Abstract
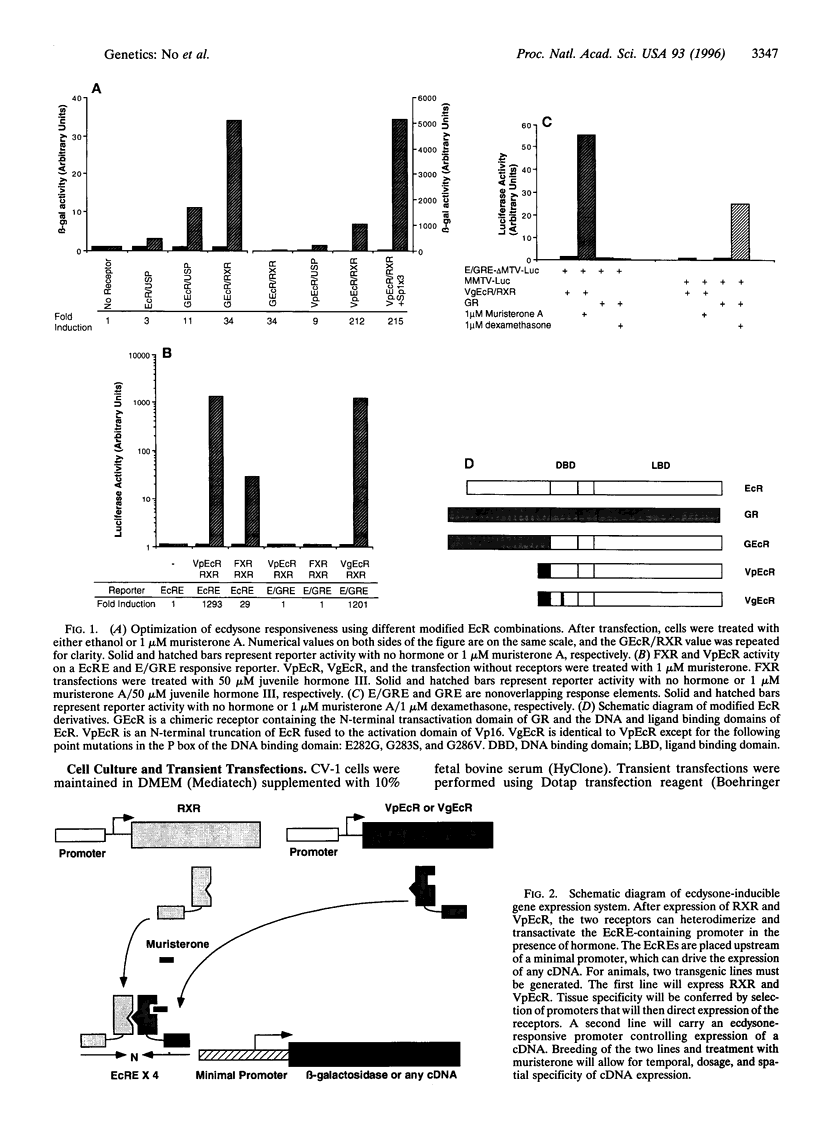
During metamorphosis of Drosophila melanogaster, a cascade of morphological changes is triggered by the steroid hormone 20-OH ecdysone via the ecdysone receptor, a member of the nuclear receptor superfamily. In this report, we have transferred insect hormone responsiveness to mammalian cells by the stable expression of a modified ecdysone receptor that regulates an optimized ecdysone responsive promoter. Inductions reaching 4 orders of magnitude have been achieved upon treatment with hormone. Transgenic mice expressing the modified ecdysone receptor can activate an integrated ecdysone responsive promoter upon administration of hormone. A comparison of tetracycline-based and ecdysone-based inducible systems reveals the ecdysone regulatory system exhibits lower basal activity and higher inducibility. Since ecdysone administration has no apparent effect on mammals, its use for regulating genes should be excellent for transient inducible expression of any gene in transgenic mice and for gene therapy.
