Abstract
Background
Startle inhibition by weak prepulses (PPI) is studied to understand the biology of information processing in schizophrenia patients and healthy comparison subjects (HCS). The Consortium on the Genetics of Schizophrenia (COGS) identified associations between PPI and single nucleotide polymorphisms in schizophrenia probands and unaffected relatives, and linkage analyses extended evidence for the genetics of PPI deficits in schizophrenia in the COGS-1 family study. These findings are being extended in a 5-site “COGS-2” study of 1800 patients and 1200 unrelated HCS to facilitate genetic analyses. We describe a planned interim analysis of COGS-2 PPI data.
Methods
Eyeblink startle was measured in carefully screened HCS and schizophrenia patients (n=1402). Planned analyses of PPI (60 ms intervals) assessed effects of diagnosis, sex and test site, PPI-modifying effects of medications and smoking, and relationships between PPI and neurocognitive measures.
Results
884 subjects met strict inclusion criteria. ANOVA of PPI revealed significant effects of diagnosis (p=0.0005) and sex (p<0.002), and a significant diagnosis × test site interaction. HCS > schizophrenia PPI differences were greatest among patients not taking 2nd generation antipsychotics, and were independent of smoking status. Modest but significant relationships were detected between PPI and performance in specific neurocognitive measures.
Discussion
The COGS-2 multi-site study detects schizophrenia-related PPI deficits reported in single-site studies, including patterns related to diagnosis, prepulse interval, sex, medication and other neurocognitive measures. Site differences were detected and explored. The target COGS-2 schizophrenia “endophenotype” of reduced PPI should prove valuable for identifying and confirming schizophrenia risk genes in future analyses.
Keywords: endophenotype, genetics, multi-site, prepulse inhibition, schizophrenia, startle
1. Introduction
The inhibition of startle by weak lead stimuli (“prepulse inhibition”: PPI) is a heritable quantitative phenotype (Greenwood et al., 2007) that is deficient in several neuropsychiatric disorders, including schizophrenia (SZ) (Braff et al., 1978; Swerdlow et al., 2008). Forebrain circuitry regulating PPI (Swerdlow et al., 2001) and genes associated with PPI in patients and healthy comparison subjects (HCS) have been identified (Greenwood et al., 2011, 2012). The NIMH Consortium on the Genetics of Schizophrenia (COGS) has pursued multi-site genetic studies of PPI and other SZ endophenotypes, first in a family study of SZ probands and unaffected family members and HCS (COGS-1 (Calkins et al., 2007)) and more recently in a larger study of patients and unrelated HCS (COGS-2). COGS-2 was designed to study the genetics of COGS-1 identified heritable endophenotypes in a large, well-characterized cohort.
Sample demands of these genetic analyses require the use of multiple, geographically dispersed data collection sites. This approach presents challenges for studies of complex phenotypes like PPI, because differences in sample demographics, methodologies or test conditions across sites introduce uncontrolled variance into experimental measures. By testing a more heterogeneous sample, multi-site studies also increase the likelihood that findings will be generalizable rather than site-specific. We reported on our efforts to carefully standardize PPI acquisition across multiple sites in studies of 196 “COGS-1” HCS (Swerdlow et al., 2007), and we now describe the outcome of this multi-site approach using measures of PPI from a planned mid-point analysis in data collection from 3000 COGS-2 subjects.
In this analysis, we characterized the ability of the COGS-2 multi-site platform to detect patterns of PPI and its deficiency in SZ patients which were previously detected in single-site studies. First, we examined the “yield” of usable data obtained from a large cohort (n=1500) representing the first 50% of the planned COGS-2 sample, to understand correctable sources of data loss. Second, we determined whether the present data collected at 5 sites reproduce findings detected in large, single-site studies within laboratories that “specialize” in PPI acquisition. Specifically, we reported (Swerdlow et al., 2006a) the impact of important moderating variables on PPI and its deficits in SZ patients, including prepulse interval (deficits at 60 ms, but not 30 or 120 ms), sex (male PPI > female PPI), medications (deficits blunted by 2nd generation antipsychotics (“SGAPs”)) and smoking (associated with higher PPI levels in patients). Each of these variables have been reported to moderate PPI or its deficits by other groups in single-site studies (Hong et al., 2008; Kumari et al., 1999, 2004; Swerdlow et al., 2006a; Weike et al., 2000). Third, we assessed the variability in these findings across the 5 sites, and attempted to identify factors contributing to this variability. Fourth, we tested for significant relationships between PPI and specific neurocognitive measures – including measures of working memory – previously detected in single-site studies of HCS and SZ patients (Bitsios and Giakoumaki, 2005; Greenwood et al., 2012; Jurado-Barba et al., 2011; Light et al., 2007, 2012; Rabin et al., 2009; Wynn et al., 2005).
2. Methods
Participants were recruited and tested at 5 sites: Mount Sinai School of Medicine, University of California Los Angeles, University of California San Diego, University of Pennsylvania and University of Washington. Participants were 18–65 years old and fluent in English. Inclusion and exclusion criteria for COGS-2 subjects are seen in Supplemental Tables 1S-2S, including criteria designed to exclude individuals whose PPI data was likely to be confounded by interfering factors in the acquisition or analysis of the startle signal (e.g. high electrode impedance, hearing impairment, “non-responder” to startle stimuli), or whose PPI may have been altered by factors unrelated to SZ per se (e.g. recent recreational drug use or history of electroconvulsive therapy). Local IRB boards of each testing site approved the study, and all participants provided signed informed consent before study participation (UCSD HRPP #080435). All participants underwent diagnostic and clinical assessments (Andreasen, 1984a, 1984b; Faraone et al., 1999; First et al., 1995, 1996; Hall, 1995) by diagnosticians trained according to a standardized procedure, as described in Supplemental Materials. An overview of clinical and neurocognitive instruments used to characterize participants was reported previously (Calkins et al., 2007).
Startle testing was initiated after completion of a specific set of diagnostic or experimental measures (Table 3S). Among the final sample for PPI analysis, testing was divided over 2 days in 86 subjects (69 of whom were from test site 2), but the test sequence was maintained. Testing methods followed previous reports (Braff et al., 2001, 2005; Swerdlow et al., 2007) to measure the eyeblink component of the acoustic startle response using an EMG system that recorded 250 1-ms epochs, starting with startle stimulus onset. The startle session included 74 active and 18 blank stimulus (“nostim”) trials (interspersed throughout the session), and lasted 23.5-min, beginning with a 5-min acclimation period with 70-dB(A) SPL noise that continued throughout the session. Startle stimuli were 40-ms 115-dB(A) SPL noise bursts (near-instantaneous rise time, est. 1 ms). Prepulses were 20 ms noise bursts 15-dB above a 70-dB(A) SPL noise background, with prepulse onset 30, 60 or 120 ms prior to pulse onset; using slightly more intense 16 dB prepulses with this startle system, prepulse-associated EMG activity is <0.5% of startle stimulus-induced levels (Swerdlow et al., 2006b). Five startle stimuli were presented at the beginning (Block 1) and end of the session (Block 4) to assess habituation. In Blocks 2–3, pulse alone and each of the 3 prepulse trial types was pseudo-randomly intermixed (9 trials per condition per blocks; inter-trial intervals 11–19 s (mean=15 s)). In 18 “nostim” trials, data were recorded without stimulus presentation, to assess basal EMG activity. Filters, amplification, calibration, scoring and training procedures were described previously (Braff et al., 1992; Calkins et al., 2007; Graham, 1975; Swerdlow et al., 2007).
Of the 1544 subjects for whom startle data were uploaded to the COGS-2 database, 1451 had any scorable startle data (i.e., in 93 subjects, waveforms were unscorable or testing was stopped mid-session at subjects’ request or testers’ discretion), and 1402 had sufficient startle data to allow calculation of the key dependent measure (60 ms PPI). Of these subjects, 884 (438 HCS, 446 patients; Table 1) met the stringent inclusion criteria for acceptable startle magnitude (mean startle magnitude for both PPI blocks ≥ 10 digital units (1.31 uV/unit)) and other inclusion criteria listed in Tables 1S-2S.
