Abstract
The National Heart, Lung, and Blood Institute has sponsored several asthma clinical networks, but the Severe Asthma Research Program (SARP) is unique, because it is not a clinical trials network, and it includes both adults and children. Investigators in SARP have comprehensively characterized 1,644 patients with asthma over the past 10 years, including 583 individuals with severe asthma and 300 children below the age of 18 years. The diversity in clinical characteristics, physiologic measures, and biomarkers in a large number of subjects across the ages provides an ideal cohort in which to investigate asthma heterogeneity. Using both biased and unbiased approaches, multiple asthma phenotypes have been described in SARP. These phenotypic analyses have improved our understanding of heterogeneity in asthma, and may provide a starting point to transform clinical practice through the evidence-based classification of disease severity. Although these new phenotypes strive to make order out of a heterogeneous group of patients, they are limited by that heterogeneity. There may be large groups of patients, especially those with milder asthma, that can be grouped into a clinical phenotype to guide therapy, but there will always be patients on the “edge” of a phenotype who will not fit into these groupings. In the SARP cluster analysis, subjects on the “edge” of a phenotype frequently had lung function that was better or worse than other subjects in the same cluster, despite similar clinical characteristics. This suggests that different pathophysiologic mechanisms may be responsible for decrements in lung function in some subjects. This is extremely important for subjects with severe asthma who may be on the “edge” of two phenotypes that may be driven by different pathobiologic mechanisms that warrant different therapeutic approaches.
Keywords: Severe Asthma Research Program, phenotype, cluster analysis, sputum
Asthma is defined as a clinical syndrome of intermittent respiratory symptoms triggered by viral upper respiratory infections, environmental allergens, or other stimuli, and is characterized by nonspecific bronchial hyperresponsiveness and airways inflammation (1). The National Asthma Education and Prevention Program and Global Initiative for Asthma guidelines classify asthma severity based on lung function (FEV1), daytime and nocturnal symptoms, and frequency of rescue bronchodilator use (2, 3). These clinical characteristics are used to assign disease severity based on four to six “steps” of asthma that range from mild, intermittent to severe, persistent disease. Treatment recommendations are based on the assignment of patients to these steps. This approach to disease classification assumes that all patients within a specific asthma severity level have similar disease characteristics and risk of future asthma exacerbations that should be managed with the same therapeutic regimen. The results of any clinical trial defy this premise; in a group of subjects recruited to be similar by strict inclusion criteria, there are responders and nonresponders to any pharmacologic intervention. Thus, the identification of groups of patients with asthma that share similar clinical, physiologic, and pathobiologic characteristics improves our understanding of asthma heterogeneity, and provides a foundation to develop personalized therapeutic approaches (4, 5).
The Severe Asthma Research Program
Asthma heterogeneity is most easily recognized in severe asthma, where patients have diverse symptom profiles and altered responses to medications (6, 7). In 2001, the National Heart, Lung, and Blood Institute initiated the Severe Asthma Research Program (SARP) to identify and characterize not only a large number of subjects with severe asthma, but also to compare these subjects with those with mild to moderate asthma. To identify subjects with severe asthma and enrich the cohort for these subjects, the definition of severe asthma developed by an American Thoracic Society (ATS) workshop on refractory asthma was used (8). The ATS definition of severe asthma requires treatment with high doses of oral or inhaled corticosteroids (major criteria), and two of seven minor criteria that represent signs of ongoing poor asthma control despite appropriate asthma therapy. Subjects who did not meet this definition were classified as not severe asthma, and further stratified into mild and moderate asthma based on prebronchodilator percent predicted FEV1 and treatment with inhaled corticosteroids in a manner consistent with the National Asthma Education and Prevention Program and Global Initiative for Asthma guidelines (2, 3). All subjects were comprehensively characterized with questionnaires designed to assess asthma symptoms, medication use, medical history, and health care utilization (HCU), complete pulmonary function testing, including maximal responsiveness to short-acting β-adrenergic agonists, evaluations for atopy (skin prick testing, serum IgE), and collection of biologic specimens for analysis (blood, sputum, and, in a subset, samples from investigative bronchoscopy) (6).
