Abstract
The experiments were designed to get some information on the metabolism controlled by variation of the NADP level, which is known to change with the variation of environmental factors.
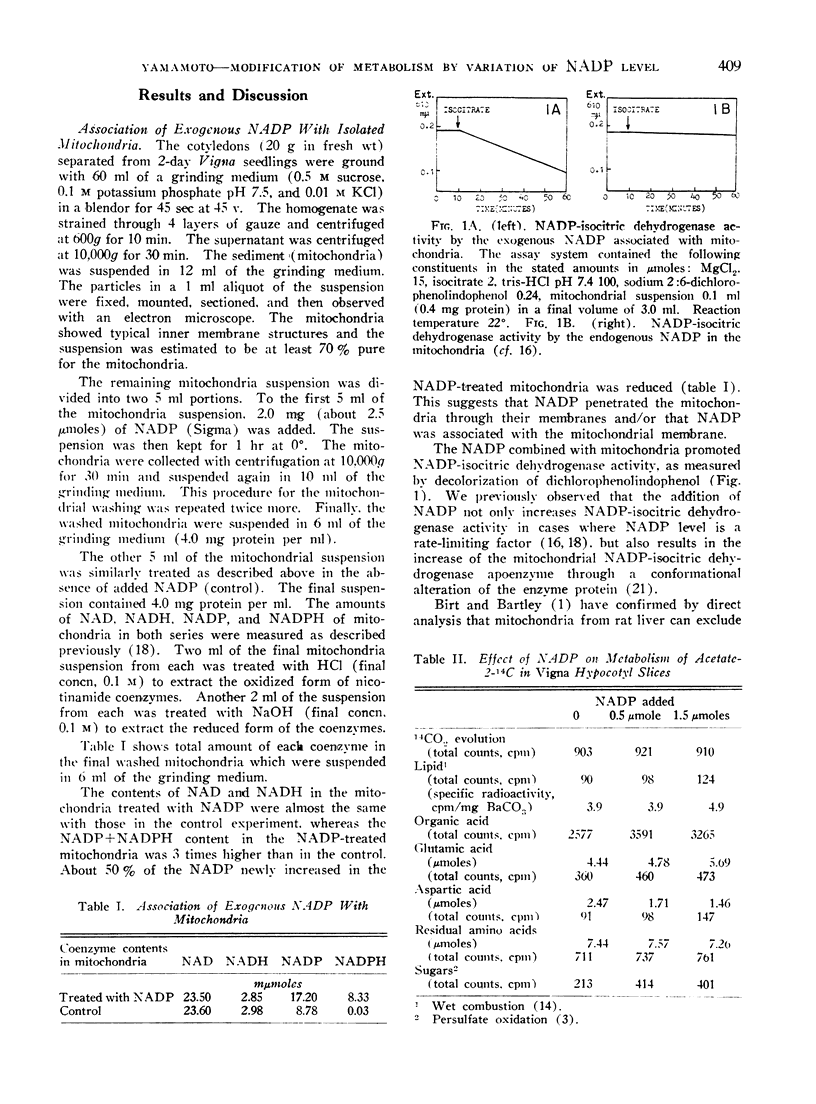
The exogenous NADP added to the mitochondria prepared from Vigna sesquipedalis cotyledons was associated with and/or penetrated into the mitochondria. The combined NADP served in the operation of the mitochondrial NADP-isocitric acid dehydrogenase.