Table 1.
| A. COGS-2 Interim PPI HCS characteristics across 5 test sites (mean (SD)) | |||||
|---|---|---|---|---|---|
| Test Site: | 1 | 2 | 3 | 4 | 5 |
| HCS (N) | 97 | 72 | 43 | 99 | 127 |
| M:F* | 40:57 | 45:27 | 17:26 | 43:56 | 58:69 |
| Age (y)@ | 39.3 (13.4) | 46.6 (7.2) | 31.0 (10.2) | 31.9 (11.8) | 35.4 (14.5) |
| Race (%) | |||||
| Nat. American | 1 | 0 | 0 | 0 | 1 |
| Asian | 10 | 4 | 9 | 2 | 7 |
| Pac. Islander | 0 | 0 | 0 | 0 | 3 |
| Af. American* | 13 | 15 | 9 | 31 | 3 |
| Caucasian* | 57 | 75 | 74 | 60 | 77 |
| More than 1 | 18 | 7 | 5 | 6 | 9 |
| Not reported | 1 | 0 | 2 | 0 | 0 |
| Smokers: | |||||
| %Never:Past:Now | 81:8:11 | 83:4:13 | 86:0:14 | 97:0:7 | 91:0:9 |
| Now # cigarettes/d | 7.1 | 11.1 | 6.5 | 6.6 | 8.0 |
| % Right Handed | 88 | 76 | 84 | 86 | 86 |
| Education (y)@ | 14.8 (2.1) | 14.6 (1.8) | 16.2 (2.2) | 14.6 (2.3) | 15.6 (2.3) |
| %Past Mood DO | 4 | 4 | 5 | 11 | 16 |
| %Past Subst DO | 8 | 17 | 2 | 13 | 17 |
| GAF@ | 91.0 (7.4) | 83.9 (6.2) | 93.5 (4.2) | 87.9 (5.1) | 83.2 (7.5) |
| MMSE score@ | 33.5 (1.5) | 33.6 (1.6) | 34.5 (1.0) | 33.3 (2.2) | 33.8 (1.4) |
| % Split Tested* | 1 | 40 | 5 | 0 | 2 |
| B. COGS-2 Interim PPI SZ patient characteristics across 5 test sites (mean (SD)) | |||||
| Patients (N) | 126 | 76 | 46 | 93 | 105 |
| M:F | 84:42 | 50:26 | 34:12 | 54:39 | 78:27 |
| Age (y)@ | 45.1 (11.9) | 47.5 (11.3) | 43.3 (9.0) | 41.8 (11.2) | 44.9 (12.2) |
| Race (%) | |||||
| Nat. American | 2 | 1 | 2 | 0 | 0 |
| Asian | 1 | 5 | 0 | 3 | 4 |
| Pac. Islander | 1 | 3 | 0 | 0 | 0 |
| Af. American* | 17 | 28 | 61 | 59 | 14 |
| Caucasian* | 61 | 57 | 30 | 33 | 65 |
| More than 1 | 17 | 7 | 4 | 5 | 17 |
| Not reported | 1 | 0 | 2 | 0 | 0 |
| Smokers: | |||||
| %Never:Past:Now* | 34:13:53 | 34:3:63 | 35:4:61 | 52:0:48 | 62:1:37 |
| Now # cigarettes/d* | 14.6 | 16.2 | 9.8 | 13.4 | 11.8 |
| % Right Handed | 87 | 87 | 83 | 90 | 81 |
| Education (y)@ | 12.6 (2.1) | 13.4 (2.0) | 12.0 (2.3) | 12.9 (2.5) | 13.3 (1.9) |
| %Past Mood DO | 28 | 36 | 28 | 38 | 30 |
| %Past Subst DO | 44 | 32 | 28 | 45 | 43 |
| GAF@ | 41.2 (4.9) | 46.7 (9.5) | 50.0 (11.5) | 44.0 (10.7) | 40.2 (4.5) |
| MMSE score@ | 31.0 (3.1) | 31.0 (3.1) | 31.2 (2.9) | 30.8 (3.8) | 32.4 (2.3) |
| % Split Tested* | 5 | 53 | 0 | 0 | 5 |
| Age, symptom onset | 22.6 (7.1) | 23.1 (7.9) | 21.4 (5.7) | 23.0 (6.8) | 23.0 (7.4) |
| # Hospitalizations | 6.9 (7.6) | 7.2 (7.7) | 6.6 (7.6) | 6.0 (11.0) | 5.5 (6.6) |
| Global SANS* | 15.7 (4.3) | 9.0 (4.3) | 3.8 (3.6) | 10.6 (3.8) | 12.5 (3.8) |
| Global SAPS* | 8.4 (4.1) | 5.3 (4.1) | 4.6 (3.3) | 7.8 (4.3) | 6.5 (3.5) |
| SOF Total Score* | 46.6 (5.3) | 49.1 (5.1) | 48.2 (6.3) | 47.6 (6.2) | 42.8 (5.4) |
| UPSA-B Score | 72.8 (15.1) | 75.2 (15.5) | 67.2 (21.4) | 70.7 (15.5) | 74.0 (13.8) |
M:F = male:female; GAF = Global Assessment of Function Scale; MMSE = Mini-Mental State Examination; SANS, SAPS, SOF, UPSA: see text; Split Tested = endophenotype testing split over 2 days
significant effect of site;
significant site × diagnosis interaction
Experimental measures (startle magnitude, habituation, latency and PPI) were analyzed using repeated measures ANOVAs and post-hoc comparisons with test site, sex and diagnosis as between-subject factors for main analyses, and other characteristics (medication, smoking status, etc.) as between-subject factors for planned analyses. %PPI was calculated as 100×(1−(magnitude of startle to pulse preceded by prepulse)/magnitude of startle to pulse without a preceding prepulse)). For PPI, the primary dependent variable was % inhibition with 60 ms prepulse intervals, which is known to differ significantly between HCS vs. samples including both medicated and unmedicated SZ patients (Swerdlow et al., 2006a), and which has been the primary dependent measure in previous studies of PPI and genetics by COGS-1 (Greenwood et al., 2007, 2011) and individual laboratories (Greenwood et al., 2012; Light et al., 2012; Swerdlow et al., 2006a, 2006b). Subsequent ANOVAs assessed the temporal characteristics of PPI and its deficits in this sample, using interval (30, 60 or 120 ms) as a within-subject variable. Analyses also compared measures of startle magnitude during PPI testing, reflex habituation (startle magnitude reduction in trial block 4 vs. 1), peak reflex latency, and latency facilitation (latency reduction on trials with a prepulse followed by pulse vs. pulse alone trials). All variables were compared across testing sites, and for simplicity given the large number of variables and factors, were collapsed across right and left eyes. Alpha for all comparisons was 0.05. Effect sizes (Cohen’s d (1988)) are reported where appropriate.
3. Results
Site differences in subject characteristics
Demographic and clinical characteristics of subjects across the 5 test sites are seen in Tables 1A-B. HCS were predominantly female in 4 out of 5 sites and predominantly male in the 5th site, and patients were predominantly male across all sites. While patient age was roughly comparable across the 5 sites, HCS age diverged across sites, with 15.6 years separating sites 2 vs. 3. Another clear site difference was evident in racial stratification: for example, the proportion of patients whose race was African American at each site ranged from 17.2% (site 5) to 66.7% (site 3). When the distribution of the 3 major single-race categories (Asian, African American, Caucasian) was compared across the 5 sites, significant site differences were detected for all subjects, and separately for HCS and patients (all p’s<0.0001). HCS clinical characteristics also differed across sites (Table 1A); for example, rates of past Major Depressive Disorders and/or Substance Abuse disorders among HCS ranged from 4.7% (site 3) to 29% (site 5) (p<0.0005). Among patients (Table 1B), significant site differences were evident in smoking status (current vs. never), current smoking levels (self-reported), SANS, SAPS and SOF scores. Site differences in both HCS and patients were evident in education, GAF scores and MMSE scores.
Startle magnitude
Startle reflex data from the final sample of 884 subjects are seen in Figures 1-2. ANOVA of startle magnitude (Figure 1A and B) revealed a significant effect of diagnosis (HCS>SZ; F=7.23, df 1,864, p<0.008), test site (F=7.58, df 1,864, p<0.0001) and trial block (F=528.28, df 1,864, p<0.0001), and significant interactions of diagnosis × block (F=6.46, df 1,864, p<0.015) and site × block (F=5.42, df 1,864, p<0.0005), but no other significant main or interaction effects. Inspection revealed substantially higher startle magnitude at test site 5 in both HCS and patients (significantly greater than sites 1-4, p’s<0.0008–0.0001), with the remaining 4 sites showing more comparable levels. Racial stratification may have contributed significantly to this site effect: when race (Asian, African American, Caucasian) was entered into the ANOVA model, startle magnitude showed a significant effect of race (F=19.99, df 2,758, p<0.0001), but not significant effects of test site or race × site interaction. Statistical descriptions of EMG activity on “no stim” trials, reflex habituation (Figure 1A,B), peak reflex latency and latency facilitation (Figure 2) are found in Supplemental Materials.
Figure 1.