Full text
PDF
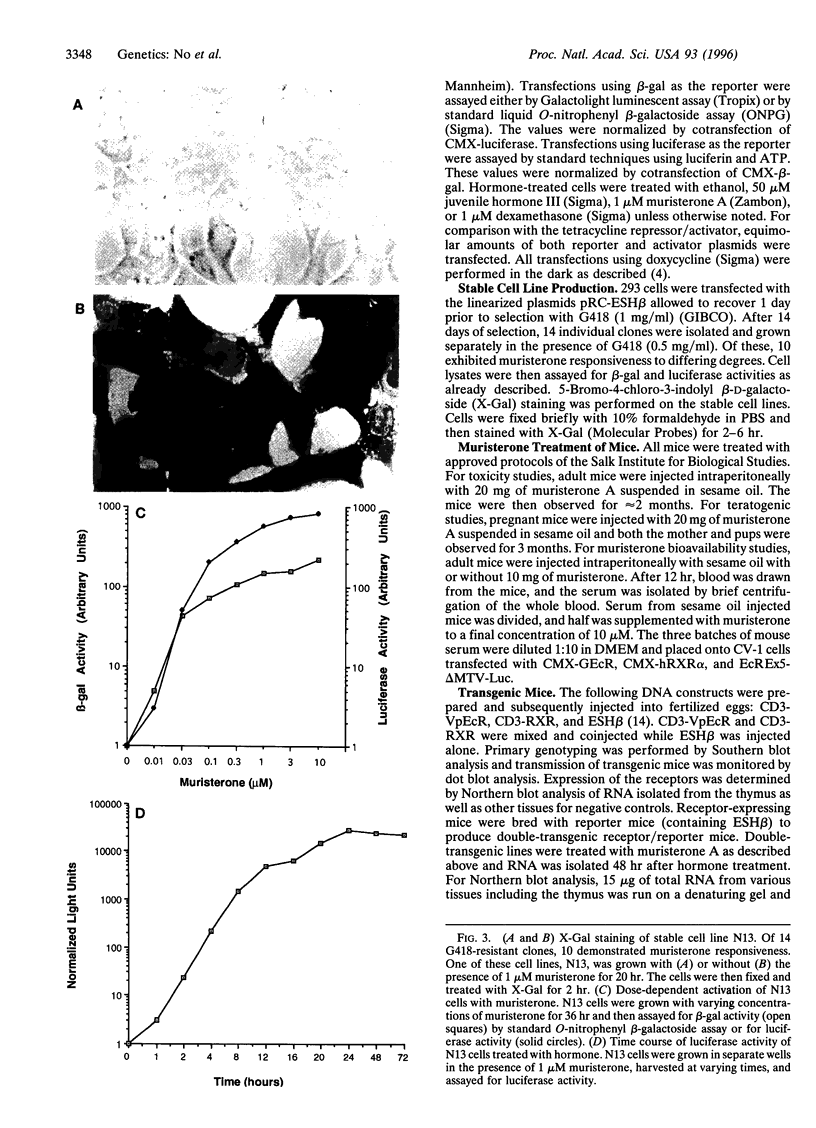
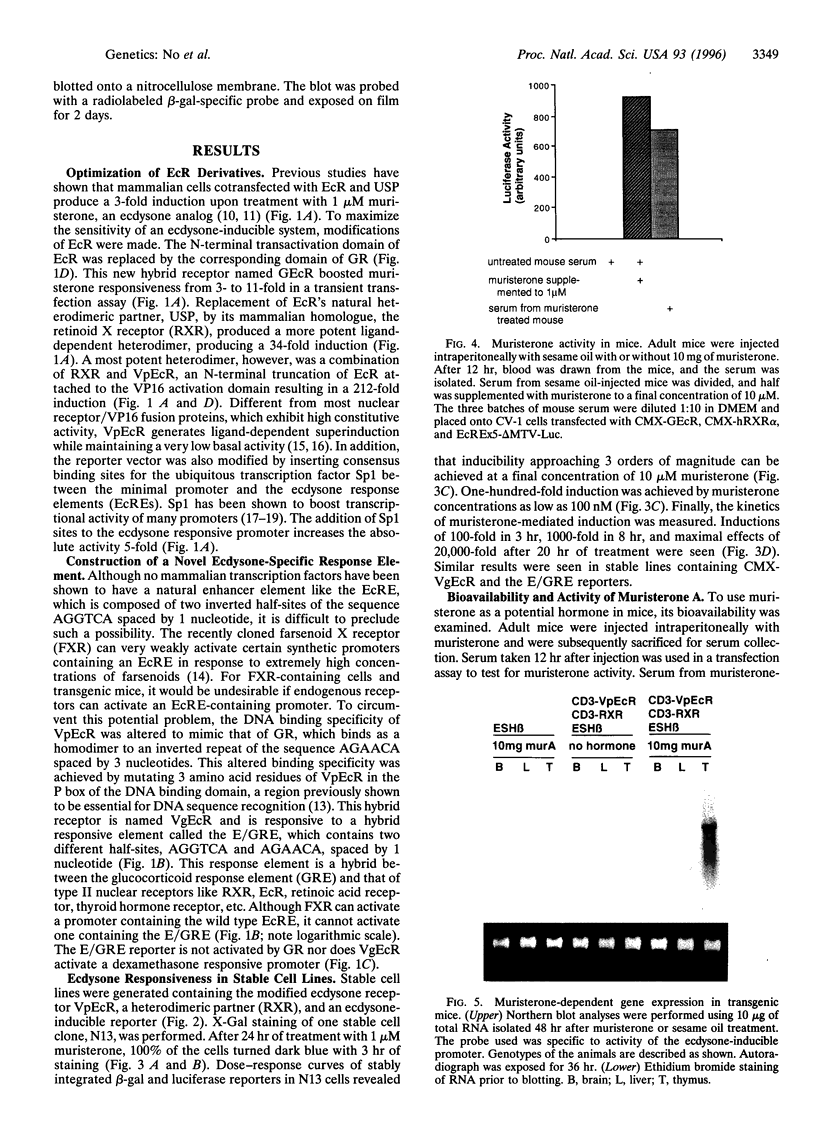
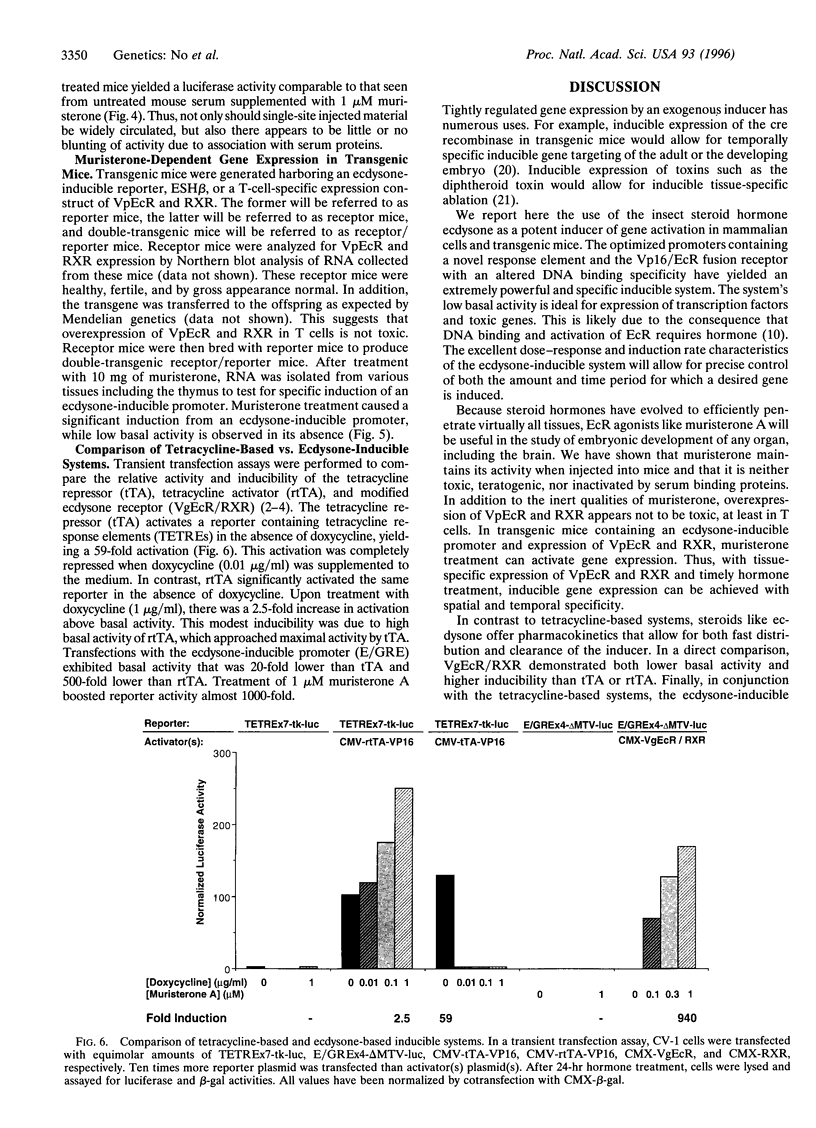

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Chihara C., Meltzer P., Richards G. Temporal control of puffing activity in polytene chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:655–662. doi: 10.1101/sqb.1974.038.01.070. [DOI] [PubMed] [Google Scholar]
- CLEVER U., KARLSON P. [Induction of puff changes in the salivary gland chromosomes of Chironomus tentans by ecdysone]. Exp Cell Res. 1960 Sep;20:623–626. doi: 10.1016/0014-4827(60)90141-5. [DOI] [PubMed] [Google Scholar]
- Forman B. M., Goode E., Chen J., Oro A. E., Bradley D. J., Perlmann T., Noonan D. J., Burka L. T., McMorris T., Lamph W. W. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995 Jun 2;81(5):687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- Furth P. A., St Onge L., Böger H., Gruss P., Gossen M., Kistner A., Bujard H., Hennighausen L. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9302–9306. doi: 10.1073/pnas.91.20.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M., Bonin A. L., Bujard H. Control of gene activity in higher eukaryotic cells by prokaryotic regulatory elements. Trends Biochem Sci. 1993 Dec;18(12):471–475. doi: 10.1016/0968-0004(93)90009-c. [DOI] [PubMed] [Google Scholar]
- Gossen M., Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M., Freundlieb S., Bender G., Müller G., Hillen W., Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995 Jun 23;268(5218):1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Transgenic animals. Science. 1988 Jun 10;240(4858):1468–1474. doi: 10.1126/science.3287623. [DOI] [PubMed] [Google Scholar]
