SUMMARY
Elevated bile acid levels increase hepatocellular carcinoma by unknown mechanisms. Here we show that mice with a severe defect in bile acid homeostasis due to loss of the nuclear receptors FXR and SHP have enlarged livers, progenitor cell proliferation, YAP (Yes Associated Protein) activation, and develop spontaneous liver tumorigenesis. This phenotype mirrors mice with loss of hippo kinases or overexpression of their downstream target YAP. Bile acids act as upstream regulators of YAP via a novel pathway dependent on induction of the scaffold protein Iqgap1. Patients with diverse biliary dysfunctions exhibit enhanced Iqgap1 and nuclear YAP expression. Our findings reveal an unexpected mechanism for bile acid regulation of liver growth and tumorigenesis via the Hippo pathway.
INTRODUCTION
Hepatocellular Carcinoma (HCC) is a leading cause for cancer mortality with poor prognosis and very little effective chemotherapy options. Several lines of evidence indicate bile acids (BAs) as promoters of hepatocarcinogenesis (Kitazawa et al., 1990; Tsuda et al., 1988; Yang et al., 2007). BAs are produced in the liver to facilitate absorption of lipids and lipid soluble nutrients from the intestine (Hofmann, 1999; Russell, 2003). BAs also function as signaling molecules and play important roles in liver regeneration (Huang et al., 2006) as well as tumor promotion (Kim et al., 2007). As detergents, they are potentially cytotoxic, and their concentrations are tightly regulated at several levels including a negative feedback loop involving the nuclear receptors Farnesoid X Receptor (FXR) and Small Heterodimer Partner (SHP) (Goodwin et al., 2000). Targeted deletion of both of these receptors, but not either individually, leads to marked elevation in hepatic BA levels and liver injury (Anakk et al., 2011). The development of spontaneous liver tumors in our Fxr−/− ;Shp−/− double knock out (DKO) mouse model which have chronically elevated BA levels enabled us to study the mechanisms that underlie BA dependent tumor promotion.
The mammalian Hippo pathway includes the serine/threonine kinase Mst1/2, which phosphorylates and activates the downstream kinase Lats1/2, and their regulators Mob1A/B and Sav1. Lats1/2 phosphorylation of the transcriptional co-activators YAP and TAZ causes them to be retained in the cytoplasm, inhibiting their ability to drive proliferation. Hippo signaling is critical in regulating liver size (Cai et al., 2010; Camargo et al., 2007; Dong et al., 2007; Lee et al., 2010; Song et al., 2010) and intestinal regeneration (Cai et al., 2010; Karpowicz et al., 2010; Staley and Irvine, 2010). Notably, down regulation of Mst1/2 or over expression of YAP in mouse liver results in hepatocellular carcinoma (HCC) (Cai et al., 2010; Dong et al., 2007). While the core components of this pathway are well defined, its upstream regulators are still being sought after. Cell-cell contact suppresses the pathway via factors including atypical cadherins (Hamaratoglu et al., 2006), α-catenin (Schlegelmilch et al., 2011) and the apical adaptor proteins Kibra-Expanded and Merlin (Cai et al., 2010; Genevet et al., 2010; Grusche et al., 2010; Hamaratoglu et al., 2006). Decreased cell density or increased extracellular matrix stiffness were shown to increase nuclear localization of YAP and TAZ (Dupont et al., 2011; Schlegelmilch et al., 2011). Recently GPCRs have been suggested as upstream regulators of the Hippo pathway in mammalian cells (Mo et al., 2012; Yu et al., 2012).
The increased liver size, hepatocyte proliferation and subsequent development of spontaneous HCC in DKO mice strongly resembled the phenotype of mammalian Hippo pathway Mst1/2 liver specific double knockouts(Anakk et al., 2011; Lee et al., 2010; Lu et al., 2010; Song et al., 2010). Consistent with this overlap we found that elevated BA levels are sufficient to activate YAP in both livers and isolated hepatocytes, and identified induction of the scaffolding protein IQGAP1 as a key intermediate in this process.
RESULTS
Fxr−/−; Shp−/− double knock out (DKO) mice phenocopy Mst1/2 liver specific knockouts
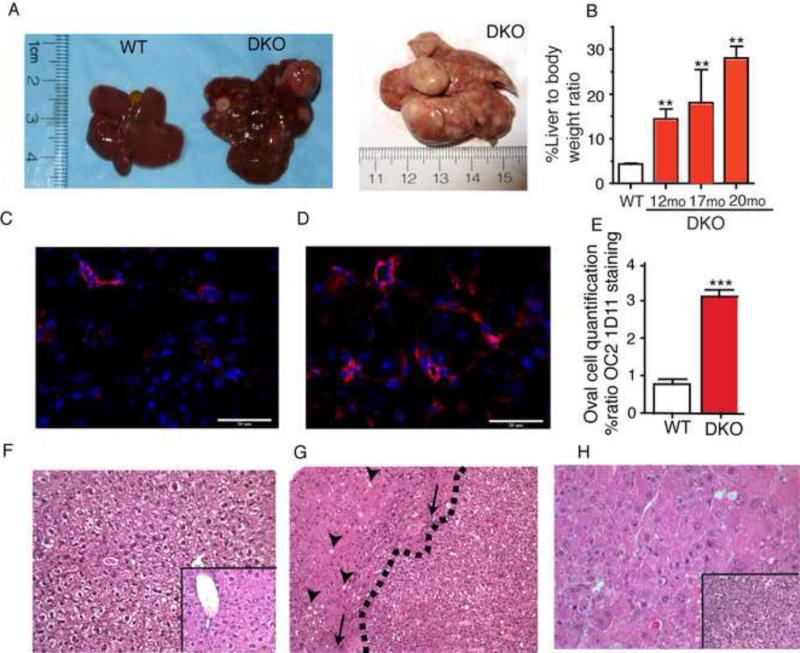
Combined loss of FXR and SHP results in early onset cholestasis (Anakk et al., 2011). In accord with the tumor promoting effects of BAs, we observed spontaneous hepatic tumorigenesis in year old DKO mice, which are maintained on the tumor resistant C57/B6 mouse background (Figure 1A). DKO mice develop tumor nodules as early as 9 months and by 12 months we can see well-developed adenomas that spread through the entire liver between 15-17 months of age (Figure 1B). Additionally, OC2-1D11, a marker for hepatic progenitor/oval cell population is dramatically increased in DKO mice compared to WT mice as early as 8-10 weeks of age (Figures 1C-1E). Histological analysis of DKO tumors revealed heterogeneous hepatocyte populations with injury, cholangiofibrosis, and multiple adenomas that can progress to hepatocellular carcinoma (Figure. 1F-H) consistent with what is observed in HCC patients (Llovet et al., 2003).