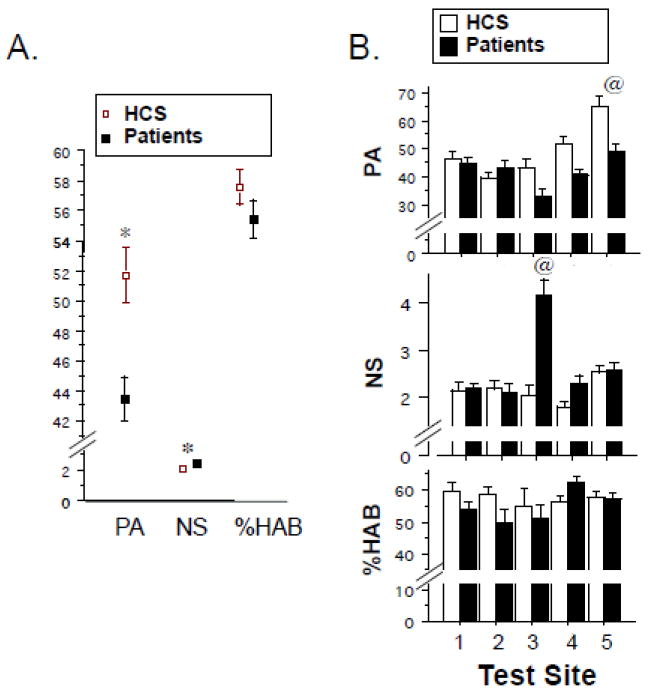
Startle measures (± SEM) in the interim COGS-2 sample. A. Startle magnitude on pulse-alone trials (PA), EMG activity during nostim trials (NS) and % startle habituation (%HAB), based on % reduction in startle magnitude in block 4 vs. block 1. Data shown are from aggregate group of N = 884 subjects. B. Same variables in “A”, shown by test site. (*) statistically significant effect of group, see text. @ significantly greater than other 4 sites (PA: site 5 HCS and patients > all other sites; NS: site 3 patients > all other sites).
Figure 2.
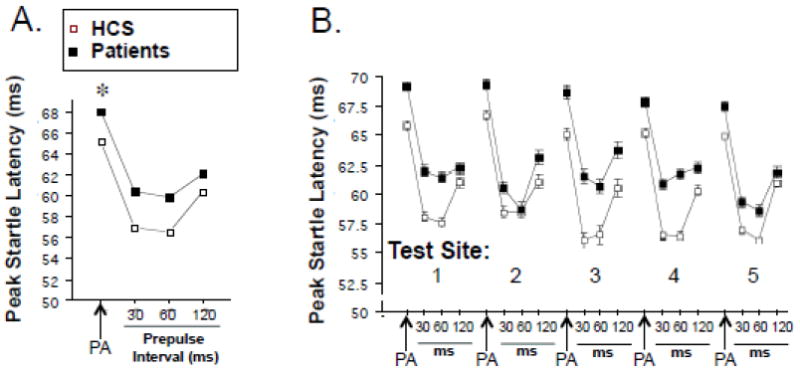
Peak startle latency on PA and 30, 60 and 120 ms prepulse trials in the aggregate sample of N = 884 subjects. B. Same variables in “A”, shown by test site. (*) statistically significant effect of group, see text. Error bars are too small to be visible.
PPI
PPI data are seen in Figure 3. ANOVA of the key dependent measure - %PPI at 60 ms intervals – revealed a significant effect of diagnosis (HCS>patient; F=12.37, df 1,864, p=0.0005; d=0.15) (Figure 3A) and sex (male>female; F=10.11, df 1,864, p<0.002), and a significant interaction of diagnosis × test site (F=3.50, df 4,864, p<0.008) (Figure 3D). Including trial block (2 vs. 3) as a within-subject factor did not change these outcomes, though the overall effect size of group differences was somewhat greater in Block 2 vs. 3 (d=0.20 vs. 0.07) (Figure 3A). While there was no significant sex × diagnosis interaction, the estimated effect size for diagnostic differences in PPI was larger in women vs. men (d=0.30 vs. 0.13). Effects of diagnosis on PPI were independent of group differences in startle magnitude: a similar pattern of PPI findings was generated using subgroups of HCS and patients matched for startle magnitude by excluding the highest startling 5% of HCS and the lowest startling 5% of patients (mean amplitudes HCS vs. patients = 45.71 vs. 45.13): analysis of PPI in these matched groups confirmed main effects of diagnosis (p<0.002) and sex (p<0.0008), and a significant diagnosis × test site interaction (p<0.015)). A similar analysis confirmed that effects of diagnosis on PPI were independent of group differences in no stim levels. Effects of diagnosis on PPI were also independent of age differences: a similar pattern of PPI findings was generated using subgroups of HCS and patients matched for age by excluding the youngest 20% of HCS and the oldest 20% of patients (mean age HCS vs. patients = 40.66 y vs. 40.19 y; PPI: main effects of diagnosis (p<0.007) and sex (p<0.006), and significant interaction of diagnosis × test site (p<0.025)). Across individual sites, HCS>patient PPI levels reached statistical significance for site 1 (group: F=6.60, df 1,219, p=0.01; sex: F=4.30, df 1,219, p<0.04; group × sex: ns), site 3 (group: F=12.42, df 1,85, p<0.0008; sex: ns; group × sex: ns) and site 4 (group: F=3.78, df 1,88, p=0.053; sex: F=10.62, df 1,188, p<0.002; group × sex: F=3.63, df 1,188, p=0.058; group × block: F=4.51, df 1,188, p<0.035; block 1 group: F=6.72, df 1,188, p=0.01), but not for sites 2 or 5.
Figure 3.
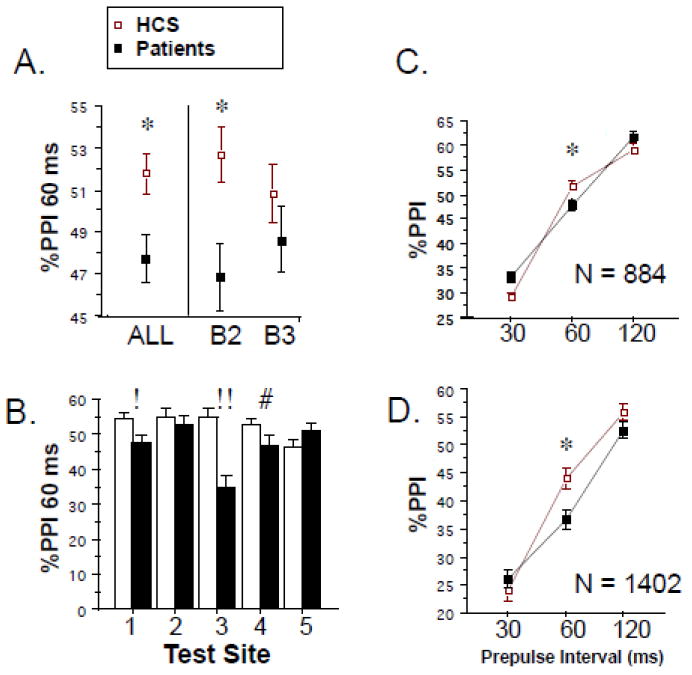
% PPI (± SEM). A. Primary dependent measure: %PPI with 60 ms prepulse intervals shown across all trials (“ALL”) and divided by trial block (B2, B3), among 884 carefully screened HCS and patients. B. Same variables in “A”, shown by test site (! p=0.01; !! p < 0.0008; # p = 0.053; group × block p<0.035; block 1 p=0.01). C. %PPI across 30, 60 and 120 ms prepulse intervals in 884 carefully screened subjects. D. Same variables in “C”, shown across the inclusive COGS-2 interim sample (n=1402). (*) statistically significant effect of group, see text.
Inspection of data from all subjects meeting inclusion criteria revealed a substantial range of effect sizes for patients vs. HCS %PPI levels across the 5 test sites, ranging from negative (higher PPI in patients vs. HCS, site 5: d=-0.16) to large and positive (higher PPI in HCS vs. patients, site 3: d=0.69), with a mean overall effect size of 0.15 (Figure 3B). Site differences were evident for PPI among patients (range: 34.75% (site 3) - 52.86% (site 2); site 3 was lower than the other 4 sites, p’s<0.002–0.03) and among HCS (range sites 1-4: 52.72%-54.86%; site 5=46.21%; site 5 was lower than the other 4 sites at or near significance levels, p’s<0.02-0.055). Based on findings with startle magnitude and no stim levels, we assessed whether racial stratification may have contributed significantly to this site effect on %PPI. Using subjects from the 3 major single-race categories, ANOVA of %PPI revealed a significant effect of race (F=3.55, df 2,758, p<0.04), but no significant effect of test site or race × site interaction. While it was not possible to complete this analysis using diagnosis as a grouping factor due to the absence of any Asian patients at site 3, ANOVA of %PPI using race, diagnosis and sex (but not test site) as grouping factors revealed a significant effect of diagnosis (F=4.03, df 1,761, p<0.05), but not race (F=1.14, df 2,761, ns) or sex (F=2.38, df 1,761, ns), and no 2- or 3-way interactions. In other words, racial stratification appears to contribute to differences across test sites, but not across diagnostic groups.