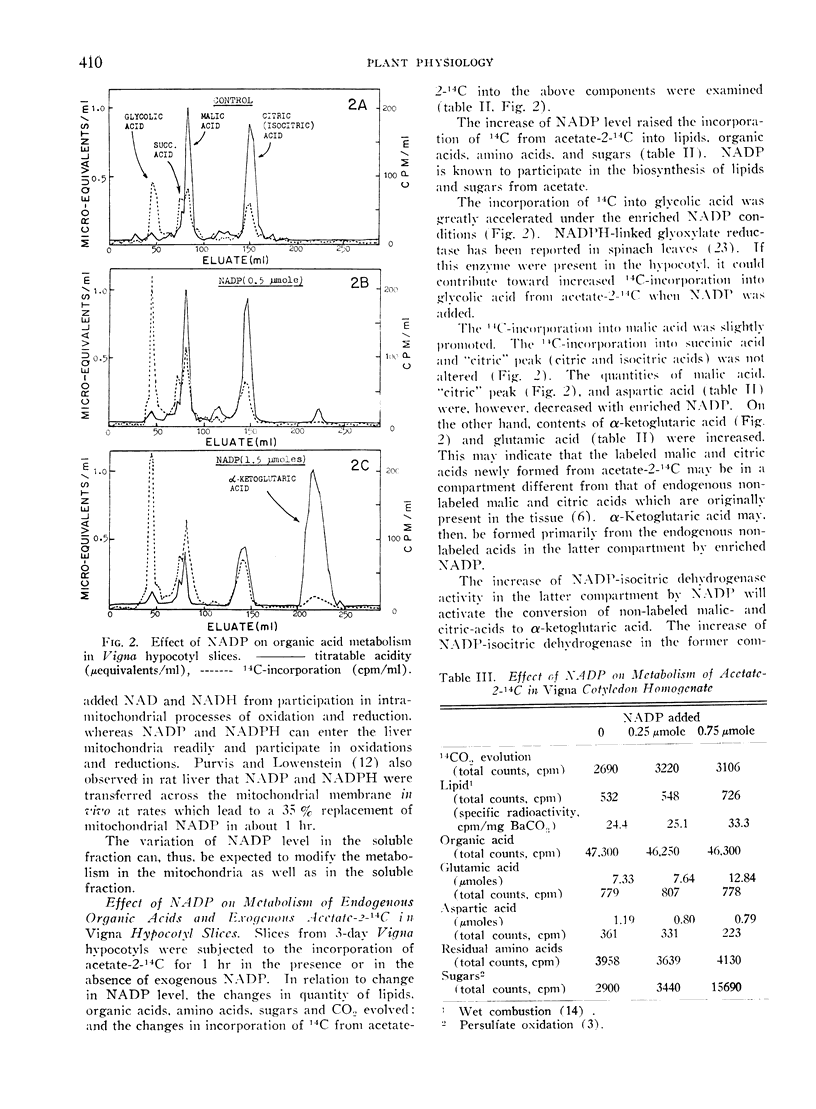
The variation of NADP level by exogenous NADP was observed to modify the rates of metabolic processes. The increase of exogenous NADP in Vigna hypocotyl slices lowered malic- and citric-acid contents and raised the α-ketoglutaric acid content. The incorporation of 14C from acetate-2-14C into lipid, organic acid, amino acid, was promoted with the exogenous NADP. The 14C-incorporation into glycolic acid, malic acid and glutamic acid was accelerated.
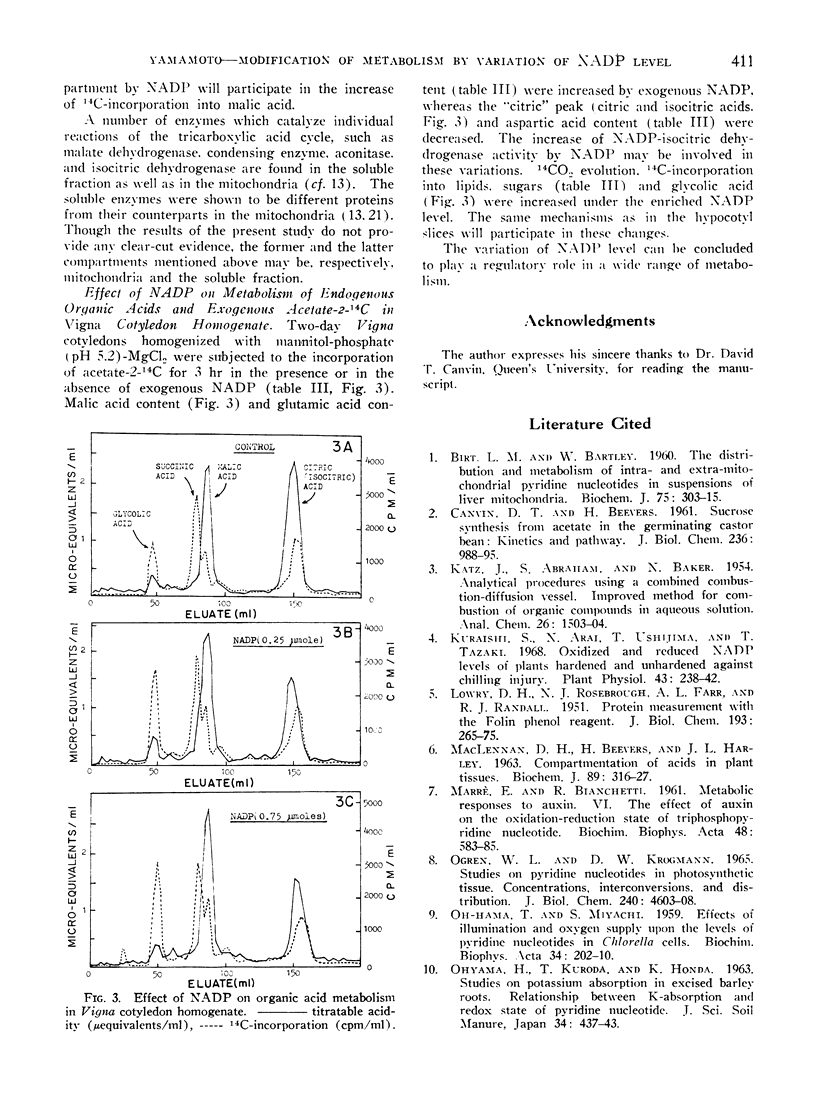
In the mannitol homogenate of Vigna cotyledon, 14CO2 evolution and 14C-incorporation into lipid, sugar, and glycolic acid from acetate-2-14C were promoted with the exogenous NADP. Endogenous citric acid content was lowered by NADP, while malic acid content was increased.
The activation of NADP-enzymes by NADP was discussed to be involved in these variations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIRT L. M., BARTLEY W. The distribution and metabolism of intra- and extra- mitochondrial pyridine nucleotides in suspensions of liver mitochondria. Biochem J. 1960 May;75:303–315. doi: 10.1042/bj0750303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANVIN D. T., BEEVERS H. Sucrose synthesis from acetate in the germinating castor bean: kinetics and pathway. J Biol Chem. 1961 Apr;236:988–995. [PubMed] [Google Scholar]