Figure 1. FXR/SHP double knockout (DKO) develop spontaneous hepato cellular carcinoma and phenocopy Mst1/2 double knockout (Mst1/2−/−) mice.
A. Gross liver examination shows presence of multiple tumor nodes with 100% penetrance compared to tumor free WT mice (n=20). B. Progressive tumor growth is evident from increasing liver to bodyweight ratio of DKO mice with age. C-D Hepatic oval cell marker, OC21D11 (red) shows increased staining in 8-10 week old DKO (right) compared to WT (left) mice and its quantification is shown in E, DAPI in blue stains the nuclei (n=4-6). F-H H&E staining of a year old normal liver WT (F) and DKO (G&H). DKO mice develop adenomas, whose growth rate exceeds the adjacent host liver (G), and Hepato Cellular Carcinoma, with marked nuclear atypia and mitotic activity (H); the inset shows cholangiofibrosis, versus the normal liver. Arrowheads show fat accumulation, arrows point to focal inflammation and dotted line demarcates the adenoma boundary from the injured liver tissue. Magnification X120 and insets are X250. *P<0.05, **P<0.001 compared to WT.
These results revealed a striking overlap of DKO and Mst1/2 knockout phenotypes including an increase in the hepatic progenitor/oval cell population and aggressive liver tumors (Lu et al., 2010; Song et al., 2010). This overlap was reinforced by the observation that Mst1/2 double knockout mice also accumulated hepatic BAs (Supplementary Figure. 1A-1C).
YAP is activated in the livers of DKO mice
Consistent with the above observation, microarray analysis revealed a significant up-regulation of YAP targets, including Connective Tissue Growth Factor (CTGF), Cyclin D1, and Osteopontin (SPP1) with no change in YAP RNA in DKO livers (Figure. 2A) and these responses were validated at the protein level (Figure. 2B). Unlike in DKO mice, YAP activation was not observed in individual FXR and SHP knockouts (Supplementary Table. 1, Supplementary Figure. 2A-2D). Moreover, a role for FXR in YAP activation is ruled out by the observation that WT mice treated with the synthetic FXR agonist GW4064, resulted in the expected induction of SHP and BSEP expression (data not shown), but did not change inactive phospho-YAP levels (Supplemental Fig. 2C-2D). These results clearly indicate that activation of YAP in the DKO livers is FXR and SHP independent.
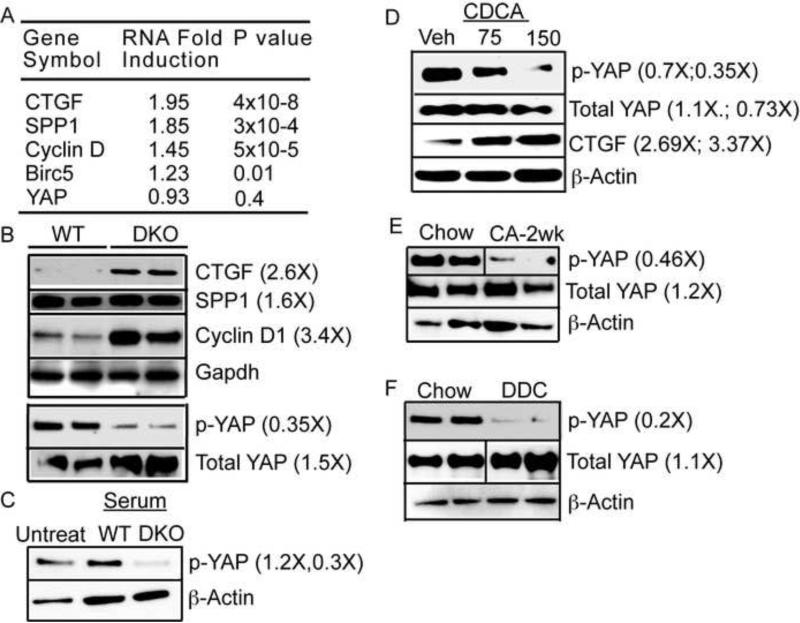
Figure 2. Bile acid overload is sufficient to induce YAP activation.
A. Gene expression analysis in DKO mice indicates induction of YAP targets. B. Immunoblot confirms reduced levels of inactive phospho YAP and activation of its targets in 5-8 weeks old DKO animals compared to WT. C. WT primary hepatocytes upon treatment with DKO serum display YAP activation, which can be mimicked with bile acid, chenodeoxycholic acid (CDCA). CDCA induces YAP target CTGF (D). WT mice fed 1% CA diet (E) or 0.1% DDC diet (F) for 2 weeks display YAP activation compared to chow fed animals. Densitometry was performed on immunoblots for n=3-5 animals per group and fold differences in comparison to control WT are written in parenthesis.
Next, we hypothesized that the YAP activation is secondary to the sharply elevated BA levels observed in the DKO mice (Supplementary Figure 3), rather than a cell autonomous effect of the loss of FXR and SHP. To test this hypothesis, we cultured WT primary hepatocytes in media containing serum from either WT or DKO mice. We observed a decrease in inactive phospho-YAP only in response to DKO serum treatment, indicating that an extrinsic circulating factor was responsible for the activation of YAP (Figure 2C). Further, treatment of WT primary hepatocyte with the DKO serum pre-incubated with the BA binding resin cholestyramine reduced DKO BA levels by 50% and increased inactive phospho-YAP levels (Supplemental Figure 4A-B). These experiments indicated BAs as putative upstream regulators of YAP activation.
High concentration of bile acids activate YAP signaling
To directly examine whether elevated BAs function as the extrinsic signal that causes YAP activation, WT primary hepatocytes and the liver cell line AML12 were treated with varying concentrations of the relatively hydrophobic BA, chenodeoxycholic acid (CDCA), which does not require transporters to enter the cell. YAP was activated and its target CTGF was induced in a dose dependent manner by CDCA at 150μM (Figure. 2D, Supplemental Figure. 4C-D). To determine whether this YAP activation is a consequence of possible CDCA toxicity we performed TUNEL staining. We find that 150μM CDCA overnight treatment had only a modest impact on cell death compared to vehicle in WT primary hepatocytes (Figure 3A-3E).