The SARP cohort exemplifies asthma heterogeneity. All levels of the asthma severity spectrum are represented in SARP from the mildest intermittent asthma to the most severe persistent disease in both children and adults (6, 9, 10). As such, the SARP cohort provides a platform to investigate asthma heterogeneity. Phenotyping analyses in SARP have been performed in a layered step-wise fashion. Initial analyses used hypothesis-driven, biased approaches with a priori separation of subjects before comparison investigating differences in not severe and severe asthma, as well as differences within the severe asthma subgroup (6, 9). Subsequent phenotyping was performed using unbiased statistical modeling approaches that group subjects without preordained hypotheses (11, 12). The two approaches are complementary techniques to explore heterogeneity in asthma.
Step 1: Identification of Phenotypes Using Biased Approaches
Severe versus Not Severe Asthma Phenotypes
Consistent with the ATS definition, children and adults with severe asthma demonstrated persistent symptoms and high HCU, despite complex medication regimens, including high doses of inhaled or oral corticosteroids (6, 9). Most adult participants with severe asthma had decreased baseline percent predicted FEV1, and some subjects showed chronic airflow obstruction with decreased responsiveness to short-acting β-adrenergic agonists. In a subset of adults who also had measurements of lung volumes, air trapping was increased in participants with severe asthma, and the response to bronchodilators was primarily in FVC (less air trapping) (13). Airflow limitation and air trapping were also distinguishing features of severe asthma in children, especially boys, albeit to a lesser degree than what was seen in the SARP adults (14, 15). Taken together, initial SARP phenotyping analyses confirmed that the ATS definition of severe asthma does identify a group of participants that differs clinically in multiple domains when compared with a group of subjects with less severe disease (6, 9, 10).
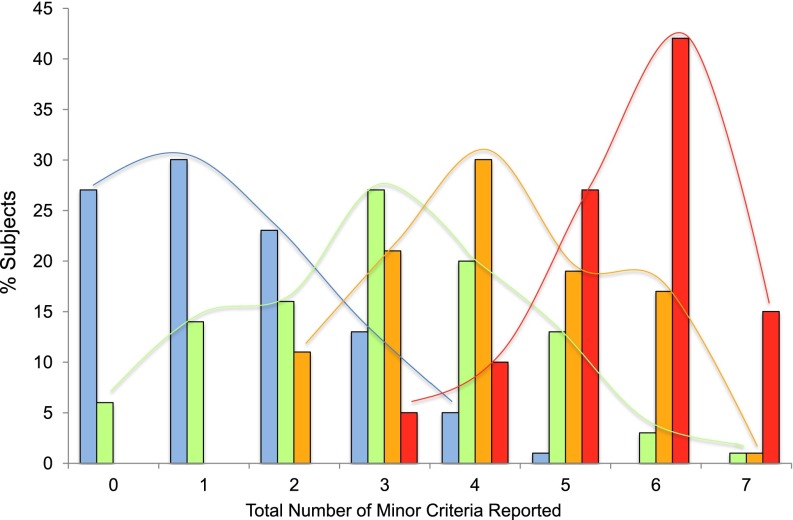
The above clinical characteristics, however, were not seen in all participants with severe asthma, and, furthermore, were seen in some subjects with milder asthma. The minor criteria of the ATS definition of severe asthma include elements of impairment (use of second controller medication, frequency of rescue albuterol use, loss of control with weaning of corticosteroids, number of exacerbations requiring oral corticosteroid) and risk (urgent care visits, near-fatal events, abnormal lung function). As such, the total number of minor criteria could be used as an indirect marker of asthma control to investigate asthma heterogeneity within a phenotypic group. Figure 1 shows the frequency of the cumulative number of minor criteria per subject in mild, moderate, and severe adult SARP subjects (6). There is appreciable overlap in this surrogate assessment of asthma control, suggesting heterogeneity in all severity groups, with subjects on both “edges” of the phenotype; there are participants classified as mild asthma with poor asthma control (two or more minor criteria) and subjects with severe asthma with better asthma control (three or fewer minor criteria). The number of minor criteria as a surrogate composite of asthma control does not distinguish asthma severity groups.
Figure 1.