- Kuraishi S., Arai N., Ushijima T., Tazaki T. Oxidized and reduced nicotinamide adenine dinucleotide phosphate levels of plants hardened and unhardened against chilling injury. Plant Physiol. 1968 Feb;43(2):238–242. doi: 10.1104/pp.43.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARRE E., BIANCHETTI R. Metabolic responses to auxin. VI. The effect of auxin on the oxidation-reduction state of triphosphopyridine nucleotide. Biochim Biophys Acta. 1961 Apr 15;48:583–585. doi: 10.1016/0006-3002(61)90056-7. [DOI] [PubMed] [Google Scholar]
- OH-HAMA T., MIYACHI S. Effects of illumination and oxygen supply upon the levels of pyridine nucleotides in Chlorella cells. Biochim Biophys Acta. 1959 Jul;34:202–210. doi: 10.1016/0006-3002(59)90248-3. [DOI] [PubMed] [Google Scholar]
- Ogren W. L., Krogmann D. W. Studies on pyridine nucleotides in photosynthetic tissue. Concentrations, interconversions, and distribution. J Biol Chem. 1965 Dec;240(12):4603–4608. [PubMed] [Google Scholar]
- ZELITCH I., GOTTO A. M. Properties of a new glyoxylate reductase from leaves. Biochem J. 1962 Sep;84:541–546. doi: 10.1042/bj0840541. [DOI] [PMC free article] [PubMed] [Google Scholar]