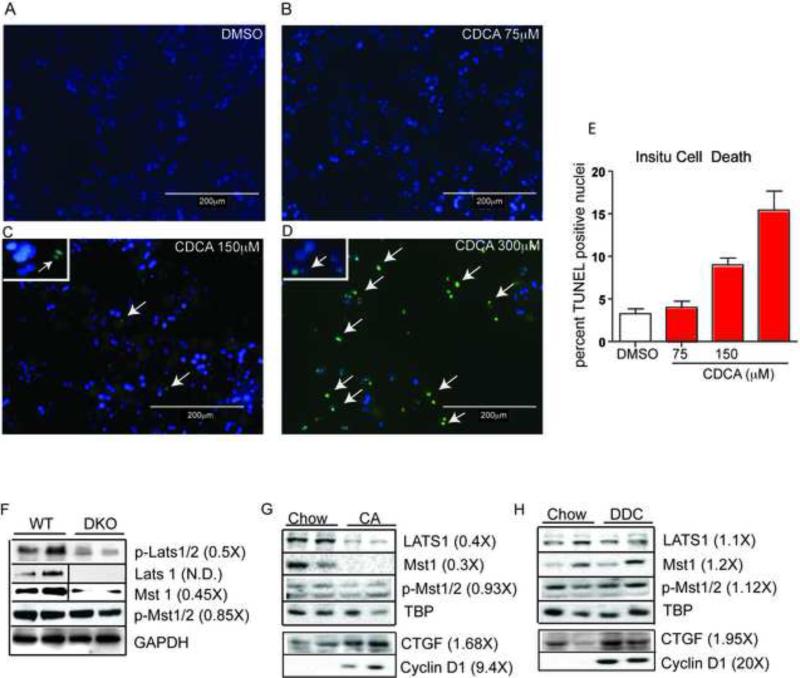
Figure 3. BA overload does not cause overt cell death but alters canonical hippo signaling.
Primary hepatocytes were cultured and treated with DMSO (A) and varying doses of CDCA (B-D) overnight. Cells were fixed and TUNEL positive green staining nuclei (marked with white arrows) were counted at least in ten different fields for each of the treatment groups to quantify cell death (E). We performed this experiment in triplicates. **P<0.001 when compared to WT, CDCA 75 and *P<0.01 when compared to CDCA 150. Magnification X120. Immunoblots for hippo pathway kinases were performed in DKO (F), WT animals treated with either 1% CA (G) or 0.1% DDC (H) for 2 weeks. Hippo kinases were altered only in DKO and in CA fed mice with no changes in DDC fed WT animals. However, YAP targets CTGF and Cyclin D1 were induced upon CA and DDC treatment clearly indicating YAP activation in these cholestatic mouse models.
We then utilized two different dietary models to increase BA levels in vivo to confirm the ability of BAs to dysregulate Hippo signaling in mice. Naïve WT mice were fed diets supplemented with either 1% cholic acid (CA), a primary BA that is relatively well tolerated by mice, or 0.1% 3,5-diethoxycarbonyl-1,4-dihydroxychollidine (DDC), an inhibitor of heme biosynthesis that induces both BA overload and oval cell proliferation (Lee et al., 2010; Wang et al., 2003). Elevated BA levels in both of these models were sufficient to robustly decrease inactive phospho-YAP (Figure 2E-2F).
Overall, these results indicate that normal physiological concentration of BAs do not activate YAP, rather this activation is dependent on pathological BA overload achieved in the cell-based models, and in DKO mice or in cholestatic models.
As an initial approach to identify the mechanism underlying the observed YAP activation, we examined the canonical hippo pathway proteins. Key hippo kinases Mst1, Lats1, and phospho-Lats1/2 were all downregulated in DKO mice compared to WT animals (Figure. 4A). Despite the decrease in Mst1 protein levels, we failed to observe any alteration in active phospho-Mst1/2 levels (Figure 3F), and the functional adaptor proteins salvador (sav1) and Mob1 (data not shown). Consistent with this result, Mst1 and Lats 1 levels were reduced upon CA feeding in WT animals (Figure 3G). However, despite activating YAP like the CA fed WT or the DKO mice, DDC diet treated mice did not show any changes in the upstream hippo pathway kinases (Figure 3H). Overall, these results indicate that modulation of the canonical Hippo pathway may be involved in BA-mediated YAP activation, but is not essential.
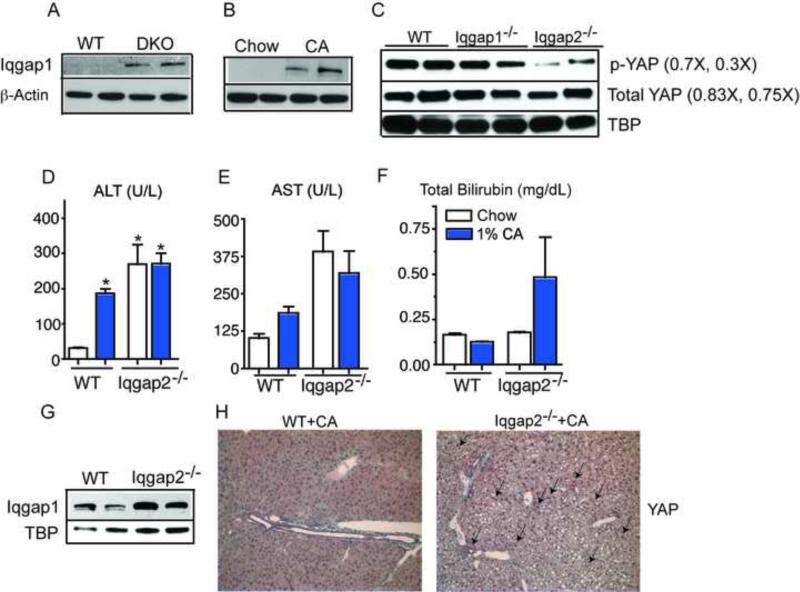
Figure 4. Increased Iqgap1 expression correlates strongly with YAP activation.
Iqgap1 is robustly induced in DKO (A), WT (B) and in Iqgap2−/− animals treated with 1%CA diet (C) compared to their respective controls. (D-F) 1% cholic acid treatment leads to elevated liver enzymes in serum of Iqgap2−/− (G) Immunoblot shows YAP activation in naïve Iqgap2−/− mice. (H) Immunohistochemistry reveals abundant YAP expression, with increased nuclear YAP in Iqgap2KO compared to WT mouse livers after 1%CA diet (Insets). Magnification X120. *P< 0.05; compared to WT mice on normal chow.
