Access Team (SER-CAT) at the Advanced Photon Source (Argonne, IL), and were processed and scaled with HKL2000 (Table 1) 30. Initial phases were determined using previously published hLRH-1 coordinates (pdb 1YOK or 1YUC respectively) as a molecular replacement search model. The structure was refined using REFMAC5 within the CCP4 suite of programs 31,32 and model building was performed in COOT 33,34.
Mass Spectrometry
Samples were analyzed using electrospray mass injection-MS in the negative-ion mode to detect and identify phospholipids. Approximately 6 mg of wild-type or mutant forms of LRH-1 LBD and Ftz-F1 LBD were extracted with a 2:1 chloroform/methanol solution, diluted in 200 μL of chloromethylene, and analyzed by negative ion ESI/MS on a Thermo LTQ FTMS using direct injection analysis with electrospray ionization (Thermo Finnigan, Somerset, NJ). All extractions were performed in duplicate. The high-resolution analyses were performed in the FTMS at a resolution of 100000 at 400 m/z. The MS/MS experiments were done in the ion trap portion of the instrument with a mass selection of 3 amu and a normalized collision energy of 30 V. The major phospholipid species were identified by accurate mass measurements and MS/MS via collisionally induced dissociation (CID), which yields product ions characteristic of the head groups and attached fatty acids. Acquisition and analyses were performed using the instrument’s Analyst QS software.
Phospholipid Quantification
Preceding phospholipid quantification, 6 mgs of protein was subjected to chloroform:methanol extraction according to the two-step Bligh and Dyer method 35 to isolate phospholipid as described previously 2. Phospholipid quantification was performed according to the improved procedures for the determination of lipid phosphorous by malachite green 36. Briefly, lipid extracts were dried completely and digested with perchloric acid (70%) at 140°C until all color disappeared. To the cooled tubes, a solution of malachite green and ammonium molybdate was added and vortexed for 20 min. The interaction between phosphomoylbdenum and malachite green was monitored at 660 nm.
Generation of apo LRH-1
Pure protein was subjected to chloroform:methanol extraction (2:1) to remove bound lipids according to the Bligh & Dyer method 35. The resulting pellet containing denatured protein was washed three times with chloroform to remove any trace lipids associated with the protein or vessel. The resulting white pellet was then dried by evaporation and resuspended in 6 M Guanidinium HCl. Empty protein was then refolded by fast dilution into a buffer containing 10mM K2HPO4, 100mM Tris (pH 7.4), 1 mM EDTA, and 500uM CTAB at 4°C. After ~ 20 hours, protein was then concentrated and purified by size exclusion chromatography to ensure a homogenous population of refolded receptors.
Transient Transfection Assay
HeLa cells were cultured in DMEM supplemented with 10% (v/v) fetal bovine serum (HyClone). The cells were seeded overnight into 96-well cell-culture plates. The cells were then transfected with a Lipofectamine (Invitrogen)/DNA mixture containing 100 ng of SHP promoter firefly luciferase reporter construct, 25 ng of pCI_LRH-1 receptor (WT or mutant), and 1ng renilla luciferase. Firefly luciferase activity was assayed using a BioTek Synergy 4 spectrophotometer and normalized to renilla luciferase activity 24 h after transfection. Transfections were performed with six replicates. The results presented are the average of two independent transfection experiments.
Cofactor binding assays
The polarization of fluorescein labeled peptides derived from human SHP NR box 1 (+H3N-QGAASRPAILYALLSSSLK-CO2−), human PGC-1α NR Box 2 (+H3N-EEPSLLKKLLLAPA- CO2−), SRC-1 NR Box 2 (+H3N- SPSSHSSLTERHKILHRLLQEGSP-CO2−), SMRT (+H3N- TNMGLEAIIRKALMGKYDQW-CO2−) or TIF2 NR Box 3 (+H3N-PVSPKKKENALLRYLLDKDDT-CO2−) containing the NR coactivator motif (LXXLL) was monitored using a BioTek Synergy 4 spectrophotometer with polarizers (Winooski, VT) as a function of protein concentration. Experiments were conducted in 150 mM sodium chloride, 20 mM Tris-HCl (pH 7.4), and 5% (v/v) glycerol unless stated otherwise. All experiments were performed in triplicate and data were fit with GraphPad Prism 5 (GraphPad, Inc.) by linear least-squares methods to a single site binding model.