The frequency of the minor criteria from the American Thoracic Society definition of severe asthma in adult subjects in the Severe Asthma Research Program (SARP). Taken together, the total number of minor criteria for each subject can be used as a surrogate of asthma control, both impairment (rescue and controller medication use) and risk (health care utilization [HCU] and pulmonary function). Each bar indicates the percent of subjects with zero to seven total minor criteria within the mild, moderate, and severe asthma groups. There is heterogeneity in all asthma severity groups, indicative of a mixture of subjects with different levels of asthma control. Blue, mild asthma; green, moderate asthma; orange, subjects with severe asthma being treated with high doses of inhaled corticosteroids; red, subjects with severe asthma being treated with chronic oral corticosteroids.
Severe Asthma Subphenotypes
Figure 1 also highlights marked heterogeneity within the subjects with severe asthma; subjects on chronic oral corticosteroids have significantly more minor criteria (poorer asthma control) than those subjects with severe asthma on high-dose inhaled corticosteroids alone, despite more complicated medication regimens. Several SARP analyses have focused on heterogeneity in the severe asthma group using hypothesis-driven approaches (10). These analyses have shown differences in the severe asthma phenotype stratified by age of onset, with a group of subjects with adult-onset with less atopy that reported frequent sinopulmonary infections (6). A subsequent analysis reported that obesity was a disease modifier in severe asthma, but only in subjects with early-onset asthma (16). These subgroup analyses describe subphenotypes of severe asthma, but in a one-dimensional dichotomous fashion that limits the ability to investigate heterogeneity. The next level of phenotyping in SARP has focused on multivariate approaches to group subjects.
Step 2: Identification of Phenotypes Using Unbiased Approaches—Cluster Analyses
SARP Clinical Cluster Analyses
Cluster analysis is a statistical modeling technique in which patients are successively grouped into “clusters” based on their similarities. This unsupervised clustering of subjects leads to groups of patients who share clinical, physiologic, and/or biologic characteristics. The SARP cohort is ideal for this modeling approach, as it consists of a large number of subjects with asthma of all severity classes who have been comprehensively characterized using standardized protocols. To explore asthma heterogeneity and perhaps develop a more pathophysiology-based classification system, two cluster analyses were performed in SARP: the first on 726 subjects over the age of 12 years (the “adult” cluster analysis), and the second on 300 children (<18 yr of age) (11, 12). These analyses were performed independently, due to expected differences in phenotypes in children as opposed to adults.
The adult cluster analysis identified five different groups of subjects with asthma who differ in clinical and pathophysiologic parameters (11). These five asthma phenotypes differ in lung function, age of asthma onset and duration, atopy, sex, symptom frequency, medication use, and HCU (see Table 1). Clusters 1, 2, and 4 reflect the spectrum of allergic asthma from mild to severe airflow obstruction. The majority of these patients have early-onset disease before puberty, with evidence of atopic sensitization by skin prick testing and elevated total serum IgE. In contrast, Clusters 3 and 5 reflect the spectrum of adult onset asthma characterized by older patients with less atopy, yet high HCU and poor quality of life. Cluster 5 patients developed asthma after puberty in their teens and 20s, and have long duration of disease. Lung function is the lowest in this group, and, although they are very responsive to β-agonists, they have fixed airflow obstruction and frequent respiratory infections. In contrast, all patients in Cluster 3 developed asthma in their 40s and are highly symptomatic, with a requirement for frequent corticosteroid tapers, but have relatively preserved lung function. Clusters 3, 4, and 5 contain the majority of the patients with severe, persistent asthma and, together, they represent adult-onset severe asthma, childhood-onset severe allergic asthma, and severe asthma with severe fixed airflow obstruction, with similarities to chronic obstructive pulmonary disease.
Table 1.