Alteration in cell adhesion is central to bile acid mediated YAP activation
Apart from FXR and SHP, BAs can signal through multiple pathways, by GPCR TGR5, by activating kinase cascade including c-Jun N-terminal kinase, JNK (Yu et al., 2005), extracellular signal-regulated kinase ERK (Dent et al., 2005), p38 MAPK (Kurz et al., 2001) and via their detergent action. To systematically address the potential mechanisms by which high levels of BA result in YAP activation, we screened hepatic lysates from DKO and WT mice using a reverse phase protein array with antibodies to 187 signaling molecules, including kinases, adaptors, extracellular matrix proteins and cyclins (Cheng et al., 2005). None of the known BA kinase targets, including p38 MAPK or ERK or JNK pathways were activated instead total ERK and EGFR were down regulated in DKO mouse livers compared to WT. Among the 29 proteins with statistically significant differences in DKO compared to WT livers, (Supplementary Figure 5A), a decrease in the cell adhesion protein E-cadherin (Supplementary Figure 5B) was consistent with a recent report that decreased E-cadherin levels increase YAP activation (Kim et al., 2011). In accord with this, CDCA treatment decreased E-cadherin protein levels in WT primary hepatocytes (Supplementary Figure 5C).
IQGAP1 is induced during biliary overload and promotes hepatic tumorigenesis
IQGAP1 (IQ motif containing GTPase activating protein1) is a key scaffolding protein that interacts with E-cadherin (Kim et al., 2011) and PP2A (Brown and Sacks, 2006). IQGAP1 modulates cell adhesion by regulating E-cadherin and β-catenin (Roy et al., 2005) and IQGAP1 over-expression reduces cellular adhesion by dissociating α-catenin from the cadherin-catenin complex (Kuroda et al., 1998). IQGAP1 is typically expressed at negligible levels in normal liver, however its potential contribution of to hepatocarcinogenesis is evident from the observations that its expression is elevated in HCC (White et al., 2010), and that loss of Iqgap1 on a Iqgap2−/− mouse background blocked development of hepatic tumorigenesis (Schmidt et al., 2008). DKO mice displayed robust induction of IQGAP1 protein compared to WT (Figure 4A), which would decrease cellular adhesion and subsequently trigger YAP activation in the livers of DKO mice. To test if elevated BA levels induce IQGAP1, we fed WT mice a diet enriched in cholic acid, which, remarkably, led to a strong induction of hepatic IQGAP1 in WT mice (Figure 4B). These data link BA mediated IQGAP1 induction to the development of spontaneous liver tumorigenesis.
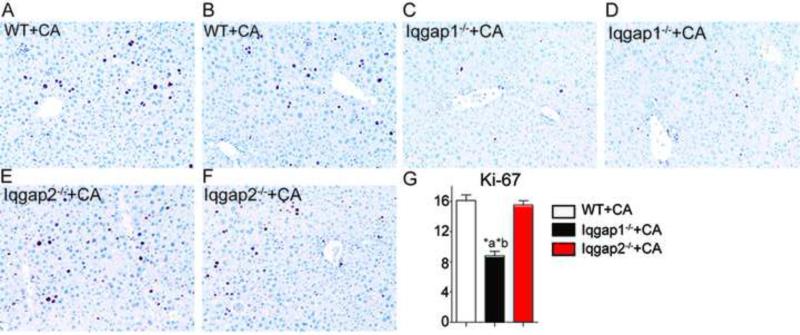
To investigate the connection between IQGAP1 induction and YAP activation, we examined phospho-YAP levels in Iqgap1−/− and Iqgap2−/− mice and found strong YAP activation in Iqgap2−/− (Figure 4C), which have elevated levels of IQGAP1. Further, this activation was blunted in Iqgap1−/− livers. Next, we tested whether IQGAP1 induction was required for BA mediated YAP activation, by challenging Iqgap1−/− and Iqgap2−/− mice with BA overload by feeding them a 1% CA diet. CA diet led to the expected increase of liver enzymes in serum (Figure 4D-4F), IQGAP1 levels (Figure 4G) along with increased nuclear YAP staining in Iqgap2−/− mice when compared to WT mice (Figure 4H). Moreover, hepatocyte proliferation upon CA diet was blunted in Iqgap1−/− mice compared to WT and Iqgap2−/− mice (Figure 5A-5G). These results indicate that IQGAP1 protein induction is crucial to BA mediated YAP activation and hepatocyte proliferation.
Figure 5. Iqgap1 is crucial for bile acid mediated hepatocyte proliferation.
WT (A-B), Iqgap1−/−(C-D) and Iqgap2−/− (E-F) mice were fed 1% CA diet for 2 weeks. Ki-67 staining was performed in these samples to assess cellular proliferation. N= 3-4 mice per group were stained with Ki-67 and the positively stained brown nuclei were quantified (G). *P< 0.001; compared to WT (a) and compared to Iqgap2−/− (b). Magnification used is X120.
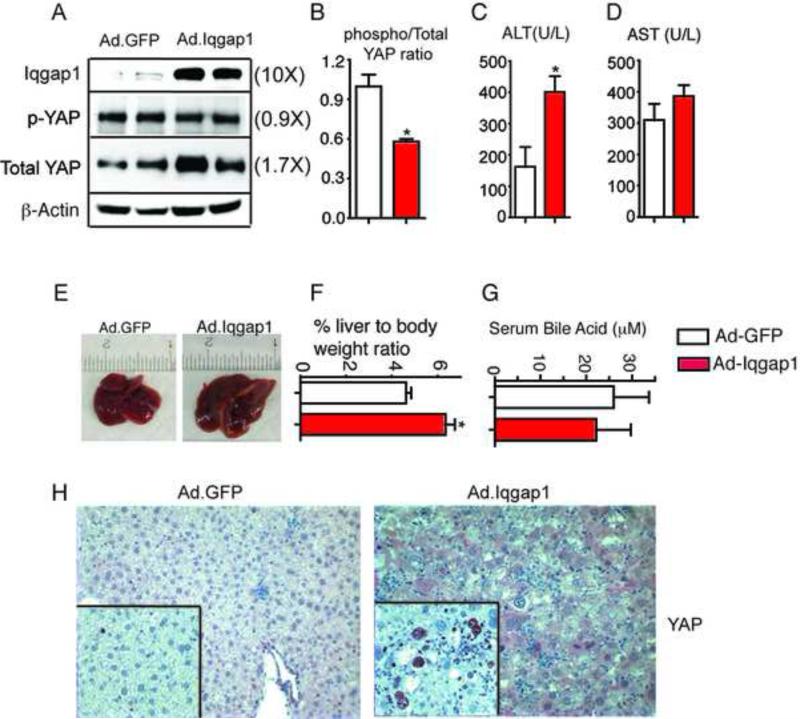
IQGAP1 over expression is sufficient to induce YAP activation
We used adenoviral IQGAP1 over expression to determine whether increased Iqgap1 was sufficient to induce YAP activation. We verified strong hepatic expression of IQGAP1and a robust increase in YAP expression in hepatocytes, which was completely absent in the control Adeno GFP (Figure 6A & 6H). These data clearly show that over expression of Iqgap1 can drive YAP expression and activation in mouse livers. Increased IQGAP1 expression resulted in modest elevation of serum ALT but not AST (Figure 6C-6D) and increased liver size (Figure 6E-6F) with no change in serum BA levels (Figure 6G). In addition, nuclear Ki-67 staining increased upon IQGAP1 over expression (inset Figure. 6H). Overall, we conclude that chronically elevated BA levels induce IQGAP1, and that the elevated IQGAP1 is required for full YAP activation.