Analyses that included the wider temporal window of 30–120 ms prepulse intervals confirmed maximal deficits in SZ patients at 60 ms intervals (Figure 3C). ANOVA yielded a significant interaction of diagnosis × prepulse interval (F=15.43, df 2,1728, p<0.0001), with no significant diagnostic differences for 30 or 120 ms intervals. For 30 ms PPI, there were significant effects of test site (F=3.27, df 4,864, p<0.015) and sex (F=5.73, df 1,864, p<0.02), while for 120 ms PPI, site effects reached marginal significance (p=0.05), while those for sex did not.
It is worth noting that the finding of HCS>patient PPI was reproduced very closely when data were included from all subjects who completed startle testing (n=1402), independent of exclusion criteria (Figure 3D). Thus, ANOVA of 60 ms PPI from these 1402 subjects revealed a significant main effect of diagnosis (F=12.83, df 1,1382, p<0.0005; d=0.16), with a larger effect size detected in the first half of PPI testing (F=19.078, df 1,1399, p<0.0001; d=0.24) and a comparable temporal pattern to that seen in the “cleaner” sample of 884 subjects, but greater group separation at the 120 ms interval.
Moderating effects of medications and smoking
Potential moderating effects of medications on group differences in PPI were assessed (Figure 4). Significant PPI deficits were detected among subgroups of patients who, by self-report: 1) were not taking any antipsychotic medications (n=52; F=5.61, df 1,488, p<0.02; d=0.35); 2) were not taking 2nd generation antipsychotics (SGAPs) (n=80; F=4.78, df 1,514, p<0.03; d=0.23); and 3) were taking SGAPs (Table 2) (n=366; F=7.58, df 1,800, p<0.007; d=0.13). Effect sizes for each of these subgroups were larger during the first half of PPI testing (d’s=0.47, 0.32 and 0.17, respectively). Among SGAPs taken by more than 5 patients, PPI rankings (mean %PPI) were: clozapine (54.82) > ziprasidone (51.27) > olanzapine (48.16) > quetiapine (47.33) > risperidone (46.58) > aripiprazole (45.14). Dose effects were not readily assessed because many subjects were taking multiple different 1st and 2nd generation antipsychotics. Nonetheless, median splits based on the primary SGAP for each subject revealed that subjects taking higher doses exhibited numerically (but not statistically) greater levels of PPI, compared to subjects taking lower doses. Site differences in self-reported antipsychotic usage and dosing are seen in Table 2. No clear patterns of site-differences in antipsychotic use were detected, though the mean dose of clozapine – the SGAP most associated with “normalized” PPI in SZ patients (Kumari et al., 1999; Weike et al., 2000) – was approximately 30-90% greater at site 5, compared to the other 4 sites. Temporal patterns of PPI deficits were comparable among patients not taking antipsychotics vs. other medication-based subgroups, though -- as described above -- the magnitude of the deficit at 60 ms intervals was somewhat larger among unmedicated patients (Figure 4).
Figure 4.
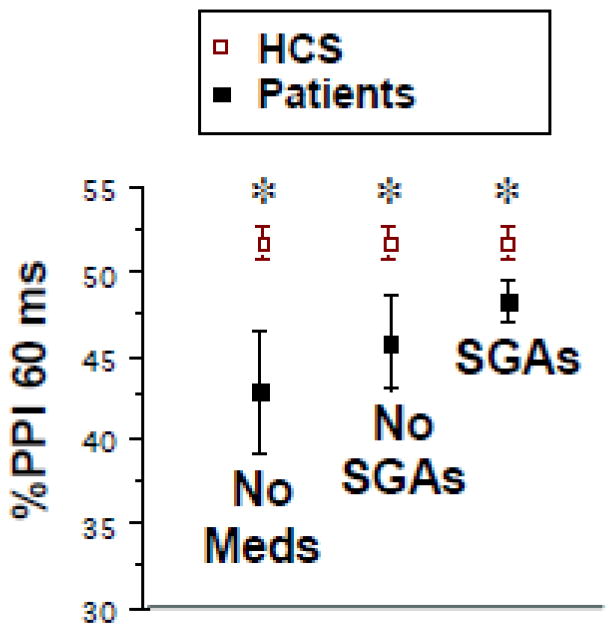
Medication effects on %PPI (± SEM) in this interim COGS-2 sample. Data shown are %PPI with 60 ms prepulse intervals in HCS vs. 3 subgroups of patients: those taking no antipsychotics (“No Meds”), those taking no second generation antipsychotics (“No SGAs”), and those taking SGA’s (“SGA’s”). (*) statistically significant effect of group, see text.
Table 2.
Self-reported antipsychotic use at 5 test sites (%subjects)
| Test Site: | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| No antipsychotics | 9.5 | 7.9 | 4.3 | 18.3 | 14.3 |
| 1st Generation | 2.4 | 5.3 | 8.7 | 8.6 | 8.6 |
| 2nd Generation | 72.2 | 80.3 | 82.6 | 60.2 | 68.6 |
| 1st & 2nd Generation | 15.9 | 6.6 | 4.3 | 12.9 | 8.6 |
| Most used 1st Generation antipsychotics (mean daily oral dose, mg): | |||||
| Haloperidol (14.7) | |||||
| Fluphenazine (20.8) | |||||
| Perphenazine (22.4) | |||||
| Most used 2nd Generation antipsychotics (mean daily oral dose, mg): | |||||
| Risperidone (4.1) | |||||
| Aripiprazole (21.8) | |||||
| Quetiapine (381.5) | |||||
| Olanzapine (19.4) | |||||
| Clozapine (390.9) | |||||
Self-reported smoking status did not moderate group differences in PPI in this sample. When smoking status (never vs. current; excluding non-cigarette tobacco use) was included as a grouping factor, ANOVA of PPI confirmed main effects of diagnosis and sex, but no main effect of smoking (F<1) or significant 2- or 3-way interactions. Compared to all HCS, PPI was deficient in SZ patients who never smoked (p<0.008) and in those who currently smoked (p<0.01). Among patients who currently smoked, there was a trend for higher PPI to be associated with higher smoking levels (mean %PPI: <0.5 packs/day = 45.65; 0.5–1.0 packs/day = 49.81; >1 pack/day = 55.60), but this relationship was not statistically significant.
Demographic, clinical and neurocognitive correlates
Effects of sex and race on PPI are described above; the relationships of PPI to other demographic variables, including those related to family structure and SZ history, are seen in Table 3, and are generally weak or absent. This was also true when PPI values were used from the first half of startle testing, where schizophrenia-linked deficits were greatest. Similarly, PPI was not significantly related to a number of clinical variables, including age of illness onset, number of hospitalizations, symptoms severity or functional impairment; PPI also did not distinguish groups based on the presence or absence of past diagnoses of mood or substance disorders. PPI was modestly related to global mental status (MMSE: r=0.08, p<0.02) and to working memory performance in the LNS (reorder) (r=0.11, p<0.0015), and these relationships were even weaker when analyses were limited to patients, due to limited range of MMSE and LNS scores. The large sample size allowed meaningful comparisons of the SZ patients whose PPI levels were in the lowest vs. highest quartiles of the SZ sample. These quartile comparisons revealed no significant group differences on any of the above demographic, functional or neurocognitive measures, except for LNS reorder score, which was significantly greater among patients in the highest vs. lowest PPI quartile (F=8.92, df 1,220, p<0.004) (Table 3). Importantly, this was not meant to be a comprehensive analysis of neurocognitive correlates of PPI in this sample, but rather was conducted as an interim assessment of the degree to which the present multi-site findings reproduce patterns described in existing reports of single-site studies. Correlates of startle magnitude and reflex latency are described in Supplemental Materials.
Table 3.