The Severe Asthma Research Program adult cluster analysis: clinical characteristics of subject groups
| Spectrum of Early-Onset Allergic Asthma |
Spectrum of Late-Onset Adult Asthma |
||||
|---|---|---|---|---|---|
| |
Mild |
Moderate |
Severe |
Fixed Airflow |
Very Late Onset, Obese |
| Cluster 1 | Cluster 2 | Cluster 4 | Cluster 5 | Cluster 3 | |
| % of all subjects in group | 15 | 44 | 17 | 16 | 8 |
| Severe, % | <25 |
70–80 |
50 | ||
| Current age, decades | 20s | 20–30s | 30–40s | 40–50s | 40–50s |
| African American, % | 30 | 30 | 30 | 20 | 20 |
| Female sex, % | 80 | 67 | 53 | 63 | 71 |
| Obese with BMI > 30, % | 24 | 31 | 44 | >50 |
|
| Asthma onset | |||||
| Age, median (IQR), yr* | 9 (5–17) | 7 (2–18) | 4 (1–13) | 20 (5–32) | 41 (33–49) |
| Relevant life events | School aged | Preschool–school aged | Toddler–school-aged | Teens–adults | Adults |
| Asthma duration, yr | 10–15 | 15–25 | >20 |
<10 | |
| Atopy measures | |||||
| ≥1 pos SPT, % | 85 | 78 | 83 | 66 | 64 |
| Baseline FEV1% predicted* | 103 (97–109) | 83 (76–90) | 59 (48–66) | 44 (32–52) | 75 (67–81) |
| Maximal FEV1% predicted* | 113 (108–117) | 95 (88–99) | 77 (68–83) | 57 (48–68) | 82 (78–90) |
| Summary Lung Function | Normal | Reversible to normal | Obstructed, more reversible | Obstructed, less reversible | Borderline normal |
| Medications, % subjects | |||||
| High-dose ICS | 10 | 28 | 63 | 78 | 49 |
| OCS | 11 | 10 | 39 | 47 | 17 |
| Taking ≥3 controllers | 19 | 29 | 56 | 67 | 54 |
| Health care utilization, past yr, % subjects | |
||||
| ≥3 exacerbations with OCS | 11 | 19 | 46 | 42 | 36 |
| Hospitalization for asthma | 7 | 9 | 23 | 28 | 15 |
Definition of abbreviations: BMI = body mass index; ICS = inhaled corticosteroids; IQR = interquartile range; OCS = oral corticosteroids; SPT = skin prick testing.
Data expressed as median (25–75% [IQR]). Table adapted with permission from Ref. 11.
The pediatric cluster analysis similarly identified four clusters of children who differed in clinical and pathophysiologic measures (see Table 2) (12). Children in Cluster 1 were differentiated by a greater age at symptom onset, shorter asthma duration, normal lung function, and minimal need for asthma controller medications. Children in Cluster 2 also had normal lung function, but were diagnosed with asthma at an earlier age, were more symptomatic, and were treated with additional asthma controller medications. In contrast, children in Clusters 3 and 4 tended to be most symptomatic, with the greatest asthma medication requirements. Children in Clusters 3 and 4 also had the longest duration of asthma, and greatest degree of airflow limitation and air trapping, which was not completely reversed with bronchodilator administration. However, in contrast to the adult cluster analysis, the degree of lung function impairment in each of the pediatric clusters was significantly less.
Table 2.
The Severe Asthma Research Program pediatric cluster analysis: clinical characteristics of subject groups
| Spectrum of Childhood Allergic Asthma |
||||
|---|---|---|---|---|
| |
Mild |
Moderate |
Severe |
Fixed Airflow |
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | |
| % of all subjects in group | 30 | 32 | 20 | 18 |
| Severe, % | <30 | 50–60 |
>80 | |
| African American, % | 31 | 48 | 59 | 83 |
| Female sex, % | 50 |
66 |
||
| Asthma onset | ||||
| Age, median (IQR), yr* | 6 (3–9) | 1 (1–4) | 1 (0–1) | 1 (0–2) |
| Relevant life events | School aged | Preschoolers | Infants | Toddlers |
| Atopy measures | ||||
| ≥1 pos SPT, % | 57 | 83 | 87 | 89 |
| Baseline FEV1 % predicted* | 96 (82–108) | 91 (80–99) | 89 (75–99) | 73 (64–88) |
| Maximal FEV1 % predicted* | 109 (95–124) | 103 (97–111) | 99 (85–109) | 91 (80–101) |
| Summary lung function | Normal | Normal | Obstructed, more reversible | Obstructed, less reversible |
| Medications, % subjects | ||||
| High-dose ICS | 33 | 59 | 63 | 83 |
| OCS | 0 | 8 | 16 | 10 |
| Taking ≥3 controllers | 31 | 71 | 66 | 83 |
| Health care utilization, past yr, % subjects | ||||
| ≥3 exacerbations with OCS | 65 | 79 | 72 | 90 |
| Hospitalization for asthma | 23 | 33 | 41 | 48 |
Definition of abbreviations: ICS = inhaled corticosteroids; IQR = interquartile range; OCS = oral corticosteroids; SPT = skin prick testing.