Proteolytic Protection Assay
DLPC or apo hLRH-1 (11.25 μg) was digested with 80 ηg of chymotrypsin (Protea Biosciences, Inc.) for 5 minutes at room temperature. The reaction was quenched with the addition of acetic acid and boiled for 5 minutes. The entire reaction was resolved by SDS-PAGE and stained by Coomassie blue. Gel regions below undigested intact protein were excised and were subjected to in-gel trypsin digestion. The digested peptides were analyzed by reverse-phase liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) as previously described 37. Briefly, peptide mixtures were loaded onto a C18 column (75 μm i.d., 30 cm long, 3 μm resin from Michrom Bioresources, Inc., Auburn, CA) and eluted over a 12-35% gradient (Buffer A: 0.1% Formic Acid, 0.005% heptafluorobutyric acid, and 5% AcN; Buffer B: 0.1% formic acid, 0.005% heptafluorobutyric acid, and 95% AcN). Eluates were monitored in a MS survey scan followed by ten data-dependent MS/MS scans on an LTQ-Orbitrap ion trap mass spectrometer (Thermo Finnigan, San Jose, CA). The LTQ was used to acquire MS/MS spectra (2 m/z isolation width, 35% collision energy, 5,000 AGC target, 300 ms maximum ion time). The Orbitrap was used to collect MS scans (300-1600 m/z, 1,000,000 AGC target, 750 ms maximum ion time, resolution 60,000). The acquired MS/MS spectra were searched against a concatenated target-decoy E.coli database (UNIPROT January 23, 2011) that included the hLRH-1 sequence using the SEQUEST Sorcerer algorithm (version 2.0, SAGE-N) 38. Searching parameters included: partially tryptic restriction, parent ion mass tolerance (± 10 ppm), product ion tolerance (± 0.5 m/z), and dynamic modifications for oxidized Met (+15.9949 Da). The peptides were classified by charge state and trypticity (fully and partial) and filtered dynamically by increasing XCorr and ΔCn values to reduce the protein false discovery rate to less than 5%. The MS/MS spectra of matched hLRH-1 peptides were manually inspected. Trypsin digest sites were removed manually and spectral counts per peptide were used to determine the relative amount of each chymotrypsin proteolysis fragment between DLPC and apo hRLH-1. The protection factor reported in Supplementary Fig. 5 and Supplementary Table S2 is the result of subtracting spectral counts for chymotryptic proteolysis fragments observed in the LRH-1-DLPC complex from the same chymotryptic proteolysis fragments generated from the apo protein. A higher protection factor indicates less chymotrypsin cleavage events upon DLPC binding.
Circular Dichroic Spectroscopy and Thermal Unfolding Studies
Circular dichroism (CD) studies were performed on a Jasco J-800 spectropolarimeter with a 1-mm cell. Proteins were dissolved at a concentration of 0.2 mg/mL in 20 mM tris (pH 7.4), 0.1 M sodium chloride, and 10% glycerol. Wavelength scans were performed at 25 °C from 200 to 250 nm at a rate of 50 nm/min. For thermal unfolding studies, ellipticity was continuously monitored at 220 nm while the temperature was raised by use of a Jasco PFD-425S temperature control unit from 25 to 80 °C at a rate of 1 °C/min. The α-helix/ β-sheet ratio was calculated using the k2d3 server http://www.ogic.ca/projects/k2d339.
HDX
Solution-phase amide HDX was performed with a fully automated system as described previously40. Briefly, 4 μL of protein was diluted to 20 μL with D2O-containing HDX buffer, and incubated at 25°C for; 10 s, 30 s, 60 s, 900 s, or 3600 s. Following on-exchange, unwanted forward or back exchange was minimized and the protein is denatured by dilution to 50 μL with 0.1% TFA in 5 M urea (held at 1 °C). Samples are then passed across an immobilized pepsin column (prepared in house) at 50μL min-1 (0.1% TFA, 15 °C) and the resulting peptides were trapped on a C8 trap cartridge (Thermo Fisher, Hypersil Gold). Peptides were then gradient eluted (4% CH3CN to 40% CH3CN, 0.3% formic acid over 5 minutes, 2°C) across a 1 mm × 50 mm C18 HPLC column (Hypersil Gold, Thermo Fisher) and electrosprayed directly into an Orbitrap mass spectrometer (LTQ Orbitrap with ETD, Thermo Fisher). Data was processed with in-house software and visualized with pyMOL (DeLano Scientific). To measure the difference in exchange rates, we calculated the average percentage deuterium uptake for native LRH-1 LBD following 10, 30, 60, 900 and 3600 seconds of on exchange. From this value, we subtract the average percent deuterium uptake measured for the DLPC bound LRH-1 LBD. Positive perturbation values means that the exchange rate is faster for these regions within LRH-1 bound to DLPC.