Figure 6. IQGAP1 overexpression is sufficient to increase YAP levels and to increase hepatocyte proliferation.
(A). Hepatic Iqgap1 over-expression results in YAP activation, this is quantitated using immunoblots (B). Iqgap1 over-expression for a period of 2 weeks results in slightly elevated serum ALT but not AST levels (C-D) and increased liver size (E-F) with no change in serum bile acid concentration (G). Robust YAP expression is observed in all hepatocytes after Iqgap1 overexpression, which correlates well with the increased proliferation observed with Ki-67 staining (inset). Densitometry on immunoblots for n=3-5 animals per group. Fold differences in comparison to control WT are in parenthesis. Magnification X120 and insets are X250. *P<0.05, compared to WT.
Cholestatic individuals and human liver tumors exhibit high expression of YAP and IQGAP1
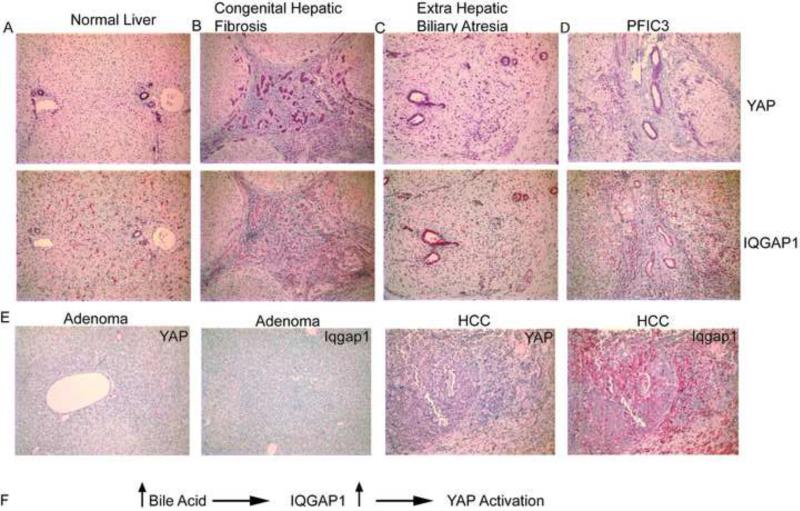
We then examined whether BA mediated activation of Hippo signaling is conserved in human livers. We screened 26 patients ranging in age from 2 weeks to 16 years with diverse biliary diseases resulting in cholestasis and liver cancer. We analyzed both YAP and IQGAP1 expression levels in these liver biopsies and found that cholestatic livers displayed robust YAP staining in hepatocytes compared to normal tissue or diseased livers without bile retention (Figure 7A-7D). YAP was also detected in a few hepatic adenomas with no detectable hepatic IQGAP1 expression whereas YAP and IQGAP1 were robustly expressed in hepatocellular carcinoma (Figure 7E). Further, bile ducts expressed YAP and IQGAP1 in normal livers and in proliferative ducts and ductules, with overall increased expression in the cholestatic situation. It is important to note that bile ducts, which are exposed to high concentration of BAs, maintain IQGAP1 and YAP expression indicating a functional interplay of BAs, IQGAP1 And YAP.
Figure 7. Human biliary disorders exhibit strong YAP and IQGAP1 expression.
A. Normal liver: bile duct epithelium has uniform cytoplasmic expression of both YAP and IQGAP1 proteins. Arterial smooth muscle is also positive. IQGAP1 is also expressed in sinusoidal Kupffer cells. B. 2 year old with congenital hepatic fibrosis: all ducts express IQGAP1 and YAP in cytoplasm and YAP in some nuclei. C. Extrahepatic biliary atresia in 2 week old: In addition to duct and ductular expression of both proteins in the cytoplasm, many ductal, ductular and some hepatocyte nuclei are positive for YAP. D. PFIC3 (Progressive Familial Intrahepatic Cholestasis 3)- The expression pattern is identical to that of extra hepatic biliary atresia. (Original magnifications X125, with purple staining for YAP (top) and red staining for IQGAP1 (bottom). E. Human adenoma and hepatocellular carcinoma samples stained with YAP and IQGAP1. F. Model illustrating how elevated BAs cause YAP activation.
DISCUSSION
Hepatocellular Carcinoma (HCC) is one of the most deadly human cancers (Han, 2012) with poor therapeutic options. It is commonly associated with HBV or HCV infection, but the underlying molecular mechanism is poorly understood. A major barrier to understanding HCC is its inherent heterogeneity with distinct subtypes linked to different potential oncogenic pathways (Lee and Thorgeirsson, 2005; Nault and Zucman-Rossi, 2011). Bile acids are intrinsic signaling molecules in hepatocytes and are elevated in HCC (Tsuboyama et al., 2010) and increase HBV (Kim et al., 2010) and HCV (Chang and George, 2007) expression. BAs are known to promote hepatic tumorigenesis (Shiota et al., 1999; Yang et al., 2007) but how this occurs is not clear. By utilizing the Fxr−/−Shp−/− double knockout mice, which have chronically elevated BA levels, we examined how BAs could function as a tumor promoter.
Our results identify BAs as upstream regulator of Hippo pathway. This pathway and its target YAP have been recently identified as crucial drivers for liver growth and tumorigenesis (Zhou et al., 2009), (Cai et al., 2010; Lu et al., 2010) (Song et al., 2010). Thus we propose that BAs function as tumor promoters by driving YAP activation. The ability of BAs to activate YAP is concentration dependent. Normal physiological or modestly elevated concentrations of BA do not result in YAP activation, however, chronically elevated pathological concentrations of more than 100 μM, which are commonly observed in cholestastic patients (Murphy et al., 1972; Neale et al., 1971), activate YAP, and could promote tumorigenesis. Recently, YAP was shown to be important for bile duct and hepatocyte proliferation after cholestatic injury, which is in accord with our data and supports interplay between BAs and YAP (Bai et al., 2012). Furthermore, the down regulation of both FXR and SHP in all the different stages of HCC (Wolfe et al., 2011; Yang et al., 2007) provides additional clinical relevance for our findings.