Data expressed as median (25–75% [IQR]). Table adapted with permission from Ref. 12.
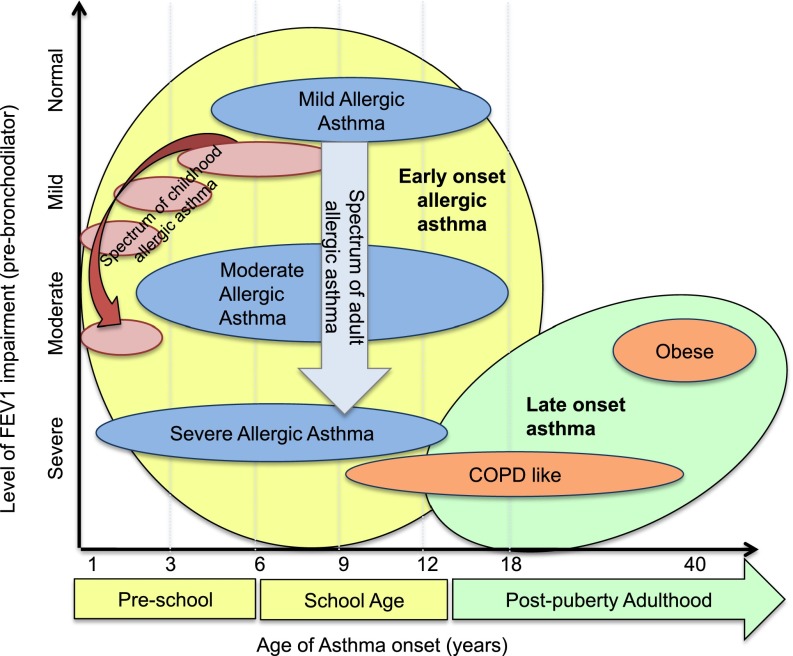
In both cluster analyses, the variables that were most influential were age and measures of lung function (11, 12). Figure 2 compares the adult and pediatric SARP clusters in a hypothetical graphic representation contrasting age of asthma onset with a subjective appraisal of FEV1 impairment. In this interpretation, nearly all children and most adults have early-onset allergic asthma. Severity of allergic asthma appears to be associated with age of asthma onset; the earlier the asthma, the worse the severity. Subjects with adult-onset asthma are very different, with disproportional decrements in lung function. In all phenotypes, especially the adults, there is heterogeneity. The width of the ovals depicts broad ranges in age of asthma onset in all groups, with some subjects in each phenotype (except Cluster 3), presenting in middle school or late adolescence. Likewise, the height of the ovals represents heterogeneity in lung function within each phenotype, emphasizing that there is a continuum of subjects within each cluster phenotype. Who are the subjects on the “edge” of these ovals, and how are they different from the rest of the group?
Figure 2.
A proposed combination of the adult and pediatric SARP clinical clusters. Generally, earlier age of asthma onset in childhood appears to be associated with progressively more severe allergic asthma in both children and adults. Adult subjects with onset of asthma after puberty have greater decrements in lung function than those with childhood onset, with the exception of subjects with severe, long-standing allergic asthma. The shape of the oval indicates heterogeneity within each phenotype; the “width” represents heterogeneity in the age of asthma onset, the “height” symbolizes heterogeneity of lung function within each group. COPD = chronic obstructive pulmonary disease.