Relationships of PPI (60 ms) to demographic and clinical variables in SZ patients
| Sex | F = 9.65, df 1,444, p<0.003 (M>F) |
| Age (y) | R = -0.10, n=446, p = 0.03 |
| Ethnicity (Caucasian vs. African American) | F = 1.08, df 1,371, NS |
| Known multiplex vs. known singleton | F = 0.75, df 1,431, NS |
| Smokers: | |
| Never vs. Current | F = 0.55, df 1,423, NS |
| Current # cigarettes/d | R = 0.07, n = 211, NS |
| Handedness (R vs. L) | F = 0.98, df 1,432, NS |
| Education (y) | R = 0.04, n = 446, NS |
| Past Mood DO | F = 0.08, df 1,444, NS |
| Past Subst DO | F = 2.33, df 1,444, NS |
| GAF | R = -0.08, n=442, NS |
| MMSE score | R = 0.07, n=434, NS |
| Age, symptom onset | R = 0.04, n=438, NS |
| # Hospitalizations | Rs = 0.02, n=445, NS |
| Global SANS | R = 0.06, n=444, NS |
| Global SAPS | R = 0.05, n=442, NS |
| SOF Total Score | R = -0.03, n=441, NS |
| UPSA-B Score | R = 0.05, n=441, NS |
| Letter Number Span (LNS), Forward | R = 0.06, n=445, NS |
| LNS Forward, lowest vs. highest quartile PPI | F = 1.38, df 1,220, NS |
| LNS, Reorder | R = 0.09, n=445, p<0.055 |
| LNS Reorder, lowest vs. highest quartile PPI | F = 8.92, df 1,220, p<0.004 |
4. Discussion
The major finding of this interim analysis is that the COGS-2 multi-site platform is capable of detecting the key schizophrenia PPI endophenotype – reduced PPI with 60 ms prepulse intervals. The moderating impact of several factors on PPI and its reduction in SZ patients was also detected. That such findings can emerge from a multi-site platform is important because genetic analyses for which this endophenotype will be used require large samples that are feasible only in multi-site designs; were these designs to add uncontrolled variance (i.e. due to site-specific idiosyncrasies in testing) or other confounding effects that prevented detection of the endophenotype, this might greatly diminish the scientific value of such an undertaking. The present findings support the utility and fidelity of the COGS-2 multi-site platform, because the general pattern of results with PPI and its moderating factors have been detected in single-site studies by a number of different investigative groups (Aggernaes et al., 2010; Braff et al., 1978, 1999, 2001; Csomor et al., 2009; Hammer et al., 2011, 2013; Hong et al., 2007; Kishi et al., 2012; Kumari et al., 1999, 2007; Kunugi et al., 2007; Light et al., 2012; Ludewig et al., 2003; Mackeprang et al., 2002; Martinez-Gras et al., 2009; Meincke et al., 2004; Molina et al., 2011; Moriwaki et al., 2009; Oranje and Glenthoj, 2013; Preuss et al., 2011; Quednow et al., 2006; Rabin et al., 2009; Takahashi et al., 2008; Wang et al., 2013; Weike et al., 2000; Xue et al., 2012), one of which (UCSD) is represented in this study (see reference data from UCSD, Figure 5).
Figure 5.
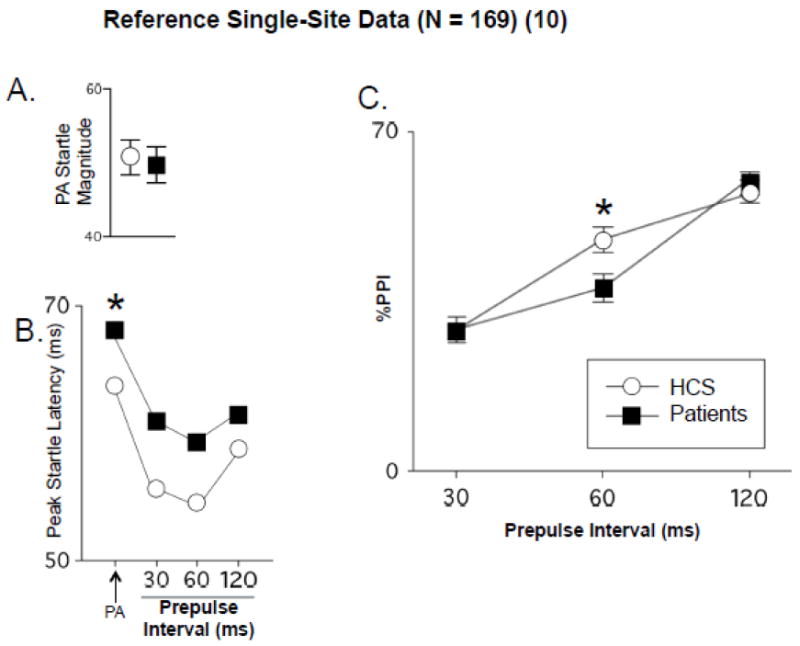
Reference data (± SEM) on identical measures from published single-site study at UCSD (Swerdlow et al., 2006a), N=169). A. Startle magnitude on PA trials (compare to Figure 1A). B. Peak startle latency (compare to Figure 2A). C. PPI across 30, 60 and 120 ms prepulse intervals (compare to Figures 3C and 3D). (*) statistically significant effect of group. At UCSD, d for 60 ms PPI, HCS vs. patients was 0.24 in (Swerdlow et al., 2006a) and 0.28 in present study (0.39 in Block 2).
Reduced PPI in SZ patients was not detected at all COGS sites. Analyses across test sites revealed that patients’ PPI at site 3 was lower, and HCSs’ PPI at site 5 was lower, compared to corresponding groups at other test sites. Among the 5 COGS sites, site 5 HCS and patients also exhibited significantly higher startle magnitude than the others, and site 3 patients exhibited significantly higher “no stim” values compared to patients at the other 4 sites. In our previous report of startle and PPI across COGS-1 test sites, levels of PPI, startle magnitude and “no stim” activity from HCS in sites 3 and 5 did not deviate significantly from those of the other sites. A well-trained “mock subject” was tested at each test site as part of our COGS-wide quality assurance process, but did not note any deviations in protocol at sites 3 or 5 that might explain the observed differences in startle measures. Conceivably, some unique characteristics of site 3 patients or site 5 subjects might account for their disparate startle values. These sites were “outliers” compared to the other 4 sites in demographic variables such as age and sex. For example, site 3 was the only site at which the sex distribution in patients (M:F=50:26) and HCS (45:22) was comparable. Site 5 HCS more often had histories of mood or substance disorders, and had the lowest GAF scores among the 5 sites; patients from sites 3 and 5 differed from those at other sites in some clinical variables (e.g. highest clozapine use at site 5), but the “direction” of these differences (suggesting more or less clinical severity) was not always consistent (Table 1).
One contributor to site differences in startle variables may have been site differences in racial stratification. We previously reported racial differences in both startle magnitude and %PPI in a single-site study (Swerdlow et al., 2005), noting that Asian vs. Caucasian differences in %PPI were obviated by matching groups for comparable startle magnitude. A full accounting for contributions of race to site differences in COGS-2 is complicated by the fact that among the 3 major race groups – Asian, African American and Caucasian – not all were represented among both HCS and patient groups at each of the 5 test sites. Nonetheless, separate ANOVAs revealed that significant HCS>patient differences in PPI remained after inclusion of race as a grouping factor, while site differences in PPI did not. Certainly, site differences in racial stratification is an important issue facing multi-site studies of SZ (Tamminga et al., 2013).
Since publication of our first multi-site COGS-1 study of PPI in HCS in 2007, at least 18 single-site Medline-listed reports of PPI deficits in SZ or prodromal (Quednow et al., 2008; Ziermans et al., 2011, 2012) patients have been published, including studies from 10 different countries. This brings the total number of published reports of such deficits to over 40. Among these recent (post-2007) single-site reports of SZ-linked PPI deficits, moderating effects prepulse interval (Csomor et al., 2009), atypical antipsychotic medications (Aggernaes et al., 2010; Wynn et al., 2007), smoking (Rabin et al., 2009; Woznica et al., 2009) and sex (Takahashi et al., 2008), and significant correlates with clinical symptoms (Martinez-Gras et al., 2009; Wang et al., 2013) and neuropsychological performance (Rabin et al., 2009) were detected in some, but not all studies. While other negative findings may have gone unpublished, one recent group reported a failure to detect reduced PPI using 120 ms prepulse intervals in a large sample of SZ patients (Ivleva et al., 2013). Such a result would be consistent with our present findings (Figure 3C and 3D), and predicted by our large, single-site studies (Light et al., 2012; Swerdlow et al., 2006a; see Figure 5) as well as other reports in medicated SZ cohorts, which demonstrated SZ-linked PPI deficits with 60 ms but not 120 ms prepulse intervals (Csomor et al., 2009; Hammer et al., 2011; Ludewig et al., 2003; Wang et al., 2013). We previously discussed the potential importance in schizophrenia of inhibitory processes active 60 ms after a lead stimulus to the flow of preconscious information into conscious awareness (Grobstein, 2005; Kanabus et al., 2002; Libet et al., 1979, 1985; Swerdlow et al., 2006a). The insensitivity of 120 ms PPI to any moderating variables (see Supplemental Materials) raises the possibility that inhibition at this interval may have reached a physiological “ceiling” in both HCS and SZ groups in this particular study, that obscured detection of group differences. Of note, PPI deficits in SZ patients have been reported by other groups using 120 ms prepulse intervals (Kumari et al., 2007; Kunugi et al., 2007; Mackeprang et al., 2002; Martinez-Gras et al., 2009; Preuss et al., 2011; Quednow et al., 2006, 2010; Xue et al., 2012).