Supplementary Material
FIGURE 4.
Acknowledgements
We thank Dr. Nicholas T. Seyfried in the Dept. of Biochemistry at the Emory University for his help with acquiring the mass spectral data.
This work was supported with start up funds from Emory University. PMM was supported by Emory-NIEHS Graduate and Postdoctoral Training in Toxicology (T32ES012870).
Abbreviations
- human LRH-1
(hLRH-1)
- mouse LRH-1
(mLRH-1)
- drosophila melanogaster Ftz-F1
(Ftz-F1)
- 1,2-diundecanoyl-sn-glycero-3-phosphocholine
(DUPC, PC 11:0/11:0)
- 1,2-dilauroyl-sn-glycero-3-phosphocholine
(DLPC, PC 12:0/12:0)
- phospholipid
(PL)
- Silencing Mediator for Retinoid and Thyroid-hormone receptors
(SMRT)
- Transcriptional Mediators/Intermediary Factor 2
(TIF2)
- Steroid Receptor Coactivator 1
(SRC-1)
- Small Heterodimer Partner
(SHP)
- dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome, gene 1
(Dax-1)
- Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
(PGC-1α)
- nuclear receptor
(NR)
Footnotes
ACCESSION NUMBERS:
Coordinates and structure factors have been deposited in the Protein Data Bank with accession numbers #4DOR and #4DOS.
References
- 1.Lee JM, et al. A nuclear-receptor-dependent phosphatidylcholine pathway with antidiabetic effects. Nature. 2011 doi: 10.1038/nature10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortlund EA, et al. Modulation of human nuclear receptor LRH-1 activity by phospholipids and SHP. Nat Struct Mol Biol. 2005 doi: 10.1038/nsmb910. [DOI] [PubMed] [Google Scholar]
- 3.Krylova IN, et al. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell. 2005;120:343–55. doi: 10.1016/j.cell.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, et al. Structural and biochemical basis for selective repression of the orphan nuclear receptor liver receptor homolog 1 by small heterodimer partner. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9505–10. doi: 10.1073/pnas.0501204102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Marcos PJ, Auwerx J, Schoonjans K. Emerging actions of the nuclear receptor LRH-1 in the gut. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbadis.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee YK, Moore DD. Liver receptor homolog-1, an emerging metabolic modulator. Front Biosci. 2008;13:5950–8. doi: 10.2741/3128. [DOI] [PubMed] [Google Scholar]
- 7.Wagner RT, Xu X, Yi F, Merrill BJ, Cooney AJ. Canonical Wnt/beta-catenin regulation of liver receptor homolog-1 mediates pluripotency gene expression. Stem Cells. 2010;28:1794–804. doi: 10.1002/stem.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu P, et al. Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development. Mol Cell Biol. 2005;25:3492–505. doi: 10.1128/MCB.25.9.3492-3505.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clyne CD, Speed CJ, Zhou J, Simpson ER. Liver receptor homologue-1 (LRH-1) regulates expression of aromatase in preadipocytes. J Biol Chem. 2002;277:20591–7. doi: 10.1074/jbc.M201117200. [DOI] [PubMed] [Google Scholar]
- 10.Clyne CD, et al. Regulation of aromatase expression by the nuclear receptor LRH-1 in adipose tissue. Mol Cell Endocrinol. 2004;215:39–44. doi: 10.1016/j.mce.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J, et al. Interactions between prostaglandin E-2, liver receptor homologue-1, and aromatase in breast cancer. Cancer Research. 2005;65:657–663. [PubMed] [Google Scholar]
- 12.Chand AL, Herridge KA, Thompson EW, Clyne CD. The orphan nuclear receptor LRH-1 promotes breast cancer motility and invasion. Endocr Relat Cancer. 2010;17:965–75. doi: 10.1677/ERC-10-0179. [DOI] [PubMed] [Google Scholar]
- 13.Annicotte JS, et al. The nuclear receptor liver receptor homolog-1 is an estrogen receptor target gene. Oncogene. 2005;24:8167–75. doi: 10.1038/sj.onc.1208950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiruchelvam PT, et al. The liver receptor homolog-1 regulates estrogen receptor expression in breast cancer cells. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-010-0994-9. [DOI] [PubMed] [Google Scholar]
- 15.Nagy L, Schwabe JW. Mechanism of the nuclear receptor molecular switch. Trends Biochem Sci. 2004;29:317–24. doi: 10.1016/j.tibs.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin B, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–26. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 17.Sablin EP, et al. The structure of corepressor Dax-1 bound to its target nuclear receptor LRH-1. Proc Natl Acad Sci U S A. 2008;105:18390–5. doi: 10.1073/pnas.0808936105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sablin EP, Krylova IN, Fletterick RJ, Ingraham HA. Structural basis for ligand-independent activation of the orphan nuclear receptor LRH-1. Mol Cell. 2003;11:1575–85. doi: 10.1016/s1097-2765(03)00236-3. [DOI] [PubMed] [Google Scholar]