Liver tumors occur in single Fxr−/− and Shp−/− mice at 15 months of age (Yang et al., 2007) and the underlying mechanism for tumor formation is either via elevated pro-inflammatory cascade (Wang et al., 2008) or by increased cellular proliferation (Zhang et al., 2008). BA concentrations do not reach more than 100 μM even at 6-9 months of age in Fxr−/− or Shp−/− (Liu et al., 2012) mice, and consistent with that we do not observe YAP activation in these knockouts.
The strong decrease in Lats1 and Mst1/2 levels and a modest decrease in terminal phospho-lats1/2 with unchanged levels of phospho-Mst1/2 in DKO mice show that the canonical Hippo pathway is affected in the DKO mice. However, similar responses were not observed in the DDC fed mice, and may not be essential for YAP activation in response to elevated BA. Non-canonical Hippo/Mst independent activation of YAP was recently described in both Drosophila and mammalian cells (Kim et al., 2013; Yin et al., 2013; Yu et al., 2013). In addition to the potential input of the Hippo pathway, we found that elevated BA levels could activate YAP by disrupting signaling at the plasma membrane. Thus the decrease in E-Cadherin levels in DKO mice concurs with the recent study linking E-cadherin activity to YAP activation (Kim et al., 2011). The signaling scaffold protein IQGAP1, which interacts with E-cadherin to decrease its expression is dramatically up regulated in DKO livers. Interestingly, IQGAP1 also interacts with the protein phosphatase PP2A, which stabilizes the interaction of IQGAP1 with E-cadherin (Takahashi and Suzuki, 2006). We have recently found that PP2A levels are also increased in the DKO livers (Jiang et al., 2013). Increased PP2A activity could also contribute to YAP activation via dephosphorylation at S127 (Schlegelmilch et al., 2011).
In gain of function studies, we demonstrated that overexpression of IQGAP1 in wild type liver is sufficient to activate YAP and induce an acute proliferative response (Figure. 6). We conclude that the induction of IQGAP1 by BAs is necessary for full YAP activation, and can drive YAP expression in the absence of the BA stimulus.
Liver cancer is strongly associated with human cholestatic syndromes (Davit-Spraul et al., 2010; Silveira et al., 2008; Strautnieks et al., 2008), with 15% incidence of HCC or cholangiocarcinoma in progressive familial intrahepatic cholestasis 2 (PFIC2) patients as a consequence of genetic loss in bile salt export pump (Bsep). Increased YAP levels are observed specifically in liver tumors and strongly correlate with poor survival (Xu et al., 2009). Thus maintaining appropriate BA levels early on in cholestasis will not only improve liver pathology but may also prevent subsequent YAP activation and hepatocarcinogenesis.
Methods
Animals
Wild-type (WT) C57BL/6 mice, Fxr−/−, Shp−/− and DKO congenic, age-matched, male mice were used throughout this study. WT control mice were purchased from Harlan Sprague Dawley, Inc. (Indianapolis, IN). Mice were sacrificed at 5 weeks, 8-10 weeks or 12 and 17 months after birth. Control, Iqgap1−/− and Iqgap2−/− age matched male mice were also used in this study. To study BA overload, mice were either fed normal, 1% cholic acid (CA) or 0.1% 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) supplemented chow for a period of two weeks. At the end of the experiment, blood was collected and liver tissue was flash frozen in liquid nitrogen. Mice were housed on a standard 12-hour light/dark cycle and were fed normal chow and water ad libitum. All animal experiments were carried out as outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (National Institutes of Health publication 86-23, revised 1985).
Histology
Liver samples were fixed in 10% formalin or snap frozen in OCT. The formalin fixed sections were used for H&E staining. Frozen liver sections were used to detect oval cell specific marker OC2-1D11 by immunofluorescence as described previously(Dorrell et al., 2008). For quantification of oval cells, three mice per group were used. Random multiple pictures 10-15 per slide were taken using axioplan fluorescent microscope and quantitated using metamorph software. Human liver biopsies were stained using YAP antibody (Epitomics) at 1:400, Iqgap1 antibody (Epitomics) at 1:50 dilution followed by a substrate-chromogen (AEC).
Tissue Bile Acid Analysis
100-mg of frozen liver tissue was weighed and shipped to Metabolon Inc for determining the bile acid levels using LC-MS.
Gene Expression Analysis
Total RNA from liver was prepared according to manufacturer's protocol. (Trizol, Invitrogen). The RNA obtained was further purified using Qiagen columns for microarray analysis. Microarray analysis was performed on the Illumina mouseRefseq-8 Expression platform as previously described(Anakk et al., 2011).
Western Blot Analysis
Protein was extracted from 100 mg of fresh liver with a dounce homogenizer in 10 mM HEPES (pH 7.5), 0.32 M sucrose, 1% SDS, 5 μM MG132, and 5 mM EDTA with protease and phosphatase inhibitors. For Western blot, 30-50μg total protein was used. The membrane was incubated with a dilution of 1:5000 p-YAP (Epitomics) or 1:1000 of CTGF (Abcam), Iqgap1 (Epitomics), total YAP, JNK, p-JNK, Erk, p-Erk, Akt, p-Akt, E-Cadherin, β-actin or 1:300 Cyclin D1, Mst1, Mst2, p-LATS1/2, LATS1, p-Mst1/2 (Cell Signaling) and developed.
Primary Hepatocyte Culture
Mouse hepatocytes were prepared using a modified two-step perfusion technique as previously described(Seglen, 1976). Primary hepatocytes were seeded at 100% confluence on to 6 well plates and treated with DMSO, CDCA (75μM, 150μM), CHAPS (50μM, 100μM, 150μM), LCA (10μM, 30μM), 20% WT or DKO serum for 16-18 hours.
Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling (TUNEL) Assay
Mouse primary hepatocytes were prepared and treated with varying concentration of CDCA as mentioned above. TUNEL (Roche) was performed as described by manufacturer's instructions.
Adenoviral Infection
Full length Iqgap1 cDNA was obtained from Origene Technologies, Rockville, MD and was cloned into pAdenoX expression system from Clone Tech, Mountain View, CA. Positive clones were identified by sequencing. Adenovirus expressing Iqgap1 or GFP was amplified and purified to perform tail vein injection in mice.