- Kamine J., Subramanian T., Chinnadurai G. Sp1-dependent activation of a synthetic promoter by human immunodeficiency virus type 1 Tat protein. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8510–8514. doi: 10.1073/pnas.88.19.8510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N. A., Loh D. Y., Lacy E. CD8 surface levels alter the fate of alpha/beta T cell receptor-expressing thymocytes in transgenic mice. J Exp Med. 1992 Apr 1;175(4):1013–1025. doi: 10.1084/jem.175.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gorman S., Fox D. T., Wahl G. M. Recombinase-mediated gene activation and site-specific integration in mammalian cells. Science. 1991 Mar 15;251(4999):1351–1355. doi: 10.1126/science.1900642. [DOI] [PubMed] [Google Scholar]
- Perlmann T., Rangarajan P. N., Umesono K., Evans R. M. Determinants for selective RAR and TR recognition of direct repeat HREs. Genes Dev. 1993 Jul;7(7B):1411–1422. doi: 10.1101/gad.7.7b.1411. [DOI] [PubMed] [Google Scholar]
- Ross S. R., Graves R. A., Spiegelman B. M. Targeted expression of a toxin gene to adipose tissue: transgenic mice resistant to obesity. Genes Dev. 1993 Jul;7(7B):1318–1324. doi: 10.1101/gad.7.7b.1318. [DOI] [PubMed] [Google Scholar]