While some groups have reported medium-to-large effect size deficits in PPI in SZ vs. HCS cohorts (Braff et al., 1999; Kunugi et al., 2007; Martinez-Gras et al., 2009), our most recent large single-site reports from UCSD have detected deficits with 60 ms prepulse intervals with effect sizes that ranged from 0.24 (Swerdlow et al., 2006a) to 0.58 (Light et al., 2012), consistent with the present COGS-2 findings at UCSD (d=0.28 overall, and 0.39 in Block 2). The effect size from the most rigorously screened subjects (n=884) in the present study (d≈0.15) is considerably lower than that from either of these recent studies, and it is parsimonious to suggest that this smaller effect size in this multi-site study may have resulted from between-site variability in either subject characteristics or data acquisition. Factors contributing to “artificially” small PPI differences between HCS and SZ patients in the present study are common to most recent reports: 1) women have lower PPI than do men (Swerdlow et al., 1993), and HCS are predominantly (1.6:1) women, while SZ patients are predominantly (2:1) men. At the one site with balanced sex distributions in the present study (site 3), the PPI deficit in patients had an effect size of 0.69; 2) PPI is generally increased by nicotine (Hong et al., 2008; Kumari et al., 2001), and smoking is both more common and heavier among SZ patients vs. HCS (Table 1); PPI is higher in medicated vs. unmedicated SZ patients, and especially in patients medicated with SGAPs (Csomor et al., 2009; Kumari et al., 1999; Swerdlow et al., 2006a; Weike et al., 2000), and the prevalent use of any (88%) or specifically SGAPs (82%) by SZ patients in the present study is representative of most recent studies of PPI in SZ. While these known effects of sex, smoking and medications on PPI diminish the magnitude of the measurable group difference between HCS and SZ patients, it is even more important that they are not easily extricated from a subject’s PPI value, and thereby complicate the signal provided by this endophenotype for identifying SZ genes.
The present findings may provide guidance for enhancing the power of future genetic studies utilizing PPI as an endophenotype. First, the PPI “signal” appears to be most robust early in the test session, before startle magnitude has been further reduced by reflex habituation. Second, use of a full temporal range of prepulse intervals may allow investigators to select the PPI interval that provides the most robust group differences for use as the primary endophenotype. It is clear that studies from different laboratories differ slightly in the precise temporal “sweet spot” for this inhibitory deficit, and this might reflect differences in stimulus characteristics or response acquisition hardware and/or software. For this reason, using a modest range of intervals make sense vs. relying on a single interval. Third, less restrictive exclusion criteria (e.g. startle magnitude, hearing threshold, history of ECT) do not appear to weaken the PPI endophenotype, and greatly increase the number of subjects eligible for genetic analyses. Whether it is preferable for genetic analyses to utilize a smaller and “cleaner” sample vs. a larger and more confounded sample may depend somewhat on the specific questions being asked and the types of genetic analyses being pursued. However, the present findings suggest that a larger, more confounded sample does not deflate the effect size of the PPI deficit in patients vs. HCS, and this may provide investigators with options in their study design.
Less consistent findings across recent single-site studies, as well as the present multi-site study, include the relationship between smoking and the magnitude of PPI deficits among SZ patients (Hong et al., 2008; Moriwaki et al., 2009), and the associations between PPI and either clinical symptoms (Braff et al., 1999; Duncan et al., 2006; Light et al., 2012; Martinez-Gras et al., 2009; Meincke et al., 2004; Quednow et al., 2010; Swerdlow et al., 2006a; Wang et al., 2013; Xue et al., 2012) or neurocognitive measures (Bitsios and Giakoumaki, 2005; Kishi et al., 2012) in SZ patients. Perhaps such variability should not be surprising: studies vary widely in the precision with which nicotine use and levels are documented, and many studies – including our own – use very blunt self-report measures of current and lifetime smoking history. In contrast to the wide range of metrics that characterize nicotine use across studies, clinical symptom scales and neurocognitive measures should be relatively more standardized, and the variability in the relationship between these measures and PPI across studies may reflect other factors, including site differences in patient characteristics or PPI methodology, and the intrinsic heterogeneity of SZ. In fact, the relative consistency of PPI deficits across numerous SZ cohorts should not be interpreted as evidence of a single, common neuropathological process: PPI deficits across different studies, and indeed across different patients, almost certainly reflect pathology at different levels of limbic cortico-striato-pallido-thalamic and pontine circuitries – any one of which contributes to the regulation of this complex phenotype, and which have been reported to be among the many brain structures involved in the heterogeneous neuropathology of the schizophrenias (cf. Swerdlow et al., 2008, 2011).
Perhaps the bigger issue raised in this interim analysis is the relative cost and benefit of acquiring and analyzing complex phenotypes like PPI using single- vs. multi-site designs. Were it not for the need to achieve adequate power for genetic analyses, the present findings might be interpreted to suggest that the costs exceed the benefits for multi-site studies of PPI: the general patterns of findings in this multi-site study reproduce most reports from single-site studies, have smaller effect sizes, and include added sources of uncontrolled variance, even after substantial efforts were allocated towards careful multi-site training and quality control. Despite these reduced effect sizes, the orderly and consistent findings detected with this multi-site platform support optimism that COGS will continue to identify PPI- and SZ-related genes upon completion of testing in the full sample of 3000 COGS-2 subjects.
Supplementary Material
Acknowledgments
This study was supported by grants R01-MH065571, R01-MH065588, R01-MH065562, R01-MH065707, R01-MH065554, R01-MH065578, R01-MH065558, R01 MH86135, and K01-MH087889 from the National Institute of Mental Health. Genotyping services were provided by the Center for Inherited Disease Research (CIDR). CIDR is fully funded through a federal contract from the National Institutes of Health to The Johns Hopkins University, Contract Number HHSN268200782096C.
Role of funding source: Other than proving support, the NIH had no further role in this manuscript.
Footnotes
Contributors: Dr. Swerdlow supervised 5-site quality assurance for PPI measures, completed all statistical analyses and wrote the first draft of this manuscript. Joyce Sprock performed quality assurance for all data and coordinated database activities. All other authors participated in aspects of study design, including subject recruitment, phenotyping, and validation of the clinical and endophenotype data. All authors were responsible for reviewing and approving the final manuscript.