Clinical Heterogeneity within the SARP Clusters
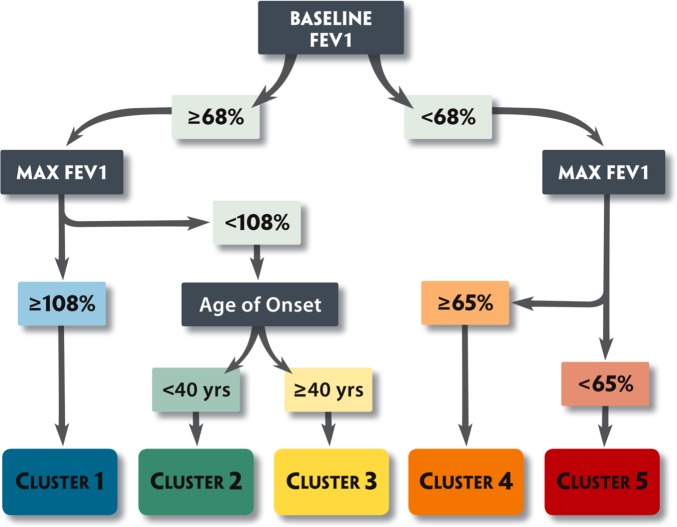
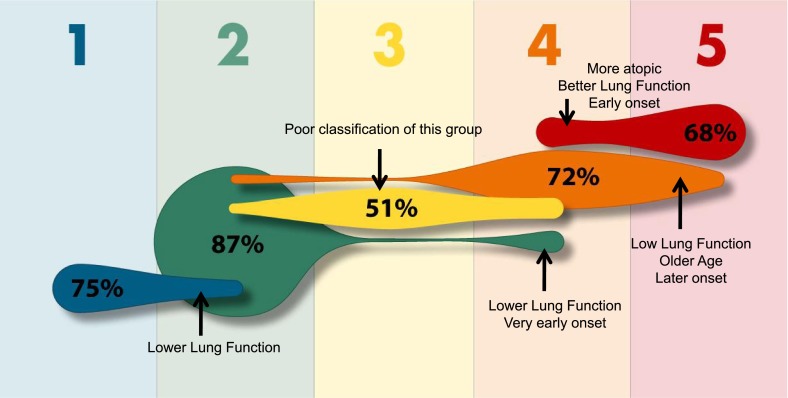
In the SARP adult clinical cluster analysis, a tree diagram was developed to predict cluster assignment in subjects using only three variables (Figure 3) (11). Using baseline FEV1 (prebronchodilator), maximum FEV1 after six to eight puffs of albuterol, and age of onset, 80% of subjects could be assigned to the correct cluster (Figure 4). The more informative subjects, however, may be the 20% of adult subjects who were incorrectly classified using this algorithm. These are the subjects on the “edge,” who represent the greatest heterogeneity within the clusters.
Figure 3.
Tree analysis. Subjects can be assigned to the five clusters that range from milder asthma (Cluster 1) to more severe disease (Clusters 4 and 5) using three variables: (1) baseline FEV1 (with a bronchodilator withhold); (2) maximal (Max) FEV1 (after six to eight puffs of albuterol); and (3) age of onset of asthma. Blue, mild atopic asthma; green, mild to moderate atopic asthma; yellow, late-onset nonatopic asthma; orange, severe atopic asthma; red, severe asthma with fixed airflow. Reproduced by permission from Reference 11.
Figure 4.
Misclassification of predicted SARP clinical cluster assignment by the tree diagram. Using the three-variable tree algorithm, the percent of subjects correctly assigned to each cluster is indicated within each shape. Cluster assignment is incorrect in a subset of subjects (indicated by arrows), due to heterogeneity in lung function or age of asthma onset within a given cluster; misclassified subjects are on the “edge” when compared with the majority of subjects within a given cluster. Blue, mild atopic asthma; green, mild to moderate atopic asthma; yellow, late-onset nonatopic asthma; orange, severe atopic asthma; red, severe asthma with fixed airflow. Individual figure size is proportional to the frequency of a specific cluster. Adapted with permission from Reference 11.
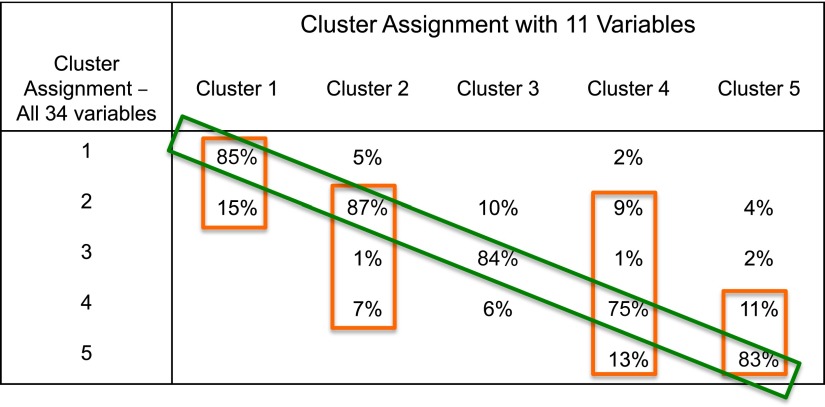
If variables are added to the tree algorithm, it becomes more complicated, but more accurate. Using all 11 variables from the discriminant analysis from the original clinical cluster analysis improves classification of subjects (Figure 5). These 11 variables include extended lung function measurements (baseline and maximal % predicted FVC, baseline FEV1:FVC ratio, maximal % change in FEV1), asthma duration, sex, frequency of β-agonist use, and intensity of corticosteroid use, in addition to baseline and maximal percent predicted FEV1 and age of asthma onset. Despite higher accuracy, however, subjects on the “edge” continue to be misclassified, especially among the three allergic asthma clusters (1, 2, and 4) and between the two most severe clusters (4 and 5).