- Shockett P., Difilippantonio M., Hellman N., Schatz D. G. A modified tetracycline-regulated system provides autoregulatory, inducible gene expression in cultured cells and transgenic mice. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6522–6526. doi: 10.1073/pnas.92.14.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strähle U., Schmid W., Schütz G. Synergistic action of the glucocorticoid receptor with transcription factors. EMBO J. 1988 Nov;7(11):3389–3395. doi: 10.1002/j.1460-2075.1988.tb03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey W. P., Schaufele F., Heslewood M., Handford C., Reudelhuber T. L., Catanzaro D. F. Distance-dependent interactions between basal, cyclic AMP, and thyroid hormone response elements in the rat growth hormone promoter. J Biol Chem. 1993 Jul 15;268(20):14906–14911. [PubMed] [Google Scholar]
- Umesono K., Evans R. M. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell. 1989 Jun 30;57(7):1139–1146. doi: 10.1016/0092-8674(89)90051-2. [DOI] [PubMed] [Google Scholar]
- Underhill T. M., Cash D. E., Linney E. Constitutively active retinoid receptors exhibit interfamily and intrafamily promoter specificity. Mol Endocrinol. 1994 Mar;8(3):274–285. doi: 10.1210/mend.8.3.8015546. [DOI] [PubMed] [Google Scholar]
- Yao T. P., Forman B. M., Jiang Z., Cherbas L., Chen J. D., McKeown M., Cherbas P., Evans R. M. Functional ecdysone receptor is the product of EcR and Ultraspiracle genes. Nature. 1993 Dec 2;366(6454):476–479. doi: 10.1038/366476a0. [DOI] [PubMed] [Google Scholar]
- Yao T. P., Segraves W. A., Oro A. E., McKeown M., Evans R. M. Drosophila ultraspiracle modulates ecdysone receptor function via heterodimer formation. Cell. 1992 Oct 2;71(1):63–72. doi: 10.1016/0092-8674(92)90266-f. [DOI] [PubMed] [Google Scholar]