Conflict of interest: Drs. Braff, Calkins, Greenwood, Light, Radant, Seidman, Siever, Silverman, Stone, Sugar, DW Tsuang, and MT Tsuang report no financial relationships with commercial interests. Dr. Green reports having been a consultant to Abbott laboratories (AbbVie), Biogen, and Roche, he is a member of the scientific board for Mnemosyne, and he has received research funds from Amgen. Drs. Gur and Turetsky have received unrelated research support for investigator-initiated grants from Pfizer and AstraZeneca. Dr. Nuectherlein has received unrelated research support from Janssen Scientific Affairs, Genentech, and Brain Plasticity, Inc., and has consulted to Genentech, Otsuka, and Brain Plasticity, Inc. Dr. Swerdlow has been a paid Consultant for Neurocrine, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggernaes B, Glenthoj BY, Ebdrup BH, Rasmussen H, Lublin H, Oranje B. Sensorimotor gating and habituation in antipsychotic-naive, first-episode schizophrenia patients before and after 6 months’ treatment with quetiapine. Int J Neuropsychopharmacol. 2010;13(10):1383–1395. doi: 10.1017/S1461145710000787. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The scale for the assessment of negative symptoms (SANS) The University of Iowa; Iowa City, IA: 1984a. [Google Scholar]
- Andreasen NC. The scale for the assessment of positive symptoms (SAPS) The University of Iowa; Iowa City, IA: 1984b. [Google Scholar]
- Bitsios P, Giakoumaki SG. Relationship of prepulse inhibition of the startle reflex to attentional and executive mechanisms in man. Int J Psychophysiol. 2005;55(2):229–241. doi: 10.1016/j.ijpsycho.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Light GA, Sprock J, Perry W, Cadenhead KS, Swerdlow NR. Impact of prepulse characteristics on the detection of sensorimotor gating deficits in schizophrenia. Schizophr Res. 2001;49(1-2):171–178. doi: 10.1016/s0920-9964(00)00139-0. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992;49(3):206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Braff DL, Light GA, Ellwanger J, Sprock J, Swerdlow NR. Female schizophrenia patients have prepulse inhibition deficits. Biol Psychiatry. 2005;57(7):817–820. doi: 10.1016/j.biopsych.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15(4):339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Swerdlow NR, Geyer MA. Symptom correlates of prepulse inhibition deficits in male schizophrenic patients. Am J Psychiatry. 1999;156(4):596–602. doi: 10.1176/ajp.156.4.596. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Dobie DJ, Cadenhead KS, Olincy A, Freedman R, Green MF, Greenwood TA, Gur RE, Gur RC, Light GA, Mintz J, Nuechterlein KH, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Braff DL. The consortium on the genetics of endophenotypes in schizophrenia: model recruitment, assessment, and endophenotyping methods for a multisite collaboration. Schizophr Bull. 2007;33(1):33–48. doi: 10.1093/schbul/sbl044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Lawrence Erlbaum Associates, Inc; Hillsdale, NJ: 1988. [Google Scholar]
- Csomor PA, Yee BK, Feldon J, Theodoridou A, Studerus E, Vollenweider FX. Impaired prepulse inhibition and prepulse-elicited reactivity but intact reflex circuit excitability in unmedicated schizophrenia patients: a comparison with healthy subjects and medicated schizophrenia patients. Schizophr Bull. 2009;35(1):244–255. doi: 10.1093/schbul/sbm146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan EJ, Bollini AM, Lewison B, Keyes M, Jovanovic T, Gaytan O, Egan G, Szilagyi S, Schwartz M, Parwani A, Chakravorty S, Rotrosen J. Medication status affects the relationship of symptoms to prepulse inhibition of acoustic startle in schizophrenia. Psychiatry Res. 2006;145(2-3):137–145. doi: 10.1016/j.psychres.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Tsuang D, Tsuang MT. Genetics of Mental Disorders: A Guide for Students, Clinicians, and Researchers. Guilford, New York: 1999. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders -- Patient Edition (SCID-I/P, Version 2.0) New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders -- Non-Patient Edition (SCID-I/NP, Version 2.0) New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Graham F. The more or less startling effects of weak prestimuli. Psychophysiology. 1975;12(3):238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Braff DL, Light GA, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Green MF, Gur RE, Gur RC, Mintz J, Nuechterlein KH, Olincy A, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Schork NJ. The Consortium on the Genetics of Schizophrenia (COGS): Initial Heritability Analyses of Endophenotypic Measures for Schizophrenia. Arch Gen Psychiatry. 2007;64(11):1242–1250. doi: 10.1001/archpsyc.64.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Lazzeroni LC, Murray SS, Cadenhead KS, Calkins ME, Dobie DJ, Green MF, Gur RE, Gur RC, Hardiman G, Kelsoe JR, Leonard S, Light GA, Nuechterlein KH, Olincy A, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Freedman R, Braff DL. Analysis of 94 candidate genes and twelve endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am J Psychiatry. 2011168(9):930–946. doi: 10.1176/appi.ajp.2011.10050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Light GA, Swerdlow NR, Radant AD, Braff DL. Association analysis of 94 candidate genes and schizophrenia-related endophenotypes. PLoS One. 2012;7(1):e1002134. doi: 10.1371/journal.pone.0029630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobstein P. Making the unconscious conscious: a bi-directional bridge between neuroscience/cognitive science and psychotherapy? Cortex. 2005;41(5):663–668. doi: 10.1016/s0010-9452(08)70283-1. [DOI] [PubMed] [Google Scholar]
- Hall RC. Global assessment of functioning. A modified scale. Psychosomatics. 1995;36(3):267–275. doi: 10.1016/S0033-3182(95)71666-8. [DOI] [PubMed] [Google Scholar]
- Hammer TB, Oranje B, Fagerlund B, Bro H, Glenthøj BY. Stability of prepulse inhibition and habituation of the startle reflex in schizophrenia: a 6-year follow-up study of initially antipsychotic-naive, first-episode schizophrenia patients. Int J Neuropsychopharmacol. 2011;14(7):913–925. doi: 10.1017/S1461145711000034. [DOI] [PubMed] [Google Scholar]
- Hammer TB, Oranje B, Skimminge A, Aggernæs B, Ebdrup BH, Glenthøj B, Baaré W. Structural brain correlates of sensorimotor gating in antipsychotic-naive men with first-episode schizophrenia. J Psychiatry Neurosci. 2013;38(1):34–42. doi: 10.1503/jpn.110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Wonodi I, Adami H, Buchanan RW, Thaker GK. Independent domains of inhibitory gating in schizophrenia and the effect of stimulus interval. Am J Psychiatry. 2007;164(1):61–65. doi: 10.1176/ajp.2007.164.1.61. [DOI] [PubMed] [Google Scholar]
- Hong LE, Wonodi I, Lewis J, Thaker GK. Nicotine effect on prepulse inhibition and prepulse facilitation in schizophrenia patients. Neuropsychopharmacology. 2008;33(9):2167–2174. doi: 10.1038/sj.npp.1301601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivleva EI, Moates AF, Hamm JP, Bernstein IH, O’Neill HB, Cole D, Clementz BA, Thaker GK, Tamminga CA. Smooth Pursuit Eye Movement, Prepulse Inhibition, and Auditory Paired Stimuli Processing Endophenotypes Across the Schizophrenia-Bipolar Disorder Psychosis Dimension. Schizophr Bull. 2013 doi: 10.1093/schbul/sbt047. [Epub ahead of print, Apr 18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado-Barba R, Morales-Muñoz I, Del Manzano BÁ, Fernández-Guinea S, Caballero M, Martínez-Gras I, Rubio-Valladolid G. Relationship between measures of inhibitory processes in patients with schizophrenia: role of substance abuse disorders. Psychiatry Res. 2011;190(2-3):187–192. doi: 10.1016/j.psychres.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Kanabus M, Szelag E, Rojek E, Poppel E. Temporal order judgement for auditory and visual stimuli. Acta Neurobiol Exp (Wars) 2002;62(4):263–270. doi: 10.55782/ane-2002-1443. [DOI] [PubMed] [Google Scholar]
- Kishi T, Fukuo Y, Okochi T, Kawashima K, Moriwaki M, Furukawa O, Fujita K, Musso GM, Correll CU, Kane JM, Iwata N. The relationship between acoustic startle response measures and cognitive functions in Japanese patients with schizophrenia. Neuromolecular Med. 2012;14(2):131–138. doi: 10.1007/s12017-012-8177-y. [DOI] [PubMed] [Google Scholar]
- Kumari V, Aasen I, Sharma T. Sex differences in prepulse inhibition deficits in chronic schizophrenia. Schizophr Res. 2004;69(2-3):219–235. doi: 10.1016/j.schres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Kumari V, Fannon D, Sumich AL, Sharma T. Startle gating in antipsychotic-naïve first episode schizophrenia patients: one ear is better than two. Psychiatry Res. 2007;151(1-2):21–28. doi: 10.1016/j.psychres.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Kumari V, Soni W, Sharma T. Normalization of information processing deficits in schizophrenia with clozapine. Am J Psychiatry. 1999;156(7):1046–1051. doi: 10.1176/ajp.156.7.1046. [DOI] [PubMed] [Google Scholar]
- Kumari V, Soni W, Sharma T. Influence of cigarette smoking on prepulse inhibition of the acoustic startle response in schizophrenia. Hum Psychopharmacology. 2001;16(4):321–326. doi: 10.1002/hup.286. [DOI] [PubMed] [Google Scholar]