Figure 5.
Predicted SARP clinical cluster assignments using 11 variables instead of 3. Overall correct classification is improved, especially in Clusters 1, 3, and 5, but subjects continue to be misclassified, due to heterogeneity of lung function within the clusters. Misclassification is the greatest among the three clusters that represent the continuum of allergic asthma (1, 2, and 4) and between the most severe clusters (4 and 5). Green rectangle, percent of subjects correctly assigned; orange rectangles, percent of subjects correctly and incorrectly assigned by the 11-variable algorithm.
It is not surprising that the misclassification of subjects using the tree diagrams is largely due to lung function heterogeneity within a cluster, given the importance of these variables in the algorithms. The question is whether the pathobiology in subjects on the “edge” resembles that of the current cluster or the misclassified cluster. This is extremely important for subjects living on the “edge” between Cluster 4 (severe allergic asthma) and Cluster 5 (chronic obstructive pulmonary disease–like with fixed airflow obstruction), as it has been hypothesized that different pathobiologic mechanisms may determine these two severe asthma phenotypes. In the original SARP clinical cluster analysis, a small Cluster 6 was identified; these subjects had severe baseline chronic airflow obstruction similar to Cluster 5 subjects, but clinical characteristics and bronchial responsiveness similar to Cluster 4 subjects (severe allergic asthma). Different pathobiologic mechanisms are likely important in this group of subjects, and, thus, different therapeutic approaches would be needed.
Inflammatory Heterogeneity within the SARP Clusters
At the time of the adult SARP clinical cluster analysis, inflammatory cellular measures were not available on all subjects and, thus, could not be used as a variable in the analysis. Evaluation of blood and sputum inflammatory cells in the subset of subjects who did have biologic specimens collected, however, suggested that sputum eosinophils were increased in Cluster 4 subjects with severe allergic asthma, whereas both eosinophils and neutrophils were increased in subjects from Cluster 5 (11). A separate analysis of a larger, single-center SARP sputum dataset stratified subjects based on sputum granulocytes (< or ≥2% eosinophils and < or ≥40% neutrophils) showed that grouping of subjects based on their sputum inflammatory cell profile identified four groups of subjects with distinguishing clinical characteristics (17). Subjects with a mixed granulocytic pattern (≥2% eosinophils and ≥40% neutrophils) had the most severe asthma with severe chronic airflow obstruction and increased symptoms with high HCU. Using this paradigm, assignment of sputum inflammatory profiles to subjects within the five clinical clusters shows all four patterns in all five cluster phenotypes without a dominant pattern in any one cluster (Figure 6). This heterogeneity in inflammatory cell measures in each cluster may explain the clinical heterogeneity observed.
Figure 6.
Sputum inflammatory profiles in SARP clinical clusters. Inflammatory profiles were determined for individual subjects based on percent eosinophils (≥2%) and percent neutrophils ≥40% in induced sputum (n = 423). All four patterns are present in each cluster, indicative of heterogeneity of cellular inflammation within each group. Blue, paucigranulocytic; red, eosinophil predominant; green, neutrophil predominant; purple, mixed granulocytic.