Data Analysis
Data was analyzed using one-way ANOVA with posthoc Bonferroni test for comparison of multiple groups or unpaired student's t test for comparison between two groups. *p<0.05 and **p<0.001 when compared to their respective age matched WT controls.
Supplementary Material
HIghlights.
Bile Acids are upstream regulators of Hippo Pathway
Signaling scaffold protein IQGAP1 is induced by bile acid overload
IQGAP1 is sufficient to increase YAP expression
BAs promote hepatocarcinogenesis via IQGAP1 induction and YAP activation
SIGNIFICANCE.
Our current results identify an underlying mechanism for BA induction of hepatocarcinogenesis via YAP activation, which is associated with hepatocellular injury and induction of oval cell proliferation. BA mediated up regulation of Iqgap1 and resultant YAP activation could drive the development of a subset of human hepatocellular carcinomas that have increased levels of YAP (Xu et al., 2009) and a characteristic progenitor cell gene expression signature (Lee et al., 2006). In this scenario, pharmacologically decreasing BA levels in severe cholestasis(Lindor, 2011; Trauner and Halilbasic, 2011) would not only alleviate acute pathologies, but could decrease progression to hepatocellular carcinoma.
Acknowledgements
The authors would like to thank the NIDDK, Digestive Disease Center Morphology Core DK56338, Houston, Texas especially Ms. Angela Major for help with immunohistochemistry. We would also like to thank the proteomics core at UT MD Anderson Cancer Center funded by NCI # CA16672. This study was supported by grants DK068804, DK62434 and CPRIT RP120138 (to DDM), the R.P. Doherty, Jr. Welch Chair in Science (DDM), HD060579 (to RLJ), American Cancer Society Research Scholar Grant RSG-09-033-01-CSM (to VAS) and start up funds from University of Illinois at Urbana-Champaign (SA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None Declared.
REFERENCES
- Anakk S, Watanabe M, Ochsner SA, McKenna NJ, Finegold MJ, Moore DD. Combined deletion of Fxr and Shp in mice induces Cyp17a1 and results in juvenile onset cholestasis. The Journal of clinical investigation. 2011;121:86–95. doi: 10.1172/JCI42846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H, Zhang N, Xu Y, Chen Q, Khan M, Potter JJ, Nayar SK, Cornish T, Alpini G, Bronk S, et al. Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology (Baltimore, Md. 2012;56:1097–1107. doi: 10.1002/hep.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MD, Sacks DB. IQGAP1 in cellular signaling: bridging the GAP. Trends in cell biology. 2006;16:242–249. doi: 10.1016/j.tcb.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes & development. 2010;24:2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Chang KO, George DW. Bile acids promote the expression of hepatitis C virus in replicon-harboring cells. Journal of virology. 2007;81:9633–9640. doi: 10.1128/JVI.00795-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KW, Lu Y, Mills GB. Assay of Rab25 function in ovarian and breast cancers. Methods in enzymology. 2005;403:202–215. doi: 10.1016/S0076-6879(05)03017-X. [DOI] [PubMed] [Google Scholar]
- Dent P, Fang Y, Gupta S, Studer E, Mitchell C, Spiegel S, Hylemon PB. Conjugated bile acids promote ERK1/2 and AKT activation via a pertussis toxin-sensitive mechanism in murine and human hepatocytes. Hepatology (Baltimore, Md. 2005;42:1291–1299. doi: 10.1002/hep.20942. [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell C, Erker L, Lanxon-Cookson KM, Abraham SL, Victoroff T, Ro S, Canaday PS, Streeter PR, Grompe M. Surface markers for the murine oval cell response. Hepatology (Baltimore, Md. 2008;48:1282–1291. doi: 10.1002/hep.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Developmental cell. 2010;18:300–308. doi: 10.1016/j.devcel.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Molecular cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- Grusche FA, Richardson HE, Harvey KF. Upstream regulation of the hippo size control pathway. Curr Biol. 2010;20:R574–582. doi: 10.1016/j.cub.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nature cell biology. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- Han ZG. Functional genomic studies: insights into the pathogenesis of liver cancer. Annual review of genomics and human genetics. 2012;13:171–205. doi: 10.1146/annurev-genom-090711-163752. [DOI] [PubMed] [Google Scholar]
- Hofmann AF. Bile acids, cholesterol, gallstone calcification, and the enterohepatic circulation of bilirubin. Gastroenterology. 1999;116:1276–1277. doi: 10.1016/s0016-5085(99)70042-9. [DOI] [PubMed] [Google Scholar]
- Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science (New York, NY. 2006;312:233–236. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development (Cambridge, England) 2010;137:4135–4145. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Cho HK, Choi YH, Lee KS, Cheong J. Bile acids increase hepatitis B virus gene expression and inhibit interferon-alpha activity. The FEBS journal. 2010;277:2791–2802. doi: 10.1111/j.1742-4658.2010.07695.x. [DOI] [PubMed] [Google Scholar]
- Kim I, Morimura K, Shah Y, Yang Q, Ward JM, Gonzalez FJ. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007;28:940–946. doi: 10.1093/carcin/bgl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Kim M, Lee S, Kuninaka S, Saya H, Lee H, Lee S, Lim DS. cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes. The EMBO journal. 2013;32:1543–1555. doi: 10.1038/emboj.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11930–11935. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa S, Denda A, Tsutsumi M, Tsujiuchi T, Hasegawa K, Tamura K, Maruyama H, Konishi Y. Enhanced preneoplastic liver lesion development under ‘selection pressure’ conditions after administration of deoxycholic or lithocholic acid in the initiation phase in rats. Carcinogenesis. 1990;11:1323–1328. doi: 10.1093/carcin/11.8.1323. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, Izawa I, Nagase T, Nomura N, Tani H, et al. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin- mediated cell-cell adhesion. Science (New York, NY. 1998;281:832–835. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- Kurz AK, Graf D, Schmitt M, Vom Dahl S, Haussinger D. Tauroursodesoxycholate-induced choleresis involves p38(MAPK) activation and translocation of the bile salt export pump in rats. Gastroenterology. 2001;121:407–419. doi: 10.1053/gast.2001.26262. [DOI] [PubMed] [Google Scholar]
- Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, Mikaelyan A, Roberts LR, Demetris AJ, Sun Z, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nature medicine. 2006;12:410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- Lee JS, Thorgeirsson SS. Genetic profiling of human hepatocellular carcinoma. Seminars in liver disease. 2005;25:125–132. doi: 10.1055/s-2005-871192. [DOI] [PubMed] [Google Scholar]
- Lee KP, Lee JH, Kim TS, Kim TH, Park HD, Byun JS, Kim MC, Jeong WI, Calvisi DF, Kim JM, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8248–8253. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindor KD. Farnesoid X receptor agonists for primary biliary cirrhosis. Current opinion in gastroenterology. 2011;27:285–288. doi: 10.1097/MOG.0b013e32834452c8. [DOI] [PubMed] [Google Scholar]
- Liu N, Meng Z, Lou G, Zhou W, Wang X, Zhang Y, Zhang L, Liu X, Yen Y, Lai L, et al. Hepatocarcinogenesis in FXR−/− mice mimics human HCC progression that operates through HNF1alpha regulation of FXR expression. Molecular endocrinology (Baltimore, Md. 2012;26:775–785. doi: 10.1210/me.2011-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, Wang Y, Halder G, Finegold MJ, Lee JS, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs). Genes & development. 2012;26:2138–2143. doi: 10.1101/gad.197582.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GM, Ross A, Billing BH. Serum bile acids in primary biliary cirrhosis. Gut. 1972;13:201–206. doi: 10.1136/gut.13.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nault JC, Zucman-Rossi J. Genetics of hepatobiliary carcinogenesis. Seminars in liver disease. 2011;31:173–187. doi: 10.1055/s-0031-1276646. [DOI] [PubMed] [Google Scholar]
- Neale G, Lewis B, Weaver V, Panveliwalla D. Serum bile acids in liver disease. Gut. 1971;12:145–152. doi: 10.1136/gut.12.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Li Z, Sacks DB. IQGAP1 is a scaffold for mitogen-activated protein kinase signaling. Molecular and cellular biology. 2005;25:7940–7952. doi: 10.1128/MCB.25.18.7940-7952.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annual review of biochemistry. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt VA, Chiariello CS, Capilla E, Miller F, Bahou WF. Development of hepatocellular carcinoma in Iqgap2-deficient mice is IQGAP1 dependent. Molecular and cellular biology. 2008;28:1489–1502. doi: 10.1128/MCB.01090-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen PO. Preparation of isolated rat liver cells. Methods in cell biology. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Shiota G, Oyama K, Noguchi N, Takano T, Ito H, Kawasaki H. Oral administration of cholic acid promotes growth of liver tumors initiated by diethylnitrosamine in rats. International journal of oncology. 1999;15:259–265. doi: 10.3892/ijo.15.2.259. [DOI] [PubMed] [Google Scholar]
- Song H, Mak KK, Topol L, Yun K, Hu J, Garrett L, Chen Y, Park O, Chang J, Simpson RM, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1431–1436. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley BK, Irvine KD. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol. 2010;20:1580–1587. doi: 10.1016/j.cub.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauner M, Halilbasic E. Nuclear receptors as new perspective for the management of liver diseases. Gastroenterology. 2011;140:1120–1125. e1121–1112. doi: 10.1053/j.gastro.2011.02.044. [DOI] [PubMed] [Google Scholar]
- Tsuboyama T, Onishi H, Kim T, Akita H, Hori M, Tatsumi M, Nakamoto A, Nagano H, Matsuura N, Wakasa K, et al. Hepatocellular carcinoma: hepatocyte-selective enhancement at gadoxetic acid-enhanced MR imaging--correlation with expression of sinusoidal and canalicular transporters and bile accumulation. Radiology. 2010;255:824–833. doi: 10.1148/radiol.10091557. [DOI] [PubMed] [Google Scholar]
- Tsuda H, Asamoto M, Kagawa M, Uwagawa S, Inoue K, Inui M, Ito N. Positive influence of dietary deoxycholic acid on development of pre-neoplastic lesions initiated by N-methyl-N-nitrosourea in rat liver. Carcinogenesis. 1988;9:1103–1105. doi: 10.1093/carcin/9.6.1103. [DOI] [PubMed] [Google Scholar]
- Wang L, Han Y, Kim CS, Lee YK, Moore DD. Resistance of SHP-null mice to bile acid-induced liver damage. The Journal of biological chemistry. 2003;278:44475–44481. doi: 10.1074/jbc.M305258200. [DOI] [PubMed] [Google Scholar]
- Wang YD, Chen WD, Wang M, Yu D, Forman BM, Huang W. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology (Baltimore, Md. 2008;48:1632–1643. doi: 10.1002/hep.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CD, Khurana H, Gnatenko DV, Li Z, Odze RD, Sacks DB, Schmidt VA. IQGAP1 and IQGAP2 are reciprocally altered in hepatocellular carcinoma. BMC gastroenterology. 2010;10:125. doi: 10.1186/1471-230X-10-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe A, Thomas A, Edwards G, Jaseja R, Guo GL, Apte U. Increased activation of the Wnt/beta-catenin pathway in spontaneous hepatocellular carcinoma observed in farnesoid X receptor knockout mice. The Journal of pharmacology and experimental therapeutics. 2011;338:12–21. doi: 10.1124/jpet.111.179390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, Zender L, Lowe SW, Poon RT, Luk JM. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576–4585. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer research. 2007;67:863–867. doi: 10.1158/0008-5472.CAN-06-1078. [DOI] [PubMed] [Google Scholar]
- Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. Spatial Organization of Hippo Signaling at the Plasma Membrane Mediated by the Tumor Suppressor Merlin/NF2. Cell. 2013;154:1342–1355. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Wang F, Jin C, Huang X, McKeehan WL. Independent repression of bile acid synthesis and activation of c-Jun N-terminal kinase (JNK) by activated hepatocyte fibroblast growth factor receptor 4 (FGFR4) and bile acids. The Journal of biological chemistry. 2005;280:17707–17714. doi: 10.1074/jbc.M411771200. [DOI] [PubMed] [Google Scholar]
- Yu FX, Mo JS, Guan KL. Upstream regulators of the Hippo pathway. Cell cycle. 2012;11:4097–4098. doi: 10.4161/cc.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhang Y, Park HW, Jewell JL, Chen Q, Deng Y, Pan D, Taylor SS, Lai ZC, Guan KL. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes & development. 2013;27:1223–1232. doi: 10.1101/gad.219402.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu P, Park K, Choi Y, Moore DD, Wang L. Orphan receptor small heterodimer partner suppresses tumorigenesis by modulating cyclin D1 expression and cellular proliferation. Hepatology (Baltimore, Md. 2008;48:289–298. doi: 10.1002/hep.22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, Lauwers GY, Thasler W, Lee JT, Avruch J, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.