- 19.Ingraham HA, Redinbo MR. Orphan nuclear receptors adopted by crystallography. Current opinion in structural biology. 2005;15:708–15. doi: 10.1016/j.sbi.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Xu PL, Kong YY, Xie YH, Wang Y. Corepressor SMRT specifically represses the transcriptional activity of orphan nuclear receptor hB1F/hLRH-1. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 2003;35:897–903. [PubMed] [Google Scholar]
- 21.Ortlund EA, et al. Modulation of human nuclear receptor LRH-1 activity by phospholipids and SHP. Nature structural & molecular biology. 2005;12:357–63. doi: 10.1038/nsmb910. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, et al. The crystal structures of human steroidogenic factor-1 and liver receptor homologue-1. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7505–10. doi: 10.1073/pnas.0409482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krylova IN, et al. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell. 2005;120:343–55. doi: 10.1016/j.cell.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, et al. Crystallographic identification and functional characterization of phospholipids as ligands for the orphan nuclear receptor steroidogenic factor-1. Mol Cell. 2005;17:491–502. doi: 10.1016/j.molcel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Fumiaki Yumoto PN, Sablin Elena P., Baxter John D., Webb Paul, Fletterick Robert J. Structural basis of coactivation of liver receptor homolog-1 by β-catenin. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1117036108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gee AC, Katzenellenbogen JA. Probing conformational changes in the estrogen receptor: evidence for a partially unfolded intermediate facilitating ligand binding and release. Mol Endocrinol. 2001;15:421–8. doi: 10.1210/mend.15.3.0602. [DOI] [PubMed] [Google Scholar]
- 27.Jasuja R, et al. Kinetic and thermodynamic characterization of dihydrotestosterone-induced conformational perturbations in androgen receptor ligand-binding domain. Mol Endocrinol. 2009;23:1231–41. doi: 10.1210/me.2008-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venteclef N, et al. GPS2-dependent corepressor/SUMO pathways govern anti-inflammatory actions of LRH-1 and LXRbeta in the hepatic acute phase response. Genes Dev. 2010;24:381–95. doi: 10.1101/gad.545110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitby RJ, et al. Small Molecule Agonists of the Orphan Nuclear Receptors Steroidogenic Factor-1 (SF-1, NR5A1) and Liver Receptor Homologue-1 (LRH-1, NR5A2) Journal of medicinal chemistry. 2011 doi: 10.1021/jm1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Macromolecular Crystallography, Pt A. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 31.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–55. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 32.Potterton E, Briggs P, Turkenburg M, Dodson E. A graphical user interface to the CCP4 program suite. Acta Crystallogr D Biol Crystallogr. 2003;59:1131–7. doi: 10.1107/s0907444903008126. [DOI] [PubMed] [Google Scholar]
- 33.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–32. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 34.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 36.Zhou X, Arthur G. Improved procedures for the determination of lipid phosphorus by malachite green. J Lipid Res. 1992;33:1233–6. [PubMed] [Google Scholar]
- 37.Xu P, Duong DM, Peng J. Systematical optimization of reverse-phase chromatography for shotgun proteomics. J Proteome Res. 2009;8:3944–50. doi: 10.1021/pr900251d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eng J, McCormack AL, Yates JR., 3rd. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 39.Louis-Jeune C, Andrade-Navarro MA, Perez-Iratxeta C. Prediction of protein secondary structure from circular dichroism using theoretically derived spectra. Proteins. 2011 doi: 10.1002/prot.23188. [DOI] [PubMed] [Google Scholar]
- 40.Chalmers MJ, et al. Probing protein ligand interactions by automated hydrogen/deuterium exchange mass spectrometry. Analytical chemistry. 2006;78:1005–14. doi: 10.1021/ac051294f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.