- Kunugi H, Tanaka M, Hori H, Hashimoto R, Saitoh O, Hironaka N. Prepulse inhibition of acoustic startle in Japanese patients with chronic schizophrenia. Neurosci Res. 2007;59(1):23–28. doi: 10.1016/j.neures.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Libet B. Unconscious cerebral initiative and the role of conscious will in voluntary action. Behav Brain Sci. 1985;8:529–566. [Google Scholar]
- Libet B, Wright EW, Jr, Feinstein B, Pearl DK. Subjective referral of the timing for a conscious sensory experience. Brain. 1979;102(1):193–224. doi: 10.1093/brain/102.1.193. [DOI] [PubMed] [Google Scholar]
- Light GA, Braff DL, Sprock J, Cadenhead KS, Swerdlow NR. Prepulse inhibition of startle is positively associated with higher order cognition in women. Abstract, Society for Neuroscience. 2007;806:17. [Google Scholar]
- Light GA, Swerdlow NR, Rissling AJ, Radant A, Sugar CA, Sprock J, Pela M, Geyer MA, Braff DL. Characterization of neurophysiologic and neurocognitive biomarkers for use in genomic and clinical outcome studies of schizophrenia. PLoS One. 2012;7(7):e39434. doi: 10.1371/journal.pone.0039434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig K, Geyer MA, Vollenweider FX. Deficits in prepulse inhibition and habituation in never-medicated, first-episode schizophrenia. Biol Psychiatry. 2003;54(2):121–128. doi: 10.1016/s0006-3223(02)01925-x. [DOI] [PubMed] [Google Scholar]
- Mackeprang T, Kristiansen KT, Glenthoj BY. Effects of antipsychotics on prepulse inhibition of the startle response in drug-naive schizophrenic patients. Biol Psychiatry. 2002;52(9):863–873. doi: 10.1016/s0006-3223(02)01409-9. [DOI] [PubMed] [Google Scholar]
- Martinez-Gras I, Rubio G, del Manzano BA, Rodriguez-Jimenez R, Garcia-Sanchez F, Bagney A, Leza JC, Borrell J. The relationship between prepulse inhibition and general psychopathology in patients with schizophrenia treated with long-acting risperidone. Schizophr Res. 2009;115(2-3):215–221. doi: 10.1016/j.schres.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Meincke U, Mörth D, Voss T, Thelen B, Geyer MA, Gouzoulis-Mayfrank E. Prepulse inhibition of the acoustically evoked startle reflex in patients with an acute schizophrenic psychosis--a longitudinal study. Eur Arch Psychiatry Clin Neurosci. 2004;254(6):415–421. doi: 10.1007/s00406-004-0523-0. [DOI] [PubMed] [Google Scholar]
- Molina V, López DE, Villa R, Pérez J, Martín C, Ballesteros A, Cardoso A, Sancho C. Prepulse inhibition of the startle reflex in schizophrenia remains stable with short-term quetiapine. Eur Psychiatry. 2011;26(5):271–275. doi: 10.1016/j.eurpsy.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Moriwaki M, Kishi T, Takahashi H, Hashimoto R, Kawashima K, Okochi T, Kitajima T, Furukawa O, Fujita K, Takeda M, Iwata N. Prepulse inhibition of the startle response with chronic schizophrenia: a replication study. Neurosci Res. 2009;65(3):259–262. doi: 10.1016/j.neures.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Oranje B, Glenthøj BY. Clonidine normalizes sensorimotor gating deficits in patients with schizophrenia on stable medication. Schizophr Bull. 2013;39(3):684–691. doi: 10.1093/schbul/sbs071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss UW, Zimmermann J, Watzke S, Langosch J, Siafarikas N, Wong JW, Hamm A, Weike A. Short-term prospective comparison of prepulse inhibition between schizophrenic patients and healthy controls. Pharmacopsychiatry. 2011;44(3):102–108. doi: 10.1055/s-0031-1271687. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Frommann I, Berning J, Kühn KU, Maier W, Wagner M. Impaired sensorimotor gating of the acoustic startle response in the prodrome of schizophrenia. Biol Psychiatry. 2008;64(9):766–773. doi: 10.1016/j.biopsych.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Wagner M, Mössner R, Maier W, Kuhn KU. Sensorimotor gating of schizophrenia patients depends on catechol O-methyltransferase Val158Met polymorphism. Schizophr Bull. 2010;36(2):341–346. doi: 10.1093/schbul/sbn088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quednow BB, Wagner M, Westheide J, Beckmann K, Bliesener N, Maier W, Kuhn KU. Sensorimotor gating and habituation of the startle response in schizophrenic patients randomly treated with amisulpride or olanzapine. Biol Psychiatry. 2006;59(6):536–545. doi: 10.1016/j.biopsych.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Rabin RA, Sacco KA, George TP. Correlation of prepulse inhibition and Wisconsin Card Sorting Test in schizophrenia and controls: effects of smoking status. Schizophr Res. 2009;114(1-3):91–97. doi: 10.1016/j.schres.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR. Are we studying and treating schizophrenia correctly? Schizophr Res. 2011;130(1-3):1–10. doi: 10.1016/j.schres.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: Current knowledge and future challenges. Psychopharmacology. 2001;156(2-3):194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Cadenhead KC, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: Relationship to medications, symptoms, neurocognition and level of function. Arch Gen Psychiatry. 2006a;63(12):1325–1335. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Monroe SM, Hartston HJ, Braff DL, Geyer MA, Auerbach PP. Men are more inhibited than women by weak prepulses. Biol Psychiatry. 1993;34(4):253–260. doi: 10.1016/0006-3223(93)90079-s. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Sprock J, Braff DL. Prepulse-elicited motor reactions do not differ between schizophrenia patients and control subjects. Behav Neurosci. 2006b;120(1):224–227. doi: 10.1037/0735-7044.120.1.224. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Sprock J, Light GA, Cadenhead K, Calkins ME, Dobie DJ, Freedman R, Green MF, Greenwood TA, Gur RE, Mintz J, Olincy A, Nuechterlein KH, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Tsuang DW, Tsuang MT, Turetsky BI, Braff DL. Multi-site studies of acoustic startle and prepulse inhibition in humans: Initial experience and methodological considerations based on studies by the Consortium on the Genetics of Schizophrenia. Schizophr Res. 2007;92(1-3):237–251. doi: 10.1016/j.schres.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Talledo JA, Braff DL. Startle modulation in Caucasian-Americans and Asian-Americans: a prelude to genetic/endophenotypic studies across the “Pacific Rim”. Psychiatr Genet. 2005;15(1):61–65. doi: 10.1097/00041444-200503000-00010. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology. 2008;199(3):331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Iwase M, Ishii R, Ohi K, Fukumoto M, Azechi M, Ikezawa K, Kurimoto R, Canuet L, Nakahachi T, Like N, Tagami S, Morihara T, Okochi M, Tanaka T, Kazui H, Yoshida T, Tanimukai H, Yasuda Y, Kudo T, Hashimoto R, Takeda M. Impaired prepulse inhibition and habituation of acoustic startle response in Japanese patients with schizophrenia. Neurosci Res. 2008;62(3):187–194. doi: 10.1016/j.neures.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, Morris DW, Bishop J, Thaker GK, Sweeney JA. Clinical Phenotypes of Psychosis in the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013 doi: 10.1176/appi.ajp.2013.12101339. [Epub ahead of print, Jul 12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZR, Tan YL, Yang FD, Zhang WF, Zou YZ, Tan SP, Song CS, Li YL, Zhang WH, Zhou DF. Impaired prepulse inhibition of acoustic startle in Chinese patients with first-episode, medication-naïve schizophrenia. Chin Med J (Engl) 2013;126(3):526–531. [PubMed] [Google Scholar]
- Weike AI, U Bauer, Hamm AO. Effective neuroleptic medication removes prepulse inhibition deficits in schizophrenia patients. Biol Psychiatry. 2000;47(1):61–70. doi: 10.1016/s0006-3223(99)00229-2. [DOI] [PubMed] [Google Scholar]
- Woznica AA, Sacco KA, George TP. Prepulse inhibition deficits in schizophrenia are modified by smoking status. Schizophr Res. 2009;112(1-3):86–90. doi: 10.1016/j.schres.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Green MF, Sprock J, Light GA, Widmark C, Reist C, Erhart S, Marder SR, Mintz J, Braff DL. Effects of olanzapine, risperidone and haloperidol on prepulse inhibition in schizophrenia patients: a double-blind, randomized controlled trial. Schizophr Res. 2007;95(1-3):134–142. doi: 10.1016/j.schres.2007.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn JK, Sergi MJ, Dawson ME, Schell AM, Green MF. Sensorimotor gating, orienting and social perception in schizophrenia. Schizophr Res. 2005;73(2-3):319–325. doi: 10.1016/j.schres.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Xue YY, Wang HN, Xue F, Tan QR. Atypical antipsychotics do not reverse prepulse inhibition deficits in acutely psychotic schizophrenia. J Int Med Res. 2012;40(4):1467–1475. doi: 10.1177/147323001204000425. [DOI] [PubMed] [Google Scholar]
- Ziermans T, Schothorst P, Magnée M, van Engeland H, Kemner C. Reduced prepulse inhibition in adolescents at risk for psychosis: a 2-year follow-up study. J Psychiatry Neurosci. 2011;36(2):127–134. doi: 10.1503/jpn.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziermans TB, Schothorst PF, Sprong M, Magnée MJ, van Engeland H, Kemner C. Reduced prepulse inhibition as an early vulnerability marker of the psychosis prodrome in adolescence. Schizophr Res. 2012;134(1):10–15. doi: 10.1016/j.schres.2011.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