Conclusions
There is marked physiologic and pathobiologic heterogeneity within the SARP cohort, even among subjects who clinically cluster together. Since the time of the original clinical cluster analyses, the SARP cohort has more than doubled in size, sputum inflammatory cellular measures are available on nearly 500 subjects, and more than 350 bronchoscopies have been performed (10). The lack of association between the clinical clusters and sputum inflammatory cell patterns emphasizes that future analyses must incorporate clinical, physiologic, and inflammatory measures into one analysis (18). There are two multivariate phenotype analyses currently being performed in SARP: one that extends the above cluster analysis through the inclusion of blood and sputum inflammatory cellular measures as variables, and a second using a machine learning–based approach with inclusion of bronchoalveolar lavage inflammatory cells as variables. These results should be available within the next several months, and both will further our understanding of asthma phenotypes and the pathobiologic mechanisms that underlie them.
In summary, there will always be heterogeneity within any phenotype in a complex disease such as asthma. The key is that phenotypes should reflect pathobiologic mechanisms that can direct therapeutic approaches (5). The challenge is to optimize and simplify phenotypes so that they can easily translate into the clinic. Understanding heterogeneity will eventually lead to usable phenotypes. As usual, the devil is in the details.
Footnotes
Supported by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 2.National Asthma Education and Prevention Program. Expert panel report 3: guidelines for the diagnosis and management of asthma [Internet]. Bethesda, MD: National Institutes of Health; National Heart, Lung, and Blood Institute; 2007: no. 07-4051 [accessed 2013 Mar 4]. Available from: http://www.nhlbi.nih.gov/guidelines/asthma
- 3.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O’Byrne P, Pedersen SE, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 4.Chanez P, Wenzel SE, Anderson GP, Anto JM, Bel EH, Boulet LP, Brightling CE, Busse WW, Castro M, Dahlen B, et al. Severe asthma in adults: what are the important questions? J Allergy Clin Immunol. 2007;119:1337–1348. doi: 10.1016/j.jaci.2006.11.702. [DOI] [PubMed] [Google Scholar]
- 5.Wenzel S. Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy. 2012;42:650–658. doi: 10.1111/j.1365-2222.2011.03929.x. [DOI] [PubMed] [Google Scholar]
- 6.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, et al. National Heart, Lung, Blood Institute’s Severe Asthma Research Program. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wener RRL, Bel EH. Severe refractory asthma: an update. Eur Respir Rev. 2013;22:227–235. doi: 10.1183/09059180.00001913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Thoracic Society. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 9.Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. Features of severe asthma in school-age children: atopy and increased exhaled nitric oxide. J Allergy Clin Immunol. 2006;118:1218–1225. doi: 10.1016/j.jaci.2006.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SA, Chung KF, Curran-Everett D, Dweik RA, Fain SB, et al. NHLBI Severe Asthma Research Program (SARP) Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med. 2012;185:356–362. doi: 10.1164/rccm.201107-1317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D’Agostino R, Jr, Castro M, Curran-Everett D, Fitzpatrick AM, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, Wenzel SE, Aujla S, Castro M, Bacharier LB, et al. National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol 2011:127:382–389.e1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorkness RL, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Chung KF, Curran-Everett D, Erzurum SC, Gaston BM, Israel E, et al. National Heart, Lung, and Blood Institute Severe Asthma Research Program. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J Appl Physiol (1985) 2008;104:394–403. doi: 10.1152/japplphysiol.00329.2007. [DOI] [PubMed] [Google Scholar]
- 14.Sorkness RL, Teague WG, Penugonda M, Fitzpatrick AM National Institutes of Health, National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Sex dependence of airflow limitation and air trapping in children with severe asthma. J Allergy Clin Immunol. 2011;127:1073–1074. doi: 10.1016/j.jaci.2010.12.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzpatrick AM, Teague WG National Institutes of Health/National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Progressive airflow limitation is a feature of children with severe asthma. J Allergy Clin Immunol. 2011;127:282–284. doi: 10.1016/j.jaci.2010.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, Fitzpatrick AM, Gaston B, Israel E, Jarjour NN, et al. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol 2011;127:1486–1493.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, Bleecker ER.National Heart, Lung, and Blood Institute Severe Asthma Research Program. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol 2010:125:1028–1036.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kupczyk M, Wenzel S. U.S. and European severe asthma cohorts: what can they teach us about severe asthma? J Intern Med. 2012;272:121–132. doi: 10.1111/j.1365-2796.2012.02558.x. [DOI] [PubMed] [Google Scholar]